Abstract
Background and aims
The use of pornography, while unproblematic for the majority, can grow into addiction-like behavior which in its extreme form is labeled as compulsive sexual behavioral disorder in the ICD-11 (WHO, 2018). The aim of this study was to investigate the addiction-specific reactivity to cues in order to better understand underlying mechanisms in the development of this disorder.
Methods
We have used an optimized Sexual Incentive Delay Task to study brain activity in reward associated brain areas during an anticipation phase (with cues predicting pornographic videos, control videos or no videos) and a corresponding delivery phase in healthy men. Correlations to indicators of problematic pornography use, the time spent on pornography use, and trait sexual motivation were analyzed.
Results
The results of 74 men showed that reward-related brain areas (amygdala, dorsal cingulate cortex, orbitofrontal cortex, nucleus accumbens, thalamus, putamen, caudate nucleus, and insula) were significantly more activated by both the pornographic videos and the pornographic cues than by control videos and control cues, respectively. However, we found no relationship between these activations and indicators of problematic pornography use, time spent on pornography use, or with trait sexual motivation.
Discussion and conclusions
The activity in reward-related brain areas to both visual sexual stimuli as well as cues indicates that optimization of the Sexual Incentive Delay Task was successful. Presumably, associations between reward-related brain activity and indicators for problematic or pathological pornography use might only occur in samples with increased levels and not in a rather healthy sample used in the present study.
Keywords: fMRI, pornography use, problematic pornography use, reward system, sexual incentive delay task, sexual motivation
Intoduction
Internet pornography use is a very widespread behavior in the general population (Blais-Lecours, Vaillancourt-Morel, Sabourin, & Godbout, 2016; Bőthe, Tóth-Király, Potenza, Orosz, & Demetrovics, 2020; Martyniuk, Okolski, & Dekker, 2019). While the vast majority shows unproblematic pornography use, in a few individuals it is accompanied by distress, a perceived lack of control, and the inability to reduce the behavior in spite of negative consequences (around 8%, depending on the criteria used; Cooper, Scherer, Boies, & Gordon, 1999; Gola, Lewczuk, & Skorko, 2016; Grubbs, Volk, Exline, & Pargament, 2015). Pornography use accompanied by masturbation is the most common problematic behavior among individuals with compulsive sexual behaviors (Kraus, Voon, & Potenza, 2016; Reid et al., 2012; Wordecha et al., 2018). For the first time, the World Health Organization (WHO) has defined specific diagnostic criteria for these symptoms in the 11th edition of the International Classification of Disorders (ICD-11) under the term Compulsive Sexual Behavior Disorder (CSBD, World Health Organization, 2018). For a better understanding of both recreational and problematic porn use, its neurobiological underpinnings must be elucidated.
Although the correct classification of problematic pornography use is a controversial topic, neuroscientific findings suggest its proximity to addiction disorders (Love, Laier, Brand, Hatch, & Hajela, 2015; Stark, Klucken, Potenza, Brand, & Strahler, 2018). Robinson and Berridge described in their Incentive Sensitization Theory for the development of addictions how repeated drug exposure leads to neuroadaptive changes within the reward circuits (Robinson & Berridge, 1993, 2008). During addiction development, the response to cues (“wanting”) increases while the desired effect of drug intake (“liking”) might even decrease. Therefore, cue reactivity which encompasses the emotional, behavioral, physiological and cognitive response to addiction-related stimuli (Berridge & Robinson, 2016; Tiffany & Wray, 2012) is an important concept to explain the transition from occasional use of a drug to addictive use (Brand et al., 2019; Koob & Volkow, 2010; Volkow, Koob, & McLellan, 2016).
Studies on patients with diverse substance-related disorders have found increased reactivity in the ventral striatum, the dorsal striatum, the anterior cingulate cortex (ACC), the orbitofrontal cortex (OFC), the insula and the amygdala to substance-related cues (Jasinska, Stein, Kaiser, Naumer, & Yalachkov, 2014; Kühn & Gallinat, 2011a; Stippekohl et al., 2010; Zilverstand, Huang, Alia-Klein, & Goldstein, 2018). With regard to behavioral addictions, there are several reviews that demonstrate increased activity in reward-associated regions to addiction-related cues (Antons, Brand, & Potenza, 2020; Fauth-Bühler, Mann, & Potenza, 2017; Starcke, Antons, Trotzke, & Brand, 2018; Van Holst, van den Brink, Veltman, & Goudriaan, 2010). Whether the processes involved in CSBD resemble those of substance use disorders and behavioral addictions is still a matter of scientific debate.
Several reviews show increased activity of the ventral and the dorsal striatum, OFC, ACC, insula, caudate nucleus, putamen, amygdala, thalamus, and hypothalamus in healthy participants when looking at visual sexual stimuli (VSS) compared to neutral stimuli (Georgiadis & Kringelbach, 2012; Poeppl, Langguth, Laird, & Eickhoff, 2014; Stoléru, Fonteille, Cornélis, Joyal, & Moulier, 2012). In addition, there are studies on neural responses to cues which predict VSS but do not contain any sexual content (e.g., Banca et al., 2016: colored patterns; Klucken, Wehrum-Osinsky, Schweckendiek, Kruse, & Stark, 2016: colored squares; Stark et al., 2019: category-describing terms). The brain responses to these cues preceding VSS (Banca et al., 2016; Klucken et al., 2016; Stark et al., 2019) were similar to responses to VSS (ventral striatum, OFC, occipital cortex, insula, putamen, thalamus). Moreover, persons with problematic pornography use (PPU) compared to control participants showed an increased amygdala reactivity to geometric figures associated with VSS (Klucken et al., 2016). Using VSS as cues, Voon et al. (2014) found higher responses in the dorsal anterior cingulate, ventral striatum and amygdala of persons with PPU. These findings of an increased reactivity towards cues predicting VSS in persons with PPU are in line with the expectations drawn from Incentive Sensitization Theory.
To study the development of addiction, the Monetary Incentive Delay Task (MIDT) is an established instrument to investigate altered neural responses to cues and stimuli (Balodis & Potenza, 2015). The MIDT starts with an anticipatory phase in which cues signal whether a monetary win or loss is possible during the succeeding delivery phase. Originally, this task was used to assess the general reward sensitivity in addiction with, however, inconsistent results regarding the anticipatory and the delivery phase (Balodis & Potenza, 2015; Beck et al., 2009; Bustamante et al., 2014; Jia et al., 2011; Nestor, Hester, & Garavan, 2010). To examine cue reactivity in PPU, a modified version of the established MIDT (Knutson, Fong, Adams, Varner, & Hommer, 2001; Knutson, Westdorp, Kaiser, & Hommer, 2000) was proposed: The Sexual Incentive Delay Task (SIDT) using sexual cues and rewards. Three studies have employed incentive delay tasks with sexual cues and rewards so far (Gola et al., 2017; Sescousse, Li, & Dreher, 2015; Sescousse, Redouté, & Dreher, 2010). Sescousse and colleagues investigated differential activity patterns regarding erotic and monetary rewards in healthy adults and identified the posterior part of the OFC and the amygdala as regions specifically activated by erotic rewards (Sescousse et al., 2010). Gola and colleagues (2017) compared men with PPU and control men with regard to their brain activity to a mixed MIDT/SIDT. Whereas PPU participants showed increased activity in the ventral striatum for cues predicting sexual rewards, they did not differ from controls regarding the brain activity to sexual rewards. Consistent with the Incentive Sensitization Theory, the authors argued for an increased “wanting” of sexual rewards in PPU participants while the “liking” of sexual stimuli remains unaffected.
Although prior studies using SIDT are highly promising regarding the examination of cue reactivity towards sexual cues and rewards in healthy persons and persons with PPU, there are some methodological aspects which have to be discussed. Regarding the external validity, previous studies used static images instead of videos, although the latter are the most widely used form of pornography (Solano, Eaton, & O'Leary, 2020). Concerning the control condition, former studies used scrambled versions of VSS as control conditions (Gola et al., 2017; Sescousse et al., 2010, 2015). Consequently, the experimental and control conditions differed with regard to several characteristics (naturalistic setting vs. abstract patterns, image resolution, human portrayal vs. non-human portrayal). It is questionable if these stimuli represent optimal control stimuli. Moreover, the researchers used pictograms of naked women as cues. In this way the cues could not only have a predictive value, but also represent sexual content. Further, it would be helpful to investigate the influence of risk factors for the development of a CSBD, where the following appear to be the most relevant: self-reported problems concerning pornography use (Brand, Snagowski, Laier, & Maderwald, 2016; Laier, Pawlikowski, Pekal, Schulte, & Brand, 2013), time spent watching pornography (Kühn & Gallinat, 2014) and trait sexual motivation (Baranowski, Vogl, & Stark, 2019; Kagerer et al., 2014; Klucken et al., 2016; Stark et al., 2018; Strahler, Kruse, Wehrum-Osinsky, Klucken, & Stark, 2018).
Therefore, the aims of the present study were the following: (1) We wanted to establish an optimized SIDT using film clips instead of static images. We expected the activity patterns during the anticipation phase and the delivery phase to be similar to results in former studies showing the involvement of ACC, OFC, thalamus, insula, amygdala, nucleus accumbens (NAcc), caudate, and putamen. (2) We wanted to investigate the extent to which risk factors for CSBD (self-reported PPU, time spent on pornography use, and trait sexual motivation) are connected to neural activity during the anticipation phase and the delivery phase in a non-clinical sample. According to the Incentive Sensitization Theory of Robinson and Berridge (1993), we expected the neural activity of the above-mentioned brain regions during the anticipation phase of the SIDT to be positively correlated with these risk factors. In accordance with the study of Gola et al. (2017), we expected the neural activity of the above-mentioned regions during the delivery phase not to be correlated with these risk factors.
Methods
Participants
Seventy-eight heterosexual healthy men between 18 and 45 years were recruited via mailing lists, postings and media press releases. Two participants had to be excluded due to technical difficulties, two because of image artifacts and one due to atypical neuroanatomy. The final sample consisted of 73 men with a mean age of 25.47 (SD = 4.44) years. Most of the participants (n = 65; 89.04%) were students. Thirty-three (45.21%) participants were singles, 36 (49.32%) lived in a romantic relationship and four (5.48%) participants were married. Twenty-four (32.88%) participants described themselves as religious (“Do you profess a religion or denomination?” “yes”/“no”). The following inclusion criteria were applied: absence of current somatic/mental diseases, no current psychotherapeutic/pharmacological treatment, no harmful use of alcohol/nicotine, no contra-indication for fMRI, and fluency in the German language.
Procedure
At study entry, the participants signed an informed consent document. The present sample comes from a larger study investigating the effects of acute stress on VSS processing by comparing a stress condition to a control condition. One other study using data from this project has been published so far. Klein et al. (2020) examined the influence of individual preference on neural reactivity to VSS. The analyses showed that several reward-associated brain areas correlate positively with the individual rating of the VSS and that this correlation correlates positively with the level of PPU. No data reported herein were previously published. Participants from the present analysis were randomly allocated to the control condition and underwent the non-stressful placebo version of the Trier Social Stress Test (placebo TSST, 15 min, Het, Rohleder, Schoofs, Kirschbaum, & Wolf, 2009) prior to MRI scanning. This test consists of two easy mental tasks (a free speech and simple mental arithmetic) that do neither induce significant mental strain nor marked physiological changes in the participants, an influence on the following SIDT is therefore not expected. Subsequent to the placebo TSST, the participants took part in the SIDT. After leaving the scanner, participants rated the film clips alone in a separate room to ensure privacy and validity of the rating. Part of the socio-demographic and non-sexual questionnaire data was already collected before the TSST started (duration about 45 min) using the Internet-based SoSci Survey platform. After the MRI scanning, the participants were given time to rate the film clips and fill in further questionnaires (about 60 min).
Measures
Sexual Incentive Delay Task
We used a SIDT derived from the established MIDT (Knutson et al., 2001). Monetary rewards were replaced in this study by six-second-long film clips that were presented without sound and either showed VSS (VSS clip), non-sexual massage videos (control clip) or a black screen (none). The use of massage videos assured comparability of visual aspects (social interaction, partial nudity, rhythmic movements, etc.) to the film clips showing VSS. In a preliminary study, all film clips were rated with respect to pleasantness (from “1” = “very unpleasant” to “9” = “very pleasant”) and sexual arousal (from “1” = “not sexually arousing at all” to “9” = “very sexually arousing”) by an independent sample of 58 non-homosexual men. Values above 5 were interpreted as high. The 21 VSS clips used in the actual study achieved average scores of high valence (M = 6.20, SD = 1.12) and high sexual arousal (M = 6.29, SD = 1.34 in the pre-study, whereas medium to high scores for valence (M = 5.44, SD = 0.97) and low scores for sexual arousal (M = 1.86, SD = 0.81) were reported for the 21 control clips. Each film clip was only presented once during the task. The experiment was realized with the Presentation software package (Version 17.0, Neurobehavioral Systems, Inc, USA) and lasted for about 20 min. The SIDT included 63 trials consisting of an anticipation phase and a delivery phase with three conditions (21 × VSS, 21 × control, 21 × none).
During the anticipation phase, three different geometric figures, were presented as cues announcing either the VSS clip (CueVSS), the control clip (CueControl) or a black screen (CueNone, see also Fig. 1). The assignment of the geometric figures to the potential outcomes (VSS clip, control clip, none) was randomized across participants. We used geometric figures as cues to ensure that there were no previous associations between these cues and VSS. The participants were informed about the associations between cues and videos before the fMRI experiment. These associations were trained in 21 exercise trials outside the scanner. After one of the cues was visible for 4 s, a fixation cross followed for a variable interstimulus interval of 1–3 s. Then the target stimulus (white square, 200 × 200 pixel) was shown between 16 ms (minimum) and 750 ms (maximum). Regardless of the previously presented cue, the instruction was to respond to the target as quickly as possible by pressing a button. If CueVSS or CueControl appeared and the participants pressed the button while the target stimulus was visible, the participants “won” a film clip. The target was followed by the presentation of another fixation cross for a variable interstimulus interval of 0–2 s. Subsequently, the participants were shown a VSS clip, a control clip or a black screen for a duration of 6 s. The exercise trials before scanning also served to calculate the individual average reaction times (meanRT) and standard deviations (SDRT) to determine the presentation times of the target stimulus (win: meanRT+2 × SDRT; no win: MeanRT–2 × SDRT). Wins were planned for approximately 71% of VSS and control trials (15 out of 21 trials), while nothing trials were never combined with a win. The first three trials presented CueControl, CueVSS, and CueNone in random order. These CueControl and CueVSS trials were always planned as winning trials. After the first three trials, subblocks of 6 trials each were formed (2 × CueControl, 2 × CueVSS and 2 × CueNone). Between winning trials (VSS winning trials or control winning trials) no more than 5 other trials (other winning trials or none trials) were allowed. The same condition could be presented a maximum of 2 times in a row. The presentation of the target stimulus was adjusted online by subtraction or addition of 20 ms each if the participants won in unplanned trials or did not win in planned trials to ensure the reinforcement rate in future trials. VSS trials and control trials, which did not result in outcomes as planned, were repeated in scheduled trials with the new duration of target presentation.
Fig. 1.
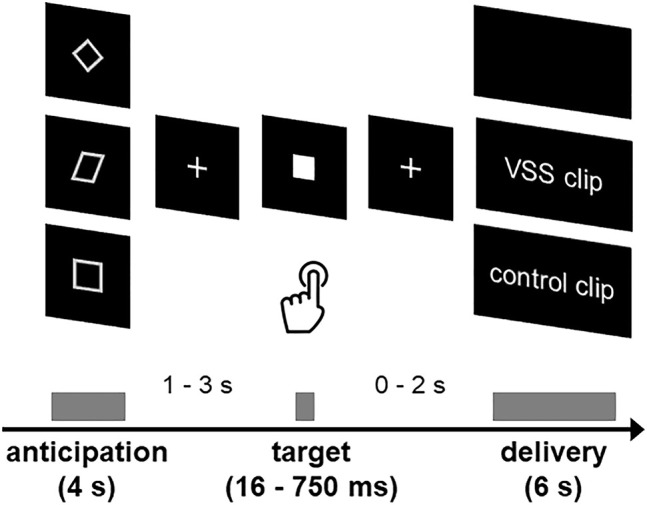
Sexual Incentive Delay Task. During the anticipation phase, the participants saw a cue (geometric figure). Following a variable time interval, a target was presented for a short time, to which the participants were asked to react as quickly as possible by pressing a button. If the cue in the anticipation phase was a CueVSS or a CueControl, a corresponding video could be obtained by reacting quickly to the target (see also Klein et al., 2020)
Assessment of psychometric data
After the SIDT, participants rated their current level of sexual arousal on a 9-point Likert-scale while still inside the scanner. The film clips were rated using Self-Assessment-Manikin scales (Bradley & Lang, 1994) for valence (from 1 = very unpleasant to 9 = very pleasant) and sexual arousal (from 1 = not sexually arousing to 9 = very sexually arousing) after leaving the scanner in a separate room.
The time spent on watching VSS in everyday life was assessed with the item “How much time did you spend on consuming pornography, basing your answer on the last month?”. The participants were able to choose hours and min “per month”, “per week” or “per day” to specify their answer. Prior to analyses, different answer formats were converted into “hours per month”.
PPU was measured by German versions of the short Internet Addiction Test (s-IAT) (Pawlikowski, Altstötter-Gleich, & Brand, 2013) modified for cybersex (s-IATsex; Laier et al., 2013) and by the Hypersexual Behavior Inventory (HBI; Reid, Garos, & Carpenter, 2011). Internal reliability of the collected questionnaire data was calculated for the current sample. Each of the twelve items of the s-IATsex is rated on a 5-point Likert scale ranging from 1 (never) to 5 (very often). The total score (s-IATsex sum, 12 Items, Cronbach's ɑ = 0.90) ranges from 12 to 60. Two subscales can be additionally calculated: loss of control (6 items, Cronbach's ɑ = 0.89) and craving (6 items, Cronbach's ɑ = 0.73). The HBI consists of 19 items rated from 1 (never) to 5 (very often) with a total score (HBIsum, 19 items, Cronbach's ɑ = 0.89) ranging from 19 to 95. Three subscales can be calculated: control (8 items, Cronbach's ɑ = 0.89), coping (7 items, Cronbach's ɑ = 0.84) and consequences (4 items, Cronbach's ɑ = 0.76). Internal consistencies were in acceptable to good ranges in the present study (see data above).
Trait sexual motivation was measured by the Trait Sexual Motivation Questionnaire (TSMQ; Stark et al., 2015). The TSMQ consists of 35 items loading on 4 subscales: solitary sexuality (10 items, Cronbach's ɑ = 0.77), importance of sex (15 items, Cronbach's ɑ = 0.89), seeking sexual encounters (4 items, Cronbach's ɑ = 0.92), and comparison with others (6 items, Cronbach's ɑ = 0.86). Further, a general index for trait sexual motivation (TSMQmean) can be calculated as the mean of all 35 items (Cronbach's ɑ = 0.91). Each item is rated on a 6-point Likert scale ranging from 0 (not at all) to 5 (very much). The participants are instructed to relate their statements to the last five years. The term “sexual motivation” used in this scale includes sexual activities with a partner as well as solitary sexual activities. Higher values indicate higher trait sexual motivation.
Behavioral data
Reaction time was defined as the time between target onset and response onset. Reaction time data was screened for outliers by excluding data below 100 ms or above mean + 1.5 × SD per condition based on sample statistical values. According to this, there were three outliers within the whole sample (one per condition). Descriptive statistics were computed excluding outliers and missing values in the data. Missing values consisted of too late reactions or no reactions to the fixation cross. Differences in the medians of the reaction times on successful trials were analyzed using Kruskal-Wallis test and Dunn-Bonferroni tests. Finally, Pearson's correlations between the reaction times of the three conditions and the risk factors for CSBD were calculated.
fMRI data acquisition and statistical analysis
Functional and anatomical images were acquired using a 3 Tesla whole-body MR tomograph (Siemens Prisma) with a 64-channel head coil. The structural image acquisition encompassed 176 T1-weighted sagittal slices (slice thickness 0.9 mm; FoV = 240 mm; TR = 1.58 s; TE = 2.3 s). For functional imaging, a total of 632 images were recorded using a T2-weighted gradient echo-planar imaging (EPI) sequence with 36 slices covering the whole brain (voxel size = 3 × 3 × 3.5 mm; gap = 0.5 mm; descending slice acquisition; TR = 2 s; TE = 30 ms; flip angle = 75; FoV = 192 × 192 mm2; matrix size = 64 × 64; GRAPPA = 2). The field of view was positioned automatically relative to the AC-PC line with an orientation of -30°. Statistical Parametrical Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, UK; 2014) implemented in Matlab Mathworks Inc., Sherbourn, MA; 2012) was used for preprocessing the raw data, as well as first and second level analysis.
Preprocessing of the EPI images comprised coregistration to a Montreal Neurological Institute (MNI) template, segmentation, realignment and unwarping, slice time correction, normalization to MNI standard space as well as smoothing with a Gaussian kernel at 6 mm FWHM. Functional data were analyzed for outlying volumes using a distribution free approach for skewed data (Schweckendiek et al., 2013). Each resulting outlying volume was later modeled within the general linear model (GLM) as a regressor of no interest. Each of the experimental conditions (CueVSS, CueControl, CueNone, DeliveryVSS, NoDeliveryVSS, DeliveryControl, NoDeliveryControl, NoDeliveryNone and target) was modelled as a regressor of interest. All regressors were convolved with the canonical hemodynamic response function. Six movement parameters were entered as covariates in addition to the regressors for the identified outlying volumes. The time series was filtered with a high pass filter (time constant = 128 s).
On the group level, two contrasts were examined: CueVSS-CueControl and DeliveryVSS-DeliveryControl. One-sample t-tests as well as linear regressions with the following variables as predictors were performed with the contrasts: s-IATsex, HBI, time spent on pornography use (hours per month), and TSMQ. For the TSMQ and for the HBI, multiple regressions containing all subscales at once were done. We used linear regressions for the amount of time spent on pornography use and for the s-IATsex.
ROI analyses on the voxel level were conducted using small volume correction (SVC) with P < 0.05 (family-wise-error corrected: FWE-corrected). Caudate, NAcc, putamen, dorsal anterior cingulate cortex (dACC), amygdala, insula, OFC, and thalamus were chosen as ROIs because they have been previously reported in studies on cue reactivity and VSS processing (Ruesink & Georgiadis, 2017; Stoléru et al., 2012). Bilateral anatomical ROI masks for OFC and dACC were created in MARINA (Walter et al., 2003); all other masks were taken from the Harvard Oxford Cortical Atlas (HOC). The left and right variants of a ROI were merged to one mask. For these eight ROIs, analyses on the voxel level were conducted with P < 0.05 FWE-corrected.
We computed linear regressions of the questionnaire scores and pornography use on the CueVSS–CueControl contrast and the DeliveryVSS–DeliveryControl contrast. Only significant (SVC, FWE-corrected) voxels from the one-sample t-tests within the ROIs where used for SVC. Therefore, smaller ROIs were used for the regression analyses. Exploratory whole brain analyses (FWE-corrected) supplemented the ROI analyses.
Ethics
The study was approved by the local ethics committee and was conducted in accordance with the 1964 declaration of Helsinki and its later amendments. All participants provided informed consent prior to any assessment. A neurological doctor was available to clarify suspect neuroanatomical abnormalities.
Results
Sample characteristics
Table 1 summarizes the descriptive statistics. Bivariate correlations between the questionnaire constructs yielded medium-strong correlations that show both content overlaps and incremental shares of the different constructs (see Fig. 2).
Table 1.
Psychometric measurements and ratings of the sexual and control videos used in the sexual incentive delay task (N = 73)
| Mean (SD) | Range | ||
| s-IATsex | Loss of control | 10.56 (4.66) | 6.00–30.00 |
| Craving | 9.60 (3.44) | 6.00–26.00 | |
| s-IATsex total score | 20.16 (7.74) | 12.00–56.00 | |
| HBI | Control | 14.86 (6.28) | 8.00–39.00 |
| Coping | 17.92 (5.48) | 7.00–32.00 | |
| Consequences | 6.71 (2.81) | 4.00–20.00 | |
| HBIsum | 39.49 (11.48) | 20.00–90.00 | |
| TimePU [h/month] | 6.49 (7.21) | 0.00–42.00 | |
| TSMQ | Solitary sexuality | 3.74 (0.68) | 1.80–5,00 |
| Importance of sex | 3.82 (0.74) | 1.27–5.00 | |
| Seeking sexual encounters | 1.50 (1.40) | 0.00–4.75 | |
| Comparison with others | 1.73 (1.10) | 0.00–4.33 | |
| TSMQmean | 2.70 (0.69) | 1.05–4.35 | |
| Ratings of the sexual stimuli | Valence | 6.35 (1.17) | 2.14–8.67 |
| Sexual arousal | 6.63 (1.16) | 2.14–8.62 | |
| Ratings of the control stimuli | Valence | 5.51 (1.27) | 2.95–8.86 |
| Sexual arousal | 2.01 (0.97) | 1.00–5.00 |
Note: s-IATsex = short version of the Internet Addiction Test modified for cybersex (Laier et al., 2013), HBI = Hypersexual Behavior Inventory (Reid et al., 2011), TimePU = Time spend on pornography use; TSMQ = Trait Sexual Motivation Questionnaire (Stark et al., 2015).
Fig. 2.
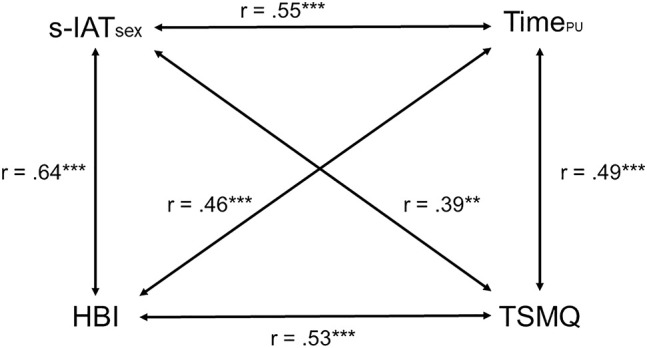
Intercorrelation of the addiction-associated characteristics (N = 73): s-IATsex and HBI = sum scores for problematic pornography use, TimePU = time spent on pornography in h/month; TSMQ = mean value for trait sexual motivation
A Kruskal–Wallis test showed significant differences between the median reaction times in response to the target in the three conditions (CueNone, CueControl, CueVSS; Χ2(2) = 12.05, P < 0.01). Table 2 summarizes the descriptive statistics of the reaction times during the SIDT. Subsequent post hoc tests (Dunn–Bonferroni tests) revealed that the reaction time to the target in the condition CueVSS was significantly faster than the reaction time in the condition CueControl (z = 2.68, P < 0.05, Cohen's d = -0.65) and in the condition CueNone (z = 3.35, P < 0.01, Cohen's d = -0.82). In contrast, the reaction times to the target stimulus in the conditions CueControl and to CueNone did not differ significantly from each other (z = 0.59, P = 0.56). No significant correlations were found between the reaction times of the three conditions and risk factors for CSBD (all r < 0.1, P > 0.10). CueNone was followed by 75 (4.89%) missing responses, CueControl was followed by 51 (3.33%) missing responses, and CueVSS was followed by 17 (1.11%) missing responses across all participants.
Table 2.
Descriptive statistics of reaction times in the sexual incentive delay task (N = 73)
| Median (SD) | |
| CueVSS | 235.11 (60.94) |
| CueControl | 296.63 (135.01) |
| CueNone | 314.42 (158.64) |
Note: CueVss = cue announcing a pornographic video, CueControl = cue announcing a massage video, CueNone = cue announcing no video.
Hemodynamic responses
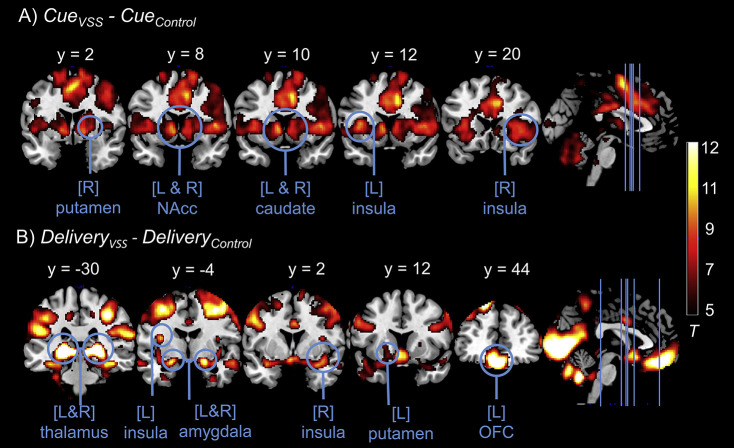
Cues signaling VSS as compared to cues signaling control clips elicited a higher blood-oxygenation-level dependent (BOLD) response in NAcc, caudate, putamen, and insula (all bilateral), as well as in the right dACC and thalamus. A higher BOLD response was also found in the left NAcc and OFC, in the bilateral caudate, putamen, dACC, insula, amygdala, and thalamus during delivery of VSS clips compared to control clips (all results see Table 3 and Fig. 3).
Table 3.
ROI results for the contrasts CueVSS–CueControl and DeliveryVSS–DeliveryControl (One Sample t-tests) with cluster size (k) and statistics (FWE-corrected; N = 73)
| Contrast | Structure | Side | x | y | z | k | T max | P corr |
| CueVSS–CueControl | NAcc | L | −6 | 8 | −4 | 77 | 8.71 | <0.001 |
| R | 8 | 10 | −4 | 65 | 7.50 | <0.001 | ||
| caudate | L | −8 | 10 | 2 | 449 | 9.66 | <0.001 | |
| R | 10 | 14 | 4 | 476 | 8.18 | <0.001 | ||
| putamen | L | −16 | 8 | −2 | 774 | 6.72 | <0.001 | |
| R | 24 | 2 | 4 | 766 | 7.42 | <0.001 | ||
| dACC | R | 12 | 16 | 36 | 1,697 | 10.77 | <0.001 | |
| insula | L | −34 | 14 | 6 | 592 | 9.43 | <0.001 | |
| R | 38 | 14 | 4 | 604 | 8.65 | <0.001 | ||
| thalamus | R | 8 | −2 | 0 | 2,164 | 8.91 | <0.001 | |
| DeliveryVSS–DeliveryControl | NAcc | L | −8 | 14 | −8 | 69 | 9.49 | <0.001 |
| caudate | L | −12 | −6 | 18 | 56 | 4.24 | <0.01 | |
| R | 16 | −16 | 22 | 71 | 5.32 | <0.001 | ||
| putamen | L | −18 | 12 | −10 | 314 | 6.58 | <0.001 | |
| R | 32 | −12 | −10 | 63 | 7.28 | <0.001 | ||
| dACC | L | −2 | 20 | 28 | 953 | 5.43 | <0.001 | |
| R | 4 | 4 | 32 | 953 | 9.19 | <0.001 | ||
| amygdala | L | −22 | −4 | −16 | 232 | 10.71 | <0.001 | |
| R | 20 | −4 | −14 | 280 | 12.20 | <0.001 | ||
| insula | L | −36 | −4 | 14 | 517 | 9.52 | <0.001 | |
| R | 38 | 2 | −16 | 476 | 9.19 | <0.001 | ||
| OFC | L | −6 | 44 | −18 | 2,825 | 17.45 | <0.001 | |
| thalamus | L | −20 | −30 | −2 | 1,747 | 25.67 | <0.001 | |
| R | 20 | −28 | 0 | 1,747 | 24.08 | <0.001 |
Fig. 3.
ROI activity for the contrasts CueVSS–CueControl (A) and DeliveryVSS–DeliveryControl (B). Lines on the sagittal slice on the right side indicate the coronal slices depicted on the left. Cues signaling VSS (CueVSS) as compared to cues signaling massage clips (CueControl) elicited a higher BOLD response in putamen, NAcc, caudate, and insula. VSS clips (DeliveryVSS) compared to massage clips (DeliveryControl) elicited a higher BOLD response in thalamus, insula, amygdala, putamen, and OFC. Displayed t-values are thresholded at t < 5
Whole brain analyses revealed higher hemodynamic responses in a continuous cluster including large parts of the brain for the contrast CueVSS compared to CueControl (Cluster extent k = 174,054 voxel) and again for the contrast DeliveryVSS compared to DeliveryControl (k = 134,654)
Risk factors for CSBD and hemodynamic responses
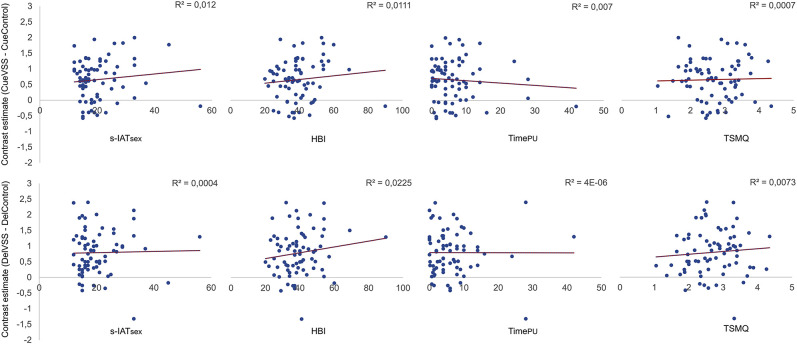
None of the regression analyses on the links between risk factors for CSBD (self-reported PPU, time spent on pornography use, and trait sexual motivation) and discriminative neural activity in any ROI during the anticipation phase (CueVSS–CueControl) or the delivery phase (DeliveryVSS–DeliveryControl) yielded any significant effects. Figure 4 presents the associations between these risk factors and left nucleus accumbens' peak voxel activity.
Fig. 4.
Correlation between the left nucleus accumbens' peak voxel activity and s-IATsex, HBI, time spent on pornography use in h/month (TimePU) and total scores of the TSMQ during the anticipation phase (upper row, NAcc [-6 8 -4]) and the delivery phase (bottom row, NAcc [-8 14 -8]) of the Sexual Incentive Delay Task (N = 73)
Discussion
The first aim of this report was to investigate the reward-related brain activity during the anticipation and the delivery phase of VSS in a large non-clinical sample using an SIDT. We found that the presentation of pornographic videos as well as the presentation of cues preceding pornographic videos was associated with higher brain activity in predefined reward-related brain areas (NAcc, amygdala, OFC, putamen, caudate nucleus, insula, thalamus, and dACC) compared to the presentation of massage videos or cues preceding massage videos, respectively. Our results are in line with the findings of Sescousse et al. (2015, 2010), who compared the neural response to VSS and non-VSS stimuli (here monetary) stimuli in a sample of healthy men during an incentive delay task. Regarding the brain responses to VSS cues, they found higher activity in the ventral striatum with increasing expected reward intensity. During delivery, they found also reward-specific brain activity to VSS in part of the OFC as well as in the bilateral amygdala. Additionally, they identified regions that were involved in the processing of both kinds of rewards (ventral striatum, midbrain, ACC, anterior insula).
The behavioral data showed that the reaction times were significantly faster to target stimuli in the condition presenting pornographic cues than in the conditions with control cues or cues that announced no video at all. This indicates that the expectation of VSS activates the motor system, which underlines the high motivational value of VSS.
The second aim was to explore the relationship between the neural responses to VSS as well as cues and risk factors for CSBD. The measured risk factors showed correlative relationships of medium strength among each other, indicating similarities as well as incremental parts of the constructs. Neither questionnaires measuring PPU (HBI and s-IATsex), nor the amount of time spend on porn, nor trait sexual motivation (TSMQ) were significantly correlated with brain activities of the reward-related brain areas during delivery and anticipation of sexual stimuli.
To appropriately discuss the missing correlation between risk factors for CSBD and neural responses to VSS, it is helpful to consult the existing literature of studies which either compare the neural responses of CSBD with control participants (group comparison approach) or analyze the correlation of risk factors for CSBD with the NAcc responses to VSS (correlational approach). Following the group comparison approach, some studies found greater neural responses towards VSS in the ventral striatum as well as in other reward-associated brain areas in participants with PPU compared to control participants (Gola et al., 2017; Seok & Sohn, 2015; Voon et al., 2014). An important result of the study by Gola et al. (2017) was that cues that predicted VSS were associated with higher striatal activity in CSBD participants than in healthy subjects. While Gola et al. (2017) investigated a mixed sexual and monetary incentive delay paradigm with pictograms of naked women as cues, Klucken et al. (2016) examined an appetitive conditioning paradigm with geometric cues. As a result, they found increased amygdala activity during conditioning for the CS+ (cue predicting VSS) versus the CS− (cue predicting nothing) in participants with CSBD compared to control participants, but no differences in the ventral striatum. In contrast, in the appetitive conditioning paradigm of Banca et al. (2016) there were no group effects between CSBD participants and control participants regarding the neural responses to different cues (colored patterns predicting VSS, monetary rewards or nothing).
Studies following the correlational approach revealed inconsistent results regarding the correlation between risk factors for CSBD and the neural responses to VSS: While Kühn and Gallinat (2014) found a negative correlation between the time spent on pornography and activity in the left putamen, Brand et al. (2016) reported no statistically significant correlation of ventral striatum responses and usual time spent on pornography. However, they found that the ventral striatum activity was positively correlated with the level of self-assessed PPU (measured by the s-IATsex). In addition, in one of our previous studies we could not find any significant influence of time spend on pornography or trait sexual motivation on the neural response to VSS (Stark et al., 2019). Accordingly, current research concerning the processing of VSS in subjects with varying degrees of risk factors for CSBD appears inconsistent. Rather uniform findings of studies employing the group comparison approach but inconsistent results from correlational studies might suggest that the neural processing of VSS in CSBD substantially differs from that in subclinical samples. This suggestion, however, is of interest in light of the Incentive Sensitization Theory of Robinson and Berridge (1993) which suggests increasing neural responses to cues during addiction development. So far, it remains unclear whether the theory applies to CSBD and if so, whether the increasing neural responses to VSS change dimensionally or whether a critical level of addictive behavior must be exceeded.
Interestingly, also in substance-related addictions the results concerning the Incentive Sensitization Theory are inconsistent. Several meta-analyses showed an increased cue reactivity in the reward system (Chase, Eickhoff, Laird, & Hogarth, 2011; Kühn & Gallinat, 2011b; Schacht, Anton, & Myrick, 2012), but some studies could not confirm these findings (Engelmann et al., 2012; Lin et al., 2020; Zilberman, Lavidor, Yadid, & Rassovsky, 2019). Also for behavioral addictions a higher cue reactivity in the reward network of addictive subjects in comparison to healthy subjects was only found in a minority of the studies as summarized in a most recent review by Antons et al. (2020). From this summary, the conclusion can be drawn that cue reactivity in addiction is modulated by several factors like individual factors and study-specific factors (Jasinska et al., 2014). Our zero findings regarding the correlations between striatal activity and risk factors of CSBD may also be due to the fact that even with our large sample we could only consider a small selection of possible influencing factors. Further large-scale studies are needed to do justice to multicausality. In terms of design, for example, the sensory modality of cues or the individualization of cues could be important (Jasinska et al., 2014).
According to our large sample size (in contrast to other studies) it is unlikely that a lack of statistical power caused the null findings with regard to the correlation of risk factors for CSBD and neural responses to VSS and cues of VSS. More probably, the evolutionary-driven, generally highly motivational value of VSS activates reward-associated brain areas highly uniformly leaving only small space for individual differences (ceiling effect). This hypothesis is supported by studies showing that there are hardly any sex differences with regard to the processing of VSS in the reward network (Poeppl et al., 2016; Stark et al., 2019; Wehrum et al., 2013). Nevertheless, the reasons for inconsistencies between the studies need to be uncovered by further studies.
Limitations and recommendations for further research
Several limitations have to be considered. In our study we only examined western-culture, heterosexual men. A replication of the study with a more diverse sample in terms of gender, sexual orientation, and socio-cultural factors seems necessary to ensure ecological validity. In addition, data was derived from a non-clinical sample, future studies will have to also consider samples with clinically relevant CSBD symptoms. The cues used in this study were described as neutral cues without any individually different previous experience. However, the price of this procedure with high internal validity might be a lack of external validity since pornography cues in everyday life are highly individualized.
Another limitation is the flexible response format (per day/per week/per month) regarding the assessment of pornography use. According to Schwarz and Oyserman (2001) responses to the same question are of limited comparability when the response format refers to different time periods. The main reason for choosing this response format was that the extent of pornography use in samples can vary greatly (from a few hours a year to several hours a day). In addition, it seemed relevant that a fixed response format would potentially impose a norm as to what level of pornography use is appropriate. Therefore, we decided to use the flexible response format for this intimate question, despite its known weakness.
Moreover, the laboratory represents an artificial setting, since pornography use in daily life is usually accompanied by masturbation. Therefore, it remains uncertain whether the reward comes from masturbation/orgasm and/or from the pornographic material itself. Gola et al. (2016) convincingly argued that sexual stimuli can be both cues and rewards. If the pornographic films are also interpreted as cues, future studies might allow masturbation to realize a true delivery phase. However, ethical and technical difficulties need to be considered to conduct such a study. To better understand the development of CSBD, studies covering the entire spectrum of CSBD symptoms (healthy, subclinical, clinical) are necessary.
Conclusions
Our study examined the processing of cues and VSS stimuli using a SIDT in a large non-clinical sample. Further, our modified SIDT optimizes previous SIDT by using film clips instead of static pictures, by using massage videos as control condition instead of scrambled pictures, and by using cues not containing sexual information. We were able to replicate the results showing the involvement of the reward system during both the processing of cues and of VSS. Contrary to our hypotheses, we could not identify effects of personal characteristics thought as risk factors for the development of CSBD on the neural responses in any ROI connected to the reward system. Future research should examine the entire spectrum of CSBD symptoms to better understand how pornography use develops into pathological behavior and which factors can predict this development.
Funding sources
This study was funded by the German research association (DFG STA 475/16-1).
Author's contribution
JS, OK, and RS conceived the study, RS obtained funding, SK and CM analyzed the data, CM drafted the article, RS, SK, JS, and OK revised the article for important intellectual content.
All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors declare no conflicts of interest.
Contributor Information
Charlotte Markert, Email: charlotte.markert@psychol.uni-giessen.de.
Sanja Klein, Email: sanja.klein@psychol.uni-giessen.de.
Jana Strahler, Email: jana.strahler@psychol.uni-giessen.de.
Onno Kruse, Email: onno.kruse@psychol.uni-giessen.de.
Rudolf Stark, Email: rudolf.stark@psychol.uni-giessen.de.
References
- Antons, S., Brand, M., & Potenza, M. N. (2020). Neurobiology of cue-reactivity, craving, and inhibitory control in non-substance addictive behaviors. Journal of the Neurological Sciences, 415, 116952. 10.1016/j.jns.2020.116952. [DOI] [PubMed] [Google Scholar]
- Balodis, I. M., & Potenza, M. N. (2015). Anticipatory reward processing in addicted populations: A focus on the monetary incentive delay task. Biological Psychiatry, 77(5), 434–444. 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banca, P., Morris, L. S., Mitchell, S., Harrison, N. A., Potenza, M. N., & Voon, V. (2016). Novelty, conditioning and attentional bias to sexual rewards. Journal of Psychiatric Research, 72, 91–101. 10.1016/j.jpsychires.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski, A., Vogl, R., & Stark, R. (2019). Prevalence and determinants of problematic online pornography use in a sample of German women. The Journal of Sexual Medicine, 16(8). 10.1016/j.jsxm.2019.05.010. [DOI] [PubMed] [Google Scholar]
- Beck, A., Schlagenhauf, F., Wüstenberg, T., Hein, J., Kienast, T., Kahnt, T., et al. (2009). Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry, 66(8), 734–742. 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berridge, K. C., & Robinson, T. E. (2016). Liking, wanting and the incentive-sensitization theory of addiction. American Psychologist, 71(8), 670–679. 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais-Lecours, S., Vaillancourt-Morel, M. P., Sabourin, S., & Godbout, N. (2016). Cyberpornography: Time use, perceived addiction, sexual functioning, and sexual satisfaction. Cyberpsychology, Behavior, and Social Networking, 19(11). 10.1089/cyber.2016.0364. [DOI] [PubMed] [Google Scholar]
- Bradley, M. M., & Lang, P. J. (1994). Measuring emotion: The Self-Assessment Manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Brand, M., Snagowski, J., Laier, C., & Maderwald, S. (2016). Ventral striatum activity when watching preferred pornographic pictures is correlated with symptoms of Internet pornography addiction. NeuroImage, 129, 224–232. 10.1016/j.neuroimage.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Brand, M., Wegmann, E., Stark, R., Müller, A., Wölfling, K., Robbins, T. W., et al. (2019). The Interaction of Person-Affect-Cognition-Execution (I-PACE) model for addictive behaviors: update, generalization to addictive behaviors beyond internet-use disorders, and specification of the process character of addictive behaviors. Neuroscience & Biobehavioral Reviews, 104, 1–10. 10.1016/j.neubiorev.2019.06.032. [DOI] [PubMed] [Google Scholar]
- Bustamante, J. C., Barrós-Loscertales, A., Costumero, V., Fuentes-Claramonte, P., Rosell-Negre, P., Ventura-Campos, N., et al. (2014). Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addiction Biology, 19(5). 10.1111/adb.12041. [DOI] [PubMed] [Google Scholar]
- Bőthe, B., Tóth-Király, I., Potenza, M. N., Orosz, G., & Demetrovics, Z. (2020). High-frequency pornography use may not always be problematic. The Journal of Sexual Medicine, 17(4), 793–811. 10.1016/j.jsxm.2020.01.007. [DOI] [PubMed] [Google Scholar]
- Chase, H. W., Eickhoff, S. B., Laird, A. R., & Hogarth, L. (2011). The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biological Psychiatry, 70(8), 785–793. 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, A., Scherer, C. R., Boies, S. C., & Gordon, B. L. (1999). Sexuality on the Internet: From sexual exploration to pathological expression. Professional Psychology: Research and Practice, 30(2), 154–164. 10.1037/0735-7028.30.2.154. [DOI] [Google Scholar]
- Engelmann, J. M., Versace, F., Robinson, J. D., Minnix, J. A., Lam, C. Y., Cui, Y., et al. (2012). Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. NeuroImage, 60(1). 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth-Bühler, M., Mann, K., & Potenza, M. N. (2017). Pathological gambling: A review of the neurobiological evidence relevant for its classification as an addictive disorder. Addiction Biology, 22(4), 885–897. 10.1111/adb.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis, J. R., & Kringelbach, M. L. (2012). The human sexual response cycle: Brain imaging evidence linking sex to other pleasures. Progress in Neurobiology, 98(1), 49–81. 10.1016/j.pneurobio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Gola, M., Lewczuk, K., & Skorko, M. (2016). What matters: Quantity or quality of pornography use? Psychological and behavioral factors of seeking treatment for problematic pornography use. The Journal of Sexual Medicine, 13(5), 815–824. 10.1016/j.jsxm.2016.02.169. [DOI] [PubMed] [Google Scholar]
- Gola, M., Wordecha, M., Marchewka, A., & Sescousse, G. (2016). Visual sexual stimuli – cue or reward? A perspective for interpreting brain imaging findings on human sexual behaviors. Frontiers in Human Neuroscience, 10, 402. 10.3389/fnhum.2016.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola, M., Wordecha, M., Sescousse, G., Lew-Starowicz, M., Kossowski, B., Wypych, M., et al. (2017). Can pornography be addictive? An fMRI study of men seeking treatment for problematic pornography use. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42(10), 2021–2031. 10.1038/npp.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs, J. B., Volk, F., Exline, J. J., & Pargament, K. I. (2015). Internet pornography use: Perceived addiction, psychological distress, and the validation of a brief measure. Journal of Sex & Marital Therapy, 41(1), 83–106. 10.1080/0092623X.2013.842192. [DOI] [PubMed] [Google Scholar]
- Het, S., Rohleder, N., Schoofs, D., Kirschbaum, C., & Wolf, O. T. (2009). Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology, 34(7), 1075–1086. 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Jasinska, A. J., Stein, E. A., Kaiser, J., Naumer, M. J., & Yalachkov, Y. (2014). Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neuroscience & Biobehavioral Reviews, 38, 1–16. 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Z., Worhunsky, P. D., Carroll, K. M., Rounsaville, B. J., Stevens, M. C., Pearlson, G. D., et al. (2011). An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry, 70(6), 553–560. 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer, S., Wehrum, S., Klucken, T., Walter, B., Vaitl, D., & Stark, R. (2014). Sex attracts: Investigating individual differences in attentional bias to sexual stimuli. PloS One, 9(9). 10.1371/journal.pone.0107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, S., Kruse, O., Markert, C., Tapia León, I., Strahler, J., & Stark, R. (2020). Subjective reward value of visual sexual stimuli is coded in human striatum and orbitofrontal cortex. Behavioural Brain Research, 393, 112792. 10.1016/j.bbr.2020.112792. [DOI] [PubMed] [Google Scholar]
- Klucken, T., Wehrum-Osinsky, S., Schweckendiek, J., Kruse, O., & Stark, R. (2016). Altered appetitive conditioning and neural connectivity in subjects with compulsive sexual behavior. The Journal of Sexual Medicine, 13(4), 627–636. 10.1016/j.jsxm.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Knutson, B., Fong, G. W., Adams, C. M., Varner, J. L., & Hommer, D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport, 12(17), 3683–3687. 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson, B., Westdorp, A., Kaiser, E., & Hommer, D. (2000). Fmri visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–27. 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Koob, G. F., & Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(1), 217–238. 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus, S. W., Voon, V., & Potenza, M. N. (2016). Should compulsive sexual behavior be considered an addiction? Addiction (Abingdon, England), 111(12), 2097–2106. 10.1111/add.13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn, S., & Gallinat, J. (2011a). Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience, 33(7), 1318–1326. 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Kühn, S., & Gallinat, J. (2011b). A quantitative meta-analysis on cue-induced male sexual arousal. The Journal of Sexual Medicine, 8(8), 2269–2275. 10.1111/j.1743-6109.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- Kühn, S., & Gallinat, J. (2014). Brain structure and functional connectivity associated with pornography consumption: The brain on porn. JAMA Psychiatry, 71(7), 827–834. 10.1001/jamapsychiatry.2014.93. [DOI] [PubMed] [Google Scholar]
- Laier, C., Pawlikowski, M., Pekal, J., Schulte, F. P., & Brand, M. (2013). Cybersex addiction: Experienced sexual arousal when watching pornography and not real-life sexual contacts makes the difference. Journal of Behavioral Addictions, 2(2), 100–107. 10.1556/JBA.2.2013.002. [DOI] [PubMed] [Google Scholar]
- Lin, X., Deng, J., Shi Le., Wang, Q., Li, P., Li, H., et al. (2020). Neural substrates of smoking and reward cue reactivity in smokers: A meta-analysis of fMRI studies. Translational Psychiatry, 10(1), 97. 10.1038/s41398-020-0775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, T., Laier, C., Brand, M., Hatch, L., & Hajela, R. (2015). Neuroscience of internet pornography addiction: A review and update. Behavioral Sciences (Basel, Switzerland), 5(3), 388–433. 10.3390/bs5030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyniuk, U., Okolski, L., & Dekker, A. (2019). Pornographic content and real-life sexual experiences: Findings from a survey of German university students. Journal of Sex & Marital Therapy, 45(5). 10.1080/0092623X.2018.1531334. [DOI] [PubMed] [Google Scholar]
- Nestor, L., Hester, R., & Garavan, H. (2010). Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage, 49(1). 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowski, M., Altstötter-Gleich, C., & Brand, M. (2013). Validation and psychometric properties of a short version of Young’s Internet Addiction Test. Computers in Human Behavior, 29(3), 1212–1223. 10.1016/j.chb.2012.10.014. [DOI] [Google Scholar]
- Poeppl, T. B., Langguth, B., Laird, A. R., & Eickhoff, S. B. (2014). The functional neuroanatomy of male psychosexual and physiosexual arousal: A quantitative meta-analysis. Human Brain Mapping, 35(4), 1404–1421. 10.1002/hbm.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl, T. B., Langguth, B., Rupprecht, R., Safron, A., Bzdok, D., Laird, A. R., et al. (2016). The neural basis of sex differences in sexual behavior: a quantitative meta-analysis. Frontiers in Neuroendocrinology, 43, 28–43. 10.1016/j.yfrne.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, R. C., Carpenter, B. N., Hook, J. N., Garos, S., Manning, J. C., Gilliland, R., et al. (2012). Report of findings in a DSM-5 field trial for hypersexual disorder. The Journal of Sexual Medicine, 9(11), 2868–2877. 10.1111/j.1743-6109.2012.02936.x. [DOI] [PubMed] [Google Scholar]
- Reid, R. C., Garos, S., & Carpenter, B. N. (2011). Reliability, validity, and psychometric development of the Hypersexual Behavior Inventory in an outpatient sample of men. Sexual Addiction & Compulsivity, 18(1), 30–51. 10.1080/10720162.2011.555709. [DOI] [Google Scholar]
- Robinson, T. E., & Berridge, K. C. (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews, 18(3), 247–291. [DOI] [PubMed] [Google Scholar]
- Robinson, T. E., & Berridge, K. C.(2008). The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society, 363, 3137–3146. 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesink, G. B., & Georgiadis, J. R. (2017). Brain imaging of human sexual response: recent developments and future directions. Current Sexual Health Reports, 9(4), 183–191. 10.1007/s11930-017-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht, J. P., Anton, R. F., & Myrick, H. (2012). Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addiction Biology, 18(1), 121–133. 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, N., & Oyserman, D. (2001). Asking questions about behavior: Cognition, communication, and questionnaire construction. American Journal of Evaluation, 22(2), 127–160. 10.1177/109821400102200202. [DOI] [Google Scholar]
- Schweckendiek, J., Klucken, T., Merz, C. J., Kagerer, S., Walter, B., Vaitl, D., et al. (2013). Learning to like disgust: Neuronal correlates of counterconditioning. Frontiers in Human Neuroscience, 7, 346. 10.3389/fnhum.2013.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok, J.-W., & Sohn, J.-H. (2015). Neural substrates of sexual desire in individuals with problematic hypersexual behavior. Frontiers in Behavioral Neuroscience, 9, 321. 10.3389/fnbeh.2015.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse, G., Li, Y., & Dreher, J. C. (2015). A common currency for the computation of motivational values in the human striatum. Social Cognitive and Affective Neuroscience, 10(4), 467–473. 10.1093/scan/nsu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse, G., Redouté, J., & Dreher, J. C. (2010). The architecture of reward value coding in the human orbitofrontal cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(39), 13095–13104. 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano, I., Eaton, N. R., & O'Leary, K. D. (2020). Pornography consumption, modality and function in a large Internet sample. Journal of Sex Research, 57(1), 92–103. 10.1080/00224499.2018.1532488. [DOI] [PubMed] [Google Scholar]
- Starcke, K., Antons, S., Trotzke, P., & Brand, M. (2018). Cue-reactivity in behavioral addictions: A meta-analysis and methodological considerations. Journal of Behavioral Addictions, 7(2), 227–238. 10.1556/2006.7.2018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, R., Kagerer, S., Walter, B., Vaitl, D., Klucken, T., & Wehrum-Osinsky, S. (2015). Trait sexual motivation questionnaire: Concept and validation. The Journal of Sexual Medicine, 12(4), 1080–1091. 10.1111/jsm.12843. [DOI] [PubMed] [Google Scholar]
- Stark, R., Klein, S., Kruse, O., Weygandt, M., Leufgens, L. K., Schweckendiek, J., et al. (2019). No sex difference found: Cues of sexual stimuli activate the reward system in both sexes. Neuroscience, 416, 63–73. 10.1016/j.neuroscience.2019.07.049. [DOI] [PubMed] [Google Scholar]
- Stark, R., Klucken, T., Potenza, M. N., Brand, M., & Strahler, J. (2018). A current understanding of the behavioral neuroscience of compulsive sexual behavior disorder and problematic pornography use. Current Behavioral Neuroscience Reports, 5(4), 218–231. 10.1007/s40473-018-0162-9. [DOI] [Google Scholar]
- Stippekohl, B., Winkler, M., Mucha, R. F., Pauli, P., Walter, B., Vaitl, D., et al. (2010). Neural responses to BEGIN- and END-stimuli of the smoking ritual in nonsmokers, nondeprived smokers, and deprived smokers. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(5). 10.1038/npp.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoléru, S., Fonteille, V., Cornélis, C., Joyal, C., & Moulier, V. (2012). Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: A review and meta-analysis. Neuroscience & Biobehavioral Reviews, 36(6), 1481–1509. 10.1016/j.neubiorev.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Strahler, J., Kruse, O., Wehrum-Osinsky, S., Klucken, T., & Stark, R. (2018). Neural correlates of gender differences in distractibility by sexual stimuli. NeuroImage, 176, 499–509. 10.1016/j.neuroimage.2018.04.072. [DOI] [PubMed] [Google Scholar]
- Tiffany, S. T., & Wray, J. M. (2012). The clinical significance of drug craving. Annals of the New York Academy of Sciences, 1248(1), 1–17. 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Holst, R. J., van den Brink, W., Veltman, D. J., & Goudriaan, A. E. (2010). Brain imaging studies in pathological gambling. Current Psychiatry Reports, 12(5), 418–425. 10.1007/s11920-010-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow, N. D., Koob, G. F., & McLellan, A. T. (2016). Neurobiologic advances from the brain disease model of addiction. New England Journal of Medicine, 374(4), 363–371. 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon, V., Mole, T. B., Banca, P., Porter, L., Morris, L., Mitchell, S., et al. (2014). Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. Plos One, 9(7), e102419. 10.1371/journal.pone.0102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, B., Blecker, C., Kirsch, P., Sammer, G., Schienle, A., Stark, R., et al. (2003, June). Marina: An easy to use tool for the creation of masks for region of interest analyses. 9th International Conference on Functional Mapping of the Human Brain, New York. [Google Scholar]
- Wehrum, S., Klucken, T., Kagerer, S., Walter, B., Hermann, A., Vaitl, D., et al. (2013). Gender commonalities and differences in the neural processing of visual sexual stimuli. The Journal of Sexual Medicine, 10(5), 1328–1342. 10.1111/jsm.12096. [DOI] [PubMed] [Google Scholar]
- Wordecha, M., Wilk, M., Kowalewska, E., Skorko, M., Łapiński, A., & Gola, M. (2018). “Pornographic binges” as a key characteristic of males seeking treatment for compulsive sexual behaviors: Qualitative and quantitative 10-week-long diary assessment. Journal of Behavioral Addictions, 7(2), 433–444. 10.1556/2006.7.2018.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (Ed.) (2018). International statistical classification of diseases and related health problems (11th ed.). [Google Scholar]
- Zilberman, N., Lavidor, M., Yadid, G., & Rassovsky, Y. (2019). Qualitative review and quantitative effect size meta-analyses in brain regions identified by cue-reactivity addiction studies. Neuropsychology, 33(3). 10.1037/neu0000526. [DOI] [PubMed] [Google Scholar]
- Zilverstand, A., Huang, A. S., Alia-Klein, N., & Goldstein, R. Z. (2018). Neuroimaging impaired response inhibition and salience attribution in human drug addiction. A systematic review. Neuron, 98(5), 886–903. 10.1016/j.neuron.2018.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]