Abstract
Background and aims: Attentional biases contribute to the maintenance of addictive behaviors. For the problematic use of online gaming – recognized as Internet Gaming Disorder (IGD) – first evidence points to a bias towards in-game stimuli. This study aimed to provide behavioral and electrophysiological evidence for a generalized bias towards computer-related stimuli, and to identify the specific attentional processes contributing to this bias: facilitated attention deployment, impaired disengagement or failed suppression. Method: Twenty participants with IGD and 23 casual gamers performed a visual search task with photographs of real-world objects. Either the target or a to-be-ignored distractor was addiction-relevant (computer-related), whereas all other items were addiction-irrelevant (related to cars or sport). Event-related potential components associated with facilitated attentional deployment to the target (NT), its post-selection processing (SPCN), and suppression of irrelevant information (PD) were analyzed. Results: Unlike casual gamers, gamers with IGD exhibited prolonged reaction times and increased SPCN amplitudes for computer-related stimuli, reflecting their continued attentional processing. At the individual level, larger SPCN amplitudes were associated with longer delays in reaction time. Discussion and Conclusions: This pattern of results indicates that the disengagement of attention from computer-related stimuli is impaired in IGD. More generally, our findings demonstrate that conditioning processes occur in IGD, and thus open up new avenues for treatment.
Keywords: Internet Gaming Disorder, behavior, addictive, attentional bias, evoked potential, visual search
Introduction
With around 2.5 billion gamers worldwide, playing video games is an extremely popular pastime (Statista, 2019). For a minority, however, it is associated with negative consequences such as sleep problems, headache, loneliness, low self-esteem, interpersonal conflicts and lower academic performance (e.g. Kiraly et al., 2014; Kuss, Griffiths, Karila, & Billieux, 2014; Torres-Rodríguez, Griffiths, Carbonell, & Oberst, 2018). Internet Gaming Disorder (IGD) is defined as “persistent and recurrent engagement in video games, often with other players, leading to clinically significant impairments or distress” (DSM-5; APA, 2013); the diagnostic criteria (e.g., preoccupation, withdrawal symptoms and tolerance) were derived from the criteria for pathological gambling and substance use disorder. In studies with representative samples and instruments based on the DSM-5 criteria, prevalence rates vary between 1.4% (Wittek et al., 2016) and 5.4% (Lemmens, Valkenburg, & Gentile, 2015).
A central hypothesis that can be derived from the most recent model of Internet-use disorders – the Interaction of Person-Affect-Cognition-Execution Model (I-PACE; Brand, Young, Laier, Wölfling, & Potenza, 2016; Brand et al., 2019) – is that attention is biased towards addiction-relevant stimuli. Visual attention selects relevant information, as determined by current goals, physical salience and past experience (Theeuwes, 2018; Wolfe & Horowitz, 2017). In general, attentional biases are beneficial for guiding behavior, even when they conflict with current goals (e.g., salient objects might alert us to potential danger). However, they can also contribute to the maintenance of addictive behaviors (Ciccarelli, Nigro, Griffiths, Cosenza, & D’Olimpio, 2016; Field & Cox, 2008). Attentional biases increase the likelihood of detecting addiction-related stimuli in the environment and subsequent addiction-related cognitions – making it difficult to disengage from these stimuli – and limit resources available to process alternative cues (Franken, 2003). Thereby, they make it harder to remain abstinent and can lead to further consumption (Field, Marhe, & Franken, 2014; Waters & Feyerabend, 2000).
As attentional biases can be targeted by interventions (Cox, Fadardi, Intriligator, & Klinger, 2014; Mogoaşe, David, & Koster, 2014), their identification opens up new avenues for treatment. However, only few studies have investigated attentional biases in IGD. They produced mixed evidence: Using classical paradigms such as Stroop or dot-probe tasks, some studies found evidence of attentional biases towards stimuli directly related to gaming (e.g., in-game screenshots; Lorenz et al., 2013; Metcalf & Pammer, 2011), whereas others did not (Van Holst et al., 2012; Zhang et al., 2016). Most recently, Kim et al. (2018) interpreted an increased Late Positive Potential (LPP; a component of the event-related potential, ERP, of the EEG) for game-related images as evidence of an attentional bias. However, the LPP is not a suitable marker of selective attention: It is more closely linked with emotional or motivational processing and associated with sustained attention to emotional content (Hajcak, Weinberg, MacNamara, & Foti, 2011).
Attentional biases presumably develop not only for the addictive substance (e.g., nicotine), but through conditioning also for addiction-related cues (e.g., lighters; Field & Cox, 2008). The prominent incentive sensitization theory of addiction (Robinson & Berridge, 1993, 2001, 2008) postulates that addictive use of a substance sensitizes the dopaminergic reward system, resulting in a hypersensitivity to the incentive effects of the substance and substance-associated stimuli. This incentive sensitization leads to an attentional bias towards substance-related stimuli.
One might thus hypothesize that individuals with IGD are also biased towards gaming-related stimuli such as computer gear. Jeromin, Nyenhuis, and Barke (2016) tested this using computer-related words: An attentional bias emerged in a Stroop, but not in a visual probe task (Jeromin et al., 2016). Experiments with web-based addiction Stroop tests (Jeromin, Rief, & Barke, 2016) likewise did not reveal attentional biases for computer-related words. This inconsistent pattern of results may be due to experimental details such as the use of words – biases towards such abstract stimuli are likely less pronounced than those towards objects, and might thus only be detected under specific conditions.
This study aimed to provide direct evidence for an attentional bias towards computer-related objects in IGD. Casual gamers served as controls to ensure that attentional biases can be specifically attributed to IGD and not to gaming per se. In a visual search task, participants reported the orientation of a target presented among non-target objects. Stimuli were photographs of real-world objects from different categories: Targets were either addiction-relevant (computer) or neutral (sport), and non-targets were always from the same neutral category (car). On distractor-present trials, one of the non-targets deviated from the others in its object category: the distractor. When the target was addiction-relevant, the distractor was neutral or vice versa.
We analyzed performance and the visual ERP. In distractor-present trials, either the target or the distractor was presented laterally, while the other was presented centrally. With this lateralization technique (Hickey, Di Lollo, & McDonald, 2009), effects in the lateralized ERP only reflect processing of the lateral stimulus (i.e., target or distractor). This allowed us to isolate three lateralized ERP components specifically associated with different attentional processes: facilitated initial deployment of attention towards relevant information (Target Negativity, NT; e.g., Feldmann-Wüstefeld, Uengoer, & Schubö, 2015; Hickey et al., 2009; Munneke, Fait, & Mazza, 2013), its subsequent post-selection processing (Sustained Posterior Contralateral Negativity, SPCN; e.g., Jolicoeur, Brisson, & Robitaille, 2008; Luck, 2012; Mazza, Turatto, Umiltà, & Eimer, 2007) and suppression of irrelevant information (Distractor Positivity, PD; e.g., Gaspar & McDonald, 2014; Heuer & Schubö, 2019; Hickey et al., 2009).
These negative or positive components can be observed over the parieto-occipital cortex contralateral to targets (NT, SPCN) or distractors (PD). NT and PD are subcomponents of a widely used marker of visual attentional selection, the N2pc (e.g., Eimer, 1996; Luck, 2012), and emerge in the N2 time range. The SPCN has been linked to a transfer into visual working memory and subsequent maintenance processes (e.g., Eimer & Kiss, 2010; Jolicoeur et al., 2008), but often emerges in visual search tasks after the N2pc (e.g., Berggren & Eimer, 2018; Kiss, Driver, & Eimer, 2009; McDonald, Green, Jannati, & Di Lollo, 2013), reflecting continued attentional processing.
Our central hypothesis is that individuals with IGD – as compared to casual gamers – exhibit an attentional bias towards computer-related stimuli. Depending on which attentional processes contribute to this bias, its presence would be indicated by different result patterns. First, if attention was drawn towards addiction-relevant stimuli, we should see (i) facilitated deployment of attention towards addiction-relevant targets, indicated by shorter reaction times and larger NT amplitudes for addiction-relevant relative to neutral targets and/or (ii) an attentional capture by addiction-relevant distractors. This would be indicated by longer reaction times to neutral targets in distractor-present trials than in distractor-absent trials and reduced PD amplitudes for addiction-relevant relative to neutral distractors (i.e., less efficient suppression) or even an NT elicited by addiction-relevant distractors (i.e., initial deployment of attention to distractors). Second, if the disengagement of attention from addiction-relevant stimuli was impaired, reaction times should be longer and SPCN amplitudes larger for addiction-relevant than for neutral targets.
Method
Participants
The study was advertised via mailing lists and the university’s outpatient clinic. In a first step, we conducted a structured telephone interview with potential participants to check their eligibility. The German version of the Internet Gaming Disorder Questionnaire (IGDQ; Jeromin et al., 2016; Petry et al., 2014) served to assess how many DSM-5 criteria of IGD were met. Participants were assigned to the casual gamer group (IGDQ score ≤2), the IGD group (IGDQ score ≥5; Petry et al., 2014) or found ineligible to participate in the study. Additional eligibility criteria were: online gaming (PC), aged between 18 and 30, German as native language, normal vision, no neurological disease and no drug abuse. Visual acuity and color vision were tested with the OCULUS Binoptometer 3 (OCULUS Optikgeräte GmbH, Wetzlar, Germany). In a second step, eligible participants (27 casual and 26 IGD gamers) were tested in the EEG experiment, for which they received a compensation (50€). Five participants were excluded from the analyses because of ocular EEG artifacts in more than 30% of trials, and four participants because of chance performance for at least one of the five stimulus sets. The results are based on the remaining 43 participants (casual gamer group: 23; IGD group: 20).
Demographic and clinical measures
We collected information about age, sex, education, occupational status, online gaming (type of games, time spent per session/week) and experience with sports and cars. After the experiment, participants rated valence, familiarity and arousal for all stimuli on a scale from 0 (“very negative”/“not at all”) to 9 (“very positive”/“very much”). The German version of the Short Internet Addiction Test (sIAT) was used to validate the IGDQ results (Pawlikowski, Altstötter-Gleich, & Brand, 2013; Young, 1998). The items were rephrased in terms of online gaming. We additionally assessed impulsivity (Barratt Impulsiveness Scale-15, BIS-15; Meule, Vögele, & Kübler, 2011; Patton, Stanford, & Barratt, 1995), ADHD symptoms (German screening version of Conners’ Adult ADHD Rating Scale, CAARS; Christiansen, Hirsch, Abdel-Hamid, & Kis, 2014; Conners, Erhardt, & Sparrow, 1999), cognitive functioning (Digit Symbol Test, Wechsler Adult Intelligence Scale –IV, WAIS-IV; Wechsler, 2008), and psychological distress (German version of the Brief Symptom Inventory, BSI; Derogatis, 1993; Franke, 2000).
Apparatus and stimuli
The experiment was conducted in a dimly-lit, electrically shielded room. Participants faced a monitor (22 inches, 1680 × 1050 pixels) at a 104 cm viewing distance. Stimulus presentation and response collection were controlled using E-Prime 2.0 (Psychology Software Tools). Participants responded with one of two buttons on the back of a gamepad.
Stimuli were photographs of objects organized in five sets of three objects, one each from the categories computer, sports and cars: laptop, dumbbell suitcase, car tool kit; computer mouse, sports shoe, model car; gaming chair, weight lifting bench, car seat; computer keyboard, fascia roll, car radio; gaming headset, boxing headguard, motorcycle helmet. Visual features (e.g., brightness, shape, color) of stimuli within the sets were matched. The search displays contained eight stimuli equally spaced on an imaginary circle (eccentricity: 5.23 degrees of visual angle, dva). On each trial, all stimuli were drawn from one stimulus set: Computer (addiction-relevant) and sports stimuli (neutral) served as targets and distractors, car stimuli constituted the remaining six items. Stimuli were rotated 45° to the left or right and embedded in diamond-shaped (target) or circle-shaped (distractor and other non-target items) outlines. Target and distractor had the same or different orientations (each 50% of distractor-present trials). The remaining item orientations were chosen randomly. Shape outlines subtended 3.31 dva, objects 2.20 dva, and the fixation dot 0.17 dva. The background was grey.
Procedure and design
Participants performed a visual search task (exemplary displays are shown in Fig. 1). They reported the orientation of the target embedded within the diamond-shaped outline. The target object was from the category computer (addiction-relevant), or sports (neutral). In distractor-absent trials, all non-target items were the same object from the category car (Fig. 1a). Sports and cars were chosen as neutral categories as these are also popular interests in the studied population of young male adults. However, the valence of these stimuli, unlike that of computer-stimuli, should not differ between IGD and casual gamers; this was confirmed by stimulus ratings. In distractor-present trials (Fig. 1b), one of the non-target items, the distractor, deviated from the others in its object category: When the target was addiction-relevant (i.e., computer-related), the distractor was neutral (i.e., sports-related), and when the target was neutral, the distractor was addiction-relevant. The search display was presented for 300 ms, followed by a blank display until response or for up to 1,600 ms. Participants reported the orientation of the target as pointing to the top left or right by pressing the left or right button on a gamepad using their index fingers. The inter-trial interval varied randomly (800–1,200 ms, steps of 100 ms).
Fig. 1.
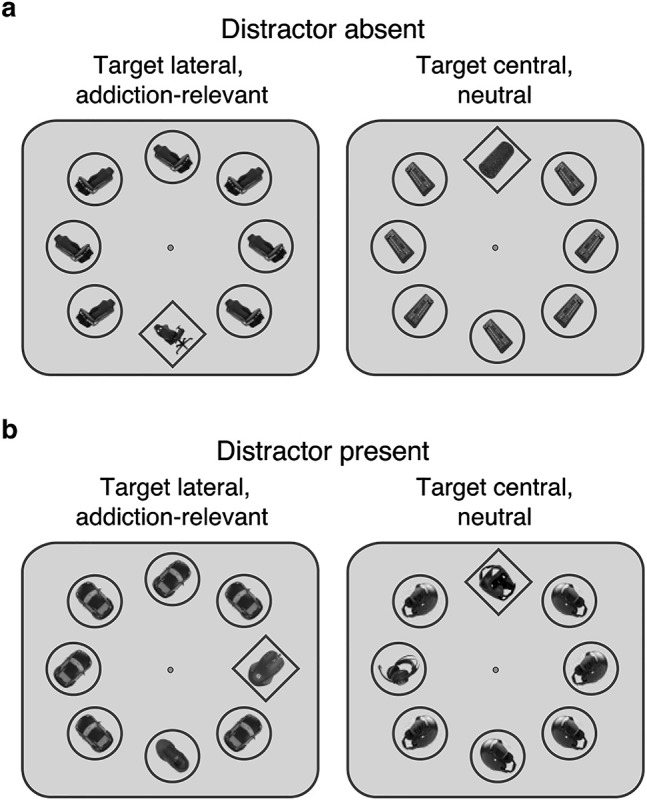
Examples of search displays und stimuli. Participants’ task was to report the orientation of the object in the diamond-shaped target. The target was either addiction-relevant (computer) or neutral (sports). (a) In distractor-absent trials, the target was presented among seven objects from a neutral category (car). (b) In distractor-present trials, one of the objects was from a different object category: When the target was addiction-relevant, this distractor was neutral, and when the target was neutral, it was addiction-relevant
The experiment consisted of 1,080 trials, equally distributed among target type conditions (neutral vs. addiction-relevant). One third of trials were distractor-absent trials; the other two thirds were distractor-present trials. Distractor-present trials were equally divided into two display configurations: target lateral, distractor central and distractor lateral, target central. Central locations were on the vertical above or below fixation, and lateral locations on the horizontal to the left or right from fixation. In distractor-absent trials, the target was also presented centrally or laterally with equal probability, but lateral targets were presented at any of the six lateral locations to ensure that the participants searched all locations. Trial types were varied randomly. Every 40 trials, participants could take a short break.
EEG recording
We recorded the EEG with Ag/AgCl active electrodes (actiCAP, Brain Products, Munich, Germany) from 64 scalp sites, placed according to the International 10–20 system. The vertical (vEOG) and horizontal electrooculogram (hEOG) were recorded as voltage differences between electrodes above and below, and to the left and right of the eyes (F9/F10). AFz served as ground electrode. All electrodes were referenced to FCz and re-referenced offline to the average of all electrodes. Impedances were kept below 5 kΩ. The signal was recorded at a sampling rate of 1,000 Hz with a BrainAmp amplifier (BrainProducts) and filtered with a high cut-off filter of 250 Hz and a low cut-off filter of 0.016 Hz.
Data analysis
To compare the groups in terms of demographic and clinical variables, independent t-tests, Welch’s test (in case of unequal variances) and Χ2 tests (in case of categorical data) were performed.
Our primary behavioral measure of interest were mean reaction times in correct trials; accuracy was analyzed to ensure that there were no speed-accuracy trade-offs. Trials in which participants did not respond (0.12% of trials) and reaction time outliers (±2 SD from individual mean reaction time, calculated for each of four blocks of 270 trials; 4.09% of trials) were removed from further analysis. Individual mean reaction times and accuracy in percent were calculated for distractor conditions and target type conditions, and submitted to three-way ANOVAs (within-factors distractor condition and target type, between-factor group).
EEG preprocessing and analyses were performed in Matlab (MathWorks) using Fieldtrip (Oostenveld, Fries, Maris, & Schoffelen, 2011) and custom scripts. The EEG was segmented into epochs of 700 ms, starting 200 ms before search display onset. The 200 ms prestimulus period served as baseline. Trials excluded from behavioral analyses and error trials were removed, including trials with blinks (vEOG > 100 μV), eye movements (hEOG > 80 μV) or absolute voltage exceeding 80 μV in channels of interest (PO3/4, PO7/8). Only distractor-present trials were analyzed for all ERP measures.
Individual contralateral and ipsilateral ERPs were calculated for a parieto-occipital electrode pool (PO3/4, PO7/8), as is standard for the ERP components we were interested in (e.g., Eimer & Kiss, 2010; Hickey et al., 2009; Luck, 2012), and averaged separately for display configurations, target types and groups. Difference waves were calculated by subtracting ipsilateral from contralateral activity. Time windows of analysis for the NT and SPCN were chosen based on a visual inspection of the grand averages (across distractor and target type conditions and participants) for the two display configurations. Mean NT amplitudes were computed from 200 to 300 ms after search display onset, and mean SPCN amplitudes from 300 to 500 ms. For trials with lateral distractors, no lateralized activity was detected. We accordingly refrained from conducting an analysis of the PD, which clearly did not emerge in this task. Individual mean amplitude measures of NT and SPCN were submitted to ANOVAs with the within-factor target type and the between-factor group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Department of Psychology at Philipps-Universität Marburg. All participants provided informed written consent.
Results
The demographic and clinical characteristics are presented in Table 1.
Table 1.
Demographic and clinical data of the participants
| IGD | Casual | Statistical analysis | |||
| (n = 20) | (n = 23) | t (f) or Χ2 | P | d | |
| Sex | 17 | 3a | 21 | 2a | 0.414 (41) | 0.520 | −0.19 |
| Education | 16 | 4b | 16 | 7b | 0.612 (41) | 0.434 | −0.24 |
| Age | 24.5 ± 3.2 | 24.2 ± 3.2 | 0.336 (41) | 0.738 | 0.10 |
| Gaming (h/session) | 3.1 ± 1.1 | 2.1 ± 0.8 | 3.382 (41) | 0.002 | 1.03 |
| Gaming (h/week) | 18.6 ± 10.7 | 13.2 ± 8.7 | 1.842 (41) | 0.073 | 0.56 |
| Sports (h/week) | 4.9 ± 3.9 | 4.4 ± 3.5 | 0.490 (41) | 0.627 | 0.15 |
| Car (h/week) | 2.1 ± 4.8 | 1.4 ± 2.3 | 0.629 (41) | 0.533 | 0.19 |
| ZST | 42.4 ± 5.3 | 43.3 ± 6.5 | −0.524 (41) | 0.603 | −0.16 |
| BIS (total score) | 32.3 ± 6.3 | 29.2 ± 4.6 | 1.825 (41) | 0.075 | 0.56 |
| CAARS (ADHS-DSM) | 58.7 ± 11.9 | 48.2 ± 4.8 | 3.642# (24.351) | <0.001 | 1.17 |
| BSI (GSI) | 53.6 ± 9.4 | 42.3 ± 9.7 | 3.864 (41) | <0.001 | 1.18 |
| IAT | 33.2 ± 7.4 | 21.8 ± 4.7 | 5.902# (31.152) | <0.001 | 1.86 |
Note: a = male | female; b = A-Levels | University degree; # = Welch’s correction.; P-values < 0.05 are highlighted bold.
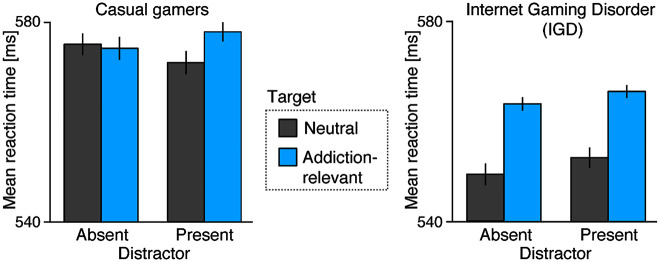
Fig. 2 shows reaction times in the visual search task for the different target types and distractor conditions, separately for casual and IGD gamers. Although IGD gamers’ responses were overall faster, the groups did not differ significantly, F(1.41) = 0.74, P = 0.395. Reaction times were not different in trials with and without a distractor, F(1.41) = 0.69, P = 0.41, but responses were slower for addiction-relevant targets than for neutral targets, F(1.41) = 30.02, P < 0.001, partial η2 = 0.423. Importantly, the interaction between group and target type, F(1.41) = 13.50, P < 0.001, partial η2 = 0.248, revealed that this was mainly driven by the IGD group. This is a critical finding, as it shows that IGD gamers differed from casual gamers in their processing of neutral and addiction-relevant targets. Indeed, the difference in reaction times to neutral and addiction-relevant targets (averaged across distractor conditions) was only significant in the IGD group, t(19) = 11.72, P < 0.001, d = 2.62), but not in the casual gamers group, t(22) = 1.04, P = 0.31. No other interactions were significant.
Fig. 2.
Behavioral results. Mean reaction times to neutral (dark grey) and addiction-relevant (blue) targets in distractor-absent and distractor-present trials, shown separately for casual gamers and IGD group. Error bars show within-subject standard errors of the means (Morey, 2008)
Accuracy was overall high (91.31 ± 0.83%). Responses were more accurate for neutral (91.72 ± 0.69%) than for addiction-relevant targets (89.86 ± 0.81%), F(1.41) = 29.10, P < 0.001, partial η2 = 0.415. An interaction of target type and group, F(1.41) = 6.03, P = 0.018, partial η2 = 0.128, revealed that this difference was larger in the IGD (2.79 ± 0.56%) than in the casual gamers group (1.05 ± 0.44%). No other effects were significant.
EEG/ERP results
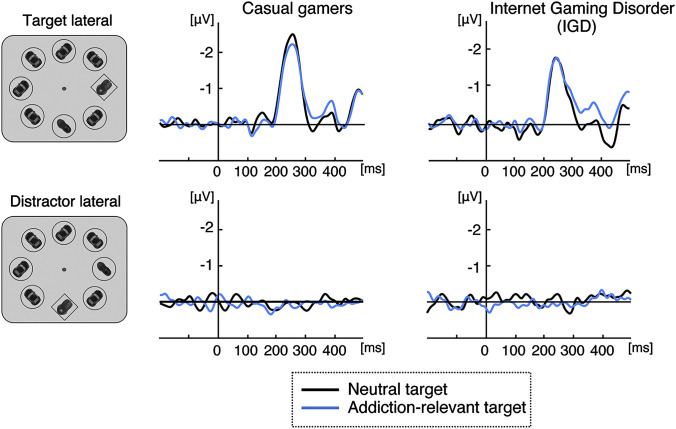
Fig. 3 shows the grand-averaged lateralized ERPs (difference waves: contralateral minus ipsilateral activity) in distractor-present trials time-locked to search display onset. No lateralized activity was observed for lateral distractors (Fig. 3, bottom). For trials with lateral targets, we analyzed the NT (200–300 ms) and the SPCN (300–500 ms). While there was overall a pronounced NT, t(42) = 8.64, P < 0.001, d = 1.32 (one-tailed t-test against zero), it did not differ between groups, F(1.41) = 1.62, P = 0.209, or between target types, F(1.41) = 0.14, P = 0.714; neither was there an interaction of the two factors, F(1.41) = 1.00, P = 0.322. The SPCN (300–500 ms) fell short of statistical significance at the 5% level overall, t(42) = 1.32, P = 0.097, d = 0.20, but was significantly larger for addiction-relevant (−0.37 ± 0.18 μV) than for neutral targets (−0.09 ± 0.18 μV), F(41) = 7.88, P = 0.008, partial η2 = 0.161. Although this difference was observed in both groups, resulting in a non-significant interaction, F(1.41) = 1.97, P = 0.168, it was larger in the IGD group (0.44 ± 0.14 μV) than in the casual gamer group (0.15 ± 0.15 μV). Indeed, planned comparisons revealed that the difference between target types was only significant in the IGD group, t(19) = 3.09, P = 0.003, d = 0.69 (one-tailed), but not in the casual gamer group, t(22) = 0.98, P = 0.17.
Fig. 3.
Grand-averaged ERPs recorded at parieto-occipital electrode sites (pool of PO3/4 and PO7/8), time-locked to the onset of the search display. Shown are the difference waves (contralateral minus ipsilateral activity) elicited by lateral targets (top) and lateral distractors (bottom), separately for trials with a neutral target (black lines) and trials with an addiction-relevant target (blue/light grey lines) and for casual gamers (left column) and the IGD group (right column). For illustration purposes, the waveforms were lowpass filtered at 35 Hz
Correlations
We computed correlations between the individual differences in SPCN amplitudes for addiction-relevant and neutral targets, and the analogous difference in reaction times. A larger difference in SPCN amplitudes, indicating prolonged attentional processing of addiction-relevant targets, should be associated with a longer delay of responses to addiction-relevant targets. Due to the negative polarity of the SPCN, this would be reflected in a negative correlation. Although this relationship should be present in both groups, we computed correlations separately for each group, because of the differences in the means. Fig. 4 shows the scatterplot. There was a significant negative correlation in the IGD group r = −0.438, P = 0.034 (one-tailed); the negative correlation in the casual gamers group was slightly smaller and fell just short of statistical significance r = −0.361, P = 0.054.
Fig. 4.
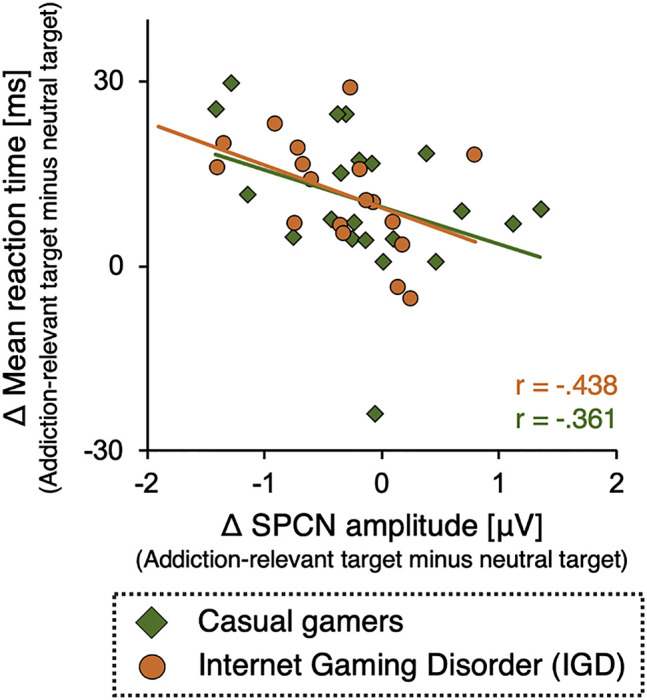
Correlation between SPCN amplitudes and mean reaction times in the casual gamers (diamonds/green) and in the IGD group (circles/orange). Shown are the differences between trials with addiction-relevant targets and trials with neutral targets. Positive values indicate longer reaction times or smaller SPCN amplitudes to addiction-relevant targets than to neutral targets. Each dot represents one participant
Discussion
With this study, we provide evidence for an attentional bias towards computer-related stimuli in gamers with IGD. Our visual search task allowed us to identify the specific attentional process that produces the bias, namely impaired disengagement of attention from addiction-relevant stimuli, but not facilitated initial deployment of attention towards addiction-relevant stimuli or failed suppression of addiction-relevant stimuli that conflict with current behavioral goals. This conclusion is based on three findings: First, in contrast to casual gamers, it took gamers with IGD longer to respond to addiction-relevant than to neutral targets. Second, they exhibited larger SPCN amplitudes – a marker of continued attentional processing – for addiction-relevant than for neutral targets. Third, individual differences between SPCN amplitudes and reaction times for addiction-relevant and neutral targets were correlated.
We found no evidence that IGD gamers’ attention was initially attracted by computer-related objects more than by neutral objects. A facilitated deployment of attention towards computer-related objects would have yielded faster rather than slower responses to addiction-relevant targets. More importantly, it would have been reflected in a modulation of the NT. But while an overall NT was observed, its magnitude did not differ between target types or gamer groups. Moreover, if gamers with IGD were always attracted to computer-related stimuli, we would have expected attentional capture by addiction-relevant distractors, as indicated by slower responses in distractor-present trials than in distractor-absent trials. Our findings do not preclude the possibility, though, that individuals with IGD are drawn towards computer-related stimuli in other contexts. The stimuli we used were carefully matched and designed so that there were no differences in overall salience (unlike in other studies on attentional capture, e.g., Bacon & Egeth, 1994; Kadel, Feldmann-Wüstefeld, & Schubö, 2017; Theeuwes, 2004). This also means that the objects were rather difficult to identify and distinguish. Possibly, the initial deployment of attention was entirely guided by the easy-to-discriminate shapes that defined targets and distractors, and not substantially affected by object identity. Under more natural conditions with less ambiguous object identities, IGD gamers’ attention might well be captured by computer-related objects. In any case, once computer-related objects are attended, individuals with IGD have more difficulty disengaging from them.
Only few studies have experimentally examined an attentional bias in IGD and their findings have remained inconclusive. Whereas most studies have used game-related stimuli to examine attentional biases (e.g. Lorenz et al., 2013; Van Holst et al., 2012), we used computer-related stimuli instead. This allowed us to address a wider range of questions and draw conclusions about conditioning processes. Computer-related (verbal) stimuli have previously only been used by studies with indirect measurements of attention biases such as reaction times in Stroop tests or visual probe tasks (Jeromin et al., 2016). Here, we complement reaction time results with neurophysiological evidence obtained by EEG recordings. This allowed us to isolate specific attentional processes and to analyze which process is involved in attentional biases in IGD. While Kim et al. (2018) also conducted an EEG study to investigate the presence of an attentional bias in IGD, they examined an ERP component that does not reflect selective attention.
Impaired disengagement of attention from addiction-related stimuli has also been demonstrated for other addictive disorders such as gambling and substance abuse (Campbell et al., 2018; Hudson, Olatunji, Gough, Yi, & Stewart, 2016), and it has been proposed to contribute to the maintenance of addictive behaviors (Brand et al., 2019; Field & Cox, 2008). Continued attentional processing of computer-related stimuli may similarly contribute to the maintenance of IGD. For instance, the difficulty in disengaging from these stimuli may make it harder to quit gaming, which leads to longer gaming sessions. Here, we observed impaired disengagement even though we did not use gaming stimuli but potential gaming cues (i.e., photographs of computer objects). This suggests that conditioning occurs in IGD, likely contributing to the development and maintenance of this disorder: By linking previously neutral stimuli (e.g., a keyboard) with reward-associated behavior (playing computer games), they become addiction-related stimuli. According to the incentive sensitization theory of addiction (Robinson & Berridge, 1993, 2001, 2008), hypersensitivity develops for these stimuli, which in turn leads to altered attention processes. Our findings thus provide further evidence (i) that the addiction concept can be applied to the problematic use of computer games and (ii) for the role of conditioning processes for addictive disorders in general (e.g., Everitt, 2014; Field & Cox, 2008).
The identification of attentional biases in IGD is clinically relevant, as bias modification is helpful in the treatment of several mental disorders (Hakamata et al., 2010; Hallion & Ruscio, 2011). There is initial evidence that it may also be helpful in the treatment of IGD (Rabinovitz & Nagar, 2015) and bias modification could become part of a standardized treatment (Dong & Potenza, 2014). Our results illuminate the specific attentional processes involved, which is crucial for designing interventions for bias modification. They further suggest that any treatment of IGD should ensure that stimulus control/abstinence also pertains to gaming cues.
To ensure that differences between groups were due to differences in the problematic use of online gaming, we controlled for a number of potential influences. First, we examined stimuli-related factors: computer stimuli, but not other stimuli, had a higher valence for the IGD group than for casual gamers; the groups did not differ with respect to time spent on sports and cars, which served as neutral stimulus categories. Second, we examined clinical characteristics. While the groups did not differ in impulsivity and cognitive processing speed, ADHD symptoms and psychological distress were more pronounced in the IGD group. Positive correlations between ADHD symptoms and problematic online gaming have been observed in previous studies (González-Bueso et al., 2018; Yen et al., 2017) and playing online games may serve as a coping strategy for psychological distress (Plante, Gentile, Groves, Modlin, & Blanco-Herrera, 2019). These group differences thus support the clinical validity of the group assignment. Importantly, however, the higher means for ADHD and psychological distress in the IGD group were not clinically significant. The observed effects can therefore not be exclusively attributed to ADHD or other mental health problems. Moreover, our findings can be specifically related to IGD and not gaming per se, as we used casual gamers as control group.
The following limitations must be considered when interpreting the results of this study. Firstly, our sample is mostly male. This, however, reflects the actual prevalence rates (Wartberg, Kriston, & Thomasius, 2017). Secondly, the assignment of participants to the IGD group and the control group relied on the IGDQ, which – although a validated and frequently used instrument – does not constitute a clinical diagnosis. Lastly, the assessment of psychopathology was based solely on the BSI, because we did not want to further prolong the already long session. Ideally, this would have been done on the basis of a clinical interview such as the CIDI (Robins et al., 1988).
In sum, our findings revealed that IGD gamers’ ability to disengage attention from computer-related stimuli is impaired, indicating that attentional biases generalize from gaming stimuli to computer-cues. These insights could potentially be useful in the development of new treatments. Future studies should investigate whether approaches such as cue-exposure therapy or attentional bias modification could be helpful as complementary treatment components for IGD. The link between attentional biases and problematic use should also be investigated in more detail: An attentional bias may constitute a measurable marker of IGD, which could prove a useful addition to self-report measures for diagnostic purposes. Overall, our findings elucidate the cognitive processes involved in the maintenance of problematic online gaming by highlighting the role of conditioning processes for IGD and identifying a mechanism that may render abstinence more difficult to maintain.
Funding sources
This study received no funding.
Authors’ contribution
AH, AB and AS conceived of and designed the study. AH programmed the experiment; MM and AH supervised data collection. MM analyzed the clinical measures; AH analyzed the behavioral and electrophysiological data. All authors interpreted the data. AH and MM wrote the first draft of the manuscript. All authors reviewed and revised the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Sophia S. Tennie, Franziska Jeromin and Hanna Kadel for their contributions during study preparation and Johannes Hauck, Lydia Ifland, Jan-Christoph Thiele and Almut Gitter for their assistance with data collection. The open access publication of this article was supported by the Open Access Fund of the Catholic University Eichstaett-Ingolstadt.
References
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Bacon, W. F., & Egeth, H. E. (1994). Overriding stimulus-driven attentional capture. Perception & Psychophysics, 55(5), 485–496. [DOI] [PubMed] [Google Scholar]
- Berggren, N., & Eimer, M. (2018). Object-based target templates guide attention during visual search. Journal of Experimental Psychology: Human Perception and Performance, 44, 1368–1382. [DOI] [PubMed] [Google Scholar]
- Brand, M., Wegmann, E., Stark, R., Müller, A., Wölfling, K., Robbins, T. W., et al. (2019). The Interaction of Person-Affect-Cognition-Execution (I-PACE) model for addictive behaviors: Update, generalization to addictive behaviors beyond internet-use disorders, and specification of the process character of addictive behaviors. Neuroscience & Biobehavioral Reviews, 104, 1–10. 10.1016/j.neubiorev.2019.06.032. [DOI] [PubMed] [Google Scholar]
- Brand, M., Young, K. S., Laier, C., Wölfling, K., & Potenza, M. N. (2016). Integrating psychological and neurobiological considerations regarding the development and maintenance of specific Internet-use disorders: An Interaction of Person-Affect-Cognition-Execution (I-PACE) model. Neuroscience & Biobehavioral Reviews, 71, 252–266. 10.1016/j.neubiorev.2016.08.033. [DOI] [PubMed] [Google Scholar]
- Campbell, D. W., Stewart, S., Gray, C. E. P., Ryan, C. L., Fettes, P., McLandress, A. J., et al. (2018). Chronic cannabis use and attentional bias: Extended attentional capture to cannabis cues. Addictive Behaviors, 81, 17–21. 10.1016/j.addbeh.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Christiansen, H., Hirsch, O., Abdel-Hamid, M., & Kis, B. (2014). Conners Skalen zur Aufmerksamkeit und Verhalten für Erwachsene (CAARS). Deutschsprachige Adaptation der Conners‘ Adult ADHD Rating Scales (CARRS) von C. Keith Conners, Drew Erhardt und Elizabeth Sparrow. Bern: Hans Huber. [Google Scholar]
- Ciccarelli, M., Nigro, G., Griffiths, M. D., Cosenza, M., & D’Olimpio, F. (2016). Attentional bias in non-problem gamblers, problem gamblers, and abstinent pathological gamblers: An experimental study. Journal of Affective Disorders, 206, 9–16. 10.1016/j.jad.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Conners, C. K., Erhardt, D., & Sparrow, E. P. (1999). Conners’ adult ADHD rating scales: Technical manual. New York: Multi-Health Systems. [Google Scholar]
- Cox, W. M., Fadardi, J. S., Intriligator, J. M., & Klinger, E. (2014). Attentional bias modification for addictive behaviors: Clinical implications. CNS Spectrums, 19(3), 215–224. 10.1017/S1092852914000091. [DOI] [PubMed] [Google Scholar]
- Derogatis, L. R. (1993). BSI: Brief symptom inventory (manual). Minneapolis: National Computer Systems. [Google Scholar]
- Dong, G., & Potenza, M. N. (2014). A cognitive-behavioral model of Internet gaming disorder: Theoretical underpinnings and clinical implications. Journal of Psychiatric Research, 58, 7–11. 10.1016/j.jpsychires.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer, M. (1996). The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology, 99, 225–234. [DOI] [PubMed] [Google Scholar]
- Eimer, M., & Kiss, M. (2010). An electrophysiological measure of access to representations in visual working memory. Psychophysiology, 47, 197—200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt, B. J. (2014). Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories--indications for novel treatments of addiction. European Journal of Neuroscience, 40(1), 2163–2182. 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld, T., Uengoer, M., & Schubö, A. (2015). You see what you have learned. Evidence for an interrelation of associative learning and visual selective attention. Psychophysiology, 52, 1483–1497. [DOI] [PubMed] [Google Scholar]
- Field, M., & Cox, W. M. (2008). Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence, 97(1–2), 1–20. 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field, M., Marhe, R., & Franken, I. H. A. (2014). The clinical relevance of attentional bias in substance use disorders. CNS Spectrums, 19(3), 225–230. 10.1017/S1092852913000321. [DOI] [PubMed] [Google Scholar]
- Franke, G. H. (2000). BSI. Brief symptom inventory – Deutsche version. Manual. Göttingen: Beltz. [Google Scholar]
- Franken, I. H. A. (2003). Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 27(4), 563–579. 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Gaspar, J. M., & McDonald, J. J. (2014). Suppression of salient objects prevents distraction in visual search. Journal of Neuroscience, 34, 5658–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Bueso, V., Santamaría, J. J., Fernández, D., Merino, L., Montero, E., & Ribas, J. (2018). Association between internet gaming disorder or pathological video-game use and comorbid psychopathology: A comprehensive review. International Journal of Environmental Research and Public Health, 15(4). 10.3390/ijerph15040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak, G., Weinberg, A., MacNamara, A., & Foti, D. (2011). ERPs and the study of emotion. In Luck S., & Kappenman E. (Eds.), Oxford handbook of event-related potential components. New York: Oxford University Press. [Google Scholar]
- Hakamata, Y., Lissek, S., Bar-Haim, Y., Britton, J. C., Fox, N. A., Leibenluft, E., …, Pine, D. S. (2010). Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry, 68(11), 982–990. 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion, L. S., & Ruscio, A. M. (2011). A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin, 137(6), 940–958. 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Heuer, A., & Schubö, A. (2020). Cueing distraction: Electrophysiological evidence for anticipatory active suppression of distractor location. Psychological Research, 84, 2111–2121. 10.1007/s00426-019-01211-4. [DOI] [PubMed] [Google Scholar]
- Hickey, C., Di Lollo, V., & McDonald, J. J. (2009). Electrophysiological indices of target and distractor processing in visual search. Journal of Cognitive Neuroscience, 21, 760–775. [DOI] [PubMed] [Google Scholar]
- Hudson, A., Olatunji, B. O., Gough, K., Yi, S., & Stewart, S. H. (2016). Eye on the prize: High-risk gamblers show sustained selective attention to gambling cues. Journal of Gambling Issues, 26(34), 153. 10.4309/jgi.2016.34.6. [DOI] [Google Scholar]
- Jeromin, F., Nyenhuis, N., & Barke, A. (2016). Attentional bias in excessive Internet gamers: Experimental investigations using an addiction Stroop and a visual probe. Journal of Behavioral Addictions, 5(1), 32–40. 10.1556/2006.5.2016.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeromin, F., Rief, W., & Barke, A. (2016). Using two web-based addiction Stroops to measure the attentional bias in adults with Internet Gaming Disorder. Journal of Behavioral Addictions, 5(4), 666–673. 10.1556/2006.5.2016.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur, P., Brisson, B., & Robitaille, N. (2008). Dissociation of the N2pc and sustained posterior contralateral negativity in a choice response task. Brain Research, 1215, 160–172. [DOI] [PubMed] [Google Scholar]
- Kadel, H., Feldmann-Wüstefeld, T., & Schubö, A. (2017). Selection history alters attentional filter settings persistently and beyond top-down control. Psychophysiology, 54(5), 736–754. 10.1111/psyp.12830. [DOI] [PubMed] [Google Scholar]
- Kim, S. N., Kim, M., Lee, T. H., Lee, J.-Y., Park, S., Park, M., et al. (2018). Increased attentional bias toward visual cues in internet gaming disorder and obsessive-compulsive disorder: An event-related potential study. Frontiers in Psychiatry, 9, 315. 10.3389/fpsyt.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Király, O., Griffiths, M. D., Urbán, R., Farkas, J., Kökönyei, G., Elekes, Z., et al. (2014). Problematic internet use and problematic online gaming are not the same: Findings from a large nationally representative adolescent sample. Cyberpsychology, Behavior, and Social Networking, 17(12), 749–754. 10.1089/cyber.2014.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, M., Driver, J., & Eimer, M. (2009). Reward priority of visual target singletons modulates ERP signatures of attentional selection. Psychological Science, 20, 245—251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss, D. J., Griffiths, M. D., Karila, L., & Billieux, J. (2014). Internet addiction: A systematic review of epidemiological research for the last decade. Current Pharmaceutical Design, 20(25), 4026–4052. 10.2174/13816128113199990617. [DOI] [PubMed] [Google Scholar]
- Lemmens, J. S., Valkenburg, P. M., & Gentile, D. A. (2015). The internet gaming disorder scale. Psychological Assessment, 27(2), 567–582. 10.1037/pas0000062. [DOI] [PubMed] [Google Scholar]
- Lorenz, R. C., Krüger, J.-K., Neumann, B., Schott, B. H., Kaufmann, C., Heinz, A., et al. (2013). Cue reactivity and its inhibition in pathological computer game players. Addiction Biology, 18(1), 134–146. 10.1111/j.1369-1600.2012.00491.x. [DOI] [PubMed] [Google Scholar]
- Luck, S. J. (2012). Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. In Kappenman E. S., & Luck S. J. (Eds.), The Oxford handbook of event-related potential components (pp. 329–360). Oxford: Oxford University Press. [Google Scholar]
- Mazza, V., Turatto, M., Umiltà, & Eimer (2007). Attentional selection and identification of visual objects are reflected by distinct electrophysiological responses. Experimental Brain Research, 181, 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. J., Green, J. J., Jannati, A., & Di Lollo, V. (2013). On the electrophysiological evidence for the capture of visual attention. Journal of Experimental Psychology: Human Perception and Performance, 39, 849–860. [DOI] [PubMed] [Google Scholar]
- Metcalf, O., & Pammer, K. (2011). Attentional bias in excessive massively multiplayer online role-playing gamers using a modified Stroop task. Computers in Human Behavior, 27(5), 1942–1947. 10.1016/j.chb.2011.05.001. [DOI] [Google Scholar]
- Meule, A., Vögele, C., & Kübler, A. (2011). Psychometrische Evaluation der deutschen Barratt Impulsiveness Scale – Kurzversion (BIS-15). Diagnostica, 57(3), 126–133. 10.1026/0012-1924/a000042. [DOI] [Google Scholar]
- Mogoaşe, C., David, D., & Koster, E. H. W. (2014). Clinical efficacy of attentional bias modification procedures: An updated meta-analysis. Journal of Clinical Psychology, 70(12), 1133–1157. 10.1002/jclp.22081. [DOI] [PubMed] [Google Scholar]
- Morey, R. D. (2008). Confidence intervals from normalized data: A correction to cousineau (2005). Tutorials in Quantitative Methods for Psychology, 4, 61–64. [Google Scholar]
- Munneke, J., Fait, E., & Mazza, V. (2013). Attentional processing of multiple targets and distractors. Psychophysiology, 50, 1104–1108. [DOI] [PubMed] [Google Scholar]
- Oostenveld, R., Fries, P., Maris, E., & Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, J. H., Stanford, M. S., & Barratt, E. S. (1995). Factor structure of the barratt impulsiveness scale. Journal of Clinical Psychology, 51(6), 768–774. . [DOI] [PubMed] [Google Scholar]
- Pawlikowski, M., Altstötter-Gleich, C., & Brand, M. (2013). Validation and psychometric properties of a short version of Young’s Internet Addiction Test. Computers in Human Behavior, 29(3), 1212–1223. 10.1016/j.chb.2012.10.014. [DOI] [Google Scholar]
- Petry, N. M., Rehbein, F., Gentile, D. A., Lemmens, J. S., Rumpf, H.-J., Mößle, T., …, O’Brien, C. P. (2014). An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction (Abingdon, England), 109(9), 1399–1406. 10.1111/add.12457. [DOI] [PubMed] [Google Scholar]
- Plante, C. N., Gentile, D. A., Groves, C. L., Modlin, A., & Blanco-Herrera, J. (2019). Video games as coping mechanisms in the etiology of video game addiction. Psychology of Popular Media Culture, 8(4), 385–394. 10.1037/ppm0000186. [DOI] [Google Scholar]
- Rabinovitz, S., & Nagar, M. (2015). Possible end to an endless quest? Cognitive bias modification for excessive multiplayer online gamers. Cyberpsychology, Behavior, and Social Networking, 18(10), 581–587. 10.1089/cyber.2015.0173. [DOI] [PubMed] [Google Scholar]
- Robinson, T. E., & Berridge, K. C. (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews, 18(3), 247–291. 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson, T. E., & Berridge, K. C. (2001). Incentive-sensitization and addiction. Addiction (Abingdon, England), 96(1), 103–114. 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson, T. E., & Berridge, K. C. (2008). Review. The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3137–3146. 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins, L. N., Wing, J., Wittchen, H. U., Helzer, J. E., Babor, T. F., Burke, J., et al. (1988). The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry, 45(12), 1069–1077. 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Statista (2019). Number of gamers worldwide 2021 | Statista. Retrieved from https://www.statista.com/statistics/748044/number-video-gamers-world/.
- Theeuwes, J. (2004). Top-down search strategies cannot override attentional capture. Psychonomic Bulletin & Review, 11(1), 65–70. 10.3758/BF03206462. [DOI] [PubMed] [Google Scholar]
- Theeuwes, J. (2018). Visual selection: Usually fast and automatic; seldom slow and volitional. Journal of Cognition, 1, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rodríguez, A., Griffiths, M. D., Carbonell, X., & Oberst, U. (2018). Internet gaming disorder in adolescence: Psychological characteristics of a clinical sample. Journal of Behavioral Addictions, 7(3), 707–718. 10.1556/2006.7.2018.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Holst, R. J., Lemmens, J. S., Valkenburg, P. M., Peter, J., Veltman, D. J., & Goudriaan, A. E. (2012). Attentional bias and disinhibition toward gaming cues are related to problem gaming in male adolescents. Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine, 50(6), 541–546. 10.1016/j.jadohealth.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Wartberg, L., Kriston, L., & Thomasius, R. (2017). The prevalence and psychosocial correlates of internet gaming disorder. Deutsches Arzteblatt International, 114(25), 419–424. 10.3238/arztebl.2017.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, A. J., & Feyerabend, C. (2000). Determinants and effects of attentional bias in smokers. Psychology of Addictive Behaviors, 14(2), 111–120. 10.1037//0893-164x.14.2.111. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. A. (2008). Wechsler adult intelligence scale (4th ed.). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wittek, C. T., Finserås, T. R., Pallesen, S., Mentzoni, R. A., Hanss, D., Griffiths, M. D., et al. (2016). Prevalence and predictors of video game addiction: A study based on a national representative sample of gamers. International Journal of Mental Health and Addiction, 14(5), 672–686. 10.1007/s11469-015-9592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, J. M., & Horowitz, T. S. (2017). Five factors that guide attention in visual search. Nature Human Behaviour, 1, 0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, J.-Y., Liu, T.-L., Wang, P.-W., Chen, C.-S., Yen, C.-F., & Ko, C.-H. (2017). Association between Internet gaming disorder and adult attention deficit and hyperactivity disorder and their correlates: Impulsivity and hostility. Addictive Behaviors, 64, 308–313. 10.1016/j.addbeh.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Young, K. S. (1998). Internet addiction: The emergence of a new clinical disorder. Cyber Psychology and Behavior, 1(3), 237–244. 10.1089/cpb.1998.1.237. [DOI] [Google Scholar]
- Zhang, Y., Lin, X., Zhou, H., Xu, J., Du, X., & Dong, G. (2016). Brain activity toward gaming-related cues in internet gaming disorder during an addiction Stroop task. Frontiers in Psychology, 7, 714. 10.3389/fpsyg.2016.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]