Abstract
Background
Although previous studies have revealed gender-related differences in executive function in internet gaming disorder (IGD), neural mechanisms underlying these processes remain unclear, especially in terms of brain networks.
Methods
Resting-state fMRI data were collected from 78 subjects with IGD (39 males, 20.8 ± 2.16 years old) and 72 with recreational game use (RGU) (39 males, 21.5 ± 2.56 years old). By utilizing graph theory, we calculated participation coefficients among brain network modules for all participants and analyzed the diagnostic-group-by-gender interactions. We further explored possible causal relationships between networks through spectral dynamic causal modeling (spDCM) to assess differences in between-network connections.
Results
Compared to males with RGU, males with IGD demonstrated reduced modular segregation of the frontal-parietal network (FPN). Male IGD subjects also showed increased connections between the FPN and cingulo-opercular network (CON); however, these differences were not found in female subjects. Further spDCM analysis indicated that the causal influence from CON to FPN in male IGD subjects was enhanced relative to that of RGU males, while this influence was relatively reduced in females with IGD.
Conclusions
These results suggest poor modular segmentation of the FPN and abnormal FPN/CON connections in males with IGD, suggesting a mechanism for male vulnerability to IGD. An increased “bottom-up” effect from the CON to FPN in male IGD subjects could reflect dysfunction between the brain networks. Different mechanisms may underlie in IGD, suggesting that different interventions may be optimal in males and females with IGD.
Keywords: internet gaming disorder, sex difference, frontal-parietal network, participation coefficient, dynamic causal modeling
Introduction
Online gaming has become a popular form of entertainment; however, some individuals game excessively. These individuals may exhibit self-control difficulties, strong cravings and gaming behavior that interferes in important domains of life functioning. Criteria for internet gaming disorder (IGD) are located in Section III of the DSM-5 (American Psychiatric Association, 2013). It has been also designated as an addictive disorder by the World Health Organization in ICD-11 (https://icd.who.int/dev11/l-m/en#/http://id.who.int/icd/entity/144859723). IGD has been associated with multiple negative consequences, including physical and psychological disorders, social impairments and poor work performance (King & Delfabbro, 2018; Petry et al., 2014).
Gender-related differences in IGD have been observed among adolescents and young adults (King & Potenza, 2020). Males more frequently develop IGD than females (Borgonovi, 2016). Among survey respondents, 87.4% of male and 45.6% of female teenagers reported playing online games in China, with more boys (6.3%) than girls (2.4%) experiencing IGD (Li, L., Yu, Q., Zhang, L., Jin, 2015). Similar findings have been reported in Korea (Ha & Hwang, 2014) and other regions (Borgonovi, 2016; Desai, Krishnan-Sarin, Cavallo, & Potenza, 2010). These differences do not appear to result from differences in gaming skills (Shen, Ratan, Cai, & Leavitt, 2016) while behavioral motivation may be a potential explanation. Gray proposed different brain systems that underlie motivated behaviors: a behavioral inhibition system (BIS) and behavioral activation system (BAS) (Gray, 1987). The BIS/BAS scale assesses these domains (Carvel & White, 1994). Gender-related differences in BIS/BAS have been reported including with respect to emotional processing and internet addiction. For example, women versus men have exhibited higher BIS scores that mediated gender-related differences in fear and sadness (Ma-Kellams & Wu, 2020). Among adolescents, BIS and impulsivity predicted internet addiction in girls and BAS and impulsivity predicted internet addiction in boys (Q. Li et al., 2019). Gender differences have also been noted in neurobiological correlates of BIS/BAS (Li et al., 2014), and BIS has been related to amygdalar and hippocampal volumes in a predominantly male sample with gambling disorder (Rahman, Xu, & Potenza, 2014). However, this has not been investigated previously in IGD.
In fact, many prior studies have included predominantly or solely male subjects, generating a relative deficiency in our understanding of females with IGD. Understanding gender-related differences in neural correlates of IGD could provide further insight into behavioral and clinical features in males and females, having important public health implications.
Gender-related differences in IGD may reflect underlying neurocognitive mechanisms (Dong, Wang, Wang, Du, & Potenza, 2019). One fMRI study found that males versus females with IGD reported stronger cue-elicited cravings and greater changes in thalamic activation following gaming (Dong, Wang, Du, & Potenza, 2018). Another study found differences in dorsolateral prefrontal cortical (DLPFC) and caudate activations during a cue-exposure task before and after gaming, with individuals with IGD showing less DLPFC activation post-gaming (particularly among females); relatively greater caudate activation among females with IGD was also observed post-gaming (Dong, Zheng, Li, Wang, & Potenza, 2018). In addition, gender-related differences have also been found in functional connectivity of cortical and subcortical regions in response to gaming cues that differed during by recency of gaming and linked to self-reported craving (Dong et al., 2019). These findings indicated that gender-related IGD differences appeared to exist in neurological responses related to cravings and were linked to executive function.
Brain function is driven not only by local properties of specific regions, but also by coordinated efforts between brain regions and effective coupling between brain networks (Marek, Hwang, Foran, Hallquist, & Luna, 2015; Reineberg, Andrews-Hanna, Depue, Friedman, & Banich, 2015; Reineberg & Banich, 2016). However, the extent to which gender-related differences in IGD are reflected in the functioning of specific brain networks has not been previously studied. Further exploring the neural mechanisms especially in terms of brain networks could provide more insight into behavioral and clinical features of IGD in males and females, which help to develop better intervention.
Brain network interactions underlie cognitive activity that may be involved in regulating emotions and motivations. Neural changes in specific brain circuits have been linked to specific cognitive functions (Grattan-Miscio & Vogel-Sprott, 2005; Warren et al., 2014). For example, graph theory analyses (Rubinov & Sporns, 2010) suggest that structural brain network modules may become increasingly segregated with age, relating to increased within-network connectivity and decreased between-network connectivity (Baum et al., 2017). Similar results have been found in resting-state brain network modules (Fair et al., 2007). Furthermore, successful modular segregation of brain networks contributes to the development of executive functions, and task-dependent brain network modules become increasingly segregated with age during executive-function fMRI tasks (Baum et al., 2017; C. Wang, Hu, Weng, Chen, & Liu, 2020). For example, modular segregation of the frontal-parietal network (FPN) significantly mediated the relationship between age and executive function performance (Wang et al., 2020). In previous studies, the FPN has been suggested as the primary substrate of executive functions, based on a strong pattern of coactivation across a wide range of executive function tasks (Owen, McMillan, Laird, & Bullmore, 2005; Wang, Dong, Zheng, Du, & Dong, 2020). Executive function deficits have been described in IGD (Dong, Li, Wang, & Potenza, 2017). Besides, males as compared to females with IGD subjects have demonstrated neural differences in cue reactivity that may involve subsequent motivational drives and executive control (Dong, Liu, Wang, Du, & Potenza, 2018). Given that executive functions are driven not only by regional properties, but also by neural network coupling (Marek et al., 2015; Reineberg & Banich, 2016), applying graph theory to examine segregation of the FPN in IGD may provide further insight into executive functioning in IGD.
Based on above questions, the present study aimed to further understand brain network processes by applying the graph theory and dynamic causal modeling (DCM) to analyze the resting state fMRI data of IGD. Contrasted with previous activation and FC analysis, participation coefficient (PC) analysis, an indicator of graph theory, can provide the degree of modular segregation in different brain networks, which give the information of intra- and inter-network connections (Baum et al., 2017; Wang et al., 2020). These information contribute to the understanding the interaction of different brain regions. Further, understanding the causal effects of the interaction is also important. DCM analysis provides such a chance to explore the effective connectivity. Effective connectivity refers to the influence that one neural system exerts over another. It is dynamic, and dependent on a model of interactions or coupling (Friston, 2011). It provides estimations for directional relationships among brain regions from fMRI time series (Friston, Kahan, Biswal, & Razi, 2014). So we conducted an exploratory analysis of the connections between the networks based on the findings of PC analysis. We used spectral dynamic causal modeling (spDCM) to investigate directionality of connection differences between networks.
We first hypothesized that 1) resting-state brain network modules would be more poorly segregated in male IGD subjects than in females. As the FPN has been suggested as a substrate of executive function and its modular segregation significantly mediates the relationship between age and executive functions, we also hypothesized that 2) differences in network modularity between male and female IGD subjects would be reflected in the FPN. We also explored relationships with BIS/BAS measures. 3) differences in directionality of connections between male and female would appeared in the FPN and other brain networks.
Methods
Participants
Seventy-eight IGD subjects (39 males) and 72 RGU subjects (39 males) were recruited from universities in Shanghai China by online advertisements. These subjects were screened by completing an electronic addiction questionnaire and were strictly evaluated by trained researchers on the day of the experiment according to the nine criteria of the DSM-5. All IGD subjects satisfied at least five of nine inclusionary criteria for IGD and indicated that they played internet game more than 20 hours per week. All subjects were evaluated by a structured psychiatric interview (MINI) and did not have any Axis I psychiatric illness.
All subjects were required to complete the Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) scale (Carvel & White, 1994) which has been related to self-control-related measures (Krmpotich et al., 2013; Laurent et al., 2018). This scale contains 20 four-point Likert-type items and is divided into BIS and BAS subscales. The BIS subscale assesses tendencies associated with inhibition/avoidance of punishment. The BAS subscale tendencies associated with seeking rewarding stimuli and outcomes. The latter is further divided into three subscales: (1) BAS-Drive, measuring persistence in pursuit of goals; (2) BAS-Fun Seeking, measuring desires for novel rewards; (3) BAS-Reward, measuring positive responses to anticipation of rewards. In each subscale, a higher score indicates a higher level of the tendency.
In addition, all subjects were also asked to fill in the following questionnaire: 1) Sensation Seeking Scale – VI; 2) Beck Depression Inventory; 3) Profile of mood states; 4) Loneliness Scale, University of California at Los Angels; 5) Self-Esteem Scale; 6) Pittsburgh sleep quality index; 7) Fagerstrom Test of Nicotine Dependence and 8) Alcohol Use Disorders Identification Test. Since these scales were not related to the subject of this study, they were not included in the analysis. The fMRI sequences collected by the subjects on the day of the scan mainly include: 1) high-resolution T1-weighted images; 2) resting-state functional images; 3) task-based functional images (craving reactivity task; instrumental learning task). The current study mainly used the resting-state functional images and part of the data has been published in previous studies (Dong, Wang, et al., 2018; Dong, Zheng, et al., 2018).
Image acquisition
Resting-state fMRI data were acquired using a 3T MRI system (Siemens Trio). During resting-state fMRI, subjects were instructed to keep their eyes closed and remain motionless and awake. Imaging parameters were as follows: repetition time (TR) = 2,000 ms, interleaved 33 slices, echo time (TE) = 30 ms, thickness = 3.0 mm, flip angle = 90°, field of view (FOV) = 220 mm × 220 mm, matrix = 64 × 64. Each fMRI scan lasted 420 s, and included 210 imaging volumes.
Preprocessing of image data
Preprocessing was conducted with DPABI_v4.3, a toolbox for Data Processing & Analysis for Brain Imaging (Yan, Wang, Zuo, & Zang, 2016), based on MATLAB’s toolbox. The first 10 volumes from each participant were discarded to minimize instability in the initial signal and ensure that participants had adapted to the scanning environment, leaving 200 volumes. The remaining processes included slice timing to correct for time differences and head-motion correction and spatial normalization using the standard EPI template. Data with head-motion exceeding a maximum 2.5 mm or 2.5° of maximum rotation during scanning were excluded from further analyses. Six motion vectors (e.g., cerebral spinal fluid, white matter) were regressed out as nuisance signals. Following this, data were detrended to remove linear trends and temporally filtered (0.01–0.08 Hz) to remove low-frequency drift and high-frequency noise.
Network construction and graph theory analysis
We applied a functional template (Dosenbach et al., 2010) to obtain defined nodes for network construction as described previously (Wang et al., 2020). This functional template can divide the brain into 160 functionally segregated regions of interest (ROIs) that cover most of the cerebral cortex and cerebellum. The set of ROIs (6 mm diameter spheres) was generated using the peak coordinates derived from a series of meta-analyses of fMRI activation studies (Dosenbach et al., 2010). Average time courses from each ROI were extracted and paiwise Pearson correlation coefficients were computed between these ROIs. To enable comparison of network properties across participants and groups, a proportional threshold (15%) was used to ensure the same number of network edges for each participant in the present study. This threshold is capable of maintaining a balance between the use of very sparse graphs and denser graphs (Reineberg & Banich, 2016).
All graph theory measures were calculated using GRETNA (https://www.nitrc.org/projects/gretna). As previously (Dosenbach et al., 2010), the 160 regions of interest (ROIs) were assigned into six functional modules, corresponding to the default-mode network (DMN), frontal–parietal network (FPN), cingulo-opercular network (CON), sensorimotor network (SMN), visual network, and cerebellum (see Fig. 1). This modular structure was used to compute the following graph theory measures. First, the participation coefficient (PC) was calculated to quantify the degree of modular segregation. For node i, PC is defined as . In this formula, m refers to a module in a set of modules M, kim refers to the number of connections between node i and module m, and ki is the total number of connections of node i in the whole brain network (Guimera & Amaral, 2005). PCi measures the proportion of inter- and intra-module connections for node i. Generally, PC will be close to zero if one node is highly integrated with other nodes in its own module but less integrated with nodes in other modules (greater modular segregation); inversely, PC will be close to one if the node is less integrated with the nodes in its own module but is highly integrated with nodes in other modules (lower modular segregation). Here we used the average PC of each module to characterize modular segmentation.
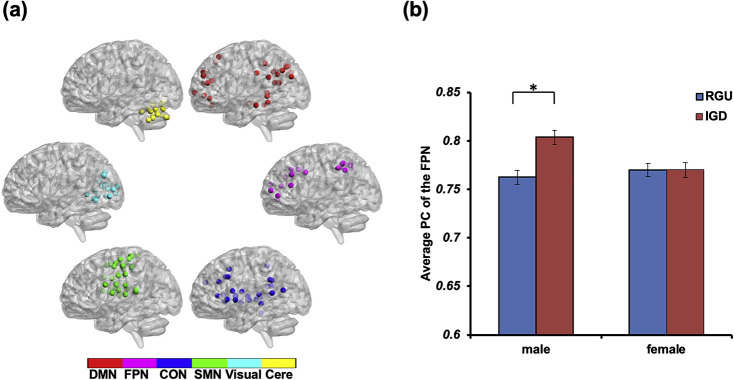
Fig. 1.
Illustration of simple effect analysis in average PC of the FPN. The subplot (a) shows module partitions: RGU, recreational gaming user; IGD, internet gaming disorder; DMN, the default-mode network; FPN, the frontal–parietal network; CON, the cingulo-opercular network; SMN, the sensorimotor network; Visual, the visual network; Cere, the cerebellum network. The subplot (b) shows the PC of FPN for the 4 subgroups. PC: participation coefficient. *: p with Bonferroni correction
In addition, as significant interactions between diagnostic and gender groups were found in the PC of the FPN (see results), we calculated the number of connections within the FPN and between the FPN and the other 5 modules. These measures provide further information regarding segregation of information processing within the FPN and the degree of integration between the FPN and other networks.
Spectral dynamic causal modeling
The spDCM analysis was implemented by DCM12.5 (revision 7487), which is based on SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) to further infer causal influences between the FPN and CON (see results for the inter-module connections). A detailed description of the DCM specification can be found in Friston, Harrison, and Penny (2003), Friston, Kahan, Biswal, and Razi (2014) and our previous work (Wang et al., 2020). Briefly, we first constructed a general linear model (GLM) for each participant’s data and extracted and corrected the principal eigenvariate from the FPN and CON masks (see Fig. 2). A single “full” model was then specified for each participant. Here, the “full” model means that a two-way influence is assumed between the FPN and CON. Then, we completed the estimation of the first-level DCM model by inverting the “full” models.
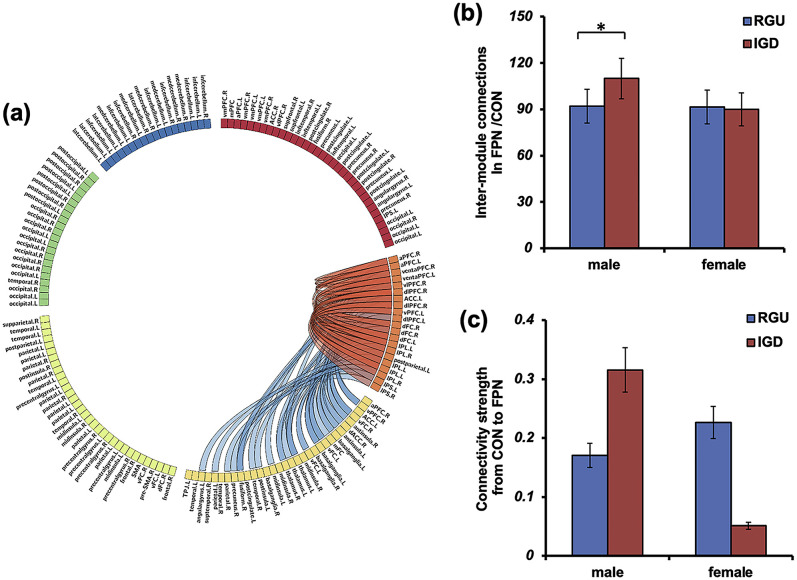
Fig. 2.
Illustration of the inter-module connections and causality between the FPN and the CON. The subplot (a) is an illustration of the inter-module and intra-module connections of the FPN. The subplot (b) shows the simple effect analysis of FPN/CON connections for the 4 subgroups. The subplot (c) shows the simple effect analysis of effective connections from the CON to the FPN. RGU, recreational gaming user; IGD, internet gaming disorder; FPN, the frontal–parietal network; CON, the cingulo-opercular network. *: p with Bonferroni correction
Statistical analyses
Two-way ANOVAs were conducted by SPSS 20.0 to test the effects of group, gender, and their interaction on the average PC of the six functional modules, in which age and education history were used as covariates. To control for multiple comparisons, Bonferroni correction was used. The significant threshold was set at α = 0.05/6 (six measures) = 0.0083. Additionally, we analyzed changes in the intra-module connections and inter-module connections of the FPN. Similarly, Bonferroni correction was used to control for multiple comparisons (α = 0.0083).
For the spDCM analysis, to enable interactions between diagnostic and gender groups to be quantified efficiently, we used the parametric empirical Bayes (PEB) framework to structure a hierarchical model over parameters (Friston et al., 2016; Henson, Flandin, Friston, & Mattout, 2010). We used the GLM to construct a group-level model based on the first-level DCM parameters. The group-level design matrix incorporates four regressors: 1) average DCM parameters of all subjects; 2) interactions between diagnostic and gender groups; 3) main effect of diagnosis; and, 4) main effect of gender. These matrix parameters were estimated and exploratory Bayesian model reduction was performed to optimize the ‘full’ model. A Bayesian model averaging of the second-level PEB models was then implemented to infer effective connectivity that best describes the interactions. The resulting parameters were thresholded at posterior probability >0.95 (based on free energy).
Finally, the BIS/BAS scores were calculated to assess correlations with the average PCs, inter-module and intra-module connections of the FPN, and effective connectivity strength for all subjects. Bonferroni correction was used to control for multiple comparisons.
Ethics
This experiment was approved by the Human Investigations Committee of Hangzhou Normal University and conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants provided written informed consent prior to participation in the study.
Results
Demographics
There were no significant differences in age, gender or education between IGD and RGU subjects. DSM-5 scores (t(148) = 20.608, p < 0.001, Cohens’d = 3.37) and gaming time (t(148) = 19.376, p < 0.001, Cohens’d = 3.17) were greater for IGD versus RGU subjects. IGD versus RGU subjects also showed higher BIS scores (t(148) = 3.415, p = 0.001, Cohens’d = 0.55) (Table 1).
Table 1.
Demographic information and diagnostic group differences
| (M ± SD) | IGD (n = 78) | RGU (n = 72) | F | P | ||
| M (n = 39) | F (n = 39) | M (n = 33) | F (n = 39) | |||
| Age | 20.9 ± 2.28 | 20.8 ± 2.08 | 21.7 ± 2.86 | 21.3 ± 2.20 | 1.224 | 0.271 |
| DSM-5 score | 5.97 ± 0.98 | 5.90 ± 1.12 | 2.00 ± 1.22 | 2.12 ± 1.32 | 417.15 | <0.001 |
| Gaming history (years) | 3.82 ± 0.49 | 3.70 ± 0.56 | 3.43 ± 0.65 | 3.78 ± 0.57 | 0.020 | 0.887 |
| Gaming per week (hours) | 29.21 ± 9.40 | 29.85 ± 10.30 | 6.64 ± 2.53 | 6.09 ± 3.17 | 370.389 | <0.001 |
| Educations (years) | 14.59 ± 1.76 | 14.65 ± 1.40 | 14.88 ± 2.08 | 14.30 ± 0.95 | 0.075 | 0.784 |
| BIS | 14.28 ± 2.92 | 14.03 ± 3.75 | 12.35 ± 2.48 | 12.42 ± 3.13 | 11.501 | 0.001 |
| BAS-total | 27.95 ± 6.20 | 29.28 ± 6.85 | 26.79 ± 6.15 | 27.88 ± 7.77 | 0.012 | 0.912 |
| BAS-reward | 9.90 ± 2.44 | 9.74 ± 2.40 | 9.29 ± 2.46 | 8.94 ± 3.26 | 0.052 | 0.820 |
| BAS-driven | 7.87 ± 2.75 | 8.23 ± 2.71 | 7.94 ± 2.57 | 7.94 ± 2.42 | 0.170 | 0.681 |
| BAS-pleasure | 10.18 ± 2.17 | 11.31 ± 2.75 | 9.56 ± 2.34 | 11.00 ± 3.24 | 0.126 | 0.912 |
IGD: Internet gaming disorder; RGU: recreational game user; IAT: Internet addiction test; DSM-5: Diagnostic and Statistical Manual of Mental Disorders-5; BIS: Behavioral Inhibition System; BAS: Behavioral Activation System; F & p value was the result of main effect of group (IGD vs. RGU).
Participation coefficient
We used a 2 (group) x2 (gender) ANOVA (age and education as the covariates) to examine the effect of gaming group and gender on the average PC of the six modules (see Table 2 and Fig. 1(a)). The results showed that the main effect of gender on the average PC of the DMN and cerebellum were significant, F(1, 149) =8.35, p uncorrected = 0.004, η2 = 0.059; F(1, 149) = 8.52, p uncorrected = 0.004, η2 = 0.060. Besides, significant interactions were found for average PC of the FPN (F(1, 149) = 6.04, p uncorrected = 0.015, η2 = 0.039). There was no difference in the PC of FPN between female IGD and RGU participants (t(70) = 0.15, p uncorrected = 0.704, Cohens’d = 0.003), but for male participants, the PC of the FPN in the IGD group was significantly higher than that in the RGU group (t(76) = 3.06, p corrected = 0.003, Cohens’d = 0.693) (see Fig. 1(b)). No main or significant interaction effects were found on PC of other functional modules. Additionally, there were no correlations between the BIS/BAS scores and the average PC of the FPN for all subjects.
Table 2.
Descriptive statistics of participation coefficient of four groups
| (M ± SD) | IGD (n = 78) | RGU (n = 72) | F | P | ||
| M (n = 39) | F (n = 39) | M (n = 33) | F (n = 39) | |||
| DMN | 0.67 ± 0.08 | 0.61 ± 0.12 | 0.66 ± 0.07 | 0.63 ± 0.08 | 0.779 | 0.379 |
| FPN | 0.80 ± 0.06 | 0.77 ± 0.07 | 0.76 ± 0.06 | 0.77 ± 0.06 | 6.041 | 0.015 |
| CON | 0.75 ± 0.05 | 0.72 ± 0.06 | 0.73 ± 0.06 | 0.73 ± 0.07 | 2.235 | 0.137 |
| SMN | 0.65 ± 0.08 | 0.65 ± 0.08 | 0.65 ± 0.08 | 0.64 ± 0.07 | 0.226 | 0.635 |
| Visual network | 0.73 ± 0.10 | 0.69 ± 0.11 | 0.72 ± 0.08 | 0.70 ± 0.85 | 0.644 | 0.424 |
| Cerebellum | 0.75 ± 0.12 | 0.79 ± 0.09 | 0.72 ± 0.16 | 0.80 ± 0.08 | 1.049 | 0.308 |
IGD: Internet gaming disorder; RGU: recreational game user; DMN: default-mode network; FPN: frontal–parietal network; CON: cingulo-opercular network; SMN: sensorimotor network; F & p value was the result of interaction effect of group and gender.
Intra-module and inter-module connections
We further tested the effects of group and gender on intra-module and inter-module connections of the FPN (see Table 3 and Fig. 2(a)) based on the significant results described above. We found the main effect of group on inter-module connections between the FPN and visual network was significant, F(1, 149) = 5.27, p uncorrected = 0.023, η2 = 0.038; The main effect of gender on inter-module connections between the FPN and CON, between the FPN and cerebellum were significant, F(1, 149) = 4.62, p uncorrected = 0.033, η2 = 0.038; F(1, 149) = 14.74, p uncorrected < 0.001, η2 = 0.099. Significant interactions between diagnostic and gender groups were found for inter-module connections between the FPN and CON, F(1, 149) = 6.03, p uncorrected = 0.015, η2 = 0.043. In terms of simple effects, there were no significant between-group differences in inter-module connections between the FPN and CON among females, t (70) = 0.549, p uncorrected = 0.462, Cohens’d = 0.116. However, for males, inter-module connections between the FPN and CON in the IGD group were higher than those in RGU group, t (76) = 2.48, p corrected = 0.048, Cohens’d = 0.562 (see Fig. 2(b)). No main effects of diagnostic or gender groups were observed for inter-module connections between the FPN and CON. No significant interaction effects were found for the other intra-module and inter-module connections of the FPN.
Table 3.
Descriptive statistics of intra-module and inter-module connections of four groups
| (M ± SD) | IGD(n = 78) | RGU (n = 72) | F | P | ||
| M (n = 39) | F (n = 39) | M (n = 33) | F (n = 39) | |||
| FPN-DMN | 102.92 ± 34.43 | 91.31 ± 39.71 | 85.92 ± 30.21 | 90.25 ± 35.11 | 1.903 | 0.167 |
| FPN-FPN | 86.51 ± 24.93 | 86.53 ± 16.31 | 75.27 ± 19.33 | 82.69 ± 22.61 | 0.982 | 0.324 |
| FPN-CON | 109.94 ± 32.20 | 86.50 ± 26.10 | 91.22 ± 32.35 | 92.41 ± 29.62 | 6.029 | 0.015 |
| FPN-SMN | 51.12 ± 25.06 | 37.38 ± 19.88 | 43.57 ± 24.35 | 42.57 ± 23.72 | 2.432 | 0.121 |
| FPN-Visual network | 30.89 ± 16.09 | 27.56 ± 23.83 | 23.24 ± 9.11 | 21.59 ± 11.82 | 0.042 | 0.838 |
| FPN-Cerebellum | 42.72 ± 23.64 | 55.84 ± 27.90 | 33.38 ± 20.27 | 52.06 ± 25.03 | 0.464 | 0.497 |
IGD: Internet gaming disorder; RGU: recreational game user; FPN-DMN: inter-modulae connection between frontal–parietal network and default-mode network; FPN-FPN: intra-module connection of frontal–parietal network; FPN-CON: inter-module connection between frontal–parietal network and cingulo-opercular network; FPN-SMN: inter-module connection between frontal–parietal network and sensorimotor network; FPN-visual network: inter-module connection between frontal–parietal network and visual network; FPN-cerebellum: inter-module connection between frontal–parietal network and cerebellum; F & p value was the result of interaction effect of group and gender.
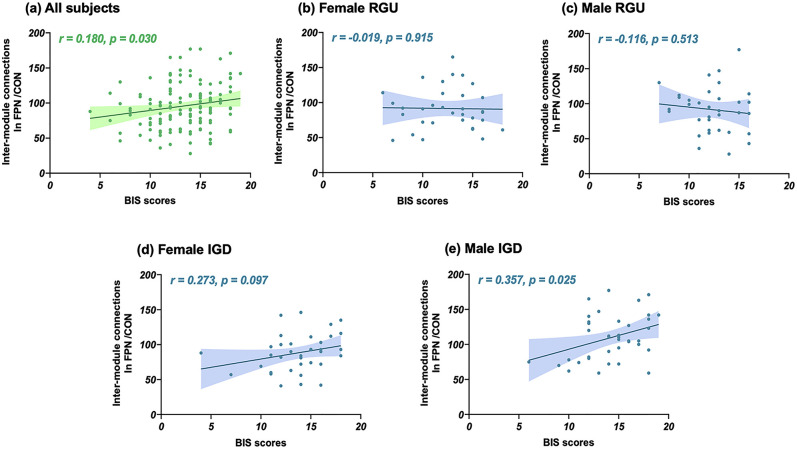
Correlation analyses showed a positive correlation between the BIS scores and the inter-module connections for the FPN/CON for all subjects (r = 0.180, p uncorrected = 0.030, Fig. 3(a)). Further subgroup analyses showed that positive correlations were found among the male (r = 0.357, p uncorrected = 0.026) but not female (r = 0.273, p uncorrected = 0.098) IGD subjects (Fig. 3(e)).
Fig. 3.
Correlations between the FPN/CON connections and BIS scores. RGU, recreational gaming user; IGD, internet gaming disorder; FPN, the frontal–parietal network; CON, the cingulo-opercular network; BIS, Barratt impulsivity scale. There are significant positive correlations between FPN/CON connections and BIS scores for all subjects. Subgroups analysis further shows positive correlations between the two only reaching statistical significance in male IGD subjects, although a “trend-level” effect was observed in females with IGD
Spectral dynamic causal modeling
Finally, we investigated causal influences between the FPN and CON. There were significant interactions between diagnostic and gender groups on the effective connectivity from the CON to the FPN (parameter effect = 0.36, posterior probability = 0.95). The simple effect indicated that male and female IGD subjects showed opposite changes in connectivity intensity as compared to RGU subjects, and the differences were significant (t (76) corrected = 2.74, p = 0.032) (see Fig. 2(c)). Main effects of diagnostic group were found in the effective connectivity from the FPN to the CON (parameter effect = 0.15, posterior probability = 0.97). There were no correlations between BIS/BAS scores and the effective connectivity strength.
Discussion
In this study, we aimed to investigate whether resting-state network integration/segregation differed by function of diagnostic group (IGD, RGU) and gender (male, female). Reduced module segmentation was observed in the FPN of males with IGD individuals. In addition, increased connections between the FPN and the CON were observed in males but not females with IGD. These FPN/CON connections were positively associated with BIS scores. Further, spDCM analyses found that the causal influence from the CON to the FPN in male IGD subjects is enhanced, while this effective connection in female IGD subjects is reduced compared with that of matched RGU subjects. Taken together, these findings suggest gender-related differences in neural network functioning underlying IGD with respect to functional integration. Implications are discussed below.
Reduced modular segregation in males but not females with IGD
IGD may involve impaired executive control over reward seeking and craving for gaming (Dong et al., 2019, 2020; Wang, Zheng, Du, & Dong, 2019; Zheng et al., 2019). However, gender-related differences have been observed. For example, our previous work has shown that males versus females with IGD exhibit stronger craving-related regional activity when facing gaming cues during forced withdrawal than females (Dong et al., 2019). At the network level, the FPN has been implicated in executive functioning (Kim, Cilles, Johnson, & Gold, 2012), including with respect to control over substance use. For example, reduced FPN function during a Go/No-Go task has been linked to alcohol consumption cross-sectionally and longitudinally, as well as to compulsivity and reward/punishment sensitivity (Worhunsky et al., 2015). FPN function has been differentially linked to cognitive control in individuals with and without cocaine use disorder (Worhunsky et al., 2012). Modular segregation of the FPN and connection with other modules is important for executive function performance (Fair et al., 2007; Wang et al., 2020). Fair et al. (2007), found demonstrably lower PC of the FPN in adults than in children. Similarly, Wang et al. (2020) found that these segregation developments significantly improved the performance on executive function tasks. Reduced functional segregation of the FPN has also been found in high-risk male offspring from families with alcohol use disorder (Holla et al., 2017). The current findings in males with IGD are consistent with those of previous studies. The reduced functional module segregation of the FPN in IGD subjects may in part underlie their disordered gaming, particularly in males.
Despite presenting with characteristics of addiction, there was no significant difference in modular segmentation in females with IGD relative to those with RGU, suggesting different mechanisms may be operating in females. Two possible explanations are as follows. First, impaired executive function may be mainly reflected in local attributes but not overall network attributes in females with IGD. Functional and structural differences in females with IGD have been reported, and it has been suggested that neural underpinnings of IGD differ across gender groups (Wang, Hu, et al., 2019; Wang, Xu, et al., 2019). Second, potential neural mechanisms may protect against IGD in females. Dong, Zheng et al. (2018) have suggested that females with IGD with may show better executive control when exposed to gaming cues than males with IGD, which may contribute to a resiliency against developing IGD. Future studies may use multi-modal imaging to investigate further modular segmentation in IGD and how these may differ in females and males.
Increased FPN/CON connections in males but not females with IGD
Further inter-module and intra-module connection analyses indicated greater FPN/CON connections in male IGD subjects compared with those in male RGU subjects, and this pattern was not observed in females. The FPN and the CON are two distinct networks that have been implicated in cognitive control (Church et al., 2009; Fair et al., 2007). Effective coupling between the two is important for executive functioning. Wang, Xu et al. (2019) indicated that good cognitive control relies on the appropriate modulation of connectivity associated with the FPN and CON. Among individuals with cocaine use disorder, greater engagement of fronto-cingular integrated activation during Stroop performance was linked to shorter time (Worhunsky et al., 2012) in treatment. In the current study, we speculate that the increased FPN/CON connections in males with versus without IGD reflects a male-IGD-specific “fuzzy boundary” between these two brain networks that may relate to relevant features of IGD in males. Correlation analysis suggested a positive association between FPN/CON connections and BIS scores only in male IGD subjects. BIS scores reflect a greater tendency to avoid punishment and have been linked to emotional processes and brain regions involved in emotion processing and regulation (Li et al., 2014), including in behavioral addictions. The “male-specific” findings are somewhat surprising in that BIS typically associates with emotional processes in females more so than males (Ma-Kellams & Wu, 2020), including in behavioral addictions (Li et al., 2019). Of note, a similar positive correlation was observed at a trend level in females with IGD, suggesting that this relationship may hold across gender groups for individuals with IGD. Also, BIS\BAS scores have also been previously linked to executive control network and cognitive control processes; however, BAS scores haven more implicated in substance addictions and BIS scores in Parkinson’s disease (Krmpotich et al., 2013; Laurent et al., 2018). Further study is needed to examine the extent to which the current findings extend to (and may be more significant in) individuals with IGD and co-occurring affective disorders, and to examine gender-related effects.
The CON has been implicated in multiple psychiatric illnesses (Cai, Griffiths, Korgaonkar, Williams, & Menon, 2019; Tu, Hsieh, Li, Bai, & Su, 2012; Vaden, Eckert, Dubno, & Harris, 2020). Patients with schizophrenia may exhibit functional disconnection within the CON (Tu et al., 2012). Cai et al. (2019) reported that differences between children with and without attention-deficit hyperactivity disorder (ADHD) could be positively predicted by multivariate patterns of task-evoked effective connectivity between brain regions in the CON and FPN, emphasizing cingulo-frontal connectivity’s relationship to a disorder characterized by deficits in cognitive control and attention. Attention control as a fundamental function of the CON has been suggested in healthy populations (Sadaghiani & D’Esposito, 2015). Thus, the increased FPN/CON connections in current male subjects with IGD in this study may reflect imbalance between attention and cognitive control systems. The extent which such neural function relates to comorbid ADHD and IGD and transitions in IGD warrants additional study (Karaca, Saleh, Canan, & Potenza, 2016), particularly as ADHD may more commonly affect males than females.
Gender-by-diagnostic-group interactions in effective connectivity from CON to FPN
Additionally, spDCM analyses demonstrated that the connection strength from the CON to the FPN of males/females with IGD showed opposing trends from those in RGU subjects. However, the most robust statistical effect appeared to be related to differences in males and females with IGD, suggesting different neural mechanisms for IGD operating across gender groups. The observed relationship may reflect a bottom-up-driven control process of the CON to the FPN in IGD that is particularly relevant to males with IGD. Dosenbach, Fair, Cohen, Schlaggar, and Petersen (2008) suggested that during top-down control processes, the FPN seems to initiate and adjust control while the CON provides a stable ‘set-maintenance’. Another prospective study has suggested that the FPN may provide top–down modulation of the sensory cortex during both preparatory attention and orientation within memory, while the CON may play a more downstream role in cognitive control (George, Mark, Helena, Mark, Nobre, 2015). These possibilities warrant additional study.
Second, the opposite trend in this effective connection may reflect differences in neural correlates of IGD in males and females (Wang, Hu et al., 2019; Wang, Xu et al., 2019). Specifically, the CON may have a more robust causal influence (i.e., excitatory effect) on the FPN in males with IGD. Combined with PC and inter-module connection findings, it is possible that excitatory effective connections in males IGD may represent a potential risk factor leading to increased FPN/CON connections. Conversely, a decreased causal effect of this connection was suggested in female IGD. The FPN and CON have shown greater dissociation when switching from rest to external task performance, and this was associated with better task accuracy (Elton & Gao, 2014). Currently, inhibitory effective connectivity from the CON to the FPN may act as a protective mechanism to maintain stability of between-network connections in females. However, it should be noted that we did not find any statistical correlations between DCM parameters and network coupling in this study. Considering the lack of more supportive evidence, these inferences should be treated cautiously.
Limitations
Several limitations should be mentioned. First, although we investigated IGD between recreational game users and addicted game users, there is no normal control group in this study. Ideally three groups should be recruited which may better explain the effective connectivity changes from non-IGD to IGD. Second, the sample only included young adult university students from China. Additional study is needed to examine the extent to which the findings may extend to other age groups and jurisdictions/cultures. Thirdly, denoising procedures, including the regression of head motion, global signal, white matter, and cerebrospinal fluid, might have unexpected effects on reliability. In this study, we mainly regressed out the head vectors, cerebral spinal fluid, and white matter signals, which might affect the reliability of results. Fourthly, the correlations between BIS/BAS and PC was uncorrected which cannot directly reflect the executive function and network modularity. Further measures by using different scales or cognitive tasks are needed to examine this relationship. Finally, we did not perform power analysis before the study. We re-conduct a power analysis and showed that the appropriate sample size is 128. The sample size of 128 participants was determined based on the medium effect size by G*power (version: 3.1; Faul, Erdfelder, Lang, & Buchner, 2007). Thirty-two participants per group were needed to detect a reliable effect (cohen f = 0.25, α = 0.05, 1–β = 0.8, ANOVA fixed effect, main effects and interaction; Faul et al., 2007). These limitations need to be improved in future research.
Conclusions
The current study found reduced modular segmentation of the FPN and increased connections between the FPN and the CON in male IGD versus RGU subjects, while no differences were observed in female IGD versus RGU subjects. Further spDCM analyses indicated that the connection strength from the CON to the FPN in males/females with IGD showed opposite trending patterns from those with RGU. These findings suggest potential neural mechanisms underlying IGD in males that may involve impaired control associated with avoidant tendencies and disordered gaming. Excitatory effective connection from the CON to the FPN in males with IGD may drive these relationships. In contrast, the inhibitory CON – FPN connectivity in females may contribute to maintaining the stability of the between-network connections. These findings suggest alternate mechanisms may underlie IGD in females, and future studies should identify these more fully. In general, these findings contribute to the understanding of the gender-related neural specificity and emphasize the importance of considering gender in IGD studies.
Authors’ contributions
Z and MW wrote the first draft of the manuscript and analyzed the data. HZ, JZ and HD contributed to fMRI data collection. GD designed this research and edited the manuscript; MP contributed in revising the manuscript. All authors contributed to and approved the final manuscript.
Conflicts of interest
The authors declare that no competing interests exist.
Supplementary Material
Acknowledgments
The current research was supported by The Cultivation Project of Province-levelled Preponderant Characteristic Discipline of Hangzhou Normal University (20JYXK008) and the Zhejiang Provincial Natural Science Foundation (LY20C090005).
References
- American Psychiatric Association (Ed.). (2013). Diagnostic and statistical manual of mental disorders: DSM-V (5th ed.). Arlington: American Psychiatric Association. [Google Scholar]
- Baum, G. L., Ciric, R., Roalf, D. R., Betzel, R. F., Moore, T. M., Shinohara, R. T., et al. (2017). Modular segregation of structural brain networks supports the development of executive function in youth. Current Biology, 27(11), 1561–1573. 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgonovi, F. (2016). Video gaming and gender differences in digital and printed reading performance among 15-year-olds students in 26 countries. Journal of Adolescence, 48, 45–61. 10.1016/j.adolescence.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Cai, W., Griffiths, K., Korgaonkar, M. S., Williams, L. M., & Menon, V. (2019). Inhibition-related modulation of salience and frontoparietal networks predicts cognitive control ability and inattention symptoms in children with ADHD. Molecular Psychiatry. 10.1038/s41380-019-0564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvel, C., & White, T. (1994). Behavior inhibition, behavior activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319–333. 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Church, J. A., Fair, D. A., Dosenbach, N. U. F., Cohen, A. L., Miezin, F. M., Petersen, S. E., et al. (2009). Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain, 132(1), 225–238. 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, R., Krishnan-Sarin, S., Cavallo, D., & Potenza, M. (2010). Video game playing in high school students: Health correlates, gender differences and problematic gaming. Pediatrics, 126(6), e1414–e1424. 10.1542/peds.2009-2706.Video. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G, Li, H., Wang, L., & Potenza, M. N. (2017). Cognitive control and reward/loss processing in Internet gaming disorder: Results from a comparison with recreational Internet game-users. European Psychiatry, 44, 30–38. 10.1016/j.eurpsy.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Dong, G, Liu, X., Wang, Y., Du, X., & Potenza, M. N. (2018). Gender-related differences in cue-elicited cravings in Internet gaming disorder: The effects of deprivation. Journal of Behavioral Addictions, 7(4), 953–964. 10.1556/2006.7.2018.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G, Wang, L., Du, X., & Potenza, M.(2018). Gender-related differences in neural responses to gaming cues before and after gaming: Implications for gender-specific vulnerabilities to Internet gaming disorder. Social Cognitive and Affective Neuroscience, 13(11), 1203–1214. 10.1093/scan/nsy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G, Wang, Z., Wang, Y., Du, X., & Potenza, M. N. (2019). Gender-related functional connectivity and craving during gaming and immediate abstinence during a mandatory break: Implications for development and progression of internet gaming disorder. Progress In Neuro-Psychopharmacology & Biological Psychiatry, 88, 1–10. 10.1016/j.pnpbp.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Dong, G, Wang, M., Zheng, H., Wang, Z., Du, X., & Potenza, M. N. (2020). Disrupted prefrontal regulation of striatum-related craving in Internet gaming disorder revealed by dynamic causal modeling: Results from a cue-reactivity task. Psychological Medicine. 10.1017/S003329172000032X. [DOI] [PubMed] [Google Scholar]
- Dong, G, Zheng, H., Li, H., Wang, Y., & Potenza, M. (2018). Individual differences in self-reported reward-approach tendencies relate to resting-state and reward-task-based fMRI measures. International Journal of Psychophysiology, 128. 10.1016/j.ijpsycho.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. F., Fair, D. A., Cohen, A. L., Schlaggar, B. L., & Petersen, S. E. (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. F., Nardos, B., Cohen, A. L., Fair, D. A., Power, J. D., Church, J. A., et al. (2010). Prediction of individual brain maturity using fMRI. Science, 329(5997), 1358–1361. 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton, A., & Gao, W. (2014). Divergent task-dependent functional connectivity of executive control and salience networks. Cortex, 51(1), 56–66. 10.1016/j.cortex.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Fair, D. A., Dosenbach, N. U. F., Church, J. A., Cohen, A. L., Brahmbhatt, S., Miezin, F. M., et al. (2007). Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America, 104(33), 13507–13512. 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Friston, K. J., (2011). Functional and effective connectivity: A review. Brain Connectivity, 1(1), 13. 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston, K. J., Harrison, L., & Penny, W. (2003). Dynamic causal modelling. NeuroImage, 19(4), 1273–1302. 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston, K. J, Kahan, J., Biswal, B., & Razi, A. (2014). A DCM for resting state fMRI. NeuroImage, 94, 396–407. 10.1016/j.neuroimage.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J., Litvak, V., Oswal, A., Razi, A., Stephan, K. E., Van Wijk, B. C. M., et al. (2016). Bayesian model reduction and empirical Bayes for group (DCM) studies. NeuroImage, 128, 413–431. 10.1016/j.neuroimage.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, W., Mark, S., Helena, J., Mark, W., & Nobre, A. C. (2015). Frontoparietal and cingulo-opercular networks play dissociable roles in control of working memory. Journal of Cognitive Neuroscience, 27(10), 2019–2034. 10.1162/jocn. [DOI] [PubMed] [Google Scholar]
- Grattan-Miscio, K. E., & Vogel-Sprott, M. (2005). Effects of alcohol and performance incentives on immediate working memory. Psychopharmacology, 181(1), 188–196. 10.1007/s00213-005-2226-2. [DOI] [PubMed] [Google Scholar]
- Gray, J.(1987). The neuropsychology of emotion and personality. Cognitive Neurochemistry, 87, 171–190. [Google Scholar]
- Guimera, R., & Amaral, L. A. N. (2005). Functional cartography of complex metabolic networks. Nature, 433(7028), 895–900. 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, Y.-M., & Hwang, W. J. (2014). Gender differences in internet addiction associated with psychological health indicators among adolescents using a national web-based survey. International Journal of Mental Health and Addiction, 12(5), 660–669. 10.1007/s11469-014-9500-7. [DOI] [Google Scholar]
- Henson, R. N., Flandin, G., Friston, K. J., & Mattout, J. (2010). A parametric empirical bayesian framework for fMRI-constrained MEG/EEG source reconstruction. Human Brain Mapping, 31(10), 1512–1531. 10.1002/hbm.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holla, B., Panda, R., Venkatasubramanian, G., Biswal, B., Bharath, R. D., & Benegal, V. (2017). Disrupted resting brain graph measures in individuals at high risk for alcoholism. Psychiatry Research – Neuroimaging, 265, 54–64. 10.1016/j.pscychresns.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Karaca, S., Saleh, A., Canan, F., & Potenza, M.(2016). Comorbidity between behavioral addictions and attention deficit/hyperactivity disorder: A systematic review. International Journal of Mental Health and Addiction, 15(3), 701–724. 10.1007/s11469-016-9660-8. [DOI] [Google Scholar]
- Kim, C., Cilles, S. E., Johnson, N. F., & Gold, B. T. (2012). Domain general and domain preferential brain regions associated with different types of task switching: A meta-analysis. Human Brain Mapping, 33(1), 130–142. 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, D. L., & Delfabbro, P. H. (2018). The concept of “harm{’’} in Internet gaming disorder. Journal of Behavioral Addictions, 7(3), 562–564. 10.1556/2006.7.2018.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, D. L., & Potenza, M. (2020). Gaming disorder among female adolescents: A hidden problem? Journal of Adolescent Health, 66, 650–652. 10.1016/j.jadohealth.2020.03.011. [DOI] [PubMed] [Google Scholar]
- Krmpotich, T. D., Tregellas, J. R., Thompson, L. L., Banich, M. T., Klenk, A. M., & Tanabe, J. L. (2013). Resting-state activity in the left executive control network is associated with behavioral approach and is increased in substance dependence. Drug and Alcohol Dependence, 129(1–2), 1–7. 10.1016/j.drugalcdep.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent, R., Van Wouwe, N. C., Turchan, M., Tolleson, C., Phibbs, F., Bradley, E., et al. (2018). Motivational sensitivities linked to impulsive motor errors in Parkinson’s disease. Journal of the International Neuropsychological Society, 24(2), 128–138. 10.1017/S1355617717000741. [DOI] [PubMed] [Google Scholar]
- Li, Q., Dai, W., Zhong, Y., Wang, L., Dai, B., Liu, X., et al. (2019). The mediating role of coping styles on impulsivity, behavioral inhibition/approach system, and internet addiction in adolescents from a gender perspective. Frontiers in Psychology, 10(October), 1–14. 10.3389/fpsyg.2019.02402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Qiao, L., Sun, J., Wei, D., Li, W., & Qiu, J. (2014). Gender-specific neuroanatomical basis of behavioral inhibition/approach systems (BIS/BAS) in a large sample of young adults: A voxel-based morphometric investigation. Behavioural Brain Research, 274, 400–408. 10.1016/j.bbr.2014.08.041. [DOI] [PubMed] [Google Scholar]
- Li, L., Yu, Q., Zhang, L., Jin, S. (2015). The gender difference on internet addictive among adolescent: The mediation effect of the differentiation of social and psychological situation in school. Chinese Journal of Clinical Psychology, 23(6), 1044–1048. 10.16128/j.cnki.1005-3611.2015.06.020. [DOI] [Google Scholar]
- Ma-Kellams, C., & Wu, M. (2020). Gender, behavioral inhibition/activation, and emotional reactions to negative natural and social events. Personality and Individual Differences, 157, 109809. 10.1016/j.paid.2019.109809. [DOI] [Google Scholar]
- Marek, S., Hwang, K., Foran, W., Hallquist, M. N., & Luna, B.(2015). The contribution of network organization and integration to the development of cognitive control. PLoS Biology, 13(12), e1002328. 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, A. M., McMillan, K. M., Laird, A. R., & Bullmore, E. T. (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging. Human Brain Mapping, 25(1), 46–59. 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry, N. M., Rehbein, F., Gentile, D. A., Lemmens, J. S., Rumpf, H.-J., Moessle, T., et al. (2014). An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction, 109(9), 1399–1406. 10.1111/add.12457. [DOI] [PubMed] [Google Scholar]
- Rahman, A. S., Xu, J., & Potenza, M. N. (2014). Hippocampal and Amygdalar volumetric differences in pathological Gambling: A preliminary study of the associations with the behavioral inhibition system. Neuropsychopharmacology, 738–745. 10.1038/npp.2013.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineberg, A. E., Andrews-Hanna, J. R., Depue, B. E., Friedman, N. P., & Banich, M. T. (2015). Resting-state networks predict individual differences in common and specific aspects of executive function. NeuroImage, 104, 69–78. 10.1016/j.neuroimage.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineberg, A. E., & Banich, M. T. (2016). Functional connectivity at rest is sensitive to individual differences in executive function: A network analysis. Human Brain Mapping, 37(8), 2959–2975. 10.1002/hbm.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sadaghiani, S., & D’Esposito, M. (2015). Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cerebral Cortex, 25(9), 2763–2773. 10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, C., Ratan, R., Cai, Y. D., & Leavitt, A. (2016). Do men advance faster than women? Debunking the gender performance gap in two massively multiplayer online games. Journal of Computer-Mediated Communication, 21(4), 312–329. 10.1111/jcc4.12159. [DOI] [Google Scholar]
- Tu, P. C., Hsieh, J. C., Li, C. T., Bai, Y. M., & Su, T. P. (2012). Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: A resting fMRI study. NeuroImage, 59(1), 238–247. 10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- Vaden, K. I., Eckert, M. A., Dubno, J. R., & Harris, K. C. (2020). Cingulo-opercular adaptive control for younger and older adults during a challenging gap detection task. Journal of Neuroscience Research, 98(4), 680–691. 10.1002/jnr.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M., Dong, H., Zheng, H. U. I., Du, X., & Dong, G. (2020). Inhibitory neuromodulation of the putamen to the prefrontal cortex in Internet gaming disorder: How addiction impairs executive control. Journal of Behavioural Addictions, 9(2), 312–324. 10.1556/2006.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Hu, Y., Zheng, H., Yuan, K., Du, X., & Dong, G. (2019). Females are more vulnerable to Internet gaming disorder than males: Evidence from cortical thickness abnormalities. Psychiatry Research – Neuroimaging, 283(March 2018), 145–153. 10.1016/j.pscychresns.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Wang, C., Hu, Y., Weng, J., Chen, F., & Liu, H. (2020). Modular segregation of task-dependent brain networks contributes to the development of executive function in children. NeuroImage, 206(June), 116334. 10.1016/j.neuroimage.2019.116334. [DOI] [PubMed] [Google Scholar]
- Wang, C., Xu, T., Geng, F., Hu, Y., Wang, Y., Liu, H., et al. (2019). Training on abacus-based mental calculation enhances visuospatial working memory in children. Journal of Neuroscience, 39(33), 6439–6448. 10.1523/JNEUROSCI.3195-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M., Zheng, H., Du, X., & Dong, G. (2019). Mapping internet gaming disorder using effective connectivity: A spectral dynamic causal modeling study. Addictive Behaviors, 90, 62–70. 10.1016/j.addbeh.2018.10.019. [DOI] [PubMed] [Google Scholar]
- Warren, D. E., Power, J. D., Bruss, J., Denburg, N. L., Waldron, E. J., Sun, H., et al. (2014). Network measures predict neuropsychological outcome after brain injury. Proceedings of the National Academy of Sciences of the United States of America, 111(39), 14247–14252. 10.1073/pnas.1322173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky, P., Dager, A., Meda, S., Khadka, S., Stevens, M., & Austad, C., et al. (2015). A preliminary prospective study of an escalation in “maximum daily drinks”, fronto-parietal circuitry and impulsivity-related domains in young adult drinkers. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41, 1637–1647. 10.1038/npp.2015.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky, P., Stevens, M., Carroll, K., Rounsaville, B., Calhoun, V., & Pearlson, G., et al. (2012). Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors, 27(2), 477–488. 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C.-G., Wang, X.-D., Zuo, X.-N., & Zang, Y.-F. (2016). DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics, 14(3), 339–351. 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Zheng, H., Hu, Y., Wang, Z., Wang, M., Du, X., & Dong, G.(2019). Meta-analyses of the functional neural alterations in subjects with Internet gaming disorder: Similarities and differences across different paradigms. Progress In Neuro-Psychopharmacology & Biological Psychiatry, 94, 109656. 10.1016/j.pnpbp.2019.109656. [DOI] [PubMed] [Google Scholar]