Abstract
While invasive meningococcal disease (IMD) is uncommon, it can result in serious sequelae and even death. In 2018 in the United States, the incidence of IMD per 100,000 people was 0.03 among adolescents 11−15 years of age, 0.10 among persons 16−23 years of age, and 0.83 among infants < 1 year of age. Serogroup B accounted for 86%, 62%, and 66% of cases, respectively, in those age groups. Currently, routine meningococcal vaccination covering serogroups ACWY (MenACWY) is recommended in the United States for all adolescents at 11−12 years of age, with a booster dose at 16 years of age, whereas a meningococcal serogroup B (MenB) vaccine series is recommended for persons 16−23 years of age under the shared clinical decision-making paradigm. The MenACWY vaccination program in adolescents has been successful in reducing disease burden, but does not prevent disease caused by serogroup B, which accounts for more than half of IMD cases. There are currently no approved vaccines that cover all of the most common disease-causing meningococcal serogroups, which are A, B, C, W, and Y. A pentavalent MenABCWY vaccine that is constituted from 2 licensed meningococcal vaccines—MenB-FHbp and MenACWY-TT—is being investigated in healthy persons ≥ 10–25 years of age. The addition of a MenABCWY vaccine is the next natural step in the incremental meningococcal immunization program in the United States to improve protection against the most common serogroup causing IMD, with no increase in the number of immunizations needed. With high uptake, routine use of MenABCWY could reduce IMD cases and associated mortality, the rate of long-term physical and psychosocial sequelae in survivors, and costs associated with controlling outbreaks, particularly on college campuses. A MenABCWY vaccine would also reduce the number of injections required for adolescents, potentially improving compliance.
Keywords: Adolescents, Infants, Invasive meningococcal disease, Meningococcal conjugate vaccine, Neisseria meningitidis, Pentavalent
Key Summary Points
| Invasive meningococcal disease morbidity and mortality persist among adolescents and infants in the United States |
| Currently, meningococcal vaccination covering serogroups ACWY (MenACWY) is routinely recommended for all adolescents 11−12 years of age, with a booster dose at 16 years of age, and meningococcal serogroup B (MenB) vaccination is recommended for persons 16−23 years of age under the shared clinical decision-making framework |
| A pentavalent meningococcal vaccine covering serogroups A, B, C, W, and Y (MenABCWY) is being investigated in healthy persons ≥ 10–25 years of age |
| The addition of a MenABCWY vaccine to the immunization schedule warrants consideration because it could simplify the schedule and increase uptake, which may reduce cases of invasive meningococcal disease, long-term sequelae, and costs associated with outbreaks |
| A MenABCWY vaccine would also reduce the number of injections required for adolescents, potentially improving compliance |
Introduction
Neisseria meningitidis is an obligate human pathogen that causes endemic and epidemic disease, including invasive meningococcal disease (IMD) [1]. Case fatality rates (CFRs) can range from 10% to 15%, and up to 20% of individuals develop long-term sequelae, including limb amputation and neurologic deficits [1–3].
The occurrence of IMD varies according to age, underlying conditions, and geographic area, and is unpredictable because of fluctuations over time and differences based on serogroup [4–6]. In the United States, infants and young children are at greatest risk of IMD, with another incidence peak occurring in adolescents and young adults [7]. Serogroup B causes a high proportion of cases in many parts of the Americas, Australasia, Europe, and North Africa, and serogroup C predominates in some regions of South America, Asia, and Africa [8]. After near-eradication of serogroup A IMD in the African meningitis belt, cases caused by serogroup C are expanding, furthering the need for multivalent vaccination [5]. Although relatively uncommon compared with other serogroups, serogroup X meningococci are responsible for outbreaks and endemic disease in parts of sub-Saharan Africa [5, 9]. Because nearly all IMD cases are caused by serogroups A, B, C, W, and Y [5], comprehensive protection against IMD requires vaccination against all 5 serogroups.
Several monovalent and polyvalent meningococcal vaccines are currently available (Table 1). Quadrivalent meningococcal conjugate (MenACWY) vaccines include capsular polysaccharides from serogroups A, C, W, and Y, individually conjugated to carrier proteins [10–13]. Because the serogroup B capsular polysaccharide is poorly immunogenic [14], vaccines based on cell surface-expressed proteins were developed and licensed for use against serogroup B disease [15–17]. There are currently no approved meningococcal vaccines that cover all 5 common disease-causing serogroups, although such vaccines are undergoing clinical investigation.
Table 1.
Currently available meningococcal vaccines
| Name | Meningococcal serogroup | Type | US licensed age range |
|---|---|---|---|
| Menactra (MenACWY-D) [10] | A, C, W, Y | Polysaccharides conjugated to diphtheria toxoid |
9 months − 55 years |
| Menveo (MenACWY-CRM) [11] | A, C, W, Y | Polysaccharides conjugated to CRM197, a (nontoxic) mutant diphtheria toxin |
2 months– 55 years |
| MenQuadfi (MenACYW-TT) [12] | A, C, W, Y | Polysaccharides conjugated to tetanus toxoid | ≥ 2 years |
| Nimenrix (MenACWY-TT) [13] | A, C, W, Y | Polysaccharides conjugated to tetanus toxoid | ≥ 6 weeksa |
| Bexsero (MenB-4C) [50] | B | Recombinant-derived outer membrane proteins NadA, NHBA, fHbp (subfamily B), plus PorA-containing outer membrane vesicles | 10 − 25 years |
| Trumenba (MenB-FHbp) [51] | B | Recombinant-derived lipidated fHbp (subfamily A and B) | 10 − 25 years |
fHbp factor H binding protein, NadA neisserial adhesin A, NHBA neisserial heparin binding antigen
aNot currently licensed in the United States
This review considers the universal need for a pentavalent meningococcal vaccine covering serogroups A, B, C, W, and Y (MenABCWY), and focuses on data and recommendations from the United States for adolescents and infants, as well as on the epidemiology and high burden of meningococcal disease in these age groups. Progress towards developing pentavalent vaccines is ongoing, and this review is focused on the rationale for a MenABCWY meningococcal vaccine, which is constituted from 2 licensed meningococcal vaccines [MenB-FHbp (Trumenba®; Pfizer, Philadelphia, PA, USA) and MenACWY-TT (Nimenrix®; Pfizer Europe, Brussels, Belgium)]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals.
Meningococcal Disease in Adolescents And Young Adults
Epidemiology and Burden of Disease in the United States
Adolescents and young adults are at increased risk of IMD. In 2018, the incidence was 0.13 per 100,000 among adolescents and young adults 11−23 years of age (Fig. 1a) [7]. While the incidence has generally decreased since 2015 (with a peak occurring in 2016), the burden of disease among young adults continues to be higher than in any other age group except for infants. Notably, serogroup B accounted for 66% of all cases of IMD in 2018 among adolescents and young adults (i.e., 86% and 62% of all cases among individuals 11−15 years of age and 16−23 years of age, respectively) (Fig. 1b) [7]. In comparison, serogroup C, W, and Y cases collectively accounted for 10% of cases in those 11–25 years of age, and the incidence for these disease-causing serogroups has generally been stable since 2015 [7, 18–20]. Serogroup A caused no IMD in the United States, and unknown or nongroupable IMD accounted for 13%–24% of cases in adolescents and young adults in the period 2015−2018 [7, 18–20].
Fig. 1.
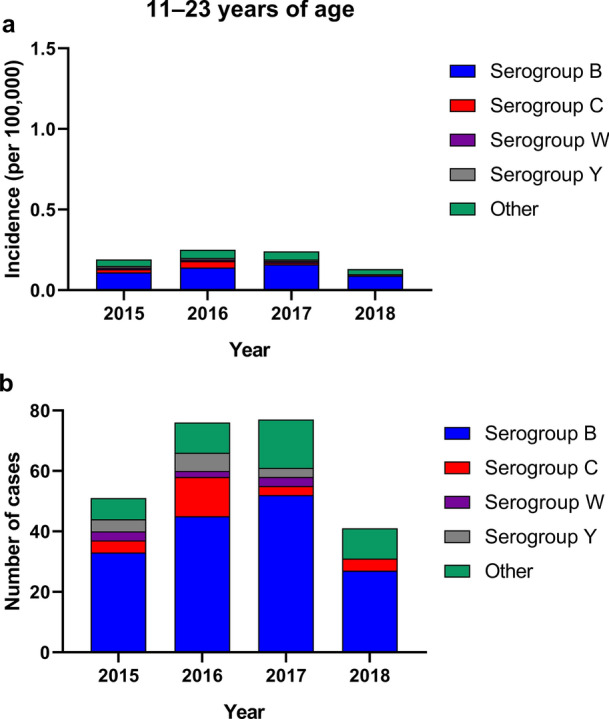
Incidence (a) and cases (b) of meningococcal disease in the United States in adolescents and young adults (2015-2018) [7, 18–20]. "Other" includes nongroupable cases
No IMD-associated deaths were reported in adolescents or young adults 11−23 years of age in 2018 (Table 2) [7]. However, from 2015 to 2017, the CFR ranged from 12.5 to 25.0% and from 4.5 to 17.4% in those 11−15 and 16−23 years of age, respectively [18–20].
Table 2.
Case fatality rates in infants and adolescents and young adults in the United States (2015–2018) [7, 18–20]
| Year | Infants (< 1 year of age) |
Adolescents (11–15 years of age) |
Young adults (16–23 years of age) |
|---|---|---|---|
| 2015 | 5.4% | 25.0% | 17.4% |
| 2016 | 11.1% | 12.5% | 8.8% |
| 2017 | 12.0% | 25.0% | 4.5% |
| 2018 | 12.9% | 0.0% | 0.0% |
Nasopharyngeal carriage of the bacterium is a prerequisite for the development of IMD [1]. Thus, it is not surprising that carriage is most common among adolescents and young adults, peaking at about 24% at 19 years of age among countries in which serogroup B and C disease dominates [21], although most carriage strains do not cause invasive disease [22]. Carriage rates in institutional settings, such as college dormitories, can be much higher, exceeding 50% in historical reports from UK universities without meningococcal vaccine programs in place at the time [23, 24]. Age-typical behaviors, such as smoking, having close or prolonged contact (e.g., through kissing), or living in close quarters (e.g., dormitories), leads to transmission from carriers to others, who may then develop disease [25]. Disease develops suddenly and progresses rapidly, and the initial symptoms can be nonspecific, together contributing to the high mortality rate. Therefore, a vaccination-based approach to prevention is justified [1, 25].
College attendance is a risk factor of IMD; from 2015 to 2017, the incidence of IMD was more than five-fold higher among 18- to 24-year-olds attending college compared with those not attending (0.22 vs. 0.04 cases per 100,000) [26]. Adolescents and young adults are also at risk of IMD because of college outbreaks [27]. Between 1994 and 2002, 57% of organization-based outbreaks (i.e., occurring in individuals with a common affiliation but with no known close contact with each other) of meningococcal disease in the United States were caused by serogroup C, with the remaining attributed to serogroups B (25%) and Y (18%); notably, these data predate the availability of routine MenACWY vaccine programs that began in 2005 [28, 29]. Between 2011 and 2019, the epidemiology of college outbreaks shifted, with all 14 college outbreaks being caused by serogroup B, including 50 cases, 2 deaths, an outbreak duration of a few weeks to more than 1 year, and a total at-risk population of more than 250,000 individuals [27].
Adolescents and young adults who have had IMD can experience high rates of long-term detrimental physical and psychosocial sequelae [3, 30]. In a matched-cohort study, case subjects who had IMD between 15 and 19 years of age had poorer mental health, social support, educational outcomes, and quality of life compared with matched control subjects [30]. Specifically, 58% of those who had IMD experienced sequelae at 18−36 months after IMD, which was most commonly skin scarring (18%), vertigo (17%), mobility and speech problems (13% each), and hearing deficits (12%). A considerable percentage of those who had IMD also experienced effects on their daily life, with about half reporting effects on their leisure activities, physical ability, academic achievement, and home life, and with more than 40% noting that their friendships and vocational choices were affected. Serogroup B disease in children and adolescents caused significantly more disabilities in a population-based case–control study from the United Kingdom compared with matched controls [3]. These disabilities included major physical or neurologic disabilities, such as limb amputations, very low intelligence quotient (IQ), seizures, and hearing loss in approximately 10% of children, as well as minor deficits (e.g., psychological disorders, borderline IQ, digit loss, minor hearing loss, or communication deficits) in more than one-third of patients at a median of 3.75 years after disease.
Therefore, although the incidence of meningococcal disease is low, prevention of meningococcal disease among adolescents and young adults is important because of the devastating effects, including mortality and long-term sequelae, and the potential for outbreaks.
Vaccine Recommendations and Uptake in the United States
The US Advisory Committee on Immunization Practices (ACIP) recommends routine MenACWY vaccination for all adolescents at 11−12 years of age, with a booster dose at 16 years of age [31]. ACIP also recommends a meningococcal serogroup B (MenB) vaccine series for persons 16−23 years of age (preferred age of 16−18 years) under the shared clinical decision-making (SCDM) paradigm, which calls for a discussion between provider and patient about risks and benefits, without a default position in terms of whether vaccination should occur [32–34]. The decision to recommend SCDM (as opposed to routine use) was based on the overall low incidence of serogroup B disease, limited data on duration of protection and effectiveness, seriousness of disease, and the availability of licensed vaccines [34]. However, this recommendation may be in part responsible for the low uptake of the MenB vaccines [35].
The MenACWY vaccination program was associated with a reduced incidence of disease due to serogroups C, W, and Y in adolescents and young adults from 2006 to 2017, and an estimated 222 cases were averted in persons 11−22 years of age [36]. There is currently high uptake for the first dose of MenACWY by US adolescents (87% in 2018), which may partially be due to state mandates requiring vaccination for school entry [37, 38]. However, uptake of the second dose is lower (51% in 2018) [37]. MenB vaccines have a low uptake (17% of adolescents in 2018 received ≥ 1 dose); it is not clear to what extent this is due to patient’s unwillingness to be vaccinated or the fact that SCDM conversations may not be taking place [37, 39–41]. In either case, these data suggest that many adolescents and young adults are not fully protected.
Meningococcal Disease In Infants
Epidemiology and Burden of Disease in the United States
The greatest burden of meningococcal disease is in infants < 1 year of age [7]. In 2018, the incidence of IMD in infants was 0.83 per 100,000 (Fig. 2a) compared with 0.10 per 100,000 in the general population. Infants 2−5 months of age appear to be at highest risk, although data are limited [6, 42–45]. Approximately 66% of cases in infants are caused by serogroup B, with serogroups C, W, and Y accounting for 28% of cases (Fig. 2b) [7]. While the incidence of meningococcal disease overall and for serogroup B disease has decreased steadily from 2015 to 2017, an increase in total cases in infants was noted in 2018, which was predominantly attributed to serogroup B and to a lesser extent serogroup C disease [7, 18–20]. As with adolescents, serogroup A caused no IMD in the United States in infants in the period 2015−2018 [7, 18–20]. Over the same period, nongroupable and unknown accounted for 2%–24% of cases in infants.
Fig. 2.
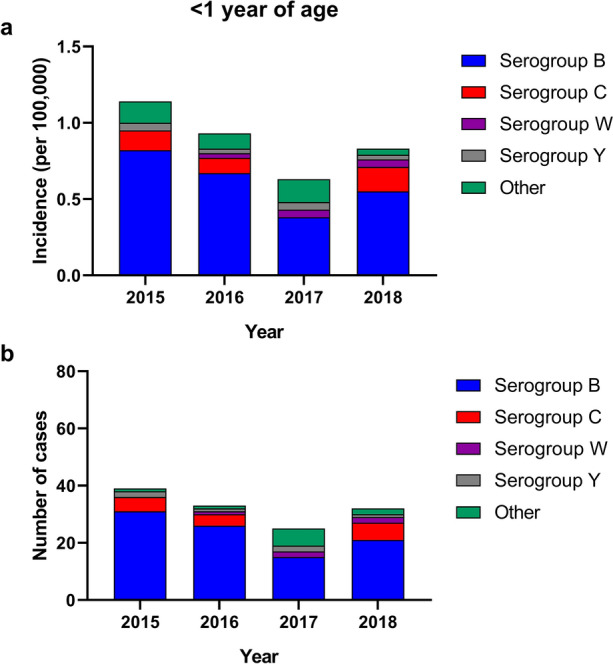
Incidence (a) and cases (b) of meningococcal disease in the United States in infants (2015–2018) [7, 18–20]. "Other" includes nongroupable cases
From 2015 to 2018, CFR in infants was 5.4%−12.9% (Table 2) [7, 18–20]. Infants may be more likely to have sequelae and have more severe sequelae than older patients who had IMD [44, 46]. For instance, hearing loss in infants with meningitis (19%) was more common than among adolescent or adult cases (12% and 8%, respectively). Neurologic complications in young infants, including hearing loss, are of concern because they may lead to developmental delay and may necessitate surgery [47].
While other risk factors of IMD in the general population are also thought to be applicable to infants, factors specifically in this population have not been well elucidated, although an association with increased IMD risk in infants and low birth weight, cigarette smoke exposure, and lower socioeconomic status is reported [42, 48]. Catabolism of transplacentally acquired antibodies may also increase the risk of disease in infants [6]. Infants acquire meningococcus from colonized adolescents and adults in their environment, and they are more susceptible to infection because of immunologic immaturity [6, 42].
Vaccine Recommendations in the United States
MenACWY vaccination is not routinely recommended by the ACIP for children 2 months to 10 years of age unless they have a high risk condition (e.g., HIV infection, anatomic asplenia, complement component deficiency, exposure in an outbreak, travel to or living in a country in which meningococcal disease is hyperendemic or epidemic) [49]. In recommending against routine vaccination in this age group, the ACIP cited the low burden of both IMD and cases that are preventable with MenACWY vaccines, which thereby was projected to limit the potential impact of a routine infant meningococcal vaccination program [42]. Of note, MenB vaccination of infants is not currently recommended [49] because no MenB vaccines are licensed in the United States for this age group [50, 51].
Development of a Pentavalent Meningococcal Vaccine
An investigational pentavalent MenABCWY vaccine is being developed, which is constituted from 2 licensed meningococcal vaccines: MenB-FHbp and MenACWY-TT. MenB-FHbp is currently licensed in the United States for administration on a 2-dose (months 0 and 6) schedule in individuals 10−25 years of age, and is supported by a clinical development program involving more than 20,000 adolescents and young adults [51, 52]. During an outbreak, a 3-dose schedule (months 0, 1−2, and 6) is recommended [53]. MenACWY-TT is a quadrivalent conjugate vaccine that uses tetanus toxoid as the carrier protein; it is licensed in the European Union and several other countries (but not the United States) for vaccination beginning at 6 weeks of age [13, 54]. A 2-dose series (given 2 months apart) is administered from 6 weeks to < 6 months of age, or a single dose from 6 to 12 months of age, with a booster dose administered at 12 months of age (> 2 months after the previous dose) [13]. The clinical development of MenACWY-TT includes several phase 2 and 3 studies evaluating the immunogenicity and safety of primary vaccination in more than 2000 adolescents and young adults, as well as antibody persistence through 10 years after primary and booster dosing [54, 55]. Clinical development also includes several phase 3 studies in more than 8000 infants and children 6 weeks to 10 years old [56].
The MenABCWY vaccine is being investigated in an ongoing phase 2/3 study in healthy adolescents and young adults 10−25 years of age, including both MenACWY vaccine-naive and -experienced subjects (NCT03135834) [57]. After administration of a 2-dose schedule given at months 0 and 6 (control subjects received MenB-FHbp at months 0 and 6 and MenACWY-CRM [Menveo®; GSK Vaccines, Sovicille, Italy] at month 0), immune responses to MenABCWY were robust and noninferior to MenB-FHbp and MenACWY-CRM at 1 month after dose 2, regardless of prior MenACWY vaccine exposure [57]. MenABCWY was also well tolerated with an acceptable safety profile [57]. Other ongoing clinical studies of the MenABCWY vaccine include phase 2 studies in healthy infants (NCT04645966) and adolescents (NCT04440176), and a phase 3 study in adolescents and young adults (NCT04440163; Table 3).
Table 3.
Ongoing clinical studies of the MenABCWY vaccine
| ClinicalTrials.gov identifier | Phase | Status | Details |
|---|---|---|---|
| NCT03135834 | 3 | Recruiting | 1590 participants (estimated) |
| 10 − 25 years of age | |||
| To assess the immunogenicity and safety of MenABCWY in MenACWY vaccine-naive healthy adolescents and young adults | |||
| To assess persistence of MenABCWY | |||
| To assess immunogenicity and safety after MenABCWY booster | |||
| NCT04440163 | 3 | Recruiting | 2413 participants (estimated) |
| 10 − 25 years of age | |||
| To assess the immunogenicity and safety of MenABCWY versus MenB-FHbp and MenACWY-CRM in both MenACWY vaccine-naive and -experienced healthy adolescents and young adults | |||
| NCT04645966 | 2 | Recruiting | 1325 participants (estimated) |
| 2 − 6 months of age | |||
| To assess the immunogenicity and safety of MenABCWY administered on a 2 + 1 schedule in healthy infants | |||
| NCT04440176 | 2 | Recruiting | 300 participants (estimated) |
| 11 − 14 years of age | |||
| To assess the safety and immunogenicity of MenABCWY administered at either months 0 and 12 or months 0 and 36 |
Data are from ClinicalTrials.gov and are current as of July 27, 2021. Another MenABCWY vaccine is in development, but this review is focused on a pentavalent vaccine that is constituted from 2 licensed meningococcal vaccines (MenB-FHbp and MenACWY-TT)
Using the current US schedule (i.e., MenACWY vaccine given at 11 and 16 years of age) and assuming current rates of vaccination uptake for MenACWY and MenB vaccines, a population-based dynamic model simulating transmission of meningococcal disease in the United States found that vaccination with 2 doses of each vaccine (total of 4 injections between 11 and 16 years of age) has the potential to avert 165 cases of IMD over a 10-year period compared with no vaccination [58]. The same model estimated that a MenABCWY vaccine has the potential to prevent up to 256 cases of IMD in this population compared with no vaccine; the higher number of cases averted with the MenABCWY vaccine was predominantly attributed to the prevention of more serogroup B cases.
Considerations for Use of a Pentavalent Meningococcal Vaccination
Several factors would need to be weighed in considering recommendations for use of a MenABCWY vaccine in the general population.
Fatality Rate and Serious Sequelae
The public health threat represented by IMD is relatively low in the context of other current public health challenges, such as the coronavirus disease 2019 (COVID-19) pandemic, the opioid crisis, the decrease in preventive healthcare visits after 15 years of age, and poor uptake of the human papillomavirus vaccine [7, 59, 60]. However, as described previously, with the existing routine recommendations for MenACWY vaccination of adolescents in the United States, a large proportion of disease continues to occur, including in the age-based populations at greatest risk (i.e., infants and adolescents/young adults), which is mostly attributed to serogroup B and to a lesser extent serogroup C disease (Fig. 1) [7]. Thus, replacing MenACWY vaccine with a pentavalent MenABCWY vaccine would reduce disease burden and simplify the current vaccination schedule, and in many ways represents the next natural step in the evolution of the US meningococcal vaccination program. In addition, among vaccine-preventable diseases, meningitis has one of the highest fatality rates, and the burden is persistently high and lagging behind other vaccine-preventable diseases [61]. For adolescents and young adults, the CFR for IMD from 2015 to 2017 was exceptionally high (e.g., a CFR of 17.4%−25.0% in 2015) [7, 18–20]. Any death, including preventable ones occurring in childhood or young adulthood, is devastating for families, loved ones, and the community, and the mortality risk of a vaccine-preventable disease should be a consideration in formulating recommendations for vaccination.
As described previously, patients who have IMD in childhood can also experience high rates of long-term effects, including detrimental physical and psychosocial sequelae that can linger into adulthood [3, 30, 62]. These can lead to adverse quality of life and psychosocial effects for both the children and their families [46, 63, 64]. Of note, the paucity of data regarding long-term outcomes for childhood survivors of IMD, particularly infants, emphasizes the need for further study, including population-based investigations.
Cost of IMD
A large proportion of IMD cases among college students occurs in the context of campus outbreaks [27]. Controlling IMD outbreaks requires coordination among numerous parties and significant human and capital resources [65]. In the absence of proactive vaccination programs, mitigating the extent of the outbreak is dependent on the ability of reactive vaccination strategies to quickly interrupt carriage and transmission. Responses to college outbreaks are also associated with high financial costs. For instance, the total cost of 2 serogroup B college outbreaks occurring in Oregon and Rhode Island was US$0.589−1.696 million with the cost per student vaccinated of US$636−2333 [27].
Traditional cost–benefit analyses may be difficult to apply to vaccination against IMD because of the unpredictable nature of IMD and the variability in the estimations of indirect disease costs (e.g., premature death, additional education, welfare needs) and vaccination benefits [66]. Of relevance to the epidemiologic situation in the United States, the quality-adjusted life-year thresholds for MenB vaccines have been outside the accepted willingness-to-pay range; however, the methodology used to assess cost effectiveness can vary and may not fully measure vaccine impact [66]. Incorporation of a MenABCWY vaccine into the recommended vaccination schedule would prevent disease due to serogroup B and reduce costs associated with individual and outbreak response.
Challenges with Vaccination
Importantly, meningococcal vaccination programs have resulted in disease reductions, emphasizing the benefits of such public health measures. For instance, countries that included routine use of the serogroup C conjugate vaccine in the routine infant vaccination program experienced dramatic decreases in IMD among infants, as well as in other age groups who were not directly vaccinated [67–70]. In the Netherlands, after the introduction of a meningococcal serogroup C vaccination program in individuals 1−18 years of age in 2002, the number of disease cases due to serogroup C rapidly decreased across all age groups [70]. Within 2 years of the introduction of routine MenACWY in the Netherlands in 2018, there was a reduction in the incidence of IMD of 85% in all vaccine-eligible ages, mainly driven by a reduction in serogroup W disease [13]. In addition, 3 years after MenB-4C was included in the UK infant immunization program, a 75% decrease in the incidence of serogroup B disease was reported among all children who were eligible for vaccination [71]. Importantly, while large observational studies have shown vaccination against serogroups A and C can affect meningococcal carriage, this effect of MenB vaccination has not been shown in adolescents with moderate-to-high vaccine uptake [72, 73]. Therefore, direct vaccination of at-risk populations will be required to reduce serogroup B disease.
To achieve these public health benefits of meningococcal vaccination, it is necessary that there be large uptake of a vaccine for the currently relevant disease-causing serogroup(s). However, challenges exist in achieving these goals.
Dynamic Nature of Meningococcal Disease Epidemiology
The variable epidemiology of IMD, including temporal fluctuations in the predominant disease-causing serogroup, can lead to challenges in ensuring that at-risk populations are appropriately protected. To address these challenges, several countries outside the United States have amended vaccine recommendations as the incidence of IMD caused by specific serogroups has changed [74–76]. Serogroup W cases have been associated with a hypervirulent ST-11 strain and an emergent ST-9316 strain predominantly affecting children younger than 4 years [74, 77–80]. A proportion of these serogroup W cases has presented with atypical clinical features, including septic arthritis, gastrointestinal symptoms, and severe respiratory tract infections, such as pneumonia, epiglottitis, and supraglottitis [74, 81]. The serogroup Y cases have varied regarding the most affected age group, and commonly manifest as septicemia and with decreased penicillin susceptibility [75, 76]. Importantly, several countries worldwide have introduced MenACWY vaccination to their immunization programs in response to this changing epidemiology [82], emphasizing that a single vaccine that provides protection against the 5 predominant disease-causing serogroups could best address the variable epidemiology of IMD for at-risk populations.
Challenges with Vaccinating Infants and Adolescents
The infant vaccination schedule is already crowded; incorporating optimal protection against IMD using separate MenACWY and MenB vaccines would add as many as 8 injections to the first year of life [11, 83], which may lead to decreased compliance [84]. Combination vaccines are generally preferred by ACIP because they reduce the number of injections that are required and improve vaccine coverage rates, among other benefits [85]. The availability of a MenABCWY vaccine may therefore offer the possibility of more efficiently vaccinating infants against the most predominant disease-causing serogroups and with a minimal number of doses. The same arguments could be made in limiting the number of injections for other age groups.
The uptake of meningococcal vaccines among adolescents can also be challenging [86]. Patient-associated factors among adolescents that could account, at least in part, for diminished vaccine uptake include less healthcare utilization compared with younger individuals and missed opportunities for vaccination (i.e., a healthcare visit in which vaccines could have been administered but were not). Other provider-/practice- and policy-related factors that are suggested to affect vaccine uptake among adolescents include competing demands among healthcare providers to address important topics at adolescent clinic visits, the lack of school entry requirements for vaccinations, and the ability to be vaccinated without parental consent [86].
Besides the availability of safe and efficacious vaccines, successful pediatric vaccination programs require sufficient parental awareness, provider knowledge, and equitable access [35]. However, notable challenges have been found in this regard. For instance, a US survey of healthcare providers’ understanding of ACIP meningococcal recommendations found a lack of understanding of the shared decision-making recommendations [40]. In addition, parents are often unaware of MenB vaccines, and racial and socioeconomic inequities exist in patient access to these vaccines [35].
Despite these challenges, the relative success of the existing MenACWY vaccination program presents an opportunity to build on the existing framework to cover all serogroups with a MenABCWY vaccine [87]. Given the persistent and dynamic nature of IMD [88], it is not likely that the current recommendations for MenACWY vaccination will be removed. Thus, providing additional coverage of serogroup B with a MenABCWY vaccine would further reduce disease incidence regardless of potential serogroup replacement in the future and the costs associated with outbreak response.
Conclusion
Although rare, IMD can have devastating clinical consequences for individuals and cause disruptive and costly outbreaks. The universal MenACWY vaccination program in US adolescents has been successful in reducing disease burden, but is incomplete in the sense that less than half of the incident disease is prevented. A MenABCWY vaccine would cover serogroup B disease and could help close this gap. Availability of such a vaccine would warrant serious consideration for addition to the routine immunization schedule, given the higher incidence of disease in adolescents and infants and the potential for life-long sequelae. Traditional cost–benefit analyses may underestimate the human impact that such a program might have.
Acknowledgements
Funding
This work was funded by Pfizer Inc. The sponsor is also funding the journal’s Rapid Service Fee.
Medical Writing and Editorial Assistance
Editorial/medical writing support was provided by Tricia Newell, PhD, and Allison Gillies, PhD, of ICON (Blue Bell, PA), and was funded by Pfizer Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conception and design: Gary S. Marshall; Jaime Fergie; Jessica Presa; Paula Peyrani. Data collection: Not applicable. Statistical analysis: Not applicable. Drafting and revising for intellectual content: Gary S. Marshall; Jaime Fergie; Jessica Presa; Paula Peyrani. Agreed to be accountable for the integrity of the work: Gary S. Marshall; Jaime Fergie; Jessica Presa; Paula Peyrani.
Disclosures
GSM has been an investigator on clinical trials and participated in advisory boards for GlaxoSmithKline, Merck, Novartis, Pfizer, Sanofi Pasteur, and Seqirus and is a speaker for Pfizer and Sanofi. JF is a speaker for Pfizer, Merck, AstraZeneca, and Sanofi; is a consultant/advisory board member for Pfizer, Merck, Sanofi, Moderna, Novavax, and Sobi; and is principal investigator or investigator for Pfizer, AstraZeneca, and Merck trials. JP and PP are employees of Pfizer Inc and may hold stock or stock options.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals.
Data Availability
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gary S. Marshall, Email: gary.marshall@louisville.edu
Jaime Fergie, Email: Jaime.Fergie@dchstx.org.
Jessica Presa, Email: jessica.presa@pfizer.com.
Paula Peyrani, Email: paula.peyrani@pfizer.com.
References
- 1.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang B, Santoreneos R, Giles L, Haji Ali-Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37:2768–2782. doi: 10.1016/j.vaccine.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Viner RM, Booy R, Johnson H, et al. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol. 2012;11:774–783. doi: 10.1016/S1474-4422(12)70180-1. [DOI] [PubMed] [Google Scholar]
- 4.Peterson ME, Li Y, Bita A, et al. Meningococcal serogroups and surveillance: a systematic review and survey. J Glob Health. 2019;9:010409. doi: 10.7189/jogh.09.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purmohamad A, Abasi E, Azimi T, et al. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: a systematic review and meta-analysis. Microb Pathog. 2019;2019:134. doi: 10.1016/j.micpath.2019.103571. [DOI] [PubMed] [Google Scholar]
- 6.Judelsohn R, Marshall GS. The burden of infant meningococcal disease in the United States. J Pediatric Infect Dis Soc. 2012;1:64–73. doi: 10.1093/jpids/pir003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2018. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2018.pdf. Accessed 24 Feb 2022.
- 8.Acevedo R, Bai X, Borrow R, et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18:15–30. doi: 10.1080/14760584.2019.1557520. [DOI] [PubMed] [Google Scholar]
- 9.Tzeng YL, Stephens DS. A narrative review of the W, X, Y, E, and NG of meningococcal disease: emerging capsular groups, Pathotypes, and Global Control. Microorganisms. 2021;2021:9. doi: 10.3390/microorganisms9030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menactra® (meningococcal [groups A, C, Y and W-135] polysaccharide diphtheria toxoid conjugate vaccine). Full Prescribing Information, Sanofi Pasteur Inc., Swiftwater, PA. 2018.
- 11.Menveo. GSK Vaccines, Srl. 2021. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Menveo/pdf/MENVEO.PDF. Accessed 24 Feb 2022.
- 12.MenQuadfiTM. Sanofi Pasteur Inc. 2021. https://www.fda.gov/media/137306/download. Accessed 24 Feb 2022.
- 13.Nimenrix SmPC. 2021. https://www.medicines.org.uk/emc/medicine/26514#gref. Accessed 24 Feb 2022.
- 14.Wyle FA, Artenstein MS, Brandt BL, et al. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126:514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 15.Feavers IM, Maiden MCJ. Recent progress in the prevention of serogroup B meningococcal disease. Clin Vaccine Immunol. 2017;2017:24. doi: 10.1128/CVI.00566-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trumenba®. Prescribing Information. Meningococcal group B vaccine. Philadelphia, PA: Wyeth Pharmaceuticals, Inc., a subsidiary of Pfizer Inc. 2017.
- 17.Bexsero (meningococcal group B vaccine). Full Prescribing Information, GlaxoSmithKline, Research Triangle Park, NC. 2017.
- 18.US Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2015. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2015.pdf. Accessed 24 Feb 2022.
- 19.US Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2016. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf. Accessed 24 Feb 2022.
- 20.US Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2017. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2017.pdf. Accessed 24 Feb 2022.
- 21.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–861. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 22.Villena R, Safadi MAP, Valenzuela MT, et al. Global epidemiology of serogroup B meningococcal disease and opportunities for prevention with novel recombinant protein vaccines. Hum Vaccin Immunother. 2018;14:1042–1057. doi: 10.1080/21645515.2018.1458175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bidmos FA, Neal KR, Oldfield NJ, et al. Persistence, replacement, and rapid clonal expansion of meningococcal carriage isolates in a 2008 university student cohort. J Clin Microbiol. 2011;49:506–512. doi: 10.1128/JCM.01322-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ala'aldeen DA, Oldfield NJ, Bidmos FA, et al. Carriage of meningococci by university students, United Kingdom. Emerg Infect Dis. 2011;17:1762–1763. doi: 10.3201/eid1709.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Meningococcal meningitis. 2021. http://www.who.int/en/news-room/fact-sheets/detail/meningococcal-meningitis. Accessed 24 Feb 2022.
- 26.Marshall GS, Dempsey AF, Srivastava A, Isturiz RE. US college students are at increased risk for serogroup B meningococcal disease. J Pediatric Infect Dis Soc. 2020;9:244–247. doi: 10.1093/jpids/piz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alderfer J, Isturiz RE, Srivastava A. Lessons from mass vaccination response to meningococcal B outbreaks at US universities. Postgrad Med. 2020;132:614–623. doi: 10.1080/00325481.2020.1766265. [DOI] [PubMed] [Google Scholar]
- 28.Brooks R, Woods CW, Benjamin DK, Jr, Rosenstein NE. Increased case-fatality rate associated with outbreaks of Neisseria meningitidis infection, compared with sporadic meningococcal disease, in the United States, 1994–2002. Clin Infect Dis. 2006;43:49–54. doi: 10.1086/504804. [DOI] [PubMed] [Google Scholar]
- 29.Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). US Centers for Disease Control and Prevention. 2013. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6202a1.htm. Accessed 24 Feb 2022. [PubMed]
- 30.Borg J, Christie D, Coen PG, Booy R, Viner RM. Outcomes of meningococcal disease in adolescence: prospective, matched-cohort study. Pediatrics. 2009;123:e502–e509. doi: 10.1542/peds.2008-0581. [DOI] [PubMed] [Google Scholar]
- 31.US Centers for Disease Control and Prevention. Recommended child and adolescent immunization schedule for ages 18 years or younger: United States. 2019. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 5 Mar 2019.
- 32.US Centers for Disease Control and Prevention. ACIP shared clinical decision-making recommendations. 2020. https://www.cdc.gov/vaccines/acip/acip-scdm-faqs.html. Accessed 24 Feb 2022.
- 33.US Centers for Disease Control and Prevention. Recommended child and adolescent immunization schedule for ages 18 years or younger: United States. 2020. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 15 May 2020.
- 34.MacNeil JR, Rubin L, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. Morb Mortal Wkly Rep. 2015;64:1171–1176. doi: 10.15585/mmwr.mm6441a3. [DOI] [PubMed] [Google Scholar]
- 35.Fergie J, Howard A, Huang L, Srivastava A. Implementation experience with meningococcal serogroup B vaccines in the United States: impact of a nonroutine recommendation. Pediatr Infect Dis J. 2021;40:269–275. doi: 10.1097/INF.0000000000003033. [DOI] [PubMed] [Google Scholar]
- 36.Mbaeyi S, Pondo T, Blain A, et al. Incidence of meningococcal disease before and after implementation of quadrivalent meningococcal conjugate vaccine in the United States. JAMA Pediatr. 2020;174:843–851. doi: 10.1001/jamapediatrics.2020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2018. Morb Mortal Wkly Rep. 2019;68:718–723. doi: 10.15585/mmwr.mm6833a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bednarczyk RA, King AR, Lahijani A, Omer SB. Current landscape of nonmedical vaccination exemptions in the United States: impact of policy changes. Expert Rev Vaccines. 2019;18:175–190. doi: 10.1080/14760584.2019.1562344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kempe A, Allison MA, MacNeil JR, et al. Adoption of serogroup B meningococcal vaccine recommendations. Pediatrics. 2018;142:e20180344. doi: 10.1542/peds.2018-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang L, Goren A, Lee LK, et al. Disparities in healthcare providers' interpretations and implementations of ACIP's meningococcal vaccine recommendations. Hum Vaccin Immunother. 2020;16:933–944. doi: 10.1080/21645515.2019.1682845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basta NE, Becker AB, Li Q, Nederhoff D. Parental awareness of meningococcal B vaccines and willingness to vaccinate their teens. Vaccine. 2019;37:670–676. doi: 10.1016/j.vaccine.2018.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacNeil JR, Bennett N, Farley MM, et al. Epidemiology of infant meningococcal disease in the United States, 2006–2012. Pediatrics. 2015;135:e305–e311. doi: 10.1542/peds.2014-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ladhani SN, Flood JS, Ramsay ME, et al. Invasive meningococcal disease in England and Wales: implications for the introduction of new vaccines. Vaccine. 2012;30:3710–3716. doi: 10.1016/j.vaccine.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Rivero-Calle I, Vilanova-Trillo L, Pardo-Seco J, et al. The burden of pediatric invasive meningococcal disease in Spain (2008–2013) Pediatr Infect Dis J. 2016;35:407–413. doi: 10.1097/INF.0000000000001048. [DOI] [PubMed] [Google Scholar]
- 45.Parikh SR, Campbell H, Beebeejaun K, et al. Meningococcal group W disease in infants and potential prevention by vaccination. Emerg Infect Dis. 2016;22:1505–1507. doi: 10.3201/eid2208.160128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olbrich KJ, Muller D, Schumacher S, et al. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7:421–438. doi: 10.1007/s40121-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu MH, Hsu JF, Kuo HC, et al. Neurological complications in young infants with acute bacterial meningitis. Front Neurol. 2018;9:903. doi: 10.3389/fneur.2018.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sørensen HT, Labouriau R, Jensen ES, Mortensen PB, Schønheyder HC. Fetal growth, maternal prenatal smoking, and risk of invasive meningococcal disease: a nationwide case-control study. Int J Epidemiol. 2004;33:816–820. doi: 10.1093/ije/dyh169. [DOI] [PubMed] [Google Scholar]
- 49.Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. Recomm Rep. 2020;69:1–41. doi: 10.15585/mmwr.rr6909a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beganovic M, McCreary EK, Mahoney MV, et al. Interplay between rapid diagnostic tests and antimicrobial stewardship programs among patients with bloodstream and other severe infections. J Appl Lab Med. 2019;3:601–616. doi: 10.1373/jalm.2018.026450. [DOI] [PubMed] [Google Scholar]
- 51.Greene E, Proctor P, Kotz D. Secure sharing of mHealth data streams through cryptographically-enforced access control. Smart Health (Amst) 2019;12:49–65. doi: 10.1016/j.smhl.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez JL, Absalon J, Beeslaar J, et al. From research to licensure and beyond: clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev Vaccines. 2018;17:461–477. doi: 10.1080/14760584.2018.1483726. [DOI] [PubMed] [Google Scholar]
- 53.Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp serogroup B meningococcal vaccine - Advisory Committee on Immunization Practices, 2016. Morb Mortal Wkly Rep. 2017;66:509–513. doi: 10.15585/mmwr.mm6619a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peyrani P, Webber C, Burman C, Balmer P, Perez JL. A review of the clinical development of MenACWY-TT, a quadrivalent meningococcal vaccine conjugated to tetanus toxoid, in adolescents [abstract] Open Forum Infect Dis. 2020;7:S23–S24. doi: 10.1093/ofid/ofaa439.048. [DOI] [Google Scholar]
- 55.Quiambao B, Peyrani P, Li P, et al. Efficacy and safety of a booster dose of the meningococcal A, C, W, Y-tetanus toxoid conjugate vaccine administered 10 years after primary vaccination and long-term persistence of tetanus toxoid conjugate or polysaccharide vaccine. Hum Vaccin Immunother. 2020;16:1272–1279. doi: 10.1080/21645515.2020.1744363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Findlow J, Knuf M. Immunogenicity and safety of meningococcal group A, C, W and Y tetanus toxoid conjugate vaccine: review of clinical and real-world evidence. Future Microbiol. 2019;14:563–580. doi: 10.2217/fmb-2018-0343. [DOI] [PubMed] [Google Scholar]
- 57.Peterson J, Drazan D, Czajka H, et al. 6. Pentavalent meningococcal (MenABCWY) vaccine is safe and well tolerated with immunogenicity noninferior to coadministered MenB-FHbp and MenACWY-CRM in a phase 2 study of healthy adolescents and young adults [abstract]. Open Forum Infect Dis. 2021;7:S25–S6.
- 58.Huang L, Snedecor SJ, Balmer P, Srivastava A. Potential public health impact of a Neisseria meningitidis A, B, C, W, and Y pentavalent vaccine in the United States. Postgrad Med. 2021;2021:1–8. doi: 10.1080/00325481.2021.1876478. [DOI] [PubMed] [Google Scholar]
- 59.US Centers for Disease Control and Prevention. Nine health threats that made headlines in 2019: A CDC Review. 2019. Available at: https://www.cdc.gov/media/releases/2019/p1218-nine-health-threats-2019-review.html. Accessed 23 Feb 2022.
- 60.Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72–S78. doi: 10.1016/j.acap.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Wright C, Blake N, Glennie L, et al. The global burden of meningitis in children: challenges with interpreting global health estimates. Microorganisms. 2021;9:1–16. doi: 10.3390/microorganisms9020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vermunt LC, Buysse CM, Joosten KF, et al. Survivors of septic shock caused by Neisseria meningitidis in childhood: psychosocial outcomes in young adulthood. Pediatr Crit Care Med. 2011;12:e302–e309. doi: 10.1097/PCC.0b013e3182192d7f. [DOI] [PubMed] [Google Scholar]
- 63.Garralda ME, Gledhill J, Nadel S, et al. Longer-term psychiatric adjustment of children and parents after meningococcal disease. Pediatr Crit Care Med. 2009;10:675–680. doi: 10.1097/PCC.0b013e3181ae785a. [DOI] [PubMed] [Google Scholar]
- 64.Judge D, Nadel S, Vergnaud S, Garralda ME. Psychiatric adjustment following meningococcal disease treated on a PICU. Intensive Care Med. 2002;28:648–650. doi: 10.1007/s00134-002-1237-2. [DOI] [PubMed] [Google Scholar]
- 65.Capitano B, Dillon K, LeDuc A, Atkinson B, Burman C. Experience implementing a university-based mass immunization program in response to a meningococcal B outbreak. Hum Vaccin Immunother. 2019;15:717–724. doi: 10.1080/21645515.2018.1547606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christensen H, Al-Janabi H, Levy P, et al. Economic evaluation of meningococcal vaccines: considerations for the future. Eur J Health Econ. 2020;21:297–309. doi: 10.1007/s10198-019-01129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meningococcal Reference Unit, Gray SJ, Trotter CL, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55:887–96. [DOI] [PubMed]
- 68.Public Health England. Health Protection Report: Invasive meningococcal disease in England: annual laboratory confirmed reports for epidemiological year 2017 to 2018. 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/751821/hpr3818_IMD.pdf. Accessed 24 Feb 2022.
- 69.Ribeiro IG, Percio J, Moraes C. Evaluation of the national meningococcal disease surveillance system: Brazil, 2007–2017. Epidemiol Serv Saude. 2019;28:e2018335. doi: 10.5123/S1679-49742019000300009. [DOI] [PubMed] [Google Scholar]
- 70.National Institute for Public Health and the Environment--Ministry of Health, Welfare and Sport. Meningococcal disease in the Netherlands: background information for the Health Council. 2017. https://www.rivm.nl/bibliotheek/rapporten/2017-0031.pdf. Accessed 24 Feb 2022.
- 71.Ladhani SN, Andrews N, Parikh SR, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382:309–317. doi: 10.1056/NEJMoa1901229. [DOI] [PubMed] [Google Scholar]
- 72.Balmer P, Burman C, Serra L, York LJ. Impact of meningococcal vaccination on carriage and disease transmission: a review of the literature. Hum Vaccin Immunother. 2018;14:1118–1130. doi: 10.1080/21645515.2018.1454570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marshall HS, McMillan M, Koehler AP, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382:318–327. doi: 10.1056/NEJMoa1900236. [DOI] [PubMed] [Google Scholar]
- 74.Booy R, Gentile A, Nissen M, Whelan J, Abitbol V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum Vaccin Immunother. 2019;15:470–480. doi: 10.1080/21645515.2018.1532248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fazio C, Neri A, Renna G, et al. Persistent occurrence of serogroup Y/sequence type (ST)-23 complex invasive meningococcal disease among patients aged five to 14 years, Italy, 2007 to 2013. Eurosurveillance. 2015;20:30061. doi: 10.2807/1560-7917.ES.2015.20.45.30061. [DOI] [PubMed] [Google Scholar]
- 76.Broker M, Emonet S, Fazio C, et al. Meningococcal serogroup Y disease in Europe: continuation of high importance in some European regions in 2013. Hum Vaccin Immunother. 2015;11:2281–2286. doi: 10.1080/21645515.2015.1051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skoczynska A, Wasko I, Kuch A, et al. Invasive meningococcal disease in Poland. Presented at: 15th Congress of the EMGM, European Meninogococcal and Haemophilus Disease Society, May 27–30, 2019; Lisbon, Portugal. 2019.
- 78.Wasko I, Golebiewska A, Kiedrowska M, et al. Emerging meningococci representing ST-9316 in Poland. Presented at: 15th Congress of the EMGM, European Meninogococcal and Haemophilus Disease Society, May 27–30 2019. Lisbon, Portugal. 2019.
- 79.Skoczynska A, Wasko I, Kuch A, et al. Current status of serogroup W meningococcal disease in Poland. Presented at: 15th Congress of the EMGM, European Meninogococcal and Haemophilus Disease Society; Lisbon, Portugal. 2019.
- 80.Deghmane A, Hong E, Taha M-K. The emergence of a new genetic lineage (ST-9316) of Neisseria meningitidis serogroup W in North France. Presented at: 15th Congress of the EMGM, European Meninogococcal and Haemophilus Disease Society; May 27–30, 2019, Lisbon, Portugal.
- 81.Stinson C, Burman C, Presa J, Abalos M. Atypical presentation of invasive meningococcal disease caused by serogroup W meningococci. Epidemiol Infect. 2020;148:e12. doi: 10.1017/S0950268819002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serra L, Knuf M, Martinón-Torres F, Yi K, Findlow J. Review of clinical studies comparing meningococcal serogroup C immune responses induced by MenACWY-TT and monovalent serogroup C vaccines. Hum Vaccin Immunother. 2021;2021:1–11. doi: 10.1080/21645515.2020.1855952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bexsero (MenB-4C) Summary of Product Characteristics. Full Prescribing Information, GlaxoSmithKline Vaccines Srl, Siena, Italy. 2017.
- 84.Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance: part 1: Childhood vaccinations. P T. 2016;41:426–436. [PMC free article] [PubMed] [Google Scholar]
- 85.Centers for Disease Control and Prevention General recommendations on immunization–recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 2011;60:3–61. [Google Scholar]
- 86.Niccolai LM, Hansen CE. Suboptimal uptake of meningococcal vaccines among older adolescents: Barriers, solutions, and future research directions. Hum Vaccin Immunother. 2020;16:3208–3212. doi: 10.1080/21645515.2020.1754052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.MacNeil JR, Blain AE, Wang X, Cohn AC. Current epidemiology and trends in meningococcal disease-United States, 1996–2015. Clin Infect Dis. 2018;66:1276–1281. doi: 10.1093/cid/cix993. [DOI] [PubMed] [Google Scholar]
- 88.Presa J, Findlow J, Vojicic J, Williams S, Serra L. Epidemiologic trends, global shifts in meningococcal vaccination guidelines, and data supporting the use of MenACWY-TT vaccine: a review. Infect Dis Ther. 2019;8:307–333. doi: 10.1007/s40121-019-0254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.


