Abstract
Background
Salmonella is one of the foodborne pathogens affecting public health around the globe. A cross-sectional bacteriological study was conducted from December 2019 to November 2020. This study aimed to isolate, molecularly detect and determine antibiotic susceptibility patterns of Salmonella from raw cows’ milk collected from dairy farms and households in Hawassa, Arsi Negele, and Dale districts.
Materials and methods
A total of 384 raw milk samples were collected using a simple random sampling technique. Standard bacteriological and biochemical tests were used to isolate Salmonella. The positive samples were further confirmed by the molecular test. Kirby-Bauer disk diffusion method was used for antimicrobial susceptibility testing of Salmonella.
Results
Using bacteriological and biochemical detection tests, Salmonella was isolated from 10.42% (N = 40) of the total sample. However, in molecular detection, only 32 of the 40 isolates were confirmed to be Salmonella using PCR test. The prevalence was 8.54, 12.69, and 10.46% in Hawassa, Dale, and Arsi Negele districts, respectively. Bacteriological prevalence did not vary significantly between the districts (P > 0.05). Likewise, no significant (P > 0.05) variation was observed in the Salmonella isolation rate between households (12.5%) and farms (8.33%) as well as between dry (8.85%) and wet (11.98%) seasons. Based on herd size, the isolation rate of Salmonella was significantly higher (P < 0.05) in large-scale farms (19.51%) than in small (5.1%) or medium (5.6%) scale dairy farms. The result of the antibiotic susceptibility test showed that Salmonella isolates were 100% resistant to ampicillin, while they were 100% sensitive to ciprofloxacin. Multi-drug resistance (MDR) was demonstrated in all isolates.
Conclusion
This study showed that Salmonella is widespread in the raw milk samples and developing MDR, which may be of public health concern in the study area. It is therefore important that dairy farmers and raw milk sellers in the study area take serious measures to avoid contamination of the milk with Salmonella spp. In addition, the active commitment of the animal health departments in the respective districts to sensitizing dairy farmers and the sensible use of antibiotics at the farm level can help to reduce the antibiotic resistance of Salmonella spp.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-022-02504-2.
Keywords: Antimicrobial susceptibility, Culture, Ethiopia, InvA gene and Salmonella
Background
Foodborne diseases are major public health problems in both developed and developing countries. More than 250 different foodborne diseases have been described. Most of these foodborne diseases are infectious diseases caused by a variety of bacteria [1]. Foodborne bacterial diseases are a critical problem to the public health [2]. Bacteria are commonly found in soil, water, plant, animals and animal products including milk, meat, cheese and yoghurt [1].
Cow milk has high water activity and nutritive value which serves as a kind medium for growth of microorganisms [3]. Microbes commonly isolated and detected from milk and milk products pose a critical problem to human health. Bacteria which are commonly isolated from milk includes Escherichia coli, Staphylococcus aureus, Salmonella spp. and Listeria monocytogens [4]. Among these pathogens Salmonella attributes, the major part of foodborne diseases [1].
Salmonella species belong to Gram negative, rod shaped, facultative intracellular bacteria that potentially infect a wide variety of hosts. Salmonella is comprised of two species, Salmonella bongori and Salmonella enterica [5]. Depending on the bacterial outer membrane somatic ‘O’ antigen, and flagellar ‘H’ antigen over 2700 different serovars of Salmonella has been characterized [6]. Out of these 2700 serovars, nearly 1500 belong to the Salmonella enterica subsp. Enterica [7]. Salmonella enterica subsp. enterica are the most common pathogenic and zoonotic bacteria causing different form of salmonellosis in human and animals [2].
Milk provided for human beings should not contain any pathogenic microorganisms [8]. However, raw milk and its products are considered as important sources of Salmonella. Milk and dairy products especially those produced from raw or unpasteurized milk have been attributed as potential vehicles for the transmission of Salmonella to humans [9, 10]. The contamination of raw milk by pathogenic microorganisms including Salmonella comes from feces of infected cattle, contaminated skin, infected udder, contaminated milking equipment, air, feed and water, and from milkers [11–13]. Salmonella is transmitted to human either through the fecal-oral route or through consumption of contaminated food (milk, eggs, and meats) and cause either typhoidal or nontyphoidal salmonellosis. In addition, milk is a potential source of multiple drug resistance, and is a potential public health concern. Antimicrobial resistance is one of the biggest global public health challenges [14].
Over the years, a number of studies have been carried out in Ethiopia that examine the occurrence of Salmonella in milk and feces of humans and cattle, as well as the development of patterns of resistance to various antibiotics in human and veterinary medicine [15–18]. However, the studies available are sparse given the serious public health threat posed by the organisms. In addition, data are not available for the city of Hawassa and its surrounding areas, which is one of the potential areas of the country for milk production and consumption associated with increasing urbanization. Further studies on Salmonella prevalence in milk and its antibiotic resistance profile are also believed to complement the baseline data in Ethiopia and worldwide. The aim of the present study was therefore to isolate and identify Salmonella from raw cow milk using cultural, biochemical and molecular methods and to determine the antimicrobial susceptibility pattern of the organism.
Materials and methods
Study area
For this study, Hawassa city and its neighboring districts (Dale and Arsi Negele) which supply milk for the big market at Hawassa were purposively selected due to their relatively larger potential for dairy cattle population and milk production. Hawassa is the capital city of the Sidama Region State, located at 275 km south of Addis Ababa. Geographically it lies between 703′1.35″ N latitude and 38029′43.81″ E longitudes at an altitude of 1750 m above sea level. The annual rainfall and temperature vary from 800 to 1000 mm and 20.1–25 °C, respectively. Dale district is one of the potential milks producing districts in Sidama Regional State. The elevation of this district ranges from 1200 m above sea level along the shores of Lake Abaya to 3200 m at its westernmost point. Arsi Negele is a town in southeastern Ethiopia, located in the West Arsi Zone of the Oromia Region northern to Shashamane. This town is situated at a longitude of 38°42′E and latitude of 7°21′N and has an elevation of 2043 m above sea level. Arsi Negele town is the administrative center of Arsi Negele woreda [19].
Source of milk samples
Raw milk samples for the present study were collected from households (households engaged in smallholder dairy farming primarily for household consumption) and dairy farms (specialized commercial dairy farms) found in Hawassa city, Dale and Arsi Negele Districts. For collection of milk from producers, appropriate number of dairy farms and households having one and more lactating cows were selected from the list of dairy farms and households in the study areas. The dairy farms and households were selected by using simple random sampling technique based on the data obtained from the district’s Livestock and Fishery Resource Development Offices. Prior to sample collection, a cooperation letter was sent to each district livestock and fishery resource development office. As a response, an animal health technician was assigned who helped during collection of milk samples from the dairy farms and households selected for the study.
Study design and sampling method
General information about the total number of households, farms, farm size, and potential milk producing Kebeles was obtained from Livestock and Fishery Offices in the study area. Accordingly, in Hawassa city, three sub cities namely, (Hawella Tula, Tabor and Addis Ketema) were found to be the major milk producing sub cities. Likewise, Melka Sheki, Melka Giltota and Mali Weyo in Arsi Negele and Berra Tedicho, Wuhalimat, Mesencho, Manche and Shafina in Dale district were the other Kebeles identified for their relatively higher dairy potential. Therefore, these productive Kebeles were selected purposively for this study. Simple random sampling technique was employed to select farms and households in each Kebele except for large-scale farms where all such farms were included in the study. Similarly, milk containers were selected by simple random sampling to take appropriate raw milk samples. The whole study was carried out from December 2019 to November 2020. However, milk samples were collected during the dry (December, January, and February) and wet (July, August, September) seasons. The season in our study area is divided into two main seasons, wet and dry seasons, based on the average rainfall and average temperature. Here the dry season is a season of the year characterized by high temperature (28–29 °C) and low rainfall (24–44 mm), while the wet season is characterized by low average temperatures (24–25 °C) and high rainfall (128–140 mm). Accordingly, December, January and February are considered the dry season and July, August, September as the wet season [20]. For the purpose of this study and ease of data analysis, the farms were categorized as small scale (1–5 cows), medium scale (6–10 cows) and large scale (> 10 cows) [21].
Sample size determination
The sample size required for this study was determined by considering a 50% expected prevalence of Salmonella in the study, 5% desired absolute precision and 95% confidence level using the formula given in [22] as follows.
Where: n = required sample size; Pexp = expected prevalence; d = desired absolute precision,
z = statistic for a level of confidence =1.96.
The expected prevalence of Salmonella was considered 50% due to lack of similar previous study in the study area. Thus, the maximum number of raw milk samples needed to determine the prevalence of Salmonella was calculated to be 384 milk samples. Using proportional sampling method 164, 134 and 86 samples were allocated to Hawassa, Arsi Negele and Dale districts, respectively. Equal number of milk samples (n = 192) were collected from both households and farms. Likewise, equal number of milk samples (n = 192) were collected during dry and wet seasons.
Milk sample collection and transportation
Utmost efforts were made to prevent contamination and cross contamination of milk in the course of sample collection. Samples were collected early in the morning around 7:00 to 8:00 AM by arranging time in communication with the milkers’ and owners of the farms. Nearly 10 ml of raw milk samples were collected into sterile screw capped bottles. The milk samples were then held in an icebox with ice packs and transported to Molecular Biotechnology Laboratory of School of Animal and Range Science, Hawassa University. All samples were clearly labeled with date of sampling, type of sample and with the name of household or farm. In the laboratory, raw milk samples were cultured immediately or stored at 4 °C for a maximum of 24 h until they were transferred into enrichment medium and inoculated onto a standard bacteriological media [23].
Bacteriological isolation of Salmonella
Bacteriological examination was done according to microbiology of food chain; horizontal method for detection, enumeration and serotyping of Salmonella [23]. Accordingly, it is standard to use three stage processes: pre-enrichment, selective enrichment and selective plating to isolate Salmonella. In primary enrichment step; in order to get better recovery of Salmonella one ml of milk sample was measured aseptically, homogenized into 9 ml of buffered peptone water (HIMEDIA BM020, India) and incubated at 37 °C for 24 h. Likewise, in secondary enrichment step Rappaport-Vassiliadis with soya (RVS) was adjusted to room temperature according to the manufacturer’s directions. The mix incubated in the primary enrichment sample was well massaged by hand for at least 10 s. Then 0.1 ml aliquot was transferred and added it to 10 ml of Rappaport-Vassiliadis with soya (RVS). Finally, the tubes were vortexed and incubated at 41.5° for 24 h. Lastly, the enriched milk sample were plated onto a Selective Agar. Xylose lysine deoxycholate (XLD) agar (HIMEDIA M031, India) was used as selective medium for isolation of Salmonella and it was adjusted to room temperature according to the manufacturer. The secondary enrichment tubes were vortexed before plating on XLD agar. After adjusting XLD the samples were streaked from secondary enrichment tubes, using 10 μl loop and incubated at 35 °C for 24 h. After the recommended incubation time the selective-differential agar plates were examined for the presence of colonies meeting the description for suspect of Salmonella colonies. Typical Salmonella spp. colonies are pink colonies with or without black centers on XLD agar. Three to five typical colonies of Salmonella were picked and streaked onto Trypton soya agar and incubated at 37 °C for 18–24 h for the further biochemical identification.
Biochemical identification
The biochemical identification was done according to [23, 24] by using indole, Methyl red, Vogas-Proskaur, urease, citrate utilization, triple sugar iron (TSI), lysine decarboxylase and hydrogen sulphide production tests.
Antimicrobial susceptibility test
The antibiotic susceptibility tests of the Salmonella isolates were performed using Kibry-Bauer disk diffusion test on Muller Hinton agar (HIMEDIA M173, India). Pure colonies from trypton soya agar were taken with a wire loop, transferred to a tube containing 5 ml of saline water, and emulsified. The emulsified broth culture was incubated at 37 °C as far as it reached the 0.5 McFarland turbidity standard. Sterile cotton head swab was soaked into the emulsified broth and the bacteria were swabbed evenly over the surface of Muller Hinton agar plate within a sterile safety cabinet hood. The plates were put at room temperature for 15 min to allow drying. Antibiotic discs with known concentration of antimicrobials were carefully placed on the plates and the plates were incubated for 24 h at 37 °C. Each isolate of Salmonella was tested for a series of nine common antimicrobials. Chloroamphenicol (C) (25 μg), ampicillin (AP) (25 μg), cefotaxime (CTX) (5 μg), gentamycin (CN) (10 μg), streptomycin (S) (10 μg), kanamycin (K) (30 μg), nalidixic acid (NA) (30 μg), ciprofloxacin (CIP) (5 μg) and trimethoprim-sulphamethaxazole (TS) (25 μg), all from Oxoid company, United Kingdom. Following incubation, the diameters of clear zones produced by antimicrobial inhibition of bacterial growth were measured to the nearest millimeter for each disc using transparent straight-line ruler and then classified as resistant, intermediate, or susceptible according to published interpretive chart of clinical laboratory standard institute [25]. The MDR index was determined for each of the isolates examined using the formula: MDR index = x/y; Where “x” is the number of antibiotics to which the strain display resistance, and “y” is the total number of antibiotics to which the test strain had been evaluated for sensitivity [26].
Molecular detection of Salmonella
DNA extraction: Genomic DNA extraction from Salmonella isolates were done by boiling and chilling as described by [27]. The DNA quality and concentration were detected using UV spectrophotometer (JENWAY Spectrophotometer, 6705).
PCR amplification: The primarily identified Salmonella was confirmed by PCR targeting invA gene of Salmonella at genus level [28, 29]. The primer used for the amplification of the highly conserved region of invA gene was Salm3 (forward): 5’GCTGCGCGCGAACGGCGAAG 3′ and Salm4 (reverse): 5’TCCCGGCAGAGTTCCCATT 3′ that produce an amplicon with expected length of 389 bp [28, 30]. A uniplex PCR condition was done to detect invA gene from Salmonella isolates as determined by [31] using a thermocycler (VWR UNO, 732–2549). Amplification was carried out in a total volume of 25 μl containing 0.7 μl of each primer (10 uM), 0.5 μl of dNTP mix (200 μM) with 1.5 mM MgCl2, 0.25 μl Taq DNA polymerase (0.05 U), 2.5 μl PCR buffer (1X), 5 μl template DNA and 20.25 ul of nuclease free water. A positive and negative control containing the template DNA from Salmonella Typhimurium ATCC 13311 (brought from Ethiopian Biodiversity Institute) and nuclease free water, respectively, was included in every experiment. The reaction condition was optimized with initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 65 °C for 1 min and extension at 72 °C for 1 min. Finally, an additional extension was achieved for 7 min at 72 °C and stored at 4 °C infinitely.
Electrophoresis of PCR products: The PCR product (amplicons) was electrophoresed on a 1.5% agarose gel. After loading the amplicons and markers in each well, an electric current of 150 mA and 100 V was applied for 40 min. Electrophoresis results were observed under gel documentation system (GelDoc-It2 310 Imager, USA). The expected positive result was indicated by the presence of 389 bp band on the gel; 100 bp DNA ladder was used as a marker. The confirmed Salmonella isolate from each positive sample were stored at − 80 °C in 20% glycerol for further testing.
Data management and analysis
The data generated from this study were entered and managed in Microsoft Excel Office 2016. All the data analysis was done using Statistical Package for Social Sciences (SPSS) software version 26. Descriptive statistics such as percentages and frequency distribution were used to describe the nature and the characteristics of data. The association of the Salmonella isolates with the source of milk samples (milk from dairy farms versus milk from households), season of sample collection, milk sample districts and farm size were analysed using Chi-square (χ2) test. In all the analyses, P - value less than 0.05 (P < 0.05) was considered as statistically significant.
Results
Overall isolation rate of Salmonella
A total of 384 raw milk samples were collected and bacteriologically examined to determine the isolation rate of Salmonella. Out of the total 384 raw milk samples examined, 164 (42.7%), 134 (34.9%) and 86 (22.4%) were collected from Hawassa, Dale and Arsi Negele, respectively. From the total of 384 raw milk samples examined, 40 (10.42%) were found to be positive for Salmonella by biochemical tests. Out of the 40 isolates, 14 (35.0%), 17(42.5%) and 9 (22.5%) were from raw milk samples collected from Hawassa, Dale and Arsi Negele districts, respectively. The isolation rate of Salmonella was relatively higher in raw milk samples collected from Dale district (12.69%) than from Arsi Negele (10.54%) and Hawassa (8.54%); however, the difference was not statistically significant (P = 0.51. Table 1).
Table 1.
Overall isolation rate of Salmonella and its association with the district of raw milk samples
| Milk sample district | Total milk sample examined | Number of positives | Isolation Rate (%) | χ2- Value | P-value |
|---|---|---|---|---|---|
| Hawassa | 164 | 14 | 8.54 | Ref. | |
| Dale | 134 | 17 | 12.69 | 1.56 | 0.23 |
| Arsi Negele | 86 | 9 | 10.46 | 1.25 | 0.51 |
| Total | 384 | 40 | 10.42 |
Ref. Reference, χ2 Chi-square.
Association of Salmonella isolation rate with the origin of milk samples
Equal number of milk samples were collected from households (n = 192) and dairy farms (n = 192). Overall, the isolation rate of Salmonella was higher in raw milk samples collected from households (12.5%) than dairy farms (8.33%); however, the difference was not statistically significant (P = 0. 24) (Table 2).
Table 2.
The association of Salmonella isolation rate with the source of raw milk samples
| Source of samples | No examined | No positive | Prevalence (%) | χ2 -Value | P -value |
|---|---|---|---|---|---|
| Households | 192 | 24 | 12.5 | 1.79 | 0.24 |
| Dairy farms | 192 | 16 | 8.33 | ||
| Total | 384 | 40 | 10.42 |
Isolation rate of Salmonella in dry and wet seasons
It was observed that out of the total 192 raw milk samples collected during the dry season, 17 (8.85%) were found to be positives for Salmonella. Out of the positive samples, 6, 6 and 5 were from Hawassa, Dale and Arsi Negele districts, respectively. On the other hand, from the 192 raw milk samples collected during the wet season, 23 (11.98%) were found to be positive for Salmonella. Out of the 23 Salmonella isolates detected during the wet season 8, 11 and 4 were from Hawassa, Dale and Arsi Negele districts respectively. Though the overall isolation rate of Salmonella was higher during the wet season than the dry season, the difference was not statistically significant (P = 0.4) (Table 3).
Table 3.
Association of the isolation rate of Salmonella with season of milk sample collection
| Milk sampling season | No milk samples | No positive | Prevalence (%) | χ2 | P Value |
|---|---|---|---|---|---|
| Dry | 192 | 17 | 8.85 | ||
| Wet | 192 | 23 | 11.98 | 1.0 | 0.4 |
| Total | 384 | 40 | 10.42 |
Isolation rate of Salmonella in relation to farm category
In this study, the isolation rate of Salmonella was determined in relation to farm categories. Based on herd size,79 small dairy farms, 72 medium scale dairy farms and 41 large-scale dairy farms were included in this study. The isolation rate of Salmonella in small, medium, and large-scale dairy farms was 5.06, 5.56 and 19.51%, respectively. The result showed that the isolation rate of Salmonella was significantly higher in large scale farms than that in small or medium scale dairy farms (P = 0.01) (Table 4).
Table 4.
Isolation rate of Salmonella in relation to herd size of the farms
| Herd size | No Examined | No positives | Prevalence (%) | χ2 -Value | P -value |
|---|---|---|---|---|---|
| Small | 79 | 4 | 5.06 | Ref. | |
| Medium | 72 | 4 | 5.56 | 1.10 | 0.8 |
| Large | 41 | 8 | 19.51 | 4.45 | 0.01 |
| Total | 192 | 16 | 8.33 |
Ref. Reference, χ2 Chi-square.
Antimicrobial susceptibility test result
The antibiotic susceptibility tests of the Salmonella isolates were performed according to the Clinical and Laboratory Standards Institute guidelines by using Kibry-Bauer disk diffusion test on Muller Hinton agar. All the 40 Salmonella isolates were tested against nine commonly used antimicrobials. All the isolates were found resistant at least to one or more antimicrobials tested. The antibiotic susceptibility profiles of the isolates showed that the isolates were 100, 92.5 and 72.5% resistant to ampicillin, streptomycin and cefotaxime, respectively. On the other hand, all the isolates were 100 and 77.5% susceptible to ciprofloxacin and Trimethoprim-Sulphamethaxazole, respectively (Table 5).
Table 5.
Antimicrobial susceptibility profile of Salmonella isolated from milk samples
| Antibiotic tested | Status of antimicrobial agent against the isolates | ||
|---|---|---|---|
| Resistant (%) | Intermediate (%) | Susceptible (%) | |
| Ampicillin | 40(100) | - | - |
| Cefotaxime | 31(77.5) | 3(7.5) | 6(15.0) |
| Chloroamphenicol | 10(25.0) | 17(42.5) | 13(32.5) |
| Ciprofloxacin | - | - | 40(100) |
| Gentamycin | 17(42.5) | 13(32.5) | 10(25.0) |
| Kanamycin | 26(65.0) | 11(27.5) | 3(7.5) |
| Nalidixic Acid | - | 23(57.5) | 17(42.5) |
| Streptomycin | 37(92.5) | 3(7.5) | - |
| Trimethoprim & Sulphamethaxazole | 9(22.5) | 0(0.0) | 31(77.5) |
Multiple antimicrobial resistances (resistance to two or more antimicrobials) were detected in 100% (40/40) of the isolates. Five different antimicrobial resistance patterns were observed (Table 6). From the total 40 isolates tested for antimicrobial sensitivity 3, 9, 8, 15 and 5 showed resistance to two, three, four, five and six antimicrobials, respectively. The highest multiple antibiotic resistance was seen in the pattern (AMP, CN, CTX, K, S) where nine isolates showed resistance to them. Furthermore, the MDR indexes of the isolates obtained from this study ranged from 0.22 to 0.66. MDR index values greater than 0.2 indicates unwise use of antibiotics.
Table 6.
Multiple antimicrobial susceptibility profile of isolated Salmonella
| Number of Antimicrobial Resistance | Antimicrobial Resistance Pattern (number of isolates) | Number of Isolates (%) |
|---|---|---|
| Two |
AMP, CTX(2) AMP, S(1) |
3(7.5) |
| Three |
AMP, CTX, S(7) AMP, C,S(1) AMP, CTX, C(1) |
9(22.5) |
| Four |
AMP, CN, K,S(4) AMP, CTX, C, S(2) AMP, TS, K, S(2) |
8(20.0) |
| Five |
AMP,CTX,K,C,S(1) AMP,CN,CTX,K,S(9) AMP, TS, K, C, S(1) AMP, CTX, TS,K,S(4) |
15(37.5) |
| Six |
AMP,CN,CTX,K,C,S(3) AMP,CN,CTX,TS,K,S(1) AMP, CTX, TS,K, C, S(1) |
5(12.5) |
Key to Abbreviations: AMP Ampicillin, C Chloramphenicol, CIP Ciprofloxacin, CN Gentamycin, CTX Cefotaxime, NA Nalidixic Acid, K Kanamycin, S Streptomycin, TS Trimethoprim-Sulphamethoxazole.
Polymerase chain reaction (PCR) confirmation test result
Using the primers Salm3 and Salm4, a 389 sized PCR amplicon was obtained from DNA of Salmonella isolates (Fig. 1). PCR yielded a 389 bp fragment when DNA from Salmonella isolates was used as a template, whereas no specific products were obtained when other microbial DNA was used as a template. The invA gene was amplified from 32 out of 40 Salmonella isolates as shown in Fig. 1 below. Gels are cropped and put together from different tests which is included in supplementary information.
Fig. 1.
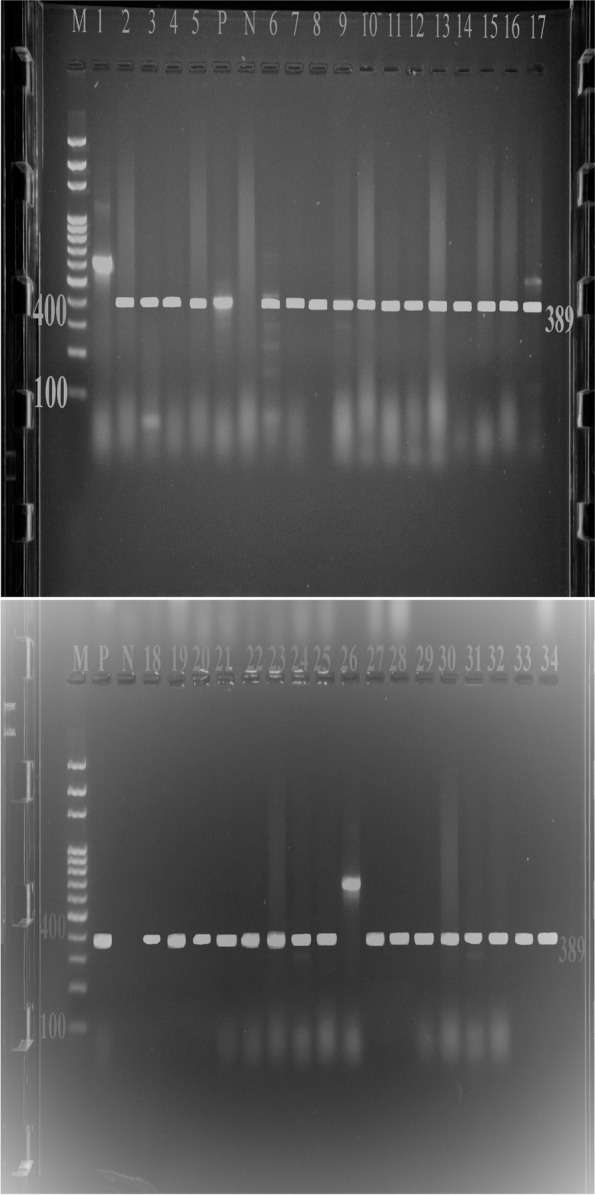
PCR amplification of biochemically identified Salmonella
As shown in Fig. 1 above, lane 1 and lane 26 brought unexpected amplification with fragment length of 600 bp. However, all the other Salmonella isolates (Lane 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 27, 28, 29, 30, 31, 32, 33 & 34) and the positive control brought the expected amplicon length of 389. Therefore, 32 Salmonella isolates were confirmed by polymerase chain reaction.
Discussions
Foods from animal origin are taken into account to be the major sources of foodborne salmonellosis. Therefore, routine detection of Salmonella in foods is an important part of public health programs. This cross-sectional bacteriological study was conducted to isolate and identify Salmonella from raw cows’ milk collected from selected dairy farms and households found in Hawassa city, Arsi Negele and Dale districts. The result of the current study shows that the overall isolation rate of Salmonella based on culture and biochemical tests was 10.42%, which is comparable to the findings of [17] who reported 10.5% in Modjo town. However, the prevalence of Salmonella in this study is relatively higher than the report of [16, 32, 33] who reported 6.0, 2.1 and 0.7% prevalence in Addis Ababa and Sebeta, respectively. On the other hand, reports from Iran (17.0%) by Hossein et al. (2013) and from Egypt (29.0%) by Omar et al. (2018) are much higher than the current investigation.
The difference in the relative occurrence of Salmonella in milk between the present and previous studies at different study areas in Ethiopia could be due to difference in the potential risk factors that contribute to the occurrence of Salmonella. For instance, milking procedure, milk handling practices, feeding strategies, hygienic and management practice, stocking density, usage of contaminated utensils, housing type, movement of animals, milking environment, and production facilities in different areas are the major risk factors that play a major role for Salmonella occurrence [18, 34]. Furthermore, the difference in the relative isolation rate of Salmonella may be due to the difference in the milk sample collected (since in the present study milk samples were taken from bulk milk whereas in the previous studies milk samples were taken from lactating cow), methods used, management and milk handling strategies in the study areas.
The study reveals that the milk sample sources is not significantly associated with the isolation rate of Salmonella. Salmonella is isolated regardless of milk sample source. The observed absence of variation might be due to similarity in hygienic and management practices, housing conditions, milking practices, feeding habits of the two farming systems. In commercial modern dairy farms, the hygienic and management practices are supposed to be better than the management practices in traditional farming system; however, in this study the isolation rate of Salmonella do not vary.
In this study, the isolation rate of Salmonella in raw milk samples were compared among milk sample districts (Hawassa, Arsi Negele and Dale). As a result, Salmonella is isolated irrespective of districts of milk sample collected without significant statistical association with Salmonella isolation. Here, the isolation rate of Salmonella from Arsi Negele, Dale and Hawassa is comparable. The possible explanation for the absence of variation among districts is supposed to be due to their comparable hygiene and management practices, housing conditions, milking and milk handling practices.
The association between the occurrence of Salmonella in milk and season of sample collection (dry and wet) were also determined in this study. Thus, the isolation rate of Salmonella is comparable in the dry and wet seasons with insignificant statistical difference. The ability of the bacteria to grow in a high range of temperature between 5 °C to 45 °C could be the possible reason why the prevalence does not vary between the two seasons.. Presumably, poor hygienic conditions on dairy farms and households have created an environment conducive for the organism to reproduce and persist. This, coupled with the ability of the bacteria to survive in wide temperature ranges, has led to contamination of the cows environment on the farms and thus of the milk regardless of the season. In contrast to the present findings, literatures show that contamination of bedding, feed and water containers, and gates and pens of the farm by the cattle dung is higher during the wet season [35].
In this study, we also further attempted to compare the isolation rate of Salmonella among farms with different herd size (small, medium and large). The isolation rate was comparable between small and medium sized farms. However, the isolation rate was significantly higher in large farms than in small or medium sized farms. This difference could be attributed to the difference in the bulkiness of milk, milk handling and management practices. Since samples were taken from bulk milk, the probability of cross contamination is considered to be high in large scale farms.
The increased score of antimicrobial resistance observed in Salmonella has become a public health concern. Antimicrobial utilization in animal production systems has long been suspected to be a cause of the emergence and spread of antimicrobial resistant Salmonella. Unwise use of antimicrobials in both human and veterinary medicine has contributed to development and dissemination of antimicrobial resistant pathogens [16, 36]. In this study, Salmonella isolates show high resistant to ampicillin, streptomycin and cefotaxime. The pattern of resistance to ampicillin observed in the current study is comparable to the reports of [16, 37]. However, the present resistance pattern to ampicillin is much higher than the findings of [17] who reported 39.5% in Modjo and that of [26] who reported 15.62% in India. This difference could be due to the differences in the habit of antimicrobials usage in animal production system in the study areas. Overall, Salmonella shows resistant to ampicillin and this is supposed to be due to long and extensive use in human and veterinary treatments over several years. In addition, the observed resistance of Salmonella to ampicillin may attribute to due to the acquired ability of the strains to produce β-lactamase enzymes that are able to degrade the chemical structure of the antimicrobial agents [38]. On the other hand, all the Salmonella isolates show highly sensitive to ciprofloxacin. This finding is in line with the reports of [16, 37] in which Salmonella isolates from human and cattle were 100% susceptible to ciprofloxacin. Though no data has indicated this, the effectiveness of ciprofloxacin may be because it is not widely used in countries like Ethiopia in the animal production system.
Over the years, bacterial pathogens have developed resistance against various antibiotics. In this study, all Salmonella isolates showed multiple drug resistance (MDR) to two or more antimicrobials. This is higher than the percentage of isolates showed MDR in previous studies in Ethiopia such as [16] (83.0%), [39] (36.4%), [40] (52.5%), [15] (31.8%), [17] (96.4%), and [37] (95.5%). This may be due to the increasing rate of inappropriate utilization of antimicrobials in the dairy animal production systems, which favor selection pressure that increased the advantage of maintaining strains of bacteria carrying resistance genes [41]. The MDR indexes of the isolates obtained from this study ranges from 0.22 to 0.66. MDR index values greater than 0.2 indicates unwise use of antibiotics [42]. Therefore, this study shows milk is a potential source of MDR, and is a potential public health concern in the study area.
The existence of invA gene in almost all Salmonella serovars and its absence from the other bacteria proved it as a genetic marker for the identification of Salmonella. In this study, the invA gene was amplified from 32 (80.0%) of the 40 Salmonella isolates. Eight isolates, which were biochemically, identified as Salmonella were excluded by polymerase chain reaction method. The possible explanation for this is; in the conventional bacterial isolation and biochemical characterization; Salmonella is confused with other related Enterobacteriaceae. However, PCR is highly sensitive because the invA gene is absent in other related bacteria. This amplification of invA gene using Salm3 and Salm4 primer is comparable with the reports of [28, 30].
The unexpected amplification in lane 1 and lane 26 is supposed to be due to attachment of the primer on non-target region which bring nonspecific product. Compared to conventional cultural and biochemical identification methods, PCR based method show better specificity, higher sensitivity, shorter analysis time, and better accuracy. Therefore, for detection of Salmonella in milk with better accuracy and specificity within short period of time polymerase chain reaction method is preferable.
Conclusion
Detection and quantification of Salmonella in food samples should be regularly performed. The present study revealed that Salmonella are important contaminants of raw milk regardless of the source of milk, season of the year and districts sampled. However, the rate of isolation was significantly higher in large sized farms. The study further showed that the isolated Salmonella had developed varying degree of resistance to commonly used antimicrobials such as ampicillin, streptomycin, cefotaxime and gentamycin. It was marked that all of the isolates are resistant to two more antibiotics. Thus, the high percentage of MDR isolates recovered indicate the potential importance of raw milk as a source of MDR Salmonella infections and a serious public health concern and global challenge. It is therefore important that dairy farmers and raw milk sellers in the study area take serious measures to avoid contamination of the milk with Salmonella spp. In addition, the active commitment of the veterinary departments in the respective districts to sensitizing dairy farmers and the sensible use of antibiotics at farm level can help to reduce the antibiotic resistance of Salmonella spp. Further molecular studies are also needed to identify the Salmonella serotypes circulating in the study area.
Supplementary Information
Acknowledgments
The authors express special thanks to owners of the dairy farms included in the study for their collaboration during the study period. The authors also appreciate Institute of Biodiversity for their dedication in giving the control strains. Authors are thankful to the editor and an anonymous reviewer for their constructive comments that helped to improve the quality of this paper.
Abbreviations
- PCR
Polymerase Chain Reaction
- MDR
Multi-drug Resistance
- invA
Invasive A gene
- XLD
Xylose lysine Desoxycholate agar
Authors’ contributions
AG collected all the required data and performed the laboratory works. MT designed the study; RA analyzed and interpreted the data. MT provided all the laboratory materials and reagents required for the research. AG drafted the manuscript. MT and RA critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Ministry of Science and Higher Education, Ethiopia.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
A local ethics committee ruled that no formal ethics approval was required to conduct this research. Before conducting the research, informed consent was obtained from the owners of the dairy farms included in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mestawet Taye and Rahmeto Abebe contributed equally to this work.
Contributor Information
Alemayehu Gebeyehu, Email: alemayehugebeyehu66@gmail.com.
Mestawet Taye, Email: danbebis@gmail.com.
Rahmeto Abebe, Email: rahmetoabe@gmail.com.
References
- 1.Center for Disease Control and Prevention (CDC) Food borne illness, January. 2018. pp. 1–13. [Google Scholar]
- 2.Forshell PL, Wierup M. Salmonella contamination: a significant challenge to the global marketing of animal food products. Revue ScientifiqueEt Technique-Office International Des Epizooties. 2006;25(2):541–554. [PubMed] [Google Scholar]
- 3.Parekh TS, Subhash R. Molecular and bacteriological examination of milk from different milk animals with special reference to Coliforms. Curr Res Bacteriol. 2008;1(2):56–63. doi: 10.3923/crb.2008.56.63. [DOI] [Google Scholar]
- 4.Abeer A, Azza SA, Dardir HA, Ibrahim AK. Prevalence of some milk borne bacterial pathogens threatening camel milk consumers in Egypt. Glob Vet. 2012;8(1):76–82. [Google Scholar]
- 5.Guibourdenche M, Roggentin P, Mikoleit M, Fields I, Bockemuhl J, Grimont P, et al. White-Kauffmann-Le Minor scheme. Suppl 2003–2007 (No. 47). Res Microbiol. 2010;161:26–9. [DOI] [PubMed]
- 6.Collazo C, Galan J. The invasion-associated type-III protein secretion system in Salmonella-a review. Gene. 1997;192:51–59. doi: 10.1016/S0378-1119(96)00825-6. [DOI] [PubMed] [Google Scholar]
- 7.Bäumler A, Tsolis R, Ficht T, Adams L. Evolution of host adaptation in Salmonella enterica. Infect Immunol. 1998;66:4579–4587. doi: 10.1128/IAI.66.10.4579-4587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertu WJ, Dapar M, Gusi AM, Ngulukun SS, Leo SL, Jwander D. Prevalence of brucella antibodies in marketed milk in Jos and environs. Afr J Food Sci. 2010;4(2):062–064. [Google Scholar]
- 9.Capita R, Álvarez-Astorga M, Alonso-Calleja C, Moreno B, García-FernÁndez MC. Occurrence of salmonellae in retail chicken carcasses and their products in Spain. Int J Food Microbiol. 2003;81:169–173. doi: 10.1016/S0168-1605(02)00195-2. [DOI] [PubMed] [Google Scholar]
- 10.Halawa M, Moawad A, Eldesouky I, Ramadan H. Detection of Antimicrobial Phenotypes, β-Lactamase Encoding Genes and Class I Integrons in Salmonella Serovars Isolated from Broilers. Int J Poult Sci. 2016;15:1–7. doi: 10.3923/ijps.2016.1.7. [DOI] [Google Scholar]
- 11.Bergonier D, de Crémoux R, Rupp R, Lagriffoul G, Berthelot X. Mastitis of dairy small ruminants. Vet Res. 2003;34(5):689–716. doi: 10.1051/vetres:2003030. [DOI] [PubMed] [Google Scholar]
- 12.Coorevits A, De Jonghe V, Vandroemme J, Reekmans R, Heyrman J, Messens W, et al. Comparative analysis of the diversity of aerobic spore-forming bacteria in raw milk from organic and conventional dairy farms. Syst Appl Microbiolo. 2008;31(2):126–40. [DOI] [PubMed]
- 13.Callon C, Gilbert FB, Cremoux RD, Montel MC. Application of variable number of tandem repeat analysis to determine the origin of S. aureus contamination from milk to cheese in goat cheese farms. Food Control. 2008;19:143–150. doi: 10.1016/j.foodcont.2007.02.014. [DOI] [Google Scholar]
- 14.Zewdu E, Cornelius P. Antimicrobial resistance pattern of Salmonella serotypes isolated from food items and personnel in Addis Ababa, Ethiopia. Trop Anim Health Prod. 2009;41:241–249. doi: 10.1007/s11250-008-9181-y. [DOI] [PubMed] [Google Scholar]
- 15.Wassie M, Bayelegn M, Daniel A, Muckle A, Cole L, Wilkie E. Occurrence and antimicrobial resistance of Salmonella serovars in apparently healthy slaughtered sheep and goats of central Ethiopia. Trop Anim Health Prod. 2006;38:455–462. doi: 10.1007/s11250-006-4325-4. [DOI] [PubMed] [Google Scholar]
- 16.Addis Z, Nigatu K, Zufan W, Haile G, Alehegne Y, Tesfu K. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BioMed Central Infect Dis. 2011;11:222. doi: 10.1186/1471-2334-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abunna F, Ashenafi D, Beyene T, Ayana D, Mamo M, Duguma R. Isolation, identification and antimicrobial susceptibility profiles of Salmonella isolates from dairy farms in and around Modjo town, Ethiopia. Vet J. 2017;21(2):92–108. [Google Scholar]
- 18.Mitike G. Occurrence and antimicrobial susceptibility of Salmonella in fecal and carcass swab samples of small ruminants at Addis Ababa livestock market and abattoir. In: MSc Thesis: Addis Ababa University, College of Veterinary Medicine and Agriculture; 2018.
- 19.Central Statistical Agency . The Federal Democratic Republic of Ethiopia, central statistical agency. Agricultural sample survey report on livestock and livestock characteristics. 2018. [Google Scholar]
- 20.National Meteorology Agency (NAM). Climate of major cities; monthly, seasonal and annual bulletin. Addis Ababa: Ethiopian National Meteorology Agency; 2019.
- 21.Abebe R, Hatiya H, Abera M, Megersa B, Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res. 2016;12:270. doi: 10.1186/s12917-016-0905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thrusfield M. Veterinary Epidemiology. 2. UK: BlackWell Science; 2007. pp. 182–198. [Google Scholar]
- 23.International Organization for Standardization (ISO) Microbiology of Food chain -Horizontal methods for detection, enumeration and serotyping of Salmonella. Geneva: ISO; 2018. pp. 6579–6571. [Google Scholar]
- 24.Quinn PJ, Carter ME, Markey BK, Carter GR. Clinical Veterinary microbiology. 5. Spain: Grafos; 2002. pp. 331–344. [Google Scholar]
- 25.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. Wayne: Sixteenth Informational Supplement; 2018. [Google Scholar]
- 26.Kumar V. Studies on isolation and molecular characterization of salmonella spp. Of public health significance in chevon and chicken meat. MSc Thesis. Durg: College of Veterinary Science and Animal Husbandry; 2014.
- 27.Ramadan H, Awad A, Ateya A. Detection of phenotypes, virulence genes and phylotypes of avian pathogenic and human diarrheagenic Escherichia coli in Egypt. JIDC. 2016;10(6):584–591. doi: 10.3855/jidc.7762. [DOI] [PubMed] [Google Scholar]
- 28.Ferretti R, Mannazzu I, Cocolin L, Comi G, Clementi F. Twelve-Hour PCR-Based Method for Detection of Salmonella spp. in Food. Appl Environ Microbiol. 2001;67:977–978. doi: 10.1128/AEM.67.2.977-978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reischl U, Youssef MT, Kilwinski J. Real time fluorescence PCR assays for detection and characterization of shiga toxin, intimin and enterohemolysin genes for shiga toxin-producing Escherichia coli. J Clin Microbiol. 2002;40:2555–2565. doi: 10.1128/JCM.40.7.2555-2565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocolin L, Manzano M, Cantoni C, Comi G. Use of Polymerase Chain Reaction and Restriction Enzyme Analysis to directly detect and identify Salmonella Typhimurium in food. J Appl Microbiol. 1998;85:673–677. doi: 10.1111/j.1365-2672.1998.00575.x. [DOI] [PubMed] [Google Scholar]
- 31.Amira H, Mohammed S, Adel A, Maha A. Prevalence and molecular characterization of Salmonella serovars in milk and cheese in Mansoura city, Egypt. J Adv Vet Anim Res. 2017;4(1):45–51. https://doi.org/10.24966/ARVS-3751/100021.
- 32.Tesfaw L, Taye B, Alemu S, Alemayehu H, Sisay Z, Negussie H. Prevalence and antimicrobial resistance profile of Salmonella isolates from dairy products in Addis Ababa, Ethiopia. Afr J Microbiol. 2013;7(43):5046–5050. doi: 10.5897/AJMR2013.5635. [DOI] [Google Scholar]
- 33.Dadi S, Matios L, Sei KM, Olani T, Yimesgen A, Mekdes L, et al. Isolation of Salmonella and E. coli (E. coli O157:H7) and its antimicrobial resistance pattern from bulk tank raw milk in Sebeta Town, Ethiopia. J Anim Res Vet Sci. 2020;4(1):021. 10.24966/ARVS-3751/100021.
- 34.Karin H, Andrea I, Martin W. Animal contact as a source of human nontyphoidal salmonellosis. J Vet Res. 2011;42:1–28. doi: 10.1186/1297-9716-42-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radostits OM, Gray CC, Hinchcliff K, Constable PD. Veterinary Medicine. A text book of the disease of Cattle, Horse, Sheep, Pigs and Goats. 10. Spain: Sounder; 2007. Mastitis; pp. 673–719. [Google Scholar]
- 36.Tibaijuka B, Molla B, Hildebrandt G, Kleer J. Occurrence of salmonellae in retail raw chicken products in Ethiopia. Berliner Munchener Tierärztiliche Wchenschrift. 2003;16:55–58. [PubMed] [Google Scholar]
- 37.Merara O. Detection and antimicrobial susceptibility test of Salmonella species along beef supply chain in Bishoftu town. MSc Thesis. Bishoftu: Addis Ababa University, College of Veterinary Medicine and Agriculture; 2018.
- 38.Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: a guide for clinicians. J Anaesthesiol Clin Pharmacol. 2017;33(3):300. doi: 10.4103/joacp.JOACP_349_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sefinew A, Bayleyegn M. Prevalence and antimicrobial resistance profiles of Salmonella enterica serovars isolated from slaughtered cattle in Bahir Dar, Ethiopia. Trop Anim Health Prod. 2012;44:595–600. doi: 10.1007/s11250-011-9941-y. [DOI] [PubMed] [Google Scholar]
- 40.Birhanu B, Mesfin A, Alemayehu D. Multiple antimicrobial–resistant Salmonella serotypes isolated from chicken carcass and giblets in Debre Zeit and Addis Ababa, Ethiopia. Ethiop J Health Develop. 2003;17(2):131–149. [Google Scholar]
- 41.McDermott PF, Tyson GH, Kabera C. Whole genome sequencing for detecting antimicrobial resistance in nontyphoidal Salmonella. Antimicrob Agents Chemother. 2016;60(9):5515–5520. doi: 10.1128/AAC.01030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Antimicrobial Monitoring System (NARMS) Antibiotic tested by NARMS for enteric bacteria, Salmonella. 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


