Abstract
Background
Previous large-scale vaccination clinics have been successful before the coronavirus disease 2019 (COVID-19) pandemic; however, owing to the strict storage requirements and pharmaceutical preparation needed for the COVID-19 vaccines, careful thought and planning were necessary to successfully deploy these clinics immediately after vaccine availability. The focus of this manuscript is to describe the development and implementation of COVID-19 vaccination clinics in a large public university, using professionals from within and outside of its health sciences schools.
Objectives
The primary objective of this project was to (1) implement COVID-19 vaccination clinics for university faculty, staff, students, and community members. Additional objectives of the clinics were to (2) actively incorporate pharmacy, nursing, and medical students into the clinic workflow; (3) promote interprofessional collaboration among faculty and students; and (4) assess patient satisfaction.
Practice description
The School of Pharmacy faculty, in conjunction with the Office of Strategic Initiatives, planned and coordinated COVID-19 vaccination clinics from December 2020 to July 2021. Students and faculty from schools of pharmacy, nursing, and medicine were used. COVID-19 vaccinations were offered to university faculty, staff, and students and community members based on the Centers for Disease Control and Prevention priority groups. The clinic processes were designed such that they could be scaled from 100 to 2,000 participants per day.
Practice innovation
The School of Pharmacy led approach was adjustable depending on the number of patients, continuously monitored and adaptable. The importance of pharmacists as part of the interprofessional health care team was exemplified by faculty and students involved.
Evaluation methods
All patients receiving COVID-19 vaccinations at the clinics were e-mailed anonymous surveys for assessment of the quality of the vaccination encounter after completion of their primary vaccine series.
Results
More than 15,000 COVID-19 vaccinations were provided through the clinics from December 2020 to July 2021. Professional staffing totaled 3352 hours for the 48 clinics. Thirty-eight percent of the vaccinated patients responded to the clinic satisfaction survey with predominately excellent ratings.
Conclusion
COVID-19 vaccination clinics can be successfully planned and implemented in a scalable fashion in a large university setting using an interprofessional team approach.
Key Points.
Background
-
•
Large-scale vaccination clinics have successfully been conducted in the past to meet the public health needs of a community; however, limited data are available in a university setting using a multidisciplinary staffing approach.
-
•
A high and immediate demand for coronavirus disease 2019 (COVID-19) vaccines was present owing to the lethality of the virus and the extremely high efficacy of the vaccines.
-
•
Few data were publicly available on the COVID-19 vaccine products before their approval to adequately prepare for large-scale vaccination clinics.
Findings
-
•
Schools of pharmacy with assistance of university administration can successfully plan and implement large-scale vaccination clinics in a university setting.
-
•
Interprofessional teams can conduct vaccination clinics with a high level of patient satisfaction.
-
•
Faculty and students were willing to volunteer an extraordinary number of hours to organize, staff, and oversee COVID-19 vaccination clinics.
Background
Since the first vaccine was discovered 225 years ago, many approaches in development and administration of vaccinations have been implemented.1 Commonly seen during influenza season, public health officials use community vaccination clinics to rapidly immunize large populations.2 , 3 They are also crucial to pandemic response efforts, as illustrated by the success of public vaccination clinics created to quell the H1N1 influenza strain in 2009.3 During the coronavirus disease 2019 (COVID-19) pandemic, one community hospital developed a pharmacist-run clinic to quickly use their first allotment of 975 COVID-19 vaccine doses for employees.4 Later in the vaccination efforts, a university implemented an interprofessional effort to administer 1582 doses to students.5 The publication of specific factors associated with successful implementation of these clinics is crucial to the overall scalability of immunization administration.
Although large-scale vaccination clinics have been successful in the past, the COVID-19 pandemic created new challenges for vaccination acceptance and administration, namely the limited time between approval and the need to disseminate in large quantities.6 Initial studies were conducted in the United States and globally to determine how factors such as cost, adverse effects, efficacy, and manufacturing location would influence patients’ vaccination intentions.7, 8, 9 The aforementioned speed of vaccine development presented a unique challenge in part owing to relatively short time intervals between product information release and vaccine availability, in addition to evolving data on vaccine pharmaceutics and administration guidance.6 In some cases, health systems and local partners had less than a week to plan and implement large-scale vaccination clinics.10 Other challenging factors included the importance of immediately identifying means to optimize flow, safety, and patient experiences; incorporation of quality improvement processes in initial plans; and assurance of patient safety in the context of a highly contagious respiratory virus.11 Several health care professions’ contributions are required to meet all of these charges illustrating the importance of sharing information on the development and deployment of impactful interprofessional teams.
Given limited previous publication of detailed logistical descriptions of vaccine clinic operations, particularly in university settings, it is important to disseminate and assess this successful approach. This manuscript adds to the rapidly evolving COVID-19 literature by describing and assessing the unique opportunities and challenges of implementation and operation of scalable COVID-19 vaccination clinics in one large university setting that incorporates interdisciplinary health professions students and providers. In addition, this work describes patient satisfaction with receipt of a COVID-19 vaccine, a topic with limited peer-reviewed publications to date.
Objectives
The primary objective is to (1) describe the implementation of COVID-19 vaccination clinics for the administration of COVID-19 vaccines to university faculty, staff, students, and community members. Additional objectives of the vaccination clinics were to (2) actively incorporate health professions students into the vaccination clinic workflow; (3) promote interprofessional collaboration among faculty, staff, and students; and (4) assess patient satisfaction.
Practice description
Clinic planning
The School of Pharmacy faculty and the West Virginia University (WVU) Office of Strategic Initiatives planned and coordinated the COVID-19 vaccination clinics from December 2020 to July 2021 for university staff, faculty, students, and community members. The Office of Strategic Initiatives comprised nonacademic staff members who work to support the university’s everyday needs, including maintaining sustainable resources, provide administrative and support programs and services, and build connections with government and business leaders. WVU has a multidisciplinary health sciences campus with schools of Dentistry, Medicine, Nursing, Pharmacy, and Public Health. Prioritization of students for vaccination within the 15 health sciences-associated experiential degree-based programs was modeled after potential exposure risk level. Students scheduled on rotations in emergency departments and intensive care units were immunized first, followed by all others with patient contact. A proportional distribution of vaccine appointments was spread across all health sciences programs for students participating in experiential rotations at higher risk sites.
Scheduling
Individual patient appointments were scheduled by inviting priority groups following guidance, as provided by the state, which was based on U.S. Centers for Disease Control and Prevention (CDC) recommendations. Initial priority was given to those employees older than 65 years of age and then progressed through the age ranges of faculty, staff, clinically active health sciences students, and, finally, the general student population. Subsequent groups also eventually included children of employees ≥ 12 years of age in addition to the surrounding community. The Moderna COVID-19 vaccine was used at most clinics based on recommendations from the state task force. Pfizer BioNTech vaccine was used toward the end of the semester to achieve full immunization of patients more quickly.
First- and second-dose clinics were conducted on separate days for the first few months to avoid confusion and minimize potential risks associated with mixing patient groups. Full clinic days were subdivided into morning and afternoon sessions to ease scheduling of both patients and volunteers. Most clinics used scheduled individual appointments through the Vaccine Administration Management System (VAMS) to verify eligibility. However, the latter clinics were conducted at a time of more widespread dose availability; thus, the team used a combined scheduled and walk-in approach to improve access. In addition, the latter clinics offered multiple vaccine products with careful attention to avoid dose preparation and administration errors.
One faculty member communicated vaccination supply requests with the West Virginia Higher Education Policy Commission coordinator who sat on the joint interagency task force for the state. Allotted vaccines (and ancillary supplies) for each clinic day were shipped from the state’s allotment to a regional hub operated by the WV National Guard where a designated School of Pharmacy faculty pharmacist collected and transported them to a monitored storage location in the WVU Medicine Student Health Services Clinic.
Volunteers and staffing
Before vaccination clinics, a pharmacy faculty member sorted and organized ancillary supplies. Another School of Pharmacy faculty pharmacist was responsible for soliciting pharmacy faculty and student pharmacists staffing volunteers using an electronic sign-up site on the Health Science Center’s online learning management system. A subsite was created for the COVID-19 vaccination clinics that included training information and volunteer sign-up lists for student pharmacist and faculty volunteers. All pharmacy faculty and student pharmacists were loaded into this site. The sign-up lists were created with a specific number of volunteer spots available based upon anticipated clinic volume. The faculty representative communicated new opportunities via e-mail with the School of Pharmacy student body and faculty who were qualified to administer vaccinations or prepare doses. Additional requests were sent if the volunteer spots were not filled a few days in advance of each clinic. Any remaining openings were filled by WVU Medicine Student Health Services nursing staff, which were scheduled outside the learning management system. When the clinic size was subsequently increased, the pharmacy faculty communicated with nursing faculty to recruit additional student nurse volunteers and faculty as needed. A School of Medicine faculty member sent volunteer requests to their student body, which included the contact information for the pharmacy faculty member volunteer coordinator. Interested medicine students e-mailed the pharmacy faculty member to sign up to assist in the postvaccine observation area as primarily first-year medical students who had not yet been trained to administer immunizations volunteered.
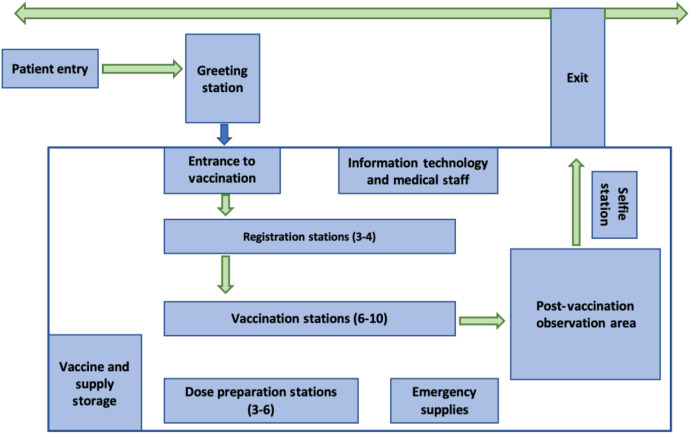
Volunteers were sent an e-mail before each clinic, which detailed their responsibilities and expectations. Pharmacy faculty vaccinators were asked to complete CDC trainings related to the COVID-19 vaccines. In addition, pharmacy faculty and student volunteers were asked to watch videos related to dose preparation, review the emergency use authorization (EUA) for all vaccines that would be used at the specific clinic, and review the clinic flow process that was developed by the School of Pharmacy faculty. All volunteers were asked to arrive 30 minutes before the start of each clinic to participate in an interdisciplinary huddle to discuss the clinic process. The appointed clinical lead pharmacist for that day would review immunization technique as needed, ensure volunteer registration in the VAMS (U.S. CDC), familiarize everyone with building and clinical logistics, flow, and address any questions or concerns. Each clinic followed a systematic flow to ensure continuity and minimize errors, which is depicted in Figure 1 .
Figure 1.
Clinic layout. Early clinics were conducted in a 19,000 square foot section of the 177,000 square foot West Virginia University Student Recreation Center building, whereas later (higher volume) clinics were in a 25,200 square foot section of the building.
Dose preparation
Vaccine doses were prepared by pharmacists and supervised student pharmacists in a distinct area of the room away from the patient flow, separated by plexiglass, yet within visual sight of the vaccination stations. Pharmacists verified appropriate preparation technique, appropriate dilution when applicable, and accuracy of doses to minimize the chance for errors. Syringes (along with other supplies) were provided by the WV National Guard along with the vaccine product. Given difficulties in supply chain, 9 different syringe types were used across the clinics, which varied by manufacturer and volume (1 or 3 mL). Syringe brand and volume affected the ability to obtain extra doses from vials with the best and most consistent being 1 mL VanishPoint (Retractable Technologies). Once vaccines were prepared, they were dispersed in plastic baskets by team members to the nearby immunization stations.
Documentation
The university’s Strategic Initiatives marketing and communication team communicated with patients via e-mail to guide them through scheduling in VAMS, which was selected as the documentation system by the state. The WVU Student Recreation Center was chosen as the main site for the vaccine clinics based on its size, ventilation, provision for unidirectional patient flow, familiarity, and access to university faculty, staff, and students, as well as reliable Internet service to allow for online, real-time documentation in VAMS.
Clinic layout
School of Pharmacy faculty and university coordinators carefully designed and planned the clinic layout and continuously re-evaluated and adjusted the site as necessary based on clinic size and patient population. Stations included greeting, check-in or registration, immunization, postvaccination observation, dose preparation, vaccine storage, and emergency supplies. Multiple signs were used to direct patient traffic and ensure unidirectional flow during the vaccination process. Information technology specialists were on-site at each clinic to troubleshoot issues with the electronic management system and patient scheduling. Additional details related to clinic layout are depicted in Figure 1.
The Strategic Initiatives marketing and communication group sent scheduled patients the EUA associated with the vaccine product to be used and also verified their eligibility requirements for vaccination before each clinic. One to 2 greeters were used at the entrance of each clinic to verify patient identification and ensure their appointment time was within 10 minutes of the check-in time. They assigned each patient to one of 4-8 check-in or registration stations (based on clinic size) where patient identification was confirmed again, checked-in VAMS, patients were offered a paper copy of the EUA fact sheet, and name and date of birth were added to vaccination cards.
Registration personnel assigned patients to a vaccination station operated by pharmacists, student pharmacists, nurses, or student nurses. In clinics where multiple COVID-19 vaccine products were available, vaccinators were assigned only one vaccine type. Vaccinators verified the patient’s name, date of birth, and address and then interviewed the patient to ensure vaccination eligibility by the use of a questionnaire checklist. Vaccinators also provided education regarding possible adverse effects, management of adverse effects, and reporting of adverse effects on V-safe and addressed patient questions and concerns. They additionally verified the vaccine product and dose to be given, prepared the patient for vaccination, and administered the vaccine. They completed the vaccination card using sticker labels preprinted with the product and lot number and then instructed patients to take a picture of the card, keep it in a safe location, and bring it back for their second dose, if applicable. The specific date for a follow-up clinic was provided to patients in their information packet.
Patients were referred to the postvaccine observation area and asked to keep track of their observation time, which was communicated by the vaccinator based on U.S. CDC guidelines and previous reactions to vaccines or vaccine components. After the observation time, patients were directed to the clinic exit. Vaccinators then documented the administered dose in the VAMS before progressing to the next patient. Pharmacist, nurse, and physician preceptors oversaw health science student volunteers to provide guidance and feedback on vaccination administration technique and were available to answer any questions from the students, employees, and patients. An interdisciplinary approach was used as preceptors assisted students within and outside of their specific discipline.
The postvaccination observation area was organized with physically distanced forward facing chairs and was staffed by a physician, medical student, nurse, pharmacist, or student pharmacists, oftentimes with an interdisciplinary mix. A movable partition was available for patients who experienced syncope or to adapt to a requested private vaccination area so as to be inclusive with social and religious convictions. When vaccinations were administered to adolescents, the postvaccination observation area was modified to have sets of chairs paired together to allow seating with a parent or guardian. Snacks and water were available for patients in the observation area on an as-needed basis. Patients were provided vaccine stickers and instructed to not use their phones in the facility except to take a photo of their vaccination card and to take a picture using a backdrop selfie wall as they exited after observation. This was located on the opposite side of the space from the entrance to ensure unidirectional flow and ensure patient privacy.
Clinic volume and extra doses
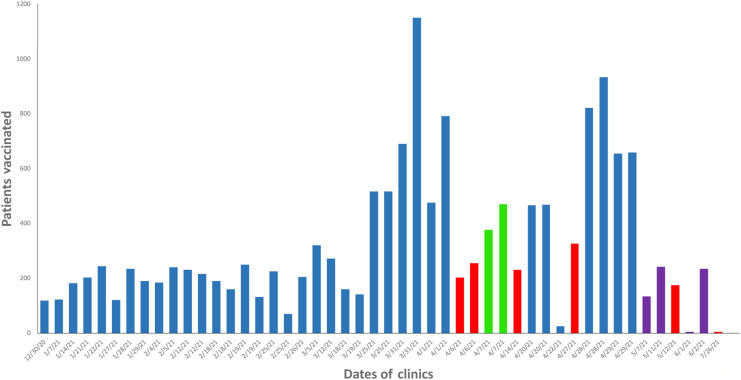
The scheduled clinic patient volumes were largely based on doses available until the later months. The initial clinic gave 118 doses starting with university employees of the oldest age, thus at the highest risk of severe COVID-19 infection. Vaccine availability increased each week, as shown in Figure 2 . By the fifth week, we were directed on a limited basis to begin vaccination of health sciences students who were on experiential clinical rotations, as previously described.
Figure 2.
Patient volume data for the university COVID-19 vaccination clinics. Bars show the number of patients vaccinated during each clinic session. The color of bars reflect the predominant COVID-19 vaccine offered in that session: blue, Moderna; red, Pfizer; green, Janssen; purple, all 3 products. The earliest clinics started with the university’s most older faculty or staff, followed by health science students, general students, and employees’ children older than 12 years, and then walk-in clinics for the general public. Abbreviation used: COVID-19, coronavirus disease 2019.
Given the limited initial stability data and low availability of the vaccine products early in the pandemic, the team prepared a list of backup patients following priority group guidance from the U.S. CDC and state who could be “on call” for extra doses to avoid waste from cancellations or from skillful preparation that allowed extra doses to be obtained from vaccine vials. In addition, clinic pharmacists were in frequent contact with other clinics in our region to both accept and transfer extra doses. All extra doses were given to eligible patients from December 2020 to the end of April 2021, after which vaccine availability became much more widespread and some limited wastage occurred in the final few clinics.
Practice innovation
The overall process for planning the university-based clinics on a scalable platform, which was adjustable from 100 to > 1000 patients per clinic session, recruiting and communicating with volunteers, prioritizing patients from thousands of employees and -students, and vaccinating patients is novel given the context of a pandemic. These processes were continuously monitored and adjusted based on clinic needs along with faculty, staff, and patient feedback. The clinic process also allowed health professions’ students and faculty to work together on interprofessional teams to prevent disease and promote the health of patients in and around WV. This experience could lead to improved interprofessional collaboration in the clinical students’ future careers. In addition, the School of Pharmacy faculty were featured on various local, state, national, and international media outlets for their vaccination efforts, which promoted the pharmacy profession and exemplified the importance of pharmacists as part of the health care team.
A reason for the success of the clinics was the team’s ability to constantly adapt. Clinic leaders and volunteers frequently implemented changes to accommodate different vaccine products, number of patients vaccinated per clinic, and patient demographic groups being vaccinated. In addition, clinic leaders and volunteers would routinely conduct postclinic brainstorming sessions to identify areas for improvement. Implementation of scalable vaccine clinics similar to those described here can be readily reproduced in other settings.
Evaluation methods
All patients receiving COVID-19 vaccinations in these clinics were sent anonymous surveys by e-mail for assessment of the quality of the vaccination encounter after completion of their primary vaccine series. The e-mail provided a hyperlink to a short survey consisting of Likert scale and open-ended questions. Areas covered included individual parts of the process (scheduling, appointment, administration, explanation, postvaccination, and overall assessment of the facility and process.) Two reminders were sent to optimize compliance with the survey.
Qualtrics XM (Qualtrics LLC) was used for all surveys. Nonpatient-specific data such as the number of volunteers, hours of individual discipline-specific volunteers served, number of vials used, lot numbers for the vaccines used, doses administered for each type of vaccine, duration of the clinic, number of syncopal reactions, and predominant population of patients served were compiled and analyzed with Microsoft Excel version 16.51 (Microsoft Corporation). Before planning larger vaccination clinics, modeling and observations were completed to determine average time spent at each station. A vast majority of the clinics used a scheduled appointment approach with the number of vaccinating volunteers based on the number appointments per hour with a goal of only a few minutes wait time before initiating the continuous process of registration, vaccination, and postvaccination observation. We used the Excel spreadsheet to track statistics from the clinics in real time. Final data were verified with VAMS numbers within 24 hours of ending the clinics.
The assessment plan conducted for this study was approved by the university’s institutional review board before its initiation.
Results
The processes described earlier enabled 15,229 vaccinations to be administered to faculty, staff, students, and community members from December 2020 to July 2021 (Figure 2). Health professional staffing (including clinical students in pharmacy, nursing, and medicine) totaled 3,352 hours for the 48 clinics with approximately 90% of those hours representing volunteered time (Table 1 ). Individual health care professionals or clinical students provided staffing at a clinic for a collective total of 842 times (sessions).
Table 1.
Discipline-specific health professionals and clinical students staffing West Virginia University COVID-19 vaccination clinics
| Clinics and hours served | School of Pharmacy faculty | School of Pharmacy students | School of Nursing students | School of Medicine students | Student Health nursesa | Other professionals |
|---|---|---|---|---|---|---|
| No. clinics attended by at least 1 health professional or clinical student | 48 | 46 | 22 | 14 | 43 | 30 |
| No. clinic sessions attended by a health professional or clinical student | 227 | 237 | 222 | 20 | 95 | 41 |
| Total no. hours served | 898 | 921 | 916 | 84 | 372 | 161 |
Abbreviation used: COVID-19, coronavirus disease 2019.
Paid employees as part of a regular work assignment.
The team’s goal was to have a short wait time at the clinic of only a few minutes to minimize contact exposures between the patients. The duration of and number of volunteer workers in clinics were scaled based on doses available (Table 2 ). The clinical staffing model was fairly consistent in the type of positions filled over the course of the clinics, as was the ratio of doses administered to health professionals + clinical students with a mean of 17; however, we underestimated the number of staff needed for the largest clinics (mean ratio 33) The later walk-in clinics tended to have the lowest ratio (i.e., overstaffing) owing to a lesser ability to accurately predict patient volumes because we started allowing for walk-ins, given improved access to the supply of vaccines.
Table 2.
Workload data for clinical staff of West Virginia University COVID-19 vaccination clinics
| Parameters | < 200 doses per clinic session | 200–500 doses per clinic session | > 500 doses per clinic session |
|---|---|---|---|
| Total no. clinics | 17 | 22 | 9 |
| Doses administered | 124 ± 62.5 | 290 ± 96.1 | 748 ± 203 |
| No. health professionals + clinical students staffing | 13.2 ± 5.5 | 18.3 ± 5.6 | 23.8 ± 4.3 |
| Doses administered/health professionals + clinical students staffing | 9.0 ± 4.6 | 16.6 ± 5.1 | 32.7 ± 11.7 |
Abbreviation used: COVID-19, coronavirus disease 2019.
Note: Data are shown as mean ± SD, except for the number of clinics.
Acute toxicities were very rare after the 15,229 doses administered, with symptomatic reactions (fainting) occurring in approximately 0.1% of patients during the 15-30-minute observation period. A vast majority of these were college students who had little or no previous food ingestion that day and experienced lightheadedness. One instance of symptoms consistent with an anaphylactic reaction was observed. After epinephrine administration in the clinic and transfer of the patient to a nearby emergency department, they fully recovered and were discharged to home.
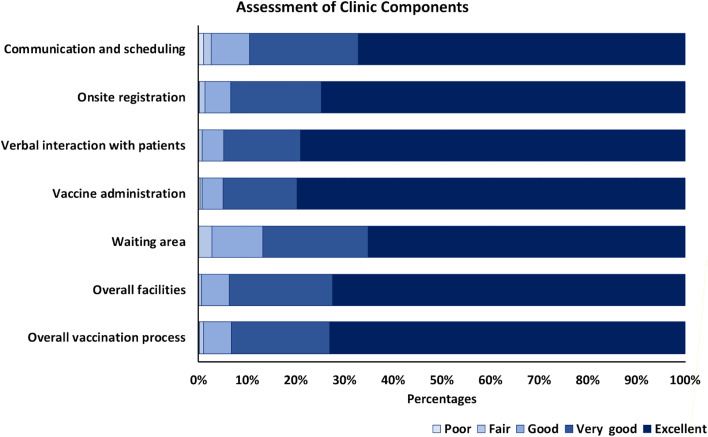
Thirty-eight percent of the 6326 vaccinated patients responded to the clinic satisfaction survey. Results demonstrated the success of the vaccination delivery program (Figure 3 ). Although all areas of assessment showed predominantly excellent ratings, the 2 questions related to the vaccine administration station received the highest scores. Open-ended questions in the survey provided responses that were very appreciative of the operations.
Figure 3.
Patient assessment of university COVID-19 vaccination clinics. All patients who registered with e-mail addresses (n = 6,326) were sent an electronic link to complete a survey-based assessment of the clinics using the categories shown in the figure. Surveys were completed by 38% of the patients. Abbreviation used: COVID-19, coronavirus disease 2019.
Practice implication
Strategies used to implement the scalable immunization clinics for university employees and students were highly successful given that all weekly available doses were efficiently delivered with a high degree of patient satisfaction. One of the important factors in the effective implementation of these clinics was communication among clinic leaders, volunteers, university staff, and students. A unique benefit to these clinics was the interprofessional approach with pharmacists, student pharmacists, nurses, nursing students, physicians, and medical students working together to perform clinic duties. In general, pharmacy roles included clinic planning, volunteer requests, dose preparation, vaccine administration, and overseeing student pharmacists and nurses. Nursing roles included vaccine administration and overseeing pharmacy and nursing students. Physician and medical student roles included observing patients in the postvaccine observation area. Physicians and medical students also worked with pharmacy and nursing faculty to address reactions to vaccines as needed. The inclusion of students in this process significantly increased the number of volunteers for each clinic. Student inclusion also provided health sciences students with the opportunity to make a difference in their community and obtain hands-on learning by applying and demonstrating what they acquired in the classroom to real life scenarios. Interactions between students and faculty were also strengthened through this opportunity as students worked one on one with faculty members. Given that many of their classes were offered via remote learning owing to the pandemic, these volunteer opportunities provided students with the chance to get to know and learn from faculty members in person.
Previously published studies have shown high levels of patient satisfaction with influenza vaccination clinics conducted by public health departments.12 , 13 Similarly, a study looked at satisfaction with student pharmacist-run influenza vaccination clinics in both community- and campus-based clinics and found that 98% of vaccine recipients were satisfied or very satisfied with their experience.14 As the pandemic continues and the need for booster doses intensifies, decision-influencing factors such as satisfaction with previous vaccination experiences are likely to be of importance and perhaps crucial to continued vaccine uptake. We hope the approaches taken here will foster continued adoption of any future recommendations for COVID-19 vaccinations.
Comments provided in the patient assessment survey suggested improvements to the clinics, including a focus on limiting the wait time for vaccination. This was facilitated by recruiting more volunteers and expanding our interdisciplinary approach. In addition, there were several comments regarding individuals passing out after their vaccines were given. Although comments suggested that the clinic did an excellent job in helping those individuals, strategies to prevent this issue could have been improved. A few recommendations included encouraging patients to eat and drink plenty of fluids before receiving their vaccine. It was also suggested we notify individuals during the appointment process of the 15-20-minute observation time after vaccine administration to help with planning and to enable student patients to get to class on time.
Many comments requested that the university use this model in the future to conduct similar vaccine clinics for efficiency. Multiple people commented on how caring vaccine administrators were in helping ease their vaccine-related anxiety.
A limitation to this study was the number of individuals who responded to the survey (38%). To improve response rates, surveys could be given during the observation period or have patients check out on a computer that provides them with a survey.
Overall, these clinics were extremely successful and demonstrate that schools of pharmacy across the country could help administer similar university-wide vaccination events.
Conclusion
Efficient COVID-19 vaccination clinics allow for mass vaccination of faculty, staff, students, and community members, and student involvement and interprofessional collaboration promote the engagement and cooperation of all members. The strategy for organization of clinics for university employees and students was highly successful such that all doses available were given each week with a high degree of patient satisfaction. Faculty and students readily volunteered an extraordinary number of hours to organize, staff, and oversee COVID-19 vaccination clinics. As described, scalable university COVID-19 vaccination clinics can be successfully planned and implemented by a School of Pharmacy. Future research should continue to assess how schools of pharmacy can assist their communities in response to public health emergencies.
Acknowledgments
The authors recognize the efforts of Joanne Watson, MSN, RN, who coordinated student nurse volunteers for the clinic. WVU Student Health nurses, particularly Nikki Thorn and Kayla Cuppett, delivered outstanding clinical care. The West Virginia National Guard, led by Major General James Hoyer, was invaluable in providing vaccine and supplies in a timely manner for each clinic. The authors are very appreciative of Steve Watkins, Tim Povenski, and Mary Jo Thompson for providing clinic logistical assistance and the guidance of Rob Alsop, Dr Jeff Coben, Dr Cynthia Persily, Dr William Ramsey, and Dr Clay Marsh. The authors also deeply appreciate all of the volunteers who were so generous with their time in support of these clinics.
Biographies
Gretchen K. Garofoli, PharmD, BCACP, CTTS, Clinical Associate Professor, Department of Clinical Pharmacy, School of Pharmacy, West Virginia University, Morgantown, WV; and Managing Network Facilitator, Community Pharmacy Enhanced Services Network, Morgantown, WV
Marina Galvez-Peralta, PharmD, PhD, FCP, Teaching Associate Professor; and Director of the Division of Education, Department of Pharmaceutical Sciences, School of Pharmacy, West Virginia University, Morgantown, WV
Ashleigh L. Barrickman, PharmD, BCACP, CTTS, Clinical Assistant Professor; and Director of Skills Development, Department of Clinical Pharmacy, School of Pharmacy, West Virginia University, Morgantown, WV
Angela L. Goodhart, PharmD, BCACP, CTTS, Assistant Professor, Department of Family Medicine, School of Pharmacy, West Virginia University, Morgantown, WV; and West Virginia University Office of the Vice President for Strategic Initiatives, Morgantown, WV
Heather Johnson, PharmD, BCACP, CTTS, Assistant Professor, Department of Family Medicine, School of Medicine, West Virginia University, Morgantown, WV; and West Virginia University Office of the Vice President for Strategic Initiatives, Morgantown, WV
Ashlee N. McMillan, PharmD, BCACP, Clinical Assistant Professor, Department of Clinical Pharmacy, School of Pharmacy, West Virginia University, Morgantown, WV
Betsy M. Elswick, PharmD, FAPhA, Clinical Associate Professor, Department of Clinical Pharmacy, School of Pharmacy, West Virginia University, Morgantown, WV; and Director, PGY-1 Community-Based Pharmacy Residency, School of Pharmacy, West Virginia University, Morgantown, WV
Erin S. Newmeyer,BS, Assistant Vice President of Strategic Initiatives, West Virginia University, Morgantown, WV
Carmen N. Burrell, DO, Associate Professor, Department of Family Medicine and Department of Emergency Medicine, School of Medicine, West Virginia University, Morgantown, WV; Division Chief of Ambulatory Operations, Department of Emergency Medicine, School of Medicine, West Virginia University, Morgantown, WV; Medical Director, West Virginia University Medicine Urgent Care, Student Health Services, and International Travel Clinic, Department of Emergency Medicine, School of Medicine, West Virginia University, Morgantown, WV
Krista D. Capehart, PharmD, BCACP, FAPhA, Clinical Associate Professor, Department of Clinical Pharmacy, School of Pharmacy (Charleston), Charleston, WV; and Director, Wigner Institute for Advanced Pharmacy Practice, Education, and Research, Department of Clinical Pharmacy, School of Pharmacy, West Virginia University, Morgantown, WV
William P. Petros, PharmD, FCCP, Professor, Department of Pharmaceutical Sciences, School of Pharmacy, West Virginia University, Morgantown, WV; and Dean, Gates Wigner Endowed Chair, School of Pharmacy, West Virginia University, Morgantown, WV
Footnotes
Disclosure: The authors declare no relevant conflicts of interest or financial relationships.
Previous presentations: Part of this work was presented as a poster at the 2021 American Association of Colleges of Pharmacy Annual Meeting virtual poster session (July 19-21, 2021) and the 2021 Appalachian Translational Research Network Virtual Health Summit poster session (October 19-20, 2021).
References
- 1.Immunize.org. Vaccine timeline. https://www.immunize.org/timeline/ Available at:
- 2.Ha C., Taylor C., Modi J.R. Mass vaccinations at the United States Naval Academy. Health Secur. 2016;14(6):382–388. doi: 10.1089/hs.2016.0030. [DOI] [PubMed] [Google Scholar]
- 3.Klaiman T., PhD M.P.H., Oʼconnell K., Stoto M. Local health department public vaccination clinic success during 2009 pH1N1. J Public Health Manag Pract. 2013;19(4):E20–E26. doi: 10.1097/PHH.0b013e318269e434. [DOI] [PubMed] [Google Scholar]
- 4.Andrade J., Slaby M., DeAngelis J., et al. Implementation of a pharmacist-led COVID-19 vaccination clinic at a community teaching hospital. Am J Health Syst Pharm. 2021;78(12):1038–1042. doi: 10.1093/ajhp/zxab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyce S., Ruff J., Galloway A., Shannon S. Implementation of a COVID-19 mass vaccination clinic to college students in Montana. Am J Public Health. 2021;111(10):1776–1779. doi: 10.2105/AJPH.2021.306435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wouters O.J., Shadlen K.C., Salcher-Konrad M., et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motta M. Can a COVID-19 vaccine live up to Americans' expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc Sci Med. 2021;272:113642. doi: 10.1016/j.socscimed.2020.113642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borriello A., Master D., Pellegrini A., Rose J.M. Preferences for a COVID-19 vaccine in Australia. Vaccine. 2021;39(3):473–479. doi: 10.1016/j.vaccine.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong D., Xu R.H., Wong E.L., et al. Public preference for COVID-19 vaccines in China: a discrete choice experiment. Health Expect. 2020;23(6):1543–1578. doi: 10.1111/hex.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longhurst C.A., Kremer B., Maysent P.S. Rapid implementation of a vaccination superstation. JAMA. 2021;325(10):931–932. doi: 10.1001/jama.2021.0801. [DOI] [PubMed] [Google Scholar]
- 11.Smith I.M., Bayliss E., Salisbury H., Wheeler A. Operations management on the front line of COVID-19 vaccination: building capability at scale via technology-enhanced learning. BMJ Open Qual. 2021;10 doi: 10.1136/bmjoq-2021-001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mobasher Z., Smith L.V., Stegall A., et al. Community-based flu outreach clinics in south Los Angeles: client satisfaction and experiences. Public Health Nurs. 2017;34(3):276–285. doi: 10.1111/phn.12313. [DOI] [PubMed] [Google Scholar]
- 13.Balbi D.A., Calcagni K. Effects of the design and practice of points-of-dispensing on patient-reported satisfaction: municipal H1N1 clinics in Rhode Island. Disaster Med Public Health Prep. 2011;5(2):106–111. doi: 10.1001/dmp.2011.37. [DOI] [PubMed] [Google Scholar]
- 14.Dang C.J., Dudley J.E., Truong H.A., Boyle C.J., Layson-Wolf C. Planning and implementation of a student-led immunization clinic. Am J Pharm Educ. 2012;76(5):78. doi: 10.5688/ajpe76578. [DOI] [PMC free article] [PubMed] [Google Scholar]