Abstract
Background and Objectives
Autoantibodies against α3-subunit–containing nicotinic acetylcholine receptors (α3-nAChRs), usually measured by radioimmunoprecipitation assay (RIPA), are detected in patients with autoimmune autonomic ganglionopathy (AAG). However, low α3-nAChR antibody levels are frequently detected in other neurologic diseases with questionable significance. Our objective was to develop a method for the selective detection of the potentially pathogenic α3-nAChR antibodies, seemingly present only in patients with AAG.
Methods
The study involved sera from 55 patients from Greece, suspected for autonomic failure, and 13 patients from Italy diagnosed with autonomic failure, positive for α3-nAChR antibodies by RIPA. In addition, sera from 52 patients with Ca2+ channel or Hu antibodies and from 2,628 controls with various neuroimmune diseases were included. A sensitive live cell-based assay (CBA) with α3-nAChR–transfected cells was developed to detect antibodies against the cell-exposed α3-nAChR domain.
Results
Twenty-five patients were found α3-nAChR antibody positive by RIPA. Fifteen of 25 patients were also CBA positive. Of interest, all 15 CBA-positive patients had AAG, whereas all 10 CBA-negative patients had other neurologic diseases. RIPA antibody levels of the CBA-negative sera were low, although our CBA could detect dilutions of AAG sera corresponding to equally low RIPA antibody levels. No serum bound to control-transfected cells, and none of the 2,628 controls was α3-CBA positive.
Discussion
This study showed that in contrast to the established RIPA for α3-nAChR antibodies, which at low levels is of moderate disease specificity, our CBA seems AAG specific, while at least equally sensitive with the RIPA. This study provides Class II evidence that α3-nAChR CBA is a specific assay for AAG.
Classification of Evidence
This study provides Class II evidence that an α3-nAChR cell-based assay is a more specific assay for AAG than the standard RIPA.
Neuronal nicotinic acetylcholine receptors (nAChRs) are a group of pentameric ligand–gated cationic channels formed by 5 subunits, selected among 12 different subunits (α2-α10, β2-β4).1 Neuronal nAChRs are ubiquitously expressed in the CNS and peripheral nervous system2,3 modulating neurotransmitter release or mediating postsynaptic neurotransmission. nAChR dysfunction has been described in several neurologic diseases, including Parkinson disease, Alzheimer disease, autism, and schizophrenia.4 In the peripheral nervous system, nAChRs are linked to autoimmunity, specifically autoimmune autonomic ganglionopathy (AAG), with antibodies against α3-subunit–containing nAChRs (α3-nAChRs).5-7
Several studies indicate that α3-nAChR antibodies may have pathogenic properties resulting in deterioration of synaptic transmission at the sympathetic, parasympathetic, and enteric ganglia.6,8,9 Such antibodies have been found in patients with AAG characterized by autonomic failure, with main clinical features: orthostatic hypotension, xerostomia, impaired pupil responses, urinary retention, anhidrosis, and gastrointestinal dysmotility.5 However, low levels of α3-nAChR antibodies have been detected in various neurologic diseases5,10-12 of unknown clinical and therapeutic implications.
The currently established diagnostic method for α3-nAChRs antibodies is a radioimmunoprecipitation assay (RIPA) with 125I-epibatidine–labeled α3-nAChR. Although this method is widely used for AAG diagnosis, low antibody levels have low specificity for AAG (∼50%), frequently identified in postural orthostatic tachycardia syndrome (POTS), encephalopathy, or other neurologic syndromes, where the role of these antibodies has not been justified.11-13 Therefore, there is a need for the development of an assay that detects only disease-specific α3-nAChR antibodies and yet with high sensitivity. Cell-based assay (CBA) is the gold standard method for the detection of potentially pathogenic antibodies against neuronal and glial antigens, usually with high disease specificity14-16
Expression of neuronal nAChRs by transfected cells is usually low, prohibiting the required high sensitivity of the corresponding CBAs. The chaperons RIC3 and NACHO and the ligand nicotine increase expression of some nAChR subtypes.17-19 Here, we optimized α3-nAChR expression and therefore the assay's sensitivity by the combined use of the 2 chaperons and nicotine. With this CBA, we studied sera of patients from a broad spectrum of neurologic diseases. Of interest, we found that, contrary to RIPA, our CBA was totally AAG specific, without compromising the high RIPA sensitivity.
In this study, we aimed to overcome the low disease specificity of the current diagnostic assays for α3-nAChR antibodies at the low titer range. To do this, we developed an assay (a CBA), which detects only antibodies to cell-exposed epitopes of the antigen (i.e., the potentially pathogenic antibodies).
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the IRBs of the Attikon University Hospital and the Fondazione IRCCS Istituto Neurologico Carlo Besta. Patients signed informed consents, approved by the ethics committees.
Patients, Sera, and mAbs
We screened sera from 2 patient groups: (1) patients with neuroimmune diseases referred to Tzartos NeuroDiagnostics (Athens) between January 2017 and August 2020; of them, 55 were referred for α3-nAChR antibody testing, 52 were positive for antibodies to voltage-gated Ca2+ channels P/Q-type (VGCC) or Hu antigen, and 2,628 patients were referred for antibodies relevant to autoimmune encephalitis, paraneoplastic syndromes, neuromyelitis optica, autoimmune peripheral neuropathies, and myasthenia gravis; and (2) 13 RIPA-positive patients with autonomic failure, identified in the Neurology Department, Carlo Besta Institute, Milan, and the Syncope and Orthostatic Disorders Unit Clinica Medica, Humanitas Research Hospital, Rozzano, Italy. Subunit-specific mAb-295 (anti-β2) and mAb-299 (anti-α4) were provided by J. Lindstrom.20
Population Sample and Clinical Features of Patients With AAG
We studied 15 patients with AAG with a typical hemodynamic profile characterized by remarkable orthostatic hypotension without any reflex increases in heart rate. The amount of orthostatic hypotension spanned between −30 and −60 mm Hg, depending on the magnitude of the supine hypertension. Additional autonomic symptoms were present, including impaired pupil response to light, early satiety and significant constipation, sweat loss, dry mouth and eyes, and bladder and erectile dysfunctions in the patients with AAG. Notably, erectile dysfunction was the earliest presenting symptom in almost all male patients, and most patients had at least 1 syncope episode on standing up during the 12 months preceding the study enrollment. Most patients also complained for interrupted, troubled, and unrestful sleep; however, no EEG recordings were performed.
Diabetes, autoimmune diseases, and other causes potentially causing dysautonomia were excluded on the basis of clinical examination, appropriate blood test, electrophysiologic testing, and brain MRI. Amyloidosis was excluded with biopsy of periumbilical fat in 3 patients.
In terms of response to immunosuppressive therapies, we obtained clinical data from 9 patients. Specifically, these patients were treated with plasma exchange (5 patients), IV immunoglobulins (2 patients), azathioprine followed by mycophenolate mofetil (1 patient), and/or corticosteroids (5 patients). As previously reported for patients with AAG,21,22 response to immunotherapies for these 9 patients significantly improved autonomic function, with most prominent the achievement of orthostatic tolerance.
Radioimmunoprecipitation Assay
RIPA was performed with 125I-epibatidine–labeled extracts of HEK293 cells transfected with α3β4-nAChR. Cells were solubilized with 0.5% Triton/PBS for 30 minutes at 4°C. Cell supernatant was incubated with 125I-epibatidine for 1 hour at room temperature (RT). Indirectly 125I-labeled α3-nAChR (∼10,000 cpm/reaction) was incubated with the patient's serum for 2 hours at RT and subsequently overnight at 4°C. Then, goat anti-human IgG (H + L) (RSR Ltd, Cardiff; product number RBA/Ig100) was added and incubated for 1.5 hours at 4°C. One milliliter buffer was added, centrifuged, pellets washed and their radioactivity was counted in a γ-counter. The cutoff for positivity was 0.05 nM (average of the values of 20 healthy controls + 4 SD). All sera tested by RIPA were previously tested either in Euro Diagnostica, Sweden (Athens sera) or in Carlo Besta (Milan sera) with 125I-epibatidine–labeled IMR32 extracts. All positive sera by the later systems were also positive by the recombinant α3β4-nAChR, with occasional differences in antibody levels. These differences may be due to differences in the specific activity of 125I-epibatidine used, the amount of antigen in the reaction, and/or to nAChR heterogeneity in the IMR32 cell extracts.
Cell Culture and Transfection
HEK293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in 5% CO2. Various parameters were tested in preliminary experiments to identify the best conditions. The final selected conditions were as follows: cells were seeded on culture dishes and transiently transfected with a mixture of plasmids pCMV6-XL4-CHRNA3 or pCMV6-XL5-CHRNA4, pCMV6-XL5-CHRNB4 or pCMV6-XL5-CHRNB2, pCMV6-XL5-TMEM35 (NACHO), and pCMV6-XL5-RIC3 (OriGene, Herford, Germany); 3.7 μg/plasmid for α3β4 or α3β2 and 2.5 μg for α4β2 per 100 mm culture dish; or control vector, using jetPRIME kit transfection reagent (Polyplus jetPRIME, France). Cells were treated with 1 mM nicotine (N3876, Sigma), 24 hours before analysis. Cells were washed to remove nicotine before incubation with the test sera.
Development of a Novel Cell-Based Assay
All sera were screened using the live CBA with HEK293 cells expressing the α3β4- or α3β2-nAChR. Forty-eight hours posttransfection, cells were washed with DMEM/0.46% w/v N-(2-hydroxyethyl)-piperazine-N’-(2-ethanesulfonic acid) (HEPES) buffer (DMEM-HEPES) in principle as described.23,24
CBA involved incubation of serum (1/10 dilution in 1% bovine serum albumin in DMEM-HEPES buffer) with transfected cells. After 1 hour, cells were washed 3 times with DMEM-HEPES buffer and fixed immediately with 4% paraformaldehyde for 10 minutes. Fixed cells incubated with rabbit anti-human IgG (Invitrogen) at 1/750 dilution for 1 hour, followed by incubation with Alexa Fluor-568 goat anti-rabbit IgG (H + L) (Invitrogen), as the third antibody, at 1/750 dilution for 1 hour (all at RT). The use of the third antibody increased signal, without increasing background, and therefore, it increased sensitivity. Microscopy was performed under blinded conditions by 2 or 3 independent observers. The Olympus microscope CKX-41 was used, and images were analyzed using Infinity Analyze-6.5 Lumenera software. As negative controls, AQP4-transfected HEK293 cells were used. Positive sera were subsequently tested at serial dilutions to determine their titer, expressed as the highest positive dilution. All positive sera were also tested with HEK293 cells transfected with α4β2- and α7-nAChRs.
Colocalization of serum α3-nAChR antibody with the rat anti-β2 mAb295 was tested by their coincubation with α3β2-nAChR–expressing cells, fixation as above, followed by incubation with rabbit anti-human IgG (Invitrogen) at 1/750 dilution for 1 hour, followed by simultaneous incubation with Alexa Fluor-488 goat anti-rat IgG (Invitrogen) (1/200 dilution), and Alexa Fluor-568 goat anti-rabbit IgG (Invitrogen) (1/750 dilution). Cell incubation with only mAb or serum, followed by incubation with only the antiantibodies to IgG of the heterologous species, showed no background binding.
The fluorescent signal of a CBA-positive serum antibodies was also counterstained with Hoechst 33258 (40044 Biotium) for cell nucleus staining: Hoechst dye at 1/40000 dilution was simultaneously incubated with the third antibody for the human serum (Alexa Fluor-568 goat anti-rabbit IgG [H + L]).
Statistical Analysis
We tested both RIPA and CBA variables for normality, and given the small data set, we used the Shapiro-Wilk test. To explore possible associations, we used the Pearson correlation coefficient and the coefficient of determination R2.
Data Availability
Reasonable requests from any qualified investigator for anonymized data will be satisfied.
Results
Identification of Sera Positive for α3-nAChR Antibodies by RIPA
Two patient cohorts were tested by RIPA for α3β4-nAChR serum antibodies: (1) 55 patients suspected for AAG referred from Greek clinics to Tzartos NeuroDiagnostics and (2) 13 RIPA-positive patients already found positive in Carlo Besta, Milan, by RIPA with 125I-labeled IMR32 nAChR extracts and with verified final clinical diagnosis.
In total, 6/55 patients of the Greek cohort were positive, 3 with high (1.2–1.5 nM) and 3 with low (0.05–0.11 nM) antibody levels (Table 1). In addition, all 13 patients of the Milan cohort were also found positive by the α3β4-nAChR RIPA: 11 of them with medium-to-high antibody concentrations (0.53–3.35 nM) and 2 with low concentrations (0.05 and 0.13 nM) (Table 1). We also tested 31 sera from patients with VGCC antibodies and 21 from patients with antibodies to the paraneoplastic antigen Hu; 6 sera were found α3-nAChR RIPA positive, all with low antibody levels, 0.06–0.11 nM (Table 1); i.e., overall, 25 sera were identified as RIPA positive.
Table 1.
RIPA and CBA α3-Subunit–Containing Nicotinic Acetylcholine Receptor Antibody Titers of All RIPA-Positive Patients and Summary of Clinical Characterizationa
Development of a Sensitive CBA for α3-nAChR Antibodies. Optimization of Conditions
Myc-flag and GFP tags reduced nAChR expression; therefore, we used untagged subunits in the following experiments, detecting their expression with RIPA-positive sera, and anti-β2 mAb-295, to determine the optimal conditions for a very sensitive CBA.
The expression of α3β4- and α3β2-nAChRs was compared at 4 conditions: (1) with only chaperon RIC3, (2) with RIC3 and NACHO, (3) with RIC3 and culture with 1 mM nicotine 24 hours after transfection, or (4) with RIC3, NACHO, and nicotine. Figure 1 and eTable 1, links.lww.com/NXI/A707, show that the combined use of both chaperons and nicotine resulted in the strongest staining for both α3-nAChRs. Therefore, we used all 3 factors in all subsequent experiments. Similarly, the use of these factors was also beneficial for α4β2-nAChR expression (not shown). None of the Greek or Italian cohorts suspected for autonomic failure had antibodies to α4β2-nAChR.
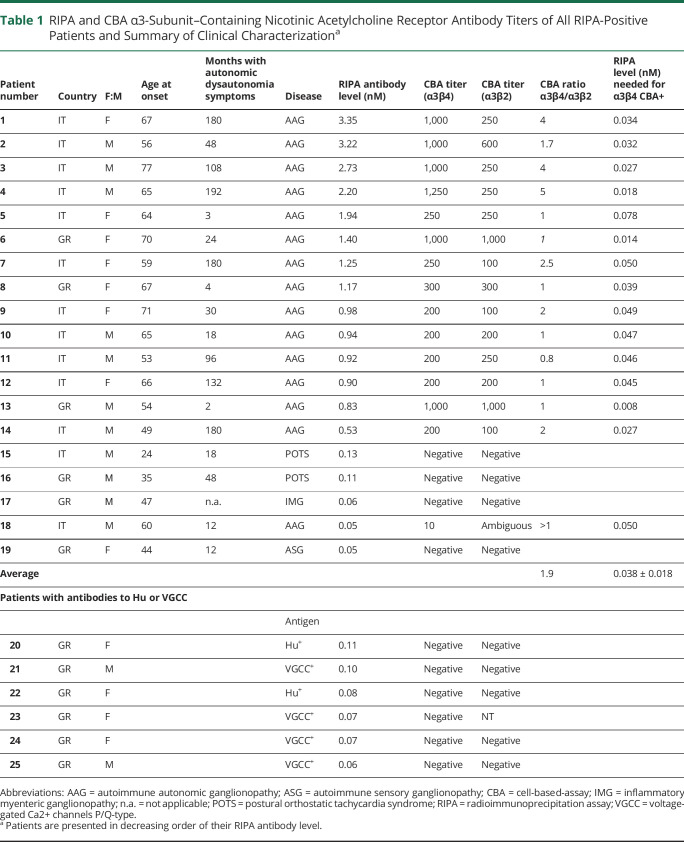
Figure 1. Optimization of the α3-nAChR Cell-Based-Assay.
(A) Treatment of α3-nAChR–transfected HEK293 cells, with RIC3, NACHO, and nicotine increases significantly the expression of these receptors. Cells expressing α3β4-, α3β2-nAChRs, or control molecules AQP4 and α4β2-nAChR were cotransfected with 1 (RIC3) or 2 (RIC3 and NACHO) chaperons and cultured in the presence or absence of 1 nM nicotine. Cells were then incubated with the serum of a patient with autoimmune autonomic ganglionopathy with positive RIPA titer for α3-nAChR. Scale bar: 20 μm. (B) Colocalization of patient 2 serum with the rat anti-β2 mAb. (C) Colocalization of α3-nAChR antibody binding and cell nucleus/DNA staining. The fluorescent signal of patient 13 antibodies was counterstained with Hoechst (for cell nucleus staining). α3-nAChR = α3-subunit–containing nicotinic acetylcholine receptor; RIPA = radioimmunoprecipitation assay.
Coincubation of α3β2-nAChR HEK293 cells with positive sera (no. 2 and 13) and the anti-β2 mAb-295 revealed excellent colocalization, confirming that serum antibodies indeed bound to nAChRs (Figure 1B). Moreover, Figure 1C shows that serum antibody bound on viable cells; antibody-labeler cells were ∼1/5 of the total cells.
Binding of Patients' Sera to Cell-Exposed α3-nAChR by CBA and Comparison With Their Binding to Solubilized nAChR by RIPA
Using the live CBAs with α3β4- and α3β2-nAChRs, with optimized conditions, we screened all 25 RIPA-positive sera and sera from the 49 patients referred for α3-nAChR antibodies but found RIPA negative. As controls we screened the remaining control 2,674 sera.
Fifteen RIPA-positive patients were α3-nAChR CBA positive (Table 1). Figure 2 shows binding of selected sera to α3β4- and α3β2-nAChRs but not to the control-transfected cells. Figure 3 shows that correlation between RIPA and CBA values exists; however, it is rather weak (R2 = 0.502), while a stronger association was observed for the log-log transformation of RIPA and CBA values (R2 = 0.77).
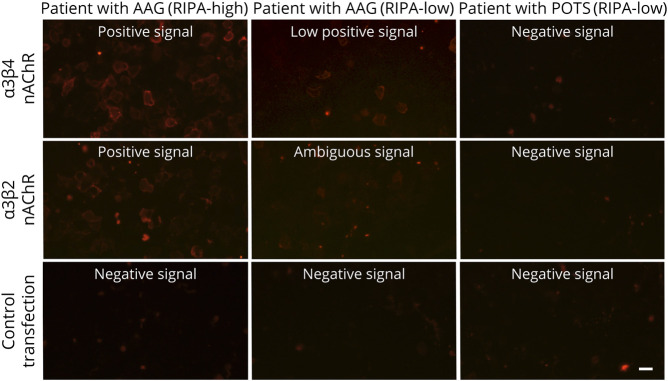
Figure 2. The Novel CBA With α3β4-nAChR–Transfected HEK293 Cells Detects Antibodies in Sera With High or Low RIPA Antibody Levels Specifically in Patients With AAG.
HEK293 cells expressing α3β4, α3β2-nAChRs, or control AQP4 molecule were incubated with sera from patients with AAG (of high or low RIPA antibody levels) or from a patient with POTS and stained with anti-human IgG (red). It is shown that both patients with AAG showed positive staining, independently of the RIPA antibody level, whereas the patient with POTS gave no staining. The AAG RIPA-high, AAG RIPA-low, and POTS RIPA-low patients are patients no. 6 (RIPA 1.4 nM, CBA 1/1,000), 18 (RIPA 0.05 nM, CBA 1/10), and 16 (RIPA 0.13 nM, CBA negative) of Table 1, respectively. Scale bar: 20 μm. AAG = autoimmune autonomic ganglionopathy; CBA = cell-based-assay; POTS = postural orthostatic tachycardia syndrome; RIPA = radioimmunoprecipitation assay.
Figure 3. Correlation of α3 nAChR Antibody Levels by RIPA vs CBA Titers for the 15 Patients With Autoimmune Autonomic Ganglionopathy.
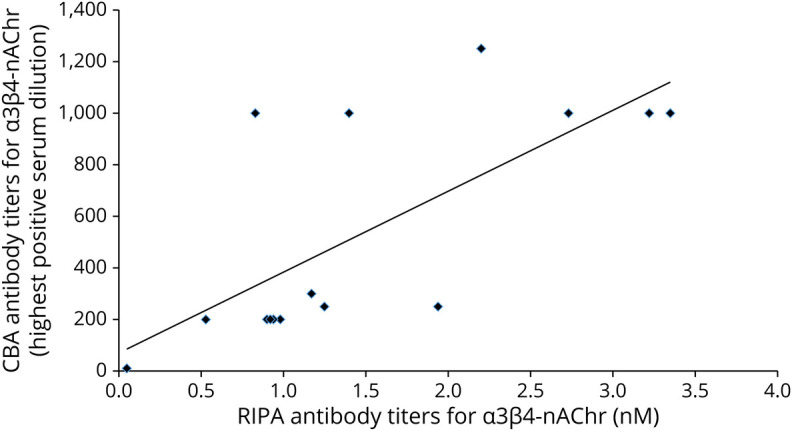
The normality Shapiro-Wilk test showed that the RIPA data follow normal distribution, whereas the CBA data do not (RIPA Shapiro-Wilk p = 0.109; CBA Shapiro-Wilk p = 0.002). Pearson correlation coefficient r = 0.71 and coefficient of determination R2 = 0.50. We also plotted the values using log-log transformation of the RIPA and CBA values, which resulted in improved r (=0.88) and R2 (=0.77). CBA = cell-based-assay; RIPA = radioimmunoprecipitation assay.
Importantly, all sera from the 2,674 controls and all RIPA-negative sera from the 49 patients referred for α3-nAChR antibodies were CBA negative, further confirming the specificity of the assay (9 sera showed nonspecific weak staining, similar to the control-transfected cells).
Although half of the CBA-positive sera had similar CBA titers for α3β4- and α3β2-nAChRs, the other half had higher titers with the α3β4-nAChR. On the average, α3β4-nAChR CBA resulted in nearly 2 times higher titers than α3β2-nAChR CBA but varying from 0.8 to 5.0 times higher (Table 1). Yet, almost all sera positive for α3β4-nAChR were also positive for α3β2-nAChR, suggesting that all sera contain anti-α3 antibodies, with or without anti-β2/β4 antibodies.
Although most CBA-positive sera had high RIPA levels, and all CBA negative had low RIPA levels, apparently antibody concentration was not the limiting factor for CBA positivity because CBA could efficiently detect low antibody concentrations in patients with AAG. Table 1 (last column) presents the calculated minimum RIPA levels, which would be needed for positive CBA (at the adopted 1/10 dilution). These minimum RIPA levels for CBA positivity are derived from the ratio: [RIPA]/[CBA] multiplied by 10 (because the adopted test serum dilution is 1/10). It is shown that an average 0.038 ± 0.018 nM RIPA values would be sufficient for CBA positivity. Taking into account that the RIPA cutoff is 0.05 nM, we conclude that CBA is at least as sensitive as the RIPA.
Clinical Characterization of Patients Whose Sera Were Positive for α3-nAChR Antibodies by RIPA and/or CBA Shows Superiority of the CBA, With High Specificity for AAG
Clinical characterization of the 19 RIPA-positive AAG-suspected patients showed that 15 had AAG (with autonomic symptoms including orthostatic hypotension, impaired pupil response to light, erectile dysfunction, constipation, bladder symptoms, loss of sweating, lightheadedness, and dry mouth and/or dry eyes, without any additional symptoms from the peripheral nervous system or CNS), whereas the remaining 4 patients had other neurologic diseases (Table 2 and eTable 2, links.lww.com/NXI/A707). However, in terms of RIPA concentration, all 14 patients with medium to high α3-nAChR antibody concentration had AAG, whereas only 1 of the 5 low RIPA patients had AAG. Among the remaining 4 low RIPA patients, 2 had POTS, 1 had autoimmune sensory ganglionopathy with Sjögren disease, and 1 had inflammatory myenteric ganglionopathy.
Table 2.
Summary of Clinical Characteristics of the α3-Subunit–Containing Nicotinic Acetylcholine Receptor Antibody–Positive Patients
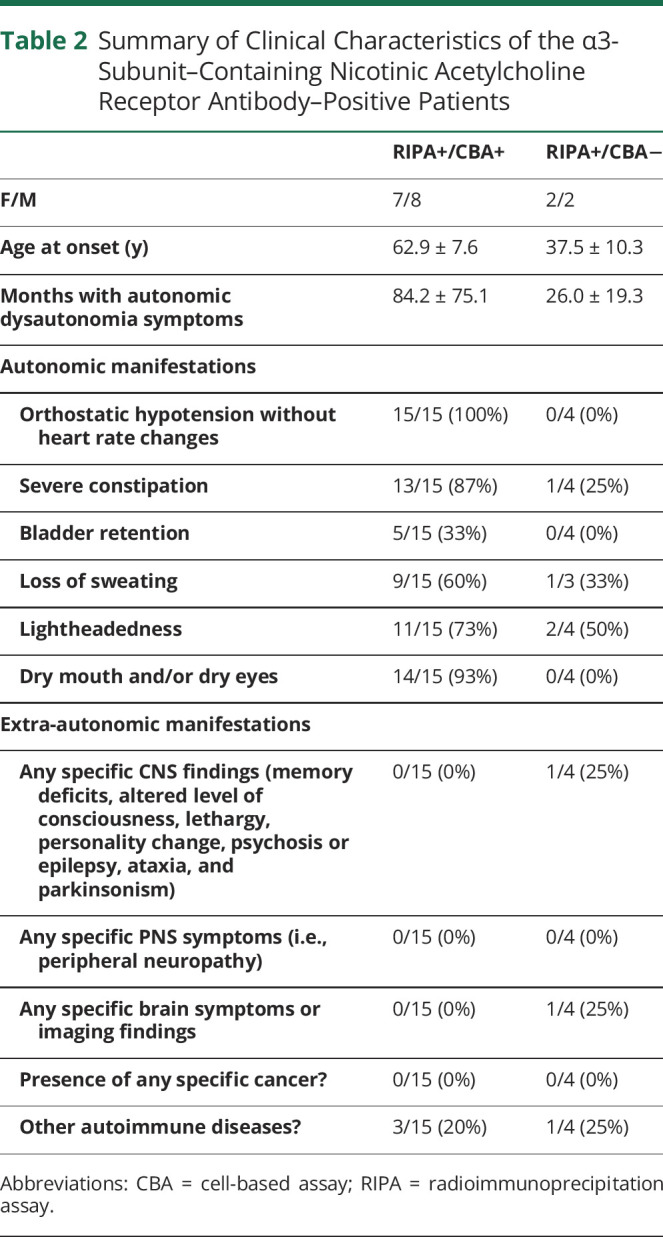
Of interest, the CBA, opposed to RIPA, detected α3-AChR antibodies selectively in all 15 patients with AAG, independently of the RIPA level, but in none of the 4 patients without AAG, despite their RIPA positivity (i.e., 100% specificity for AAG). Patient 18, with low RIPA level and marginal CBA positivity, had a limited form of AAG (loss of sweating, dry eyes and mouth, and lightheadedness with only a transient episode of hypotension). In addition, none of the 6 RIPA-positive patients with VGCC or Hu antibodies was CBA positive; it should be noted that none of these patients had mentioned any features of dysautonomia.
Ig Class and Subclass of the α3-nAChR Antibodies Determined by CBA
Finally, we determined the Ig class/subclass of the α3-nAChR antibodies by CBA because this characteristic may be related to their pathogenicity. eTable 3, links.lww.com/NXI/A707, shows that all tested CBA-positive sera contain α3-nAChR antibodies of the complement-binding IgG1 subclass. In addition, 4 sera also contained IgG2 and/or IgG3 or IgM antibodies.
Discussion
In this study, we introduce a novel assay (a sensitive live CBA) for the detection of α3-nAChR antibodies, at least as sensitive as the currently established RIPA, but, importantly, apparently AAG specific. Detection of autoantibodies against nAChRs containing the α3-subunit (α3-nAChR/ganglionic nAChR) for the serologic diagnosis of AAG has been usually performed by a RIPA developed by Vernino et al.7 who have discovered the presence of α3-nAChR antibodies in AAG using solubilized α3-AChR (from extracts of α3-nAChR–bearing cells) preincubated with 125I-epibatidine. This assay has revolutionized diagnosis and therefore the proper treatment of AAG.5,6 In addition, another group10 developed a luciferase immunoprecipitation system (LIPS) for the detection of antibodies to individually expressed α3 or β4 nAChR subunits.10 Recently, a FACS assay has been described25 with IMR32 cells, detecting potentially pathogenic α3-nAChR antibodies in high RIPA samples, in agreement with an earlier study on the immunomodulating capacity of the α3-nAChR antibodies.26 This assay may prove an attractive diagnostic assay, especially if it can substitute the IMR32 cells with α3-nAChR–transfected and control-untransfected cells and confirm a high specificity and sensitivity.
Although the RIPA and secondarily the LIPS have been invaluable for AAG diagnosis, they are not without caveats. Important limitations are as follows: (1) about 50% of the low antibody concentration seem nonspecific, present in various neurologic disease11,27; (2) both assays, using detergent-solubilized nAChR or subunits, cannot discriminate between antibodies to cell-exposed nAChR (i.e., the potentially pathogenic) and antibodies to in vivo inaccessible nAChR sites; (3) RIPA can be performed only in laboratories eligible to use radioactivity; and (4) LIPS uses individual nAChR subunits, which probably lack several intact epitopes.
Over the last decade, it has been shown that CBAs are superior for detecting autoantibodies of clinical significance.16,23,24 In live CBAs, the detected antibodies bind to extracellular epitopes of the antigens in native conformation, that is, they are potentially pathogenic. By excluding the detection of nonpathogenic antibodies, CBA is generally more disease specific than other assays. In addition, live CBA preserves the intact conformation of the epitopes, contrary to fixed-cell CBAs and other assays, improving the specificity of the assay. Thus, live CBAs are now the gold standard assays for the detection of several autoantibodies to cell surface antigens involved in neuroimmune diseases.14,15
Until recently, the development of sensitive and reliable CBAs for antibodies to neuronal nAChRs was too difficult because of their low surface expression.17,26 However, the chaperons RIC3 and NACHO and nicotine have shown to increase expression of some nAChR subtypes.17,19,28 Herein, we tested the effect of these 3 factors on α3-nAChR expression by the transfected HEK293 cells. In addition, nAChR subunits were untagged because the use of myc-flag or GFP tags reduced nAChR expression. We found that the combined use of chaperons, nicotine, and untagged subunits resulted in the most sensitive live CBA.
The major advantage of our α3-nAChR CBA is on its disease specificity. As described above, with the currently used assays, about 50% of the patients with low α3-nAChR antibody levels present a variety of disorders other than AAG, including POTS,13 small- and large-fiber neuropathy, encephalitis, LEMS, and Hu-related paraneoplastic disorders, of unknown clinical significance.5,7,11 Therefore, the significance of a low RIPA antibody level is questionable. Of interest, we observed that our CBA is AAG specific. Specifically, all 15 patients with AAG were both RIPA and CBA positive (Table 1). Although the majority of these patients had medium to high RIPA levels (i.e., considered AAG specific), 1 patient had a borderline RIPA (0.05 nM) and yet was also CBA positive. Of interest, this patient had a limited form of AAG, probably due to the very low α3-nAChR antibody titers (by RIPA and CBA) in agreement with Vernino et al.21 conclusions for limited forms of AAG in patients with intermediate antibody levels. Further studies should define how marginal α3-CBA titers should be evaluated. In contrast, all 4 patients without AAG, with low RIPA levels (0.05–0.13 nM), were CBA negative. The predominant IgG subclass of the α3-nAChR antibodies of the CBA-positive patients was IgG1, very efficient in inducing effector mechanisms and therefore damaging the α3-nAChR–bearing cells. IgM antibodies were a minority, detected in only 1 patient. Similarly, preliminary RIPA experiments with high-titer sera and anti–Fc-IgG for precipitation suggested that the majority of the RIPA-detected α3-nAChR antibodies are IgG.
Importantly, none of the 2,680 control sera from patients verified or suspected for various neuroimmune diseases was found α3-CBA positive. These included the 52 patients with anti-VGCC or anti-Hu antibodies, 6 of which were found α3-nAChR RIPA positive but CBA negative.
All sera of Table 1 were tested with CBA with both ganglionic nAChR subtypes, α3β4 and α3β2. All CBA-positive sera bound to both nAChRs, strongly suggesting that all had antibodies to the α3 subunit. This is further supported by the fact that none of these sera bound to the α4β2- nAChR; therefore, any binding to α3β2-nAChR should be due to binding to the α3 subunit. However, there was very considerable variation in the titer ratio between α3β4- and α3β2-nAChRs among the different sera, from 0.8 to 5 (average 1.9). The high α3β4/α3β2 CBA ratios in several sera (6/15 sera with ratio ≥2) could be attributed to several possible reasons: (1) some sera may also contain anti–β antibodies, especially anti-β4; (2) several mostly α3-subunit antibodies may bind to epitopes on the different α3β2/α3β4 subunit interphases; (3) the 2 different β-subunits may impose different conformational changes on the α3 epitopes; or (4) the likely higher expression of α3β4 than α3β219 could have affected the CBA titers. Although anti–β4 antibodies may exist,10,29 Nakane et al.10 showed that anti–β4 antibodies are generally much fewer than the anti–α3 antibodies, which cannot justify the large heterogeneity in α3β4/α3β2 CBAs. Any difference in expression efficiency between the 2 nAChRs would be expected to have similar effect on the binding of all sera. Therefore, we suggest that differences in subunit interphases and/or conformational differences on the α3 subunits between the 2 nAChRs are the most likely reasons for the different CBA titers between the 2 nAChRs.
In conclusion, our findings suggest that the novel CBA for α3-nAChR antibodies is at least equally sensitive with the currently established RIPA, and, importantly, contrary to RIPA, it is highly AAG specific. Furthermore, in contrast to RIPA, which can be performed only in laboratories eligible to use radioactivity, this CBA, following our suggested conditions for high α3-nAChR expression, can be performed by many laboratories worldwide. Because of the limited number of patients with AAG of the current investigation, further studies on larger patient cohorts are needed to evaluate the exact specificity and sensitivity of this assay and to better determine AAG as a clinical entity.
Acknowledgment
The authors thank Prof. Sotirios Giannopoulos, Prof. Konstantinos Kotsis, and Dr. Christos Stergiou for valuable discussions and suggestions. They also thank Aliki Papakonstantinou, Christiana Vasileiadi, and Vassiliki Papadopoulou for technical assistance in some experiments.
Glossary
- AAG
autoimmune autonomic ganglionopathy
- AQP4
aquaporin-4
- CBA
cell-based assay
- DMEM
Dulbecco's modified Eagle's medium
- HEPES
0.46% w/v N-(2-hydroxyethyl) piperazine-N’-(2-ethanesulfonic acid)
- LIPS
luciferase immunoprecipitation system
- mAb
monoclonal antibody
- nAChR
nicotinic acetylcholine receptor
- POTS
postural orthostatic tachycardia syndrome
- RIPA
radioimmunoprecipitation assay
- RT
room temperature
- VGCC
voltage-gated Ca++ channels P/Q-type
- α3-nAChR
α3-subunit–containing nAChR
Appendix. Authors
Contributor Information
Katerina Karagiorgou, Email: aik.karagiorgou@gmail.com.
Maria Dandoulaki, Email: dandoulakimaria90@gmail.com.
Renato Mantegazza, Email: renato.mantegazza@istituto-besta.it.
Francesca Andreetta, Email: francesca.andreetta@istituto-besta.it.
Raffaello Furlan, Email: raffaello.furlan@hunimed.eu.
Jon Lindstrom, Email: jslkk@pennmedicine.upenn.edu.
Paraskevi Zisimopoulou, Email: pzisim@gmail.com.
Elisabeth Chroni, Email: echroni@yahoo.com.
Panagiotis Kokotis, Email: pkokotis@med.uoa.gr.
Evangelos Anagnostou, Email: eanagnost@eginitio.uoa.gr.
Dimitrios Tzanetakos, Email: tzanetakosdim@yahoo.com.
Marianthi Breza, Email: marianthibr@gmail.com.
Zoe Katsarou, Email: katsarouzoe@gmail.com.
Georgios Amoiridis, Email: amoirid@gmail.com.
Vasileios Mastorodemos, Email: vasmast@yahoo.com.
Marianna Bregianni, Email: marianna.breg@gmail.com.
Anastasios Bonakis, Email: bonakistasos@gmail.com.
Georgios Tsivgoulis, Email: tsivgoulisgiorg@yahoo.gr.
Konstantinos Voumvourakis, Email: cvoumvou@outlook.com.
Socrates Tzartos, Email: stzartos@gmail.com.
Study Funding
(1) By the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation (EPANEK), under the call Research Create Innovate (project code: T1EDK- 05024, MIS 5032815); (2) by a grant from ERA-NET NEURON JTC 2019 and Greek national funds through the EPANEK, under the priority axis: Enhancing entrepreneurship with sectoral priorities (project code: MIS 5075033); (3) by a grant from the Stavros Niarchos Foundation; and (4) by a grant from the Italian Ministry of Health (grant RF-2013-02355242).
Disclosure
S. Tzartos has shares in the research and diagnostic laboratory Tzartos NeuroDiagnostics. J. Tzartos and S. Tzartos are coinventors in a patent that relates to the detection of antibodies to aquaporin 1. All other authors declare no conflict of interest. Go to Neurology.org/NN for full disclosures.
References
- 1.Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8(9):733-750. [DOI] [PubMed] [Google Scholar]
- 2.Clementi F, Fornasari D, Gotti C. Neuronal nicotinic receptors, important new players in brain function. Eur J Pharmacol. 2000;393(1-3):3-10. [DOI] [PubMed] [Google Scholar]
- 3.Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53(2):199-237. [DOI] [PubMed] [Google Scholar]
- 4.Graham AJ, Martin-Ruiz CM, Teaktong T, Ray MA, Court JA. Human brain nicotinic receptors, their distribution and participation in neuropsychiatric disorders. Curr Drug Targets CNS Neurol Disord. 2002;1(4):387-397. [DOI] [PubMed] [Google Scholar]
- 5.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847-855. [DOI] [PubMed] [Google Scholar]
- 6.Vernino S, Lindstrom J, Hopkins S, Wang Z, Low PA, Muscle Study G. Characterization of ganglionic acetylcholine receptor autoantibodies. J Neuroimmunol. 2008;197(1):63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernino S, Adamski J, Kryzer TJ, Fealey RD, Lennon VA. Neuronal nicotinic ACh receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology. 1998;50(6):1806-1813. [DOI] [PubMed] [Google Scholar]
- 8.Lennon VA, Ermilov LG, Szurszewski JH, Vernino S. Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest. 2003;111(6):907-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vernino S, Ermilov LG, Sha L, Szurszewski JH, Low PA, Lennon VA. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24(32):7037-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakane S, Higuchi O, Koga M, et al. Clinical features of autoimmune autonomic ganglionopathy and the detection of subunit-specific autoantibodies to the ganglionic acetylcholine receptor in Japanese patients. PLoS One. 2015;10(3):e0118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeon A, Lennon VA, Lachance DH, Fealey RD, Pittock SJ. Ganglionic acetylcholine receptor autoantibody: oncological, neurological, and serological accompaniments. Arch Neurol. 2009;66(6):735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamakawa M, Mukaino A, Kimura A, et al. Antibodies to the alpha3 subunit of the ganglionic-type nicotinic acetylcholine receptors in patients with autoimmune encephalitis. J Neuroimmunol. 2020;349:577399. [DOI] [PubMed] [Google Scholar]
- 13.Bryarly M, Raj SR, Phillips L, et al. Ganglionic acetylcholine receptor antibodies in postural tachycardia syndrome. Neurol Clin Pract. 2021;11(4):e397-e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh EA, Nakashima I. Live-cell based assays are the gold standard for anti-MOG-Ab testing. Neurology. 2019;92(11):501-502. [DOI] [PubMed] [Google Scholar]
- 15.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15(2):89-102. [DOI] [PubMed] [Google Scholar]
- 16.Waters PJ, McKeon A, Leite MI, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78(9):665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu S, Matta JA, Lord B, et al. Brain alpha7 nicotinic acetylcholine receptor assembly requires NACHO. Neuron. 2016;89(5):948-955. [DOI] [PubMed] [Google Scholar]
- 18.Kuryatov A, Mukherjee J, Lindstrom J. Chemical chaperones exceed the chaperone effects of RIC-3 in promoting assembly of functional alpha7 AChRs. PLoS One. 2013;8(4):e62246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matta JA, Gu S, Davini WB, et al. NACHO mediates nicotinic acetylcholine receptor function throughout the brain. Cell Rep. 2017;19(4):688-696. [DOI] [PubMed] [Google Scholar]
- 20.Whiteaker P, Cooper JF, Salminen O, et al. Immunolabeling demonstrates the interdependence of mouse brain alpha4 and beta2 nicotinic acetylcholine receptor subunit expression. J Comp Neurol. 2006;499(6):1016-1038. [DOI] [PubMed] [Google Scholar]
- 21.Vernino S. Autoimmune autonomic disorders. Continuum (Minneap Minn). 2020;26(1):44-57. [DOI] [PubMed] [Google Scholar]
- 22.Barbic F, Dipaola F, Andreetta F, et al. Long-term cardiovascular autonomic and clinical changes after immunoglobulin G immunoadsorption therapy in autoimmune autonomic ganglionopathy. J Hypertens. 2017;35(7):1513-1520. [DOI] [PubMed] [Google Scholar]
- 23.Leite MI, Jacob S, Viegas S, et al. IgG1 antibodies to acetylcholine receptors in “seronegative” myasthenia gravis. Brain. 2008;131(pt 7):1940-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzartos JS, Karagiorgou K, Tzanetakos D, et al. Deciphering anti-MOG IgG antibodies: clinical and radiological spectrum, and comparison of antibody detection assays. J Neurol Sci. 2020;15410:116673. [DOI] [PubMed] [Google Scholar]
- 25.Urriola N, Spies JM, Blazek K, Lang B, Adelstein S. A flow cytometric assay to detect functional ganglionic acetylcholine receptor antibodies by imunomodulation in autoimmune autonomic ganglionopathy. Front Immunol. 2021;12:705292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi S, Yokoyama S, Maruta T, et al. Autoantibody-induced internalization of nicotinic acetylcholine receptor alpha3 subunit exogenously expressed in human embryonic kidney cells. J Neuroimmunol. 2013;257(1-2):102-106. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Jammoul A, Mente K, et al. Clinical experience of seropositive ganglionic acetylcholine receptor antibody in a tertiary neurology referral center. Muscle Nerve. 2015;52(3):386-391. [DOI] [PubMed] [Google Scholar]
- 28.Papke RL, Lindstrom JM. Nicotinic acetylcholine receptors: conventional and unconventional ligands and signaling. Neuropharmacology. 2020;168:108021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakane S, Mukaino A, Higuchi O, et al. Autoimmune autonomic ganglionopathy: an update on diagnosis and treatment. Expert Rev Neurother. 2018;18(12):953-965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Reasonable requests from any qualified investigator for anonymized data will be satisfied.