Abstract
Background
DAV132 (colon-targeted adsorbent) has prevented antibiotic-induced effects on microbiota in healthy volunteers.
Objectives
To assess DAV132 safety and biological efficacy in patients.
Patients and methods
An open-label, randomized [stratification: fluoroquinolone (FQ) indication] multicentre trial comparing DAV132 (7.5 g, 3 times a day, orally) with No-DAV132 in hospitalized patients requiring 5–21 day treatment with FQs and at risk of Clostridioides difficile infection (CDI). FQ and DAV132 were started simultaneously, DAV132 was administered for 48 h more, and patients were followed up for 51 days. The primary endpoint was the rate of adverse events (AEs) independently adjudicated as related to DAV132 and/or FQ. The planned sample size of 260 patients would provide a 95% CI of ±11.4%, assuming a 33% treatment-related AE rate. Plasma and faecal FQ concentrations, intestinal microbiota diversity, intestinal colonization with C. difficile, MDR bacteria and yeasts, and ex vivo resistance to C. difficile faecal colonization were assessed.
Results
Two hundred and forty-three patients (median age 71 years; 96% with chronic comorbidity) were included (No-DAV132, n = 120; DAV132, n = 123). DAV132- and/or FQ-related AEs did not differ significantly: 18 (14.8%) versus 13 (10.8%) in DAV132 versus No-DAV132 patients (difference 3.9%; 95% CI: −4.7 to 12.6). Day 4 FQ plasma levels were unaffected. DAV132 was associated with a >98% reduction in faecal FQ levels (Day 4 to end of treatment; P < 0.001), less impaired microbiota diversity (Shannon index; P = 0.003), increased ex vivo resistance to C. difficile colonization (P = 0.0003) and less frequent FQ-induced VRE acquisition (P = 0.01).
Conclusions
In FQ-treated hospitalized patients, DAV132 was well tolerated, and FQ plasma concentrations unaffected. DAV132 preserved intestinal microbiota diversity and C. difficile colonization resistance.
Introduction
Antibiotics constitute a landmark of modern medicine. However, their use disrupts the colonic microbiota. The non-absorbed part of orally administered antibiotics, and the fraction of oral and parenteral antibiotics excreted into the bile that reach the colon, induce dysbiosis and a decrease in richness and diversity of the microbiota. Short-term consequences1–6 include antibiotic-associated diarrhoea (AAD), Clostridioides difficile infection (CDI) and selection of resistant bacteria.7–9 Long-term consequences include impacts on immune4,6 and metabolic regulations.5,6 Exacerbation of graft-versus-host disease in allogeneic HSCT recipients and increased mortality in cancer patients have been associated with antibiotic use.10,11 Various strategies to protect the intestinal microbiota have been developed.12 Oral administration of β-lactamases hydrolysing β-lactams in the colon appears promising in Phase 2 studies,13–17 although limited to β-lactam antibiotics. Filling this gap, DAV132 has been developed for use with a broad range of antibiotics. It is made of millimetric beads consisting of a core of a specific activated charcoal surrounded by a polymer coating that is insoluble during transit through the stomach and most of the small intestine. In the Phase 1 studies programme, it was shown to dissolve in the distal ileum to liberate the charcoal, which then adsorbs and thereby inactivates antibiotics in the caecum/colon.18 In another Phase 1 study in healthy volunteers treated with oral moxifloxacin, DAV132 reduced faecal antibiotic concentrations by 99% and preserved intestinal microbiota diversity.19
As the charcoal contained in DAV132 is delivered in the late ileum and not before, there is no adsorption of oral medications in the upper part of the GI tract. Indeed, blood levels of drugs absorbed in the upper part of the small intestine, such as antibiotics like amoxicillin18 and moxifloxacin,19 or other medications like narrow therapeutic index drugs such as warfarin and clonazepam20 are not impacted by co-administration of DAV132. Here, we report the safety of DAV132 in an open-label, randomized, Phase 2 clinical trial in hospitalized patients, mostly elderly with comorbidities, receiving systemic fluoroquinolones (FQs) for acute infections and DAV132 efficacy to protect intestinal microbiota diversity and preserve colonization resistance.
Materials and methods
Study design and oversight
We performed a parallel-arm randomized open-label multicentre clinical trial in hospitalized patients receiving FQs. The primary endpoint was the safety of DAV132 based on the occurrence of events of interest (definition in the Supplementary methods, available as Supplementary data at JAC Online). All other clinical and biological endpoints were secondary (Table S1). Patients were randomized to receive DAV132 or No-DAV132, and the 1:1 randomization was stratified according to FQ indication. The trial was performed in an open-label fashion, since no placebo with the ability to blacken the stools in the same manner as DAV132 could be identified. In response, an independent adjudication committee (IAC), made up of external pharmacologists and pharmacovigilance experts, performed a blinded assessment of the causality of predefined types of events (see Safety assessment section and Supplementary methods). Approvals from Health Authorities and Ethics Committees from each of the participating countries where the trial was run were obtained before study initiation. All analytical assays were performed in central laboratories blinded to the treatment arm.
Ethics
The study was conducted in agreement with Good Clinical Practice and registered appropriately (NCT03710694 and EUDAMED #CIV-18-03-023465). Patients signed an informed consent prior to inclusion and randomization. Regulatory authority approvals were obtained on the first version and the final protocol in the participating countries (for details, see Supplementary methods).
Patients
Eligible patients were ≥18 years old, hospitalized (excluding ICUs) for an expected stay of ≥3 days, and treated for 5–21 days with a FQ monotherapy (moxifloxacin, levofloxacin or ciprofloxacin; oral or IV) for either a lower respiratory tract infection (LRTI), a complicated urinary tract infection (cUTI) or for prophylaxis of febrile neutropenia. Inclusion criteria also included risk factors for CDI (Table S2). Exclusion criteria included antibiotic exposure during the week preceding randomization, suspected or confirmed CDI at screening or anti-C. difficile treatment, use of probiotics or intestinal adsorbents, a history of faecal transplantation, or diarrhoea of any cause (Table S2). As the duration of the FQ treatment was variable, study visits were planned at fixed days following randomization, and at days based on the end of FQ treatment (see Visits in Supplementary methods and Figure S1).
Products and treatments
In the DAV132 arm, the product was given orally three times a day before meals, at a unitary dose of 7.5 g (i.e. 5.1 g of activated charcoal), for the entire duration of the antibiotic treatment, and for 2 days thereafter.
Study populations
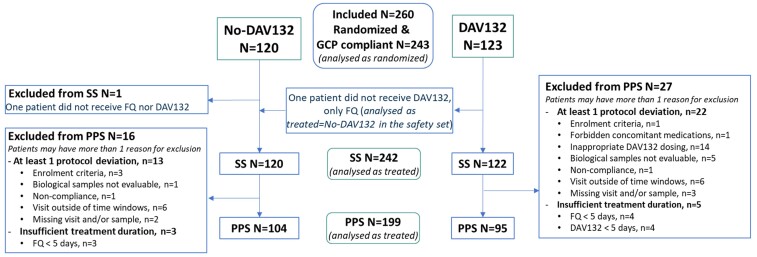
The intent-to-treat set (ITTS) included all randomized patients managed in compliance with Good Clinical Practice throughout the study (Figure 1). The safety set (SS), on which safety was assessed, included patients from the DAV132 arm having received at least one dose of DAV132 and one dose of FQ for the No-DAV132 arm. The per protocol set (PPS), on which efficacy was assessed, included patients having received at least 5 days of DAV132 (if in the DAV132 arm) and of FQ, and without any major protocol deviations.
Figure 1.
Study populations flowchart. GCP, good clinical practice. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Safety assessment
Clinical examinations were performed at each study visit and as needed according to the investigator’s judgment. Management of comorbidities and compliance with DAV132 treatment were documented throughout the study. Adverse events (AEs), serious AEs (SAEs) and treatment-emergent AEs (TEAEs) were described according to their pre-specified definitions (see Supplementary methods). For predefined events such as those reported as related to DAV132 and/or to FQ by the investigators, AAD and modification of concomitant treatments of comorbidities, i.e. events of interest for the study, their causality was independently adjudicated by the IAC whilst blinded to treatment allocation. Assessment of the absence of interaction of DAV132 with antibiotic efficacy was based on the clinical cure of the original infection, and relied on the investigator’s judgement and on the duration of hospitalization. The acceptability of DAV132 was assessed through questionnaires administered to patients, which used 1 (very bad) to 9 (very good) scales for the evaluation of taste, texture, ease of intake, ease of compliance with reconstitution and intake instructions, and ease of reconstitution.
Efficacy assessment
CDI and AAD were defined as described.21,22 Blood, rectal swabs and stool were sampled at defined intervals after inclusion (Figure S1). Plasma and free faecal FQ concentrations were measured by reversed-phase HPLC coupled with tandem MS detection. Faecal carriage of enterobacteria producing ESBL, of VRE and of yeasts was assessed qualitatively and semi-quantitatively, while that of C. difficile was assessed qualitatively only (all methods in Supplementary methods). Intestinal microbiota analysis was carried out by 16S rRNA gene sequencing on an Illumina MiSeq platform; α- and β-diversity indices were computed as described.23 Resistance to C. difficile colonization in patients’ faeces was assessed ex vivo, as described.24
Study endpoints
The primary endpoint was the proportion of patients with at least one event of interest with causality considered related to DAV132 and/or to FQ as assessed by the IAC. Secondary endpoints included other safety endpoints, and clinical and biological efficacy endpoints such as the rate of AAD, the plasma and faecal levels of FQ, intestinal colonization with predefined bacteria, and diversity of the intestinal microbiota.
Statistics
The trial sample size, 260 patients, was based on the precision of the estimated AE rate. Assuming an observed IAC-adjudicated AE rate of 33% in each arm, the difference in rates would be 0%, with a 95% CI of −11.4% to +11.4%. This was considered a reasonable precision for a Phase 2a study. A blocked randomization list stratified by indication of FQ treatment was generated by computer. Randomization results were communicated through a Web Response System.
All data were managed blindly prior to database lock and statistical analyses performed with SAS® version 9.4 (Cary, NC, USA) or R version 3.6.1.25 While the ITTS was predefined to be analysed as randomized, SS and PPS populations were predefined to be analysed as treated, i.e. considering the treatments actually received by the patient. Safety endpoints were analysed on the SS, and clinical and biological efficacy endpoints were analysed on the PPS. For the primary endpoint, the difference between treatments was calculated and the 95% CI for the difference presented using the method of Miettinen and Nurminen. Descriptive statistical analyses were performed for the AEs reported by the investigators. Statistical tests were two-sided with a 5% level of significance. For the analysis of each intestinal microbiota index, correction for multiple testing at different timepoints was used as described.26 No missing data were expected for the primary endpoints as the IAC reviewed relevant information on all patients. For secondary and exploratory endpoints, data were classified as missing and not imputed, and the number of missing values was clearly stated.
Results
Patients
From October 2018 to August 2019, 260 patients were enrolled at 24 study sites in Serbia (n = 114 patients; 5 sites), Romania (n = 34; 10 sites), Bulgaria (n = 110; 8 sites) and Germany (n = 2; 1 site). All 17 patients from one specific site were excluded, based on the site’s non-compliance with Good Clinical Practice (mostly unavailability of patient charts for monitoring). The ITTS included 243 patients with 120 in the No-DAV132 arm and 123 in the DAV132 arm (Figure 1). For safety purposes, the SS analysed patients as actually treated; therefore, one patient from the No-DAV132 arm who did not receive FQs was excluded from the SS, whereas one patient from the DAV132 arm who did not receive DAV132 was considered for safety analysis in the No-DAV132 arm. Therefore, the SS included 242 patients from 23 participating centres. The PPS eventually comprised 199 patients. Baseline characteristics were similar between both arms (Table 1); in particular, the mean duration of treatment with FQs was 7.4 days in the No-DAV132 arm and 7.6 days in the DAV132 arm (median 7.0 days in both arms), and the treatment was initially administered by the IV route for 79.1% and 78.1% of patients, respectively. The duration of treatment and the ratio of IV route versus oral route of administration were similar between FQs (see Table S3).
Table 1.
Main characteristics of patients at baseline in the SS and PPS
| Variables | SS | PPS | ||
|---|---|---|---|---|
| No-DAV132 (n = 120) | DAV132 (n = 122) | No-DAV132 (n = 104) | DAV132 (n = 95) | |
| Age, years, median (SD) | 72.2 (7.8) | 72.0 (8.1) | 72.8 (5.9) | 72.6 (7.9) |
| patients aged ≥65 years, n (%) | 114 (95.0) | 113 (92.6) | 101 (97.1) | 88 (92.6) |
| Male sex, n (%) | 60 (50.0) | 60 (49.2) | 53 (51.0) | 43 (45.3) |
| FQ indication, n (%) | ||||
| LRTI | 96 (80.0) | 96 (78.7) | 84 (80.8) | 73 (76.8) |
| cUTI | 20 (16.7) | 22 (18.0) | 17 (16.3) | 19 (20.0) |
| febrile neutropenia (prophylaxis) | 4 (4.2) | 4 (3.3) | 3 (2.9) | 3 (3.2) |
| Fluoroquinolone administereda, n (%) | ||||
| moxifloxacin | 25 (20.8) | 17 (13.9) | 23 (22.1) | 16 (16.8) |
| levofloxacin | 48 (40.0) | 57 (46.7) | 43 (41.3) | 47 (49.5) |
| ciprofloxacin | 47 (39.2) | 48 (39.3) | 38 (36.5) | 32 (33.7) |
| Chronic comorbidities | ||||
| at least one comorbidity, n (%) | 113 (94.2) | 118 (96.7) | 99 (95.2) | 93 (97.9) |
| charlson comorbidity index, median (min–max) | 2.0 (0–10) | 2.0 (0–9) | 2.0 (0–10) | 2.0 (0–9) |
| Comorbidities, n (%) | ||||
| severe cardiopulmonary conditionb | 85 (70.8) | 91 (74.6) | 70 (72.2) | 69 (80.2) |
| congestive heart failure | 62 (51.7) | 66 (54.1) | 52 (53.6) | 53 (61.6) |
| COPD | 71 (59.2) | 63 (51.6) | 68 (65.4) | 50 (52.6) |
| diabetes mellitus | 35 (29.2) | 49 (40.2) | 29 (29.9) | 38 (44.2) |
| cerebrovascular disease | 15 (12.5) | 10 (8.2) | 13 (12.5) | 5 (5.3) |
| solid tumour or haematological malignancy | 12 (10.0) | 9 (7.3) | 11 (10.6) | 8 (8.4) |
| moderate to severe chronic kidney disease | 9 (7.5) | 4 (3.3) | 7 (6.7) | 2 (2.1) |
| cirrhosis | 3 (2.5) | 4 (3.3) | 3 (2.9) | 3 (3.2) |
| Recent history of CDIc, n (%) | ||||
| patients without any CDI | 97 (80.8) | 96 (78.7) | 85 (81.7) | 74 (77.9) |
| 1 or 2 recent episodes | 4 (3.3) | 9 (7.4) | 3 (2.9) | 8 (8.4) |
| Previous hospitalization of more than 72 h and/or receiving long-term nursing care for more than 1 month within the last 90 days, n (%) | 47 (39.2) | 45 (36.9) | 40 (38.5) | 34 (35.8) |
| Previous cumulated exposure of at least 5 days to any antibiotic within the last 90 days, n (%) | 107 (89.2) | 114 (93.4) | 98 (94.2) | 89 (93.7) |
| β-Lactams, penicillins | 20 (16.7) | 15 (12.3) | 18 (17.3) | 10 (10.5) |
| β-Lactams, cephalosporins and carbapenems | 52 (43.3) | 53 (43.4) | 43 (41.3) | 42 (44.2) |
| quinolones | 28 (23.3) | 32 (26.2) | 24 (23.1) | 24 (25.3) |
| macrolides, lincosamides and streptogramins | 27 (22.5) | 28 (23.0) | 26 (25.0) | 24 (25.3) |
Most commonly used FQ dose regimen: (a) moxifloxacin 400 mg once a day, IV route; (b) levofloxacin 500 mg once a day, IV route; (c) levofloxacin 500 mg once a day, oral route; (d) ciprofloxacin 200 mg twice a day, IV route.
Severe cardiopulmonary conditions included chronic congestive heart failure and severe arterial hypertension.
The time interval for recent history of CDI was within the last 6 months prior to study inclusion.
Safety
Primary endpoint
The proportions of patients with events of interest related to DAV132 and/or FQ, as adjudicated by the IAC, were not different between arms [20 events in 13 (10.8%) No-DAV132 patients, versus 25 events in 18 (14.8%) DAV132 patients; difference of the proportions: 3.9%; 95% CI: −4.7% to 12.6%]. No event of interest could be related to DAV132 only.
Secondary endpoints
Clinical safety
Overall, 135 AEs were reported by the investigators in 74/242 patients (30.4%), without a clinically meaningful difference between arms (Table 2). None of the TEAEs leading to withdrawal from the study led to modification of the regimens of any concomitant drug. Overall, SAEs were reported in 17 patients, with a similar incidence between treatment arms, and none were considered related to DAV132 or FQs (Table 2). All patients with non-fatal SAEs recovered, except for one with a lung neoplasm. The most frequent TEAEs were gastrointestinal, mostly constipation in DAV132 patients (5.7% versus 0.8% of No-DAV132 patients), whereas diarrhoea events were more frequent in No-DAV132 patients (6.7% versus 3.3% in DAV132 patients). Most TEAEs were mild to moderate. The incidence of TEAEs leading to FQ discontinuation was similar between arms (Table 2).
Table 2.
Number of AEs (TEAE or not) and as reported by the investigators, and number of patients affected by these AEs/TEAEs in patients not receiving (No-DAV132) or receiving DAV132 in the SS
| Characteristics | No-DAV132 (n = 120) | DAV132 (n = 122) | ||
|---|---|---|---|---|
| number of patients (%) | number of events | number of patients (%) | number of events | |
| At least one AE | 33 (27.5) | 62 | 41 (33.6) | 73 |
| At least one TEAE | 33 (27.5) | 62 | 40 (32.8) | 71 |
| AE leading to study withdrawal | 2 (1.7) | 2 (3.2) | 4 (3.3) | 4 (5.5) |
| SAE | 8 (6.7) | 8 (12.9) | 9 (7.4) | 9 (12.3) |
| AE leading to death | 2 (1.7) | 2 (3.2) | 2 (1.6) | 2 (2.7) |
| Intensity of the AE | ||||
| mild | 24 (20.0) | 35 | 29 (23.8) | 44 |
| moderate | 13 (10.8) | 23 | 12 (9.8) | 19 |
| severe | 3 (2.5) | 4 | 6 (4.9) | 8 |
| At least one TEAE related to DAV132 | NA | NA | 8 (6.6) | 8 (11.3) |
| At least one TEAE related to FQ | 9 (7.5) | 9 (14.5) | 11 (9.0) | 11 (15.5) |
| At least one TEAE related to DAV132 only | NA | NA | 0 | 0 |
| Any TEAE | 33 (27.5) | 62 | 40 (32.8) | 71 |
| Gastrointestinal disorders | 15 (12.5) | 23 | 17 (13.9) | 22 |
| diarrhoea | 8 (6.7) | 8 | 4 (3.3) | 4 |
| constipation | 1 (0.8) | 1 | 7 (5.7) | 7 |
| nausea | 2 (1.7) | 2 | 4 (3.3) | 4 |
| abdominal pain | 4 (3.3) | 4 | 1 (0.8) | 1 |
| General disorders and administration site conditions | 5 (4.2) | 6 | 8 (6.6) | 8 |
| Infections and infestations | 5 (4.2) | 5 | 6 (4.9) | 6 |
| Vascular disorders | 3 (2.5) | 3 | 5 (4.1) | 6 |
| Cardiac disorders | 4 (3.3) | 4 | 4 (3.3) | 5 |
| Nervous system disorders | 3 (2.5) | 3 | 4 (3.3) | 4 |
| Psychiatric disorders | 0 | 0 | 2 (1.6) | 3 |
| Respiratory, thoracic and mediastinal disorders | 7 (5.8) | 7 | 3 (2.5) | 3 |
| Musculoskeletal and connective tissue disorders | 1 (0.8) | 1 | 2 (1.6) | 2 |
| Ear and labyrinth disorders | 0 | 0 | 2 (1.6) | 2 |
| Skin and subcutaneous tissue disorders | 0 | 0 | 2 (1.6) | 2 |
| Investigations | 3 (2.5) | 4 | 2 (1.6) | 4 |
| Metabolism and nutrition disorders | 3 (2.5) | 5 | 1 (0.8) | 1 |
| Renal and urinary disorders | 1 (0.8) | 1 | 1 (0.8) | 1 |
| Neoplasms benign, malignant and unspecified | 0 | 0 | 1 (0.8) | 1 |
| Reproductive system and breast disorders | 0 | 0 | 1 (0.8) | 1 |
Several AEs may have occurred in a single patient. An AE is any untoward medical occurrence, unintended disease or injury, or untoward clinical signs (including abnormal laboratory findings) in a patient participating in a clinical study whether or not the event is related to a treatment or procedure. A TEAE is an AE that occurs on or after the first administration or that is present prior to dosing but is exacerbated on or after the first administration. An SAE is defined as any untoward medical occurrence that, at any dose, results in death, is life-threatening, requires hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect. NA, not applicable.
No patient with DAV132 required modification of the regimen of concomitantly administered drugs.
The rate of cure of LRTI or cUTI was not different between the No-DAV132 and DAV132 arms (95.9% versus 94.2%). Hospitalization duration was similar between arms: median (IQR): 8 (7−9) days in No-DAV132 versus 8 (7−10) in DAV132 patients.
The compliance with DAV132, defined as the proportion of patients who took 100% of the doses, was high (86.9%). Product acceptability was good with a median score of 6–7 for the various items (details in Table S4).
Biological safety
Trough and peak plasma concentrations of the three tested FQs were not significantly different between arms for any regimen (Figure S2 and Table S5).
Vitamin K blood levels at the end of FQ treatment were similar between groups: median (min–max): 180 (30–1449) versus 159 ng/L (30–1776) in No-DAV132 versus DAV132 patients, respectively, as were blood electrolytes (data not shown).
Efficacy
Stools and rectal swabs were collected at predefined timepoints, e.g. 86.9% of faecal samples at end-of-FQ visit and 87.9% at 10 ± 1 days for the pharmacokinetics analysis (Figure S1).
Faecal levels of fluoroquinolones
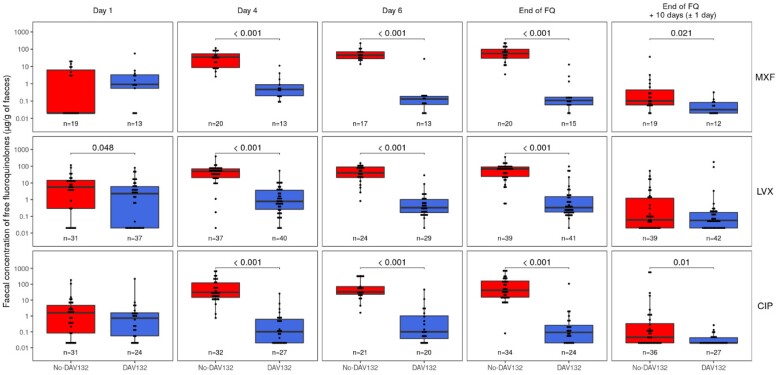
Co-administration of DAV132 led to a major reduction in the faecal levels of all FQs, at all times, with the geometric mean of free FQ faecal concentrations at the end-of-FQ visit reduced by 99.6%, 98.6% and 99.8% for levofloxacin, moxifloxacin and ciprofloxacin, respectively (Figure 2).
Figure 2.
Faecal free concentrations of FQs at successive timepoints since study initiation. Faecal free concentrations of FQs (mean ± SEM) in PPS patients treated in the absence (red signs) or presence (blue signs) of DAV132 are shown. At each timepoint, the values from both arms were compared using a Wilcoxon rank sum test. MXF, moxifloxacin; LVX, levofloxacin; CIP, ciprofloxacin (by FQ, all dose regimens together for each FQ). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Clinical and biological endpoints
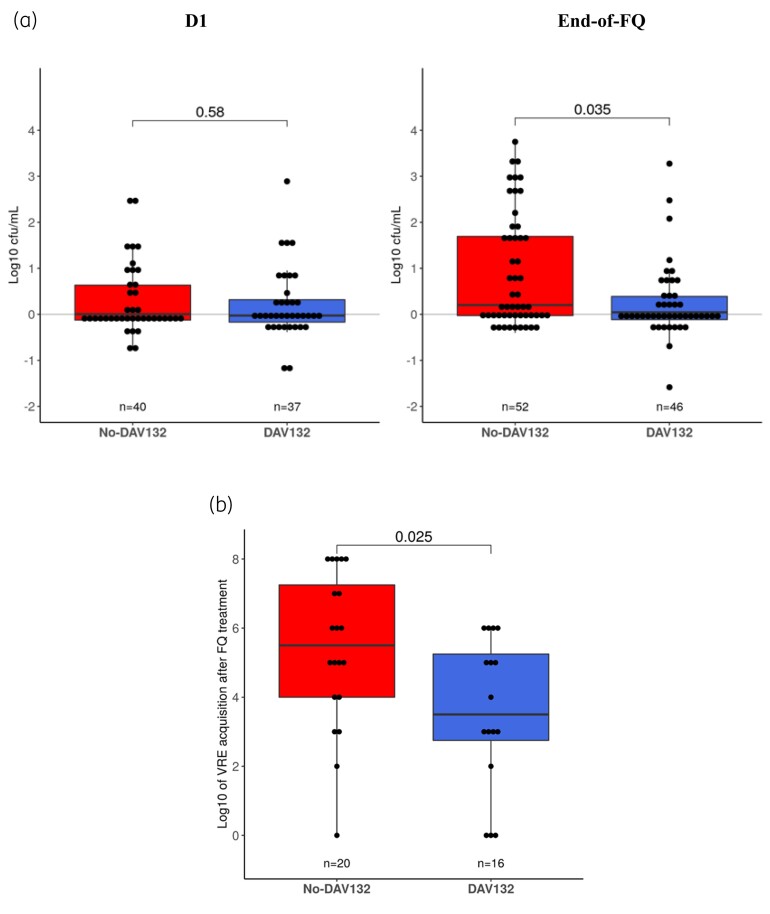
No CDI occurred in any patient. The proportion of AAD was not significantly different between treatment arms [between-treatment-arm difference in proportions of AAD: 2.6 (95% CI: −2.1 to 8.1); 5 patients (4.2%) in No-DAV132 arm versus 2 (1.6%) patients in DAV132 arm]. There was a trend towards decreased newly acquired C. difficile colonization during FQ treatment in DAV132 patients (5/101 versus 0/88; P = 0.06). Furthermore, the ex vivo-tested resistance of patient faeces to proliferation of inoculated C. difficile, while similar between both arms at baseline, was significantly better preserved in patients who received DAV132: mean (95% CI) proliferation: 0.25 log cfu/mL (0.01–0.48) in DAV132 (n = 46) versus 0.87 (0.53–1.21) in No-DAV132 (n = 52); P = 0.035 (Figure 3a).
Figure 3.
Microbiological assessment of the effect of DAV132 on the intestinal colonization of patients from the PPS in the DAV132 (blue) or No DAV132 (red) arm. (a) Ability to prevent the growth of C. difficile inoculated ex vivo into stools of patients collected at baseline (D1) or at the end of FQ treatment. (b) Quantification of the acquisition of VRE colonization at the end of FQ treatment among those not colonized at baseline. Each box shows the IQR (the bottom is Q1; the top is Q3) and the inner line is the median. In (a) and (b), values from both arms were compared using a Wilcoxon rank sum test. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The proportion of patients who acquired VRE during FQ treatment was similar between arms (No-DAV132, n = 20 versus DAV132, n = 16). However, in those newly colonized patients, the mean faecal counts of VRE per gram of faeces was reduced by >98% in those who received DAV132: mean (95% CI) log10 of VRE count: 5.4 (4.41–6.39) in the No-DAV132 arm versus 3.6 (2.49–4.64) in the DAV132 arm; P = 0.025 (Figure 3b). By contrast, there was no statistically significant difference between arms in terms of acquisition of ESBL-producing Enterobacteriaceae or yeasts (data not shown).
Protection of intestinal microbiota diversity
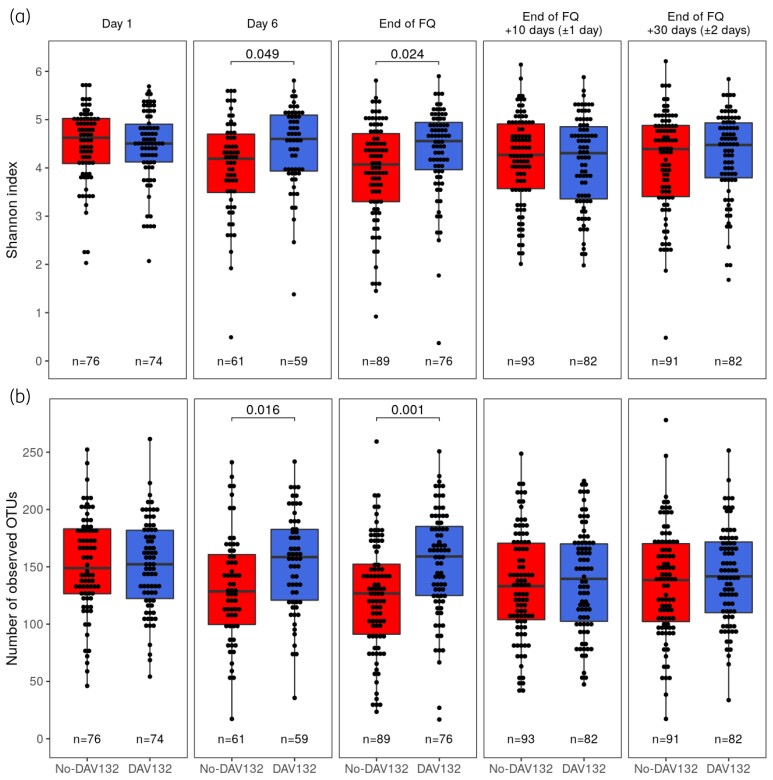
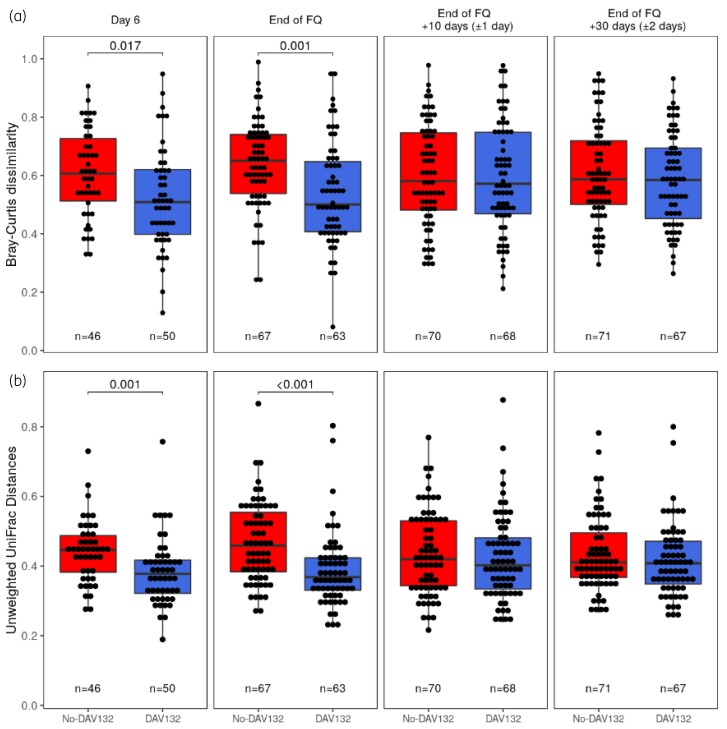
In the No-DAV132 arm, FQ treatment induced a decreased intestinal microbiota α-diversity and richness, as assessed by the change at the end of FQ versus baseline for the Shannon index (mean ± SEM: −0.44 ± 0.14) and the number of observed operational taxonomic units (OTUs) (mean ± SEM: −23.5 ± 6.1). These were highly significantly prevented by DAV132, both for the Shannon index [difference of means (95% CI) for No-DAV132 versus DAV132: −0.42 (−0.71 to −0.12); P = 0.024] and the number of observed OTUs [difference of means (95% CI) for No-DAV132 versus DAV132: −28.1 (−42.6 to −13.7); P = 0.001], respectively (Figure 4). In DAV132 patients, the mean (SEM) changes at the end of FQ versus baseline were −0.02 (0.10) for the Shannon index and 2.4 (5.1) for the number of observed OTUs. Ten and 30 days after the end of FQ, microbiota diversity and richness were not significantly different between arms. Differences in composition of the microbiota were analysed by computing two β-diversity indices, Bray–Curtis dissimilarity and unweighted UniFrac distances (Figure 5). Both metrics show that the composition of the microbiota was significantly less altered when patients were co-administered DAV132 together with antibiotics.
Figure 4.
Microbiota diversity at different days after the start of the FQ treatment in patients without (red) or with DAV132 (blue) in the PPS. (a) Shannon diversity index (mean ± SEM) and (b) the number of observed OTUs (mean ± SEM). At each timepoint, the values from both arms were compared using a Wilcoxon rank sum test and P values are reported for the significant differences. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 5.
Microbiota β-diversity at different days after start of the FQ treatment in patients without (red) or with DAV132 (blue) in the PPS. (a) Bray–Curtis dissimilarity and (b) unweighted UniFrac distances. Each box shows the IQR (the bottom is Q1; the top is Q3) and the inner line is the median. At each timepoint, the values from both arms were compared using a Wilcoxon rank sum test and P values are reported for the significant differences. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
Our most important result in this first-in-patient trial with DAV132 was that the number of AEs was similar between both arms, providing reassurance for the safe use of the product. This result was observed in a challenging setting of mostly elderly patients with comorbidities, which is reassuring. The safety of DAV132 was confirmed by several secondary endpoints. First, none of the investigators felt it necessary to adjust any of the regimens of the concomitant treatments prescribed for comorbidities. This may suggest an absence of clinically meaningful drug interactions in the intestinal tract between DAV132 and other treatments, because of DAV132’s localized site of action in the caecum and colon. Nearly all drugs used here for the care of patients are absorbed proximally to the distal ileum; thus, their pharmacokinetics should not be modified, as shown for some probes in volunteers.18–20 Second, treatment with DAV132 was not modified by investigators after initiation, demonstrating good safety and acceptability. Third, antibiotic treatment success rates, as well as hospitalization duration, were not different between arms, regardless of the FQ type and regimen. FQ pharmacokinetics in plasma were preserved and similar in patients receiving DAV132 or not. Fourth, no biological parameter was significantly modified in patients receiving DAV132. In particular, the serum level of vitamin K, which is produced by the intestinal microbiota and is instrumental for blood coagulation,27 was not affected.
The efficacy of DAV132 in protecting the intestinal microbiota from FQ-induced changes was another important result: the impairment of metagenomic α-diversity indices, which are recognized as the major descriptive element of a healthy microbiota,28 was significantly prevented. This is likely related to the almost complete elimination of FQ residues from the colon by DAV132, thereby sparing the microbiota from antibiotic exposure, as previously shown in volunteers.19
The trial results also suggest a functional benefit of DAV132 in preserving resistance to colonization by potentially pathogenic microorganisms. First, among patients who acquired VRE during FQ, VRE counts were significantly lower at the end of FQ in DAV132 patients. Nosocomial infections can be preceded by VRE colonization and overgrowth,29,30 which may also promote spread to other patients,31 resulting in complications and costs.32 Thus, DAV132 may limit the spread of and subsequent infections with VRE. We did not observe the same effect for ESBL-producing Enterobacteriaceae. However, in vivo results in mice treated with a third-generation cephalosporin suggest that it might be different in other epidemiological settings.33 Of note, a Phase 2 study assessing colonization resistance during co-administration of an orally administered β-lactamase together with an IV cephalosporin treatment yielded similarly discrepant results between Gram-negative and Gram-positive pathogens,17 just like faecal microbiota transfer treatments in eradicating VRE and/or MDR Gram-negative pathogens from the human gut.34,35 Gram-negative pathogens seem to occupy an ecological niche that is more difficult to modify than that of VRE.36,37
Second, the co-administration of DAV132 conferred improved colonization resistance against C. difficile. Patients taking DAV132 tended to be less frequently colonized by C. difficile and the ex vivo assay demonstrated the maintained growth-suppressive effect of faeces recovered from DAV132 recipients. This is in agreement with results in hamsters, which demonstrated the ability of activated charcoal to prevent CDI after a FQ38,39 or clindamycin.23 Hence, there is consistency between the prevention of antibiotic-induced dysbiosis by DAV132 (assessed by α- and β-diversity indices) and the concomitant maintenance of resistance to colonization by potentially pathogenic bacteria such as VRE and C. difficile.
Our study has limitations. One is the lack of a placebo that can uniformly blacken the stools as DAV132 does. We minimized the consequences of this by keeping the IAC blinded to the randomization arm for analysing the causality of events of interest, and by performing all biological assays blind. Another limitation is the focus on FQs only. This enabled us to obtain a more homogeneous patient population than that of a study open to all antibiotics. Importantly, in vitro19 and in vivo studies23,33 have shown that DAV132 efficiently adsorbs antibiotics from non-FQ classes, hence the conclusions may be applicable to other antimicrobials. Finally, we conducted our study in four countries only; however, no country-specific effect was observed.
In conclusion, DAV132 was safe in mostly elderly hospitalized patients with comorbidities who received FQs for the treatment or prevention of infection, and effective in preventing antibiotic-induced changes in the intestinal microbiota. Given the increasing concern that antibiotic-induced intestinal changes are major drivers of a broad range of acute and chronic infectious and non-infectious diseases, as well as a factor that limits the efficacy of some major anticancer therapies, the results of this trial underline the importance of further clinical development of DAV132.
Supplementary Material
Acknowledgements
In memoriam of Annie Ducher MD who was the initiator of the medical development strategy for DAV132, and more specifically of the trial described here. She was instrumental in its accomplishment, and we are very sad that her premature death prevented her from participating in the preparation of this manuscript and witnessing its publication. We would like to thank Enago for the English language review.
Funding
This study was funded by Da Volterra.
Transparency declarations
Authors affiliated with the sponsor wrote the first draft of the manuscript. All the authors vouch for the accuracy and completeness of the data reported.
Annie D., M.V. and F.V. are (or were) employees of Da Volterra. Annie D., J.d.G. and A.A. are (or were) shareholders of Da Volterra. T.L., C.F., O.A.C., Aaron D., J.d.G., A.A., F.M. and M.H.W. are consultants of Da Volterra. M.H.W. and F.M. have received grants from Da Volterra. M.J.G.T.V. has received research funds from 3M, Astellas Pharma, BioNtech, Da Volterra, Evonik, Gilead Sciences, Glycom, Immunic, MaaT Pharma, Merck/MSD, Organobalance, Seres Therapeutics, Takeda Pharmaceutical; speaker’s fees from Astellas Pharma, Basilea, Gilead Sciences, Merck/MSD, Organobalance and Pfizer; and has been a consultant to Alb Fils Kliniken GmbH, Ardeypharm, Astellas Pharma, bioMérieux, Da Volterra, Farmak International Holding GmbH, Ferring, Immunic AG, MaaT Pharma, Merck/MSD and SocraTec R&D GmbH. T.L. has received research grants from Seres Therapeutics, Rebiotix/Ferring, Finch Therapeutics, Vedanta Biosciences, Actelion, Summit Therapeutics PLC, MGB Biopharma and Immunimed. O.A.C. reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer and Scynexis; consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis and Seres; honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, Medscape, medupdate, Merck/MSD, Mylan and Pfizer; payment for expert testimony from Cidara; participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Janssen, Medpace, Paratek, PSI and Shionogi; a pending patent currently reviewed at the German Patent and Trade Mark Office; and other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC and Wiley. Aaron D. has acted as a consultant for Achaogen, Allecra, Amicrobe, Amplyx, Artizan, Cidara, ContraFect, Correvio, CARB-X, Da Volterra, Destiny, F2G, Geom, GSK, Gyroscope, Kymab, Mironid, Modis, Nabriva, Orca, Pfizer, Phico, Pled, Roche, Scynexis, Sinovent, SNIPR Biome, Spero, TenNor, tranScrip, Venatorx and Zavante. M.H.W. has had grants and personal fees from Actelion, Alere, Astellas, Cubist, EnteroBiotix, European Tissue Symposium, Merck, Sanofi Pasteur, Seres and Summit Therapeutics; personal fees from AstraZeneca, Basilea, Bayer, Durata, Idorsia, J&J, Menarini, Nabriva, Pfizer and Roche; and grants from Abbott, bioMérieux, Cerexa, The Medicines Company and QIAGEN, outside the submitted work. F.M. has received research funding from Roche, Sanofi and Servier and has been a consultant for Servier and IPSEN.
Supplementary data
Supplementary methods, Tables S1 to S5 and Figures S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Jernberg C, Löfmark S, Edlund Cet al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010; 156: 3216–23. [DOI] [PubMed] [Google Scholar]
- 2. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 2011; 108Suppl 1: 4554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pérez-Cobas AE, Gosalbes MJ, Friedrichs Aet al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 2013; 62: 1591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014; 157: 121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol 2015; 11: 182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aversa Z, Atkinson EJ, Schafer MJet al. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin Proc 2021; 96: 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Lastours V, Chau F, Tubach Fet al. Independent behavior of commensal flora for carriage of fluoroquinolone-resistant bacteria in patients at admission. Antimicrob Agents Chemother 2010; 54: 5193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johanesen P, Mackin K, Hutton Met al. Disruption of the gut microbiome: Clostridium difficile infection and the threat of antibiotic resistance. Genes 2015; 6: 1347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372: 1539–48. [DOI] [PubMed] [Google Scholar]
- 10. Shono Y, Docampo MD, Peled JUet al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016; 8: 339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peled JU, Gomes ALC, Devlin SMet al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med 2020; 382: 822–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andremont A, Cervesi J, Bandinelli P-Aet al. Spare and repair the gut microbiota from antibiotic-induced dysbiosis: state-of-the-art. Drug Discov Today 2021; 26: 2159–63. [DOI] [PubMed] [Google Scholar]
- 13. Leonard F, Andremont A, Leclerq Bet al. Use of beta-lactamase-producing anaerobes to prevent ceftriaxone from degrading intestinal resistance to colonization. J Infect Dis 1989; 160: 274–80. [DOI] [PubMed] [Google Scholar]
- 14. Stiefel U, Pultz NJ, Harmoinen Jet al. Oral administration of β-lactamase preserves colonization resistance of piperacillin-treated mice. J Infect Dis 2003; 188: 1605–9. [DOI] [PubMed] [Google Scholar]
- 15. Pitout JDD. IPSAT P1A, a class A β-lactamase therapy for the prevention of penicillin-induced disruption to the intestinal microflora. Curr Opin Investig Drugs 2009; 10: 838–44. [PubMed] [Google Scholar]
- 16. Connelly S, Bristol JA, Hubert Set al. SYN-004 (ribaxamase), an oral β-lactamase, mitigates antibiotic-mediated dysbiosis in a porcine gut microbiome model. J Appl Microbiol 2017; 123: 66–79. [DOI] [PubMed] [Google Scholar]
- 17. Kokai-Kun JF, Roberts T, Coughlin Oet al. Use of ribaxamase (SYN-004), a β-lactamase, to prevent Clostridium difficile infection in β-lactam-treated patients: a double-blind, phase 2b, randomised placebo-controlled trial. Lancet Infect Dis 2019; 19: 487–96. [DOI] [PubMed] [Google Scholar]
- 18. de Gunzburg J, Ducher A, Modess Cet al. Targeted adsorption of molecules in the colon with the novel adsorbent-based medicinal product, DAV132: a proof of concept study in healthy subject. J Clin Pharmacol 2015; 55: 10–6. [DOI] [PubMed] [Google Scholar]
- 19. de Gunzburg J, Ghozlane A, Ducher Aet al. Protection of the human gut microbiome from antibiotics. J Infect Dis 2018; 217: 628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinquier J-L, Varastet M, Meyers Det al. A colon-targeted adsorbent (DAV132) does not affect the pharmacokinetics of warfarin or clonazepam in healthy subjects. Clin Pharmacol Drug Dev 2021; 10: 908–17. [DOI] [PubMed] [Google Scholar]
- 21. Crobach MJT, Planche T, Eckert Cet al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 2016; 22Suppl 4: S63–81. [DOI] [PubMed] [Google Scholar]
- 22. Allen SJ, Wareham K, Wang Det al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013; 382: 1249–57. [DOI] [PubMed] [Google Scholar]
- 23. Burdet C, Sayah-Jeanne S, Nguyen TTet al. Antibiotic-induced dysbiosis predicts mortality in an animal model of Clostridium difficile infection. Antimicrob Agents Chemother 2018; 62: e00925-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris HC, Best EL, Normington Cet al. Optimization of an assay to determine colonization resistance to Clostridioides difficile in fecal samples from healthy subjects and those treated with antibiotics. Antimicrob Agents Chemother 2020; 65: e01401-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. R Core Team . R: A Language and Environment for Statistical Computing. 2020. https://www.r-project.org/. [Google Scholar]
- 26. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 1995; 57: 289–300. [Google Scholar]
- 27. LeBlanc JG, Milani C, de Giori GSet al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol 2013; 24: 160–8. [DOI] [PubMed] [Google Scholar]
- 28. Kim B-R, Shin J, Guevarra RBet al. Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol 2017; 27: 2089–93. [DOI] [PubMed] [Google Scholar]
- 29. Liss BJ, Vehreschild JJ, Cornely OAet al. Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection 2012; 40: 613–9. [DOI] [PubMed] [Google Scholar]
- 30. Freedberg DE, Zhou MJ, Cohen MEet al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med 2018; 44: 1203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donskey CJ, Chowdhry TK, Hecker MTet al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000; 343: 1925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lucet J-C, Armand-Lefevre L, Laurichesse J-Jet al. Rapid control of an outbreak of vancomycin-resistant enterococci in a French university hospital. J Hosp Infect 2007; 67: 42–8. [DOI] [PubMed] [Google Scholar]
- 33. Grall N, Massias L, Nguyen TTet al. Oral DAV131, a charcoal-based adsorbent, inhibits intestinal colonization by β-lactam-resistant Klebsiella pneumoniae in cefotaxime-treated mice. Antimicrob Agents Chemother 2013; 57: 5423–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huttner BD, de Lastours V, Wassenberg Met al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: a randomized clinical trial. Clin Microbiol Infect 2019; 25: 830–8. [DOI] [PubMed] [Google Scholar]
- 35. Seong H, Lee SK, Cheon JHet al. Fecal microbiota transplantation for multidrug-resistant organism: efficacy and response prediction. J Infect 2020; 81: 719–25. [DOI] [PubMed] [Google Scholar]
- 36. Dubberke ER, Mullane KM, Gerding DNet al. Clearance of vancomycin-resistant Enterococcus concomitant with administration of a microbiota-based drug targeted at recurrent Clostridium difficile infection. Open Forum Infect Dis 2016; 3: ofw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuijper EJ, Vendrik KEW, Vehreschild MJGT. Manipulation of the microbiota to eradicate multidrug-resistant Enterobacteriaceae from the human intestinal tract. Clin Microbiol Infect 2019; 25: 786–9. [DOI] [PubMed] [Google Scholar]
- 38. Burdet C, Sayah-Jeanne S, Nguyen TTet al. Protection of hamsters from mortality by reducing fecal moxifloxacin concentration with DAV131A in a model of moxifloxacin-induced Clostridium difficile colitis. Antimicrob Agents Chemother 2017; 61: e00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saint-Lu N, Burdet C, Sablier-Gallis Fet al. DAV131A protects hamsters from lethal Clostridioides difficile infection induced by fluoroquinolones. Antimicrob Agents Chemother 2019; 64: e01196-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.