Abstract
Objective
This study examined the relationship of the Sharp score with demographic factors and clinical immune-inflammatory markers in patients with anemia in rheumatoid arthritis (RA).
Methods
The clinical data of 1057 patients with RA and anemia and 1006 patients with RA without anemia were retrospectively analyzed. Spearman’s correlation coefficient analysis, association rule analysis, and logistic regression were used to study the relationships between the Sharp score and influencing factors in patients with RA and anemia.
Results
The incidence of anemia was 51.24% (1057/2063), and mild anemia accounted for 81.93% (866/1057) of cases. Spearman’s correlation coefficient and association rule analyses revealed that the Sharp score of patients with RA and anemia was correlated with immune-inflammatory response and anemia. Logistic regression analysis illustrated that advanced age (>60 years), female, low serum iron levels, C-reactive protein positivity, and immunoglobulin A positivity were risk factors for a high Sharp score (>28.25) in patients with RA and anemia.
Conclusion
The Sharp score is closely related to clinical disease activity and anemia, and it should be considered in the treatment strategy of patients with RA and anemia.
Keywords: Rheumatoid arthritis, anemia, Sharp score, inflammation, risk factors, disease activity, immune-inflammatory response, X-ray imaging
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that causes joint pain and structural damage.1,2 RA is treated by achieving clinical remission and reducing inflammation and joint destruction.3,4 RA usually involves multiple systems throughout the body, and anemia is a common extra-articular manifestation of RA, affecting 30% to 70% of patients. 5 However, anemia has not received sufficient attention from clinicians. Anemia is strongly related to the disease activity of RA, and it can be used to predict early joint injury of RA. 6
Among the factors correlated with poor prognosis in patients with RA, the degree of bone erosion detected by joint imaging is considered a pivotal reference index. 7 Preventing joint destruction in the early stages of the disease is one of the most important goals of RA treatment. X-ray examination of both hands is the most frequently used imaging method to assess bone destruction in RA. Clinically, RA (stages I–IV) is graded according to the presence of joint space stenosis and bone erosion,8,9 but there are certain defects because of the lack of a specific quantitative evaluation. The modified Sharp/van der Heijde score can quantify the degree of joint space narrowing and bone erosion in both hands with good intra-observer reliability and sensitivity to changes,10,11 and it is used as a scoring method in clinical practice for structural damage in RA.12,13 Several studies used the Sharp score to assess disease progression and clinical efficacy in patients with RA.14–15 Compared with the Larsen/Scott method, the smallest detectable difference of the Sharp/van der Heijde method more accurately reflects the minimal clinically important difference of early RA joint damage. 16 However, few studies have reported the factors affecting the Sharp score of patients with RA and anemia, and these studies were limited by small sample sizes. 6
This research team has long been committed to the investigation of multisystem lesions in RA.17–20 Through a retrospective analysis of previously hospitalized patients with RA and anemia, this study examined the diagnostic value of the Sharp score in patients with RA and anemia and its influencing factors, thus providing a reference for the clinical evaluation of disease progression and treatment efficacy.
Materials and methods
Patients and study design
Our study retrospectively analyzed the clinical data of patients with RA hospitalized at the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (Hefei, China) from January 2012 to December 2019. All subjects met the 2010 American College of Rheumatology/European Alliance of Associations for Rheumatology diagnostic criteria for RA. 21 All patients with RA and anemia met the diagnostic criteria for anemia defined by the World Health Organization. The exclusion criteria of this study were as follows: 1) Sjögren’s syndrome, adult-onset Still’s disease, ankylosing spondylitis, and other autoimmune diseases; 2) anemia secondary to tumors, infections, or other diseases; and 3) incomplete or non-standard imaging data. This research application was reviewed by the Ethics Institution Committee of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (2019AH-12), and its protocol complies with the ethical guidelines of the 1975 Declaration of Helsinki. The ethics committee waived the requirement for informed consent because this was a retrospective study, only the clinical data of the patients were collected, the patient’s treatment plan was not altered, and the design of the study plan and the data collection process fully protected personal privacy.
Study population and data collection
The data and information of all patients were obtained from the Hospital Information System, which consisted of demographic, peripheral blood laboratory examination, and imaging examination data. Demographic data comprised age, gender, and length of hospital stay. To protect patient privacy, the data extraction and collection process de-identified all patient details. The reporting of this study conforms to the EQUATOR and STROBE guidelines.22,23
Laboratory indicators of patients
The examined laboratory examination indicators of enrolled patients were as follows: red blood cell (RBC) count, hemoglobin (Hb), serum calcium (Ca), serum iron (Fe), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), α1-acid glycoprotein (α1-AGP), rheumatoid factor (RF), anti-cyclic citrullinated peptide (anti-CCP) antibody, immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM). The aforementioned indicators were all measured in the inspection center of the hospital.
X-ray examination of both hands
The Sharp score was calculated by two radiologists blinded to our research. Bone erosion scores were measured in 16 areas of both hands, and joint space narrowing scores were determined for 15 areas. The joint bone erosion score ranged from 0 to 5 points, and the joint space stenosis score ranged from 0 to 4 points. The total bone erosion score of each area was 160 points, and the total joint space stenosis score of each area ranged from 0 to 120 points. The total Sharp scores were the sum of these two scores, ranging from 0 to 280 points.
Association rule analysis
Indicators, including ESR, CRP, RF, anti-CCP, IgA, IgG, IgM, complement component 3 (C3), C4, and α1-AGP, with increased levels were set as “T,” whereas indicators with normal or reduced levels were set as “F.” Indicators, including the RBC count, serum Fe, and serum Ca, with decreased levels were designated “T,” whereas indicators with normal or elevated levels were set as “F.” A length of hospital stay exceeding the median value (>16.95 days) was set as “T.” Sharp score >28.25 was set as “T,” whereas Sharp score ≤28.25 was designated “F.” IBM SPSS modeler 18.0 software (IBM, Armonk, NY, USA) was used to analyze the correlation among observation indicators. The formula of association rules was employed by referring to the published literature of Fang et al. 24
Binary logistic regression analysis
Indicators, including ESR, CRP, RF, anti-CCP, IgA, IgG, IgM, C3, C4, and α1-AGP, with increased levels were set as “1,” whereas indicators with normal or diminished levels were set as “0.” Indicators, including the RBC count, serum Fe, and serum Ca, with lower levels were set as “1,” and indicators with normal or increased levels were set as “0.” Length of hospital stay > 16.95 days (median duration) was set as “1.” Sharp score > 28.25 was set as “1,” and Sharp score ≤ 28.25 was set as “0.” In the current research, the Sharp score was adopted as the dependent variable, and other factors were used as independent variables. First, univariate analysis was performed to obtain the results of the preliminary study. Based on positive univariate results, multivariate analysis was performed to investigate risk factors for high Sharp scores.
Statistical analysis
Enumerated data were expressed as the number of cases or percentages, and the chi-squared test was applied for comparisons between the two groups. Continuous variables were represented as the median and interquartile range (IQR), and the rank-sum test was used to compare data between the two groups. The Spearman correlation coefficient method and association rules were adopted to assess the correlations of the Sharp score with various factors. The factors influencing the Sharp score were analyzed using a binary logistic regression model. SPSS software (version 24.0, IBM, Armonk, NY, USA) and GraphPad software (version 8.0, GraphPad Software Inc., San Diego, CA, USA) were applied for data statistics and mapping. P < 0.05 was considered statistically significant.
Results
Characteristics of all patients
The study sample consisted of 1057 patients with RA and anemia and 1006 patients with RA without anemia, giving an incidence of anemia of 51.24%. Among the patients with anemia, 883 (83.84%) were women and 174 (16.46%) were men, and their median age was 56 years (IQR = 49–66). The median hospital stay was 16.95 days (IQR = 11.97–22.73), and the median Sharp score was 34.00 (IQR = 13.00–82.00). Among the patients without anemia, 832 (82.70%) were women and 174 (17.30%) were men, and their median age was 55 years (IQR = 48–65). The median hospital stay was 15.14 days (IQR = 10.89–20.00), and the median Sharp score was 13.00 (IQR = 5.38–27.50). Compared with patients with RA without anemia, patients with RA and anemia had longer hospital stays (P < 0.01) and higher Sharp scores (P < 0.01; Table 1).
Table 1.
Baseline characteristics of the participants.
| Characteristic | RA with anemia (N = 1057) | RA without anemia (N = 1006) | P |
|---|---|---|---|
| Age, years, median (IQR) | 56 (49, 66) | 55 (48, 65) | 0.09 |
| Male, n (%) | 174 (16.46%) | 174 (17.30%) | 0.61 |
| Female, n (%) | 883 (83.84%) | 832 (82.70%) | |
| Length of hospital stay,days, median (IQR) | 16.95 (11.97–22.73) | 15.14 (10.89–20.00) | <0.01 |
| Sharp score, median (IQR) | 34.00 (13.00–82.00) | 13.00 (5.38–27.50) | <0.01 |
| Length of hospital stay (days) | |||
| >2 to ≤15 days, n (%) | 388 (36.71) | 462 (45.92) | <0.01 |
| ≥15 to ≤30 days, n (%) | 582 (55.06) | 489 (48.61) | |
| >30 days, n (%) | 87 (8.23) | 55 (5.47) | |
| Sharp score | |||
| 0, n (%) | 11 (1.04) | 38 (3.78) | <0.01 |
| >0 to ≤50, n (%) | 628 (59.41) | 853 (84.79) | |
| >50 to ≤100, n (%) | 208 (19.68) | 105 (10.44) | |
| >100 to ≤150, n (%) | 124 (11.73) | 6 (0.60) | |
| >150, n (%) | 86 (8.14) | 4(0.40) | |
IQR, interquartile range.
Comparison of clinical indicators among subjects
The indicators were mainly classified as anemia-related hematological indicators (RBC and Hb), inflammatory indicators (ESR, CRP, and α1-AGP), and immune indicators (RF, anti-CCP, IgA, IgG, and IgM), in addition to serum Fe and Ca. Compared with patients with RA without anemia, the RBC count and Hb, serum Fe, and serum Ca levels were lower in patients with anemia (all P < 0.01), whereas the ESR and CRP, α1-AGP, RF, IgA, IgG, and IgM levels were higher (all P < 0.05). Conversely, there was no significant difference in anti-CCP levels between these two groups (Table 2).
Table 2.
Clinical laboratory parameters in patients with RA.
| Index | RA with anemia(N = 1057) | RA without anemia | P | Normal rangeof the index |
|---|---|---|---|---|
| RBC (1012/L, IQR) | M: 3.71 (3.49–3.97)(n = 174) | M: 4.67 (3.49–4.96)(n = 174) | <0.01 | M: 4.3–5.8 |
| F: 3.73 (3.45–3.98)(n = 883) | F: 4.18 (3.99–4.41)(n = 832) | <0.01 | F: 3.8–5.1 | |
| Hb (g/L, IQR) | M: 103 (94–111)(n = 174) | M: 139 (134–146)(n = 174) | <0.01 | M: 130–175 |
| F: 104 (94–111)(n = 883) | F: 122 (118–128)(n = 832) | <0.01 | F: 115–150 | |
| Fe (µmol/L, IQR) | 7.21 (4.83–10.62) | 12.23 (9.16–16.26)(n = 636) | <0.01 | 8.9–32.3 |
| Ca (mmol/L, IQR) | 2.19 (2.12–2.27) | 2.28 (2.20–2.37)(n = 980) | <0.01 | 2.11–2.52 |
| ESR (mm/hour, IQR) | M: 49 (26–70)(n = 174) | M: 24 (11–38)(n = 168) | <0.01 | M: 2–6 |
| F: 54 (34–77)(n = 883) | F: 29 (17–45)(n = 820) | <0.01 | F: 2–12 | |
| CRP (mg/L, IQR) | 27.45 (9.38–58.99) | 7.60 (2.27–22.04)(n = 1004) | <0.01 | <5 |
| α1-AGP (mg/mL, IQR) | 137 (103–172) | 104 (81–130)(n = 981) | <0.01 | 50–120 |
| RF (U/mL, IQR) | 109.20 (34.70–272.95) | 101.40 (33.20–208.50)(n = 1005) | 0.04 | ≤14 |
| anti-CCP (U/mL, IQR) | 203.73 (41.58–482.00) | 176.00 (38.42–482.00)(n = 976) | 0.65 | <4 |
| IgA (g/L, IQR) | 2.61 (1.97–3.44) | 2.36 (1.83–3.04)(n = 998) | <0.01 | 0.7–4 |
| IgG (g/L, IQR) | 13.85 (11.03–17.11) | 12.96 (10.72–15.20)(n = 832) | <0.01 | 7–16 |
| IgM (g/L, IQR) | 1.29 (2.12–2.27) | 1.22 (0.90–1.62)(n = 832) | 0.02 | 0.4–2.5 |
IQR, interquartile range; M, male; F, female; RBC, red blood cell; Hb, hemoglobin; Fe, serum iron; Ca, serum calcium; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; α1-AGP, α1-acid glycoprotein; RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptide antibody; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
The diagnostic value of the Sharp score in patients with RA and anemia
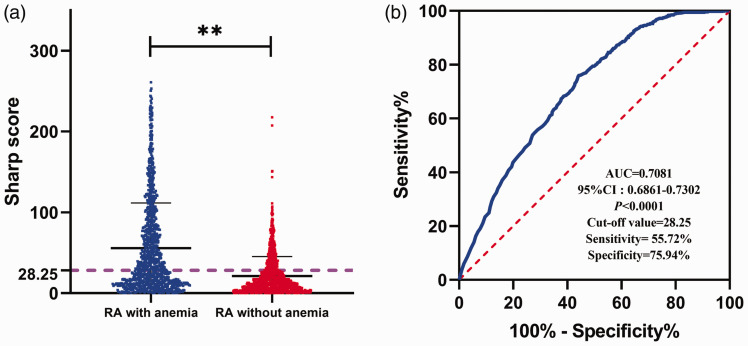
Compared with patients with RA without anemia, patients with RA and anemia had a higher Sharp score (Figure 1a). Receiver operating characteristic (ROC) curve analysis was performed to further determine the ability of the Sharp score to distinguish patients without and with anemia. The area under the ROC curve was 0.7081 (95% confidence interval [CI] = 0.6861–0.7302), and the best cut-off was 28.25. The optimal cut-off for the Sharp score for differentiating RA without and with anemia based on Youden’s index had a sensitivity of 55.72% and specificity of 75.94%, indicating that the Sharp score had good auxiliary diagnostic value (Figure 1b).
Figure 1.
Identification of the diagnostic value of the Sharp score for patients with RA and anemia. (a) The Sharp scores of the study subjects. **P < 0.01. (b) Receiver operating characteristic curve analysis to evaluate the diagnostic value of Sharp scores.
RA, rheumatoid arthritis; AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Summary of indicators in 1057 patients with RA and anemia
Further analysis of laboratory indicators of patients with RA and anemia revealed that 866 patients (81.93%) had mild anemia, 175 patients (16.56%) had moderate anemia, and 16 patients (1.51%) had severe anemia. In addition, 445 (42.10%) patients had an advanced age (>60 years), 528 (49.95%) patients had prolonged hospital stays (>16.95 days), 324 (30.65%) patients had low RBC counts, 678 (64.14%) patients had low Fe levels, and 244 (23.08%) patients had low Ca levels. The results for immune-inflammatory indicators were as follows: 1026 (97.07%) patients were ESR+, 876 (82.88%) patients were CRP+, 669 (63.29%) patients were α1-AGP+, 917 (86.75%) patients were RF+, 1002 (94.80%) patients were anti-CCP+, 158 (14.95%) patients were IgA+, 347 (32.83%) patients were IgG+, and 63 (5.96%) patients were IgM+ (Table 3).
Table 3.
Summary of laboratory indicators of all patients with RA and anemia.
| Indicator | Subjects (n) | % |
|---|---|---|
| Advanced age (>60 years) | 445 | 42.10 |
| Long hospital stay (>16.95 days) | 528 | 49.95 |
| Low RBC (M: <4.3 × 1012/L, F: <3.8 × 1012/L) | 324 | 30.65 |
| Mild anemia (Hb: ≥90 g/L) | 866 | 81.93 |
| Moderate anemia (Hb: o60 g/L to <90 g/L) | 175 | 16.56 |
| Severe anemia (Hb: ≥30 g/L to <60 g/L) | 16 | 1.51 |
| Extremely severe anemia (Hb: <30 g/L) | 0 | 0 |
| Low Fe (<8.9 µmol/L) | 678 | 64.14 |
| Low Ca (<2.11 mmol/L) | 244 | 23.08 |
| ESR+ (M: >6 mm/hour; F: >12 mm/hour) | 1026 | 97.07 |
| CRP+ (>5 mmol/L) | 876 | 82.88 |
| α1-AGP+ (>120 mg/mL) | 669 | 63.29 |
| RF+ (>14 U/mL) | 917 | 86.75 |
| anti-CCP+ (>4 U/mL) | 1002 | 94.80 |
| IgA+ (>4 g/L) | 158 | 14.95 |
| IgG+ (>16 g/L) | 347 | 32.83 |
| IgM+ (>2.5 g/L) | 63 | 5.96 |
RBC, red blood cell; Hb, hemoglobin; Fe, serum iron; Ca, serum calcium; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; α1-AGP, α1-acid glycoprotein; RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptide antibody; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
Spearman’s correlation coefficient analysis between the Sharp score and laboratory indicators
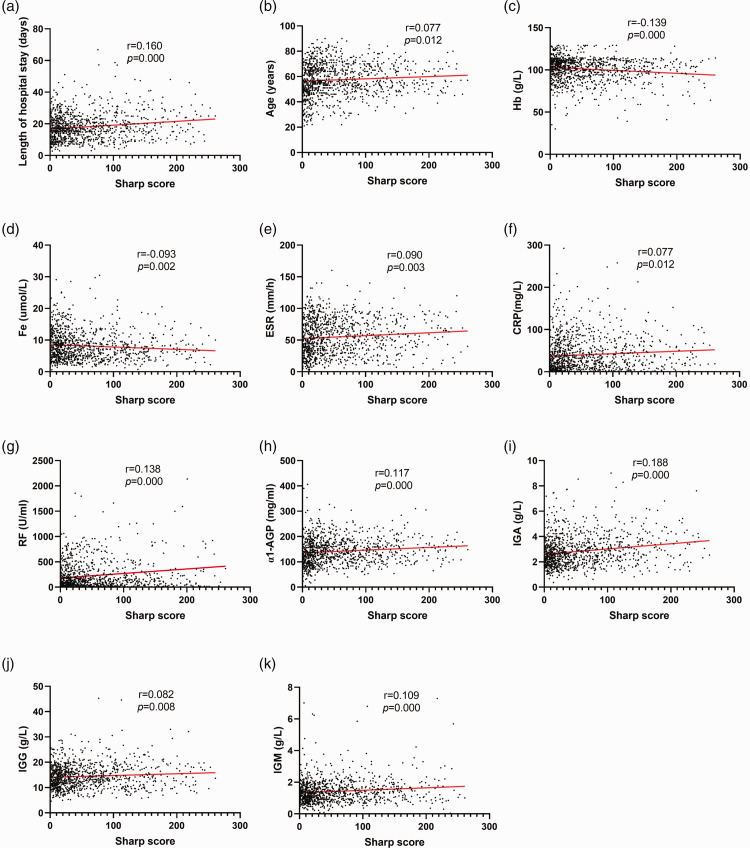
Spearman’s correlation coefficient analysis was conducted to dissect whether the Sharp score was associated with clinical indicators. The results documented that the Sharp score was positively correlated with length of hospital stay; age; ESR; and CRP, RF, α1-AGP, IgA, IgG, and IgM levels but negatively correlated with Hb and Fe levels (all P < 0.05; Figure 2).
Figure 2.
Spearman’s correlation coefficient analysis of the Sharp score and laboratory indicators. Correlation analyses were conducted between the Sharp score and (a) length of hospital stay, (b) age, (c) Hb, (d) Fe, (e) ESR, (f) CRP, (g) RF, (h) α1-AGP, (i) IgA, (j) IgG, and (k) IgM.
Hb, hemoglobin; Fe, serum iron; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RF, rheumatoid factor; α1-AGP, α1-acid glycoprotein; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
Association rule analysis between the Sharp score and laboratory indicators
The antecedent of the association rule was a potential influencing factor, and the consequent item was a high Sharp score (>28.25). Through association rule analysis, 10 association results were obtained. Association rule analysis revealed that the Sharp score (>28.25) was strongly associated with female, advanced age, long hospital stay, low RBC counts, low Fe and Ca levels, CRP+, α1-AGP+, RF+, and IgG+. The support degree of the aforementioned results was greater than 20%, and their confidence degree was greater than 50% (Table 4).
Table 4.
Association rule analysis of the Sharp score with laboratory indicators.
| Number | Antecedent | Consequent | Support (%) | Confidence (%) | χ 2 | P |
|---|---|---|---|---|---|---|
| 1 | Female | Sharp↑ | 83.54 | 57.08 | 193.36 | <0.01 |
| 2 | Advanced age | Sharp↑ | 42.10 | 59.78 | 33.98 | <0.01 |
| 3 | Long hospital stay | Sharp↑ | 49.95 | 58.90 | 7.06 | 0.01 |
| 4 | Low RBC | Sharp↑ | 30.65 | 54.63 | 135.39 | <0.01 |
| 5 | Low Fe | Sharp↑ | 64.14 | 60.18 | 15.6 | <0.01 |
| 6 | Low Ca | Sharp↑ | 23.08 | 61.07 | 235.8 | <0.01 |
| 7 | CRP+ | Sharp↑ | 82.88 | 60.16 | 183.14 | <0.01 |
| 8 | α1-AGP+ | Sharp↑ | 63.29 | 61.58 | 12.56 | <0.01 |
| 9 | RF+ | Sharp↑ | 86.75 | 56.60 | 248.38 | <0.01 |
| 10 | IgG+ | Sharp↑ | 32.83 | 60.23 | 112.28 | <0.01 |
Sharp↑, Sharp score greater than 28.25; Advanced age, age greater than 60 years; Long hospital stay, the length of hospital stay was more than 16.95 days; Low RBC, the RBC count was lower than 4.3 × 1012/L for male patients and lower than 3.8 × 1012/L for female patients; Low Fe, the Fe level was lower than 8.9 µmol/L; Low Ca, the Ca level was lower than 2.11 mmol/L; CRP+, the CRP level was 5 mmol/L or more; α1-AGP+, the α1-AGP level was more than 120 mg/mL; RF+, the RF level was more than 14 U/mL; IgG+, the IgG level was more than 16 g/L.
RBC, red blood cell; Fe, serum iron; Ca, serum calcium; CRP, C-reactive protein; α1-AGP, α1-acid glycoprotein; RF, rheumatoid factor; IgG, immunoglobulin G.
Binary logistic regression analysis of high Sharp scores and variables
To further determine the risk factors of the Sharp score, binary logistic regression analysis of single and multiple factors was conducted. Univariate analysis indicated that older age, female, long hospital stay, low Fe levels, CRP+, α1-AGP+, IgA+, and IgG+ were associated with increased risk of a high Sharp score (all P < 0.05). Based on these results, multivariate analysis was performed, and the results identified advanced age (odds ratio [OR] = 1.307; 95% CI = 1.001–1.708; P = 0.049), female (OR = 1.598; 95% CI = 1.126–2.269; P = 0.009), low Fe levels (OR = 1.331; 95% CI = 1.010–1.754; P = 0.042), CRP+ (OR = 2.269; 95% CI = 1.530–3.364; P = 0.001), and IgA+ (OR = 1.967; 95% CI = 1.333–2.901; P = 0.001) were risk factors for high Sharp scores (Table 5).
Table 5.
Binary logistic regression analysis of the Sharp score and influencing factors.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Advanced age | 1.330 (1.038–1.702) | 0.024 | 1.307 (1.001–1.708) | 0.049 |
| Female | 1.392 (1.005–1.929) | 0.046 | 1.598 (1.126–2.269) | 0.009 |
| Long hospital stay | 1.294 (1.105–1.650) | 0.038 | 1.213 (0.941–1.562) | 0.136 |
| Low RBC | 0.938 (0.721–1.220) | 0.643 | – | – |
| Low Fe | 1.653 (1.283–2.130) | <0.001 | 1.331 (1.010–1.754) | 0.042 |
| Low Ca | 1.330 (0.993–1.780) | 0.056 | – | – |
| ESR+ | 1.355 (0.663–2.769) | 0.406 | – | – |
| CRP+ | 2.289 (2.072–4.054) | <0.001 | 2.269 (1.530–3.364) | <0.001 |
| α1-AGP+ | 1.911 (1.483–2.462) | <0.001 | 1.240 (0.911–1.687) | 0.171 |
| RF+ | 1.304 (0.913–1.862) | 0.144 | – | – |
| anti-CCP+ | 0.767 (0.439–1.340) | 0.351 | – | – |
| IgA+ | 2.154 (1.492–3.110) | <0.001 | 1.967 (1.333–2.901) | 0.001 |
| IgG+ | 1.315 (1.103–1.707) | 0.039 | 1.044 (0.789–1.382) | 0.762 |
| IgM+ | 1.634 (0.954–2.800) | 0.074 | – | – |
Advanced age, age greater than 60 years; Long hospital stay, the length of hospital stay was more than 16.95 days; Low RBC, the RBC count was lower than 4.3 × 1012/L for male patients and lower than 3.8 × 1012/L for female patients; Low Fe, the Fe level was lower than 8.9 µmol/L; Low Ca, the Ca level was lower than 2.11 mmol/L; ESR+, the ESR was higher than 6 mm/hour in male patients and higher than 12 mm/hour in female patients; CRP+, the CRP level was 5 mmol/L or more; α1-AGP+, the α1-AGP level was more than 120 mg/mL; RF+, the RF level was more than 14 U/mL; anti-CCP+, the anti-CCP level was equal to or greater than 4 U/mL; IgA+, the IgA level was more than 4 g/L; IgG+, the IgG level was more than 16 g/L; IgM+, the IgM level was more than 2.5 g/L.
RBC, red blood cell; Fe, serum iron; Ca, serum calcium; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; α1-AGP, α1-acid glycoprotein; RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptide antibody; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
Discussion
RA is characterized by progressive joint destruction, and imaging is of great significance for the diagnosis and evaluation of RA. 25 This research was a large sample-based retrospective controlled analysis that explored the factors influencing the Sharp score for X-ray examination of both hands in patients with RA and anemia. The relevant indicators of anemia, clinical immune-inflammatory markers, and demographic factors were acquired as potential determinants of Sharp scores. This study unraveled that patients with RA and anemia had a higher Sharp score than their counterparts without anemia. In addition, age, length of hospital stay, ESR, CRP, RF, α1-AGP, IgA, IgG, and IgM were positively correlated with Sharp scores in RA patients with anemia, whereas Hb and Fe levels were inversely correlated. Meanwhile, advanced age, female, low Fe levels, CRP+, and IgA+ were risk factors for a high Sharp score (>28.25) in patients with RA and anemia.
RA-induced anemia is mostly inflammatory anemia, which can be triggered by the increased release of inflammatory cytokines. 26 Singh et al. 27 observed that decreased Hb levels were highly correlated with the disease activity of RA. Wilson et al. 28 found in a retrospective analysis of 19 studies of anemia associated with RA that the prevalence of mild anemia ranged from 33% to 60% and that patients with RA and anemia were more likely to experience more severe joint diseases. Cross-sectional and case-control research conducted by Chen et al. 29 elaborated that the incidence of anemia was 53.03% (472/890), and the rate of mild anemia was 71.19% (336/472). Moreover, they noted that in contrast to patients with RA without anemia, patients with RA and anemia had elevated disease activity and structural damage and worse joint function. In our research, the incidence of anemia in patients with RA was 51.24% (1057/2063), which was similar to that in prior studies. In addition, the proportion of patients with mild anemia was 81.93% (866/1057) in all patients with anemia, a slightly higher rate than those in prior studies, which may be related to the disease activities of the patients.
X-ray is a crucial evaluation method for the imaging examination of RA, and it is convenient, rapid, and inexpensive. 30 X-ray can quickly and integrally reveal joint lesions, such as joint space narrowing, bone erosion, joint subluxation or joint stiffness, deformity, and other lesions.31,32 This research unveiled that hospital stay was lengthened and Sharp scores; ESR; and CRP, α1-AGP, RF, IgA, IgG, and IgM levels were enhanced in patients with RA and anemia in comparison to the findings in patients with RA without anemia, whereas the RBC count and Hb, Fe, and Ca levels were diminished. Moreover, the results of ROC curve analysis manifested that the Sharp score had good value in the identification of anemia. Vegh et al. 33 conducted multicenter retrospective research that defined rapid radiation progression (RRP) as an increase of the Sharp score greater than or equal to 5 within 1 year and demonstrated that 18% of patients with RA had a high risk of RRP. In prospective cohort research, Pereira et al. 34 noted that leflunomide or the anti-TNFα antibody adalimumab repressed anemia in patients with RA and found a close association between anemia and enhanced ESR. Recent work indicated that serum Fe and ferritin levels are strongly correlated with osteoporosis in patients with RA. 35
In our study, the Sharp score was positively correlated with the length of hospital stay; age; ESR; and CRP, RF, α1-AGP, IgA, IgG, and IgM levels but negatively correlated with Hb and Fe levels. In addition, association rule analysis also indicated that the augmentation of Sharp scores was strongly correlated with female, older age, prolonged hospital stay, CRP+, α1-AGP+, RF+, IgG+, low Ca and Fe levels, and low RBC counts. Additionally, Chen et al. 29 observed inverse associations of serum Hb levels with a visual analog score, ESR, CRP, RF, the 28-joint disease activity score (DAS28), X-ray staging, and the Sharp score. Möller et al. 6 revealed that a low Hb level was an independent influencing factor of DAS28 for the progression of radiological joint injury in patients with early RA who received methotrexate. Our results suggested that advanced age, female, low Fe levels, CRP+, and IgA+ were risk factors for high Sharp scores in patients with RA and anemia. Our team’s previous studies identified gender, age, DAS28, RF, and the hand imaging grade as the influencing factors of the Sharp score in patients with RA. 36 However, the current study focused on patients with RA and anemia and newly identified low Fe levels as a potential risk factor. Anemia caused by RA is inflammatory anemia, which can lead to disturbances of Fe metabolism and inhibition of RBC production. 37
This research had several limitations. First, the proportion of female patients exceeded 80% in our study, indicating that the results may be biased and that our conclusions may not be completely applicable to male patients. Second, the patients were all recruited from a single hospital, and thus, larger multicenter studies are needed. Third, there were no analyses of the drugs used to treat patients in our research. Despite these limitations, the current research also had several advantages. First, we focused on secondary anemia, which is an extra-articular lesion in patients with RA. A retrospective analysis was conducted in the present research to explore the factors influencing Sharp scores in 1057 patients with RA and anemia, which has rarely been studied in China. Second, Spearman’s correlation analysis, association rule analysis, and logistic regression analysis were utilized to assess the correlations of different variables with Sharp scores, thus revealing that joint structure damage may be related to immune-inflammatory response and low Fe levels. In addition, this study could provide a valuable reference for evaluating the clinical significance of RA imaging examination, inflammation, and anemia.
In conclusion, the Sharp score was significantly higher in patients with RA and anemia, and a high Sharp score was closely related to clinical disease activity and anemia. In addition, advanced age, female, low Fe levels, CRP+, and IgA+ may be potential risk factors for high Sharp scores in patients with RA and anemia. Therefore, the Sharp score may be considered a potential factor in the treatment strategy of RA with anemia or used in monitoring disease progression.
Acknowledgements
We thank the Information Center, Imaging Examination Center, and Laboratory Center of the First Affiliated Hospital of Anhui University of Chinese Medicine for their support of this study.
Footnotes
Declaration of conflicting interests: The authors declare that they have no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by grants from the Ministry of Science and Technology National Key Research and Development Program Chinese Medicine Modernization Research Key Project (2018YFC1705204); National Nature Fund Program (82074373), The University Synergy Innovation Program of Anhui Province (GXXT-2020-025), Anhui Key Research and Development Program Foreign Science and Technology Cooperation Project (201904b11020011), Anhui Famous Traditional Chinese Medicine Liu Jian Studio Construction Project (Traditional Chinese Medicine Development Secret [2018] No. 11), Key Research and Development Plan Project of Anhui Province (201904a07020004), and The Key Laboratory of Xin’an Ministry of Medical Education (No. 2020xayx10).
Author contributions: Yanqiu Sun and Jian Liu were involved in the experimental design. Ling Xin, Yanqiu Sun, Jianting Wen, Qin Zhou, Xianheng Zhang, Xiang Ding, and Xiaolu Chen retrieved and analyzed the data. Yanqiu Sun wrote the manuscript. All authors reviewed and confirmed the final manuscript.
ORCID iD: Yanqiu Sun https://orcid.org/0000-0002-8361-5865
Data availability statement
The data in this study can be obtained from the corresponding author under reasonable request.
References
- 1.Arias DLRI, Escudero-Contreras A, Ruiz-Ponce M, et al. Molecular Changes in the Adipose Tissue Induced by Rheumatoid Arthritis: Effects of Disease-Modifying Anti-Rheumatic Drugs. Front Immunol 2021; 12: 744022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao F, Huang C, Cheng J, et al. β-arrestin-2 alleviates rheumatoid arthritis injury by suppressing NLRP3 inflammasome activation and NF-κB pathway in macrophages. Bioengineered 2022; 13: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthrit Care Res (Hoboken) 2016; 68:1–25. [DOI] [PubMed] [Google Scholar]
- 5.Song SN, Iwahashi M, Tomosugi N, et al. Comparative evaluation of the effects of treatment with tocilizumab and TNF-alpha inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther 2013; 15: R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moller B, Everts-Graber J, Florentinus S, et al. Low Hemoglobin and Radiographic Damage Progression in Early Rheumatoid Arthritis: Secondary Analysis From a Phase III Trial. Arthritis Care Res (Hoboken) 2018; 70: 861–868. [DOI] [PubMed] [Google Scholar]
- 7.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012; 64: 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukharani N, Dev K, Rahul F, et al. Association Between Rheumatoid Arthritis and Serum Vitamin D Levels. Cureus 2021; 13: e18255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirata S, Tanaka Y. [Imaging assessment of bone and cartilage destruction in rheumatoid arthritis]. Clin Calcium 2015; 25: 1777. [PubMed] [Google Scholar]
- 10.Heijde DD, Leeuwen M, Riel P, et al. Radiographic progression on radiographs of hands and feet during the first 3 years of rheumatoid arthritis measured according to Sharp's method (van der Heijde modification). J Rheumatol 1995; 22: 1792–1796. [PubMed] [Google Scholar]
- 11.Van DHD, Dankert T, Nieman F, et al. Reliability and sensitivity to change of a simplification of the Sharp/van der Heijde radiological assessment in rheumatoid arthritis. Rheumatology 1999: 941–947. [DOI] [PubMed] [Google Scholar]
- 12.Van der Heijde D, Kartman CE, Xie L, et al. Radiographic Progression of Structural Joint Damage Over 5 Years of Baricitinib Treatment in Patients With Rheumatoid Arthritis: Results From RA-BEYOND. J Rheumatol 2022; 49: 133–141. [DOI] [PubMed] [Google Scholar]
- 13.Mekic M, Hadzigrahic E, Dzubur A. Relation Between Anti-CCP Antibodies and Sharp Score in Rheumatoid Arthritis. Mater Sociomed 2020; 32: 172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis 2021; 80: 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combe B, Rincheval N, Berenbaum F, et al. Current favourable 10-year outcome of patients with early rheumatoid arthritis: data from the ESPOIR cohort. Rheumatology (Oxford) 2021; 60: 5073–5079. [DOI] [PubMed] [Google Scholar]
- 16.Bruynesteyn K, Van der Heijde D, Boers M, et al. Determination of the minimal clinically important difference in rheumatoid arthritis joint damage of the Sharp/van der Heijde and Larsen/Scott scoring methods by clinical experts and comparison with the smallest detectable difference. Arthritis Rheum 2002; 46: 913–920. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Cao Y. Effects of Xinfeng capsule on the Fas/FasL-mediated apoptotic pathway in patients with rheumatoid arthritis. J Tradit Chin Med 2018; 38: 601–609. [PubMed] [Google Scholar]
- 18.Cao Y, Guo Y, Wang Y, et al . Drug-containing serum of Xinfeng capsules protect against H9C2 from death by enhancing miRNA-21 and inhibiting toll-like receptor 4/phosphorylated p-38 (p-p38)/p-p65 signaling pathway and proinflammatory cytokines expression. J Tradit Chin Med 2018; 38: 359–365. [PubMed] [Google Scholar]
- 19.Jiang H, Liu J, Wang T, et al. Mechanism of Xinfeng Capsule on Adjuvant-Induced Arthritis via Analysis of Urinary Metabolomic Profiles. Autoimmune Dis 2016; 2016: 5690935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Wang Y, Huang C, et al. Efficacy and safety of Xinfeng capsule in patients with rheumatoid arthritis: a multi-center parallel-group double-blind randomized controlled trial. J Tradit Chin Med 2015; 35: 487–498. [DOI] [PubMed] [Google Scholar]
- 21.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 22.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 23.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 24.Fang Y, Liu J, Xin L, et al. Identifying Compound Effect of Drugs on Rheumatoid Arthritis Treatment Based on the Association Rule and a Random Walking-Based Model. Biomed Res Int 2020; 2020: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lautenbach M, Zach A, Eisenschenk A. Das Skaphoid bei Rheuma: Klassifikation anhand einer retrospektiven Röntgenanalyse [The scaphoid and rheumatoid arthritis: Classification by retrospective X-ray analysis]. Orthopade 2016; 45: 985–993. [DOI] [PubMed] [Google Scholar]
- 26.Favalli EG. Understanding the Role of Interleukin-6 (IL-6) in the Joint and Beyond: A Comprehensive Review of IL-6 Inhibition for the Management of Rheumatoid Arthritis. Rheumatol Ther 2020; 7: 473–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh H, Arya S, Talapatra P, et al. Assessment of fatigue in rheumatoid arthritis (by Functional Assessment of Chronic Illness Therapy-Fatigue score) and its relation to disease activity and anemia. J Clin Rheumatol 2014; 20: 87–90. [DOI] [PubMed] [Google Scholar]
- 28.Wilson A, Yu HT, Goodnough LT, et al. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med 2004; 116 Suppl 7A: 50S–57S. [DOI] [PubMed] [Google Scholar]
- 29.Chen YF, Xu SQ, Xu YC, et al. Inflammatory anemia may be an indicator for predicting disease activity and structural damage in Chinese patients with rheumatoid arthritis. Clin Rheumatol 2020; 39: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 30.Forslind K, Eberhardt K, Svensson B. Repair of Erosions in Patients with Rheumatoid Arthritis. J Rheumatol 2019; 46: 670–675. [DOI] [PubMed] [Google Scholar]
- 31.Nada D, Gaber R, Mahmoud AS, et al. Hyperuricemia Among Egyptian Rheumatoid Arthritis Patients. Is It an Association or an Inflammatory Marker? A Cross-Sectional Observational Study. Open Access Rheumatol 2021; 13: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid AB, Wiese M, Mcwilliams L, et al. Repeat serological testing for anti-citrullinated peptide antibody after commencement of therapy is not helpful in patients with seronegative rheumatoid arthritis. Intern Med J 2020; 50: 818–822. [DOI] [PubMed] [Google Scholar]
- 33.Vegh E, Gaal J, Geher P, et al . Assessing the risk of rapid radiographic progression in Hungarian rheumatoid arthritis patients. BMC Musculoskelet Disord 2021; 22: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira I, Sousa N, Pereira D, et al. Treatment with either leflunomide or adalimumab reduces anaemia in patients with rheumatoid arthritis. An Acad Bras Cienc 2018; 90: 2161–2166. [DOI] [PubMed] [Google Scholar]
- 35.Sato H, Takai C, Kazama JJ, et al. Serum hepcidin level, iron metabolism and osteoporosis in patients with rheumatoid arthritis. Sci Rep 2020; 10: 9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen J, Liu J, Xin L, et al. Effective factors on Sharp Score in patients with rheumatoid arthritis: a retrospective study. BMC Musculoskelet Disord 2021; 22: 865. Published 2021 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352: 1011–1023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study can be obtained from the corresponding author under reasonable request.