Abstract
Background:
Urinary tract infection (UTI) is the most common bacterial infection in pregnancy. Known risk factors for UTI in pregnancy include diabetes and certain urologic conditions. Other maternal characteristics might also be associated with risk and could provide clues to the etiology of UTI in pregnancy. Our objective was to identify maternal characteristics associated with UTI in pregnancy.
Methods:
We used data from pregnant women participating in the National Birth Defects Prevention Study, a population-based study of risk factors for major structural birth defects in 10 U.S. sites, from 1997–2011. In cross-sectional analyses, we used multivariable log-binomial regression to estimate prevalence ratios (PRs) and 95% confidence intervals (CIs) for associations between self-reported maternal characteristics and UTI in pregnancy.
Results:
In our sample of 41,869 women, the overall prevalence of reported UTI in pregnancy was 18% but ranged from 11% to 26% between study sites. In adjusted models, diabetes was moderately associated with higher UTI prevalence (PR 1.39, 95% CI: 1.24, 1.57). Higher UTI prevalence was associated even more strongly with low educational attainment (PR 2.06, 95% CI: 1.77, 2.40 for some high school versus graduate school), low household income (PR 1.64, 95% CI: 1.46, 1.84 for <$10,000 versus ≥$50,000), and race/ethnicity (PR 1.45, 95% CI: 1.13, 1.80 for American Indian or Alaska Native versus White women).
Conclusions:
About one in six women reported UTI in pregnancy but the prevalence varied markedly by geography and maternal characteristics. This variability could provide clues to the causes of UTI in pregnancy.
Keywords: Cystitis, Pregnancy Complications, Pyelonephritis, Socioeconomic Factors, Urinary Tract Infections
INTRODUCTION
Urinary tract infection (UTI) is the most common bacterial infection in pregnancy.1 In pregnancy, the definition of UTI often includes symptomatic infections of the bladder (cystitis) or kidney (pyelonephritis) as well as asymptomatic bacteriuria, the presence of bacteria in the urine without symptoms. Review articles cite a prevalence of 2–10% for asymptomatic bacteriuria, 1–4% for cystitis, and 1–2% for pyelonephritis in pregnancy.1–3 UTIs are commonly caused by ascending movement of bacteria that colonize the lower gastrointestinal and genitourinary tract, particularly E. coli or other gram-negative bacteria.3
Asymptomatic bacteriuria in pregnancy is treated primarily to prevent progression to pyelonephritis, which carries special risks during pregnancy.4 Pyelonephritis in pregnancy can lead to preterm labor, anemia, septicemia, respiratory insufficiency, and rarely, maternal death.5–7 UTIs in pregnancy are also associated with pre-eclampsia and birth defects.8–10 Clinical guidelines recommend that healthcare providers screen pregnant women for asymptomatic bacteriuria and prescribe a short course of antibiotics if bacteriuria is found.11–15 About two-thirds of women with a UTI in pregnancy take an antibiotic.16, 17
The etiology of UTI in pregnancy is poorly understood, which means there are few methods to prevent the occurrence of UTIs, as opposed to screening and treating women after they have already been infected. Women at high risk for UTI in pregnancy include those with medical comorbidities such as diabetes, polycystic kidney disease, congenital abnormalities of the urinary tract, sickle cell disease, and recurrent UTI.2, 13 Less is known about other maternal factors associated with UTI in pregnancy, which have been inconsistently reported in the literature.1, 18 For example, maternal age, race, socioeconomic status (SES), and parity are associated with UTI in pregnancy in some studies, but not others.2, 6, 7, 18–21
Understanding the etiology of UTI in pregnancy could lead to new opportunities for its prevention—not only reducing maternal and fetal morbidity, but also decreasing antibiotic use in pregnancy. Sulfonamides and nitrofurantoin are commonly used to treat UTI in pregnancy, but these types of antibiotics are associated with birth defects in some studies.16, 22–24 Our objective was to identify maternal factors associated with UTI in pregnancy in a large population-based sample of women from 10 U.S. sites to provide new clues to the etiology of UTI in pregnancy.
MATERIALS AND METHODS
Participant Selection
We used data from the National Birth Defects Prevention Study (NBDPS), a multi-site, population-based, case-control study of risk factors for major structural birth defects.25 Cases were infants or fetuses with one or more major birth defects identified through population-based birth defects surveillance systems statewide in Arkansas, Iowa, and Utah and in select regions of California, Georgia, Massachusetts, New Jersey, New York, North Carolina, and Texas. All sites ascertained cases among live births and some additionally ascertained cases among stillbirths and terminations of pregnancy. Clinical geneticists excluded cases of birth defects likely caused by genetic abnormalities or other recognized syndromes.26 Controls were liveborn infants without major birth defects identified from birth certificates or hospital delivery logs in the same areas. Eligible mothers had pregnancies ending on or after October 1, 1997, and due dates on or before December 31, 2011. All women provided informed consent for participation. Institutional review boards at all participating study sites approved the study.
Participating mothers completed a computer-assisted telephone interview in English or Spanish six weeks to two years after the estimated due date. The interview included questions on parental sociodemographics, medications and illnesses during pregnancy, and other potentially teratogenic exposures. Sixty-seven percent of eligible case mothers and 65% of eligible control mothers participated in the interview.25
Although the NBDPS is a case-control study, we analyzed the data as a cross-sectional study by pooling all participating mothers into a single study population. Pooling the participants is unlikely to bias results if sociodemographic factors associated with UTI in pregnancy are similar in case and control mothers, the prevalence of these factors is similar between groups, and each group has similar accuracy in reporting UTI in pregnancy. Our study data suggest that the first two conditions might be true. The third cannot be tested in our data, but we assume that reporting accuracy does not differ markedly between the groups given that the self-reported UTI prevalence is similar between case and control mothers.
Assessment of UTI in Pregnancy
Interviewers asked: “Between 3 months before pregnancy and the baby’s birth, did you have a kidney, bladder, or urinary tract infection?” Women who said yes were asked when the UTI occurred (before pregnancy, or during pregnancy in the first, second, or third trimester), if their UTI was diagnosed by a doctor, and what medications they used to treat the UTI. We also included UTIs reported in other sections of the questionnaire, such as those asking about the causes of fevers or medication use. The NBDPS included no questions on UTI symptoms or screening. If women reported UTI in pregnancy but listed a yeast infection medication as the only medication used for their UTI, we assumed these women had a vaginal yeast infection, not a UTI.
Maternal Characteristics
We included a variety of maternal characteristics, including sociodemographics, reproductive history, medical conditions, substance use, and variables that had been inconsistently associated with UTI in pregnancy in prior studies. Variables that had been included in previous studies included age at conception; race/ethnicity; household income; education; pregestational or gestational diabetes; UTI in the three months before pregnancy; number of prior pregnancies; multivitamin use in pregnancy; smoking or alcohol use during pregnancy; and marijuana, cocaine, or methamphetamine use during pregnancy or in the three months before.19, 20, 27–29 We also included variables related to the NBDPS study design (birth defect status [if the mother was originally selected as a case or control], study site, year of due date); pregnancy-related variables (prepregnancy body mass index [BMI], plurality); and usual caffeine intake in the year before pregnancy. Higher caffeine intake is associated with greater urinary frequency and urgency—symptoms also seen in UTIs—in previous studies of nonpregnant women.30 Caffeine intake was estimated based on reported coffee, tea, soda, and chocolate intake.
Exclusion Criteria
We excluded women who did not answer the question about UTI in pregnancy. We also excluded pregnancies that ended in stillbirth or termination of pregnancy to improve comparability between study sites—some sites included only live births.
Statistical Analyses
Statistical analyses were conducted in SAS 9.4 and R 4.0.4. We used log-binomial regression to investigate associations between sociodemographic characteristics and UTI in pregnancy, reporting prevalence ratios (PRs) and 95% confidence intervals (CIs) and adjusting multivariable models for birth defect status, study site, and maternal age at conception (continuous variable). For each sociodemographic characteristic, we chose additional potential confounders using a combination of directed acyclic graphs, literature review, and associations between variables in the NBDPS. Potential confounders included in the models were: birth defect status (case or control in the original NBDPS study design), study site (Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, Utah), maternal age at conception, maternal race/ethnicity (Hispanic [all races], and non-Hispanic Asian or Pacific Islander, Black, White, and all other races), and maternal educational attainment (less than high school, high school or equivalent, post-high school training, college degree or more). We additionally adjusted for smoking during pregnancy (yes, no) for the models examining multivitamin use, alcohol use, caffeine intake, and illicit drug use. The model for race/ethnicity was adjusted for only birth defect status and study site because the other variables were likely on the causal pathway between race/ethnicity and UTI in pregnancy.
When analyzing year of due date, we restricted to study sites and time periods without changes in study catchment areas: Arkansas, California, Iowa, Massachusetts, and Georgia, 2000–2011. When analyzing education and household income, we restricted to women aged ≥25 years because these variables are less predictive of socioeconomic status in young women who have not yet completed their education or started earning an income.
Sensitivity Analyses
We conducted five sensitivity analyses. First, we conducted probabilistic bias analyses to investigate how misclassification of self-reported UTI could have affected our results. This analysis was prompted by a previously published study that found low sensitivity (Se) and specificity (Sp), around 49% and 88%, for self-reported UTI in pregnancy.31 We provide a full description of our probabilistic bias analyses in Supplementary Materials 1. Briefly, we used a freely-available tool (https://sites.google.com/site/biasanalysis/) that uses estimates of Se and Sp to back-calculate what the crude PR would have been in the absence of bias. We conducted three bias analysis: one assuming nondifferential misclassification of outcome, one assuming differential misclassification with exposed mothers having more accurate self-report, and one assuming differential misclassification with unexposed mothers having more accurate self-report. We conducted each bias analysis 1,000 times using trapezoidal distributions for Se and Sp and incorporated sampling (random) error into the simulations using the tool’s built-in function. This tool does not incorporate confounding and therefore our sensitivity analysis produces crude PRs. Probabilistic bias analyses produce a distribution of PRs—not a single PR—as its result. We therefore present results as the median crude PR and 95% simulation interval (an interval containing 95% of the PRs from simulation).
In our second sensitivity analysis, we separately analyzed the three racial and ethnic groups with relatively large sample sizes: non-Hispanic White (n = 24,599), Hispanic (n = 10,159), and non-Hispanic black (n = 4,331) women. A previous study found that risk factors for bacteriuria at the first prenatal visit differed by race.27 In our third sensitivity analysis, we analyzed data separately from mothers who had offspring with or without birth defects to determine if combining the participants affected our results. In our fourth sensitivity analysis, we excluded participants who reported pregestational or gestational diabetes. In the fifth sensitivity analysis, we assumed that participants with missing values for UTI in pregnancy all had UTIs in pregnancy or all had no UTI in pregnancy to estimate the potential impact of missing outcome data on our results.
RESULTS
The NBDPS included 43,846 women (32,017 mothers of offspring with birth defects, 11,829 mothers of infants without birth defects). We excluded 1,257 women whose pregnancies ended in stillbirth or termination of pregnancy and 720 women with missing values for UTI in pregnancy. This left 41,869 women in the analysis. We reclassified 186 women as having vaginal yeast infections instead of UTIs in pregnancy based on reported medication use.
In our population, 18% of women (7,742) reported UTIs in one or more trimesters of pregnancy (19% of case mothers, 18% of control mothers). Almost all (96%) said their UTI was diagnosed by a doctor. Among women who reported the trimester of their UTI, UTI prevalence was 8% in the first, 8% in the second, and 7% in the third trimester. These statistics include 1,272 women who reported UTIs in more than one trimester (17% of all women who had complete data on the timing of their UTI).
We found no difference in UTI prevalence in pregnancy by birth defect status and year of conception, but a strong association with study site (Table 1). Women in Arkansas and Texas had the highest prevalence of UTI in pregnancy (26% each) and women in Massachusetts had the lowest (11%). Geographic differences persisted after adjusting for potential confounders.
Table 1.
Associations between study design variables and urinary tract infection in pregnancy—cross-sectional analysis of the National Birth Defects Prevention Study, 1997–2011.
| Total number | Reported UTI prevalence (%) | Crude prevalence ratio (95% CI) | Adjusted prevalence ratioa (95% CI) | |
|---|---|---|---|---|
|
| ||||
| Birth defect status | ||||
| Offspring with birth defects | 30,212 | 19 | 1.07 (1.02, 1.12) | 1.05 (1.01, 1.10) |
| Offspring without birth defects | 11,657 | 18 | 1.00 (Reference) | 1.00 (Reference) |
| Year of due dateb | ||||
| 2000–2002 | 5,527 | 18 | 1.00 (Reference) | 1.00 (Reference) |
| 2003–2005 | 5,845 | 18 | 1.03 (0.95, 1.11) | 1.03 (0.95, 1.11) |
| 2006–2008 | 4,793 | 18 | 1.05 (0.97, 1.14) | 1.04 (0.96, 1.13) |
| 2009–2011 | 4,432 | 18 | 1.03 (0.95, 1.13) | 1.10 (1.01, 1.20) |
| Study site | ||||
| Texas (selected regions) | 4,666 | 26 | 2.51 (2.29, 2.76) | 1.68 (1.52, 1.86) |
| Arkansas (statewide) | 5,505 | 26 | 2.44 (2.23, 2.67) | 1.74 (1.59, 1.91) |
| California (selected regions) | 4,913 | 22 | 2.12 (1.93, 2.33) | 1.43 (1.30, 1.58) |
| North Carolina (selected regions) | 3,306 | 20 | 1.89 (1.70, 2.10) | 1.53 (1.37, 1.70) |
| Utah (statewide) | 4,277 | 16 | 1.53 (1.38, 1.70) | 1.27 (1.14, 1.41) |
| Iowa (statewide) | 4,040 | 15 | 1.47 (1.32, 1.63) | 1.23 (1.11, 1.37) |
| Georgia (selected regions) | 4,620 | 15 | 1.45 (1.31, 1.61) | 1.23 (1.11, 1.37) |
| New York (selected regions) | 3,101 | 15 | 1.45 (1.30, 1.63) | 1.27 (1.14, 1.43) |
| New Jersey (selected regions) | 2,184 | 14 | 1.29 (1.13, 1.47) | 1.13 (0.99, 1.29) |
| Massachusetts (selected regions) | 5,257 | 11 | 1.00 (Reference) | 1.00 (Reference) |
CI, confidence interval; UTI, urinary tract infection.
Adjusted for birth defect status, study site, age at conception, race/ethnicity, and educational attainment.
Restricted to study sites and time periods without changes to study catchment areas: Arkansas, California, Iowa, Massachusetts, and Georgia between 2000 and 2011.
Sociodemographic characteristics were also associated with UTI prevalence (Table 2). UTI prevalence was highest among mothers aged <19 years at conception (30%; Figure 1), American Indian or Alaska Native women (27%), women with 9–11 years of education (25%), and women with annual household income <$10,000 (24%). After adjusting for potential confounders, associations between these sociodemographic variables and UTI remained.
Table 2.
Associations between maternal sociodemographic characteristics and urinary tract infection in pregnancy—cross-sectional analysis of the National Birth Defects Prevention Study, 1997–2011.
| Total number | Reported UTI prevalence (%) | Crude prevalence ratio (95% CI) | Adjusted prevalence ratioa (95% CI) | |
|---|---|---|---|---|
|
| ||||
| Age at conception | ||||
| ≤19 | 5,468 | 30 | 1.78 (1.68, 1.88) | 1.37 (1.29, 1.46) |
| 20–24 | 9,998 | 24 | 1.44 (1.36, 1.52) | 1.24 (1.17, 1.31) |
| 25–29 | 11,423 | 17 | 1.00 (Reference) | 1.00 (Reference) |
| 30–34 | 9,747 | 12 | 0.73 (0.68, 0.78) | 0.81 (0.76, 0.87) |
| ≥35 | 5,233 | 11 | 0.68 (0.62, 0.74) | 0.77 (0.71, 0.84) |
| Race/ethnicityb | ||||
| American Indian or Alaska Native | 192 | 27 | 1.67 (1.29, 2.08) | 1.45 (1.13, 1.80) |
| Hispanic | 10,159 | 24 | 1.51 (1.44, 1.58) | 1.31 (1.24, 1.38) |
| Black | 4,331 | 21 | 1.31 (1.23, 1.40) | 1.28 (1.20, 1.37) |
| White | 24,599 | 16 | 1.00 (Reference) | 1.00 (Reference) |
| Asian or Pacific Islander | 1,172 | 10 | 0.64 (0.53, 0.75) | 0.68 (0.57, 0.80) |
| All other races | 1,403 | 22 | 1.35 (1.22, 1.50) | 1.27 (1.14, 1.40) |
| Missing | 13 | |||
| Educational attainment at deliveryc | ||||
| ≤8 years of education | 1,268 | 20 | 2.53 (2.16, 2.95) | 1.58 (1.33, 1.87) |
| 9–11 years of education | 1,419 | 25 | 3.14 (2.72, 3.62) | 2.06 (1.77, 2.40) |
| High school diploma or equivalent | 4,615 | 17 | 2.06 (1.81, 2.35) | 1.55 (1.36, 1.77) |
| Post-high school education | 7,144 | 15 | 1.91 (1.69, 2.17) | 1.54 (1.36, 1.75) |
| College degree | 7,914 | 11 | 1.38 (1.21, 1.57) | 1.26 (1.11, 1.43) |
| Graduate degree | 3,592 | 8 | 1.00 (Reference) | 1.00 (Reference) |
| Missing | 451 | |||
| Household income (US dollars)c | ||||
| <$10,000 | 2,598 | 24 | 2.43 (2.23, 2.65) | 1.64 (1.46, 1.84) |
| $10,000 to <$20,000 | 2,346 | 21 | 2.12 (1.92, 2.33) | 1.50 (1.34, 1.68) |
| $20,000 to <$30,000 | 2,787 | 18 | 1.80 (1.63, 1.98) | 1.37 (1.23, 1.53) |
| $30,000 to <$40,000 | 2,479 | 15 | 1.47 (1.32, 1.64) | 1.21 (1.07, 1.35) |
| $40,000 to <$50,000 | 2,184 | 12 | 1.18 (1.04, 1.34) | 1.02 (0.90, 1.16) |
| ≥$50,000 | 12,190 | 10 | 1.00 (Reference) | 1.00 (Reference) |
| Missing | 1,819 | |||
CI, confidence interval; UTI, urinary tract infection.
Adjusted for birth defect status, study site, age at conception, race/ethnicity, and educational attainment.
Only adjusted for birth defect status and study site. Hispanic women are those with Hispanic ethnicity, regardless of race. All other categories include only non-Hispanic women.
Restricted to women aged ≥25 years at conception.
Figure 1.
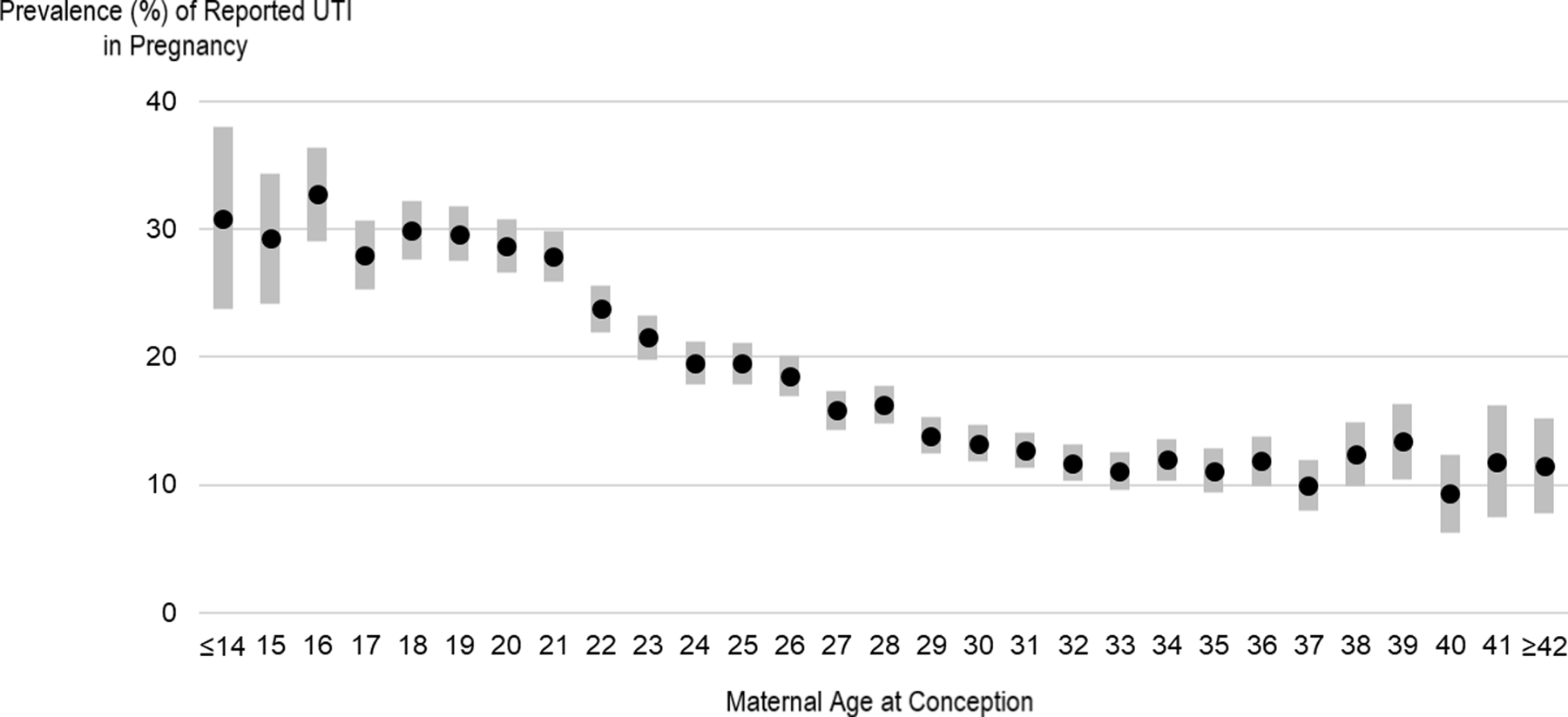
The prevalence of self-reported urinary tract infection (UTI) in pregnancy was highest among teenage mothers and decreased with maternal age at conception.
Women with UTI in the three months before pregnancy and women with pregestational diabetes reported high prevalence of UTI in pregnancy (28% and 25%, respectively). After adjusting for confounders, the associations weakened (PR 1.31, 95% CI: 1.19, 1.44 for prior UTI and PR 1.39, 95% CI: 1.24, 1.57 for pregestational diabetes) (Table 3). Although in crude analyses we found little difference in the prevalence of UTI by number of previous pregnancies, after adjusting for potential confounders, having more pregnancies was associated with higher UTI prevalence. Prepregnancy BMI was only weakly associated with UTI in pregnancy after adjustment for potential confounders.
Table 3.
Associations between medical and reproductive factors and urinary tract infection in pregnancy—cross-sectional analysis of the National Birth Defects Prevention Study, 1997–2011.
| Total number | Reported UTI prevalence (%) | Crude prevalence ratio (95% CI) | Adjusted prevalence ratioa (95% CI) | |
|---|---|---|---|---|
|
| ||||
| UTI before pregnancyb | ||||
| Yes | 1,112 | 28 | 1.54 (1.40, 1.70) | 1.31 (1.19, 1.44) |
| No | 40,719 | 18 | 1.00 (Reference) | 1.00 (Reference) |
| Missing | 38 | |||
| Diabetes | ||||
| Pregestational | 786 | 26 | 1.43 (1.26, 1.61) | 1.39 (1.24, 1.57) |
| Gestational | 2,118 | 21 | 1.14 (1.05, 1.25) | 1.18 (1.09, 1.29) |
| None | 38,908 | 18 | 1.00 (Reference) | 1.00 (Reference) |
| Missing | 57 | |||
| Previous pregnancies | ||||
| 0 | 12,694 | 20 | 1.00 (Reference) | 1.00 (Reference) |
| 1 | 11,651 | 17 | 0.87 (0.82, 0.92) | 0.98 (0.93, 1.03) |
| 2 | 8,067 | 17 | 0.88 (0.83, 0.93) | 1.05 (0.99, 1.12) |
| 3 | 4,657 | 19 | 0.94 (0.87, 1.00) | 1.17 (1.09, 1.26) |
| 4 | 2,422 | 19 | 0.97 (0.89, 1.06) | 1.26 (1.15, 1.39) |
| 5 | 1,178 | 18 | 0.92 (0.81, 1.04) | 1.26 (1.10, 1.43) |
| 6 | 521 | 21 | 1.06 (0.89, 1.25) | 1.41 (1.17, 1.66) |
| ≥7 | 616 | 19 | 0.93 (0.78, 1.09) | 1.35 (1.13, 1.60) |
| Missing | 63 | |||
| Plurality | ||||
| Singleton | 39,623 | 19 | 1.00 (Reference) | 1.00 (Reference) |
| Twins or more | 2,192 | 17 | 0.90 (0.82, 0.99) | 1.06 (0.96, 1.16) |
| Missing | 54 | |||
| Prepregnancy body mass index category (kg/m2) | ||||
| Underweight (<18.5) | 2,167 | 23 | 1.38 (1.27, 1.50) | 1.13 (1.04, 1.23) |
| Normal weight (18.5–24.9) | 20,733 | 17 | 1.00 (Reference) | 1.00 (Reference) |
| Overweight (25.0–29.9) | 9,183 | 18 | 1.08 (1.02, 1.13) | 1.06 (1.01, 1.12) |
| Obesity (≥30.0) | 8,000 | 21 | 1.24 (1.18, 1.30) | 1.17 (1.11, 1.24) |
| Missing | 1,786 | |||
CI, confidence interval; UTI, urinary tract infection.
Adjusted for birth defect status, study site, age at conception, race/ethnicity, and educational attainment.
UTI in the three months before pregnancy.
Women who did not take multivitamins, who smoked, or who used marijuana, cocaine, or methamphetamines appeared to have high prevalence of UTI in pregnancy in crude analyses, but these differences weakened or disappeared after adjusting for potential confounders (Table 4). We saw an apparent dose-response association with caffeine intake that persisted after adjustment for potential confounders.
Table 4.
Associations between multivitamin use, caffeine intake, alcohol use, smoking, and drug use and urinary tract infection in pregnancy—cross-sectional analysis of the National Birth Defects Prevention Study, 1997–2011.
| Total number | Reported UTI prevalence (%) | Crude prevalence ratio (95% CI) | Adjusted prevalence ratioa (95% CI) | |
|---|---|---|---|---|
|
| ||||
| Multivitamin use | ||||
| Yes | 38,612 | 18 | 1.00 (Reference) | 1.00 (Reference) |
| No | 2,973 | 21 | 1.16 (1.07, 1.24) | 0.99 (0.92, 1.07) |
| Missing | 284 | |||
| Caffeine intake (mg/day)b | ||||
| <10 | 6,934 | 16 | 1.00 (Reference) | 1.00 (Reference) |
| 10–99 | 14,531 | 18 | 1.15 (1.08, 1.23) | 1.06 (0.99, 1.13) |
| 100–199 | 9,856 | 18 | 1.15 (1.08, 1.24) | 1.10 (1.02, 1.17) |
| 200–299 | 5,180 | 19 | 1.19 (1.10, 1.29) | 1.16 (1.07, 1.26) |
| ≥300 | 4,477 | 22 | 1.38 (1.28, 1.50) | 1.26 (1.16, 1.36) |
| Missing | 891 | |||
| Alcohol use | ||||
| Yes | 9,836 | 18 | 0.95 (0.91, 1.00) | 1.02 (0.97, 1.07) |
| No | 31,055 | 19 | 1.00 (Reference) | 1.00 (Reference) |
| Missing | 978 | |||
| Smoking | ||||
| Yes | 7,113 | 24 | 1.42 (1.36, 1.49) | 1.24 (1.18, 1.30) |
| No | 33,954 | 17 | 1.00 (Reference) | 1.00 (Reference) |
| Missing | 802 | |||
| Marijuana use | ||||
| Yes | 2,169 | 26 | 1.44 (1.34, 1.55) | 1.05 (0.97, 1.13) |
| No | 38,875 | 18 | 1.00 (Reference) | 1.00 (Reference) |
| Missing | 825 | |||
| Cocaine use | ||||
| Yes | 319 | 30 | 1.64 (1.37, 1.93) | 1.16 (0.97, 1.36) |
| No | 40,732 | 18 | 1.00 (Reference) | 1.00 (Reference) |
| Missing | 818 | |||
| Methamphetamine usec | ||||
| Yes | 250 | 29 | 1.57 (1.27, 1.88) | 1.05 (0.85, 1.27) |
| No | 41,131 | 18 | 1.00 (Reference) | 1.00 (Reference) |
| Missing | 488 | |||
CI, confidence interval; UTI, urinary tract infection.
Adjusted for birth defect status, study site, age at conception, race/ethnicity, educational attainment, and smoking during pregnancy.
Usual caffeine intake in the year before pregnancy.
This variable was collected from free text. The number of missing values is the number of women who did not answer the question leading to the free text response.
Sensitivity Analyses
Our probabilistic bias analyses for self-reported UTI misclassification suggested that many of the associations we observed between maternal characteristics and UTI may be biased towards the null because of inaccurate self-report, meaning that the true association may be stronger than what we observed (Supplementary Materials 2). Given our assumptions in the bias analyses, adjusting for all three types of outcome misclassification (nondifferential and two types of differential misclassification) usually produced stronger median crude PRs than the observed crude PR.
When we stratified our main analysis results by race/ethnicity, results were similar, with a few exceptions (Supplementary Materials 2). The strong associations we found with UTI prevalence in the overall population were attenuated for non-Hispanic Black women with respect to household income, and for Hispanic women with respect to study site, age, and number of previous pregnancies. Despite these differences, most confidence intervals overlapped, and we cannot exclude random error as an explanation for these differences.
Results were similar when we excluded mothers with diabetes (Supplementary Materials 2), when we analyzed mothers with and without offspring with birth defects separately, and when we reassigned mothers with missing outcome status to have UTI or no UTI in pregnancy (results not shown).
COMMENT
In this large study of women from 10 U.S. sites, 18% reported at least one UTI in pregnancy. UTIs were not distributed uniformly in the study population. UTI in pregnancy was most common among younger mothers and women with characteristics associated with low SES. We also found geographic differences in UTI prevalence that had no clear longitudinal or latitudinal pattern. These sociodemographic and geographic associations were as strong or stronger than associations with known medical risk factors such as diabetes. Excluding participants with diabetes from the analysis did not change our results, suggesting the existence of additional strong risk factors aside from diabetes that could indicate that a woman is at high risk for UTI in pregnancy.
The UTI prevalence in our study was higher than in other U.S. reports. In a study of privately-insured women, 7% had a UTI diagnosed as an outpatient during pregnancy.17 The low prevalence in that study could be attributable to privately-insured women having higher SES—and therefore lower UTI prevalence—and the study’s exclusion of women with pyelonephritis and recurrent UTI.17, 20 Other claims-based studies found 9% UTI prevalence in the Pacific Northwest and 12% prevalence in Georgia—similar to the 15% prevalence reported by Georgia NBDPS participants.32, 33 If UTI prevalence varies by region and sociodemographic characteristics, we might not expect to see the same prevalence between studies conducted in different locations. In fact, some studies have found that UTI prevalence in pregnancy differs between healthcare facilities in the same region.20, 27 Systematic differences in healthcare access, urine collection procedures, or specimen handling may also contribute to this variability.
We and others have found that teenagers have high prevalence of UTI in pregnancy.6, 7, 21, 32 For example, Finnish teenagers were more likely than women aged 25–29 years to have UTI in pregnancy (adjusted odds ratio 2.9, 95% CI: 1.8, 4.8).21 However, some studies have found no association between age and UTI in pregnancy.27, 28, 34 We observed that the relationship between age and UTI prevalence was weaker in Hispanic women compared to non-Hispanic White and Black women, suggesting that the age-UTI association might not be constant across populations.
Several epidemiologic studies in the U.S. have found that Asian or Pacific Islander women have a relatively low prevalence of UTI in pregnancy compared to women of other races and ethnicities.6, 7, 32, 33 We found the same. However, we did not have sufficient sample size to disaggregate Asian subgroups, which might have hidden differences between these groups. Several studies have found a higher prevalence of UTI in pregnancy among women with higher parity.19, 20, 27 We saw a similar pattern, although not among Hispanic women.
We included caffeine intake as a potential risk factor because of its associations with urinary frequency and urgency, often experienced with symptomatic UTIs. However, we had no information on UTI symptoms in our study, and we could not tell if the association we observed with caffeine intake was driven by women with high caffeine intake being more likely to report symptomatic UTI. Our measurement of caffeine intake was likely subject to measurement error because we used self-reported intake and intake before pregnancy; many women reduce their caffeine intake in pregnancy.35, 36
Characteristics associated with SES, such as educational attainment and household income, were strongly associated with UTI in pregnancy in our population. Associations with these and other markers of SES, like health insurance type or coverage, were seen in previous studies in the United States.20, 28, 32 Low SES is also associated with higher UTI prevalence in pregnancy in studies from other countries such as Bangladesh, Egypt, Ethiopia, and Pakistan.37–41 Although we identified characteristics associated with low SES as being associated with UTI in pregnancy, we could not determine which aspects of low SES were driving the association. The association could be mediated by variables linked to development of UTI that were unavailable in our dataset, such as frequent sexual intercourse, infrequent voiding, and low fluid intake.39, 42–46 Higher frequency of intercourse is weakly or moderately associated with sociodemographic indicators such as younger age, lower educational attainment, and race/ethnicity.47 Women’s fluid intake and ability to void regularly during the day can be affected by their workplace conditions, which are in turn associated with SES.48 Other variables potentially associated with low SES, such as diet or healthcare access, might also mediate the association.
The NBDPS questionnaire collected a broad range of information, including sociodemographic characteristics, maternal exposures, and reproductive history. This data availability allowed us to control for potential confounders and to examine UTI prevalence in relation to characteristics not always available in administrative databases or medical records. Our sample size of over 41,000 participants gave precise estimates for our effect estimates. An important advantage of this large sample size was our ability to estimate prevalence in small population subgroups such as less prevalent racial and ethnic groups, single-maternal year categories, and women with infrequent exposures such as methamphetamine use.
Another strength of this study was the bias analysis we conducted because of concerns about low validity for self-reported UTI compared to medical records.31 The direction and magnitude of bias is often difficult to predict without conducting quantitative bias analysis. Our bias analysis showed that outcome misclassification is an unlikely explanation for our results, and that the unbiased associations may be even stronger than what we observed. Women participated in NBDPS six week to two years after their estimated date of delivery. Although it is possible that women interviewed later might have recalled pregnancy events less accurately, in our study population, UTI prevalence was similar regardless of time to interview, suggesting that women interviewed longer after delivery recalled UTIs just as frequently (results not shown).
In the NBDPS questionnaire, women likely reported a combination of asymptomatic bacteriuria, cystitis, and pyelonephritis when reporting UTIs, but we could not distinguish between the three. Cystitis and pyelonephritis cause symptoms that lead women to seek medical care. Asymptomatic bacteriuria, the most prevalent type of UTI in pregnancy, is typically detected through screening.11, 15 Its detection is therefore dependent on women’s access to and attendance at prenatal care. We might expect that some of the differences in UTI prevalence by sociodemographic factors are attributable to differences in screening uptake. However, if that were true, women least likely to access consistent prenatal care (young women with low SES) would have the lowest screening rates and therefore the lowest prevalence. We found the opposite—these women had the highest UTI prevalence—suggesting that differences in screening access do not explain our results.
Misclassification of variables such as smoking or use of marijuana, cocaine, or methamphetamines during pregnancy was possible, especially if women were reluctant to disclose their use. Most of our SES variables—like age, race/ethnicity, and educational attainment—were likely accurately reported.
CONCLUSIONS
In this large study of pregnant women from 10 U.S. sites, one in six reported a UTI in pregnancy. Younger women and women with characteristics of low SES were at highest risk. Identifying why UTI in pregnancy is associated with low SES and other maternal factors could lead to a better understand of the etiology of UTI pregnancy and the eventual development of interventions to prevent its occurrence.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Mir Ali for replicating our analyses. Coding of drug information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under license from the Slone Epidemiology Center of Boston University.
FUNDING
This project was supported through Centers for Disease Control and Prevention (CDC) cooperative agreements under PA #96043, PA #02081, FOA #DD09-001, FOA #DD13-003, and NOFO #DD18-001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study (NBDPS) and/or the Birth Defects Study To Evaluate Pregnancy exposureS (BD-STEPS).
Footnotes
AUTHOR DISCLOSURES
All authors declare no conflicts of interest.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
PREVIOUS PRESENTATION
Results from this manuscript were presented in part at the annual meetings of the Society for Epidemiologic Research and Society for Pediatric and Perinatal Epidemiologic Research, June 17–18 and June 18–21, 2019, Minneapolis, MN.
DATA AVAILABILITY
Instructions for accessing the National Birth Defects Prevention Study data can be found at https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html.
REFERENCES
- 1.Schnarr J, Smaill F: Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur J Clin Invest. 2008;38:50–57. [DOI] [PubMed] [Google Scholar]
- 2.Le J, Briggs GG, McKeown A, Bustillo G: Urinary tract infections during pregnancy. Ann Pharmacother. 2004;38:1692–1701. [DOI] [PubMed] [Google Scholar]
- 3.Sheffield JS, Cunningham FG: Urinary tract infection in women. Obstet Gynecol. 2005;106:1085–1092. [DOI] [PubMed] [Google Scholar]
- 4.Angelescu K, Nussbaumer-Streit B, Sieben W, Scheibler F, Gartlehner G: Benefits and harms of screening for and treatment of asymptomatic bacteriuria in pregnancy: a systematic review. BMC Pregnancy Childbirth. 2016;16:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill JB, Sheffield JS, McIntire DD, Wendel GDJ: Acute pyelonephritis in pregnancy. Obstet Gynecol. 2005;105:18–23. [DOI] [PubMed] [Google Scholar]
- 6.Jolley JA, Kim S, Wing DA: Acute pyelonephritis and associated complications during pregnancy in 2006 in U.S. hospitals. J Matern Fetal Neonatal Med. 2012;25:2494–2498. [DOI] [PubMed] [Google Scholar]
- 7.Wing DA, Fassett MJ, Getahun D: Acute pyelonephritis in pregnancy: an 18-year retrospective analysis. Am J Obstet Gynecol. 2014;10:219.e211–219.e216. [DOI] [PubMed] [Google Scholar]
- 8.Feldkamp ML, Arnold KE, Krikov S, et al. : Risk of gastroschisis with maternal genitourinary infections: the US National Birth Defects Prevention Study 1997–2011. BMJ Open. 2019;9:e026297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Easter SR, Cantonwine DE, Zera CA, Lim KH, Parry SI, McElrath TF: Urinary tract infection during pregnancy, angiogenic factor profiles, and risk of preeclampsia. Am J Obstet Gynecol. 2016;214:387.e381–387.e387. [DOI] [PubMed] [Google Scholar]
- 10.Howley MM, Feldkamp ML, Papadopoulos EA, et al. : Maternal genitourinary infections and risk of birth defects in the National Birth Defects Prevention Study. Birth Defects Res. 2018;110:1443–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolle LE, Gupta K, Bradley SF, et al. : Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68:1611–1615. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Preventive Services Task Force: Screening for asymptomatic bacteriuria in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2008;149:43–47. [DOI] [PubMed] [Google Scholar]
- 13.Moore A, Doull M, Grad R, et al. : Recommendations on screening for asymptomatic bacteriuria in pregnancy. CMAJ. 2018;190:E823–E830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widmer M, Lopez I, Gülmezoglu AM, Mignini L, Roganti A: Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database Syst Rev. 2015;11:CD000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Academy of Family Physicians: Summary of recommendations for clinical preventive services. 2017; https://www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/cps-recommendations.pdf.
- 16.Ailes EC, Gilboa SM, Gill SK, et al. : Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011. Birth Defects Res A Clin Mol Teratol. 2016;106:940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ailes EC, Summers AD, Tran EL, et al. : Antibiotics dispensed to privately insured pregnant women with urinary tract infectsion -- United States, 2014. MMWR Morb Mortal Wkly Rep. 2018;67:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matuszkiewicz-Rowińska J, Małyszko J, Wieliczko M: Urinary tract infections in pregnancy: old and new unresolved diagnostic and therapeutic problems. Arch Med Sci. 2015;11:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaitz AL, Hodder EW: Bacteriuria and pyelonephritis of pregnancy: a prospective study of 616 pregnant women. N Engl J Med. 1961;265:667–672. [DOI] [PubMed] [Google Scholar]
- 20.Turck M, Goffe BS, Petersdorf RG: Bacteriuria of pregnancy: relation to socioeconomic factors. N Engl J Med. 1962;266:857–860. [DOI] [PubMed] [Google Scholar]
- 21.Leppälahti S, Gissler M, Mentula M, Heikinheimo O: Is teenage pregnancy an obstetric risk in a welfare society? A population-based study in Finland, from 2006 to 2011. BMJ Open. 2013;3:e003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Obstetricians and Gynecologists: ACOG educational bulletin: antimicrobial therapy for obstetric patients, number 245, March 1998. Int J Gyanecol Obstet. 1998;61:299–308. [PubMed] [Google Scholar]
- 23.Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ: Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163:978–985. [DOI] [PubMed] [Google Scholar]
- 24.Committee on Obstetric Practice: Committee opinion no. 717: sufonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130:e150–e152. [DOI] [PubMed] [Google Scholar]
- 25.Reefhuis J, Gilboa SM, Anderka M, et al. : The National Birth Defects Prevention Study: a review of the methods. Birth Defects Res A Clin Mol Teratol. 2015;103:656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen SA, Olney RS, Holmes LB, et al. : Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67:193–201. [DOI] [PubMed] [Google Scholar]
- 27.Pastore LM, Savitz DA, Thorp JMJ: Predictors of urinary tract infection at the first prenatal visit. Epidemiology. 1999;10:282–287. [PubMed] [Google Scholar]
- 28.Pastore LM, Savitz DA, Thorp JMJ, Koch GG, Hertz-Picciotto I, Irwin DE: Predictors of symptomatic urinary tract infection after 20 weeks’ gestation. J Perinatol. 1999;19:488–493. [DOI] [PubMed] [Google Scholar]
- 29.Schneeberger C, Erwich JJHM, van den Heuvel ER, Mol BWJ, Ott A, Geerlings SE: Asymptomatic bacteriuria and urinary tract infection in pregnant women with and without diabetes: cohort study. Eur J Obstet Gynecol Reprod Biol. 2018;222:176–181. [DOI] [PubMed] [Google Scholar]
- 30.Bradley CS, Erickson BA, Messersmith EE, et al. : Evidence of the impact of diet, fluid intake, caffeine, alcohol, and tobacco on lower urinary tract symptoms: a systematic review. J Urol. 2017;198:1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietz P, Bombard J, Mulready-Ward C, et al. : Validation of self-reported maternal and infant health indicators in the Pregnancy Risk Assessment Monitoring System. Matern Child Health J. 2014;18:2489–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruce FC, Berg CJ, Hornbrook MC, et al. : Maternal morbidity rates in a managed care population. Obstet Gynecol. 2008;111:1089–1095. [DOI] [PubMed] [Google Scholar]
- 33.Bruce FC, Berg CJ, Joski PJ, et al. : Extent of maternal morbidity in a managed care population in Georgia. Paediatr Perinat Epidemiol. 2012;26:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conde-Agudelo A, Belizán JM, Lammers C: Maternal-perinatal morbidity and mortality associated with adolescent pregnancy in Latin America: cross-sectional study. Am J Obstet Gynecol. 2005;192:342–349. [DOI] [PubMed] [Google Scholar]
- 35.Lawson CC, LeMasters GK, Wilson KA: Changes in caffeine consumption as a signal of pregnancy. Reprod Toxicol. 2004;18:625–633. [DOI] [PubMed] [Google Scholar]
- 36.Bracken MB, Triche E, Grosso L, Hellenbrand K, Belanger K, Leaderer BP: Heterogeneity in assessing self-reports of caffeine exposure: implications for studies of health effects. Epidemiology. 2002;13:165–171. [DOI] [PubMed] [Google Scholar]
- 37.Lee ACC, Mullany LC, Koffi AK, et al. : Urinary tract infection in pregnancy in a rural population of Bangladesh: population-based prevalence, risk factors, etiology, and antibiotic resistance. BMC Pregnancy Childbirth. 2019;20:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Aziz Elzayat M, Barnett-Vanes A, Dabour MF, Cheng F: Prevalence of undiagnosed asymptomatic bacteriuria and associated risk factors during pregnancy: a cross-sectional study at two tertiary centres in Cairo, Egypt. BMJ Open. 2017;7:e013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emiru T, Beyene G, Tsegaye W, Melaku S: Associated risk factors of urinary tract infection among pregnant women at Felege Hiwot Referral Hospital, Bahir Dar, North West Ethiopia. BMC Res Notes. 2013;6:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tadesse S, Kahsay T, Adhanom G, et al. : Prevalence, antimicrobial susceptibility profile and predictors of asymptomatic bacteriuria among pregnant women in Adigrat General Hospital, Northern Ethiopia. BMC Res Notes. 2018;11:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haider G, Zehra N, Munir AA, Haider A: Risk factors of urinary tract infection in pregnancy. J Pak Med Assoc. 2010;60:213–216. [PubMed] [Google Scholar]
- 42.Su SB, Wang JN, Lu CW, Wang HY, Guo HR: Prevalence of urinary tract infections and associated factors among pregnant workers in the electronics industry. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:939–945. [DOI] [PubMed] [Google Scholar]
- 43.Hooton TM, Vecchio M, Iroz A, et al. : Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178:1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amiri FN, Rooshan MH, Ahmady MH, Soliamani MJ: Hygiene practices and sexual activity associated with urinary tract infection in pregnant women. East Mediterr Health J. 2009;15:104–110. [PubMed] [Google Scholar]
- 45.Thakre SS, Dhakne SN, Thakre SB, Ughade SN: Hygiene practices and sexual activity associated with urinary tract infection in rural pregnant women of Nagpur, India. Ind J Med Microbiol. 2015;33:177–178. [DOI] [PubMed] [Google Scholar]
- 46.Badran YA, El-Kashef TA, Abdelaziz AS, Ali MM: Impact of genital hygiene and sexual activity on urinary tract infection during pregnancy. Urol Ann. 2015;7:478–481. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Eisenberg ML, Shindel AW, Smith JF, Breyer BN, Lipshultz LI: Socioeconomic, anthropomorphic, and demographic predictors of adult sexual activity in the United States: data from the national survey of family growth. The journal of sexual medicine. 2010;7:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer MH, Athanasopoulos A, Lee KS, Takeda M, Wyndaele JJ: Sociocultural and environmental influences on bladder health. Int J Clin Pract. 2012;66:1132–1138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Instructions for accessing the National Birth Defects Prevention Study data can be found at https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html.


