Abstract
Objective
Our prospective study aims to define the correlation of EGFR(epidermal growth factor receptor) mutations with major histological subtypes of lung adenocarcinoma from resected and non-resected specimens, according to the WHO 2015 classification, in Moroccan North East Population.
Methods
Epidermal growth factor receptor mutations of 150 primary lung adenocarcinoma were performed using Real-Time PCR or SANGER sequencing. SPSS 21 was used to assess the relationship between histological subtypes of lung adenocarcinoma and EGFR mutation status.
Results
25 mutations were detected in the series of 150 lung adenocarcinomas, most of which were found in cases with papillary, acinar, patterns than without these patterns and more frequently occurred in the cases without solid pattern than with this pattern. A significant correlation was observed between EGFR mutation and acinar (P = 0,024), papillary pattern (P = 0,003) and, negative association with a solid pattern (P < 0,001). In females, EGFR mutations were significantly correlated with the acinar pattern (P = 0,02), whereas in males with the papillary pattern (P = 0,01). Association between the histologic component and exon 19 deletions and exon 21 mutations were also evaluated and, we found a significant correlation between the papillary major pattern with exon 19 mutations (P = 0,004) and, ex21 with the acinar component (P = 0,03).
Conclusion
An analysis of resected and non-resected lung ADC specimens in 150 Moroccan Northeast patients, revealed that acinar and papillary patterns may predict the presence of a mutation in the EGFR gene. While the solid major pattern may indicate a low mutation rate of the EGFR gene.
Keywords: EGFR mutations, histology, adenocarcinoma, biomarker, cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death worldwide. Non–small-cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers. Among the 3 major cell types of NSCLC (adenocarcinoma, squamous cell carcinoma, and large cell carcinoma), adenocarcinoma (ADC) is the most frequent histologic type of primary lung cancer. It is well known that lung ADCs have heterogeneous morphologic aspects and diverse new properties with the discovery of driver mutations, 1 which may require to promote tumor growth. The most common driver oncogene is the epidermal growth factor receptor (EGFR), and mutations in its intracellular domain have been observed in 47% of cases. 2 Mutant status of EGFR is predicting factor for the response to EGFR tyrosine kinase inhibitors (TKIs) and, the good prognosis of advanced NSCLC patients.3,4 Most patients with a metastatic disease whose tumors harbor EGFR activating mutations have substantial clinical and radiographic responses to EGFR tyrosine kinase inhibitors (TKIs) osimertinib, gefitinib, erlotinib, and afatinib.3,5,6 EGFR-mutated lung ADCs define a unique group with special features. EGFR mutations affect the EGFR tyrosine kinase domain, in its first 4 exons (18 through 21), they are more common in females, never smokers, and patients of Asian ethnicity. However, in the past few years, several studies correlated adenocarcinoma subtypes with EGFR mutations. Some authors report that the presence of EGFR mutations7,8 is related to the lepidic subtype. Others suggest that the acinar predominant,9,10 papillary predominant,11,12 and micropapillary predominant,8,11 subtypes had higher rates of EGFR gene mutations. For patients with advanced disease, the diagnosis of lung tumors is most often performed on small biopsies such as bronchoscopic biopsy or percutaneous fine-needle aspiration. The 2015 World Health Organization (WHO) classification of non resected lung adenocarcinoma has contained a major change. 13 In this context, the IASLC/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS), has proposed a new subclassification of lung adenocarcinoma in non resected specimens (small biopsy and cytological specimens), which will provide a powerful new tool for the characterization of tumor pathology and appropriate management. 14
Our prospective study aims to define the correlation of EGFR mutations with major histological subtypes of lung adenocarcinoma from resected and non-resected specimens, according to the WHO 2015 classification, in Moroccan North East Population.
Methods
Ethics
Ethics committee approval for this study was approved by the faculty of Medicine and Pharmacy Ethics Committee of Casablanca, Morocco, according to Helsinki Declaration under reference 17/15. Written informed consent was obtained for all patients and, all their relative data were de-identified.
Patients
A prospective study was conducted among 150 formalin-fixed paraffin-embedded (FFPE) tumors, obtained from resected (4,7%), and non-resected specimens (95,3%). Patients diagnosed with primary lung ADC were included between November 2017 and October 2020, in the department of pathology of Hassan II University Hospital, Fez, Morocco. Patients and tumor characteristics, such as age, gender, smoking status, histology, and tumor sample type were extracted from the medical records of patients. Smoking classes were defined as: Light smokers = patients who had smoked 10 or less than 10 cigarettes per day. Heavy smokers = patients who had smoked more than 20 cigarettes/day. Never smokers = patients who had never smoked. 15 The reporting of this study conforms to STROBE guidelines. 16
Histopathological Examination
All specimens were obtained from primary sites. Hematoxylin and eosin-stained slides of FFPE tumors were reviewed from 95,3% of bronchial biopsies and 4,7% of resection specimens. Histological subclassification of lung ADC was registered by a pneumatological pathologist according to the WHO 2015 classification for small biopsies. The growth pattern of lung adenocarcinoma may differ between primary tumors and metastatic sites, for that, the histological pattern of lung adenocarcinoma was only determined for biopsies taken from the primary tumor, metastatic biopsies were excluded. The pure tumor was referred for samples with a single major pattern. Tumors with 2 or more patterns were defined as combined tumors. The cellular atypia, tumor-infiltrating lymphocytes, stromal fibrosis, and tumor necrosis were also studied. Cellular atypia was described in terms of the shape, color, or size of abnormal cells compared to normal cells in the same sample. Minimal atypia was defined when cells appear 1.5-fold abnormal than normal cells; moderate atypia was referred to cells that are twice the size of normal cells; high atypia was described for pleomorphic cells.
The degree of tumor-infiltrating lymphocytes was defined by the density and the extent of the lymphocytes cells population invading tumor cells. The methodology for counting tumor-infiltrating lymphocytes was somewhat based on the scoring method used in breast cancer specimens. Lymphocytic infiltrate was defined as minimal: number of TILs <10 per tumor section; moderate: TILs 10-40 and, high: TILs> 40 per tumor section. Stromal fibrosis was defined by the density of connective tissue that directly surrounds tumor cells. Minimal stromal fibrosis meant that the sample was composed of a large number of tumor foci surrounded by a small amount of fibrosis (<25% per tumor section). Moderate stromal fibrosis intermediated minimal stromal fibrosis and high stromal fibrosis (25 – 50% per tumor section). High stromal fibrosis meant that the sample mainly consisted of fibrosis (>50% per tumor section) surrounding a few tumor cells. Tumor necrosis was determined by its presence or absence in the tumor tissue.
Mutational Analysis of EGFR Gene
Epidermal growth factor receptor mutation testing was performed in formalin-fixed paraffin-embedded tissue samples obtained from the primary lung tumor.
DNA extraction: The percentage of tumor cells was performed by a pathologist on hematoxylin, safran, and eosin-stained slides. DNA was extracted from only the surrounded tumor area. QIAamp DNA FFPE Tissue Kit (Invitrogen) was used for DNA isolation according to the manufacturer’s instructions.
The presence of EGFR mutations was determined for 150 patients. According to the percentage of tumor cells, 2 different methods were used for the detection of EGFR mutations. For tumors with a low percentage of tumor cells <30%, a Real Time PCR was performed using the therascreen EGFR RGQ PCR Kit which is designed to detect the most commonly reported 29 EGFR mutations. DNA amplification was performed using PCR for exon 18, 19, 20, and 21 with specific primers. Automatic direct sequencing (the current gold standard) with BigDye Terminator V3.1 Cycle Sequencing Kit (ABI Prism) was used only for samples with tumor cells >30%.
Statistical Analysis
All analyses were recorded using SPSS (version 21; SPSS Inc., Chicago, IL). The correlation of EGFR mutations and several parameters were analyzed using the Chi-square test or Fisher's exact test. Tests were statistically significant when P < 0 .05
Results
Epidermal Growth Factor Receptor Mutations and Demographics
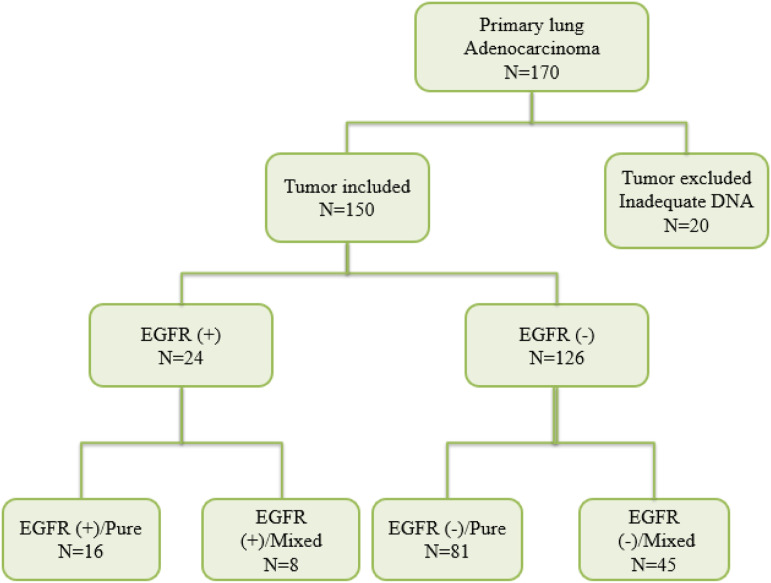
20 out of 170 tumors were excluded due to lack of tumor material or degraded DNA extracted, so 150 patients were finally enrolled (Figure 2). The clinical characteristics of patients with tumors harboring EGFR mutations are summarized in (Table 1). One patient showed multiple mutations in different exons (p. L858 R in exon 21 plus p. S768I in exon 20), so 25 mutations were detected in the series of 150 (16,6%) patients. The mean age of mutation positive was 63,38 years (range, 43-79) and was somewhat higher than that of negative cases (60,12). Out of 42 females included in the study, 26,19% (11/42) showed positive EGFR mutation compared to 11,9% (13/109) of males and the association between mutations and female gender was statistically significant (P = 0,034). As expected, most patients whose tumors harbor EGFR positive mutation were never smokers (60,8%) (P < 0.0001) as compared to (30,02%) in patients with EGFR wild-type tumors.
Figure 2:
The flowchart used to divide tumors into different groups. N: number of tumors; EGFR (+): EGFR positive mutation; EGFR (−): EGFR negative mutation.
Table 1:
Demographics of patients with EGFR mutation.
| N | EGFR positive | EGFR negative | P value | |
|---|---|---|---|---|
| Gender | ||||
| Female | 42 | 11 (45.8%) | 31 (24.6%) | P = 0.03 |
| Male | 108 | 13 (54.2%) | 95 (75.4%) | |
| Age | ||||
| <60 years | 72 | 9 (37.5%) | 63 (50%) | P = 0.1 |
| >60 years | 78 | 15 (62.5%) | 63 (50%) | |
| Smoking status | ||||
| Never | 55 | 17 (70.8%) | 38 (30.2%) | P < 0.0001 |
| Ever | 95 | 7 (29.2%) | 88 (69.8%) | |
| Sampling techniques | ||||
| Endoscopic | 80 | 15 (62.5%) | 65 (51.6%) | P = 0.1 |
| Computed tomography | 48 | 5 (20.8%) | 43 (34.1%) | |
| Surgical biopsy | 15 | 1 (4.2%) | 14 (11.1%) | |
| Lobectomy | 7 | 3 (12.5%) | 4 (3.2%) | |
| Cellular atypia | ||||
| Mild | 10 | 2 (8.3%) | 8 (6.3%) | |
| Moderate | 93 | 19 (79.2%) | 74 (58.7%) | P = 0.04 |
| Marked | 47 | 3 (12.5%) | 44 (34.9%) | |
| Fibrous stroma | ||||
| Mild | 39 | 6 (25%) | 33 (26.2%) | P = 0.08 |
| Moderate | 84 | 17 (70.8%) | 67 (53.2%) | |
| Marked | 27 | 1 (4.2%) | 26 (20.6%) | |
| Lymphocytes infiltration | ||||
| Low | 79 | 11 (45.8%) | 68 (54%) | P = 0.1 |
| Moderate | 55 | 12 (50%) | 43 (34.1%) | |
| High | 16 | 1 (4.2%) | 15 (11.9%) | |
| Tumor necrosis | ||||
| Present | 42 | 7 (29.2%) | 35 (27.8%) | P = 0.1 |
| Absent | 108 | 17 (70.8%) | 91 (72.2%) | |
| Tumor size | ||||
| <2 cm | 6 | 3 (15%) | 3 (2.5%) | P = 0.03 |
| >2 to <3 cm | 9 | 1 (5%) | 8 (6.5%) | |
| >3 to <5 cm | 33 | 3 (15%) | 33 (25%) | |
| >5 cm | 93 | 13 (65%) | 80 (66%) | |
| Pathological stage | ||||
| Ia/Ib or IIa/IIb | 7 | 2 (9.5%) | 5 (4.2%) | P = 0.2 |
| IIIa or IIIb | 13 | 2 (9.5%) | 11 (9.2%) | |
| IV | 119 | 17 (81%) | 103 (86.6%) |
Epidermal Growth Factor Receptor Mutations and Histologic Subtypes
Regarding histological subtypes, most tumors that we evaluated (n = 150; 60,7%) had a single growth pattern. 53 adenocarcinomas (35,3%) had ≥2 growth patterns (mixed tumors), Figure 1. In our study, there were 7 lobectomies, out of which 5 had mixed tumors. Out of the 150 tumors included, the acinar growth pattern was the most frequently occurring major pattern, constituting 59,3%, followed by solid 46%, papillary 22,7%, mucinous 4%, micropapillary 2,7%, lepidic and enteric 1,3%. Furthermore, the rates of EGFR mutations in acinar, papillary, solid, and lepidic subtypes were 79,2%, 45,8%, 4,2%, and 4,2%, respectively (Table 2). Significant correlation was revealed between EGFR mutation and papillary (P = 0,003), acinar (P = 0,024) component. In females, EGFR mutations were significantly correlated with the acinar pattern (P = 0,02), whereas in males with a papillary pattern (P = 0,01). EGFR mutations were more frequently found in specimens with acinar or papillary patterns than without these patterns (Table 3). We also analyzed the relationship between EGFR mutations and cellular atypia, fibrous stroma and, tumor-infiltrating lymphocytes. Moderate atypia was found in 79.2% of the EGFR mutated group, as compared to 58% in the WT group. 70,8% of EGFR positive tumors had moderate fibrous stroma versus 53,2% in the WT group. Tumor-infiltrating lymphocytes were moderate in 50% of EGFR mutated-tumors, while 54% of the WT EGFR group were minimally infiltrated. A significant correlation between EGFR mutations and moderate atypia (P = 0,04) was registered. The correlation between the histologic component and exon 19 deletions and exon 21 mutations were also evaluated and, we found a significant correlation between the papillary major pattern with exon 19 mutations (P = 0,004) and, ex21 with the acinar component (P = 0,03).
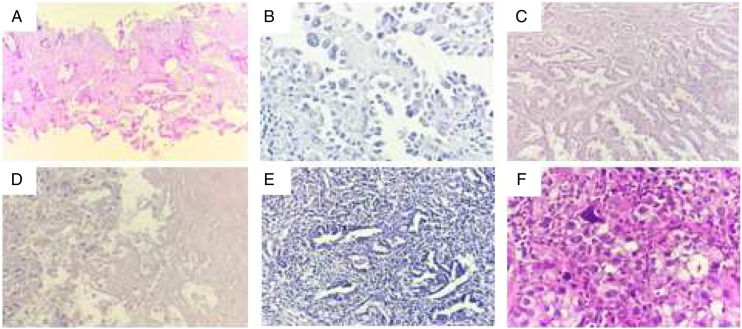
Figure 1:
Histological features of lung ADC: A. Acinar subtype; B. Papillary subtype; C. Mixed subtype « acinar and papillary »; D. Tumor necrosis; E. Tumor-infiltrating lymphocytes; F. Cellular atypia.
Table 2.
Epidermal growth factor receptor mutation in the major pattern of lung ADC.
| Main Histology | Female | Male | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | EGFR Positive | EGFR Negative | N | EGFR Positive | EGFR Negative | N | EGFR Positive | EGFR Negative | ||
| Acinar | 89 | 19 (21.3%) | 70 (78.6%) | 22 | 9 (40.9%) | 13 (59.09%) | 67 | 10 (14.9%) | 57 (85.0%) | |
| Papillary | 34 | 11 (32.3%) | 23 (67.6%) | 7 | 4 (57.1%) | 3 (42.8%) | 27 | 7 (25.9%) | 20 (74%) | |
| Solid | 69 | 1 (1.4%) | 68 (98.5%) | 19 | 0 (0%) | 19 (100%) | 50 | 1 (0.02%) | 49 (98%) | |
| Mixed tumor | 25 | 6 (24%) | 19 (76%) | 3 | 2 (66.6%) | 1 (33.3%) | 22 | 4 (18.18%) | 18 (81.8%) | |
| Acinar and papillary | ||||||||||
Table 3.
Association between EGFR mutation and histological component in lung ADC.
| Female | Male | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histological subtype of ADC | N | EGFR positive | EGFR negative | P-value | N | EGFR positive | EGFR negative | P-value | N | EGFR positive | EGFR negative | P-value |
| Acinar | ||||||||||||
| With | 89 | 19 (79.2%) | 70 (55.6%) | P = 0.02 | 22 | 9 (81.8%) | 13 (41.9%) | P = 0.02 | 67 | 10 (76.9%) | 57 (60%) | P = 0.2 |
| Without | 61 | 5 (20.8%) | 56 (44.4%) | 20 | 2 (18.2%) | 18 (58.1%) | 41 | 3 (23.1%) | 38 (40%) | |||
| Papillary | ||||||||||||
| With | 34 | 11 (45.8%) | 23 (18.3%) | P = 0.003 | 7 | 4 (36.4%) | 3 (9.7%) | P = 0.06 | 27 | 7 (53.8%) | 20 (21.1%) | P = 0.01 |
| Without | 116 | 13 (54.2%) | 103 (81.7%) | 26 | 7 (63.6%) | 28 (90.3%) | 81 | 6 (42.2%) | 75 (87.9%) | |||
| Solid | ||||||||||||
| With | 69 | 1 (4.2%) | 68 (54%) | P < 0.001 | 19 | 0 (0%) | 19 (61.3%) | P < 0.001 | 50 | 1 (7.7%) | 49 (51.6%) | P = 0.003 |
| Without | 81 | 23 (95.8%) | 58 (46%) | 23 | 11 (100%) | 12 (38.7%) | 58 | 12 (92.3%) | 46 (48.4%) | |||
| Mixed acinar and papillary | ||||||||||||
| With | 25 | 6 (25%) | 19 (15.1%) | P = 0.1 | 3 | 2 (18.2%) | 1 (3.2%) | P = 0.09 | 22 | 4 (30.8%) | 18 (18.9%) | P = 0.2 |
| Without | 125 | 18 (75%) | 107 (84.9%) | 39 | 9 (81.8%) | 30 (96.8%) | 86 | 9 (69.2%) | 77 (81.1%) | |||
Epidermal Growth Factor Receptor Mutations Distribution
Regarding the distribution by exons of EGFR mutations, the main types were in-frame deletions in exon 19 (17/25, 68%), followed by the missense substitutions in exon 21 (6/25, 24%). The only 1 mutation detected in exon 20 (1/25.4%) was found in the patient with combined mutations. Just 1 patient had an exon 18 mutation (4%).
Discussion
It has been largely reported that EGFR mutations are associated with specific features, such as female gender, never-smoking history, ethnicity, and lung ADC.17-19 The relationship between histological subtypes of lung adenocarcinoma and EGFR mutations has been extensively investigated.11,20-23 However, most studies were performed only on surgically resected tumors. For patients with advanced disease, the diagnosis is most often performed on a small biopsy, so the histological features of EGFR mutation in inoperably advanced lung ADC have not been well defined. 2015 WHO classification has proposed a new subclassification of lung adenocarcinoma in non resected specimens, which will provide a powerful new tool for the characterization of tumor pathology and appropriate management. 14 In this study, 16,6% of 150 primary lung ADC had positive mutations in the EGFR gene. The mutations tend to be more common among women than men and never-smokers versus ever smokers, they were more likely to be female. These profiles and prevalences are consistent with findings noted in prior studies from Asia including Korea, China, and Japan.24-26
Some studies described that patients characterized by papillary, micropapillary, and lepidic patterns carried EGFR mutations more frequently than others.23,27,28 According to the new classification, Campos-Parra et al reported a positive correlation between EGFR mutations and the presence of acinar or lepidic components through evaluating 313 sample tissue of lung adenocarcinoma obtained by a biopsy. 29 In a series of 186 specimens, including 135 small biopsy and 51 cell blocks made from cytology specimens, Maturu et al showed a higher frequency of EGFR mutation in the lepidic predominant pattern and a negative correlation with solid-predominant adenocarcinoma. 30 However, Weng et al found a higher prevalence in moderately differentiated (acinar or papillary) ADCs. 31
The relationship between EGFR mutations and histological subtype in metastatic lung adenocarcinoma was firstly reported by Clay et al. In this study of 100 metastatic specimens, the EGFR mutations were most frequently found in major acinar and micropapillary pattern tumors and were rarely found in major solid pattern tumors. 32 Similar results have been reported in a series of 59 brain metastases samples, the wild-type EGFR gene was significantly more frequent in cases with solid components compared with acinar and papillary subtypes. 33
In line with our results, Kim et al, in their study reported on 356 biopsies and 3 resections, patients with the papillary, lepidic, and acinar patterns were more likely to have EGFR positive mutations. 34 In our series, the histological subtype of primary lung ADC based on the 2015 WHO classification was carried out in 150 patients. Our results showed that EGFR mutation was more frequently found in the acinar major pattern, followed by papillary, lepidic, and solid components. EGFR mutation was the most frequently found in cases with papillary, acinar, patterns than without these patterns and more frequently occurred in the cases without solid pattern than with this pattern. A significant correlation was observed between EGFR mutation and acinar (P = 0,024), papillary pattern (P = 0,003) and, negative association with a solid pattern.
Similar results have been reported by Wang et al, which showed that EGFR mutation was significantly correlated with the presence of an acinar component (P < 0.001), papillary component (P = 0.034) in a series of 395 resected samples. 35 In contrast with what has been reported by Campos-Parra et al, Maturu et al, and Kim et al, a low frequency of EGFR mutations was seen in the lepidic major pattern. A possible reason for this difference was that there were just 2 lepidic major pattern adenocarcinomas in our series, and one of them displayed E746_A750del Exon 19 deletion. The results of this study suggest that the rate of EGFR mutations varies not only by geographic region, demographic factors, and ethnicity but also among histological subtypes.
Because EGFR mutations are more frequent in the female gender, we separated women from men, to reveal the difference in histological subtypes with EGFR mutation by gender. In females, EGFR mutations were more frequently existed in samples with an acinar component than without it. While in males, EGFR mutations were more frequently found in the papillary major pattern. In contrast with what has been reported by Chen et al, 36 the difference of histological subtype with EGFR mutation in gender was significant.
In a large variety of cancers, tumor infiltration lymphocytes are considered as one of the most important indicators of tumor prognosis. 37 In NSCLC, the presence of EGFR mutation is involved in changing the tumor microenvironment. 38 Feng et al found an association between low density of tumor infiltration lymphocyte and sensitive EGFR mutation, the density of lymphocyte infiltration was lower in the EGFR mutated cases (42.9% vs 53.7%). 38 In contrast with what has been reported by Feng et al in our series, the number of patients who had a moderate density of tumor infiltration lymphocytes in the EGFR positive tumors was higher than that observed in the WT group (50% vs 34,1%). This difference could be explained by the use of different scoring methods or different types of samples (biopsies vs resection specimens).
Epidermal Growth Factor Receptor exon mutation distribution in our population was similar to that generally reported in the Middle East and Africa literature. 39 It has been well reported that mutations in exon 19 and 21 are the most frequent of all mutations (80-90%).40,41 In our subjects, the EGFR mutation most frequently detected was a short in-frame deletion in exon 19 (68%), the missense substitution L858R and L861Q in exon 21 was observed only in 24% of cases which show similarity with Noronha et al and Gaura et al studies.42,43 The present study also supports a significant correlation between EGFR mutations and moderate cellular atypia (P = 0,02), exon 19 deletions with papillary major pattern (P = 0,004) and, ex21 mutations with acinar growth pattern (P = 0,03).
The present study has several limitations. First, it is a prospective study performed in a single-institution which limits reproducibility and generalization to other populations. Second, the small sample size may have affected the results, limiting the extrapolation of the findings to the general population, so the results should be confirmed in a larger series in the future. Third, tumor specimens were mostly obtained from small biopsies, which may have influenced the accuracy of pathological interpretation.
In summary, an analysis of resected and non resected lung ADC specimens in 150 Moroccan Northeast patients, revealed that female gender, non-smokers, acinar, and papillary patterns may predict the presence of a mutation in the EGFR gene. However, the EGFR test must be performed for all patients with primary lung ADC, since the histology of ADC cannot fully predict the presence of EGFR mutations.
Acknowledgments
This project is a joint initiative of the Moffitt Cancer Center and the Hassan II University Hospital.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Ethics committee approval for this study was approved by the faculty of Medicine and Pharmacy Ethics Committee of Casablanca, Morocco, under reference 17/15.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the in accordance with Declaration Helsinki approved protocols.
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
ORCID iD
Sara Boukansa https://orcid.org/0000-0003-4297-3380
References
- 1.Teng P, Darcy N, Hamilton K, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. Gynecol Oncol 2015;136(3):554-561. doi: 10.1016/j.ygyno.2014.12.035.Pharmacologic.25560806 [DOI] [Google Scholar]
- 2.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-Small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res. 2015;5(9):2892-2911. [PMC free article] [PubMed] [Google Scholar]
- 3.Takamochi K, Oh S, Matsunaga T, Suzuki K. Prognostic impacts of EGFR mutation status and subtype in patients with surgically resected lung adenocarcinoma. J Thorac Cardiovasc Surg. 2017;154(5):1768-1774. e1, doi: 10.1016/j.jtcvs.2017.06.062. [DOI] [PubMed] [Google Scholar]
- 4.Hong W, Wu Q, Zhang J, Zhou Y. Prognostic value of EGFR 19-del and 21-L858R mutations in patients with non-small cell lung cancer. Oncol Lett 2019;18(4):3887-3895. doi: 10.3892/ol.2019.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liam C, The role of osimertinib in epidermal growth factor receptor ( EGFR ) -mutant non-small cell lung cancer. J. Thrac. Dis 2019;11(6):448–452, doi: 10.21037/jtd.2018.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuda H, Park E, Yun C-H. et al. Structural , Biochemical , and Clinical Characterization of Epidermal Growth Factor Receptor ( EGFR ) Exon 20 Insertion Mutations in Lung Cancer. Sci Transl Med. 2013;5(216):216ra177, doi: 10.1126/scitranslmed.3007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonobe M, Manabe T, Wada H, Tanaka F. Mutations in the epidermal growth factor receptor gene are linked to smoking-independent, lung adenocarcinoma. Br J Cancer. 2005;93(3):355-363. doi: 10.1038/sj.bjc.6602707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol 2013;30(3):1-4. doi: 10.1007/s12032-013-0645-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res. 2012;18(7):1947-1953. doi: 10.1158/1078-0432.CCR-11-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell PA, Barnett SA, Walkieeicz M, et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol. 2013;8(4):461-468. doi: 10.1097/JTO.0b013e3182828fb8. [DOI] [PubMed] [Google Scholar]
- 11.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: Analysis of 440 Japanese patients. J Thorac Oncol. 2013;8(1):52-61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 12.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: Modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32(6):810-827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 13.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10(9):1240-1242. doi: 10.1097/JTO.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 15.Okuyemi KS, Ahluwalia JS, Richter KP, Mayo MS, Resnicow K. Differences among African American light, moderate, and heavy smokers. Nicotine Tob Res. 2001;3(1):45-50. doi: 10.1080/14622200020032097. [DOI] [PubMed] [Google Scholar]
- 16.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbrouckef JP. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies*. Bull World Health Organ. 2007;85(11):867. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee K, Ray A, Chattopadhyay B. Incidence and characteristics of epidermal growth factor receptor (EGFR) mutation in non-small-cell lung cancer (Adenocarcinoma histology): A report of 106 patients from Kolkata. Indian J Cancer. 2017;54(1):305-307. doi: 10.4103/ijc.IJC_239_17. [DOI] [PubMed] [Google Scholar]
- 18.Xia J, Li H, Ji Y, et al. Clinicopathologic characteristics and EGFR mutations in lung cancer patients aged below 45 years. Curr Probl Cancer. 2018;43(4):363-370. doi: 10.1016/j.currproblcancer.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Zhou S, Hu X, Wang Y, et al. Clinicopathologic characteristics and outcome of patients with different EGFR mutations. Asia Pac J Clin Oncol. 2019;15(3):166-171. doi: 10.1111/ajco.13072. [DOI] [PubMed] [Google Scholar]
- 20.Mansuet-Lupo A, Babbio A, Blons H, et al. The new histologic classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: The experience of a French cohort. Chest. 2014;146(3):633-643. doi: 10.1378/chest.13-2499. [DOI] [PubMed] [Google Scholar]
- 21.Hung JJ, Jeng W-J, Chou T-Y, et al. Prognostic value of the new international association for the study of lung cancer/American thoracic society/European respiratory society lung adenocarcinoma classification on death and recurrence in completely resected stage i lung adenocarcinoma. Ann Surg. 2013;258(6):1079-1086. doi: 10.1097/SLA.0b013e31828920c0. [DOI] [PubMed] [Google Scholar]
- 22.Hu H, Pan Y, Li Y. et al. Oncogenic mutations are associated with histological subtypes but do not have an independent prognostic value in lung adenocarcinoma. OncoTargets Ther. 2014;7:1423-1437. doi: 10.2147/OTT.S58900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuta K, Kwago M, Inoue E. et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer. 2013;81(3):371-376. doi: 10.1016/j.lungcan.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Noronha V, Dikshit R, Raut N. et al. Epidemiology of lung cancer in India: Focus on the differences between non-smokers and smokers: A single-centre experience. Indian J Cancer. 2012;49(1):74-81. doi: 10.4103/0019-509X.98925. [DOI] [PubMed] [Google Scholar]
- 25.Wu YL, Zhong W-Z, Li L-Y. et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: A meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007;2(5):430-439. doi: 10.1097/01.JTO.0000268677.87496.4c. [DOI] [PubMed] [Google Scholar]
- 26.Yang P-C, Shi Y, Au JS-K, et al. Molecular epidemiological prospective study of EGFR mutations from Asian patients (pts) with advanced lung adenocarcinoma (PIONEER). J. Clinc. Oncol. 2012;30(15_suppl l):1534. doi: 10.1200/JCO.2012.30.15_SUPPL.1534. [DOI] [Google Scholar]
- 27.Li H, Pan Y, Li Y, et al. Frequency of well-identified oncogenic driver mutations in lung adenocarcinoma of smokers varies with histological subtypes and graduated smoking dose. Lung Cancer. 2013;79(1):8-13. doi: 10.1016/j.lungcan.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Warth A, Penzel R, Lindenmaier H, et al. EGFR, KRAS, BRAF and ALK gene alterations in lung adenocarcinomas: Patient outcome, interplay with morphology and immunophenotype. Eur Respir J. 2014;43(3):872-883. doi: 10.1183/09031936.00018013. [DOI] [PubMed] [Google Scholar]
- 29.Campos-parra AD, Avile A, Contreras-reyes S, Rojas-marı CE, Relevance of the novel IASLC/ATS/ERS classification of lung adenocarcinoma in advanced disease. Eur Respir J. 2014;43(5);1439–1447, doi: 10.1183/09031936.00138813. [DOI] [PubMed] [Google Scholar]
- 30.Maturu VN, Singh N, Bal A, Gupta N, Das A, Behera D. Relationship of epidermal growth factor receptor activating mutations with histologic subtyping according to International sssociation for the study of lung cancer/American thoracic society/European respiratory society 2011 adenocarcinoma classificatication and their impact on overall survival. Lung India; 2016;33:257-266. 10.4103/0970-2113.180801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng CF, Huang C-J, Huang SH, et al. New international association for the study of lung cancer (IASLC) pathology committee grading system for the prognostic outcome of advanced lung adenocarcinoma. Cancers. 2020;12(11):1-14. doi: 10.3390/CANCERS12113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clay TD, Do H, Sundararajan V, et al. The clinical relevance of pathologic subtypes in metastatic lung adenocarcinoma. J Thorac Oncol 2014;9(5):654-663. doi: 10.1097/JTO.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi H, Hamasaki M, Morishita T, Yoshimura M. Clinicopathological and genetic characteristics associated with brain metastases from lung adenocarcinoma and utility as prognostic factors. Oncol Lett 2018;16(4):4243-4252. 10.3892/ol.2018.9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HJ, Choi EY, Jin HJ, Shin KC. Relationship between EGFR mutations and clinicopathological features of lung adenocarcinomas diagnosed via small biopsies. Anticancer Res. 2014;34(6):3189-3196. [PubMed] [Google Scholar]
- 35.Wang T, Zhang Y, Liu B, Hu M, Zhou N, Zhi X. Associations between epidermal growth factor receptor mutations and histological subtypes of lung adenocarcinoma according to the IASLC/ATS/ERS classification in Chinese patients. Thorac. Cancer. 2017;8(6):600-605. doi: 10.1111/1759-7714.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Liu X, Zhao J, Yang H, Teng X. Correlation of EGFR mutation and histological subtype according to the IASLC/ATS/ERS classification of lung adenocarcinoma. Int J Clin Exp Pathol. 2014;7(11):8039-8045. [PMC free article] [PubMed] [Google Scholar]
- 37.Biomarkers II, Group W. HHS Public Access. 2018;24(6).
- 38.Feng W, Fu X, Cai X, Zhang Q, Li Y, Shen L. Association of tumor In filtrating lymphocytes quanti fi cation with EGFR mutations in completely resected stage IIIA ( N2 ) lung adenocarcinoma mutational features associated with immunoreactivity in non-small cell lung cancer regulation of glycodelin E. J Thorac Oncol;12(1):S820. doi: 10.1016/j.jtho.2016.11.1109. [DOI] [Google Scholar]
- 39.Benbrahim Z, Antonia T, Mellas N. EGFR mutation frequency in Middle East and African non-small cell lung cancer patients: A systematic review and meta-analysis. BMC Cancer. 2018;18(1):1-6. doi: 10.1186/s12885-018-4774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol. 2009;4(1):22-29. doi: 10.1097/JTO.0b013e3181914111. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka T, Matsuoka M, Sutani A, et al. Frequency of and variables associated with the EGFR mutation and its subtypes. Int J Cancer. 2010;126(3):651-655. doi: 10.1002/ijc.24746. [DOI] [PubMed] [Google Scholar]
- 42.Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: Clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):1-5. doi: 10.1371/journal.pone.0061561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaur P, Bhattacharya S, Kant S, Kushwaha RAS, Singh G, Pandey S. EGFR mutation detection and its association with clinicopathological characters of lung cancer patients. World J Oncol. 2018;9(5–6):151-155. doi: 10.14740/wjon1167. [DOI] [PMC free article] [PubMed] [Google Scholar]