Abstract
The main physiological function of the lung is gas exchange, mediated at the interface between the alveoli and the pulmonary microcapillary network and facilitated by conducting airway structures that regulate the transport of these gases from and to the alveoli. Exposure to microbial and environmental factors such as allergens, viruses, air pollution, and smoke contributes to the development of chronic lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and lung cancer. Respiratory diseases as a cluster are the commonest cause of chronic disease and of hospitalization in children and are among the three most common causes of morbidity and mortality in the adult population worldwide. Many of these chronic respiratory diseases are associated with inflammation and structural remodelling of the airways and/or alveolar tissues. They can often only be treated symptomatically with no disease-modifying therapies that normalize the pathological tissue destruction driven by inflammation and remodelling. In search for novel therapeutic strategies for these diseases, several lines of evidence revealed the WNT pathway as an emerging target for regenerative strategies in the lung. WNT proteins, their receptors, and signalling effectors have central regulatory roles under (patho)physiological conditions underpinning lung function and (chronic) lung diseases and we summarize these roles and discuss how pharmacological targeting of the WNT pathway may be utilized for the treatment of chronic lung diseases.
Keywords: Ageing, Asthma, COPD, Fibrosis, Lung, Lung cancer, Regeneration, WNT
1. Introduction
The main physiological function of the lung is gas exchange of O2 and CO2, mediated at the interface between the alveoli and the pulmonary microcapillary network and facilitated by conducting airway structures that regulate the transport of these gases from and to the alveoli. Being in direct contact with the outside world, the lung needs to be resilient against microbial and environmental exposures such as bacteria, viruses, fungi, air pollution, and many other factors. Unfortunately, microbial exposures not seldomly trigger respiratory infections, whereas exposure to environmental factors such as allergens, air pollution, and smoke contributes to asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and lung cancer. Respiratory diseases as a cluster are the commonest cause of chronic disease and of hospitalization in children and are among the three most common causes of morbidity and mortality in the adult population. Many of these chronic respiratory diseases are associated with inflammation and structural remodelling of the airways and/or alveolar tissues and can often only be treated symptomatically with no disease-modifying therapies that are able to stop or even reverse the pathological inflammation and remodelling of lung tissue.
In search for novel therapeutic strategies for these diseases, the WNT pathway is increasingly in the focus as a major contributor to physiological repair but further to pathophysiological lung inflammation and remodelling (Skronska-Wasek et al. 2018; Vladar and Konigshoff 2020; Burgy and Konigshoff 2018). WNT signalling is explained in more detail elsewhere in this handbook. Briefly, the WNT family of secreted glycoproteins consists of 19 members, some of which with (partially) overlapping binding and signalling characteristics involving both the FZD family of G protein coupled receptors and non-FZD receptors such as the receptor tyrosine kinases ROR2, RYK, and PTK7 (Burgy and Konigshoff 2018). The WNT pathway is classically subdivided in a branch dependent on WNT/FZD-mediated stabilization of the intracellular signalling effector β-catenin through mechanisms involving the co-receptors LRP5/6 and inactivation of the β-catenin destruction complex composed of Axin2, APC, GSK-3, and CK1. β-catenin is a cofactor of several transcription factors, leading to the activation of transcriptional programs in cell-type specific manners. In addition, β-catenin-independent signalling functions exist, involving activation of Ca2+ signalling, ROCK signalling, JNK signalling among other pathways associated with cytoskeletal rearrangements and gene transcription (Baarsma et al. 2013a). These two branches are often referred to as β-catenin-dependent and β-catenin-independent.
Here we summarize the central regulatory roles that WNT proteins, their receptors, and signalling effectors have in the physiological and pathophysiological processes underpinning lung function and (chronic) lung disease (Skronska-Wasek et al. 2018; Baarsma and Konigshoff 2017; Lehmann et al. 2016) and discuss how pharmacological targeting of the WNT pathway may be utilized for the treatment of these diseases. In view of the detailed descriptions of the biochemistry and intracellular signalling of these WNT/FZD interactions elsewhere in this handbook, our focus will be on the (patho)physiological roles of WNT signalling in the lung across all stages of life, and in the major respiratory diseases including asthma, COPD, IPF, lung cancer, as well as pulmonary vascular diseases.
2. Physiology of the Lung: Role of WNT Signalling
2.1. Anatomy of the Lung
The human respiratory system is a complex ensemble which guarantees intake of oxygenated air through inhalation and expulsion of CO2-enriched air through exhalation. It can be generally divided into upper and lower respiratory tract. The upper respiratory tract serves as point of entry, conduction and later exit for the air through the nose, pharynx, larynx, and trachea.
The lungs are part of the lower respiratory tract. The human left lung is comprised of two lobes – superior and inferior, while the right lung includes a middle lobe and therefore comprises three lobes. The lungs start from a bifurcation of the trachea into two primary bronchi. These extend into bronchial trees, branching into progressively smaller ramifications defined as secondary and tertiary bronchi, and bronchioles which lead through the alveolar ducts to the prime site for gas exchange, the alveoli (Fig. 1a).
Fig. 1.
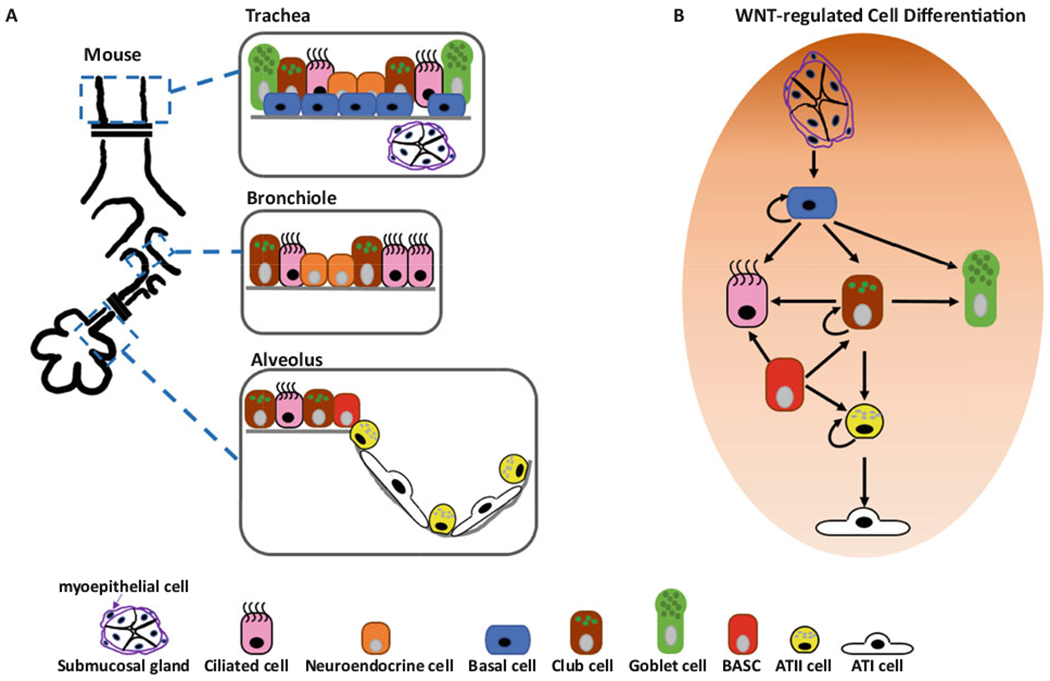
WNT signalling controls lung epithelial cell differentiation. (a) Mouse lung epithelial structure and cell types in trachea, bronchiole, and alveolus. (b) Lung epithelial cell differentiation processes under control of WNT signalling pathways
Starting from the upper conductive airways into the bronchi and the bronchioles, we observe a pseudostratified epithelium with distinct spatial composition and function. Together with neuroendocrine and basal cells, the airways comprise of multi-ciliated and secretory cells that have the important role of maintaining mucociliary clearance. Among the secretory cells, goblet cells are predominantly found in the upper larger airways, while club cells are mainly found in the smaller airways. Separated from the epithelium by a basal membrane, the mesenchyme contains submucosal glands, fibroblasts, smooth muscle cells, nerves, and blood and lymphatic vessel (Wansleeben et al. 2013; Hogan et al. 2014; Nikolic et al. 2018; Tirouvanziam et al. 2009). The terminal bronchioles are defined as the respiratory airway, a region with a monolayer of cuboidal epithelium that leads into the alveoli (Hogan et al. 2014; Basil et al. 2020).
The alveoli represent the most distal part of the lung and are characterized by a specific lobular structure surrounded by a network of capillaries that maximizes the surface available for gas exchange. Here we find a thin epithelium comprised of at least two key cell types: alveolar type I (ATI) and alveolar type II (ATII) cells. ATI are flat and elongated cells specialized in gas exchange; they share the basal lamina with the underlying pulmonary capillary endothelial cells (PCEC) to optimize the efficiency of gas exchange. ATII cells are cuboidal cells which produce and secrete pulmonary surfactant, thereby reducing the surface tension of the alveolar surface area to prevent the lungs from collapsing while breathing. Further, ATII cells serve as progenitor cells for ATI cells (Nikolic et al. 2018; Tirouvanziam et al. 2009; Basil et al. 2020; Ruaro et al. 2021).
There are several types of stromal cells in the interstitial region, including mesenchymal cells, pericytes, endothelial cells, and immune cells. These cells constitute the alveolar niche, a supporting microenvironment for epithelial progenitor cells which plays a major role during both homeostasis and injury (Ruaro et al. 2021; Hogan 2020). The mesenchyme is comprised of different cell types involved in a cross-talk with the epithelium through secretion of growth factors and other key signalling pathways, including WNT. Several types of fibroblasts have been described mainly in mouse, including myofibroblasts, matrix fibroblasts, and lipofibroblasts-like alveolar niche fibroblasts (MANCs); these niche cells produce WNT proteins, which act mainly through WNTβ-catenin signalling to replenish the AXIN2+ epithelial progenitor pool during development and repair (Frank et al. 2016; Lee et al. 2017; Zepp et al. 2017; Nabhan et al. 2018; Ushakumary et al. 2021). Our knowledge of the cell types that populate the various sections of the human respiratory system is continuously evolving, as new subtypes of cells are discovered through single-cell RNA-sequencing of the lung in steady state and disease.
2.2. Cell Type: Specific Expression Patterns of WNTs/FZDs
WNT signalling is a key mediator of cellular cross-talk in the lung. Specific interactions between epithelial, mesenchymal, immune, and endothelial cells have been described in development, homeostasis, and disease, and in the recent years, the advent of single-cell RNA-sequencing has allowed us to identify cell-specific expression of WNT proteins and receptors (Adams et al. 2020; Habermann et al. 2020; Reyfman et al. 2019; Travaglini et al. 2020).
While some WNT proteins and receptors are widely expressed in all cell types, it is important to highlight the ones that seem rather tissue- or cell-specific or have been described to be involved in signalling in specific cellular compartments: Expression of β-catenin-dependent WNT proteins such as WNT-7B or WNT-3A is mostly restricted to the epithelium, with WNT-3A highly enriched in ATI cells (Adams et al. 2020; Habermann et al. 2020; Reyfman et al. 2019; Travaglini et al. 2020). Recently, it was described that ATI cells in the early lung express WNT-3A, WNT-7A, and WNT-7B, which interact with FZD1 and LRP6 on transient secondary crest myofibroblast (Zepp et al. 2021).
In the mouse alveoli, a specific WNT niche has been proposed comprising of a single fibroblast constitutively expressing WNT-5A (and potentially other WNT proteins) to provide juxtacrine signals to the neighboring ATII cell, thereby maintaining stem cell homeostasis and cell fate (Nabhan et al. 2018). In human, within the ATII population, a WNT-responsive lineage has recently been identified to be positive for the WNT target gene Axin2. These cells, named alveolar epithelial progenitors (AEP), comprised 29% of the human ATII population and contributed to functional alveolar epithelial regeneration after injury (Zacharias et al. 2018).
WNT-5A and WNT-5B expression has been primarily linked to fibroblasts, although recent human single-cell data suggest further expression in other cell types in healthy lungs as well, e.g. some epithelial cells (Adams et al. 2020; Habermann et al. 2020); for example, a subset of ATII cells expressing, among others, WNT-5A, LRP5, TCF4, was identified in the adult human lung (Travaglini et al. 2020). WNT-5A and WNT-2 were further identified in subsets of mesenchymal cells at postnatal day 1 in mouse, including smooth muscle and fibroblasts (Guo et al. 2019).
Notably, it has been observed that secretion of WNT proteins and activation of WNT target genes can be mutually exclusive in some cells. For example, a recent study showed through single-cell RNA-seq and in situ RNA hybridization that in both human and mouse, expression of the WNT7B gene is abundant in small airway epithelial cells, such as club cells, but expression of the WNT target gene AXIN2 was not found in these cells, while it was detected only in the alveolar region (Reyfman et al. 2019). In human, among FZDs, FZD1 appears to be mostly expressed in the mesenchyme, FZD4 is highly expressed in the vascular compartment, but also, similarly to FZD5 and FZD6, enriched in ATI and ATII cells (Habermann et al. 2020; Travaglini et al. 2020). Additionally, co-receptors LRP5 and LRP6 are widely expressed in the human lung epithelium, mesenchyme, and endothelium; ROR1 and ROR2 are co-expressed in several subtypes of human lung fibroblasts, while ROR1 is also enriched in ATI cells (Habermann et al. 2020; Reyfman et al. 2019; Travaglini et al. 2020).
LGR5 and LGR6 have been identified as markers of mesenchymal cells in the adult murine lung. LGR6+ cells, including a sub-population of smooth muscle cells in the airway epithelium, promote airway differentiation of epithelial progenitors via WNT-FGF10 cooperation. In the alveoli LGR5+ cells are found, which produce WNT-3A and WNT-5A, promoting alveolar differentiation of both club and ATII cells (Lee et al. 2017; Raslan and Yoon 2020).
2.3. Lung Development
While our understanding of the homeostatic role of WNT signalling in the adult lung is limited, our knowledge about its involvement in orchestrating lung development in mouse has been extensively documented. Numerous WNT genes, including Wnt2, Wnt2b, Wnt5a, Wnt7b, and Wnt11 are expressed at various stages in the developing lung (Harris-Johnson et al. 2009; Lako et al. 1998; Li et al. 2002; Rajagopal et al. 2008). WNT signalling interplays with other key developmental signalling pathways, such as fibroblast growth factor (FGF), bone morphogenetic protein (BMP), and sonic hedgehog (SHH), in a spatial-temporal manner that allows lung specification and full formation of the respiratory system (Rock and Hogan 2011).
Lung development is historically divided into five stages which are similar in human and rodents, although they have been more extensively defined in mouse. Today, these stages are condensed into two main ones: a branching stage that corresponds to the pseudoglandular stage, which in mouse starts at embryonic day (E) 9.5 and ends around E16.5, during which distal epithelial progenitors give rise to the conducting airway epithelium, and an alveolar differentiation stage that starts around E16.5 until several weeks after birth in mouse, and 2–3 years in human, during which distal epithelial progenitors give rise to bipotential alveolar epithelial progenitors that will differentiate directly into alveolar type I (ATI) and ATII cells (Volckaert and De Langhe 2015).
Lung specification begins before the embryonic stage, around E9 in mouse and gestation week 5 in human, from a respiratory primordium which is comprised of endodermal cells that start expressing the respiratory lineage factor Nkx2.1 (Chen 2015; Herriges and Morrisey 2014). These cells are localized in the ventral wall of the anterior foregut endoderm, and by E9.5 in mouse and around 28 days in human, they evaginate and form the two primary lung buds, leading into the embryonic stage (Herriges and Morrisey 2014; Morrisey and Hogan 2010). These buds are comprised of three cell layers: in addition to the inner endoderm-derived epithelial layer, there is a thin outer mesothelium, and a mesoderm layer which will give rise to the mesenchyme and is in continuous cross-talk with the endoderm to direct branching and cell differentiation (Volckaert and De Langhe 2015; Herriges and Morrisey 2014; Hines and Sun 2014; Rawlins 2011).
At this initial stage, β-catenin-dependent WNT signalling is required to define the respiratory fate in the anterior foregut: in fact, mutant mice with combined loss of the Wnt2 and Wnt2b genes lose the Nkx2.1+ lung endoderm progenitors population which leads to impaired branching of the lung buds and trachea formation; at the same time, specification of other foregut derived tissues is not affected, indicating that WNT-2 and WNT-2B regulation is specific to lung (Goss et al. 2009). A similar result is achieved with conditional inactivation of Ctnnb1 (β-catenin) in mouse foregut endoderm (Harris-Johnson et al. 2009).
During the pseudoglandular stage, the lung buds undergo a process defined as “branching morphogenesis,” to create a tree-like structure. At this stage there’s a differential expression of specific markers which will control the proximal-distal axis and cell fate; WNT/β-catenin signalling is also involved in this axis determination, as inactivation of Ctnnb1 in the mouse pseudoglandular stage causes enhanced formation of the conducting airways while restricting formation and differentiation of the peripheral lung (Mucenski et al. 2003). Furthermore, WNT signalling is required for bud formation by inducing apical constriction, while loss of WNT signalling is necessary for air sac formation in the canalicular-saccular stages (Fumoto et al. 2017). Additionally, WNT/β-catenin signalling is involved in the upstream regulation of other essential pathways including N-myc, BMP4, and FGF signalling, acting as a regulator of a molecular hierarchy that promotes distal while repressing proximal airway development (Shu et al. 2005). Interestingly, specific combinations of WNT proteins, such as the interaction between mesenchymal WNT-2 and epithelial WNT-7B, play an essential role in branching morphogenesis, proximal-distal patterning, and development of distal lung progenitors (McCulley et al. 2015; Miller et al. 2012). WNT-7B is also involved in regulating mesenchymal proliferation, late epithelial maturation, and pulmonary vascular smooth muscle differentiation and/or survival (Shu et al. 2005).
During the branching morphogenesis phase, the β-catenin-independent WNT planar cell polarity (PCP) pathway is also involved, as mice mutant for PCP proteins such as Vangl2 (Van Gogh homolog) and Celsr1 (Flamingo homolog) form hypoplastic distal lungs with reduced airway branching (Vladar and Konigshoff 2020; Yates et al. 2010).
Recently, an active role for ATI cells in lung development has been described, with several WNT proteins, such as WNT-7A and WNT-3A, produced primarily by ATI cells that are required for myofibroblasts and alveolar formation (Zepp et al. 2021).
Other WNT proteins which signal through the β-catenin-independent pathway cause aberrant lung development in mutant mice: for example, in Wnt5a(−/−) mice there’s an increased proliferation of both epithelial and mesenchymal cell compartments, causing overexpansion of the distal airways and thickened intersaccular interstitium that leads to delayed lung maturation and causes alterations in the FGF-10, BMP4, and SHH signalling, once again proving the interplay between different epithelial-mesenchymal signalling pathways (Skronska-Wasek et al. 2018; Li et al. 2002; Li et al. 2005; Yin et al. 2008). Furthermore, WNT-5A signalling through ROR1/ROR2 is essential for the later stage of alveologenesis, as postnatal WNT-5A inactivation in mice results in hypoalveolarization of the lungs, due to myofibroblast defects (Li et al. 2020).
In conclusion, a vast array of WNT proteins and receptors are expressed in specific cells and phases of lung development and promote epithelial-mesenchymal cross-talk with other signalling pathways, initiating key steps in the developmental process; our knowledge of these interactions provides a precious insight into the understanding of how these same processes may be involved in regeneration of the adult human lung.
2.4. Epithelial Cell Specification/Regeneration in Adulthood
Unlike organs with high epithelial turnover, such as skin and intestine, the lung is relatively quiescent with slow turnover at homeostasis, however, it exhibits a potential rapid repair upon acute injuries (Rawlins and Hogan 2006; Leeman et al. 2014). Mounting evidence showed that multiple regional specific lung epithelial progenitor cells can differentiate to other epithelial cells to maintain homeostasis or repair in response to injuries (Rawlins and Hogan 2006; Leeman et al. 2014). Remarkably, collective data demonstrated that both WNT/β-catenin and β-catenin-independent WNT signalling pathways are crucial for the functions of nearly all of these progenitor cell function, including self-renewal and differentiation, at homeostasis and during regeneration (Raslan and Yoon 2020) (Fig. 1).
Basal cells, marked by expressions of transcription factor TRP63, cytokeratin 5 (KRT5), and the nerve growth factor receptor (NGFR), are found in human airways proximal to the respiratory airways, and in murine airways proximal to main bronchi (Basil et al. 2020). They maintain airway epithelial structure by self-renewing and differentiating to goblet cells, club cells, or ciliated cells (Fig. 1b). Previous studies demonstrated that activation of WNT/β-catenin signalling was required for basal cell proliferation and promoted their differentiation to ciliated cells, instead of secretory cells (goblet and club cells) (Brechbuhl et al. 2011; Giangreco et al. 2012). In the submucosal gland, a population of basal like progenitor cells called myoepithelial cells were identified to regenerate basal cells and subsequently differentiate to other epithelial cell types upon activation of transcription factor Lef1/TCF7 of WNT/β-catenin signalling pathway (Lynch et al. 2018). Therefore, WNT/β-catenin signalling controls the stemness and differentiation of the proximal airway epithelial progenitor cells. The role of β-catenin-independent WNT signalling in basal cells, however, remains unknown.
In the distal airways, club cells are dome-shaped airway epithelial progenitor cells, which represent ~20% of the bronchiolar epithelium in humans and 50–70% in mice (Okuda et al. 2019; Ito et al. 2000; Boers et al. 1999). Club cells can be identified by expression of a secreted protein called CCSP (also known as CC10, secretoglobin 1A1, or uteroglobin). They possess host defense, barrier maintenance, and progenitor functions (Zuo et al. 2018). In homeostasis, club cells maintain airway epithelial structure by self-renewing followed by differentiation to daughter club cells and ciliated cells (Rawlins et al. 2009). In addition, several studies demonstrated that the club cells formed alveolar organoids expressing ATII cell marker Surfactant Protein C (SPC) in vitro, suggesting the potential of murine club cells to differentiate to ATII cells at homeostasis.
Furthermore, the functional heterogeneity of murine proximal and distal airway club cells has been confirmed in several disease models (Chen et al. 2012; Kim et al. 2005; Liu et al. 2019). Club cells in the trachea are able to differentiate to ciliated cells upon tracheal injury (Rawlins et al. 2009). Cells at the bronchioalveolar-duct junction that express CCSP and SPC were identified as bipotential progenitor cells (Kim et al. 2005). These CCSPpos/SPCpos bronchioalveolar stem cells (BASCs) contribute to airway epithelial repair by replenishing club cells after naphthalene treatment, and they also contribute to alveolar repair by replacing ATII cells after bleomycin challenge (Kim et al. 2005; Liu et al. 2019). In addition, a distinct sub-population of Uroplakin3a+ club cells are resistant to naphthalene and regenerate both airway and alveolar epithelia after bleomycin-induced injury (Guha et al. 2017). Hence, there is strong support for critical club cell functions in lung repair.
The role of WNT signalling in the progenitor function of club cells remains largely unknown. With deficiency of a WNT/β-catenin co-receptor RYK, the club cells were shown to contribute to goblet cell hyperplasia following airway injury, suggesting a potential role of WNT/RYK signalling in the airway repair by club cells (Kim et al. 2019). However, another study showed that deletion of β-catenin did not affect airway regeneration by club cells, arguing whether RYK controls club cell fate through β-catenin pathway or other unrelated signalling pathways. Importantly, using organoid culture, two studies showed that activating WNT/β-catenin signalling with WNT-3A and the GSK3β inhibitor CHIR99021 promoted alveolar but not bronchiolar differentiation of club cells (Lee et al. 2017; Hu et al. 2020). Intriguingly, a study showed that overexpression of an active form of β-catenin leads to abnormal ATII cell differentiation in the conducting airways (Mucenski et al. 2005). These data suggest the critical role of WNT/β-catenin signalling in fate decision of club cells (Lee et al. 2017; Hu et al. 2020). Future studies are need to dissect the potential heterogeneous roles of WNT signalling pathways in different subsets of club cells in homeostasis and diseases.
In the alveoli, ATII cells function as progenitor cells to proliferate and differentiate into ATI cells in vivo, however, infrequently at steady state (Barkauskas et al. 2013; Desai et al. 2014). Importantly, most of ATII cells do not show active WNT signalling at homeostasis. Upon alveolar injury, several distinct ATII sub-populations were reported to be activated to repair the damaged alveolar tissue, including the aforementioned BASC cells, a sub-population of Axin2-positive ATII cells, and an integrin α6β4-positive SPC-negative cell (Nabhan et al. 2018; Zacharias et al. 2018; Kim et al. 2005; Liu et al. 2019; Chapman et al. 2011). Among these progenitor sub-populations, the Axin2-positive ATII cells require activation of WNT/β-catenin signalling for proliferation but not for differentiation into ATI cells (Nabhan et al. 2018). Further, a study showed that ATII progenitor cells required WNT/β-catenin signalling for cell survival and migration post bleomycin-induced injury (Flozak et al. 2010; Tanjore et al. 2013). For ATII-ATI trans-differentiation, both WNT/β-catenin and β-catenin-independent WNT signalling were demonstrated to be critical. The β-catenin-independent WNT-5A/protein kinase C (PKC) signalling enhances β-catenin/p300 interactions to promote ATII cell differentiation (Rieger et al. 2016). Furthermore, both WNT-5A and WNT-5B were recently shown to inhibit WNT/β-catenin signalling and repress proliferation of ATII cells. Unlike WNT-5A, WNT-5B also inhibits ATII-ATI trans-differentiation (Wu et al. 2019). These studies suggested that a fine-tuned temporal control of WNT/β-catenin and β-catenin-independent WNT signalling balance is required during alveolar progenitor proliferation and differentiation.
2.5. Mesenchymal Cell Specification/Differentiation in Adulthood
Airway and alveolar mesenchymal cells such as smooth muscle cells and fibroblasts express high levels of particularly WNT-5A and WNT-5B, which appear to contribute to the maintenance of mesenchymal features such as contractility and ECM production (Koopmans et al. 2016a). These WNT proteins cooperatively interact with TGF-β in the regulation of these functions, in part because TGF-β augments the expression of both WNT-5A and WNT-5B in airway smooth muscle and in lung fibroblasts (Kumawat et al. 2013). The effects of TGF-β on WNT-5A expression are mediated by TGF-β-activated kinase 1 (TAK1), which regulates recruitment of the transcription factor SP1 to the WNT-5A promoter to induce gene expression (Kumawat et al. 2013).
WNT-5A/B and TGF-β subsequently regulate expression of mesenchymal marker proteins such as sm-α-actin, by cooperating at the level of actin polymerization in a RhoA/ROCK controlled manner (Koopmans et al. 2016a). In turn, actin polymerization drives the expression of contractile apparatus genes via myocardin-related transcription factor A (MRTFA) (Wang et al. 2002). In addition to WNT-5A, WNT-11 has similar functions (Kumawat et al. 2016).
Although these effects of WNT-5A/5B/11 are not mediated by classical β-catenin-dependent pathways (Kumawat et al. 2013), this does not mean that β-catenin signalling does not play a role in mesenchymal cells. In fact, β-catenin expression is inducible in response to TGF-β and plays a role in ECM production by TGF-β (Baarsma et al. 2011; Lam et al. 2011). This is not mediated by FZD/AXIN2-dependent β-catenin stabilization, but rather by GSK-3 phosphorylation-dependent activation of β-catenin, redirecting β-catenin to SMAD and CBP dependent gene expression (Gottardi and Konigshoff 2013; Koopmans et al. 2016b) and in that respect, distinct from “classical” WNT/β-catenin signalling. A similar role for β-catenin-dependent, yet WNT/FZD independent signalling was described for PDGF-induced cell proliferation of airway smooth muscle cells (Nunes et al. 2008).
Mesenchymal cells exert important secretory functions with important roles in physiology and disease. The proinflammatory cytokines IL-6 and IL-8 are secreted from lung fibroblasts in a WNT-5B dependent manner through interactions with the FZD2 and FZD8 (van Dijk et al. 2016), whereas smooth muscle derived WNT-5A contributes to Th2 cytokine production and allergic inflammation (Koopmans et al. 2020). Moreover, mesenchymal cells contribute secreted growth factors such as WNTs and FGFs, which support epithelial plasticity and repair. Crucially, there appears to be a distinct segregation of mesenchymal cell functions based on WNT-responsiveness and -activity. In line with what has been outlined above, mesenchymal cells that do not express high levels of “classical” WNT/β-catenin signalling markers are TGF-β responsive and typically enriched in smooth muscle markers such as sm-α-actin and ECM-markers such as collagen 1 (Zepp et al. 2017). In contrast, there appears to be a sub-population co-expressing the WNT/β-catenin marker AXIN2 at high levels along with PDGFR-α with supportive roles in epithelial cell plasticity and repair (Zepp et al. 2017). A distinct segregation of mesenchymal cells supportive of repair can also be made on the basis of expression of LGR subtypes with LGR5+ cells preferentially supporting alveolar epithelial repair and LGR6+ cells preferentially supporting airway repair (Lee et al. 2017). In fact, singlecell sequencing of mouse lung mesenchymal cells identified at least five sub-populations of cells each with distinct WNT and LGR expression profiles (Lee et al. 2017). We lack a good understanding at present of the distinct features of each of these mesenchymal lineages, their functions and dependence on WNT, which will need to be addressed in future studies.
3. Pathology: Role of WNT Signalling
3.1. Normal Lung Ageing
Ageing is characterized by cellular changes that are summarized by nine hallmarks (López-Otín et al. 2013), many of which are influenced by the WNT pathway. Several studies suggest that WNT/β-catenin signalling actively contributes to ageing: Mice deficient for the negative WNT regulator klotho demonstrated increased cellular senescence in skin, intestine, and testis accompanied by reduced stem cell numbers leading to an accelerated ageing phenotype (Liu et al. 2007). Similarly, Brack et al. detected active WNT/β-catenin signalling in aged myogenic progenitors and showed a critical role for WNT signalling in lineage conversion from a myogenic to a fibrogenic lineage (Brack et al. 2007).
While WNT signalling has been investigated in the context of chronic lung disease (see later paragraphs), detailed studies on WNT signalling during normal human lung ageing are scarce. There are a couple of reports describing a change of WNT expression in the aged lung, many of which focused on mouse tissue. The expression of WNT-3A decreased along with increased WNT inhibitor, FRZB in ageing mouse lung (Hofmann et al. 2014). Moreover, WNT-2 exhibited reduced expression in lung mesenchymal stromal cells isolated from older mice (12 months old) when compared to young mice (3 months old) (Paxson et al. 2013). In contrast to this, airway epithelial cell isolated from aged mice showed higher expression of WNT-2 and lower expression of WNT-7A (Aros et al. 2020). Wu et al. describe an upregulation of the β-catenin-independent WNT-5A and WNT-5B in aged whole mouse lungs (Wu et al. 2019). In contrast to the initial studies discussed above that linked active β-catenin signalling to ageing, these latter studies suggest a (potentially cell-type specific) shift from β-catenin-dependent to β-catenin-independent WNT proteins in the aged lung. In addition to the shift of WNT proteins in lung ageing, there is also evidence showing ageing-associated dysregulation of WNT target genes. Several WNT target genes including Tle1, Lef1, Nkd1 (Hofmann et al. 2014), Ccnd2 (Cyclin D2), Ccna1 (Cyclin A1) (Watson et al. 2020) were found to be reduced in the ageing mouse lung, while c-Myc (Zhang et al. 2012a) and Wisp-1 (Chanda et al. 2021) show increased expression. Another study exploring differential expression of WNT target genes in the ageing mouse lung (Hofmann et al. 2014) also observed a decrease in WNT/β-catenin signalling with age on a whole lung level. However, these studies focus on whole lung tissue, and thus cell-specific changes might be diluted. Along these lines, Lehmann et al. described an increased population of epithelial cells displaying high β-catenin activity in the ageing lung (Lehmann et al. 2020a). In contrast, Paxson et al. reported declined β-catenin gene expression in mesenchymal stromal cells from murine aged lungs (Paxson et al. 2013). Data on normal human lung ageing is even more scarce but some reports suggest a regulation similar to the mouse (Kovacs et al. 2014; Zhou et al. 2018). Interestingly, Baarsma et al. demonstrated enhanced expression of β-catenin-independent WNT-5A in senescent primary human lung fibroblasts linked to impaired endogenous lung repair in COPD (Baarsma et al. 2017). These current data indicate a spatiotemporally controlled shift in WNT signalling during ageing, similar to what has been described in other ageing processes, such as in hematopoietic stem cells (Florian et al. 2013).
Ageing-related alterations in cells and cell niches trigger stem cell exhaustion, one hallmark of lung ageing (Meiners et al. 2015), leading to impaired regenerative capacity of the lung. As mentioned above, WNT signalling has been implicated as a contributor to cellular senescence: WNT-5A is found in senescent lung fibroblasts (Baarsma et al. 2017), while WNT/β-catenin is able to induce lung epithelial senescence (Lehmann et al. 2020a). Additionally, Kovacs et al. reported WNT-5A and WNT-11 expression to be reduced in epithelial (EpCAM1+) and increased in nonepithelial (EpCAM1−) cells during senescence, further highlighting a potential WNT signal shift and cellular senescence (Kovacs et al. 2014).
Mitochondrial dysfunction and metabolic distress along with increased ROS production is another hallmark of ageing implicated in lung diseases (Bueno et al. 2015; Braidy et al. 2011). Active WNT/β-catenin signalling during ageing has been linked to increased production of reactive oxygen species (ROS) in mesenchymal stem cells (Zhang et al. 2013; Zhang et al. 2011). Inhibition of the WNT signalling pathway via overexpression of the secreted frizzled-related protein 2 (SFRP2) in A549 (a human lung epithelial cell line) led to mitochondrial dysfunction (Li et al. 2019). The cofactor nicotinamide adenine dinucleotide (NAD+) and its reduced form NADH are key regulator of metabolism, and an age-dependent decline in NAD+ levels and NAD+/NADH ratio was detected in the lung (Braidy et al. 2011; Massudi et al. 2012).
Telomere shortening, believed to drive ageing phenotypes, is associated with lung function decline (Birch et al. 2018) and lung alveolar integrity depends on proper telomere maintenance (Lee et al. 2009). Even though it is not clear how WNT signalling in the lung contributes to telomere length, a regulatory loop connecting WNT signalling and telomerase activity has been suggested in other organs (Zhang et al. 2012b; Park et al. 2009). On the one hand, transient activation of the WNT/β-catenin pathway might induce telomerase reverse transcriptase expression and elevates telomerase activity in different cell lines (Zhang et al. 2012b). On the other hand, telomerase, by serving as a cofactor, might directly modulate WNT/β-catenin in mouse embryonic stem cells (Park et al. 2009).
In summary, although reports of WNT signalling activity in the ageing (human) lung are still limited, there is an emerging concept of a cell-type specific regulation and shift in WNT signalling probably contributing to multiple hallmarks of lung ageing. These findings further warrant cell-type specific studies of β-catenin-dependent and independent WNT signalling in the aged human lung and the modulation of WNT signalling as a potential therapeutic option to target/reverse cellular ageing processes.
3.2. Asthma
Asthma is an obstructive airways disease that affects around 300 million people worldwide. Characteristic clinical features of asthma include airway hyperresponsiveness to specific (e.g., histamine, methacholine) and non-specific (e.g., allergens, cold air) stimuli, and associated with airway inflammation and remodelling. Asthma is a highly heterogeneous disease. Whereas most asthma patients experience symptoms resulting from Th2 dependent eosinophilic inflammation associated with allergies, additional inflammatory phenotypes of asthma exist, often resulting in severe disease. A detailed literature review on the role of WNT signalling in asthma was published previously (Koopmans and Gosens 2018). Here, we will focus on key findings and on findings contributed since this review was published.
Genetic association studies have often been used to predict the functional involvement of genes or pathways of genes in the etiology of asthma. Genomewide association studies have identified two single nucleotide polymorphisms in the WNT11 gene region (El-Husseini et al. 2020). Candidate gene approach studies identified additional roles for genetic variations in the FAM13A gene in asthma (Kerkhof et al. 2014), which was originally identified to associate with COPD (Cho et al. 2010). Additional genetic associations were found for WNT-1-inducible-signalling pathway protein-1 (WISP-1) and WNT inhibitory factor-1 (WIF-1) in children diagnosed with mild to moderate persistent asthma, though both of these were identified on the basis of candidate gene approaches (Sharma et al. 2010). The nature and pathophysiological roles of these WNT pathway genes in asthma are not fully elucidated. WNT-11 is expressed in airway smooth muscle and regulates contractile genes (Kumawat et al. 2016), whereas FAM13A is a protein that supports the degradation of β-catenin (Jiang et al. 2016). WISP1 and WIF1 are both secreted proteins capable of modulating WNT signalling directly. The exact roles of these genes could well reach beyond direct functional effects on airway inflammation or remodelling, however, as these genetic associations may also be explained on the basis of changes in lung development with subsequent impact on asthma susceptibility (Sharma et al. 2010).
Nonetheless, evidence does point to a direct role for WNT signalling in asthmatic inflammation and remodelling. In line with what has been outlined above in the section on mesenchymal cells, WNT-5A appears to play a role in remodelling of the airway smooth muscle as smooth muscle-specific overexpression of WNT-5A aggravates sm-α-actin expression in combination with allergen exposure. Smooth muscle-specific WNT-5A overexpression augments the expression of Th2 cytokines as well, and in fact direct application of WNT-5A to Th2 cells obtained from asthma patients revealed regulation of the Th2 cytokine IL-31 by this WNT protein (Koopmans et al. 2020). Moreover, eosinophils isolated from asthma patients activate WNT5A gene expression in airway smooth muscle more than eosinophils obtained from healthy subjects (Januskevicius et al. 2016). In contrast, whole lung application of recombinant WNT-5A does not impact on allergic inflammation, whereas application of recombinant WNT-1 represses Th2 dependent inflammation in a mouse model of asthma (Beckert et al. 2018), possibly indicating distinct roles for WNT/β-catenin-dependent (protective) and WNT/β-catenin-independent (detrimental) signalling in the development of asthma, or for cell-specific roles of WNT signalling in individual features of the disease. The latter possibility seems most likely as WNT/β-catenin-dependent signalling is not protective towards all features of asthma and induces both β-catenin stabilization and the secretion of proinflammatory cytokines such as IL-8 and CCL8 from human mast cells.
3.3. COPD
Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of mortality worldwide (Barnes et al. 2015; Anne et al. 2015). Although preventable and treatable, COPD remains incurable in part because of an incomplete understanding of its cellular mechanisms driving the disease. COPD comprises two major clinical conditions: chronic bronchitis (CB) and emphysema. CB is characterized by chronic inflammation, airway epithelial remodelling, and mucus obstruction. It is typically thought of as condition affecting the central airways even though small airways with internal diameters of <2 mm serve as the main site of airflow limitation (Hogg et al. 1968). With more recent advances in high resolution computed tomography, small airway disease (SAD) is now recognized as an event in early COPD, with 40–56% of terminal airways in early GOLD I and II stage patients (Capron et al. 2019; Higham et al. 2019; Burgel et al. 2011). On the other hand, emphysema is characterized by irreversible destruction of parenchymal lung tissue and airspace enlargement associated with abnormal inflammation and subsequent protease/antiprotease imbalance. COPD is caused by several stressors, such as tobacco smoke and air pollution (Barnes et al. 2015; Tuder and Petrache 2012). In both CB and emphysema, the WNT/β-catenin signalling is generally found to be down-regulated, whereas the β-catenin-independent WNT signalling is upregulated (Qu et al. 2019). These data suggest a similar WNT signal shift as observed in the ageing lung, which is in line with an emphysema phenotype developing in the normal human lung upon ageing.
In CB, β-catenin was shown to be reduced in the small airway epithelial cells (Wang et al. 2011), while several components of the β-catenin-independent WNT signalling pathway were found to be increased in CB patients, including WNT-4, WNT-5B, and Frizzled-8 (FZD8) (Qu et al. 2019; Wang et al. 2011; Durham et al. 2013; Spanjer et al. 2016a; Heijink et al. 2016). In addition, a WNT inhibitor, secreted frizzled-related protein 2 (SFRP2), was found to be increased in both healthy smokers and COPD patients (Qu et al. 2019; Wang et al. 2011). Similarly, previous studies have demonstrated that WNT-3A and FZD4 were down-regulated in the alveolar epithelium in murine and human emphysema, associated with elevated β-catenin-independent WNT protein WNT-5A expressed by lung fibroblasts (Baarsma and Konigshoff 2017; Desai et al. 2014; Qu et al. 2019; Kneidinger et al. 2011; Skronska-Wasek et al. 2017; Beers and Morrisey 2011; Stabler and Morrisey 2017).
Notably, ectopic activation of WNT/β-catenin signalling using GSK3β inhibitors induced intrinsic alveolar repair in mouse models of emphysema and human precision cut lung slices derived from COPD patients (Kneidinger et al. 2011; Uhl et al. 2015). These studies demonstrated that tissue regeneration can be initiated in adult human emphysematous lungs and that WNT/β-catenin signalling pathway serves as a potential therapeutic target to initiate tissue regeneration in emphysema. However, the identity of the progenitor cells that respond to WNT/β-catenin activation upon chronic injury to regenerate alveoli, and the role of WNT/β-catenin signalling during their transition from quiescence to activation in homeostasis and disease remains largely unknown. Studies using transgenic mouse models with WNT activity reporters have identified a sub-population of ATII cells labeled by WNT/β-catenin target gene Axin2 that can be activated to regenerate alveolar tissue after acute injuries (Nabhan et al. 2018; Zacharias et al. 2018). Whether these cells can be activated in emphysema to repair lung tissue is unknown. Furthermore, another study has identified a distal lung epithelial progenitor population containing club cells and a small population of ATII cells, which respond to ectopic WNT activation to form alveolar organoids in mouse emphysema model induced by porcine pancreatic elastase, suggesting a potential role of both club and ATII cells in WNT/β-catenin induced tissue repair in COPD (Hu et al. 2020). In a more recent study, using a cigarette smoke induced mouse COPD model, Conlon et al. identified lymphotoxin β-receptor (LTβR) on epithelial cells interacts with TNF superfamily members expressed by the immune cells of a tertiary lymphoid structure in COPD known as inducible bronchus-associated lymphoid tissue (iBALT). LTβR signalling functions as an inhibitor of WNT/β-catenin signalling by activating non-canonical NF-κB signalling. Strikingly, blockade of LTβR activated WNT/β-catenin signalling in the alveolar epithelial progenitor cells and promoted tissue repair (Conlon et al. 2020). Although these studies support the notion that activating WNT/β-catenin signalling is beneficial for tissue regeneration in COPD, it is important to point out that WNT/β-catenin signalling was found upregulated in idiopathic lung fibrosis and lung cancer, as discussed below. Therefore, further studies are needed to identify disease-specific targeting WNT/β-catenin driven tissue repair. The recent development of “WNT agonist surrogates” that allows the functional study of WNT signalling and the delineation of FZD subtype specific effects can serve as an important tool in our understanding of cell-type specific WNT signalling outcomes in the lung (Janda et al. 2017).
3.4. Lung Cancer
Lung cancer is the second most commonly diagnosed cancer and the leading cause of mortality among all cancer types, which accounts for 18% of the total cancer death worldwide (Sung et al. 2021). The 5-year survival rate of lung cancer patients post diagnosis is only about 10–20% (Sung et al. 2021). Lung cancer is classified into two major types: small cell lung cancer (SCLC, 15% of all lung cancers) and non-small cell lung cancer (NSCLC, 85%) (Leeman et al. 2014; Herbst et al. 2008; Travis et al. 2013). Regardless of its smaller proportion, SCLC is one of the most aggressive and lethal forms of malignant diseases, which is characterized by aberrant neuroendocrine marker expression, genomic instability, rapid tumor growth, and high metastatic potential (Schwendenwein et al. 2021). Unfortunately, most SCLC patients already showed metastatic spread at the time of diagnosis, due to lack of early detection strategy (Schwendenwein et al. 2021). On the other hand, the most common lung cancers, NSCLCs, are further classified into three distinct histological subtypes: adenocarcinoma (ADC; 50% of NSCLC), squamous cell carcinoma (SCC; 40% of NSCLC), and large cell carcinoma (10% of NSCLC) (Leeman et al. 2014; Chen et al. 2014). ADCs are identified by glandular histology and expression of thyroid transcription factor 1 (TTF1; also known as NKX2–1) and keratin 7 (KRT7), which are markers found in the distal lung (Leeman et al. 2014; Travis et al. 2013; Chen et al. 2014; Imielinski et al. 2012). However, SCCs are characterized by expression of markers of pseudostratified epithelial cells in the proximal airways, including cytokeratin 5 (CK5), cytokeratin 6 (CK6), transcription factors SRY-box 2 (SOX2), and p63. Lastly, large cell carcinoma does not express either markers of ADC or SCC and remains largely unstudied (Leeman et al. 2014; Travis et al. 2013; Chen et al. 2014; Imielinski et al. 2012).
Studies using single-cell RNA-sequencing and numerous genetically engineered mouse models of specific lung cancer subtypes have suggested that distinct lung epithelial progenitor cells may undergo malignant transformation in lung cancer subtypes. For instance, neuroendocrine cells were shown to form SCLC tumor with Tp53 and Rb knockout (Leeman et al. 2014; Song et al. 2012; Noguchi et al. 2020). Further, both BASC and ATII cells were demonstrated to contribute to Kras-activated ADC (Leeman et al. 2014; Kim et al. 2005; Xu et al. 2012). For SCC, basal cells were proposed to be the cellular origin (Giangreco et al. 2012; Lu et al. 2010). Genetic mutations in the progenitor cells were believed to contribute to cancer initiation. High-throughput NGS studies have identified multiple oncogenic mutations in NSCLC, including KRAS and epidermal growth factor receptor (EGFR) in ADCs, discoidin domain-containing receptor 2 (DDR2), FGFR1, FGFR2, FGFR3 and genes in the PI3K pathway for SCC (Chen et al. 2014; Cancer Genome Atlas Research Network 2014). Many clinical inhibitors targeting these oncogenes have been used and significantly improved the treatment of NSCLC in the past decade, however, many patients developed resistance to these inhibitors within 9–12 months of treatments (Chen et al. 2014).
Intriguingly, mutations in genes of WNT/β-catenin signalling are predominant in many cancer types but rarely found in lung cancers (Skronska-Wasek et al. 2018; Stewart 2014). However, overexpression of β-catenin, WNT proteins, and receptors, as well as inactivation of β-catenin destruction complex components, such as APC and AXIN2, was commonly identified in resected NSCLC samples (Stewart 2014; Garcia Campelo et al. 2011). Nguyen et al. suggested that activation of WNT/β-catenin/TCF pathway was a determinant of metastasis to brain and bone during lung adenocarcinoma progression (Nguyen et al. 2009). Moreover, accumulating evidence demonstrated that abnormal activation of WNT signalling was associated with increased resistance factors, and that inhibiting WNT signalling can overcome the resistance to certain drugs against NSCLC (Song et al. 2019). Single-cell RNA-sequencing analysis revealed that samples from patients exhibiting drug resistance under therapy showed relative low expression of WNT/β-catenin-associated pathway genes SUSD2 and CAV1 (Travis et al. 2013). Altogether, these data indicate that aberrant activation of WNT/β-catenin signalling plays a role in both, lung tumorigenesis and drug resistance under anti-cancer therapies. However, whether inhibiting β-catenin is beneficial to lung cancer treatment remains controversial. Some studies indicated that activation of β-catenin may inhibit metastasis through regulating E-cadherin and increase cell–cell contact (Skronska-Wasek et al. 2018; Schwendenwein et al. 2021). In addition to WNT/β-catenin signalling, overexpression of β-catenin-independent WNT protein WNT-5A was found to elevate cell proliferation predominantly in SCC, which is highly associated with cigarette smoke (Skronska-Wasek et al. 2018; Janda et al. 2017; Chen et al. 2014). Importantly, WNT-5A was shown to activate β-catenin signalling in the stromal cells and to induce vascularization, and therefore enhance the tumor-stromal interaction (Chen et al. 2014). In contrast, another WNT protein, WNT-7A functions as an tumor suppressor, and was found down-regulated in NSCLC (Janda et al. 2017). Therefore, dysregulation of β-catenin-dependent and independent WNT signalling plays a pivotal role in NSCLC. The precise mechanisms regulating the functions of specific WNT proteins and their interactions with other oncogenes in each subtype of NSCLC remain largely unknown and require more studies.
3.5. IPF
Idiopathic Pulmonary Fibrosis (IPF) is a chronic, age-associated lung disease with poor prognosis and limited therapeutic options (Lederer and Martinez 2018). To date, lung transplantation remains as the only curative treatment and novel therapies, targeting pathogenic pathways are urgently needed. IPF is characterized by the excessive accumulation of extracellular matrix which leads to the destruction of functional lung tissue. Accumulating evidence suggests a role of epithelial cell reprogramming in disease initiation by inhibiting endogenous repair capacities of stem cells (Selman and Pardo 2020). Unbiased screening approaches and several follow-up studies unequivocally revealed an aberrant WNT signature in IPF and experimental lung fibrosis (Konigshoff et al. 2008; Konigshoff and Eickelberg 2010; Konigshoff et al. 2009; Chilosi et al. 2003; Yang et al. 2007; Lam et al. 2014; Martin-Medina et al. 2018; Vukmirovic et al. 2017). In human as well as experimental disease, an increase in active WNT/β-catenin signalling was described (Konigshoff et al. 2008; Konigshoff and Eickelberg 2010; Konigshoff et al. 2009; Chilosi et al. 2003; Yang et al. 2007; Lam et al. 2014) (Sucre et al. 2018), likely as an early event during pathogenesis with changes observed already in rather normallooking parenchyma of the human diseased lung (Rydell-Tormanen et al. 2016). Importantly, low-density lipoprotein receptor-related protein 5 was discovered as a genetic marker of IPF and furthermore proved to have a prognostic value (Lam et al. 2014). Recent single-cell sequencing approaches led to the transcriptional characterization of lung cells in unprecedented detail. These unbiased approaches further led to the identification of epithelial cells population enriched in β-catenin-dependent WNT signalling in both experimental and human lung fibrosis (Adams et al. 2020; Reyfman et al. 2019; Strunz et al. 2020; Xu et al. 2016). In experimental lung fibrosis, the appearance of transitional stem cell states, such as transitional Krt8+ ATII (ADI) cells, has been identified, which exhibit an active β-catenin signature. Further studies also suggest distinct WNT-expressing versus WNT-responsive epithelial cells in the IPF lung, suggesting the control of the WNT pathway by distinct niches in the lung (Nabhan et al. 2018).
Mechanistically, aberrant WNT/β-catenin signalling led to impaired epithelial cell function such as increased cellular senescence, expression of fibrotic markers, induction of Krt8, and increased proliferation (Selman and Pardo 2020; Konigshoff and Eickelberg 2010; Chilosi et al. 2003; Lehmann et al. 2020b; Chilosi et al. 2013). Importantly, also WNT-5A is upregulated in IPF and experimental lung fibrosis and contributes to increased proliferative capacity of primary human lung fibroblasts (Martin-Medina et al. 2018). This represents an important difference to other disease scenarios in the lung, such as COPD or the ageing lung, where a shift of β-catenin-dependent and independent WNT signalling has been observed (Baarsma et al. 2017; Kneidinger et al. 2011; Skronska-Wasek et al. 2017). Importantly, WNT-5A was found on extracellular vesicles in the BALF of IPF patients as well as secreted by fibrotic primary human lung fibroblasts, suggesting that extracellular vesicles in the IPF lung function as carriers for WNT proteins thereby extending their ability for intercellular communication (Parimon et al. 2019).
Targeting WNT/β-catenin pathway has also therapeutic potential in IPF. Inhibition of β-catenin signalling by pharmacological agents was shown to attenuate bleomycin-induced lung fibrosis (Henderson Jr. et al. 2010; Ulsamer et al. 2012; Wang et al. 2014). Similarly, administration of an siRNA directed against β-catenin resulted in reduced bleomycin-induced fibrosis load (Kim et al. 2011). In addition, the WNT/β-catenin target gene WNT inducible signalling protein (WISP) 1 has been identified as a profibrotic protein and inhibition of WISP1 attenuated the development of experimental lung fibrosis in vivo (Konigshoff et al. 2009; Klee et al. 2016).
As illustrated by these in vivo studies, targeting the WNT pathway and its downstream mediators might be a promising therapeutic approach. It will be important to develop treatments that do not interfere with normal wound healing or regeneration, illustrated by the worsening of lung fibrosis observed after epithelial-specific removal of β-catenin in murine fibrosis (Tanjore et al. 2013). Additionally, ATII cells display active WNT signalling needed for regeneration (Nabhan et al. 2018). This underlines that it is crucial to target disease-specific WNT signalling which is characterized by the involvement of additional cofactors and interactions with other profibrotic signalling pathways such as TGFβ/SMAD (Gottardi and Konigshoff 2013; Ulsamer et al. 2012; Zhou et al. 2012).
In summary, (re-) shifting the disease- and cell-specific parts of the WNT/β-catenin pathway thereby restoring the “right amount and the right flavor” of WNT signalling is an important although challenging therapeutic strategy for IPF (Gottardi and Konigshoff 2013).
3.6. Pulmonary Vascular Diseases
Pulmonary vascular disease (PVD) is a medical term for a large and diverse group of pathologies that affects the blood vessels within the lungs. PVD is characterized by remodelling of microvessels and abnormal angiogenesis. WNT signalling has been implicated in several PVDs.
There are two main types of PVDs: pulmonary embolism (PE) and pulmonary hypertension (PH). PE is a blockage of an artery in the lungs and causes lung injury due to obstructed blood flow, decreased oxygen levels in the blood. The majority of PE initials from other parts of the body and travels through the bloodstream to the lung. Thereby, we will only discuss the PH here. PH is caused by high blood pressure in the pulmonary arteries. It can be progressive, even turning to fatal disease if untreated early.
PH is characterized by vascular narrowing and increased pulmonary vascular resistance, due to pulmonary injury and abnormal pulmonary vascular remodelling (Humbert et al. 2019). Given the role of WNT signalling in angiogenesis (Olsen et al. 2017) and accumulating evidence showing WNT signalling involved in PH pathologies, the development of WNT-based therapeutics becomes amenable. The defection of WNTless (Wls), a gene that encodes for a transmembrane protein that mediates WNT protein secretion, impaired pulmonary vascular differentiation and peripheral lung morphogenesis (Cornett et al. 2013), whereas embryos and newborn mice with Wnt7a knockout exhibit severe defects in mesenchymal proliferation and vascular development in lung (Shu et al. 2002).
Pulmonary arterial hypertension (PAH), characterized by high blood pressure in the arteries of the pulmonary artery, is a type of PVD. A study investigating genetic signatures across multiple cell types in pulmonary arterial hypertension showed the activation of the WNT/β-catenin pathway in both heritable PAH (HPAH) and idiopathic PAH (IPAH) (West et al. 2014), indicating the role of WNT pathway in maintaining pulmonary vascular homeostasis. The activation of β-catenin-independent WNT/PCP pathway was demonstrated in IPAH, in particular in the endothelial layer, with significant upregulation of WNT-11, DAAM1, DSV, and RHO-kinase (Laumanns et al. 2009). WNT-5A, a known WNT/PCP pathway activating protein, was further identified as a key mediator for the recruitment of pericyte and for the establishment of epithelium–pericyte interactions to promote pulmonary angiogenesis (Yuan et al. 2019). Furthermore, de Jesus Perez et al. demonstrated both β-catenin-dependent and independent (WNT/RHOA/RAC1) WNT pathways are required in BMP-2–mediated protection of pulmonary artery endothelial cells (de Jesus Perez et al. 2009). However, inhibition of WNT/β-catenin signalling by LRP5/6 inhibitors attenuates hyperoxia-induced PH and decreased pulmonary vascular smooth muscle cell proliferation in neonatal rats (Alapati et al. 2013). Further study conducted by Sklepkiewicz et al. suggested that glycogen synthase kinase 3 beta (GSK3β), a member of β-catenin destruction complex, promoted vascular remodelling processes with proliferation of arterial smooth muscle cells in pulmonary hypertension (Sklepkiewicz et al. 2011). In conclusion, the regulation of WNT signalling pathway in vascular remodelling in PA pathologies varies from ligand-receptor combination, as well as cell types.
Apart from vascular remodelling via endothelial cells and smooth muscle cells, activation of WNT/β-catenin signalling has been implicated in the suppression of mesenchymal progenitor cells (MPC) differentiation into functional pericytes, thereby driving a phenotype of persistent microvascular dysfunction and abnormal angiogenesis (Gaskill et al. 2017). Similarly, Summers et al. suggested activation of WNT/β-catenin signalling in MPC induced emphysema-like distal lung remodelling and exacerbated vascular injury (Summers et al. 2020).
Moreover, proinflammatory activation is another major mechanism involved in PA, which has been linked to WNT signalling. Various perivascular inflammatory cells including monocytes, macrophages, T lymphocytes were identified in plexi-form lesion (Tuder et al. 1994), while several of cytokines including interleukin (IL)-1, IL-1β, IL-6, connexin 36, fractalkine (FKN) were implicated on the lesion of PAH (Tuder et al. 1994; Itoh et al. 2006; Simonneau et al. 2013). WNT signalling pathways were suggested in both proinflammatory and anti-inflammatory process. In general, WNT-5A pathway is a crucial trigger of inflammatory activation in macrophages, with upregulated expression of the proinflammatory genes IL6, IL1B, CXCL8, and CCL4 (Pereira et al. 2009). In contrast, WNT-3A/β-catenin signalling mediates anti-inflammatory effects (Schaale et al. 2011). There are rare reports linking WNT pathway with inflammation in lung, in particular in PA pathologies. It is reported that β-catenin-independent WNT-5B signalling induces IL-6 and CXCL8 secretion in human lung fibroblasts (van Dijk et al. 2016). Li et al. suggest that WNT/β-catenin signalling acts as a negative feedback loop, attenuated cell injury from excessive inflammatory reactions by suppressing inflammation in alveolar epithelial cells (Li et al. 2014). However, further in-depth investigations on how WNT pathway contributes to inflammatory-medicated PA pathologies are needed.
4. Conclusions and Perspectives
The studies summarized above support major roles for WNT signalling in lung physiology and pathophysiology and highlight a number of emerging key concepts. Probably the most compelling concept is the WNT signalling switch from WNT/β-catenin signalling to β-catenin-independent signalling in ageing, in chronic lung disease and in response to TGF-β. In light of the supportive roles of WNT/β-catenin signalling in epithelial repair and regeneration, such studies call for approaches to inhibit WNT/β-catenin-independent signalling or activate WNT/β-catenin-dependent signalling as regenerative pharmacological approaches towards restoration of lung architecture in these chronic diseases. Such approaches have been considered, mostly using GSK-3 inhibitors, which successfully prevent and restore changes in lung remodelling in animal models of LPS-induced lung injury (Baarsma et al. 2013b), in animal models of elastase-induced emphysema (Kneidinger et al. 2011), and in lung slices of COPD patients (Uhl et al. 2015). Additional data indicate that targeted agonism of FZD4 (Skronska-Wasek et al. 2017) or antagonism of FZD8 (Spanjer et al. 2016a; Spanjer et al. 2016b) is beneficial in restoration of lung injury. Such subtype selective FZD agonists and antagonists are not yet widely available but are on the horizon in view of the recent development of surrogate WNT proteins that function as FZD-LRP5/LRP6 heterodimers to activate WNT/β-catenin signalling in mammalian cells (Janda et al. 2017). Furthermore, WNT agonists may be used in concert with in vitro expanded stem cells for transplantation purposes (Fig. 2). Though not yet demonstrated for lung injury, organoid transplantation of salivary gland epithelial cells is enhanced by WNT proteins to functionally restore salivary gland function in response to irradiation injury (Maimets et al. 2016) thus serving as a promising proof-of-concept study. Finally, it is likely that to successfully intervene in these complex diseases, it will be necessary to move away from the approach to target a singular signalling pathway, but further take into account additional pathways that drive distinct disease processes. These approaches remain challenging within the translational pipeline and for potential clinical trials. Thus, integration of these concepts of early multi-pathway targeting will be essential (Fig. 2).
Fig. 2.
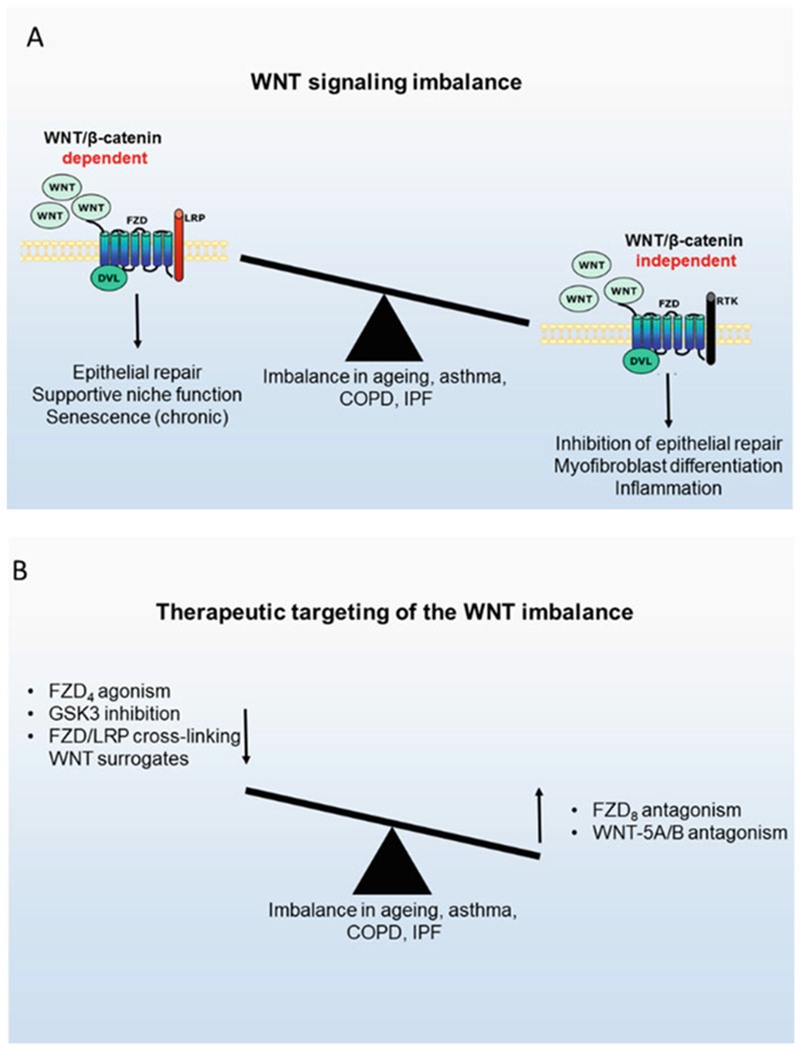
The nature and therapeutic targeting of the WNT signalling imbalance in chronic lung diseases. (a) Lung ageing and chronic lung diseases such as asthma, COPD, and IPF are characterized by reduced WNT/β-catenin-dependent signalling and increased WNT/β-catenin-inde-pendent signalling, which contributes to impaired epithelial repair, myofibroblast differentiation, and inflammation. (b) Restoration of this imbalance may hold therapeutic promise, either by amplifying WNT/β-catenin-dependent signalling or by reducing WNT/β-catenin-independent signalling
Funding:
NIH F32HL149290 (YH), Netherlands Lung Foundation LF2018.5.2 BREATH (MK, RG), NIH R01 HL141380 (MK), BfR 60-0102-01.P588 (ML), Netherlands Lung Foundation 5.1.17.166 (CC, RG, MK).
Contributor Information
Yan Hu, Division of Pulmonary Sciences and Critical Care Medicine, School of Medicine, University of Colorado, Aurora, CO, USA.
Chiara Ciminieri, Division of Pulmonary Sciences and Critical Care Medicine, School of Medicine, University of Colorado, Aurora, CO, USA; Department of Molecular Pharmacology, Groningen Research Institute for Asthma and COPD, University of Groningen, Groningen, The Netherlands.
Qianjiang Hu, Lung Repair and Regeneration Unit, Helmholtz-Zentrum Munich, Ludwig-Maximilians-University, University Hospital Grosshadern, Munich, Germany.
Mareike Lehmann, Lung Repair and Regeneration Unit, Helmholtz-Zentrum Munich, Ludwig-Maximilians-University, University Hospital Grosshadern, Munich, Germany.
Melanie Königshoff, Lung Repair and Regeneration Unit, Helmholtz-Zentrum Munich, Ludwig-Maximilians-University, University Hospital Grosshadern, Munich, Germany; Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Reinoud Gosens, Department of Molecular Pharmacology, Groningen Research Institute for Asthma and COPD, University of Groningen, Groningen, The Netherlands.
References
- Adams TS, Schupp JC, Poli S et al. (2020) Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv 6:eaba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alapati D, Rong M, Chen S, Lin C, Li Y, Wu S (2013) Inhibition of LRP5/6-mediated WNT/β-catenin signalling by Mesd attenuates hyperoxia-induced pulmonary hypertension in neonatal rats. Pediatr Res 73:719–725 [DOI] [PubMed] [Google Scholar]
- Anne G, Wheaton TJC, Earl S, Ford MD, Croft JB (2015) Employment and activity limitations among adults with chronic obstructive pulmonary disease—United States, 2013. MMWR Morb Mortal Wkly Rep 64(11):289–295 [PMC free article] [PubMed] [Google Scholar]
- Aros CJ, Vijayaraj P, Pantoja CJ et al. (2020) Distinct spatiotemporally dynamic WNT-secreting niches regulate proximal airway regeneration and aging. Cell Stem Cell 27:413–29.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarsma HA, Konigshoff M (2017) ‘WNT-er is coming’: WNT signalling in chronic lung diseases. Thorax 72:746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarsma HA, Menzen MH, Halayko AJ, Meurs H, Kerstjens HA, Gosens R (2011) Beta-catenin signalling is required for TGF-beta1-induced extracellular matrix production by airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 301:L956–L965 [DOI] [PubMed] [Google Scholar]
- Baarsma HA, Konigshoff M, Gosens R (2013a) The WNT signalling pathway from ligand secretion to gene transcription: molecular mechanisms and pharmacological targets. Pharmacol Ther 138:66–83 [DOI] [PubMed] [Google Scholar]
- Baarsma HA, Bos S, Meurs H et al. (2013b) Pharmacological inhibition of GSK-3 in a Guinea pig model of LPS-induced pulmonary inflammation: I. effects on lung remodelling and pathology. RespirRes 14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarsma HA, Skronska-Wasek W, Mutze K et al. (2017) Noncanonical WNT-5A signalling impairs endogenous lung repair in COPD. J Exp Med 214:143–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR et al. (2013) Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123:3025–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Burney PG, Silverman EK et al. (2015) Chronic obstructive pulmonary disease. Nat Rev Dis Primers 1:15076. [DOI] [PubMed] [Google Scholar]
- Basil MC, Katzen J, Engler AE et al. (2020) The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell 26:482–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckert H, Meyer-Martin H, Buhl R, Taube C, Reuter S (2018) The canonical but not the noncanonical WNT pathway inhibits the development of allergic airway disease. J Immunol 201:1855–1864 [DOI] [PubMed] [Google Scholar]
- Beers MF, Morrisey EE (2011) The three R’s of lung health and disease: repair, remodelling, and regeneration. J Clin Invest 121:2065–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch J, Barnes PJ, Passos JF (2018) Mitochondria, telomeres and cell senescence: implications for lung ageing and disease. Pharmacol Ther 183:34–49 [DOI] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB (1999) Number and proliferation of Clara cells in normal human airway epithelium. Am J Respir Crit Care Med 159:1585–1591 [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S et al. (2007) Increased WNT signalling during aging alters muscle stem cell fate and increases fibrosis. Science 317:807–810 [DOI] [PubMed] [Google Scholar]
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R (2011) Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in Wistar rats. PLoS One 6:e19194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brechbuhl HM, Ghosh M, Smith MK et al. (2011) Beta-catenin dosage is a critical determinant of tracheal basal cell fate determination. Am J Pathol 179:367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno M, Lai YC, Romero Y et al. (2015) PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125:521–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel PR, Bourdin A, Chanez P et al. (2011) Update on the roles of distal airways in COPD. Eur Respir Rev 20:007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgy O, Konigshoff M (2018) The WNT signalling pathways in wound healing and fibrosis. Matrix Biol 68–69:67–80 [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network (2014) Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron T, Bourdin A, Perez T, Chanez P (2019) COPD beyond proximal bronchial obstruction: phenotyping and related tools at the bedside. Eur Respir Rev 28:190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda D, Rehan M, Smith SR et al. (2021) Mesenchymal stromal cell aging impairs the self-organizing capacity of lung alveolar epithelial stem cells. bioRxiv. 10.1101/2021.03.05.434121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA, Li X, Alexander JP et al. (2011) Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest 121:2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J (2015) The regulation of branching morphogenesis in the developing lung. In: Bertoncello I (ed) Stem cells in the lung: development, repair and regeneration. Springer, Cham, pp 3–16 [Google Scholar]
- Chen H, Matsumoto K, Brockway BL et al. (2012) Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 30:1948–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK (2014) Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14:535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M, Poletti V, Zamo A et al. (2003) Aberrant WNT/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162:1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M, Carloni A, Rossi A, Poletti V (2013) Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res 162:156–173 [DOI] [PubMed] [Google Scholar]
- Cho MH, Boutaoui N, Klanderman BJ et al. (2010) Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat Genet 42:200–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon TM, John-Schuster G, Heide D et al. (2020) Inhibition of LTbetaR signalling activates WNT-induced regeneration in lung. Nature 588:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett B, Snowball J, Varisco BM, Lang R, Whitsett J, Sinner D (2013) WNTless is required for peripheral lung differentiation and pulmonary vascular development. Dev Biol 379:38–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus Perez VA, Alastalo TP, Wu JC et al. (2009) Bone morphogenetic protein 2 induces pulmonary angiogenesis via WNT-beta-catenin and WNT-RhoA-Rac1 pathways. J Cell Biol 184:83–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai TJ, Brownfield DG, Krasnow MA (2014) Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507:190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham AL, McLaren A, Hayes BP et al. (2013) Regulation of WNT4 in chronic obstructive pulmonary disease. FASEB J 27:2367–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini ZW, Gosens R, Dekker F, Koppelman GH (2020) The genetics of asthma and the promise of genomics-guided drug target discovery. Lancet Respir Med 8:1045–1056 [DOI] [PubMed] [Google Scholar]
- Florian MC, Nattamai KJ, Dörr K et al. (2013) A canonical to non-canonical WNT signalling switch in haematopoietic stem-cell ageing. Nature 503:392–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flozak AS, Lam AP, Russell S et al. (2010) Beta-catenin/T-cell factor signalling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem 285:3157–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DB, Peng T, Zepp JA et al. (2016) Emergence of a wave of WNT signalling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep 17:2312–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumoto K, Takigawa-Imamura H, Sumiyama K, Kaneiwa T, Kikuchi A (2017) Modulation of apical constriction by WNT signalling is required for lung epithelial shape transition. Development 144:151–162 [DOI] [PubMed] [Google Scholar]
- Garcia Campelo MR, Alonso Curbera G, Aparicio Gallego G, Grande Pulido E, Anton Aparicio LM (2011) Stem cell and lung cancer development: blaming the WNT, Hh and notch signalling pathway. Clin Transl Oncol 13:77–83 [DOI] [PubMed] [Google Scholar]
- Gaskill CF, Carrier EJ, Kropski JA et al. (2017) Disruption of lineage specification in adult pulmonary mesenchymal progenitor cells promotes microvascular dysfunction. J Clin Invest 127:2262–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Lu L, Vickers C et al. (2012) Beta-catenin determines upper airway progenitor cell fate and preinvasive squamous lung cancer progression by modulating epithelial-mesenchymal transition. J Pathol 226:575–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T et al. (2009) WNT2/2b and beta-catenin signalling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell 17:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Konigshoff M (2013) Considerations for targeting beta-catenin signalling in fibrosis. Am J Respir Crit Care Med 187:566–568 [DOI] [PubMed] [Google Scholar]
- Guha A, Deshpande A, Jain A, Sebastiani P, Cardoso WV (2017) Uroplakin 3a(+) cells are a distinctive population of epithelial progenitors that contribute to airway maintenance and postinjury repair. Cell Rep 19:246–254 [DOI] [PubMed] [Google Scholar]
- Guo M, Du Y, Gokey JJ et al. (2019) Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat Commun 10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann AC, Gutierrez AJ, Bui LT et al. (2020) Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv 6:eaba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X (2009) Beta-catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A 106:16287–16292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijink IH, de Bruin HG, Dennebos R et al. (2016) Cigarette smoke-induced epithelial expression of WNT-5B: implications for COPD. Eur Respir J 48:504–515 [DOI] [PubMed] [Google Scholar]
- Henderson WR Jr, Chi EY, Ye X et al. (2010) Inhibition of WNT/beta-catenin/CREB binding protein (CBP) signalling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A 107:14309–14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Heymach JV, Lippman SM (2008) Lung cancer. N Engl J Med 359:1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M, Morrisey EE (2014) Lung development: orchestrating the generation and regeneration of a complex organ. Development 141:502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham A, Quinn AM, Cançado JED, Singh D (2019) The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res 20:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines EA, Sun X (2014) Tissue crosstalk in lung development. J Cell Biochem 115:1469–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JW, McBryan T, Adams PD, Sedivy JM (2014) The effects of aging on the expression of WNT pathway genes in mouse tissues. Age (Dordr) 36:9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BLM (2020) The alveolar stem cell niche of the mammalian lung. Springer, Singapore, pp 7–12 [Google Scholar]
- Hogan BL, Barkauskas CE, Chapman HA et al. (2014) Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15:123–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC, Macklem PT, Thurlbeck WM (1968) Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 278:1355–1360 [DOI] [PubMed] [Google Scholar]
- Hu Y, Ng-Blichfeldt JP, Ota C et al. (2020) WNT/beta-catenin signalling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema. Stem Cells 38 (11):1467–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M, Guignabert C, Bonnet S et al. (2019) Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 53(1):1801887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imielinski M, Berger AH, Hammerman PS et al. (2012) Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 150:1107–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Udaka N, Yazawa T et al. (2000) Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 127:3913–3921 [DOI] [PubMed] [Google Scholar]
- Itoh T, Nagaya N, Ishibashi-Ueda H et al. (2006) Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology 11:158–163 [DOI] [PubMed] [Google Scholar]
- Janda CY, Dang LT, You C et al. (2017) Surrogate WNT agonists that phenocopy canonical WNT and beta-catenin signalling. Nature 545:234–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januskevicius A, Vaitkiene S, Gosens R et al. (2016) Eosinophils enhance WNT-5a and TGF-beta1 genes expression in airway smooth muscle cells and promote their proliferation by increased extracellular matrix proteins production in asthma. BMC Pulm Med 16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Lao T, Qiu W et al. (2016) A chronic obstructive pulmonary disease susceptibility gene, FAM13A, regulates protein stability of beta-catenin. Am J Respir Crit Care Med 194:185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhof M, Boezen HM, Granell R et al. (2014) Transient early wheeze and lung function in early childhood associated with chronic obstructive pulmonary disease genes. J Allergy Clin Immunol 133:68–76.e1–4 [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE et al. (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121:823–835 [DOI] [PubMed] [Google Scholar]
- Kim TH, Kim SH, Seo JY et al. (2011) Blockade of the WNT/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med 223:45–54 [DOI] [PubMed] [Google Scholar]
- Kim HT, Yin W, Nakamichi Y et al. (2019) WNT/RYK signalling restricts goblet cell differentiation during lung development and repair. Proc Natl Acad Sci U S A 116:25697–25706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee S, Lehmann M, Wagner DE, Baarsma HA, Konigshoff M (2016) WISP1 mediates IL-6-dependent proliferation in primary human lung fibroblasts. Sci Rep 6:20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneidinger N, Yildirim AO, Callegari J et al. (2011) Activation of the WNT/beta-catenin pathway attenuates experimental emphysema. Am J Respir Crit Care Med 183:723–733 [DOI] [PubMed] [Google Scholar]
- Konigshoff M, Eickelberg O (2010) WNT signalling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol 42:21–31 [DOI] [PubMed] [Google Scholar]
- Konigshoff M, Balsara N, Pfaff EM et al. (2008) Functional WNT signalling is increased in idiopathic pulmonary fibrosis. PLoS One 3:e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigshoff M, Kramer M, Balsara N et al. (2009) WNT1-inducible signalling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119:772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans T, Gosens R (2018) Revisiting asthma therapeutics: focus on WNT signal transduction. Drug Discov Today 23:49–62 [DOI] [PubMed] [Google Scholar]
- Koopmans T, Kumawat K, Halayko AJ, Gosens R (2016a) Regulation of actin dynamics by WNT-5A: implications for human airway smooth muscle contraction. Sci Rep 6:30676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans T, Crutzen S, Menzen MH et al. (2016b) Selective targeting of CREB-binding protein/beta-catenin inhibits growth of and extracellular matrix remodelling by airway smooth muscle. Br J Pharmacol 173:3327–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans T, Hesse L, Nawijn MC et al. (2020) Smooth-muscle-derived WNT5A augments allergen-induced airway remodelling and Th2 type inflammation. Sci Rep 10:6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs T, Csongei V, Feller D et al. (2014) Alteration in the WNT microenvironment directly regulates molecular events leading to pulmonary senescence. Aging Cell 13:838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumawat K, Menzen MH, Bos IS et al. (2013) Noncanonical WNT-5A signalling regulates TGF-beta-induced extracellular matrix production by airway smooth muscle cells. FASEB J 27:1631–1643 [DOI] [PubMed] [Google Scholar]
- Kumawat K, Koopmans T, Menzen MH et al. (2016) Cooperative signalling by TGF-beta1 and WNT-11 drives sm-alpha-actin expression in smooth muscle via rho kinase-actin-MRTF-A signalling. Am J Physiol Lung Cell Mol Physiol 311:L529–L537 [DOI] [PubMed] [Google Scholar]
- Lako M, Strachan T, Bullen P, Wilson DI, Robson SC, Lindsay S (1998) Isolation, characterisation and embryonic expression of WNT11, a gene which maps to 11q13.5 and has possible roles in the development of skeleton, kidney and lung. Gene 219:101–110 [DOI] [PubMed] [Google Scholar]
- Lam AP, Flozak AS, Russell S et al. (2011) Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol 45:915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AP, Herazo-Maya JD, Sennello JA et al. (2014) WNT coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 190:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumanns IP, Fink L, Wilhelm J et al. (2009) The noncanonical WNT pathway is operative in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol 40:683–691 [DOI] [PubMed] [Google Scholar]
- Lederer DJ, Martinez FJ (2018) Idiopathic pulmonary fibrosis. N Engl J Med 378:1811–1823 [DOI] [PubMed] [Google Scholar]
- Lee J, Reddy R, Barsky L et al. (2009) Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. Am J Physiol Lung Cell Mol Physiol 296:L57–L70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Tammela T, Hofree M et al. (2017) Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell 170:1149–63.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman KT, Fillmore CM, Kim CF (2014) Lung stem and progenitor cells in tissue homeostasis and disease. Curr Top Dev Biol 107:207–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Baarsma HA, Konigshoff M (2016) WNT signalling in lung aging and disease. Ann Am Thorac Soc 13(Suppl 5):S411–S4S6 [DOI] [PubMed] [Google Scholar]
- Lehmann M, Hu Q, Hu Y et al. (2020a) Chronic WNT/β-catenin signalling induces cellular senescence in lung epithelial cells. Cell Signal 70:109588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Hu Q, Hu Y et al. (2020b) Chronic WNT/beta-catenin signalling induces cellular senescence in lung epithelial cells. Cell Signal 70:109588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P (2002) WNT5a participates in distal lung morphogenesis. Dev Biol 248:68–81 [DOI] [PubMed] [Google Scholar]
- Li C, Hu L, Xiao J et al. (2005) WNT5a regulates Shh and Fgf10 signalling during lung development. Dev Biol 287:86–97 [DOI] [PubMed] [Google Scholar]
- Li Y, Shi J, Yang J et al. (2014) A WNT/β-catenin negative feedback loop represses TLR-triggered inflammatory responses in alveolar epithelial cells. Mol Immunol 59:128–135 [DOI] [PubMed] [Google Scholar]
- Li P, Zhao S, Hu Y (2019) SFRP2 modulates non-small cell lung cancer A549 cell apoptosis and metastasis by regulating mitochondrial fission via WNT pathways. Mol Med Rep 20:1925–1932 [DOI] [PubMed] [Google Scholar]
- Li C, Smith SM, Peinado N et al. (2020) WNT5a-ROR signalling is essential for alveologenesis. Cell 9:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM et al. (2007) Augmented WNT signalling in a mammalian model of accelerated aging. Science 317:803–806 [DOI] [PubMed] [Google Scholar]
- Liu Q, Liu K, Cui G et al. (2019) Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet 51:728–738 [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Futtner C, Rock JR et al. (2010) Evidence that SOX2 overexpression is oncogenic in the lung. PLoS One 5:e11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TJ, Anderson PJ, Rotti PG et al. (2018) Submucosal gland myoepithelial cells are reserve stem cells that can regenerate mouse tracheal epithelium. Cell Stem Cell 22:653–67.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimets M, Rocchi C, Bron R et al. (2016) Long-term in vitro expansion of salivary gland stem cells driven by WNT signals. Stem Cell Rep 6:150–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Medina A, Lehmann M, Burgy O et al. (2018) Increased extracellular vesicles mediate WNT-5A signalling in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 198 (12):1527–1538 [DOI] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ (2012) Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One 7:e42357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley D, Wienhold M, Sun X (2015) The pulmonary mesenchyme directs lung development. Curr Opin Genet Dev 32:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners S, Eickelberg O, Konigshoff M (2015) Hallmarks of the ageing lung. Eur Respir J 45:807–827 [DOI] [PubMed] [Google Scholar]
- Miller MF, Cohen ED, Baggs JE, Lu MM, Hogenesch JB, Morrisey EE (2012) WNT ligands signal in a cooperative manner to promote foregut organogenesis. Proc Natl Acad Sci U S A 109:15348–15353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL (2010) Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 18:8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM et al. (2003) Beta-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 278:40231–40238 [DOI] [PubMed] [Google Scholar]
- Mucenski ML, Nation JM, Thitoff AR et al. (2005) Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol 289:L971–L979 [DOI] [PubMed] [Google Scholar]
- Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ (2018) Single-cell WNT signalling niches maintain stemness of alveolar type 2 cells. Science 359:1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Chiang AC, Zhang XH et al. (2009) WNT/TCF signalling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic MZ, Sun D, Rawlins EL (2018) Human lung development: recent progress and new challenges. Development 145(16):dev163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M, Furukawa KT, Morimoto M (2020) Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease. Dis Model Mech 13(12):dmm046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes RO, Schmidt M, Dueck G et al. (2008) GSK-3/beta-catenin signalling axis in airway smooth muscle: role in mitogenic signalling. Am J Physiol Lung Cell Mol Physiol 294:L1110–L1118 [DOI] [PubMed] [Google Scholar]
- Okuda K, Chen G, Subramani DB et al. (2019) Localization of secretory mucins MUC5AC and MUC5B in normal/healthy human airways. Am J Respir Crit Care Med 199(6):715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JJ, Pohl S, Deshmukh A et al. (2017) The role of WNT signalling in angiogenesis. Clin Biochem Rev 38:131–142 [PMC free article] [PubMed] [Google Scholar]
- Parimon T, Yao C, Habiel DM et al. (2019) Syndecan-1 promotes lung fibrosis by regulating epithelial reprogramming through extracellular vesicles. JCI Insight 5(17):e129359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JI, Venteicher AS, Hong JY et al. (2009) Telomerase modulates WNT signalling by association with target gene chromatin. Nature 460:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxson JA, Gruntman AM, Davis AM, Parkin CM, Ingenito EP, Hoffman AM (2013) Age dependence of lung mesenchymal stromal cell dynamics following pneumonectomy. Stem Cells Dev 22:3214–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira CP, Bachli EB, Schoedon G (2009) The WNT pathway: a macrophage effector molecule that triggers inflammation. Curr Atheroscler Rep 11:236–242 [DOI] [PubMed] [Google Scholar]
- Qu J, Yue L, Gao J, Yao H (2019) Perspectives on WNT signal pathway in the pathogenesis and therapeutics of chronic obstructive pulmonary disease. J Pharmacol Exp Ther 369:473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal J, Carroll TJ, Guseh JS et al. (2008) WNT7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development 135:1625–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raslan AA, Yoon JK (2020) WNT signalling in lung repair and regeneration. Mol Cells 43:774–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL (2011) The building blocks of mammalian lung development. Dev Dyn 240:463–476 [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL (2006) Epithelial stem cells of the lung: privileged few or opportunities for many? Development 133:2455–2465 [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y et al. (2009) The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyfman PA, Walter JM, Joshi N et al. (2019) Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 199:1517–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger ME, Zhou B, Solomon N et al. (2016) p300/beta-catenin interactions regulate adult progenitor cell differentiation downstream of WNT5a/protein kinase C (PKC). J Biol Chem 291:6569–6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Hogan BL (2011) Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 27:493–512 [DOI] [PubMed] [Google Scholar]
- Ruaro B, Salton F, Braga L et al. (2021) The history and mystery of alveolar epithelial type II cells:focus on their physiologic and pathologic role in lung. Int J Mol Sci 22:2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydell-Tormanen K, Zhou XH, Hallgren O et al. (2016) Aberrant nonfibrotic parenchyma in idiopathic pulmonary fibrosis is correlated with decreased beta-catenin inhibition and increased WNT5a/b interaction. Physiol Rep 4(5):e12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N (2011) WNT signalling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur J Cell Biol 90:553–559 [DOI] [PubMed] [Google Scholar]
- Schwendenwein A, Megyesfalvi Z, Barany N et al. (2021) Molecular profiles of small cell lung cancer subtypes: therapeutic implications. Mol Ther Oncolytics 20:470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman M, Pardo A (2020) The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell Signal 66:109482. [DOI] [PubMed] [Google Scholar]
- Sharma S, Tantisira K, Carey V et al. (2010) A role for WNT signalling genes in the pathogenesis of impaired lung function in asthma. Am J Respir Crit Care Med 181:328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE (2002) WNT7b regulates mesenchymal proliferation and vascular development in the lung. Development 129:4831–4842 [DOI] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z et al. (2005) WNT/beta-catenin signalling acts upstream of N-myc, BMP4, and FGF signalling to regulate proximal-distal patterning in the lung. Dev Biol 283:226–239 [DOI] [PubMed] [Google Scholar]
- Simonneau G, Gatzoulis MA, Adatia I et al. (2013) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62:D34–D41 [DOI] [PubMed] [Google Scholar]
- Sklepkiewicz P, Schermuly RT, Tian X et al. (2011) Glycogen synthase kinase 3beta contributes to proliferation of arterial smooth muscle cells in pulmonary hypertension. PLoS One 6:e18883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skronska-Wasek W, Mutze K, Baarsma HA et al. (2017) Reduced frizzled receptor 4 expression prevents WNT/beta-catenin-driven alveolar lung repair in COPD. Am J Respir Crit Care Med 196(2):172–185 [DOI] [PubMed] [Google Scholar]
- Skronska-Wasek W, Gosens R, Konigshoff M, Baarsma HA (2018) WNT receptor signalling in lung physiology and pathology. Pharmacol Ther 187:150–166 [DOI] [PubMed] [Google Scholar]
- Song H, Yao E, Lin C, Gacayan R, Chen MH, Chuang PT (2012) Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci U S A 109:17531–17536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Wang H, Zhang S (2019) Negative regulators of WNT signalling in non-small cell lung cancer: theoretical basis and therapeutic potency. Biomed Pharmacother 118:109336. [DOI] [PubMed] [Google Scholar]
- Spanjer AI, Menzen MH, Dijkstra AE et al. (2016a) A pro-inflammatory role for the Frizzled-8 receptor in chronic bronchitis. Thorax 71:312–322 [DOI] [PubMed] [Google Scholar]
- Spanjer AI, Baarsma HA, Oostenbrink LM et al. (2016b) TGF-beta-induced profibrotic signalling is regulated in part by the WNT receptor Frizzled-8. FASEB J 30:1823–1835 [DOI] [PubMed] [Google Scholar]
- Stabler CT, Morrisey EE (2017) Developmental pathways in lung regeneration. Cell Tissue Res 367:677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DJ (2014) WNT signalling pathway in non-small cell lung cancer. J Natl Cancer Inst 106: djt356. [DOI] [PubMed] [Google Scholar]
- Strunz M, Simon LM, Ansari M et al. (2020) Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun 11:3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucre JMS, Deutsch GH, Jetter CS et al. (2018) A shared pattern of beta-catenin activation in bronchopulmonary dysplasia and idiopathic pulmonary fibrosis. Am J Pathol 188:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers ME, Richmond BW, Menon S et al. (2020) Resident mesenchymal vascular progenitors modulate adaptive angiogenesis and pulmonary remodelling via regulation of canonical WNT signalling. FASEB J 34:10267–10285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71 (3):209–249 [DOI] [PubMed] [Google Scholar]
- Tanjore H, Degryse AL, Crossno PF et al. (2013) Beta-catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am J Respir Crit Care Med 187:630–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirouvanziam R, Makam M, Péault B (2009) Lung. In: Masters JR, Palsson BØ (eds) Human adult stem cells. Springer, Dordrecht, pp 91–112 [Google Scholar]
- Travaglini KJ, Nabhan AN, Penland L et al. (2020) A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Riely GJ (2013) New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 31:992–1001 [DOI] [PubMed] [Google Scholar]
- Tuder RM, Petrache I (2012) Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest 122:2749–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuder RM, Groves B, Badesch DB, Voelkel NF (1994) Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144:275–285 [PMC free article] [PubMed] [Google Scholar]
- Uhl FE, Vierkotten S, Wagner DE et al. (2015) Preclinical validation and imaging of WNT-induced repair in human 3D lung tissue cultures. Eur Respir J 46:1150–1166 [DOI] [PubMed] [Google Scholar]
- Ulsamer A, Wei Y, Kim KK et al. (2012) Axin pathway activity regulates in vivo pY654-beta-catenin accumulation and pulmonary fibrosis. J Biol Chem 287:5164–5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushakumary MG, Riccetti M, Perl AT (2021) Resident interstitial lung fibroblasts and their role in alveolar stem cell niche development, homeostasis, injury, and regeneration. Stem Cells Transl Med 10(7):1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EM, Menzen MH, Spanjer AI, Middag LD, Brandsma CA, Gosens R (2016) Noncanonical WNT-5B signalling induces inflammatory responses in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 310:L1166–L1176 [DOI] [PubMed] [Google Scholar]
- Vladar EK, Konigshoff M (2020) Noncanonical WNT planar cell polarity signalling in lung development and disease. Biochem Soc Trans 48:231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T, De Langhe SP (2015) WNT and FGF mediated epithelial-mesenchymal crosstalk during lung development. Dev Dyn 244:342–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmirovic M, Herazo-Maya JD, Blackmon J et al. (2017) Identification and validation of differentially expressed transcripts by RNA-sequencing of formalin-fixed, paraffin-embedded (FFPE) lung tissue from patients with idiopathic pulmonary fibrosis. BMC Pulm Med 17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DZ, Li S, Hockemeyer D et al. (2002) Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci U S A 99:14855–14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ahmed J, Wang G et al. (2011) Down-regulation of the canonical WNT beta-catenin pathway in the airway epithelium of healthy smokers and smokers with COPD. PLoS One 6: e14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhu H, Sun Z et al. (2014) Inhibition of WNT/beta-catenin signalling promotes epithelial differentiation of mesenchymal stem cells and repairs bleomycin-induced lung injury. Am J Physiol Cell Physiol 307:C234–C244 [DOI] [PubMed] [Google Scholar]
- Wansleeben C, Barkauskas CE, Rock JR, Hogan BL (2013) Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol 2:131–148 [DOI] [PubMed] [Google Scholar]
- Watson JK, Sanders P, Dunmore R et al. (2020) Distal lung epithelial progenitor cell function declines with age. Sci Rep 10:10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JD, Austin ED, Gaskill C et al. (2014) Identification of a common WNT-associated genetic signature across multiple cell types in pulmonary arterial hypertension. Am J Physiol Cell Physiol 307:C415–C430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, van Dijk EM, Ng-Blichfeldt JP et al. (2019) Mesenchymal WNT-5A/5B signalling represses lung alveolar epithelial progenitors. Cell 8(10):1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rock JR, Lu Y et al. (2012) Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci U S A 109:4910–4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Mizuno T, Sridharan A et al. (2016) Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1:e90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IV, Burch LH, Steele MP et al. (2007) Gene expression profiling of familial and sporadic interstitial pneumonia. Am J Respir Crit Care Med 175:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates LL, Schnatwinkel C, Murdoch JN et al. (2010) The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet 19:2251–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, White AC, Huh SH et al. (2008) An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol 319:426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Shamskhou EA, Orcholski ME et al. (2019) Loss of endothelium-derived WNT5a is associated with reduced pericyte recruitment and small vessel loss in pulmonary arterial hypertension. Circulation 139:1710–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias WJ, Frank DB, Zepp JA et al. (2018) Regeneration of the lung alveolus by an evolution-arily conserved epithelial progenitor. Nature 555:251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp JA, Zacharias WJ, Frank DB et al. (2017) Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 170:1134–48.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp JA, Morley MP, Loebel C et al. (2021) Genomic, epigenomic, and biophysical cues controlling the emergence of the lung alveolus. Science 371(6534):eabc3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Wang HJ, Tan YZ (2011) WNT/β-catenin signalling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PLoS One 6:e21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu H, Davies KJ et al. (2012a) Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic Biol Med 52:2038–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Toh L, Lau P, Wang X (2012b) Human telomerase reverse transcriptase (hTERT) is a novel target of the WNT/β-catenin pathway in human cancer. J Biol Chem 287:32494–32511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Pan Y, Zhang C et al. (2013) WNT/β-catenin signalling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem 374:13–20 [DOI] [PubMed] [Google Scholar]
- Zhou B, Liu Y, Kahn M et al. (2012) Interactions between beta-catenin and transforming growth factor-beta signalling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J Biol Chem 287:7026–7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhang H, Davies KJA, Forman HJ (2018) Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol 14:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo WL, Shenoy SA, Li S et al. (2018) Ontogeny and biology of human small airway epithelial club cells. Am J Respir Crit Care Med 198:1375–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]


