Abstract
Background.
Respiration-induced tumor motion compensation using a treatment couch requires moving the patient at non-trivial speeds. The purpose of this work was to investigate motion sickness and stability of the patient’s external surface due to a moving couch with respiration-comparable velocities and accelerations.
Material and methods.
A couch was designed to move with a peak-peak displacement of 5 cm and 1 cm in the S-I and A-P directions, respectively, and a period of 3.6 s. Fifty patients completed a 16-question motion sickness assessment questionnaire (MSAQ) prior to, during, and after the study. Seven optical reflectors affixed to the abdomen of each patient were monitored by infrared cameras. The relationship between reflector positions under stationary and moving conditions was evaluated to assess the stability of the patient’s external surface.
Results and discussion.
Among the 4800 responses, 95% were 1 (no discomfort) of 9, and there were no scores of 6 or higher. Mild discomfort (scores of 4–5) was similar during couch motion and before couch motion (p=0.39). Mild discomfort was less common after couch motion (p=0.039) than before or during couch movement. There was a near 1:1 correspondence between marker-pair regression coefficients and phase offset values during couch-stationary and couch-moving conditions. Our results show that patients do not suffer motion sickness or external surface instability on a moving couch.
Keywords: Intra-fraction motion, treatment couch, motion sickness, inertial stability, optical sensors
Motion management has become an important issue in the reliable delivery of highly conformal and hypofractionated delivery of radiation to lung and abdominal tumors. Advanced motion correction strategies include gating [1–5] and real-time motion compensation [6–14]. The only clinically available real-time motion compensation device is the Cyber-knife system, in which an X-band linear accelerator mounted on an industrial robot is used to compensate for 3D tumor motion [6–9]. Additional laboratory systems for real-time motion compensation are in development. These systems involve tracking the tumor using either the MLC [10–12] or the treatment couch [13,14]. The proposed couch-based system involves translating the patient support structure.
While the technical feasibility of a treatment couch to compensate in real time for respiration-induced tumor motion has been previously demonstrated [13,14], the key to clinical development and implementation is its clinical feasibility. Sweeney et al. have shown that patients and healthy subjects tolerated movements on a couch at a speed of 0.7 cm/s (maximum speed of 1.5 cm/s) very well [15]. However, our previous work [14] has shown that typical maximum velocities and accelerations needed to achieve adequate control of realistic tumor motion are approximately 5 cm/s and 15 cm/s2 for peak-peak tumor displacements of 2 cm. Thus, since a treatment couch-based compensation system will involve moving a patient at non-trivial velocities and accelerations, two critical questions must be investigated: (1) do patients get motion sick on a moving couch, and (2) does the abdomen of the patient experience any “wobbling” due to the motion? The motivation for the latter question arises is that inference of the tumor position will rely on external surrogates, whose inter-relationship must be invariant under stationary and moving conditions. In this paper we seek to answer these questions.
Material and methods
Study design
Fifty patients undergoing radiation therapy at the University of Maryland Medical Center were recruited into the study under Institutional Review Board approval. Half of the patients were male, 56% had concurrent chemotherapy, and the median age was 60 years (range 24–86). Patients were primarily non-Hispanic Caucasian (56%) and non-Hispanic Black (40%) with 4% Hispanic participants. Patient primary cancer site and chemotherapy history were collected by medical record abstraction. In a pre-experiment survey 16 (32%) of 50 patients reported some history of motion sickness from motion travel in a car, bus, boat, ship or airplane at some point within the last 10 years.
Motion sickness survey
Patients were given a previously validated Motion Sickness Assessment Questionnaire (MSAQ) [16]. The MSAQ was designed to assess 16 different symptoms of motion sickness including nausea, dizziness, unease, sweatiness, queasiness, etc. Participants were asked to rank whether they felt each symptom on a scale from 1 (not at all) to 9 (severe).
Data acquisition
An experimental couch (3DLine Medical Systems, Milan, Italy) was designed to move in the superior-inferior (S-I) and anterior-posterior (A-P) directions according to a pre-programmed trajectory. We did not consider rotation of the couch (tilting motion). The shape of the motion trajectory was derived from a smoothed 4D CT scan of a lung cancer patient in our clinic. Seppenwoolde et al. have reported that the mean extent of tumor motion was 1.2±0.2 cm in the S-I direction and 0.2 cm±0.1 cm in the A-P and medial-lateral (M-L) directions, respectively. The same study reported an average breathing cycle period of 3.6±0.8 s [17]. The peak-peak displacement of the couch was scaled to exceed these observed clinical data. The peak-to-peak extents of the motion in the S-I and A-P directions were 5 cm and 1 cm, respectively and the period of couch motion was 3.6 s. Figure 1 shows the time-varying displacement, velocity and acceleration of the couch. Medial-lateral motion was ignored since it was considered insignificant based on previous literature estimates. Patients were asked to lie on the couch with their arms crossed above their head (simulating the treatment position).
Figure 1.
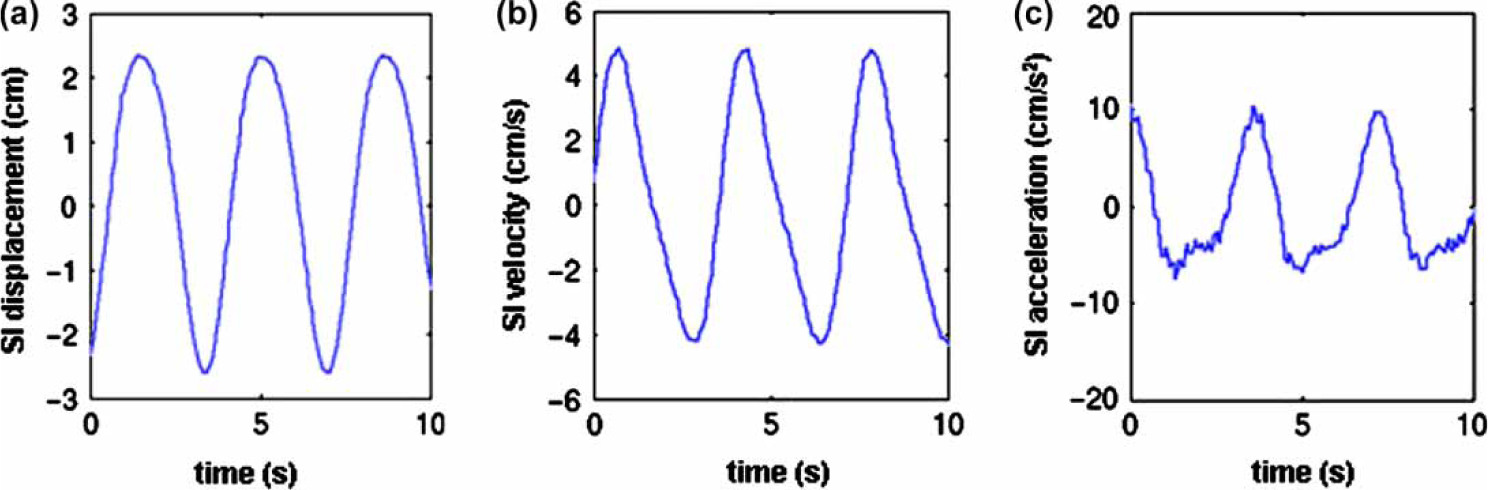
Display of the time-varying couch (a) displacement, (b) velocity and (c) acceleration used in the study.
Seven optical reflectors were affixed to the skin of the study participants at the following locations: xyphoid process (M1), halfway between the xyphoid process and the umbilicus (M2), approximately 5–7 cm lateral to M2 on either side of the patient’s midline (M3 and M4), just above the umbilicus (M5), and approximately 5–7 cm lateral to M5 on either side of the patient’s midline (M6 and M7). In addition a reflector was attached to the couch. The positions of the reflectors were monitored using the Dynatrac™ system (3DLine Medical Systems, Reston, VA), which uses three infra-red cameras to detect the 3D coordinates of each of the reflectors.
Upon initiation of the study, the reflector displacements were recorded for a 3 min period while the couch was stationary. Next, the patients were administered the MSAQ to establish a baseline response. The couch was then moved according to the pre-programmed 3D trajectory for 3 min. Following the interval of couch motion, patients were administered the MSAQ. The couch was moved for three additional 3 min intervals, each followed by the MSAQ during a period of time when the couch was stationary. After the last couch movement interval and MSAQ, the displacement of the markers was again recorded on a non-moving couch. Finally, the MSAQ was administered again at least 30 min after completion of the study.
Statistical analysis
Motion sickness
Survey responses were evaluated both separately and in combination for each of the six time-points (0, 3, 6, 9, 12 min of motion on the couch and 30 min after getting off the couch). These time-points are identified as T0, T3, T6, T9, T12, and T30, respectively, henceforth. Motion sickness was defined as a score of 7 or more (on a 9 point scale) on any of the 16 survey questions or as a cumulative score of 80 or more (of 144) for the 16 questions. The Wilcoxen matched-pairs rank-sum test was used to evaluate trends in discomfort during successive surveys.
Patient inertial stability
Data preparation.
Three-dimensional data from the seven optical reflectors placed on the abdomen of patients was separated into data frames according to the state of the couch: stationary or moving. Each frame was 120–170 s in duration.
The instantaneous position of the reflector attached to the couch was subtracted from the instantaneous position of each of the seven reflectors placed on the patient (Figure 2). The resulting motion traces were resampled from approximately 20 Hz to even 40 Hz intervals through linear interpolation. Each direction of motion (S-I, A-P, M-L) of each reflector was considered to be a separate marker.
Figure 2.
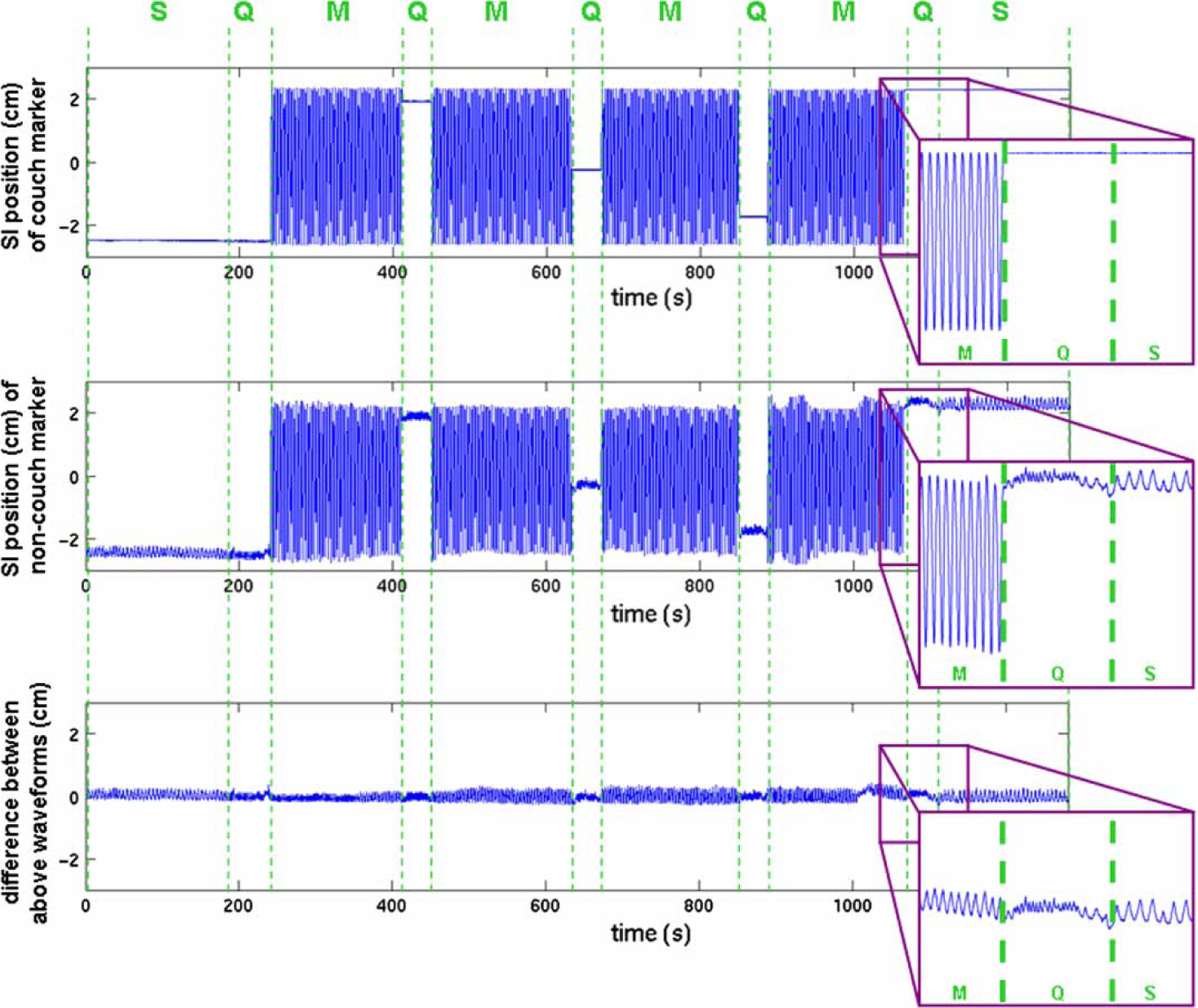
Determining patient-induced marker motion in a typical tracking session. The top two panels show the one-dimensional (S-I) motion of a marker attached to the treatment couch and the S-I motion of a marker attached to the patient during couch stationary (S) and moving (M) conditions. The bottom is the difference between the first two waveforms, or the patient-induced marker motion alone. Periods of couch motion, questionnaire in progress, and couch stationary (with no questionnaire in progress) are labeled as M, Q, and S, respectively.
Analysis of marker pair relationships
The magnitude of motion for each marker was measured by determining peak inhalation and peak exhalation of each respiratory cycle using an algorithm similar to those published by Lu et al and Suh et al. [18,19]. Specifically, a moving average of the marker motion waveform with window of 2.5 s, approximately half the period of a respiratory cycle, was calculated.
To investigate the stability of the patient’s surface while on a couch, the relationship between positions of pairs of reflectors was characterized. The goal was to determine whether this relationship was consistent under the two couch conditions of stationary and moving.
The following parameters were measured for each pair of markers in each frame:
Regression coefficient: Simple linear regression was performed on pairs of markers, where a marker is defined as reflector motion in one direction (M-L, S-I, or A-P). The regression coefficient is the slope of the least-squares-fit line relating the positions of one marker to those of the other.
Phase offset: The phase offset between the two markers is defined as the temporal shift between marker motion datasets that results in the best correlation between positions of the two markers. To facilitate peak alignment, baseline drift in each motion trace was removed by subtracting a moving average with a window larger than 1.5 times the period of a respiratory cycle from each marker dataset before the phase offsets were determined.
In addition to considering regression coefficient and phase offset values for the entire dataset, we also considered these parameters for those patient subsets of (1) patients weighing >85 kg and ≤85 kg and (2) patients with a weight-to-height ratio of >0.49 kg/cm and ≤0.49 kg/cm.
Results
Motion sickness analysis
None of the study participants reported motion sickness during or after the experiment (Table I). Twenty nine (58%) of 50 subjects reported a score of 1 for all 16 questions on all 6 survey administrations, indicating no discomfort before, during or after the experiment. The highest score reported to any question on any of the six times the survey was administered to each participant was a score of 5 (of 9) suggesting couch motion is not associated with motion sickness either during or after treatment on a moving couch.
Table I.
Frequency summary of the scores reported on the motion sickness survey at each of the survey administrations. Surveys were administered at T0 (before couch movement), T3 (after 3 minutes of couch movement), T6 (after 6 minutes of couch movement), T9 (after 9 minutes of couch movement), T12 (after 12 minutes of couch movement), and T30 (30 minutes after getting off the couch). Each time-point has 800 responses (16 questions for each of 50 subjects).
| Survey Score | Timepoints N (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T3 | T6 | T9 | T12 | T30 | |||||||
|
| ||||||||||||
| 1 | 771 | 96.4% | 762 | 95.3% | 765 | 95.6% | 754 | 94.3% | 752 | 94.0% | 770 | 96.3% |
| 2–3 | 20 | 2.5% | 29 | 3.6% | 29 | 3.6% | 41 | 5.1% | 43 | 5.4% | 29 | 3.6% |
| 4–5 | 9 | 1.1% | 9 | 1.1% | 6 | 0.8% | 5 | 0.6% | 5 | 0.6% | 1 | 0.1% |
| 6–9 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
Table I underscores that the vast majority of responses to each survey were a score of 1 indicating no discomfort. Mild discomfort (defined as a score of 4–5) was reported at some time during the experiment by seven (14%) subjects. No scores above 5 were reported and reports of mild discomfort were similar during and before couch motion (0.78% vs. 1.1%, p=0.39) and less discomfort after than before the experiment (0.12%, p=0.039) suggesting no discomfort from the moving couch. Scores of 5 were only reported by three subjects and they reported no discomfort after the study suggesting mild transient discomfort.
Inertial stability analysis
The distributions of regression coefficients and phase offset values measured while the couch was stationary and those measured while the couch was in motion were found to be virtually indistinguishable (Figure 3). Furthermore, the regression coefficients and phase offsets for each combination of a stationary frame and a motion frame within a patient dataset were compared. When the results from all patients were analyzed there was a near 1:1 correspondence between couch stationary and couch in-motion regression coefficient and phase offset values. The R2 values comparing these points against the 1:1 correspondence lines were 0.99 and 0.81 for regression coefficient and phase offset, respectively.
Figure 3.
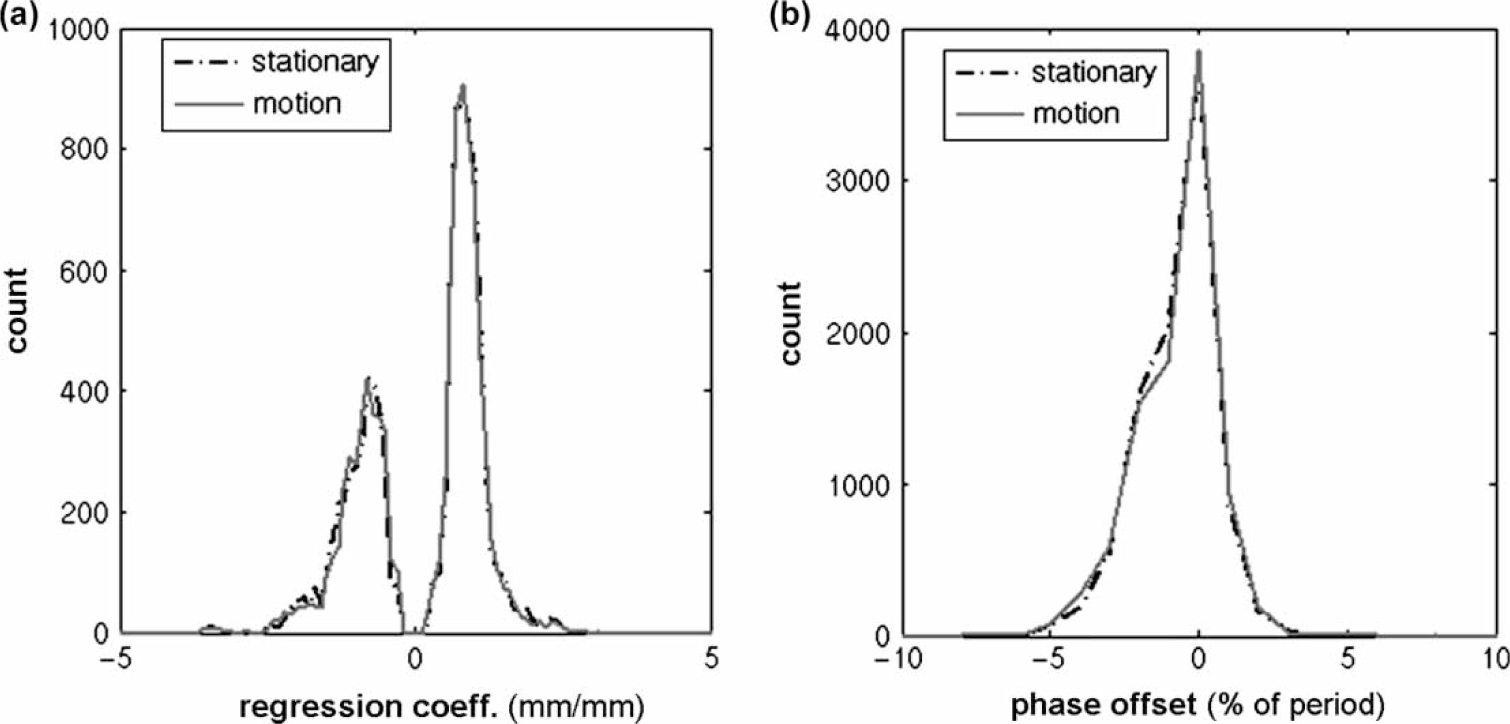
Histograms showing distributions of parameter values (a) regression coefficient and (b) phase offset used to characterize the relationship between pairs of markers while the couch is stationary and while the couch is in motion.
Of those patients for whom we recorded external marker motion and weight and height data (n=48), 11 patients weighed >85 kg, and 12 patients had a weight-to-height ratio >0.49 kg/cm. The R2 values for the regression coefficients (0.99) and phase offsets (0.81) were unchanged when data was stratified by weight (≤85 kg and >85 kg) (see Figure 4) or unstratified.
Figure 4.
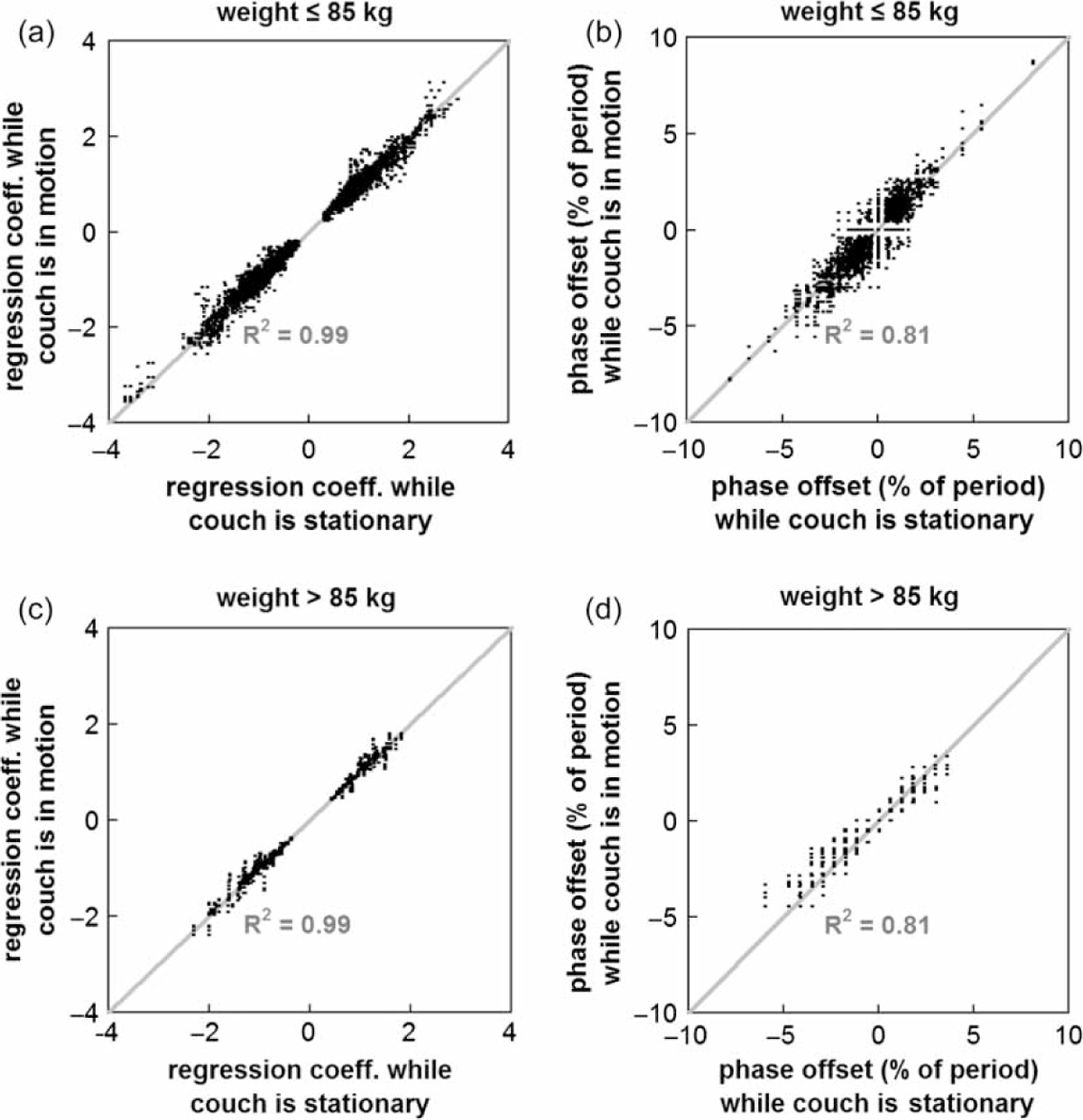
Scatterplots showing the consistency of the (a) and (c) regression coefficients and (b) and (d) phase offsets between marker pairs when data was stratified by weight (≤ 85 kg versus >85 kg) for the conditions of couch stationary and couch in motion.
Discussion
The purposes of this study were to (1) investigate if patients on a moving couch with respiration comparable speeds and accelerations suffer motion sickness and (2) investigate if the patients’ abdomen is disturbed under couch-moving conditions compared with couch-stationary conditions.
Our study demonstrates that a moving couch does not induce any motion sickness or sustained discomfort. While almost a third of the patients enrolled in this study reported having experienced some form of motion sickness during their lifetime, no patient reported any signs of motion sickness during or immediately after the study. We did not consider rotation of the couch in our study because it has been shown that the primary impact of normal respiration is translational motion. The extent of rotation and deformation was determined to be less than the tumor contouring uncertainty [20,21]. However, it is possible that under some circumstances, rotational motion may be needed.
The second portion of the study was prompted by concern that moving the couch to counter respiratory-correlated tumor motion would result in “wobbling” of the external patient surface. Real-time inference of the tumor position using the position of external surrogates is based on a predetermined relationship between the (internal) tumor position and the external surrogates’ positions. If the internal-external relationship changes due to couch motion, then resulting differences in the internal-external relationship will lead to errors in the inferred tumor position. Our results confirm that the relationship between marker positions does not change significantly due to couch motion any more than due to normal respiration, even for the third of patients with the highest weights and weight-to-height ratios.
Conclusions
In this study, we have shown that moving a treatment couch through a realistic lung tumor trajectory does not cause motion sickness, and we were unable to find evidence that a moving couch causes significant inertia-related abdominal distortion. This study supports the feasibility of using a treatment couch to compensate in real time for respiration-induced tumor motion.
Acknowledgements
This work was supported in part by grants from the NIH/NCI CA 122403 and CA 124766 and from 3DLine Medical Systems/Elekta Oncology Systems. No conflict of interest.
References
- [1].Ohara K, Okumura T, Akisada M, Akisada M, Inada T, Mori T, et al. Irradiation synchronized with respiration gate. Int J Radiat Oncol Biol Phys 1989;17:853–7. [DOI] [PubMed] [Google Scholar]
- [2].Kubo HD, Hill BC. Respiration-gated radiotherapy treatment: A technical study. Phys Med Biol 1996;41:83–91. [DOI] [PubMed] [Google Scholar]
- [3].Minohara S, Kanai T, Endo M, Noda K, Kanazawa M. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:1097–103. [DOI] [PubMed] [Google Scholar]
- [4].Kubo HD, Len PM, Minohara S, Mostafavi H. Breathing synchronized radiotherapy program at the University of California Davis Cancer Center. Med Phys 2000;27:346–53. [DOI] [PubMed] [Google Scholar]
- [5].Vedam SS, Keall PJ, Kini VR, Mohan R. Determining parameters for respiration-gated radiotherapy. Med Phys 2001;28:2139–46. [DOI] [PubMed] [Google Scholar]
- [6].Schweikard A, Glosser G, Bodduluri M, Murphy MJ, Adler JR. Robotic motion compensation for respiratory movement during radiosurgery. Comput Aided Surg 2000;5:263–77. [DOI] [PubMed] [Google Scholar]
- [7].Chang SD, Adler JR. Robotics and radiosurgery – the cyberknife. Stereotact Funct Neurosurg 2001;76:204–8. [DOI] [PubMed] [Google Scholar]
- [8].Ozhasoglu C, Murphy MJ. Issues in respiratory motion compensation during external-beam radiotherapy. Int J Radiat Oncol Biol Phys 2002;52:1389–99. [DOI] [PubMed] [Google Scholar]
- [9].Schweikard A, Shiomi H, Adler J. Respiration tracking in radiosurgery. Med Phys 2004;31:2738–41. [DOI] [PubMed] [Google Scholar]
- [10].Keall PJ, Kini VR, Vedam SS, Mohan R. Motion adaptive x-ray therapy: A feasibility study. Phys Med Biol 2001;46: 1–10. [DOI] [PubMed] [Google Scholar]
- [11].Webb S The effect on IMRT conformality of elastic tissue movement and a practical suggestion for movement compensation via the modified dynamic multileaf collimator (dMLC) technique. Phys Med Biol 2005;50:1163–90. [DOI] [PubMed] [Google Scholar]
- [12].Keall PJ, Cattell H, Pokhrel D, Dieterich S, Wong KH, Murphy MJ, et al. Geometric accuracy of a real-time target tracking system with dynamic multileaf collimator tracking system. Int J Radiat Oncol Biol Phys 2006;65:1579–84. [DOI] [PubMed] [Google Scholar]
- [13].D’Souza WD, Naqvi SA, Yu CX. Real-time intra-fraction motion tracking using the treatment couch: A feasibility study. Phys Med Biol 2005;50:4021–33. [DOI] [PubMed] [Google Scholar]
- [14].D’Souza WD, McAvoy TJ. An analysis of the treatment couch and control system dynamics for respiration-induced motion compensation. Med Phys 2006;33:4701–9. [DOI] [PubMed] [Google Scholar]
- [15].Sweeney RA, Arnold W, Steixner E, Nevinny-Stickel M, Lukas P. Compensating for tumor motion by a 6-degree-of-freedom treatment couch: Is patient tolerance an issue? Int J Radiat Oncol Biol Phys 2009;74:168–71. [DOI] [PubMed] [Google Scholar]
- [16].Gianaros PJ, Muth ER, Mordkoff JT, Levine ME, Stern RM. A questionnaire for the assessment of the multiple dimensions of motion sickness. Aviat Space Environ Med 2001;72:115–9. [PMC free article] [PubMed] [Google Scholar]
- [17].Seppenwoolde Y, Shirato H, Kitamura K, Shimizu S, van Herk M, Lebesque JV, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys 2002;53:822–34. [DOI] [PubMed] [Google Scholar]
- [18].Lu W, Nystrom MM, Parikh PJ, Fooshee DR, Hubenschmidt JP, Bradley JD, et al. A semi-automatic method for peak and valley detection in free-breathing respiratory waveforms. Med Phys 2006;33:3634–6. [DOI] [PubMed] [Google Scholar]
- [19].Suh Y, Dieterich S, Cho B, Keall PJ. An analysis of thoracic and abdominal tumour motion for stereotactic body radiotherapy patients. Phys Med Biol 2008;53:3623–40. [DOI] [PubMed] [Google Scholar]
- [20].Liu HH, Balter P, Tutt T, Choi B, Zhang J, Wang C, et al. Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 2007;68:531–40. [DOI] [PubMed] [Google Scholar]
- [21].Wu J, Lei P, Shekhar R, Li H, Suntharalingam M, D’Souza WD. Do tumors in the lung deform during normal respiration? An image registration investigation. Int J Radiat Oncol Biol Phys (in press). [DOI] [PubMed] [Google Scholar]


