Abstract
Objective
A better understanding of the current characteristics of clinical trials in oral squamous cell carcinoma (OSCC) is important to improve trial design and identify neglected areas of research. The objective is to summarize the current characteristics and publication status of OSCC trials registered on ClinicalTrials.gov.
Methods
The ClinicalTrials.gov database was searched on November 9, 2020, for trials containing the term “oral squamous cell carcinoma”. We assessed the characteristics of the included trials and searched the publication status of the primary completed trials by using PubMed and Google Scholar.
Results
Our search identified 325 studies for analysis, including 279 interventional (85.8%) and 46 observational (14.2%) studies. Interventional studies were mostly single-group (50.9%), single-center (57.0%), non-randomized (59.1%), open-label (81.4%), and early-phase (70.6%) trials. The vast majority of the studies (82.8%) focused on the treatment of OSCC. Chemotherapy (36.8%) was the most common treatment, followed by targeted therapy (15.2%) and immunotherapy (15.2%). Furthermore, 58.9% of the primary completed interventional studies were published by January 8, 2021, and the median time to publication was 48.0 months. Studies registered before this study began (HR = 1.69, 95% CI = 1.02–2.80, P = .040) and in phases 1/2 or 2 (HR = 2.10, 95% CI = 1.09–4.06, P = .026) were associated with an improved likelihood of publication.
Conclusions
OSCC clinical trials usually have small sample size and are open-label and non-randomized studies. Advanced OSCC and immunotherapy-oriented therapy are becoming the focus of current trials. Researchers should perform standardized registration and publish their results in a timely manner.
Keywords: oral squamous cell carcinoma, OSCC, clinicaltrials.gov, publication status, immunotherapy
Introduction
Approximately 880 000 patients worldwide develop head and neck cancer each year, and approximately 440 000 will die. 1 Head and neck cancer is the eighth most common tumor worldwide in 2020,1,2 among which oral squamous cell carcinoma (OSCC) is the most common malignant tumor in the oral and maxillofacial region.3,4 It differs from other head and neck cancers in its epidemiology, clinical characteristics, and treatment therapy. OSCC originates in the alveolar ridge, buccal mucosa, floor of the mouth, upper jaw, tongue, and other parts of the oral cavity. 2 The development of OSCC is related to lifestyle habits such as smoking, excessive drinking, and chewing betel nuts. Although researchers believe that there is a high correlation between head and neck cancer and HPV infection, only approximately 25% of OSCC patients are HPV positive. 5 The global incidence of OSCC is 3.90 per 100 000, while the global mortality of oral cancer is 1.94 per 100 000.6,7 The 5-year overall survival rate for OSCC related to carcinogens is no more than 60%. 8 Except for some early-stage OSCC, which is only treated with surgery, the treatment of most OSCC cases requires a multi-modal approach. The improvement of surgery, the application of radiotherapy and chemotherapy, and the research on emerging immunotherapy are increasingly permeating trial design and clinical trial endpoints. With the advancement of immunotherapy and combination therapy strategies of radiotherapy and chemotherapy, the prognosis of OSCC has greatly improved. 4 However, immunotherapy represented by immune checkpoint blockade is currently only effective for a small number of patients, and there are still a large number of OSCC patients whose treatment options are limited. 5
Clinical trials have been the foundation of evidence-based medicine and the driving force in the development of medicine. 9 The U.S. Food and Drug Administration Modernization Act (FDAMA) created ClinicalTrials.gov in 1997. FDAMA required the U.S. Department of Health and Human Services (HHS), through the National Institutes of Health (NIH), to establish a registry of clinical trials to provide the public with easy access to clinical trial information. NIH and the U.S. Food and Drug Administration (FDA) worked together to develop the site, which was made available to the public in February 2000. 10 In 2004, the International Committee of Medical Journal Editors (ICMJE) advocated that clinical trials should be recorded in a public registry before recruiting patients to ensure the transparency of clinical research.9,11 The website is currently the world’s largest clinical trial registration website, including almost all interventional human trials conducted in the United States as well as international studies from 185 countries. 10
Although there have been some studies summarizing the characteristics of cancer clinical trials,9,12 there is currently no formal or latest OSCC clinical trial evaluation. At present, doctors still lack a thorough understanding of OSCC clinical research. Therefore, in view of the need for better and more effective clinical trials, it is necessary to clarify the current problems in OSCC clinical trials to guide future trial design. We investigated OSCC clinical trials registered on ClinicalTrials.gov to gain a deeper understanding. The purpose is to (1) summarize the current status and trends of ongoing OSCC trials; (2) propose the focus of future OSCC clinical trial design; and (3) identify and prioritize the weak areas of OSCC research.
Methods
Searching the ClinicalTrials.gov Database
We performed a search on the ClinicalTrials.gov database on November 9, 2020, using the search term “oral squamous cell carcinoma”. All of the available results were downloaded in the form of a csv file and then imported into a database to facilitate further data filtering, classification, and management. After reviewing the trial summary, studies that were non-OSCC relevant were excluded. The remaining studies were selected for further manual classification analysis.
Study Variables of the Registered Studies
Based on the information provided by the ClinicalTrial.gov database, the following variables of the registered studies were categorized by 2 investigators independently: recurrent or metastatic OSCC (no/yes), disease clinical stages, register (before study start/after study start), sample size/enrollment (≤100/>100), age (without child/with child), study design of observational studies (case-only/cross-sectional/case–control/cohort), study design of interventional studies (single arm/parallel/sequential/factorial/crossover), countries where the study was carried out (US or Canada/European/Asian/Others), centers (single-center/multi-center), and funder (industry/NIH/others). For interventional studies, there additional variables were considered: purpose (treatment/others), treatment types, phases (phase 1/phase 1/2 or 2/phase 2/3 or 3 or 4/NA), blind (open label/blind), and randomized (no/yes). If the country, location, or sponsor contained subjects equal to or greater than 2, the study was assigned to the multi-center category. In the event of an industry being listed as the main investor, the study was classified as being funded by the industry. By the same principle, the other studies can be divided into the corresponding categories. Primary outcomes were classified into biological (including biomarker, gene, pharmacokinetic (PK), pharmacodynamics (PD), and apoptosis), safety (including dose limited toxicity (DLT), maximum tolerance dose (MTD), and safety), short-term effect (including tumor response rate (TRR), local response rate (LRR), and disease control rate (DCR)), long-term effect (including survival time and rate), and other outcomes (including mucositis, oral function, infection, quality of life, and feasibility). The time to primary completion was defined as the interval between the study initiation point and the accomplishment of the primary endpoint. The study duration was defined as the time interval between the study initiation point and the completion of the entire study.
OSCC is divided into disease types: Only OSCC, OSCC-precancerous lesions-epithelial hyperplasia, Multi-HNSCC (head and neck squamous cell carcinoma) (including patients with OSCC and oropharyngeal squamous cell carcinoma), and Multi-Cancer (including patients with other kinds of cancer, but not limited to OSCC). Clinical stages of disease were determined according to the eighth edition of the American Joint Committee on Cancer (AJCC) TNM (tumor, nodes, and metastases) classification system. 13 We categorized the type of treatment as follows: chemotherapy, radiotherapy, surgery, combination therapy, immunotherapy (including PD-1, PD-L1, CTLA-4 blockade, CAR-T therapy, and vaccine therapy), and targeted therapy (defined as treatment that attacks specific features of cancer cells, excluding those whose main mechanism is through immune stimulation, such as PD-1, PD-L1, and CTLA-4 blockade).14,15 An intervention that cannot fit into these classifications was classified as others.
Searching for the Publication Status
We used a standardized scheme to search for peer-reviewed publications of studies under primary completion. First, two investigators independently reviewed the “publications” field in the ClinicalTrials.gov database to search for possible matching publications. Then, PubMed and Google Scholar were searched using NCT numbers or the main titles of the clinical trials. By comparing the study characteristics, published articles that matched the registered information on ClinicalTrials.gov were screened out. Study protocols, commentaries, interim analysis, and other non-relevant publication types were excluded. If multiple publications were obtained from a study, the earliest article reporting the primary outcome was selected. Another investigator independently confirmed the publication search for the studies that were found to be unpublished. Differences were resolved by consensus. For each published article, the publication date, sample size, primary results (negative/positive), impact factor (IF, <5/5-9.99/≥10), power calculation (yes/no), and analysis method description (yes/no) were collected. We then compared the results registered on ClinicalTrials.gov and the published results to confirm whether discrepancies existed. The retrieval of publication status was constantly updated until January 8, 2021. The time to publication was defined as the time from the date of study primary completion to the date of study publication or the date of last search.
Statistical Methods
The study search, variable extraction, classification, and publication search were independently conducted by two investigators. The kappa scores of the variables between the reviewers ranged from .5 to 1.0. The differences were resolved by consensus with the third investigator. The number (percentage) for categorical variables and median (interquartile range [IQR]) for continuous variables were calculated. Fisher’s exact tests were used to compare the categorical variables between the different groups. Mann–Whitney U tests were used to compare the continuous variables. Cox regression analysis was performed to analyze the influential factors of time to publication. The hazard ratios (HRs) and 95% confidence intervals (CIs) for the factors were calculated. The variables with P < .05 in the univariate analysis were included in the multi-variate model. The time to publication was estimated using the Kaplan–Meier method.
All statistical tests were performed using Stata/MP version 14.0 (Stata Corporation LP, College Station, TX, USA), and a two-sided P < .05 was considered statistically significant.
Results
Distribution of Oral Squamous Cell Carcinoma Relevant Trials on ClinicalTrials.gov
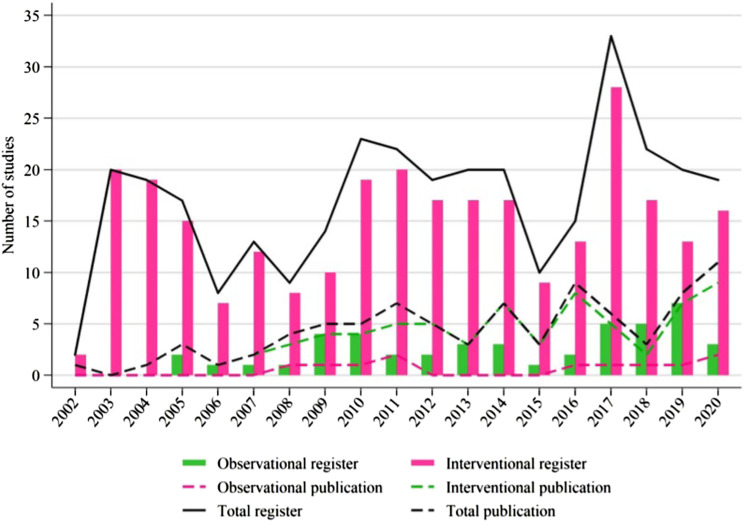
Our search identified a total of 330 studies. Five non-OSCC-relevant studies were excluded, and the remaining 279 interventional (85.8%) and 46 observational (14.2%) studies were included in the analysis. The distribution of interventional and observational studies by registered year is summarized in Figure 1. Overall, the number of registered studies has fluctuated obviously since 2003. More than 15 studies were registered in most years, and the number increased more than 30 in 2017. The number of registered interventional studies fluctuated in the same way as the total number of studies. Observational studies began in 2005 and fluctuated below 5 studies in most years.
Figure 1.
Study distributions of register and publication for observational and interventional studies according to the registered or published years.
General Characteristics of Oral Squamous Cell Carcinoma-Related Clinical Trials
The comparisons of study characteristics between interventional and observational studies are shown in Table 1. Compared to observational studies, interventional studies had longer duration of primary completion and longer duration of study completion (P < .05). More interventional studies were registered before study start, were concentrated more on Multi-HNSCC and advanced patients, and were more often conducted in the US/Canada at multiple centers and funded by the NIH/industry (P < .05). These studies had more results available and were characterized by smaller sample sizes that included fewer children (P < .05). In recent years, more studies have focused on Only OSCC patients (Supplementary Figure 1).
Table 1.
Comparisons of Study Characteristics Between Interventional and Observational Studies.
| Interventional Studies (n=279) | Observational Studies (n=46) | P-value | |
|---|---|---|---|
| Study status | .192 | ||
| Ongoing | 96 (34.4) | 17 (37.0) | |
| Completed | 109 (39.1) | 17 (37.0) | |
| Terminated | 34 (12.2) | 2 (4.3) | |
| Withdrawn | 18 (6.5) | 2 (4.3) | |
| Unknown status | 22 (7.9) | 8 (17.4) | |
| Duration of study completed (mo.) | 65.1 (38.0, 89.9) | 19.0 (8.0, 59.0) | .001 |
| Primary completed | .526 | ||
| No | 155 (55.6) | 28 (60.9) | |
| Yes | 124 (44.4) | 18 (39.1) | |
| Duration of primary completed (mo.) | 39.5 (26.6, 64.0) | 11.0 (5.1, 43.0) | .001 |
| Results of primary completed studies | .045 | ||
| No available results | 90 (72.6) | 17 (94.4) | |
| Had results | 34 (27.4) | 1 (5.6) | |
| Register before study start | .016 | ||
| No | 153 (54.7) | 34 (71.7) | |
| Yes | 126 (45.3) | 12 (28.3) | |
| Sample size | .037 | ||
| ≤100 | 222 (79.6) | 30 (65.2) | |
| >100 | 57 (20.4) | 16 (34.8) | |
| Age | .004 | ||
| Only adults | 263 (94.3) | 37 (80.4) | |
| Adults and children | 16 (5.7) | 9 (19.6) | |
| Disease type | <.001 | ||
| Only OSCC | 59 (21.1) | 21 (45.7) | |
| OSCC and precancerous lesions and epithelial hyperplasia | 12 (4.3) | 12 (26.1) | |
| Multi-HNSCC | 191 (68.5) | 12 (26.1) | |
| Multi-cancers | 17 (6.1) | 1 (2.2) | |
| Recurrent or metastatic cancer | .001 | ||
| No | 194 (69.5) | 42 (91.3) | |
| Yes | 85 (30.5) | 4 (8.7) | |
| Disease clinical stage | <.001 | ||
| I/II/I–II | 17 (6.1) | 1 (2.2) | |
| I–III/I–IV/II–IV | 95 (34.1) | 28 (60.9) | |
| III/III–IV/IV | 117 (41.9) | 3 (6.5) | |
| NA | 50 (17.9) | 14 (30.4) | |
| Study design | |||
| Single arm | 142 (50.9) | — | |
| Parallel | 122 (43.7) | — | |
| Sequential | 11 (3.9) | — | |
| Factorial/crossover | 4 (1.4) | — | |
| Basic | 3 (6.5) | ||
| Case-only | 6 (13.0) | ||
| Case–control | 12 (26.1) | ||
| Cohort | 23 (50.0) | ||
| Diagnostic | 2 (4.3) | ||
| Time series | |||
| Retrospective | 8 (17.4) | ||
| Cross-sectional | 8 (17.4) | ||
| Prospective | 279 (100.0) | 30 (65.2) | |
| Purpose | |||
| Treatment | 231 (82.8) | — | |
| Others a | 48 (17.2) | — | |
| Treatment | |||
| Radiotherapy | 15 (6.5) | — | |
| Chemotherapy | 85 (36.8) | — | |
| Surgery | 16 (6.9) | — | |
| Targeted therapy | 35 (15.2) | — | |
| Immunotherapy | 35 (15.2) | — | |
| Combination therapy | 30 (13.0) | — | |
| Others | 15 (6.5) | — | |
| Multi-center | <.001 | ||
| No | 159 (57.0) | 39 (84.8) | |
| Yes | 120 (43.0) | 7 (15.2) | |
| Phase | |||
| Phase 1 | 77 (27.6) | — | |
| Phase 1/2 or 2 | 120 (43.0) | — | |
| Phase 2/3 or 3 or 4 | 37 (13.3) | — | |
| NA | 45 (16.1) | — | |
| Blind | |||
| Open label | 227 (81.4) | — | |
| Blind | 52 (18.6) | — | |
| Randomized | |||
| No | 165 (59.1) | — | |
| Yes | 114 (40.9) | — | |
| Primary outcome | |||
| Biomarker/gene/PK/PD/apoptosis | 27 (9.7) | — | |
| DLT/MTD/safety | 58 (20.8) | — | |
| TRR/LRR/DCR | 57 (20.4) | — | |
| Survival | 92 (33.0) | — | |
| Others b | 45 (16.1) | — | |
| Country | <.001 | ||
| US/Canada | 201 (72.0) | 14 (30.4) | |
| European | 42 (15.1) | 12 (26.1) | |
| Asian | 24 (8.6) | 12 (26.1) | |
| Others | 12 (4.3) | 8 (17.4) | |
| Funder | <.001 | ||
| Industry | 69 (24.7) | 2 (4.3) | |
| NIH | 104 (37.3) | 5 (10.9) | |
| Others | 106 (38.0) | 39 (84.8) | |
| Publication of primary completed studies | 1.000 | ||
| No | 51 (41.1) | 7 (38.9) | |
| Yes | 73 (58.9) | 11 (61.1) | |
| Primary results of published studies | .277 | ||
| Negative | 20 (27.4) | 1 (9.1) | |
| Positive | 53 (72.6) | 10 (90.9) | |
| Impact factors of the published journals | .016 | ||
| <5 | 25 (34.3) | 7 (63.6) | |
| 5–9.99 | 16 (21.9) | 0 (0) | |
| ≥10 | 30 (41.1) | 2 (18.2) | |
| NA | 2 (2.7) | 2 (18.2) | |
Except where indicated otherwise, values are the number (%). mo, months; PK, pharmacokinetic; PD, pharmacodynamics; DLT, dose limited toxicity, MTD, maximum tolerance dose; TRR, tumor response rate; LRR, local response rate; DCR, disease control rate, NA, not available.
aincluded basic science, diagnostic, prevention, and supportive care.
bincluded oral function, oral mucositis, infection, quality of life, and feasibility et al.
For the study design, interventional studies were mostly single-group (142, 50.9%), single-center (159, 57.0%), non-randomized (165, 59.1%), open-label (227, 81.4%), and early-phase (197, 70.6%). Only 37 late-phase trials (phase 2/3 or 3) were registered, accounting for 13.3% of all interventional studies. The most common primary outcome for all trials was survival outcome (92, 33.0%), followed by safety (58, 20.8%) and short-term effect (57, 20.4%). The majority (65.2%) of observational studies were prospective. A total of 50.0% of observational studies were cohort studies. Fifty-two interventional studies and 4 observational studies were terminated or withdrawn. Forty-seven studies gave detailed reasons for termination or withdrawal (Supplementary Table 1). In the midst of the multifarious reasons, recruitment and funding were the top 2 reasons, constituting 26.8% and 10.7% of all reasons, respectively. Compared to other statuses, terminated or withdrawn studies included fewer children, more Multi-HNSCC and advanced patients, and smaller sample size. They were focused more on targeted therapy, were less randomized, and were conducted more often in the US/Canada and funded by the NIH/industry (P < .05, Supplementary Table 2).
Focusing on the purpose, trials based on treatments were the most common, comprising 82.8% (231/279) of all interventional studies. Among the treatment-focusing interventional studies, 36.8% (85/231) focused on chemotherapy. The number of targeted therapy and immunotherapy studies was equal, accounting for 15.2% (35/231) each. Combination therapy, radiotherapy, surgery, and others accounted for 13.0%, 6.5%, 6.9%, and 6.5%, respectively, (Table 1). Study characteristics between treatments among interventional studies are shown in Supplementary Table 3. Therapy distribution by registration year showed that trials focusing on immunotherapy had increased rapidly and targeted therapy had decreased rapidly since 2015 (Figure 2). Overall, chemotherapy has always been the main research direction of OSCC treatment. Therapy distribution by disease showed that chemotherapy was the most common treatment for Only OSCC and Multi-HNSCC, especially Multi-Cancer which accounted for 80% of the studies (Supplementary Figure 2). More than half of OSCC patients with precancerous lesions or epithelial hyperplasia studies have focused on targeted therapy
Figure 2.
Therapy distribution by registered years among interventional studies with the purpose of treatment.
Publication Status of Primary Completed Studies
Among primary completed studies, 58.9% (73/124) of interventional studies and 61.1% (11/18) of observational studies were published by January 8, 2021 (Table 1). Since 2008, approximately 5 studies have been published every year (Figure 1).
Study characteristics of the primary completed interventional studies are shown in Table 2. Among 73 published studies, the median duration of primary completion was 37.9 months, 7 months less than the trials with no published studies (P = .277). Compared to the no published studies, published studies had more results available, and were more likely to be phase 1/2 or phase 2 and multi-centered studies (P < .05). In addition, of the published interventional studies, 6.8% (5/73) of trials provided different outcomes in their papers compared to information in the registry (Supplementary Table 4).
Table 2.
Study Characteristics of Primary Completed Interventional Studies.
| No Publication (n=51) | Publication (n=73) | P-value | |
|---|---|---|---|
| Duration of primary completed (mo.) | 45.0 (29.9, 73.0) | 37.9 (25.0, 60.3) | .277 |
| Results of primary completed studies | <.001 | ||
| No available results | 48 (94.1) | 42 (57.5) | |
| Had results | 3 (5.9) | 31 (42.5) | |
| Register before study start | .429 | ||
| No | 38 (74.5) | 49 (67.1) | |
| Yes | 13 (25.5) | 24 (32.9) | |
| Age | 1.000 | ||
| Only adults | 49 (96.1) | 70 (95.9) | |
| Adults and children | 2 (3.9) | 3 (4.1) | |
| Disease type | .631 | ||
| Only OSCC | 6 (11.8) | 10 (13.7) | |
| OSCC and precancerous lesions and epithelial hyperplasia | 2 (3.9) | 1 (1.4) | |
| Multi-HNSCC | 38 (74.5) | 58 (79.5) | |
| Multi-cancers | 5 (9.8) | 4 (5.5) | |
| Recurrent or metastatic cancer | .571 | ||
| No | 34 (66.7) | 44 (60.3) | |
| Yes | 17 (33.3) | 29 (39.7) | |
| Disease clinical stage | .142 | ||
| I/II/I–II | 2 (3.9) | 2 (2.7) | |
| I–III/I–IV/II–IV | 17 (33.3) | 16 (21.9) | |
| III/III–IV/IV | 26 (51.0) | 35 (47.9) | |
| NA | 6 (11.8) | 20 (27.4) | |
| Sample size | .105 | ||
| ≤100 | 45 (88.2) | 55 (75.3) | |
| >100 | 6 (11.8) | 18 (24.7) | |
| Study design | .977 | ||
| Single arm | 28 (54.9) | 38 (52.1) | |
| Parallel | 20 (39.2) | 31 (42.5) | |
| Sequential | 2 (3.9) | 2 (2.7) | |
| Factorial/Crossover | 1 (2.0) | 2 (2.7) | |
| Purpose | 1.000 | ||
| Treatment | 41 (80.4) | 58 (79.5) | |
| Others c | 10 (19.6) | 15 (20.5) | |
| Treatment | .735 | ||
| Radiotherapy | 1 (2.4) | 4 (6.9) | |
| Chemotherapy | 18 (43.9) | 23 (39.7) | |
| Surgery | 0 (.0) | 2 (3.4) | |
| Targeted therapy | 6 (14.6) | 13 (22.4) | |
| Immunotherapy | 3 (7.3) | 3 (5.2) | |
| Combination therapy | 8 (19.5) | 8 (13.8) | |
| Others | 5 (12.2) | 5 (8.6) | |
| Phase | .012 | ||
| Phase 1 | 19 (37.3) | 12 (16.4) | |
| Phase 1/2 or 2 | 16 (31.4) | 40 (54.8) | |
| Phase 2/3 or 3 or 4 | 7 (13.7) | 14 (19.2) | |
| NA | 9 (17.6) | 7 (9.6) | |
| Randomized | .268 | ||
| No | 33 (64.7) | 39 (53.4) | |
| Yes | 18 (35.3) | 34 (46.6) | |
| Blind | 1.000 | ||
| Open label | 40 (78.4) | 56 (76.7) | |
| Blind | 11 (21.6) | 17 (23.3) | |
| Multi-center | .041 | ||
| No | 36 (70.6) | 37 (50.7) | |
| Yes | 15 (29.4) | 36 (49.3) | |
| Primary outcome | .688 | ||
| Biomarker/gene/PK/PD/apoptosis | 3 (5.9) | 6 (8.2) | |
| DLT/MTD/safety | 15 (29.4) | 14 (19.2) | |
| TRR/LRR/DCR | 9 (17.6) | 17 (23.3) | |
| Survival | 13 (25.5) | 22 (30.1) | |
| Othersd | 11 (21.6) | 14 (19.2) | |
| Country | .847 | ||
| US/Canada | 41 (80.4) | 58 (79.5) | |
| European | 6 (11.8) | 8 (11.0) | |
| Asian | 4 (7.8) | 5 (6.8) | |
| Others | 0 (.0) | 2 (2.7) | |
| Funder | .518 | ||
| Industry | 12 (23.5) | 13 (17.8) | |
| NIH | 22 (43.1) | 39 (53.4) | |
| Others | 17 (33.3) | 21 (28.8) | |
Except where indicated otherwise, values are the number (%). mo., months; PK, pharmacokinetic; PD, pharmacodynamics; DLT, dose limited toxicity, MTD, maximum tolerance dose; TRR, tumor response rate; LRR, local response rate; DCR, disease control rate, NA, not available.
Cincluded diagnostic, prevention, and supportive care.
dincluded oral function, oral mucositis, infection, quality of life, feasibility et al.
The median publication time of interventional studies was 48.0 months (95% CI: 29.9–77.1 months), and the 1-, 2-, 3-, and 5-year cumulative publication rates were 17.2%, 37.5%, 43.6%, and 54.6%, respectively, (Figure 3). The factors influencing the time to publication from primary completion of interventional studies are shown in Tables 3 and 4. Factors, such as registration before study start, study phases, and multi-center design, significantly influenced the time to publication in the univariable Cox regression analysis. However, in multi-variate Cox analysis, only the registered time and the study phases were significant factors. Compared to those trials registered after study start, the studies registered before study start were more often published on time, and the median time to publication was 23.4 months (HR = 1.69, 95% CI = 1.02–2.80, P = .040). Compared to phase 1 studies, phase 1/2 or 2 studies had less time to publication (HR = 2.10, 95% CI = 1.09–4.06, P = .026) (Table 4). Thirty (41.1%) of the primary completed interventional studies were published in IF ≥ 10 journals, and 53 (72.6%) articles reported positive primary results. Meanwhile, no significant factor was found between studies published in high-impact (IF ≥ 10) and low-impact (IF < 10) journals (Supplementary Table 5).
Figure 3.
Cumulative publication rate curve of primary completed studies.
Table 3.
Univariable Cox Regressions for Time to Publication.
| Variable | Median TTP e (mo.) | HR | 95% CI | P-value |
|---|---|---|---|---|
| Duration of primary completed (mo.) | 1.00 | (.99, 1.00) | .306 | |
| Register before study start | ||||
| No | 62.5 | 1.00 | ||
| Yes | 23.4 | 1.77 | (1.08, 2.90) | .023 |
| Sample size | ||||
| ≤100 | 49.8 | 1.00 | ||
| >100 | 33.3 | 1.55 | (.91, 2.64) | .107 |
| Age | ||||
| Only adults | 48.0 | 1.00 | ||
| Adults and children | 42.4 | .82 | (.26, 2.61) | .738 |
| Disease types | ||||
| OSCC | 42.6 | 1.00 | ||
| Multi-cancer | 48.0 | 1.03 | (.54, 1.95) | .937 |
| Recurrent or metastatic cancer | ||||
| No | 51.5 | 1.00 | ||
| Yes | 24.3 | 1.20 | (.75, 1.92) | .442 |
| Disease clinical stage | ||||
| I/II/I–II | 62.5 | 1.00 | ||
| I–III/I–IV/II–IV | 71.5 | 1.21 | (.28, 5.27) | .799 |
| III/III–IV/IV | 49.8 | 1.45 | (.35, 6.02) | .612 |
| NA | 34.0 | 2.21 | (.52, 9.45) | .286 |
| Purpose | ||||
| Others | 42.4 | 1.00 | ||
| Treatment | 48.0 | .91 | (.52, 1.61) | .753 |
| Study design | ||||
| Single group | 48.8 | 1.00 | ||
| 2 or more groups | 42.6 | 1.15 | (.73, 1.82) | .548 |
| Phase | ||||
| Phase 1 | - | 1.00 | ||
| Phase 1/2 or 2 | 40.6 | 2.18 | (1.14, 4.17) | .018 |
| Phase 2/3 or 3 or 4 | 48.7 | 1.76 | (.81, 3.82) | .150 |
| NA | 71.5 | 1.04 | (.41, 2.64) | .939 |
| Randomized | ||||
| No | 51.5 | 1.00 | ||
| Yes | 40.6 | 1.41 | (.89, 2.23) | .147 |
| Blind | ||||
| Open label | 48.0 | 1.00 | ||
| Blind | 45.4 | 1.07 | (.62, 1.84) | .814 |
| Multi-center | ||||
| No | 71.5 | 1.00 | ||
| Yes | 42.3 | 1.64 | (1.03, 2.60) | .035 |
| Primary outcome | ||||
| Biomarker/gene/PK/PD/Apoptosis | 41.4 | 1.00 | ||
| DLT/MTD/Safety | 63.9 | .64 | (.24, 1.68) | .364 |
| TRR/LRR/DCR | 42.6 | .98 | (.39, 2.51) | .971 |
| Survival | 48.8 | .95 | (.38, 2.35) | .908 |
| Others f | 48.7 | .80 | (.31, 2.09) | .646 |
| Country | ||||
| US/Canada | 42.6 | 1.00 | ||
| European | 51.5 | .85 | (.41, 1.79) | .675 |
| Asian or others | 89.3 | 1.08 | (.49, 2.36) | .853 |
| Funder | ||||
| Industry | 45.4 | 1.00 | ||
| NIH | 41.4 | 1.11 | (.59, 2.08) | .743 |
| Others | 62.5 | .89 | (.44, 1.77) | .734 |
TTP, time to publication; mo., months; PK, pharmacokinetic; PD, pharmacodynamics; DLT, dose limited toxicity, MTD, maximum tolerance dose; TRR, tumor response rate; LRR, local response rate; DCR, disease control rate, NA, not available.
eThe median time to publication was estimated by Kaplan–Meier method.
fOthers included oral function, oral mucositis, infection, quality of life, feasibility et al.
Table 4.
Multivariable Cox Regression for Time to Publication.
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Register before study start | |||
| No | 1.00 | ||
| Yes | 1.69 | (1.02, 2.80) | .040 |
| Phase | |||
| Phase 1 | 1.00 | ||
| Phase 1/2 or 2 | 2.10 | (1.09, 4.06) | .026 |
| Phase 2/3 or 3 or 4 | 1.40 | (.61, 3.18) | .424 |
| NA | 1.22 | (.47, 3.14) | .679 |
| Multi-center | |||
| No | 1.00 | ||
| Yes | 1.41 | (.84, 2.36) | .197 |
NA, not available
Discussion
Our results show that OSCC clinical trials are usually small sample size (<100), single-armed, open-label, and non-randomized studies. Currently, interventional studies are focusing on the treatment of OSCC, especially chemotherapy, targeted therapy, and immunotherapy, and we identified a growing number of immunotherapy-oriented trials. After primary trial completion, only 58.9% of the primary completed interventional studies were published, and the median time to publication was 48.0 months. In addition, 72.6% of the published results were positive.
Current research results indicated that approximately 40% of studies tested stage III/IV or IV OSCC, and 70 studies focused on recurrent or metastatic OSCC (R/M OSCC). This may reflect the poor prognosis and late diagnosis of OSCC.16,17 A report showed that approximately 30% of patients usually wait more than 3 months before medical consultation after self-discovering the signs and symptoms of OSCC. 18 After primary treatment, more than half of the patients (80% of cases within the first 2 years) found recurrences and/or metastases,16,18 the risk of local recurrence was higher than 50%, and the risk of distant metastases was approximately 20%. 19 Therefore, more attention should be given to these subgroups to optimize future clinical studies and improve OSCC patient prognosis.
In the present study, the purpose of most interventional trials was treatment-oriented. More than half of the studies examined therapeutic drugs, including chemotherapy, immunotherapy, targeted therapy, and combination therapy. Currently, decisions on therapeutic drugs are based on factors such as toxicity and patient prognosis. 12 We found that the primary outcome was mostly survival outcome, safety, and short-term effect, which showed strong motivation to find better ways to guide OSCC clinical therapies. Immunotherapy has promoted survival among patients with cancers over the past decade.20-23 Our study showed a similar trend in which immunotherapy-oriented trials had increased over the past 5 years. Anti-PD-1 immune checkpoint inhibitors have significantly increased OSCC patients’ overall survival (OS) and progression-free survival (PFS).20,24 In 2016, the FDA approved 2 anti-PD-1 immune checkpoint inhibitors, nivolumab (CheckMate 141, NCT02105636) and pembrolizumab (KEYNOTE-012, NCT01848834), for the treatment of OSCC patients. Then, in 2019, pembrolizumab (KEYNOTE-048, NCT02358031) was approved as the first-line treatment of patients with unresectable R/M OSCC.25,26 Currently, there are 35 registered studies focusing on immunotherapy and 26 of them are still ongoing.
The current study results revealed that most OSCC trials were open-label and non-randomized. Previous studies have shown that open-label trials may overstate the benefits of interventions, whereas blind trials improve the objectivity of data collection or reporting while reducing the risk of measurement bias. 27 Randomization is also important to determine whether there is a causal relationship between treatment and outcome and to assess the cost-effectiveness of treatment, whereas non-randomized trials cannot rule out the possibility that this association is caused by a third factor. 28 However, our study showed that 59.1% of the trials were non-randomized. Future researchers should consider the principles of blinding and randomization to obtain more accurate clinical results. In addition, our research found that 77.5% of OSCC trials had small sample size (<100). In oncology, there is a growing consensus that small clinical trials based on genetics or biomarkers can yield definitive results. 29 However, small trials are unlikely to provide information about some treatment aspects, such as determining the effectiveness of treatments with modest effects.29,30 Thus, multiple small clinical trials need to be aggregated and systematically evaluated to solve the existing problem.
We also found that the majority of studies were conducted in the US/Canada. We thought this may reflect the fact that all studies in the US are required to be registered with the ICMJE at ClinicalTrials.gov, 11 and trials conducted outside of the US have different guidelines. 12 We searched for OSCC-related clinical trials on the WHO International Clinical Trials Registry Platform (ICTRP) and found 45 registrations in EudraCT (which is used in the European Union) and 42 registrations in Clinical Trials Registry-India (CTRI). As our search scope was limited to ClinicalTrials.gov, our findings on location may show some selection bias. However, as the largest and most comprehensive clinical trial registry database, ClinicalTrials.gov can still offer us representative OSCC research.
Studies registered on ClinicalTrials.gov had a higher probability of publication, and approximately 60% of primary completed OSCC studies were published. Our study found that the registration time and study phases were significant factors that influenced publication status. Phase 1/2 or 2 clinical trials were more likely to be published, probably because early-stage clinical trials usually have small sample size and lower costs, and are single-armed and conducted in one institution or a few centers.31,32 Therefore, starting and completing these trials are less time-consuming and much easier than late-phase studies, which is consistent with our findings. However, phase 3/4 clinical trials are the gold standard of clinical practice in medicine, and investigators should actively publish these late-phase results despite the high attrition rates. 33
Among the published interventional studies, only 37.5% of the studies were published within 2 years of completing the primary study. The time from primary completion to publication was 48.0 months, which was quite long. Study results could not be reported and introduced into clinical practice in a timely manner. 9 Moreover, we estimated that although the registration of trials had increased, nearly 40% of the completed interventional studies remained unpublished, which limited results. Compared to the non-publication rates of completed studies in other fields of medicine (approximately 35%), 34 much more effort needs to be made to supervise the publication of OSCC articles. Timely publication results provide the best scientific evidence and yield maximum benefits for public health and scientific progress. 35 However, there are reasons for non-publication of completed interventional trials. In principle, trials that produce negative results that are not conductive or even contradictory to the researcher’s hypothesis are likely to be inhibited.34,36 In addition, editors are usually more interested in positive results, while negative tests are less likely to be published. 37 Compared to a cross-sectional study that reported that 70% of the published articles were positive, 38 our study showed a similar result, that 72.6% (53/73) of the published interventional study results were positive. Although we did not know the proportion of positive or negative results in the non-publication trials, it seemed that negative results still influenced the publication status of completed studies.
Another noteworthy phenomenon is that more than half of the studies were registered after the trials began; however, we found that the published studies were more likely to be registered before the trials began. And we noticed that some published studies lacked power calculations and found discrepancies compared to registered outcomes. This is known as selective outcome reporting, which may make the published results misleading, thereby threatening the validity of the trial. 39 To increase the transparency of OSCC research execution and reporting, the rules for clinical trial registries need to be standardized. 9
Our research had several limitations. We lacked a systematic framework since we focused only on ClinicalTrials.gov, which does not include all clinical trials. In addition, the datasets of all trials in the database are not always complete and up to date. There is a possibility that errors may be caused because some studies were not captured for analysis and may have been misclassified during the selection process. Another limitation is that even though we used OSCC as our search strategy, we found that Only OSCC accounted for approximately a quarter of the disease type, and the vast majority of clinical studies mainly focused on Multi-HNSCC. As HNSCC develops from the mucosal epithelium in the oral cavity, pharynx, and larynx which have anatomical continuity in common, 4 and according to National Comprehensive Cancer Network (NCCN) Guidelines, 14 squamous cell carcinoma in the oral cavity and oropharynx shares similar principles of systemic therapy. Therefore, we believe that these Multi-HNSCC trials can represent the research characteristics and status of OSCC and provide a representative study result. Finally, as the data for all clinical trials on ClinicalTrials.gov were provided by investigator reports, we cannot ensure the validity of all trial information.
Conclusion
Our study outlined the characteristics and publication status of all OSCC clinical trials registered on ClinicalTrials.gov. It appeared that most OSCC clinical trials were small sample size, single-armed, open-label, and non-randomized studies. Advanced OSCC is the focus of current research, and the number of immunotherapy-oriented studies has increased over the past 5 years. Most published studies reported positive results and lacked timely publication. To ensure the transparency of OSCC clinical trials, researchers should perform standardized registration and timely publication for both positive and negative clinical findings.
Supplemental Material
Supplement Material, sj-pdf-1-ccx-10.1177_10732748221080348 for The Current Landscape of Oral Squamous Cell Carcinoma: A Comprehensive Analysis from ClinicalTrials.gov by Zhaolei Zou, Bin Li, Shuqiong Wen, Dongjia Lin and Qiannan Hu, Zhi Wang and Juan Fang in Cancer Control
Supplement Material, sj-pdf-2-ccx-10.1177_10732748221080348 for The Current Landscape of Oral Squamous Cell Carcinoma: A Comprehensive Analysis from ClinicalTrials.gov by Zhaolei Zou, Bin Li, Shuqiong Wen, Dongjia Lin and Qiannan Hu, Zhi Wang and Juan Fang in Cancer Control
Appendix
Abbreviations
- AJCC
American Joint Committee on Cancer
- CAR-T
Chimeric Antigen Receptor T-Cell
- CIs
confidence intervals
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- DCR
disease control rate
- DLT
dose limited toxicity
- FDA
Food and Drug Administration
- FDAMA
Food and Drug Administration Modernization Act
- HHS
Health and Human Services
- HNSCC
head and neck squamous cell carcinoma
- HRs
hazard ratios
- ICMJE
International Committee of Medical Journal Editors
- ICTRP
International Clinical Trials Registry Platform
- IF
impact factor
- IQR
interquartile range
- LRR
local response rate
- MTD
maximum tolerance dose
- NA
not available
- NCCN
National Comprehensive Cancer Network
- NIH
National Institutes of Health
- OS
overall survival
- OSCC
oral squamous cell carcinoma
- PD
pharmacodynamics
- PD-1
programmed cell death protein-1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- PK
pharmacokinetic R/M OSCC recurrent or metastatic OSCC
- TNM
tumor, nodes, and metastases
- TRR
tumor response rate
Authors’ Contributions: Zhaolei Zou and Bin Li contributed equally to this article. Juan Fang designed and supervised the study.
Zhaolei Zou, Bin Li, and Shuqiong Wen collected the data, conducted the data analysis, and wrote the draft manuscript.
Dongjia Lin performed critical revision on the manuscript.
Qiannan Hu, Shuqiong Wen, Zhi Wang, and Juan Fang participated in the critical revision of the manuscript.
All authors approved the final version before submission.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This article was supported by National Natural Science Foundations of China (No. 82101017, 81972532); Guangdong Basic and Applied Basic Research Foundation (2020A1515110741).
Availability of Data and Materials: All data analyzed during this study are included in this published article and its supplementary information files.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Zhaolei Zou https://orcid.org/0000-0001-7577-3436
Juan Fang https://orcid.org/0000-0002-0207-3436
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Chow LQM. Head and neck cancer. N Engl J Med. 2020;382(1):60-72. doi: 10.1056/NEJMra1715715 [DOI] [PubMed] [Google Scholar]
- 3.Ghapanchi J, Ranjbar Z, Mokhtari MJ, et al. The LncRNA H19 rs217727 polymorphism is associated with oral squamous cell carcinoma susceptibility in iranian population. BioMed Res Int. 2020;2020:1, 6. doi: 10.1155/2020/1634252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim 2020;6(1):92. doi: 10.1038/s41572-020-00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai AWY, Lim KP, Cheong SC. Translational genomics and recent advances in oral squamous cell carcinoma. Semin Cancer Biol. 2020;61:71-83. doi: 10.1016/j.semcancer.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 6.Global Burden of Disease Cancer. Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncology. 2019;5(12):1749-1768. doi: 10.1001/jamaoncol.2019.2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin X, Wu X, Gomaa A, et al. Analysis of risk factors for multiple primary oral squamous cell carcinoma: A cohort study. Clin Oral Invest 2020;24(9):3147-3155. doi: 10.1007/s00784-019-03189-0 [DOI] [PubMed] [Google Scholar]
- 8.Ling Z, Cheng B, Tao X. Epithelial‐to‐mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities. Int J Cancer. 2021;148(7):1548-1561. doi: 10.1002/ijc.33352 [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Li B, Zheng Q, et al. The current landscape of clinical studies focusing on thyroid cancer: A comprehensive analysis of study characteristics and their publication status. Front Endocrinol. 2020;11:575799. doi: 10.3389/fendo.2020.575799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States National Institutesof Health. ClinicalTrials.gov: Background . 2021. (accessed June 17, 2021) https://clinicaltrials.gov/ct2/about-site/background
- 11.DeAngelis CD, Drazen JM, Frizelle FA, et al. Clinical trial registration. JAMA. 2004;292(11):1363-1364. [DOI] [PubMed] [Google Scholar]
- 12.Zibelman M, Barth P, Handorf E, et al. A review of interventional clinical trials in renal cell carcinoma: A status report from the ClinicalTrials.gov WebSite. Clin Genitourin Cancer 2015;13(2):142-149. doi: 10.1016/j.clgc.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 13.Amin MB, Greene FL, Edge SB, et al. The eighth edition ajcc cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA A Cancer J Clin. 2017;67(2):93-99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 14.Pfister DG, Spencer S, Adelstein D, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2020;18(7):873-898. doi: 10.6004/jnccn.2020.0031 [DOI] [PubMed] [Google Scholar]
- 15.Barth P, Vale C, Chambers AB, Reagan JL. The next generation of therapy for multiple myeloma: A review of ongoing clinical trials utilizing ClinicalTrials.gov. Future Oncol. 2018;14(19):1965-1976. doi: 10.2217/fon-2017-0722 [DOI] [PubMed] [Google Scholar]
- 16.Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, Panzarella V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front Physiol. 2019;10:1476. doi: 10.3389/fphys.2019.01476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez I, Seoane J, Varela-Centelles P, Diz P. Is diagnostic delay related to advancedstage oral cancer? A meta-analysis. Eur J Oral Sci. 2009;117:541-546. [DOI] [PubMed] [Google Scholar]
- 18.Panzarella V, Pizzo G, Calvino F, Compilato D, Colella G, Campisi G. Diagnostic delay in oral squamous cell carcinoma: the role of cognitive and psychological variables. Int J Oral Sci. 2014;6(1):39-45. doi: 10.1038/ijos.2013.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grobe A, Blessmann M, Hanken H, et al. Prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of patients with squamous cell carcinoma of the oral cavity. Clin Cancer Res. 2014;20(2):425-433. doi: 10.1158/1078-0432.CCR-13-1101 [DOI] [PubMed] [Google Scholar]
- 20.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang F, Markovic SN, Molina JR, et al. Association of sex, age, and eastern cooperative oncology group performance status with survival benefit of cancer immunotherapy in randomized clinical trials: A systematic review and meta-analysis. JAMA Netw Open. 2020;3(8):e2012534. doi: 10.1001/jamanetworkopen.2020.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422. doi: 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 23.Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor t-cell therapy. N Engl J Med. 2016;375(26):2561-2569. doi: 10.1056/NEJMoa1610497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. doi: 10.1016/s1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 25.Cohen EEW, Bell RB, Bifulco CB, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7(1):184. doi: 10.1186/s40425-019-0662-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi: 10.1016/s0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609-613. [DOI] [PubMed] [Google Scholar]
- 28.Sibbald B, Roland M. Understanding controlled trials. Why are randomised controlled trials important? BMJ. 1998;316(7126):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov. JAMA. 2012;307(17):1838-1847. doi: 10.1001/jama.2012.3424 [DOI] [PubMed] [Google Scholar]
- 30.Califf RM, DeMets DL. Principles from clinical trials relevant to clinical practice: Part I. Circulation. 2002;106(8):1015-1021. [DOI] [PubMed] [Google Scholar]
- 31.Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of Investigational Drugs in Late-Stage Clinical Development and Publication of Trial Results. JAMA Intern Med. 2016;176(12):1826-1833. doi: 10.1001/jamainternmed.2016.6008 [DOI] [PubMed] [Google Scholar]
- 32.Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. 2018;11:156-164. doi: 10.1016/j.conctc.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arrowsmith J, Miller P. Trial watch: phase II and phase III attrition rates 2011-2012. Nat Rev Drug Discov. 2013;12(8):569. doi: 10.1038/nrd4090 [DOI] [PubMed] [Google Scholar]
- 34.Chen YP, Liu X, Lv JW, et al. Publication status of contemporary oncology randomised controlled trials worldwide. Eur J Cancer. 2016;66:17-25. doi: 10.1016/j.ejca.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 35.Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ. 2012;344:d7292. doi: 10.1136/bmj.d7292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumenthal D, Campbell EG, Anderson MS, Causino N, Louis KS. Withholding research results in academic life science: Evidence from a national survey of faculty. JAMA. 1997;277(15):1224-1228. [PubMed] [Google Scholar]
- 37.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337(8746):867-872. doi: 10.1016/0140-6736(91)90201-y [DOI] [PubMed] [Google Scholar]
- 38.Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: A cross-sectional analysis. PLoS Med. 2009;6(9):e1000144. doi: 10.1371/journal.pmed.1000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braakhekke M, Scholten I, Mol F, Limpens J, Mol BW, van der Veen F. Selective outcome reporting and sponsorship in randomized controlled trials in IVF and ICSI. Hum Reprod. 2017;32(10):2117-2122. doi: 10.1093/humrep/dex273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Material, sj-pdf-1-ccx-10.1177_10732748221080348 for The Current Landscape of Oral Squamous Cell Carcinoma: A Comprehensive Analysis from ClinicalTrials.gov by Zhaolei Zou, Bin Li, Shuqiong Wen, Dongjia Lin and Qiannan Hu, Zhi Wang and Juan Fang in Cancer Control
Supplement Material, sj-pdf-2-ccx-10.1177_10732748221080348 for The Current Landscape of Oral Squamous Cell Carcinoma: A Comprehensive Analysis from ClinicalTrials.gov by Zhaolei Zou, Bin Li, Shuqiong Wen, Dongjia Lin and Qiannan Hu, Zhi Wang and Juan Fang in Cancer Control