Abstract
Background
Both transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) are effective methods for the treatment of recurrent hepatocellular carcinoma (RHCC). Thus far, it is unclear which method is more satisfactory in short- and long-term survival benefits.
Purpose
To compare the overall survival (OS) and complications of TACE and RFA used for the management of RHCC.
Material and Methods
A literature search was carried out using PubMed, the Cochrane Library and, Embase databases, and Google Scholar, keywords including “RHCC,” “TACEC,” and “RFA” with a cutoff date of 30 April 2021. Used Review Manager software was to calculate short- and long-term OS. The clinical outcomes are major complications and complete response (CR).
Results
Finally, nine clinical trials met the research standard, including 1326 subjects, of which 518 received RFA and 808 received TACE. The analysis showed that patients who underwent RFA had significantly higher 1-, 3-, and 5-year OS (OR1-year = 1.92, 95% confidence interval (CI) = 1.27–2.91, p = .002; OR3-year = 1.64, 95% CI = 1.30–2.08, p <.0001; OR5-year = 3.22, 95% CI = 1.34–7.72, p=.009). Besides, the patients who chose RFA had an obvious higher rate of CR than those receiving TACE (OR = 33.75, 95% CI = 1.73–658.24, p = .002). However, the major complications were consistency between these two groups.
Conclusion
Our study discovered that RFA had greater CR and incidence in both the short-term and long-term OS than TACE. In addition, obvious difference was not found in major complications in these two methods.
Keywords: Recurrent hepatocellular carcinoma, radiofrequency ablation, transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death in men worldwide, and it is noteworthy that China accounts for half of all global liver cancer cases. 1 Even after hepatectomy with negative resection margins (R0 hepatectomy), the recurrence rate at 5 years is still more than 70%, resulting in a reduction in overall survival (OS). 2 Although we can obtain authoritative European and American expert consensus for the treatment of primary HCC,3,4 there is no clear guideline for managing recurrent lesions. Recurrent hepatocellular carcinoma (RHCC) differs from primary HCC in that RHCC has a smaller residual liver size and lower liver function. 5 Therefore, few individuals with RHCC can accept further surgery. Furthermore, the scarcity of liver donors and exorbitant medical costs significantly limit the use of liver transplantation in RHCC.
Interventional methods such as radiofrequency ablation (RFA) and transarterial chemoembolization (TACE), are widely carried out in clinical practice because of their high reproducibility, low complications, and definite efficacy.6–8
However, previous trials comparing RFA and TACE for RHCC have been characterized by small sample sizes, inconsistent findings. Meta-analysis is considered to be a credible tool to solve controversial clinical problems. Therefore, we performed this study in order to provide a basis for patients with RHCC to select a more optimal therapy.
Methods
Search strategy
Two authors carried out an independent and careful search of published researches using PubMed, the Cochrane Library, the Embase database, and Google Scholar for prospective cohort studies (PCS), retrospective cohort studies (RCS), and randomized controlled trials (RCT) that studied effectiveness and complications about RFA and TACE on RHCC. The last search was performed on 30 April 2021. Search words included “recurrent hepatocellular carcinoma,” “recurrent liver cancer,” “RHCC,” “transarterial chemoembolization,” “TACE,” “radiofrequency ablation,” and “RFA”. This study only searched the published English literature. The included studies were later reviewed to judge whether they met our inclusion standard.
Inclusion and exclusion criteria
The inclusion criteria for all studies: (i) imaging diagnosis of RHCC after hepatectomy, RFA or liver transplantation; (ii) for interventions, RFA was used in the experimental group, and TACE was used in the control group; (iii) the study must clearly state at least one item of the OS, complete response (CR) and any major complications (including tumor bleeding, pleural effusion, hypotension, abscess, urinary retention, fever, wound infection, ascites). The exclusion criteria are the following: (i) case reports, comments, letters, or experiments; (ii) studies without a matched control group; (iii) a meeting summary without full text; (ii) patients received TACE and RFA for bridging to liver transplantation or rehepatectomy.
Extraction and evaluation of quality
Basic information extracted for each research was the name of the first author, year of publication, type of study, country or region of the first author, type of TCAE, sample size, gender, Child-Pugh stage, number of lesions, time to the first recurrence, and tumor size. The clinical outcomes extracted were major complications, OS and CR. Because all the included studies here are non-randomized controlled trials, this quality assessment adopted the Newcastle–Ottawa scale (NOS). 9
Statistical analysis
For each included study, odds ratios (OR) obtained 95% confidence intervals (CI) of statistical variables for OS, CR, and major complications were obtained. The heterogeneity of the experiment was evaluated by I2 test. The random-effect model was used when I2> 50% or otherwise, the fixed-effect model was used. Publication bias was evaluated via a funnel plot, and asymmetry of the graph indicated the possible publishing bias. We used the Cochrane Review Manager software for this meta-analysis. We chose whether p < .05 as the statistical difference criterion.
Results
Study screen
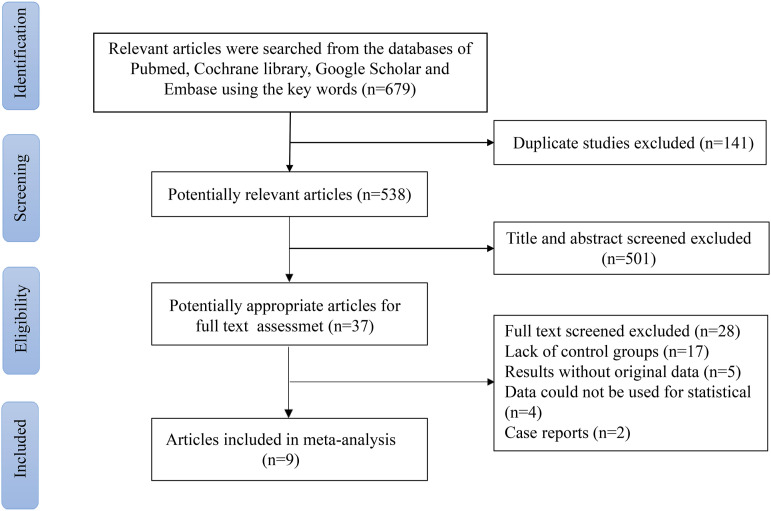
Two authors independently conducted the searches. Finally, a total of 679 articles were found through the initial search. Because of duplication, 141 articles were excluded; and 501 articles were excluded after browsing their heading and summary. Among these trials, 17 were removed due to the lack of a control group, five studies lacked the original data, four were not available for statistical analysis, and two were case reports. In the end, nine clinical trials met the inclusion standard in this meta-analysis (Fig 1). A total of 1326 samples (518 received RFA and 808 received TACE) were involved in this analysis. Eight were retrospective cohort studies,10–17 and one was a prospective cohort study. 18 The baseline data for nine included clinical trials are given in Table 1; information about all patients with RHCC is shown in Table 2; Table 3 lists the patients' eligibility criteria and the median number of TACE repetitions for each study.
Fig. 1.
Flow diagram of the literature selection.
Table 1.
Basic characteristics of the studies.
| Author | Year | Country | Type of study | Type of TCAE | Treatment | No. of patients | Median age | Sex (M/F) | Score (NOS) |
|---|---|---|---|---|---|---|---|---|---|
| Ueno 10 | 2009 | Japan | RCS | cTACE | RFA | 10 | 68 | 10/0 | 6 |
| TACE | 13 | 72 | 8/5 | ||||||
| Qkuwaki 11 | 2009 | Japan | RCS | cTACE | RFA | 30 | 69 | 16/14 | 6 |
| TACE | 19 | 65 | 12/7 | ||||||
| Koh 12 | 2016 | China | RCS | cTACE | RFA | 42 | 63 | 32/10 | 8 |
| TACE | 60 | 57 | 51/9 | ||||||
| Chen 13 | 2016 | China | RCS | cTACE | RFA | 32 | NA | 28/4 | 9 |
| TACE | 78 | NA | 71/7 | ||||||
| Kim 14 | 2017 | Korea | RCS | cTACE | RFA | 6 | 50 | 5/1 | 6 |
| TACE | 21 | 57.1 | 20/1 | ||||||
| Joliat 15 | 2017 | Switzerland | RCS | NA | RFA | 18 | 64 | 13/5 | 7 |
| TACE | 16 | 67 | 14/2 | ||||||
| Wang 16 | 2020 | China | RCS | cTACE DEB-TACE |
RFA | 47 | 60.6 | 34/13 | 8 |
| TACE | 32 | 61 | 28/4 | ||||||
| Kim 17 | 2020 | Korea | RCS | NA | RFA | 171 | 56 | 137/34 | 7 |
| TACE | 230 | 56 | 187/43 | ||||||
| Wang 18 | 2015 | China | PCS | NA | RFA | 162 | 52.7 | 148/14 | 8 |
| TACE | 339 | 51 | 301/38 |
RCS, retrospective cohort study; PCS, prospective cohort study; NA, not applicable; NOS, Newcastle-Ottawa scale; RFA, radiofrequency ablation; TACE, transarterial chemoembolization, cTACE, conventional TACE; DEB-TACE, drug-eluting bead TACE.
Table 2.
Characteristic of patients with RHCC.
| Study | The first treatment | Treatment | Tumor size (cm) | Tumor number (1/>1) | Child-pugh’ score (A/B) | Time to first recurrence (months) |
|---|---|---|---|---|---|---|
| Ueno 10 | Resection | RFA | 1.8(1.0–2.4) | 10/0 | 7/3 | 30(16–60) |
| TACE | 1.9(1.2–2.8) | 13/0 | 11/2 | 29(9–49) | ||
| Qkuwaki 11 | RFA | RFA | 1.6±0.6 | NA | 23/7 | 23.6 ± 13.7 |
| TACE | 1.4±0.4 | NA | 11/8 | 21.4 ± 14.4 | ||
| Koh 12 | Resection | RFA | 2.0(1.0–4.9) | 31/11 | 39/3 | 8.92(1.48–83.31) |
| TACE | 1.5(0.5–4.5) | 35/20 | 60/0 | 5.92(0.85–58.10) | ||
| Chen 13 | Resection | RFA | 1.9±0.6 | 22/6 | 30/2 | 17.6±15.4 |
| TACE | 1.9±1.0 | 55/23 | 75/3 | 9.7±21.5 | ||
| Kim 14 | Transplantation | RFA | 1.8(1.1–3.0) | 6/0 | NA | 27.7(11.8–38.6) |
| TACE | 1.4(1.6–7.7) | 21/0 | NA | 19.0(3.5–108.7) | ||
| Joliat 15 | Resection | RFA | NA | NA | NA | NA |
| TACE | NA | NA | NA | NA | ||
| Wang 16 | RFA | RFA | 2.18 ± 0.5 | 33/14 | 39/8 | 10.76 |
| TACE | 2.08 ± 0.5 | 17/15 | 25/7 | 10.04 | ||
| Kim 17 | Resection | RFA | 1.4(0.2–4.8) | 170/1 | 171/0 | 18(1–85) |
| TACE | 1.3(0.5–4.1) | 228/2 | 230/0 | 13.5(1–116) | ||
| Wang 18 | Resection | RFA | 2.3 ± 0.7 | 107/55 | NA | NA |
| TACE | 2.2 ± 0.9 | 204/135 | NA | NA |
RFA, radiofrequency ablation; TACE, transarterial chemoembolization; NA, not applicable.
Table 3.
The eligibility criteria and the median number of TACE repetitions for each study.
| Author, year | The selection criteria of RFA | The selection criteria of TACE | The median number of TACE repetitions |
|---|---|---|---|
| Ueno, 2009 10 | Single nodule with tumor diameter ≤3 cm | Same as RFA | NA |
| Qkuwaki, 2009 11 | Tumor number ≤3, each≤3 cm | >3 cm or unsuitable locations for RFA | NA |
| Koh, 2016 12 | Tumor size ≤5 cm, tumor number ≤3, Child’s A or selected patients with Child’s B cirrhosis and no gross ascites | Same as RFA | NA |
| Chen, 2016 13 | A single tumor ≤5 cm or tumor number ≤3, each≤3 cm; Child-Pugh class A or B | Same as RFA | 3(range, 1–9) |
| Kim, 2017 14 | Tumor number ≤3, each≤3 cm | Nodules >3, ≥3 cm in size | 1(range, 1–4) |
| Joliat, 2017 15 | Unique, <3 cm, and in one lobe | Multilobar and only intrahepatic | NA |
| Wang, 2020 16 | Tumor size ≤3 cm, ≤3 tumors, Child’s A or selected Child’s B cirrhosis, and no gross ascites | Same as RFA | 2 |
| Kim, 2020 17 | NA | NA | NA |
| Wang, 2015 18 | The tumors met the Milan criteria, Child-Pugh class score ≤8 | Same as RFA | NA |
PEI, percutaneous ethanol injection; TB, total bilirubin; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; NA, not applicable.
Overall survival
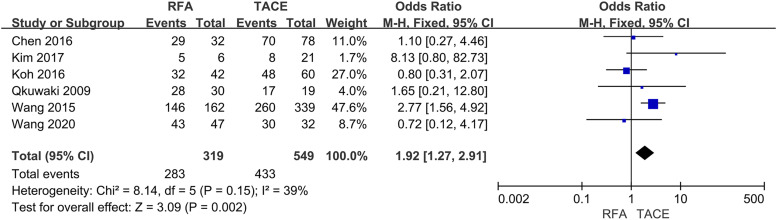
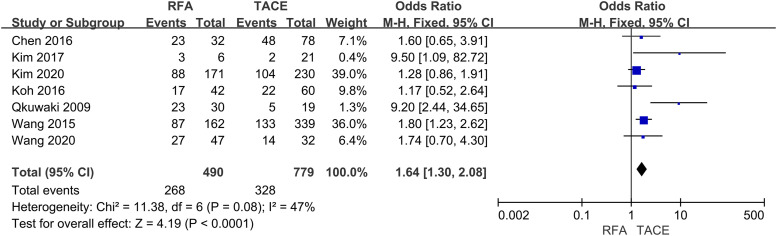
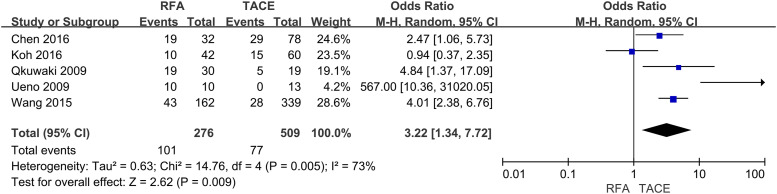
Six studies reported 1-year OS, seven trials described 3-years OS, and five trials described 5-year OS. This meta-analysis indicated that the RFA group was significantly associated with higher 1-year (OR = 1.92, 95% CI = 1.27–2.91, p = .002) (Fig 2), 3-years (OR = 1.64, 95% CI = 1.30–2.08, p < .0001) (Fig 3) and 5-years (OR = 3.22, 95% CI = 1.34–7.72, p = .009) OS (Fig 4) compared with the TACE group.
Fig. 2.
Forest plot on 1-year OS.
Fig. 3.
Forest plot on 3-year OS.
Fig. 4.
Forest plot on 5-year OS.
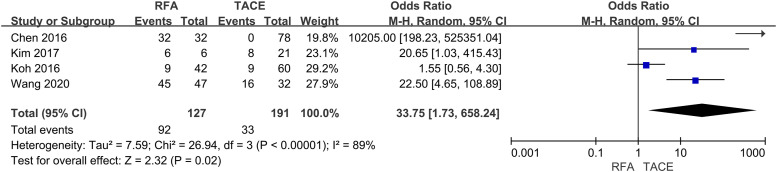
Complete response
Of the nine studies, four provided data on CR. Statistical calculation of four trials was conducted by using the random effects model showed CR was higher in patients underwent RFA (OR= 33.75, 95% CI: 1.73–658.24, p < .05) (Fig 5).
Fig. 5.
Forest plot on CR.
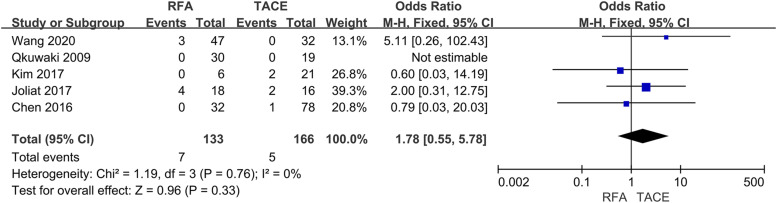
Major complications
Five studies involving 299 subjects reported major complications, and there was no statistical difference between RFA and TACE (OR = 1.78, 95% CI = 0.55–5.78, p =0.33) (Fig 6).
Fig. 6.
Forest plot on major complications.
Publication bias
Because only nine studies were included in study, funnel plots were not used to evaluate publication bias.
Discussion
To our knowledge, this is the first meta-analysis to compare the effectiveness and complications of RFA and TACE on the management of RHCC. Most patients with RHCC cannot undergo liver resection or salvage liver transplantation to achieve a cure because they commonly have low liver function due to cirrhosis and there is a worldwide shortage of livers available for transplantation.19–21 Currently, RFA and TACE are widely used in patients with unresectable recurrent liver lesions.
RFA was first used for recurrent liver cancer in 1999, and therefore, patients with RHCC have more therapy options in addition to rehepatectomy. 22 It is an effective method to ablate tumors by one or several electrodes inserted into the tumor’s interior to generate heat. Interestingly, liver cirrhosis can increase the local control rate of radiofrequency ablation, because the fibrotic tissue around the tumor can effectively isolate heat by reducing the blood flow rate, which is called the “oven effect”. 23 A recent meta-analysis shows no statistical difference in OS and progression-free survival between RFA and Rehepatectomy when recurrent tumors are less than 3 cm in diameter, but RFA has less complications. 24 The advantages of RFA include short operation time, short postoperative hospital stay, fewer surgical complications, clear curative effect, and accurate tumor location.25,26 Using more advanced RFA systems or overlapping ablation strategy, 4–5 cm tumors can be wholly ablated . 27
TACE is a well-proven treatment that can improve the OS of patients with liver malignancies. It blocks the important blood supply arteries of the neoplasm to lead to necrosis. Therefore, the surgeon must use imaging to fully observe the tumor’s blood supply to achieve the best treatment result. Furthermore, chemotherapeutic drugs can be delivered into the hepatic artery branches to increase the antitumor impact while having less side effects than systemic chemotherapy. 18 TACE can be conducted in conventional TACE (cTACE) and drug-eluting bead TACE (DEB-TACE). Previous meta-analysis has shown that cTACE and DEB-TACE have similar efficacy and complications. 28 Xiao et al., studied the effectiveness of rehepatectomy/RFA compared with TACE on the remedy for RHCC and concluded when the recurrent lesions were in the stage of 0–A in Barcelona Clinical Liver Cancer (BCLC), the patients who received rehepatectomy/RFA would have a longer OS than TACE. In contrast, in BCLC stage B–C, a noticeable difference was not obtained between TACE and rehepatectomy, and so did RFA compared with TACE. 29 However, Jin et al., discovered that in BCLC stage 0–A, there were more satisfactory OS and recurrence-free survival with TACE as contrasted to rehepatectomy/RFA. 30
The outcome of this study indicated that RFA conferred a clear advantage in terms of enhancing 1–3, and 5-year OS and CR, while there was no significant difference in major complications when comparing RFA with TACE. These findings are similar to a previous study comparing TACE and RFA for primary HCC . 31 The following explanations may assist in comprehending these findings. RFA is a physical method that can completely destroys tumor tissue; yet, by restricting the tumor’s major blood supply artery, TACE is unable to achieve similar results.
It is considered that RFA is suitable for single tumor ≤5 cm, or 2–3 tumors with a maximum diameter ≤3 cm; Child-Pugh A or B and no vascular invasion and distant metastasis.32–34 Although RFA can be as effective as rehepatectomy when the tumor is ≤3 cm in diameter , 24 the volume of coagulation necrosis induced by RFA is limited. Livraghi et al. first discovered when the diameter is 3–5 cm, the rate of complete necrosis induced by RFA has been much lower than that of ≤3 cm . 35 Therefore, it is considered that RFA alone is not suitable for the treatment of large or medium-sized liver lesions. Apart from this, RFA has a low efficacy when the tumor margins are irregular or adjacent to essential anatomical structures such as some key branches of portal vein and common hepatic artery, diaphragm, and stomach. On the other hand, TACE is applicable to the most of RHCC patients and it is the first-choice treatment for nodules ≥4 or in multiple lobes. 11 In addition, TACE should be conducted as a repetitive procedure due to the incomplete necrotic effect. 11 TACE is also often used as an alternative to RFA in certain situations, including tumor locations near critical structures, poor visualization of lesions on ultrasound, hard to reach lesions percutaneously, inadequate safety margins, and patient desires and costs. 13 However, TACE is absolutely contraindicated in severe liver dysfunction (Child-Pugh C) including jaundice, hepatic encephalopathy, refractory ascites, and uncorrectable coagulopathy.
This is the first meta-analysis to compare the survival benefit and complications of RFA with TACE for RHCC. Without a doubt, our meta-analysis still has some flaws. Above all, eight of nine included trials were RCS, and the remaining one belongs to PCS. Second, in this meta-analysis, different initial therapies were received by patients prior to recurrence, including hepatectomy, RFA, and liver transplantation, which may reduce the credibility of the research. Third, the number of TACE performed could have a great impact on the OS; however, only three of nine studies reported such data (Table 3). Furthermore, the treatment strategy for RHCC is routinely multidisciplinary, involving immunotherapy, targeted therapy, while we only looked at RFA with TACE.
In conclusion, our study discovered that RFA had greater CR and incidence in both the short-term and long-term OS than TACE. In addition, obvious difference was not found in major complications in these two methods. RFA may be the preferred option when recurrent lesions ≤5 cm in size, or 2–3 tumors with a maximum diameter ≤3 cm. In contrast, TACE has a broader indication and is recommended when tumor locations near critical structures and lesions are hard to reach for percutaneous RFA. In addition, Child-Pugh stage should be considered when performing these treatments.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Nos. 81802778) and the Key Research and Development Project of the Science & Technology Department of Sichuan Province (Nos.2021YFS0231).
ORCID iD
Haoxian Gou https://orcid.org/0000-0002-6529-9125
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. American association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver . European association for the study of the liver. EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 4.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018; 68: 723–750. [DOI] [PubMed] [Google Scholar]
- 5.Marasco G, Colecchia A, Colli A, et al. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol 2019; 70(3): 440–448. [DOI] [PubMed] [Google Scholar]
- 6.Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015; 261: 947–955. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol 2015; 41: 236–242. [DOI] [PubMed] [Google Scholar]
- 8.Ho CM, Lee PH, Shau WY, et al. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery 2012; 151: 700–709. [DOI] [PubMed] [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25(9): 603–605. [DOI] [PubMed] [Google Scholar]
- 10.Ueno M, Uchiyama K, Ozawa S, et al. Prognostic impact of treatment modalities on patients with single nodular recurrence of hepatocellular carcinoma. Surg Today 2009; 39: 675–681. [DOI] [PubMed] [Google Scholar]
- 11.Okuwaki Y, Nakazawa T, Kokubu S, et al. Repeat radiofrequency ablation provides survival benefit in patients with intrahepatic distant recurrence of hepatocellular carcinoma. Am J Gastroenterol 2009; 104: 2747–2753. [DOI] [PubMed] [Google Scholar]
- 12.Koh PS, Chan AC, Cheung TT, et al. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford) 2016; 18: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, Gan Y, Ge N, et al. Transarterial chemoembolization versus radiofrequency ablation for recurrent hepatocellular carcinoma after resection within barcelona clinic liver cancer stage 0/a: a retrospective comparative study. J Vasc Interv Radiol 2016; 27: 1829–1836. [DOI] [PubMed] [Google Scholar]
- 14.Kim SS, Kang TW, Song KD, et al. Radiofrequency ablation and transarterial chemoembolisation as first-line treatment for recurrent hepatocellular carcinoma or isolated intrahepatic recurrent hepatocellular carcinoma in transplanted livers. Clin Radiol 2017; 72: 141–149. [DOI] [PubMed] [Google Scholar]
- 15.Joliat GR, Allemann P, Labgaa I, et al. Treatment and outcomes of recurrent hepatocellular carcinomas. Langenbecks Arch Surg 2017; 402: 737–744. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Liang H, Lu Z. Efficacy of transarterial chemoembolization compared with radiofrequency ablation for the treatment of recurrent hepatocellular carcinoma after radiofrequency ablation. Minim Invasive Ther Allied Technol 2020; 29: 344–352. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Joh JW, Yi NJ, et al. Living donor liver transplantation should be cautiously considered as initial treatment in recurrent hepatocellular carcinoma within the Milan criteria after curative liver resection. Ann Transl Med 2020; 8: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol 2015; 41: 236–242. [DOI] [PubMed] [Google Scholar]
- 19.Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg 2003; 238: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim C, Shinkawa H, Hasegawa K, et al. Salvage liver transplantation or repeat hepatectomy for recurrent hepatocellular carcinoma: An intent-to-treat analysis. Liver Transpl 2017; 23: 1553–1563. [DOI] [PubMed] [Google Scholar]
- 21.Fang JZ, Xiang L, Hu YK, et al. Options for the treatment of intrahepatic recurrent hepatocellular carcinoma: Salvage liver transplantation or rehepatectomy? Clin Transpl 2020; 34: 13831. [DOI] [PubMed] [Google Scholar]
- 22.Kainuma O, Asano T, Aoyama H, et al. Recurrent hepatocellular carcinoma successfully treated with radiofrequency thermal ablation. J Hepatobiliary Pancreat Surg 1999; 6: 190–194. [DOI] [PubMed] [Google Scholar]
- 23.Livraghi T, Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 1999; 210: 655–661. [DOI] [PubMed] [Google Scholar]
- 24.Yang D, Zhuang B, Wang Y, et al. Radiofrequency ablation versus hepatic resection for recurrent hepatocellular carcinoma: an updated meta-analysis. BMC Gastroenterol 2020; 20: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izzo F, Granata V, Grassi R, et al. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019; 24: e990–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Kang T W, Cha D DI, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: Propensity score analyses of long-term outcomes. J Hepatol 2018; 69: 70–78. [DOI] [PubMed] [Google Scholar]
- 27.Rhim H, Goldberg SN, Dodd GD, 3rd, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics 2001; 21: S17–S19. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Cao C, Wei X, et al. A comparison between drug-eluting bead-transarterial chemoembolization and conventional transarterial chemoembolization in patients with hepatocellular carcinoma: A meta-analysis of six randomized controlled trials. J Cancer Res Ther 2020; 16: 243–249. [DOI] [PubMed] [Google Scholar]
- 29.Xiao H, Chen ZB, Jin HL, et al. Treatment selection of recurrent hepatocellular carcinoma with microvascular invasion at the initial hepatectomy. Am J Transl Res 2019; 11: 1864–1875. [PMC free article] [PubMed] [Google Scholar]
- 30.Jin Y-J, Lee J-W, Lee OHH, et al. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol 2014; 29: 1056–1064. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Deng T, Zeng L, et al. Efficacy and safety of radiofrequency ablation and transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma: A meta-analysis. Hepatol Res 2016; 46: 58–71. [DOI] [PubMed] [Google Scholar]
- 32.Chen M-S, Li J-Q, Zheng YY, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006; 243: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57: 794–802. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa K, Aoki T, Ishizawa T, et al. Comparison of the therapeutic outcomes between surgical resection and percutaneous ablation for small hepatocellular carcinoma. Ann Surg Oncol 2014; 21: 348–355. [DOI] [PubMed] [Google Scholar]
- 35.Livraghi T, Lazzaroni S, Meloni F. Radiofrequency thermal ablation of hepatocellular carcinoma. Eur J Ultrasound 2001; 13: 159–166. [DOI] [PubMed] [Google Scholar]