Abstract
West Nile Virus (WNV) infections are increasingly detected in birds and horses in central Europe, with a first mosquito-borne autochthonous human infection detected in Germany in 2019. Human infections are typically asymptomatic, with occasional severe neurological disease. Because of a low number of cases in central Europe, awareness regarding potential cases is low and WNV diagnostic is neglected.
We tested cerebrospinal fluid (CSF) samples from unsolved encephalitis and meningitis cases from Berlin from 2019 and 2020, and describe a WNV-encephalitis case in a 33-year old kidney transplant recipient. The infectious course was resolved by serology, RT-PCR, and sequencing of stored samples. Phylogenetic sequence analysis revealed a close relationship of the patient’s WNV strain to German sequences from 2019 and 2020. A lack of travel history and patient self-isolation during the SARS-CoV-2 pandemic suggest the infection was acquired in the patient’s home or garden. Serological tests of four people sharing the living space were negative. Retrospective RT-PCR and WNV-IgM testing of 671 CSF samples from unsolved encephalitis and meningitis cases from Berlin detected no additional infections.
The recent increase of WNV cases illustrates the importance of considering WNV in cases of meningoencephalitis, especially in immunocompromised patients, as described here. Proper education and communication and a revised diagnostic strategy will help to raise awareness and to detect future WNV infections.
Keywords: West Nile virus, encephalitis, Germany, emerging disease, Arboviruses, Diagnostics
Introduction
West Nile virus (WNV) is a mosquito-borne RNA arbovirus of the genus Flavivirus, affecting a wide range of vertebrates on all continents except Antarctica. In recent decades, WNV has further spread, is considered among the most important causative agents of viral encephalitis, and became the leading cause of mosquito-borne disease in the United States (Chancey 2015, Davis 2006). WNV infections remain asymptomatic in ~80 % of cases, with ~20 % resulting in fever and mild symptoms such as headache, joint pains, body aches, or rash. Severe illness, manifesting as neuroinvasive disease, is rare (<1 %) (Hart 2014, Sejvar 2014).
The frequency and geographic distribution of WNV outbreaks has increased in Europe since the 1990s (Tsai 1998), including an outbreak with 2083 reported human cases across southern and central Europe in 2018 (ECDC). Since then, increasing numbers of infections have been described in birds and horses in central Europe, including Germany. However, the detection of WNV is heterogeneous throughout Europe. While some countries, especially in southern Europe, have detected WNV in birds, mosquitoes, and humans for many years, WNV has not yet been detected in other countries. Although the European Centre for Disease Prevention and Control published guidelines to support the implementation of surveillance for mosquito species of public health relevance, provided up to date risk assessments and weekly reports during the WNV transmission season (June-November), no proactive surveillance program is applied across Europe ( https://www.ecdc.europa.eu/en and Gossner 2017). While in Germany, in 2018 WNV was detected in 12 birds and two horses, it was already detected in 76 birds, 36 horses, and several mosquitoes, including seven mosquito pools captured in a wildlife park in Berlin a year later. In 2018, a veterinary pathologist was infected while dissecting birds from another wildlife park in southern Germany, and the first probable German mosquito-borne autochthonous human WNV infection occurred (Kampen 2020, Pietsch 2020, Ziegler 2020). WNV surveillance activities for birds, horses, and mosquitoes started throughout many regions of Germany in recent years. As a result, the detection of WNV in mosquitoes and birds was also reported for areas near the residence of the patient in 2018–2020 (Kampen 2020, Ziegler 2019, Ziegler 2020).
Diagnosis of human WNV infection is notifiable according to the Protection against Infection Act in Germany and infection control is the responsibility of local and state health departments. WNV infections are likely underdiagnosed in Germany because, among other reasons, there is no routine testing for WNV in encephalitis cases. Seroprevalence studies are lacking to assess the extent of human WNV infection and undercoverage in the German surveillance system. The Robert Koch-Institute (RKI), as the German federal institute responsible for disease control and prevention, recommends WNV testing for (I) patients returning from WNV endemic regions with encephalitis of unclear origin and (II) clustered cases of fever of unknown origin. The responsibility for requesting WNV testing, nevertheless devolves solely to the treating physicians. Routine testing normally includes only common pathogens, such as herpes simplex virus or, in severe cases, multiplex PCR testing of up to 14 common pathogens, but not WNV. Given the recent increase in cases and the detection in mosquito populations, clinicians need to be aware of potential presence of WNV in cases where travel history is absent, and a revised diagnostic strategy for encephalitis cases needs to be implemented to routinely include WNV testing. In Berlin, the identification of the first autochthonous WNV case has led the State Office for Health and Social Affairs (SOHSA) to enhance surveillance. This includes standardized case interviews to determine whether infections are autochthonous and where the infection was acquired, and environmental investigations to identify and assess possible places of infection. Additionally, the collection of mosquitoes at suspected places of infection has been conducted since 2021 in Berlin.WNV is commonly transmitted by mosquitoes, with species from the genus Culex playing the most important role, but infections also occur due to medical treatment, such as blood transfusion (Anesi 2019). Furthermore, enhanced susceptibility to WNV infection has been specifically linked to solid organ transplantation (SOT), prompting the American Society of Transplantation Infectious Diseases Community of Practice to recently issue a guideline (Anesi, Silveira 2019). Compared to the general population, SOT recipients have a higher likelihood of developing symptomatic WNV infection and an unfavorable outcome (Granerod 2010, Kumar 2004). Furthermore, to prevent blood-donation associated WNV cases, the German federal agency for vaccines and biomedicines, the Paul Ehrlich Institute (PEI), declines stem cell, blood, and plasma donations from individuals who have visited a WNV-endemic area for at least two consecutive days in the period from June 1st to November 30th until at least four weeks after their return. Alternatively, blood donations taken during the same time period need to be screened for the presence of WNV RNA.Here, we report WNV testing of stored CSF samples from unsolved encephalitis and/or meningitis cases from Berlin in 2019 and 2020 and describe the course of a WNV encephalitis in an immunosuppressed kidney transplant recipient in 2020.
Subjects and Methods
Retrospective testing of CSF samples from routine diagnostic, years 2019 and 2020
To investigate undetected neurotropic WNV cases, we retrospectively screened 671 CSF samples from 638 patients with suspected meningitis and/or encephalitis obtained during June-October in 2019 and 2020 with a specific WNV RT-PCR and a WNV IgM enzyme-linked immunosorbent assay (ELISA) (Anti-West-Nile-Virus-ELISA (IgM), Euroimmun). For RNA purification, up to 200 µl of CSF were used in the Roche MagNAPure 96 system and eluted in 100 µl elution buffer. RNA extracts of up to nine samples were pooled and 5 µl pooled RNA were used for RT-PCR (Eiden 2010) in a 25 µl reaction volume using the SuperScript® III One-Step RT-PCR System with Platinum® Taq DNA (Invitrogen, Karlsruhe, Germany). CSF samples were obtained from patients living in the urban area of Berlin and sent to our diagnostic department for broad pathogen diagnostics, but showed no detection in the applied multiplex PCR panel. This panel (BioFire ® FilmArray ® Multiplex PCR-Systeme Meningitis/Encephalitis (ME) Panel) detects 14 common bacterial and viral pathogens (Escherichia coli K1, H. influenzae, Listeria monocytogenes, Neisseria meningitis, Streptococcus agalactiae, Streptococcus pneumoniae, Cytomegalovirus, Enterovirus, Herpes simplex virus 1&2, Human herpesvirus 6, Human parechovirus, Varicella zoster virus, and Cryptococcus sp.).
Case, contacts, public health management, and routine diagnostic testing
On 20th September 2020, a 33 year-old man was admitted to the Department of Nephrology, Charité - Universitätsmedizin Berlin, with fever (38.8 °C) and dysuria.
The patient had undergone laparoscopic bilateral native nephrectomy ten days earlier with combined general and epidural anesthesia due to recurrent urinary tract infections (UTI). He had received a deceased-donor transplant in 2012 for kidney failure due to nephrocalcinosis. Maintenance immunosuppression therapy consisted of tacrolimus (target trough level of 4–6 ng/mL), mycophenolate mofetil (MMF) 2 g/day, and prednisone 5 mg/day. There was no travel history during the weeks before symptom onset. Physical examination was unremarkable. Blood testing showed a slightly increased C-reactive protein 13.4 mg/L (normally <5.0 mg/L) with a normal white blood cell count of 10019/µl. Urine analysis and culture were unremarkable. Chest X-ray showed no cardiopulmonary abnormalities.
Initially, a recurrent UTI was suspected, for which antibiotic therapy with piperacillin/tazobactam was administered. However, fever persisted and the patient subsequently developed phono-, and photophobia, headache with neck stiffness, mild confusion and extreme lethargy, raising the possibility of meningoencephalitis. Computer tomography excluded elevated intracranial pressure. Cerebrospinal fluid (CSF) showed a predominantly lymphocytic pleocytosis with 68 white cells/µL (normally <5/µL) and with an increased protein concentration of 1.3 g/L (normally <0.45 g/L). CSF glucose and lactate levels were in normal ranges. CSF cytology revealed lymphocytes, few activated lymphocytes, monocytes and neutrophils. Contrast-enhanced brain and spinal magnetic resonance imaging revealed no significant abnormalities, including no signs of meningitis, encephalitis, myelitis, or spondylodiscitis. Due to unequivocal clinical symptoms consistent with meningoencephalitis in a severely immunocompromised patient, the anti-infective regimen was changed to ampicillin, ceftriaxone, and acyclovir. Immunosuppressive therapy was adjusted with MMF withdrawal. Microorganisms typically causing community-acquired meningitis were examined using a commercial multiplex PCR (BioFire ® FilmArray ® Multiplex PCR-Systeme Meningitis/Encephalitis (ME) Panel) as described above. The results were negative for all 14 pathogens. In addition, routine cultures of CSF and blood for bacterial and fungal pathogens remained negative.
Subsequently, other neurotrophic pathogens such as Epstein-Barr virus, SARS-CoV-2, JC and BK polyomaviruses, tick-borne encephalitis virus, Toxoplasma, Treponema, and Borrelia sp. were tested and excluded as causative pathogens. Furthermore, WNV RNA in CSF and serum was tested using commercially available kits (RealStar® WNV RT-PCR Kit, Altona Diagnostics) and an in-house assay (Eiden, Vina-Rodriguez 2010). For testing of IgG antibodies we applied an immunofluorescence test (IFT) following the manufacturer’s instructions (Anti-West-Nile-Viren-IFT; Euroimmun). IgM testing was done by applying the same IFT format as well as two commercial ELISA systems (IgM Capture DxSelect™, Focus Diagnostics; Anti-West-Nile-Virus-ELISA (IgM), Euroimmun). After WNV diagnosis and notification, the patient was routinely interviewed by the local health department. Subsequently, the State Office for Health and Social Affairs explored possible places of infection by a) administering a specifically designed questionnaire and b) inspecting the immediate surroundings of his living place. Furthermore, six weeks after initial diagnosis of the patient, serum samples were taken from his roommate and three close neighbours living in the same house and tested in two WNV IgM ELISAs.
Ethics
The patient and the close contact persons gave informed consent for their participation in this study. The ethical committee at Charité-Universitätsmedizin Berlin was notified of the retrospective testing of CSF samples and acknowledged receipt under file number EA4/169/21. The use of anonymised clinical data is further covered by section 25 of the Berlin Hospital Law and did not require ethical or legal clearance.
Sequencing and phylogenetic analysis of WNV
We used high-throughput sequencing (HTS) for genome sequencing of WNV. RNA extraction was performed using the ViralRNA kit (Qiagen) and MagNA Pure DNA and viral NA small volume kit (Roche), following manufacturer’s instructions. Due to the low WNV RNA concentration in positive samples (ct-values of 38.47 and 39.7), an amplicon-based HTS approach was adopted. Based on an alignment of 78 WNV lineage II sequences (the most common lineage in animals in Germany in the last years), we designed hemi-nested primer sets (available upon request). Amplification of WNV RNA was performed in two rounds. First, using the Superscript III one-step RT-PCR system (Invitrogen), second using the Platinum Taq Polymerase Kit (Invitrogen).
Subsequently, we pooled all PCR products and performed a 2-fold clean-up using KAPA PureBeads (Roche). A library was prepared using the PCR-Sequencing Kit (Oxford Nanopore Technologies) following the manufacturer’s instructions and sequenced on a MinION Flongle. Resulting reads were trimmed using porechop, mapped to reference sequence LR743458 using minimap2, and manually inspected using Geneious Prime. A phylogenetic tree was constructed using the PhyML plug-in in Geneious Prime, a GTR+I+G substitution model, and 5000 replicates.
Results
Testing for a broad range of bacterial, fungal, and viral pathogens remained negative for the encephalitis patient at the time of hospitalization. Subsequently, testing for WNV antibodies and RNA in serum samples led to a positive test result. Due to the medical history of the patient, stored blood samples were available and the investigation was expanded to consider disease progression. Fifty days prior to hospitalization, no WNV RNA or WNV IgG antibodies were detectable by RT-PCR or IFT. IgM antibody testing resulted in a negative ELISA, but an increased signal in the 1:10 dilution of the IFT, likely due to nonspecific binding, was observed. In contrast, IgM and IgG antibodies (IFT and ELISA) and WNV RNA were found in blood samples taken 19 days before hospitalization. Detection of viral RNA confirmed an acute replicative disease stage. Upon hospitalization, an IgG and IgM titer of 1:100 was observed, and was still detectable 160 days later (Figure 1).
Fig. 1:
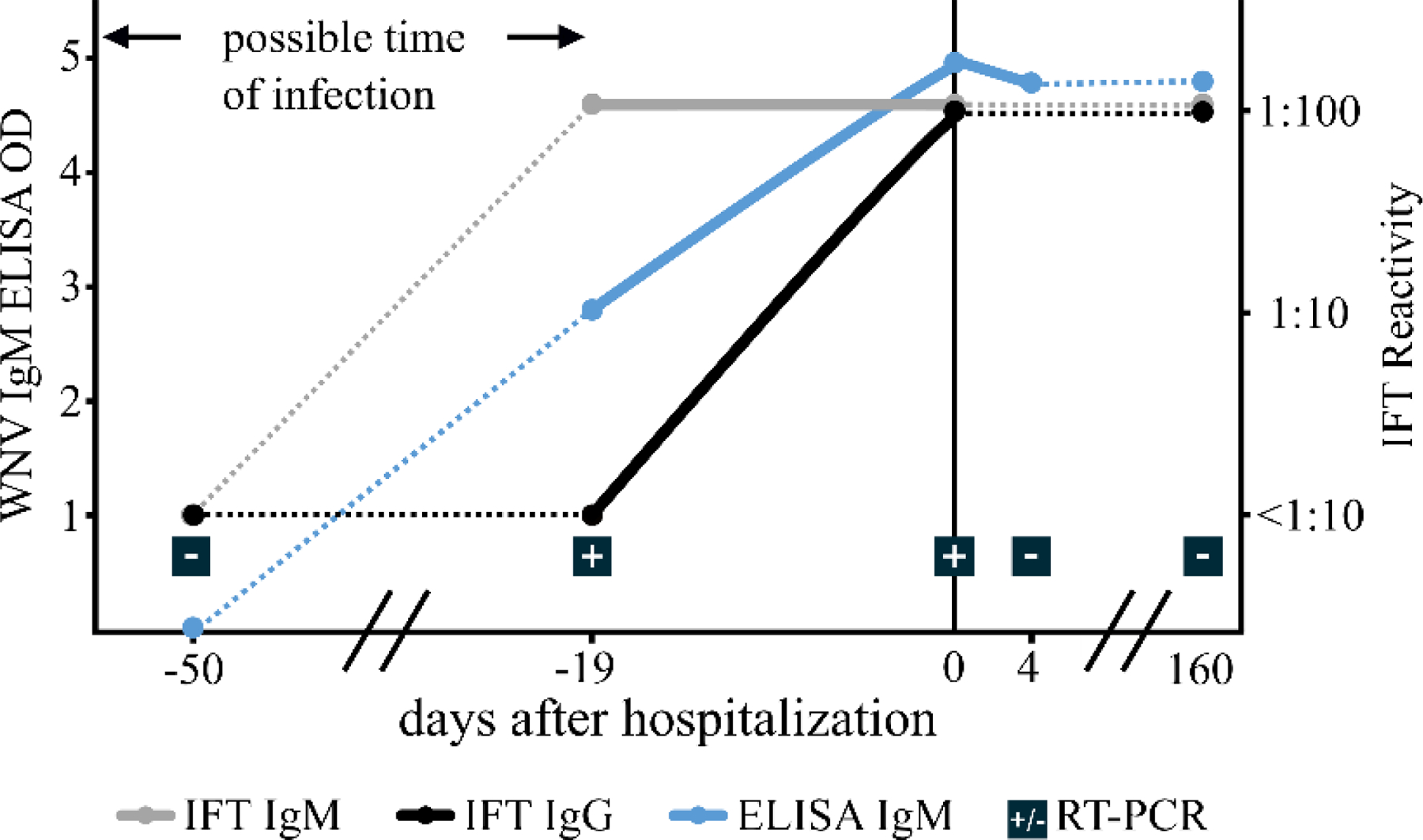
Results of WNV-specific ELISA (Focus Diagnostics), IFT (Euroimmun), and RT-PCR of serum samples. Boxed +/− signs indicate positive/negative PCR test results.
Supportive treatment resulted in gradual improvement of symptoms over seven days. WNV RT-PCR testing of blood samples turned negative four days after hospitalization. The patient was discharged in stable condition and presented to the transplant outpatient clinic for follow-up. At the last follow-up (about six months post-episode), the patient recovered, without neurological residues. A follow-up CSF examination performed at this point in time was consistent with past viral encephalitis, showing 5 cells/µl, a mildly elevated total protein (0.6 g/L), CSF-specific oligoclonal IgG bands and an intrathecal total IgM production with 83% of the total IgM in CSF being produced within the CNS.
Given the rising numbers of WNV positive birds and horses in Germany in 2018 (Michel 2019), cases of autochthonous human WNV infection in 2019 and 2020 (Ziegler, Santos 2020), and questioning the patient, we carried out an environmental investigation looking for possible sources of infection such as a local water reservoir for mosquitoes or close habitants who had asymptomatic infections. In the backyard of his flat, a rain barrel was noted near where the patient gardened and rested during the summer. Unfortunately, no mosquitoes were collected. Due to the SARS-CoV-2 pandemic during the same time period, the patient had avoided any frequent contacts, including outdoor parties. Serum samples, taken six weeks after WNV diagnosis, from the patient’s roommate and three close neighbours that used the same garden were negative for WNV-RNA by RT-PCR, and two IgM ELISAs showed no signs of past infection. No further public health measures, such as vector control or information campaigns, were undertaken.
To investigate an autochthonous infection, we compared the obtained partial WNV sequence to WNV reference sequences belonging to lineage II from Germany and other European countries. A phylogenetic analysis suggested the close relationship to WNV sequences from Germany from 2019 and 2020 (Figure 2). The partial WNV sequence was uploaded to GenBank (accession number MW072297). RT-PCR and antibody testing by ELISA (Euroimmun) of 671 CSF samples from 638 patients with suspected meningitis and/or encephalitis sent for routine diagnostics resulted in no additional WNV RNA or IgM detection.
Fig. 2:

Phylogenetic analysis of the complete coding region of WNV lineage II genomes and the partial WNV sequence of the patient (2023 concatenated nucleotides, highlighted in bold). Reference sequence AY532665 was used to root the tree. Black circles at nodes indicate bootstrap support of >90 % and white circles >75 % (5000 replicates).
Discussion
Here, we report a rare case of WNV-lineage II neuroinvasive disease in a kidney transplant recipient living in Berlin, Germany. Due to the eight-year latency since transplantation and a lack of recent blood transfusions, transmission of WNV was likely due to mosquito bite, likely in his own garden. Unfortunately, due to the fact that autumn had already begun and temperatures had dropped, catching positive mosquitoes was highly unlikely. Thus, further investigations focussed on testing serum of four individuals living in the same house, resulting in no further WNV cases.
Two years earlier, in 2018, 11 human WNV cases were reported in Germany, of which 10 were travel-associated. One additional case was a veterinarian, who likely became infected during necropsy of a WNV-infected bird in southern Germany. However, already in 2019, five of a total of twelve reported cases were likely infected locally. WNV RNA was detected in mosquito pools from the same year and their sequences were nearly identical to those obtained from birds in 2018, suggesting possible local overwintering of the virus e.g. in hibernating mosquitoes. In 2020 these numbers further rose, with 23 cases affecting five federal German states with seven autochthonous cases reported in Berlin. Three human and several WNV cases among birds and horses from different federal states have been reported for 2021 through September 25th in Germany (https://promedmail.org/promed-post/?id=20210901.8620709; https://survstat.rki.de/, and https://tsis.fli.de both accessed on 25.09.2021). According to assumptions about the clinical spectrum of WNV infections (<1 % neurological symptoms, ~20 % mild symptoms) and given at least five cases of West Nile neuroinvasive disease in Leipzig (Pietsch, Michalski 2020) and the case described here, theoretically more than 100 asymptomatic cases can be assumed for 2020. However, screening of CSF samples from our cohort of 671 samples from 638 patients with unsolved meningitis and/or encephalitis cases did not result in the detection of further severe WNV infections. In 2019 and 2020, WNV PCR testing of CSF was requested at our facility for only 19 and 22 patients respectively, against a background of approximately 1000 requests sent annually for multiplex testing of CSF samples. These low numbers of WNV diagnostic requests suggest the need for increased awareness in treating physicians of WNV.A limitation of this study is the retrospective nature that limited access to different sample types and larger volumes. We focussed on patients with suspected viral encephalitis due to the availability of CSF samples, as other sample types from these patients (e.g. urine or full blood) were not available. A further limitation may be decreased sensitivity due to RT-PCR testing of pooled CSF samples. However, we chose a highly sensitive RT-PCR assay (Eiden 2010) and also individually tested CSF samples of 41 patients (from 2019 and 2020) submitted for specific WNV PCR diagnostics, and this did not lead to the detection of any further case. Additionally, we also tested for WNV-IgM in CSF. Due to the limitations of our study we can’t fully exclude the possibility that a WNV virus case was missed, but we believe our testing strategy is a pragmatic and cost-efficient approach to test for WNV encephalitis cases retrospectively.
Our WNV case echoes findings from the small number of published cases, in which the patient rapidly deteriorated with severe neurologic symptoms requiring ICU admission and tested negative for typical infectious agents upon CSF examination. In our case, concomitant dysuria as well as a recent surgical intervention initially suggested various other possible causes of his febrile disease, and a history of an inserted epidural catheter only days before the current admission suggested a nosocomial CNS infection. It is well-recognized that a substantial number of pathogens responsible for encephalitis may go unrecognized due to a newly emerging geography of infection distribution (Kleinschmidt-DeMasters 2004). Diagnosing transplant patients is even more challenging due to their immunosuppressed status. This is where transplant specialists and an unbiased and comprehensive diagnostic testing strategy can make a difference. Following a negative screen for 14 microorganisms in a setting where lymphocytic pleocytosis and symptoms were indicative of viral meningoencephalitis, a specific RT-PCR analysis was performed, revealing WNV RNA in the patient’s plasma. Subsequent HTS, bioinformatic, and phylogenetic analysis confirmed infection with WNV lineage II. Closely related sequences have been detected in humans and birds in Germany in recent years, also in areas close to the residence of the patient, hinting at a locally established and overwintering WNV host population. A unified surveillance program for mosquitoes, birds, and humans would be of great benefit in better understanding and elucidating geographic clusters, endemic outbreaks, and sporadic occurrences of the virus.
Lacking specific therapy, the infection was treated by supportive measures and immediate reduction of immunosuppressive medication. Due to the potential for long-term sequelae in SOT recipients infected by WNV (Mostashari 2001), minimal delay in the diagnosis might be crucial to the outcome. We conclude that a non-specific additional immunosuppressive state due to the recent nephrectomy along with triple immunosuppression rendered our patient susceptible to a severe WNV infection course. While the patient recovered, he was intermittently compromised by difficulties in concentrating for several months. Of note, even with reduction of MMF, graft function remained stable.
Conclusion:
Since the first mosquito-transmitted autochthonous WNV cases in Germany in 2019, an increasing number of cases have been observed. Even though cases of WNV in central Europe are still rare, the increase in numbers and the described case here emphasize the need to consider WNV in cases of non-bacterial meningoencephalitis, especially in immunocompromised patients and to include WNV testing in the routine diagnostic work-up. In addition, we would like to emphasize the importance of WNV surveillance in birds, mosquitoes, and humans. Seroprevalence studies can additionally help to track WNV occurrence and spread in Germany. Screening of all blood donations during WNV season and information campaigns to avoid mosquito breeding will help to reduce transmission.
Acknowledgement:
We thank Petra Mackeldanz, Peter Menzel and Tatjana Schwarz for excellent laboratory assistance and help with sample and data logistics.
Funding:
Parts of this work were funded by European Union DG Research through projects Prepare (GA602525) to CD and by the German Ministry of Research through the projects VARIPath (01KI2021) to VMC. VMC is a participant in the BIH-Charité Clinician Scientist Program funded by the Charité-Universitaetsmedizin Berlin and the Berlin Institute of Health. TCJ is in part funded through NIAID-NIH CEIRS contract HHSN272201400008C.
Footnotes
Conflict of interest:
KR received research support from Novartis Pharma, Merck Serono, German Ministry of Education and Research, European Union (821283–2), Stiftung Charité (BIH Clinical Fellow Program) and Arthur Arnstein Foundation; and received speaker honoraria from Bayer and travel grants from Guthy Jackson Charitable Foundation. The other authors have no conflict of interest to declare.
References
- Anesi JA, Silveira FP, Practice A.S.T.I.D.C.o. Arenaviruses and West Nile Virus in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33(9):e13576; 10.1111/ctr.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancey C, Grinev A, Volkova E, Rios M The global ecology and epidemiology of West Nile virus. Biomed Res Int 2015;2015:376230; 10.1155/2015/376230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL West Nile virus neuroinvasive disease. Ann Neurol 2006;60(3):286–300; 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- ECDC. European Centre for Disease Prevention and Control (ECDC) Epidemiological update: West Nile virus transmission season in Europe 2018. [Available from: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc.
- Eiden M, Vina-Rodriguez A, Hoffmann B, Ziegler U, Groschup MH Two new real-time quantitative reverse transcription polymerase chain reaction assays with unique target sites for the specific and sensitive detection of lineages 1 and 2 West Nile virus strains. J Vet Diagn Invest 2010;22(5):748–53; 10.1177/104063871002200515. [DOI] [PubMed] [Google Scholar]
- Gossner CM, Marrama L, Carson M, Allerberger F, Calistri P, Dilaveris D, Lecollinet S, Morgan D, Nowotny N, Paty MC, Pervanidou D, Rizzo C, Roberts H, Schmoll F, Van Bortel W, Gervelmeyer A West Nile virus surveillance in Europe: moving towards an integrated animal-human-vector approach. Euro Surveill 2017;22(18); 10.2807/1560-7917.ES.2017.22.18.30526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granerod J, Tam CC, Crowcroft NS, Davies NW, Borchert M, Thomas SL Challenge of the unknown. A systematic review of acute encephalitis in non-outbreak situations. Neurology 2010;75(10):924–32; 10.1212/WNL.0b013e3181f11d65. [DOI] [PubMed] [Google Scholar]
- Hart J Jr., Tillman G, Kraut MA, Chiang HS, Strain JF, Li Y, Agrawal AG, Jester P, Gnann JW Jr., Whitley RJ, Team N.C.A.S.G.W.N.V.P. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis 2014;14:248; 10.1186/1471-2334-14-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampen H, Holicki CM, Ziegler U, Groschup MH, Tews BA, Werner D West Nile Virus Mosquito Vectors (Diptera: Culicidae) in Germany. Viruses 2020;12(5); 10.3390/v12050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Marder BA, Levi ME, Laird SP, McNutt JT, Escott EJ, Everson GT, Tyler KL Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol 2004;61(8):1210–20; 10.1001/archneur.61.8.1210. [DOI] [PubMed] [Google Scholar]
- Kumar D, Prasad GV, Zaltzman J, Levy GA, Humar A Community-acquired West Nile virus infection in solid-organ transplant recipients. Transplantation 2004;77(3):399–402; 10.1097/01.TP.0000101435.91619.31. [DOI] [PubMed] [Google Scholar]
- Michel F, Sieg M, Fischer D, Keller M, Eiden M, Reuschel M, Schmidt V, Schwehn R, Rinder M, Urbaniak S, Muller K, Schmoock M, Luhken R, Wysocki P, Fast C, Lierz M, Korbel R, Vahlenkamp TW, Groschup MH, Ziegler U Evidence for West Nile Virus and Usutu Virus Infections in Wild and Resident Birds in Germany, 2017 and 2018. Viruses 2019;11(7); 10.3390/v11070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet 2001;358(9278):261–4; 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- Pietsch C, Michalski D, Munch J, Petros S, Bergs S, Trawinski H, Lubbert C, Liebert UG Autochthonous West Nile virus infection outbreak in humans, Leipzig, Germany, August to September 2020. Euro Surveill 2020;25(46); 10.2807/1560-7917.ES.2020.25.46.2001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ Clinical manifestations and outcomes of West Nile virus infection. Viruses 2014;6(2):606–23; 10.3390/v6020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI West Nile encephalitis epidemic in southeastern Romania. Lancet 1998;352(9130):767–71; 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- Ziegler U, Luhken R, Keller M, Cadar D, van der Grinten E, Michel F, Albrecht K, Eiden M, Rinder M, Lachmann L, Höper D, Vina-Rodriguez A, Gaede W, Pohl A, Schmidt-Chanasit J, Groschup MH West Nile virus epizootic in Germany, 2018. Antiviral Res 2019;162; 10.1016/j.antiviral.2018.12.005 [DOI] [PubMed] [Google Scholar]
- Ziegler U, Santos PD, Groschup MH, Hattendorf C, Eiden M, Hoper D, Eisermann P, Keller M, Michel F, Klopfleisch R, Muller K, Werner D, Kampen H, Beer M, Frank C, Lachmann R, Tews BA, Wylezich C, Rinder M, Lachmann L, Grunewald T, Szentiks CA, Sieg M, Schmidt-Chanasit J, Cadar D, Luhken R West Nile Virus Epidemic in Germany Triggered by Epizootic Emergence, 2019. Viruses 2020;12(4); 10.3390/v12040448. [DOI] [PMC free article] [PubMed] [Google Scholar]


