Abstract
Yeast strains disrupted in the PDR1, PDR3, or PDR5 gene, but not in SNQ2, exhibited higher sensitivity to mucidin (strobilurin A) than did the isogenic wild-type strains. Different gain-of-function mutations in the PDR1 and PDR3 genes rendered yeast mutants resistant to this antibiotic. Mucidin induced PDR5 expression, but the changes in the expression of SNQ2 were only barely detectable. The results indicate that PDR5 provides the link between transcriptional regulation by PDR1 and PDR3 and mucidin resistance of yeast.
The antifungal antibiotic mucidin (strobilurin A) has been successfully used in clinical treatment of dermatomycoses (7) and has become a valuable tool in biochemical and molecular genetic studies with yeasts (10). Mucidin specifically inhibits electron transport in the cytochrome bc1 complex of the mitochondrial respiratory chain (16). Resistance to mucidin in the yeast Saccharomyces cerevisiae results from mutations mapping in either mitochondrial or nuclear genes (17). The nuclear mucidin-resistant mutants displayed a pleiotropic drug resistance phenotype due to the mutations in the PDR3 gene (17, 18). This gene has been cloned, sequenced (4), and subjected to mutational analysis (13). It specifies a transcriptional activator homologous to that encoded by the PDR1 gene (4). PDR1 and PDR3 together with the drug efflux pump-encoding genes like PDR5 and SNQ2 create the PDR network of genes (8) which, together with another network of stress response genes activated by the transcription factor YAP1 (11), are involved in multiple drug resistance in S. cerevisiae. Their homologues were also identified in the yeast Candida albicans and contribute significantly to the fungicide resistance of this most prominent fungal pathogen of humans (15).
The aim of the present study was to analyze the molecular mechanisms of mucidin susceptibility in S. cerevisiae using different mutants of the PDR network of genes involved in yeast multidrug resistance.
The strains of S. cerevisiae used in this study were derived from the wild-type strains FY1679-28C (MATa ura3-52 trp1Δ63 leu2Δ1 his3Δ200) (1, 4) and YPH500 (MATα ura3-52 trp1Δ63 leu2Δ1 his3Δ200 lys2-801amb ade2-101ochr) (9). The PDR3 gene and its mutant alleles were cloned on centromeric plasmid pFL38-PP3 (ARS1 CEN4 URA3 PDR3) under the control of its promoter (13). Six mutant alleles (pdr3-4 to pdr3-11) contained gain-of-function mutations in the N terminus (13), and another six mutant alleles (pdr3-15 to pdr3-20) contained mutations in the C-terminal moiety of PDR3 (J. Subik, A. Nourani, A. Delahodde, T. Simonic, V. Subikova, and C. Jacq, Abstr. Sixth Int. Mycol. Congr., p. 19, 1998). Yeast strains were grown in YPGE medium (1% Bacto Peptone, 1% yeast extract, 2% glycerol, and 2% ethanol) or in a minimal medium containing 0.67% yeast nitrogen base without amino acids and 2% glucose or 2% glycerol plus 2% ethanol. The appropriate nutritional requirements and drugs were added at the indicated concentrations. Solid media were prepared with 2% agar. Bacterial strains were grown in Luria-Bertani medium supplemented with ampicillin at 50 μg/ml.
Plasmid DNA from Escherichia coli TG1 cells was prepared by the alkaline lysis method and used to transform S. cerevisiae as described previously (13). The sensitivity of yeast cells to mucidin was assayed by measuring the MICs (13) and by determination of growth inhibition zones on the solid YPGE medium (17). Mucidin resistance of a set of independent transformants was scored for each of the DNA constructs after 5 to 12 days of growth at 30°C.
The abundance of Pdr5p and Snq2p in total yeast extracts was determined by Western blot analysis as described previously (5). Equivalent protein loading in each lane was verified by probing the immunoblots with polyclonal antibodies against Pgk1p. The level of Pdr5p after mucidin response was quantitated by densitometric scanning of the developed film using Pharmacia Biotech ImageMaster 1D software.
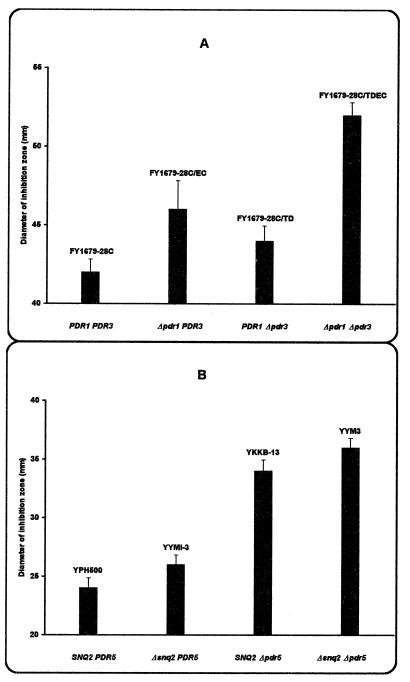
The susceptibility of the yeast S. cerevisiae to mucidin was studied using the isogenic sets of mutants derived from two wild-type strains, FY1679-28C and YPH500. It was found that the strains with the disrupted PDR1 or PDR3 gene were significantly more sensitive to mucidin than a corresponding wild-type strain (Fig. 1A). The disruption of both PDR1 and PDR3 led to the highest level of mucidin sensitivity, indicating that the two transcriptional activators play a significant role in the control of mucidin susceptibility of yeast cells.
FIG. 1.
Sensitivity to mucidin of yeast strains with deletions of PDR1 and PDR3 (A) or of PDR5 and SNQ2 (B). The amount of mucidin applied to the paper disk was 1 μg (A) and 0.5 μg (B). The bars represent the standard deviations.
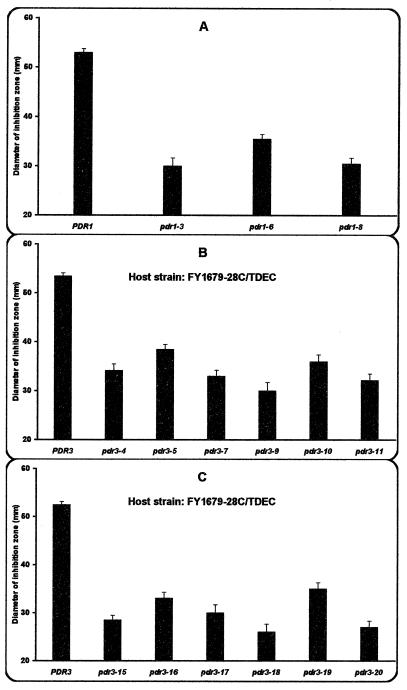
Whereas the disruption of the PDR1 and/or the PDR3 gene led to an increased sensitivity of cells to mucidin, all independently isolated gain-of-function mutations in the PDR1 gene or in the PDR3 gene resulted in mucidin resistance. In a genetic background of Δpdr3 strains (1), the most resistant was the strain containing the chromosomal pdr1-3 allele and less resistant was the strain with the pdr1-6 allele (Fig. 2A). Different levels of mucidin resistance were also observed in the isogenic transformants of the Δpdr1Δpdr3 host strain FY1679-28C/TDEC bearing on a centromeric plasmid the wild-type PDR3 gene or the pdr3 mutant alleles containing mutations either in the central regulatory domain (Fig. 2B) or in the C-terminal activation domain (Fig. 2C).
FIG. 2.
Resistance to mucidin of the isogenic strains bearing different pdr1 (A) and pdr3 (B and C) mutant alleles. The amount of mucidin applied to the disk was 5 μg. The bars represent the standard deviations.
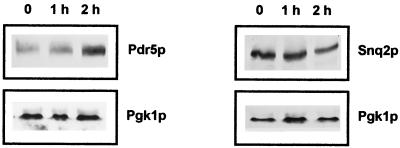
Mucidin added to the wild-type strain FY1979-28C grown in minimal glucose medium at a concentration (0.4 μg/ml) that inhibits cell growth on nonfermentable carbon sources but does not affect the growth on glucose induced an increased expression of PDR5. The abundance of Pdr5p in cells grown in the presence of mucidin for 1 and 2 h was 2.0 and 2.7 times higher, respectively, than that in the control cells (Fig. 3). Under similar conditions, no significant changes were observed in the level of Snq2p, indicating that mucidin is an inducer and apparently also a substrate of Pdr5p in S. cerevisiae. A similar induction of expression of the drug transporter genes like PDR5 (12), SNQ2 (12), YOR1 (2), and PDR12 (14) was already demonstrated by their respective substrates—cycloheximide, cations, 4-nitroquinoline-N-oxide, reveromycin, and sorbate.
FIG. 3.
Immunological detection of Pdr5p and Snq2p in the wild-type strain FY1679-28C exposed to mucidin.
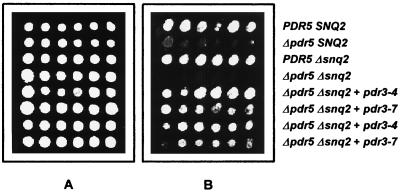
To assess whether mucidin is a substrate for Pdr5p, the susceptibility to mucidin was determined in the strains with deletion either of single PDR5 and SNQ2 or simultaneously of both genes (9). As shown in Fig. 1B, in zone-of-inhibition assays the mucidin sensitivity of Δsnq2 disruptant YYMI-3 was only slightly higher than that of the wild-type strain YPH500. Such a small change in mucidin sensitivity was not observed in the spot test experiments (Fig. 4). On the other hand, in both experiments the Δpdr5 strain YKKB-13 exhibited clear hypersensitivity to mucidin which was faintly elevated when the strain YYM3 possessed the disruptions in both genes (Δpdr5 and Δsnq2) (Fig. 1B). However, the transformants of this strain containing the pdr3-4 or the pdr3-7 allele on the centromeric plasmid were still able to grow in the presence of 0.05 μg of mucidin per ml, which already prevented the growth of the host strain with deletion simultaneously of both PDR5 and SNQ2 (Fig. 4). Under the same conditions, no growth of the wild-type strain YPH500, Δpdr5Δsnq2 double mutant YYM3, and its transformants was observed at a mucidin concentration of 0.1 μg per ml (data not shown).
FIG. 4.
Mucidin susceptibility of the strain YYM3 with deletions of PDR5 and SNQ2 and transformed with the pdr3 mutant alleles. Cells were grown for 5 days at 30°C on YPGE medium containing mucidin at concentrations of 0.025 (A) and 0.05 (B) μg/ml.
These results indicate that mucidin-inducible Pdr5p is a primary membrane transporter responsible for mucidin resistance. Nevertheless, another as-yet-unidentified drug transporter protein(s) different from that of Pdr5p and Snq2p but overexpressible by the mutated Pdr3p can also modulate mucidin sensitivity. In fact, 29 members of the superfamily of ATP binding cassette transporters (3) and 28 members of the major facilitator superfamily (6) were identified in the complete S. cerevisiae genome. However, in comparison with Pdr5p their contribution to mucidin efflux seems to be only marginal.
Acknowledgments
We thank C. Jacq, A. Delahodde, E. Balzi, A. Goffeau, Y. Mahe, and K. Kuchler for plasmids, strains, antibodies, and many helpful discussions.
This work was supported by grants from the Slovak Grant Agency of Science (VEGA) and from the Slovak Ministry of Education. The research of J.S. was also supported by an International Research Scholars Grant from the Howard Hughes Medical Institute (U.S.A.).
REFERENCES
- 1.Carvajal E, Van Den Hazel H B, Cybularz-Kolaczkowska A, Balzi E, Goffeau A. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol Gen Genet. 1997;256:406–415. doi: 10.1007/s004380050584. [DOI] [PubMed] [Google Scholar]
- 2.Cui Z, Hirata D, Tsuchiya E, Osada H, Miyakawa T. The multidrug resistance-associated protein (MRP) subfamily (Yrs1/Yor1) of Saccharomyces cerevisiae is important for the tolerance to a broad range of organic anions. J Biol Chem. 1996;271:14712–14716. doi: 10.1074/jbc.271.25.14712. [DOI] [PubMed] [Google Scholar]
- 3.Decottignies A, Goffeau A. Complete inventory of the yeast ABC proteins. Nat Genet. 1997;15:137–145. doi: 10.1038/ng0297-137. [DOI] [PubMed] [Google Scholar]
- 4.Delaveau T, Delahodde A, Carvajal E, Subik J, Jacq C. PDR3, a new yeast regulatory gene, is homologous to PDR1 and controls the multidrug resistance phenomenon. Mol Gen Genet. 1994;244:501–511. doi: 10.1007/BF00583901. [DOI] [PubMed] [Google Scholar]
- 5.Egner R, Mahe Y, Pandjaitan R, Kuchler K. Endocytosis and vacuolar degradation of the plasma membrane-localized Pdr5 ATP-binding cassette multidrug transporter in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:5879–5887. doi: 10.1128/mcb.15.11.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goffeau A, Park J, Paulsen I T, Jonniaux J L, Dinh T, Mordant P, Saier M H., Jr Multidrug-resistant transport proteins in yeast: complete inventory and phylogenetic characterization of yeast open reading frames within the major facilitator superfamily. Yeast. 1997;13:43–54. doi: 10.1002/(SICI)1097-0061(199701)13:1<43::AID-YEA56>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Kejda J. Mucidin—ein neues antimyzetisches antibiotikum. Castellania. 1976;4:193–194. [Google Scholar]
- 8.Kolaczkowski M, Goffeau A. Active efflux by multidrug transporters as one of the strategies to evade chemotherapy and novel practical implications of yeast pleiotropic drug resistance. Pharmacol Ther. 1997;76:219–242. doi: 10.1016/s0163-7258(97)00094-6. [DOI] [PubMed] [Google Scholar]
- 9.Mahe Y, Lemoine Y, Kuchler K. The ATP binding cassette transporters Pdr5 and Snq2 of Saccharomyces cerevisiae can mediate transport of steroids in vivo. J Biol Chem. 1996;271:25167–25172. doi: 10.1074/jbc.271.41.25167. [DOI] [PubMed] [Google Scholar]
- 10.Michalkova-Papajova D, Subik J. Mucidin—clinically used antifungal antibiotic and a tool in the biological research. Biol Listy. 1999;64:137–156. . (In Slovak.) [Google Scholar]
- 11.Miyahara K, Hirata D, Miyakawa T. yAP-1- and yAP-2-mediated, heat shock-induced transcriptional activation of the multidrug resistance ABC transporter genes in Saccharomyces cerevisiae. Curr Genet. 1996;29:103–105. doi: 10.1007/BF02221572. [DOI] [PubMed] [Google Scholar]
- 12.Miyahara K, Mizunuma M, Hirata D, Tsuchiya E, Miyakawa T. The involvement of the Saccharomyces cerevisiae multidrug resistance transporters Pdr5p and Snq2p in cation resistance. FEBS Lett. 1996b;399:317–320. doi: 10.1016/s0014-5793(96)01353-1. [DOI] [PubMed] [Google Scholar]
- 13.Nourani A, Papajova D, Delahodde A, Jacq C, Subik J. Clustered amino acid substitutions in the yeast transcription regulator Pdr3p increase pleiotropic drug resistance and identify a new central regulatory domain. Mol Gen Genet. 1997;256:397–405. doi: 10.1007/s004380050583. [DOI] [PubMed] [Google Scholar]
- 14.Piper P, Mahe Y, Thompson S, Pandjaitan R, Holyoak C, Egner R, Muhlbauer M, Coote P, Kuchler K. The Pdr12 ABC transporter is required for the development of weak organic acid resistance in yeast. EMBO J. 1997;17:4257–4265. doi: 10.1093/emboj/17.15.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanglard D, Ischer F, Calobrese D, de Micheli M, Bille J. Multiple resistance mechanisms to azole antifungals in yeast clinical isolates. Drug Resist Updates. 1998;1:255–265. doi: 10.1016/s1368-7646(98)80006-x. [DOI] [PubMed] [Google Scholar]
- 16.Subik J, Behun M, Musilek V. Antibiotic mucidin, a new antimycin A-like inhibitor of electron transport in rat liver mitochondria. Biochem Biophys Res Commun. 1974;57:17–22. doi: 10.1016/s0006-291x(74)80351-7. [DOI] [PubMed] [Google Scholar]
- 17.Subik J, Kovacova V, Takacsova G. Mucidin resistance in yeast. Isolation, characterization and genetic analysis of nuclear and mitochondrial mucidin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1977;73:275–286. doi: 10.1111/j.1432-1033.1977.tb11317.x. [DOI] [PubMed] [Google Scholar]
- 18.Subik J, Ulaszewski S, Goffeau A. Genetic mapping of nuclear mucidin resistance mutations in Saccharomyces cerevisiae. A new pdr locus on chromosome II. Curr Genet. 1986;10:665–670. doi: 10.1007/BF00410914. [DOI] [PubMed] [Google Scholar]