Abstract
Cryptococcus neoformans is a nonfermentative yeast that requires oxygen for growth. The shaking of culture media achieves good oxygenation, promoting the growth of cryptococci. In this study, three test media (RPMI 1640, RPMI 1640–2% glucose, and buffered yeast nitrogen base [BYNB]) recommended in the National Committee for Clinical Laboratory Standards M27A standard were examined. Growth abilities and minimum inhibitory concentrations (MICs) in microplates incubated at 35°C for 48 h were determined. The results indicated that shaking and an inoculum size of 105 CFU/ml yielded optimal growth of this yeast. Compared to RPMI 1640, supplementation of RPMI 1640 with 2% glucose did not significantly improve growth of C. neoformans and resulted in an 8.7-h delay of exponential growth. Cryptococcal growth in RPMI 1640 at 24 h was notably better than that in RPMI–2% glucose, although by 48 h the growths were comparable. The MIC range of amphotericin B observed for the C. neoformans strains grown in RPMI 1640 with or without glucose was too narrow to allow the separation of susceptible and resistant strains based on clinical outcome. The widest ranges of MICs of flucytosine and fluconazole were obtained with BYNB. This work demonstrates the need for a new antifungal susceptibility test for C. neoformans.
Several methodologies for evaluating the antifungal susceptibility of Cryptococcus neoformans have been proposed, but only three are included in the National Committee for Clinical Laboratory Standards (NCCLS) M27A document (1, 4, 9). The NCCLS standard recommends the use of RPMI 1640 buffered to a pH of 7.0 as the medium for testing by either the macrodilution or the microdilution method and an initial inoculum size of 0.5 × 103 to 2.5 × 103 (4). As Cryptococcus neoformans grows slowly in liquid culture medium, an incubation period of 70 to 74 h at 35°C was recommended (4). Ghannoum et al. (1) advise the use of a microdilution method with buffered yeast nitrogen base (BYNB) medium (pH 7.0), a higher inoculum size (104 CFU/ml), and a spectrophotometric determination of a 50% inhibition endpoint; this is an alternative method outlined in the M27A document.
Odds et al. (5) demonstrated that oxygen is a limiting factor for C. neoformans grown in liquid media, and they recommended cultivation under constant agitation. Others reported (6, 9, 10) that RPMI 1640 with 2% glucose supported better growth of yeasts than RPMI 1640 alone. The purpose of this study is to compare the NCCLS M27A, BYNB, and RPMI–2% glucose methodologies (1, 4, 9) in static versus agitated microtiter plates to ascertain the influence of agitation in C. neoformans growth and minimum inhibitory concentration (MIC) determination.
Organisms.
A collection of 35 C. neoformans isolates was included. A total of 27 were clinical isolates obtained from blood cultures or cerebrospinal fluid. A total of 15 were obtained from 15 Argentinean AIDS patients with cryptococcal meningitis, of whom 10 responded to treatment with 0.7 mg of amphotericin B/kg of body weight/day and could be classified as putatively susceptible, while 5 failed to respond clinically to 0.7 mg of amphotericin B/kg/day and could be considered putatively resistant. Two subjects died within 24 to 48 h of treatment, while the remaining three died despite a cumulative dose of 500 mg of amphotericin B. Six strains (CN5, CN6, CN7, CN8, CN10, and CN12) were kindly provided by John Rex (University of Texas Medical School). CN5 and CN8 are considered to be amphotericin B susceptible and resistant, respectively, based on clinical observations (7). Isolates CN6 and CN7 were from AIDS patients with cryptococcal meningitis who were responsive to amphotericin B treatment (2). CN10 and CN12 were from an Australian human immunodeficiency virus-infected patient who developed resistance during treatment (D. J. E. Marriot, R. Hardiman, S. Chen, J. L. Harkness, and R. Pennry, 3rd Int. Conf. Cryptococcus Cryptococcosis, abstr. 3.21, 1996). C. neoformans ATCC 90112 (3), C. neoformans CNML3405, and Candida albicans ATCC 64548 (8) were included as internal control strains. The quality control (QC) strains, Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 (4), were also included in all experiments.
Antifungal agents.
The antifungal agents used in the study were as follows: amphotericin B (Sigma Aldrich Quimica, S.A., Madrid, Spain), flucytosine (Sigma Aldrich Quimica, S.A.), fluconazole (Pfizer S.A., Madrid, Spain), and itraconazole (Janssen S.A., Madrid, Spain). Amphotericin B, fluconazole, and itraconazole were dissolved in 100% dimethyl sulfoxide (Sigma Aldrich Quimica, S.A.). Flucytosine was dissolved in sterile distilled water. All drug stock solutions were frozen at −70°C as 100× stocks until used.
Test media.
RPMI 1640 medium, without sodium bicarbonate and with l-glutamine (lot 28H83051; Sigma Aldrich Quimica, S.A.), was buffered to a pH of 7.0 with 3-(N-morpholino) propanesulfonic acid (MOPS) (lot 18H5404; Sigma Aldrich Quimica, S.A.), achieving a final concentration of 0.165 mol/liter. RPMI–2% glucose is RPMI 1640 supplemented with 18 g of glucose per liter to reach a final concentration of 2%. This medium was prepared as a 2× solution. Yeast nitrogen base (lot 71893JB; Difco Laboratories, Madrid, Spain) was prepared following the manufacturer's instructions. After reconstitution, it was supplemented with glucose to obtain a final concentration of 0.5 g/liter and buffered to a pH of 7.0 with MOPS (lot 18H5404; Sigma Aldrich Quimica, S.A.), achieving a final concentration of 0.05 mol/liter. All media were filter sterilized by passage through a 0.22-μm-pore-size filter system (Nalgene, Madrid, Spain).
Susceptibility testing. (i) NCCLS M27A methodology.
The methodology used strictly followed the NCCLS recommendations for the microdilution procedure (4), including RPMI 1640 buffered to a pH of 7.0 and a yeast inoculum of 0.5 × 103 to 2.5 × 103 CFU/ml.
(ii) RPMI–2% glucose methodology.
A starting inoculum of 1 × 106 to 5 × 106 CFU/ml was made following the NCCLS recommendation (4) and then diluted 1:10 with sterile distilled water. The final inoculum contained 0.5 × 105 to 2.5 × 105 CFU/ml.
(iii) Yeast nitrogen base methodology.
The procedure rigorously followed the instructions designed by Ghannoum et al., using BYNB and yeast inoculum size of 104 CFU/ml (1).
The final ranges of antifungal concentrations were as follows: amphotericin B, from 8 to 0.015 μg/ml; flucytosine and fluconazole, from 64 to 0.12 μg/ml; and itraconazole, from 4 to 0.007 μg/ml. All microplates were incubated at 35°C for 48 h. One set of microplates was wrapped with film sealer to prevent the medium from evaporating, attached to an electrically driven wheel inside the incubator, and agitated at 350 rpm. A corresponding set was incubated statically with their corresponding plastic covers.
Endpoint determination.
MICs were determined after 48 h of incubation by measuring the absorbance at 540 nm (A540) with a plate reader (model iEMS reader MF, Labsystems, Madrid, Spain). After the incubation, the microplates were mechanically agitated at 1,400 rpm for 30 s inside the plate reader and then read. The mean A540 of eight blank wells (column 12) was subtracted from the absorbance value obtained from each well.
Two MIC endpoints were defined as follows: (i) the lowest drug concentration exhibiting reduction in growth of 80% or more compared with that of the control growth as recommended by NCCLS M27A for macrodilution (4) (MIC80) and (ii) the lowest drug concentration exhibiting a reduction in growth of 50% or more compared with that of the control growth as recommended by Ghannoum et al. for C. neoformans (1) (MIC50). Only MIC80 of amphotericin B was calculated, while both the MIC80s and the MIC50s of flucytosine and the azoles were determined.
Growth curves.
The growth curves for 16 of the 35 C. neoformans isolates and the control strains C. albicans ATCC 64548, C. parapsilosis ATCC 22019, and C. krusei ATCC 6258 were determined by using the microdilution format. The final yeast inoculum was approximately 105 CFU/ml. The microplates were incubated for 48 h at 35°C inside a plate set at a wavelength of 540 nm with agitation at 1,400 rpm (5 min of agitation, 5 min stopped) or statically. All procedures were repeated with three different inoculum preparations.
Statistical analysis.
The A540 in the growth control well must be >0.09 U to calculate the spectrophotometric MIC80 or MIC50 endpoints. The beginning of exponential phase was marked by a change in A540 of ≥0.02 U, with continued exponential increase in A540.
The significance of the differences in A540 in static versus agitated cultures and the start of the exponential phase in different media were determined by the Student t test (unpaired, unequal variance) or by Mann-Whitney U test or Wilcoxon test. Differences in proportions were determined by Fisher's exact test or by chi-square analysis.
Results and discussion. (i) Growth of C. neoformans isolates in antifungal susceptibility testing microdilution trays.
After 48 h of incubation the microplates were spectrophotometrically read for determination of the MICs. The A540 values from the control growth wells of each isolate were recorded and the means, standard deviations (SD), ranges, and 95% confidence intervals (CI95%) are shown in Table 1. C. neoformans ATCC 90112 was tested seven times on different days. Independent of the test media, the 33 C. neoformans isolates grew better in shaken cultures. Their growth in static cultures was limited in all media, particularly in that of the NCCLS M27A method (RPMI 1640 with final inoculum size of 0.5 × 103 to 2.5 × 103 CFU/ml). With this methodology, only 6.1% of the isolates reached an A540 of >0.09 U and none reached an A540 of >0.199 U (Table 1). With static RPMI–2% glucose (inoculum size, 0.5 × 105 to 2.5 × 105 CFU/ml), 66.7% of isolates reached an A540 of >0.09 U and none exceeded 0.199 U. The A540 in static BYNB (inoculum size, 104 CFU/ml) was higher, with 93.9% of the isolates attaining A540 values of >0.09 U and 15.2% of the isolates attaining A540 values of >0.199 U. Thus, in static cultures, C. neoformans grew best in RPMI–2% glucose or BYNB.
TABLE 1.
A540 values of Cryptococcus neoformans ATCC 90112 and 33 Cryptococcus neoformans isolates reached after 48 h of growth at 35°C in static or 350-rpm-agitated culture media
| Methodology, no. of isolates tested, and value determined | Growth with shaking
|
Static growth
|
||
|---|---|---|---|---|
| C. neoformans ATCC 90112 | C. neoformans | C. neoformans ATCC 90112 | C. neoformans | |
| NCCLS M27A | ||||
| n | 7a | 33 | 7a | 33 |
| A540 | ||||
| Mean | 0.19 | 0.17 | 0.06 | 0.06 |
| SD | 0.01 | 0.06 | 0.07 | 0.019 |
| Range | 0.18–0.21 | 0.05–0.28 | 0.06–0.08 | 0.02–0.1 |
| CI95% | 0.18–0.2 | 0.15–0.19 | 0.05–0.07 | 0.06–0.07 |
| RPMI–2% glucose | ||||
| n | 7 | 33 | 7 | 33 |
| A540 | ||||
| Mean | 0.4 | 0.36 | 0.12 | 0.09 |
| SD | 0.01 | 0.06 | 0.01 | 0.02 |
| Range | 0.38–0.43 | 0.24–0.48 | 0.11–0.14 | 0.06–0.19 |
| CI95% | 0.38–0.41 | 0.34–0.38 | 0.1–0.13 | 0.09–0.1 |
| BYNB | ||||
| n | 7 | 33 | 7 | 33 |
| A540 | ||||
| Mean | 0.58 | 0.45 | 0.15 | 0.14 |
| SD | 0.04 | 0.13 | 0.01 | 0.04 |
| Range | 0.53–0.62 | 0.17–0.71 | 0.13–0.17 | 0.08–0.31 |
| CI95% | 0.54–0.62 | 0.41–0.5 | 0.13–0.16 | 0.13–0.16 |
Seven repetitions on different days.
For agitated cultures, the best growth was obtained with BYNB (inoculum size, 104 CFU/ml) followed by RPMI–2% glucose (inoculum size, 0.5 × 105 to 2.5 × 105 CFU/ml). Again, the NCCLS M27A methodology (RPMI 1640, inoculum size of 0.5 × 103 to 2.5 × 103 CFU/ml) yielded poorer growth. The CI95% for the 33 C. neoformans isolates growing in agitated microplates was very narrow in RPMI–2% glucose, thus showing good reproducibility growth. All isolates reached A540 values of ≥0.24 U (Table 1). With BYNB, one isolate did not reach an A540 of 0.2 U and the CI95% was 0.41 to 0.5 U. With the NCCLS M27A method, 57.6% of the isolates did not attain an A540 of >0.199 U.
(ii) Growth curves.
The results obtained with the antifungal susceptibility testing suggested that the size of inoculum could have a great influence on the C. neoformans growth. For this reason, we designed the growth curve experiments with the same starting inoculum size of 105 CFU/ml. Figure 1 shows the mean A540 values of 16 individual growth curves generated for 16 C. neoformans isolates in each medium. Despite an inoculum size of 105 CFU/ml, the growth in static cultures was very poor compared to growth in agitated cultures. The highest A540 values (mean ± SD) reached in each medium at 48 h of incubation were as follows: RPMI 1640, 0.13 ± 0.03 U (CI95%, 0.10 to 0.15 U); RPMI–2% glucose, 0.15 ± 0.04 U (CI95%, 0.13 to 0.18 U); and BYNB, 0.21 ± 0.06 U (CI95%, 0.18 to 0.25 U). Growth in all media was statistically greater with the agitated counterparts (P < 0.0001). The best growth was obtained with BYNB (mean ± SD at 48 h, 0.77 ± 0.07; CI95%, 0.73 to 0.81). In RPMI 1640 with or without glucose the final growths reached were similar (in RPMI 1640, the mean ± SD at 48 h was 0.41 ± 0.09 U and the CI95% was 0.36 to 0.46 U; in RPMI–2% glucose, the mean ± SD at 48 h was 0.43 ± 0.08 U and the CI95% was 0.38 to 0.47 U; P = 0.623), though the lag phase in RPMI 1640 was shorter than that in RPMI–2% glucose (lag phase, 7.6 ± 1.4 h for RPMI 1640 versus 16.3 ± 2.4 h for RPMI–2% glucose [values are means ± SD]; P < 0.0001). Also, the strains grown in RPMI 1640 reached the final stage of the exponential phase at 24 h (A540 [mean ± SD] at 24 h, 0.35 ± 0.13 for RPMI 1640 [CI95%, 0.28 to 0.42] versus 0.27 ± 0.09 for RPMI–2% glucose [CI95%, 0.22 to 0.32]; P = 0.012).
FIG. 1.
Mean growth curve of 16 C. neoformans isolates incubated at 35°C in static or shaken culture media at 1,400 rpm.
(iii) MICs for C. neoformans.
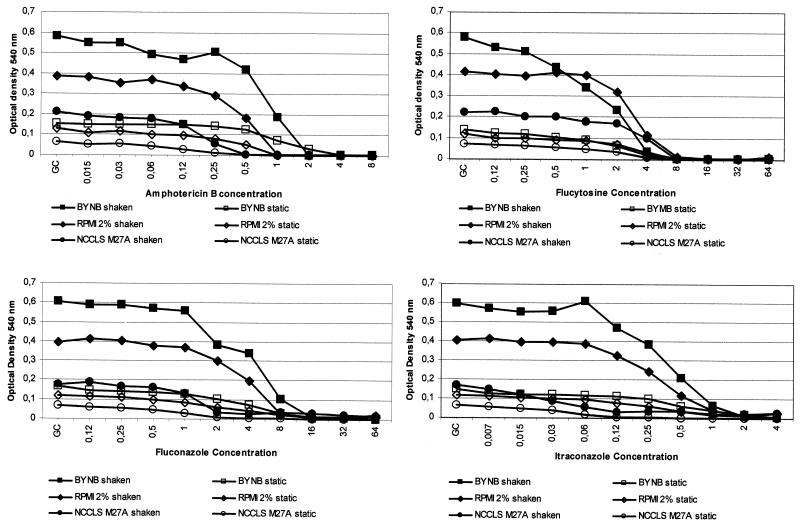
The inhibition curves of C. neoformans ATCC 90112 produced by amphotericin B, fluconazole, itraconazole, and flucytosine are shown in Fig. 2. In this figure, each inhibition curve is the mean of the seven MIC determinations. As stated above, agitated BYNB showed the best growth and also the best inhibitory curve, followed by agitated RPMI–2% glucose. In both media the endpoint determination could be performed objectively. The inhibitory curves produced by the NCCLS M27A methodology (with or without agitation), static RPMI–2% glucose, and static BYNB were very difficult to interpret due to the gentle slope of the curve. Therefore, the identification of a MIC endpoint could not be performed with C. neoformans growing under static antifungal susceptibility testing conditions. Only the MICs obtained with shaken microplates are shown in Table 2. As stated above, some strains grew poorly following NCCLS M27A methodology, so that their MICs could not be calculated. None of the three methods allowed a distinction to be made between strains as putatively susceptible or resistant to amphotericin B based on clinical outcome. For flucytosine and fluconazole, wider MIC ranges were observed with BYNB (Table 2).
FIG. 2.
Mean curve of seven inhibition curves of C. neoformans ATCC 90112 produced by amphotericin B, flucytosine, fluconazole, and itraconazole in static and 350-rpm-agitated microplates incubated for 48 h at 35°C.
TABLE 2.
MIC range obtained from shaken microplates of 33 C. neoformans isolates classified according to their clinical behavior
| Methodology and antifungal agent | MIC endpoint | MIC range (μg/ml) for isolates classified as:
|
|||
|---|---|---|---|---|---|
| Unknown | Putatively susceptible to amphotericin B | Putatively resistant to amphotericin B | All | ||
| NCCLS M27A | |||||
| Amphotericin Ba | MIC80 | 0.25–0.5 | 0.25–0.5 | 0.5–1 | 0.25–1 |
| Flucytosineb | MIC80 | 4–8 | 2–16 | 8–8 | 2–16 |
| MIC50 | 1–8 | 2–8 | 4–8 | 1–8 | |
| Fluconazolec | MIC80 | 4–8 | 2–8 | 4–8 | 2–8 |
| MIC50 | 0.5–4 | 2–8 | 2–4 | 0.5–8 | |
| Itraconazoled | MIC80 | 0.12–2 | 0.12–8 | 0.12–2 | 0.12–8 |
| MIC50 | 0.03–0.5 | 0.06–0.5 | 0.06–0.25 | 0.03–0.5 | |
| RPMI–2% glucose | |||||
| Amphotericin B | MIC80 | 1–2 | 1–1 | 1–2 | 1–2 |
| Flucytosine | MIC80 | 4–16 | 4–32 | 8–128 | 4–128 |
| MIC50 | 4–8 | 2–8 | 4–128 | 2–128 | |
| Fluconazole | MIC80 | 2–16 | 8–32 | 2–32 | 2–32 |
| MIC50 | 1–16 | 4–16 | 1–16 | 1–16 | |
| Itraconazole | MIC80 | 1–2 | 0.5–2 | 0.5–2 | 0.5–2 |
| MIC50 | 0.25–1 | 0.5–1 | 0.25–1 | 0.25–1 | |
| BYNB | |||||
| Amphotericin B | MIC80 | 1–2 | 1–2 | 1–8 | 1–8 |
| Flucytosine | MIC80 | 4–32 | 2–8 | 2–128 | 2–128 |
| MIC50 | 2–16 | 0.12–8 | 2–128 | 0.12–128 | |
| Fluconazole | MIC80 | 1–16 | 8–32 | 4–16 | 1–32 |
| MIC50 | 1–16 | 0.25–8 | 2–8 | 0.25–16 | |
| Itraconazole | MIC80 | 0.25–2 | 1–2 | 1–2 | 0.25–2 |
| MIC50 | 0.25–2 | 0.25–1 | 1–1 | 0.25–2 | |
Four isolates did not grow.
Three isolates did not grow.
Six isolates did not grow.
Five isolates did not grow.
(iv) MICs for QC strains.
The NCCLS M27A document recommends a visual endpoint for the microdilution method (4). Thus, the MIC of amphotericin B is defined as the lowest concentration exhibiting a complete inhibition of growth. The MICs of flucytosine and the azoles are defined as the lowest concentrations at which a prominent decrease in turbidity is detected (4). Furthermore, the MIC ranges for QC strains were determined by the macrodilution method (4). Despite this fact, we have compared the results obtained by the spectrophotometric determination of the MICs with the reference range obtained by the NCCLS M27A method (4). The spectrophotometric MIC values obtained at 48 h are shown in Table 3. For NCCLS M27A and RPMI–2% glucose methodologies, all MIC50s, static or shaken, of C. krusei ATCC 6258 were within the published range (4). With NCCLS M27A methodology and C. parapsilosis only the MIC80s of shaking cultures were in the reference range (4). For the QC strains, static or shaken, all amphotericin B MICs were within the published range (4).
TABLE 3.
MIC ranges for QC strains
| Methodology and antifungal agent | MIC range (μg/ml) for:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
C. krusei ATCC 6258
|
C. parapsilosis ATCC 22019
|
|||||||||
| Referencea | MIC80
|
MIC50
|
Reference | MIC80
|
MIC50
|
|||||
| Shaken | Static | Shaken | Static | Shaken | Static | Shaken | Static | |||
| NCCLS M27A | ||||||||||
| ABb | 0.5–2 | 1–2 | 1–1 | NDg | ND | 0.25–1 | 0.25–0.5 | 0.25–0.5 | ND | ND |
| 5Fc | 4–16 | 16–16 | 16–16 | 16–16 | 8–16 | 0.12–0.5 | 0.25–0.5 | 0.5–0.5 | 0.25–0.5 | 0.25–0.5 |
| FZd | 16–64 | 32–32 | 32–32 | 32–32 | 32–32 | 2–8 | 2–4 | 2–4 | 2–2 | 1–1 |
| IZe | 0.12–0.5 | 0.5–1 | 0.5–0.5 | 0.25–0.25 | 0.12–0.25 | 0.06–0.25 | 0.12–0.25 | 0.03–0.12 | 0.03–0.06 | 0.015–0.06 |
| RPMI–2% glucose | ||||||||||
| AB | NAf | 2–2 | 1–2 | ND | ND | NA | 1–1 | 1–1 | ND | ND |
| 5F | NA | 16–16 | 8–8 | 8–8 | 4–4 | NA | 1–1 | 0.5–1 | 0.5–1 | 0.25–0.5 |
| FZ | NA | 64–64 | 64–128 | 64–64 | 64–64 | NA | 16–16 | 4–8 | 4–8 | 4–4 |
| IZ | NA | 1–2 | 1–1 | 0.25–0.5 | 0.12–0.25 | NA | 0.12–0.5 | 0.5–0.5 | 0.12–0.25 | 0.25–0.5 |
| BYNB | ||||||||||
| AB | NA | 2–8 | 2–2 | ND | ND | NA | 2–4 | 2–2 | ND | ND |
| 5F | NA | 32–32 | 32–32 | 16–32 | 16–16 | NA | 0.5–1 | 0.12–0.5 | 0.5–0.5 | 0.12–0.25 |
| FZ | NA | 128–128 | 64–128 | 64–128 | 64–64 | NA | 4–8 | 4–8 | 4–4 | 4–4 |
| IZ | NA | 2–2 | 1–2 | 0.5–1 | 0.5–1 | NA | 0.5–1 | 0.5–1 | 0.5–0.5 | 0.25–0.5 |
Following the data published in reference 4 for macrodilution broth.
AB, amphotericin B.
5F, flucytosine.
FZ, fluconazole.
IZ, itraconazole.
NA, not available.
ND, not done.
In summary, this work demonstrates that agitation plus a higher inoculum size is necessary for performing antifungal susceptibility testing of C. neoformans. The growth of C. neoformans in static cultures is very poor. Furthermore, susceptibility tests are generally designed to measure the antimicrobial activity under conditions yielding adequate growth; for C. neoformans, this means agitation of cultures. Thus, for future development of antifungal susceptibility testing inclusion of agitation during incubation, particularly with C. neoformans, should be considered. Our preliminary results suggest that RPMI or BYNB, an inoculum size of 104 or 105 CFU/ml, and agitation of microplates may be a starting point for this development.
REFERENCES
- 1.Ghannoum M A, Ibrahim A S, Fu Y, Shafiq M C, Edwards J E, Jr, Criddle R S. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol. 1992;30:2881–2886. doi: 10.1128/jcm.30.11.2881-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen R A, Bozzette S A, Jones B E, Haghighat D, Leal M A, Forthal D, Bauer M, Tilles J G, McCutchan J A, Leedom J M. Fluconazole combined with flucytosine for treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1994;19:741–745. doi: 10.1093/clinids/19.4.741. [DOI] [PubMed] [Google Scholar]
- 3.Lozano-Chiu M, Paetznick V L, Ghannoum M A, Rex J H. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodologies. J Clin Microbiol. 1998;36:2817–2822. doi: 10.1128/jcm.36.10.2817-2822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. 1997. Approved standard. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 5.Odds F C, De Backer T, Dams G, Vranckx L, Woestenborghs F. Oxygen as limiting nutrient for growth of Cryptococcus neoformans. J Clin Microbiol. 1995;33:995–997. doi: 10.1128/jcm.33.4.995-997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odds F C, Vranckx L, Woestenborghs F. Antifungal susceptibility testing of yeasts: evaluation of technical variables for test automation. Antimicrob Agents Chemother. 1995;39:2051–2060. doi: 10.1128/aac.39.9.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powderly W G, Keath E J, Sokol-Anderson M, Kitz D, Kobayashi G. Amphotericin-B resistant Cryptococcus neoformans in a patient with AIDS. Infect Dis Clin Pract. 1992;1:314–316. [Google Scholar]
- 8.Rodríguez-Tudela J L, Berenguer J, Martínez-Suárez J V, Sanchez R. Comparison of a spectrophotometric microdilution method with RPMI–2% glucose with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro susceptibility testing of amphotericin B, flucytosine, and fluconazole against Candida albicans. Antimicrob Agents Chemother. 1996;40:1998–2003. doi: 10.1128/aac.40.9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Tudela J L, Martinez-Suarez J V. Improved medium for fluconazole susceptibility testing of Candida albicans. Antimicrob Agents Chemother. 1994;38:45–48. doi: 10.1128/aac.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez Tudela J L, Martinez Suarez J V. Defining conditions for microbroth antifungal susceptibility tests: influence of RPMI and RPMI-2% glucose on the selection of endpoint criteria. J Antimicrob Chemother. 1995;35:739–749. doi: 10.1093/jac/35.6.739. [DOI] [PubMed] [Google Scholar]