Abstract
Background and aims
Deficits in cognitive control represent a core feature of addiction. Internet Gaming Disorder (IGD) offers an ideal model to study the mechanisms underlying cognitive control deficits in addiction, eliminating the confounding effects of substance use. Studies have reported behavioral and neural deficits in reactive control in IGD, but it remains unclear whether individuals with IGD are compromised in proactive control or behavioral adjustment by learning from the changing contexts.
Methods
Here, fMRI data of 21 male young adults with IGD and 21 matched healthy controls (HC) were collected during a stop-signal task. We employed group independent component analysis to investigate group differences in temporally coherent, large-scale functional network activities during post-error slowing, the typical type of behavioral adjustments. We also employed a Bayesian belief model to quantify the trial-by-trial learning of the likelihood of stop signal – P(Stop) – a broader process underlying behavioral adjustment, and identified the alterations in functional network responses to P(Stop).
Results
The results showed diminished engagement of the fronto-parietal network during post-error slowing, and weaker activity in the ventral attention and anterior default mode network in response to P(Stop) in IGD relative to HC.
Discussion and conclusions
These results add to the literatures by suggesting deficits in updating and anticipating conflicts as well as in behavioral adjustment according to contextual information in individuals with IGD.
Keywords: bayesian learning, post-error adjustment, functional brain networks, independent component analysis, Internet gaming disorder, stop-signal task
Introduction
Internet gaming disorder (IGD) represents a putative behavioral addiction that has been included as a condition deserving further studies by the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) (American Psychiatric Association, 2013). Recently, gaming disorder was also included in 11th Edition of International Classification of Diseases (ICD-11) (World Health Organization, 2020). Relatively free from the confounding effects of substance use, IGD offers an ideal model to study the neural mechanisms of addiction (Cho et al., 2014). Deficits in cognitive control are central to etiological processes of addiction, including IGD. Cognitive control has been studied with a myriad of behavioral paradigms and comprises both reactive and proactive processes (Hu, Ide, Zhang, & Li, 2016; Wang et al., 2018). Extant research has largely focused on the behavioral and neural bases of reactive processes, such as response inhibition (Argyriou, Davison, & Lee, 2017; Chen et al., 2015), and few studies investigated whether contextual learning and behavioral adjustment, a core process of proactive control (Reed, Vile, Osborne, Romano, & Truzoli, 2015; Verbruggen, Stevens, & Chambers, 2014), may be compromised in IGD.
In experimental psychology, the stop signal task (SST) is one of the most widely used paradigms to study behavioral adjustment. In the SST, participants make trial-by-trial adjustment in reaction time (RT) in order to negotiate the conflicting needs to respond to go signal quickly and to avoid making a response in stop trials (Ide, Shenoy, Yu, & Li, 2013). Participants typically slow down in RT in the go trials following a stop error as compared to those following another go trial, and this RT adjustment has been called post-error slowing (PES) (Li et al., 2008). PES reflects behavioral adjustment to the immediate experience of an error, anticipation of a higher likelihood of a stop signal, and consequently a more cautious approach in executing a motor response.
Many studies have focused on the effect of drug use on behavioral and neural response to PES. For example, diminished PES was found after acute injections of alcohol (Bombeke, Schouppe, Duthoo, & Notebaert, 2013) and in opioid (Liao et al., 2014) and cocaine (Li, Milivojevic, Kemp, Hong, & Sinha, 2006) dependent individuals. By comparing post-error go trials with increased RT (pSEi) and those that did not increase in RT (pSEni), Li and colleagues demonstrated decreased activation in the right dorsolateral prefrontal cortex during PES in alcohol dependent as compared to control participants (Li, Luo, Yan, Bergquist, & Sinha, 2009). Other studies showed that long-term alcohol consumption compromised frontal-striatal circuit function, leading to poor post-error behavior adjustment (Lawrence, Luty, Bogdan, Sahakian, & Clark, 2009). Thus, PES may represent a robust behavioral marker of addiction, and it is of importance to examine whether PES is altered in individuals with IGD.
A Bayes-optimal decision-making model has been proposed to incorporate learning in examining behavioral adjustment in the SST. In this model, participants choose to go or stop by combining sensory evidence in a trial and prior belief about the likelihood of a stop trial (Shenoy & Yu, 2011). Specifically, each trial contributes to one’s belief about the likelihood of encountering a subsequent stop trial, P(stop), and one strategically modulates RT accordingly: an increase in P(Stop) predicts a prolonged go trial RT – a sequential effect (Hu, Ide, Zhang, & Li, 2015; Ide et al., 2013; Ide, Hu, Zhang, Yu, & Li, 2015; Shenoy & Yu, 2011). It is likely that deficits in behavioral adjustment reflect a specific instance of impaired ability to use contextual information to anticipate stop signals and adjust behavior accordingly. Indeed, an earlier work showed that PES was less correlated with the Bayesian account of sequential effect in cocaine dependent relative to control subjects (Ide et al., 2015). Previous imaging studies have found that bilateral inferior frontal gyrus, inferior parietal lobule and anterior pre-supplementary motor area are modulated by the probability of stop trials (Chikazoe et al., 2009; Hu, Ide, Zhang, Sinha, & Li, 2015). Neural deficits have also been shown in the left insula, bilateral inferior frontal gyrus, and inferior parietal lobule in response to P(Stop) in occasional stimulant including methylphenidate users and in alcohol dependent individuals performing the SST (Harle et al., 2014; Hu, Ide, Zhang, Sinha, et al., 2015). Thus, examining Bayesian learning would advance our understanding of the psychological processes of behavioral adjustment in IGD.
General linear modeling (GLM) represents a major approach in identifying regional brain activations during cognitive performance and has been widely used in studies of the SST (Hu, Ide, Zhang, & Li, 2015; Li et al., 2009). In contrast, as a data-driven approach, independent component analysis (ICA) reveals “hidden” factors from a set of measurements such that the sources of the observed data are maximally independent. Requiring no prior knowledge, ICA helps in identifying functional networks rather than individual brain regions of importance to psychological processes (Zhang & Li, 2012). Group ICA (gICA) may identify functional networks that participate in multiple cognitive processes (Xu et al., 2013) that elude detection by GLM-based analyses (Xu, Calhoun, & Potenza, 2015). Previous research using gICA has revealed functional networks underlying SST performance (Zhang & Li, 2012). For instance, an earlier study demonstrated disengagement of a network comprising the inferior frontal cortex and intraparietal sulcus during PES in substance-abusing individuals (Elton et al., 2014).
In the current study, we employed gICA to examine fMRI data of the SST and compared IGD subjects and healthy controls (HCs) in neural responses during post-error behavioral adjustment. We also investigated the neural representation of probabilistic expectations of the stop signal during the SST. According to previous findings, we hypothesized that IGD subjects relative to HCs would show deficient activity in the fronto-parietal network (FPN), a circuit widely implicated in action withholding/cancellation (Zhang, Geng, & Lee, 2017), during PES. We also posited attenuated activity in the ventral attention network (VAN), a network that partakes in monitoring environmental information (Congdon et al., 2010; Zhang, Hu, Bednarski, Erdman, & Li, 2014), in response to P(Stop) in individuals with IGD.
Materials and methods
Participants and clinical assessments
Considering the higher occurrence of IGD among men and to avoid the potential confounds of gender (Ko et al., 2009), we recruited 22 male participants with IGD and 22 HC for the current study. Inclusion criteria included 18–30 years of age, right-handed, not currently taking any psychotropic medications, no contraindications for MRI, and no current or previous psychiatric or neurological illness as assessed with the Chinese version of the MINI International Neuropsychiatric Interview (Si et al., 2009). Additional inclusion criteria for the IGD group included: (i) meeting at least 5 of the 9 diagnostic criteria for IGD as proposed in the DSM-5 (American Psychiatric Association, 2013; Ko et al., 2014); (ii) scores ≥50 on Young’s online Internet addiction test (IAT) (Young, 2016); (iii) engagement in Internet gaming for >20 hours per week for a minimum of 1 year; and (iv) Internet gaming as their primary online activity. Additional inclusion criteria for HC included: (i) meeting fewer than 5 of the 9 diagnostic criteria for IGD in the DSM-5; (ii) <50 on the IAT; and (iii) never engaging in Internet gaming. The Beck Anxiety Inventory (BAI) (Beck, Brown, Epstein, & Steer, 1988) and Beck Depression Inventory (BDI) (Beck, Erbaugh, Ward, Mock, & Mendelsohn, 1961) were used to assess anxiety and depressive symptoms.
Imaging sessions with head movements exceeding 3 mm in translation or 3° in rotation were removed. In addition, performance with a more than 50% rate of response in the go trials, 40–60% stop success rate was deemed compliant with instructions (Jilka et al., 2014). Imaging data of two subjects were excluded for these reasons from subsequent data analysis, and the final sample comprised 21 IGDs and 21 HCs.
Stop-signal task and behavioral data analyses
Participants performed a stop signal task (Zhang & Li, 2012). They were instructed to quickly press the button when they saw the go signal, while in 25% of these trials, a stop signal would come up after the go signal (stop trials), and they should inhibit the response to go signal when they encountered the stop signal. Go and stop trials were inter-mixed randomly in presentation. A fixation dot appeared on the screen to begin in a trial, and after a fore-period varying from 1 s to 5 s, the dot became a circle – the go signal – instructing a response. The circle disappeared at button press or after 1 s if the participant failed to respond. In approximately one quarter of trials, the circle was followed by a ‘cross’ – the stop signal – prompting participants to withhold button press. The trial terminated at button press or after 1 s if participant successfully inhibited the response. The time between the go and stop signals, the stop signal delay (SSD), started at 200 ms and varied from one stop trial to the next according to a staircase procedure, increasing or decreasing by 64 ms each after a successful or failed stop trial. Participants were trained briefly out of the scanner before imaging to ensure that they understood the task. To balance the statistic power and fatigue that may result from a lengthy task, we shorted the duration of each session (relative to (Zhang & Li, 2012)), with three 8-min sessions of the task. The total number of trials varied slightly between subjects, based on the subjects’ response rate and actual inter-stimulus-interval. The average trial numbers of each session of each individual ranged from 78 to 82 (77.77 ± 1.75).
We computed the RT difference between the go trials that followed a stop error (pSE) and those that followed another go trial (pG) to quantify the extent of PES (PES effect). We used a dynamic Bayesian model to estimate the probability of stop signal, or P(Stop). The model assumes that subjects anticipate an impending stop signal on each trial, based on prior stimulus history. They believe that stop signal frequency rk on trial k has probability α of being the same as rk−1, and probability (1 − α) of being re-sampled from a prior distribution π(rk) (Angela & Cohen, 2009). Previous studies found that individual learning rates are typically too unstable to produce robust regressors for imaging data analyses (Daw & Doya, 2006; Schonberg, Daw, Joel, & O’Doherty, 2007; Wimmer, Daw, & Shohamy, 2012). Thus, following previous work (Harle et al., 2014; Ide et al., 2013; Shenoy & Yu, 2011), we employed the standard model-based fMRI analyses with a fixed set of parameters across all subjects, the mean of the prior distribution = 0.25, scale = 10 and a learning parameter α = 0.8, to characterize P(Stop) and regional responses to P(Stop) (Harle et al., 2014; Ide et al., 2013; Shenoy & Yu, 2011). A sequential effect, was quantified by Pearson correlation between P(Stop) and RT on go trials for individual subjects (Ide et al., 2015).
Image acquisition and preprocessing
MR imaging was conducted with a Siemens Trio 3-Tesla scanner (Siemens, Erlangen, Germany). Thirty-four 3.0 mm axial fMRI data were acquired using an echo-planar imaging (EPI) sequence with TR = 2,000 ms; TE = 30 ms; flip angle = 85°; matrix size = 64 × 64; resolution= 3 × 3 mm2; FOV = 220*220 mm2. One hundred forty-four high-resolution slices were obtained using T1-weighted sagittal 3-D magnetization prepared rapid gradient-echo sequence: TR = 2,530 ms, TE = 3.39 ms, TI = 1,100 ms, FA= 7°, FOV= 256 × 256 mm, thickness = 1.33 mm, in-plane resolution = 1 × 1 mm2.
First five TRs of each session were discarded, and the images were analyzed using the standard process in DPABI version 4.2 (Yan, Wang, Zuo, & Zang, 2016), including slice timing correction, realignment, normalization to 3 × 3 × 3 mm3 MNI space, and smoothing with a Gaussian kernel of 8 mm at full width half maximum (FWHM).
Generalized linear model for post-error slowing and stop signal anticipation
Generalized linear models were conducted using SPM12 (Welcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm12). In the first GLM (GLM 1, G model), to explore the neural responses to post-error slowing, go signal onsets in each trial type were convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF. Four trial outcomes were distinguished: go success (G), go error (F), stop success (SS), and stop error (SE). G trials were then divided into those that followed a G (pG), and SE (pSE) trial. The pG/pSE trials were further divided into those that increased in RT (pGi/pSEi) and those that did not increase in RT (pGni/pSEni), as compared with the mean RT of all preceding pG trials during each session (Li et al., 2008). The motion parameters were included as regressors of no interest. We also included RT of G trials, SSD of SS and SE trials as parametric modulators (Li et al., 2009).
In the second GLM (GLM 2, F model), to examine the neural correlates of stop signal anticipation, we modeled BOLD signals by convolving the onsets of the fixation point of each G, SS, and SE trial with a canonical hemodynamic response function (HRF) and the temporal derivative of the canonical HRF, with motion parameters included as regressors of no interest. The P(Stop) of G trials [G x P(Stop)], P(Stop) of SS trials [SS x P(Stop)], SSD of SS trials, P(Stop) of SE trials [SE x P(Stop)], SSD of SE trials were included as parametric modulators (Harle et al., 2014; Hu, Ide, Zhang, & Li, 2015).
Group independent component analysis and temporal sorting
The spatially processed images were analyzed with group ICA (GIFT, http://mialab.mrn.org/software/gift, version 4.0b). Briefly, data from all sessions of participants were concatenated into a single dataset and reduced in dimensionality using two stages of principal component analysis (PCA) (Zhang & Li, 2012). The data were then separated with an infomax algorithm into 29 independent components, determined by the modified minimal description length (MDL) criteria (Li, Adali, & Calhoun, 2007). Extraction was repeated 20 times using ICASSO to assess the stability of independent components (Himberg, Hyvärinen, & Esposito, 2004). The time course and spatial map for each component were back-reconstructed for each participant. We visually identified a priori networks for our hypotheses (i.e. FPN and VAN) according to previous findings (Laird et al., 2011; Smith et al., 2009).
To examine the relevance of each component to PES, we performed multiple regression temporal sorting to compare component time courses with the modeled event time courses in GLM 1. The resulting beta weights indicated the extent to which a given network was temporally associated with, or ‘engaged’ in each event. Beta weights were averaged across runs for each event for each subject.
Similarly, a multiple regression temporal sorting was performed to compare component time courses with the modeled event time courses in GLM 2. The parametric modulator of P(Stop) ([G x P(Stop)], [SS x P(Stop)], [SE x P(Stop)]) were weighted by the proportion of trial number each of G, SS, and SE trials, respectively, to examine the relevance to P(Stop) of each component ([f x P(Stop)]). A positive value for a certain functional network indicated that the activation of this functional network increased along with the likelihood that a stop signal would appear (Hu, Ide, Zhang, & Li, 2015).
Group differences of functional network activities
For PES, we examined the beta weight contrast for ‘pSEi-pSEni’ and ‘(pSEi-pSEni)- (pGi-pGni)’ of the FPNs (the left fronto-parietal network (lFPN) and right fronto-parietal network (rFPN), corresponding to the IC34 and IC60 of Allen et al. (2011) with two-sample t tests. To offer exploratory results, we performed t tests for each component and evaluated the findings with a Bonferroni-corrected threshold. Similarly, the beta weight of [f x P(Stop)] for VAN (corresponding to the IC71 of Allen et al. (2011)) was examined with a two-sample t test to investigate the group difference, and, to offer exploratory results, we performed t tests for each component and evaluated the findings at the same Bonferroni-corrected threshold.
Given the small sample size, we computed the post-hoc power of two-sample t test (two groups) using G*Power (Faul, Erdfelder, Lang, & Buchner, 2007). In post-hoc analyses, Power (1-β) is computed as a function of α (0.05), the population effect size, and the sample size in our study.
Ethics
This study was approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University (study number: ICBIR_A_0075_002). All participants providing written informed consent prior to the study.
Results
Demographic and behavioral characteristics
The demographic characteristics of the IGD and HC are shown in Table 1. Compared with HC, IGD reported higher YIAS score and more frequent Internet gaming, as well as higher levels of anxiety and higher proportion of Alcohol use. IGD and HC both showed post-error slowing (one-t test; IGD: t(20) = 2.29, p = 0.033; HC: t(20) = 2.95, p = 0.008) and sequential effect (one-t test; IGD: t(20) = 3.73, p = 0.001; HC: t(20) = 2.96, p = 0.008), but IGD and HC showed no difference in SST performance measures.
Table 1.
Participants' characteristics and behavioral performance in the SST
| IGD (n = 21) | HC (n = 21) | t/χ 2 /U | P | |
| mean ± SD | mean ± SD | |||
| Clinical assessments | ||||
| Age (years) | 22.29 ± 1.42 | 21.82 ± 1.85 | 0.84 | 0.405 |
| Education (years) | 16.14 ± 1.32 | 16.00 ± 1.82 | 0.29 | 0.772 |
| YIAT score | 69.62 ± 12.48 | 25.19 ± 6.74 | 14.35 | <0.001*** |
| Internet gaming (h/wk) | 31.83 ± 9.64 | – | 5.59a | <0.001*** |
| Alcohol use (at least once/month) | 6b/0.29c | 13b/0.62c | 4.71d | 0.030* |
| Cigarette use (at least once/month) | 0b/0.00c | 0b/0.00c | – | – |
| BAI score | 4.57 ± 4.82 | 1.81 ± 2.80 | 2.27 | 0.030* |
| BDI score | 6.38 ± 7.69 | 3.38 ± 4.50 | 1.54 | 0.131 |
| SST performance | ||||
| Mean number of trials (per session) | 78.09 ± 1.92 | 77.46 ± 1.55 | 1.16 | 0.252 |
| Mean number of G (per session) | 52.39 ± 5.70 | 48.35 ± 9.36 | 1.69 | 0.099 |
| Mean number of F (per session) | 5.14 ± 3.91 | 8.88 ± 7.63 | −2.00 | 0.053 |
| Mean number of SS (per session) | 11.02 ± 1.03 | 11.21 ± 1.33 | −0.54 | 0.591 |
| Mean number of SE (per session) | 9.54 ± 1.81 | 9.02 ± 1.07 | 1.14 | 0.261 |
| GS % | 91.10 ± 6.91 | 84.41 ± 13.62 | 2.00 | 0.054 |
| SS % | 53.78 ± 4.39 | 55.76 ± 2.32 | −2.71 | 0.073 |
| Mean GoRT (ms) | 690.42 ± 109.94 | 721.94 ± 77.37 | −1.07 | 0.289 |
| Critical SSD | 473.63 ± 133.07 | 495.89 ± 113.83 | −0.58 | 0.563 |
| SSRT | 212.41 ± 56.12 | 226.80 ± 61.77 | −0.79 | 0.434 |
| PES effect (ms) | 32.92 ± 61.54 | 47.8 ± 81.67 | −0.66 | 0.512 |
| Sequential effect | 0.15 ± 0.19 | 0.09 ± 0.14 | 1.23 | 0.225 |
| Mean FD | 0.13 ± 0.05 | 0.12 ± 0.06 | 0.38 | 0.704 |
Abbreviations: SD = standard deviation; IGD = Internet gaming disorder; HC = healthy control; YIAT = Young's online Internet addiction test; Internet gaming (hrs/wk) = Internet gaming hours per week; BAI = Back Anxiety Inventory; BDI = Beck Depression Inventory; Mean number of trials (per session) = the average number of trials per session; Mean number of G (per session) = the average number of successful go trials per session; Mean number of F (per session) = the average number of failed go trials per session; Mean number of SS (per session) = the average number of successful stop trials per session; Mean number of SE (per session) = the average number of stop error trials per session; GS (%) and SS (%) = percentage of successful go and stop trials; Mean GoRT (ms) = the mean reaction time of successful go trials; PES effect (ms) = the RT difference between the go trials that followed a stop error (pSE) and those that followed another go trial (pG); Critical SSD = Critical stop-signal delay; SSRT = Stop-signal reaction time; Sequential effect= Pearson correlation between P(Stop) and RT on go trials for individual subjects; Mean FD = the frame-wise displacement (FD) of head position.
U value.
the number of participants.
the rate of alcohol and cigarette use.
χ2 value.
Group differences in neural response
The activity of two functional networks during post-error slowing and two functional networks during Bayesian learning anticipation of stop signal differed between IGD and HC. Functional networks were labelled based on the primary regions of positive spatial integration in each network according to the known intrinsic connectivity of the networks (Allen et al., 2011; Laird et al., 2011; Smith et al., 2009).
Altered functional networks in post-error slowing
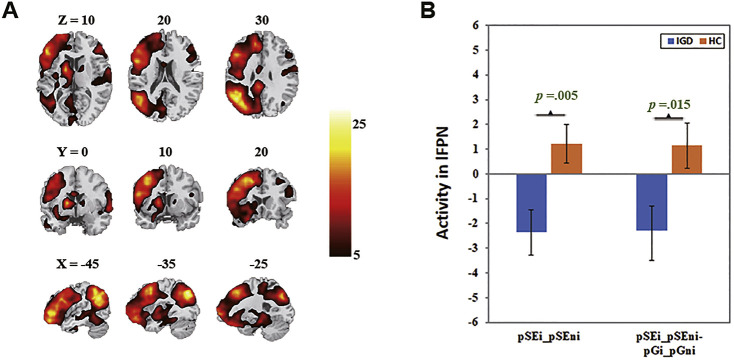
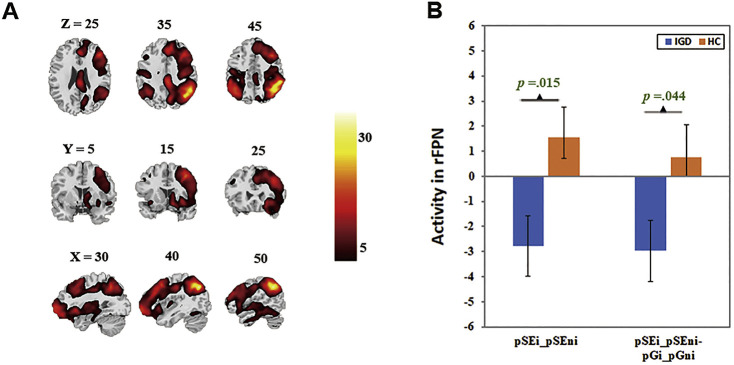
The left (Fig. 1) and right fronto-parietal network (Fig. 2) encompassed the dorsolateral prefrontal cortices and posterior parietal cortex. Beta weights in these networks were lower for the ‘pSEi-pSEni’ contrast (lFPN: t(1,40) = −2.97, p = 0.005, Cohen’s d = −0.92, r = −0.42, power = 0.90; rFPN: t(1,40) = −2.54, p = 0.015, Cohen’s d = −0.78, r = −0.36, power = 0.80) and for the ‘(pSEi-pSEni)- (pGi-pGni)’ contrast (lFPN: t(1,40) = −2.55, p = 0.015, Cohen’s d = −0.79, r = −0.37, power = 0.81; rFPN: t(1,40) = −2.08, p = 0.044, Cohen’s d = −0.64, r = −0.31, power = 0.65) in IGD, as compared to HC. Exploratory results showed no significant finding with Bonferroni correction.
Fig. 1.
The left fronto-parietal network (lFPN). A: Spatial map displayed at a threshold at p = 0.00001; B: Lower engagement in lFPN during post-error slowing in IGD
Fig. 2.
The right fronto-parietal network (rFPN). A: Spatial map displayed at a threshold at p = 0.00001; B: Lower engagement in rFPN during post-error slowing in IGD
Altered functional networks responding to P(Stop)
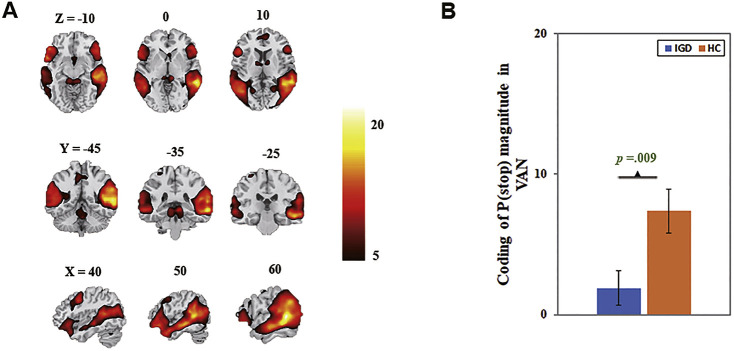
The ventral attention network, comprising the temporo-parietal junction, insula, supplementary motor area, inferior frontal gyrus and inferior parietal lobule, showed lower beta weight for [f x P(Stop)] in IGD relative to HC (t(1,40) = −2.76, p = 0.009, Cohen’s d = −0.85, r = −0.39, power = 0.86; Fig. 3).
Fig. 3.
The ventral attention network (VAN) coding of P(Stop). A: Spatial map displayed at a threshold at p = 0.00001; B: Lower engagement in ventral attention network coding of P(Stop) in IGD
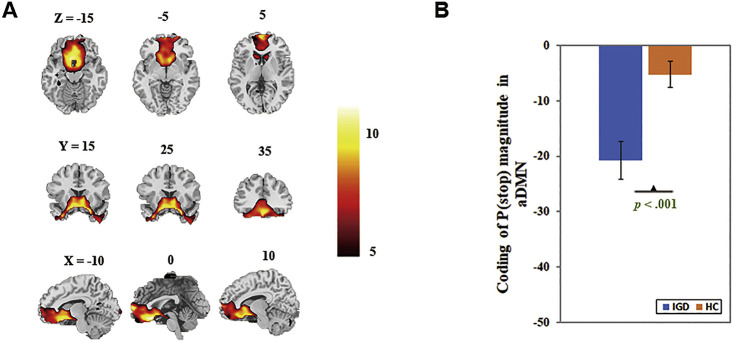
Exploratory results showed that the beta weight for [f x P(Stop)] of anterior default mode network (aDMN), corresponding to IC25 in Allen et al. (2011) and comprising the medial frontal gyrus, was decreased in IGD relative to HC (Bonferroni corrected, t(1,40) = −3.76, p < 0.001, Cohen’s d = −1.16, r = −0.50, power = 0.98; Fig. 4).
Fig. 4.
The coding of P(Stop) in anterior default mode network (aDMN). A: Spatial map displayed at a threshold at p = 0.00001; B: Higher disengagement in aDMN coding of P(Stop) in IGD
Because IGD reported higher levels of anxiety and higher proportion of alcohol use, we also examined group differences with anxiety score and alcohol use state (1= use, 0 = no use) as covariates. The results were similar with current findings (Supplementary Information Table S1).
Discussion
To our knowledge, this is the first report to identify alterations in functional network activities for proactive control in IGD, a behavioral addiction independent of drug effects. We found, despite indistinguishable behavioral performance, lower engagement of the fronto-parietal network during post-error slowing and weaker activity in the ventral attention network and the anterior default mode network during Bayesian learning in IGD as compared to HC. These results suggested altered neural activity not only during post-error behavioral adjustment, but also during probabilistic expectations of the stop signal. We highlighted the main findings in discussion.
Altered functional networks in post-error slowing
The left and right fronto-parietal network showed lower engagement during PES in IGD. Frontal and parietal cortices respond to cued attention allocation (Luks, Simpson, Dale, & Hough, 2007) and preparatory control of response switching (Rushworth, Paus, & Sipila, 2001). An extensive body of work also provides evidence that activation of middle frontal regions reflects response adjustment strategies to balance the opposing go and stop demands (Chevrier, Noseworthy, & Schachar, 2007), following conflicts or errors (Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). Further, fronto-parietal circuit (e.g. dorsolateral prefrontal cortices) dysfunction compromised post-signal behavioral adjustment in cocaine and alcohol addicted individuals (Chevrier et al., 2007; Hester, Simoes-Franklin, & Garavan, 2007; Li et al., 2009).
The lFPN and rFPN may support different functional dimensions of cognitive control. Previous studies have proposed that the rFPN is involved in high attentional load stop trials, hence in support of the restraint of actions, while lFPN is involved in low attentional load go trials, hence in support of the cancellation of actions, in the SST (Schachar et al., 2007; Stevens, Kiehl, Pearlson, & Calhoun, 2009; Xu, Calhoun, Pearlson, & Potenza, 2014). Indeed, consistent with the temporal organization of these functional roles, Zhang and Li (2012) also found that the activity of the lFPN significantly lagged in time behind the rFPN during the SST.
Thus, decreased activity in the fronto-parietal network in IGD may indicate that individuals with IGD are unable to utilize this network to adjust response strategies to balance the opposing demands of the go and stop trials to restrain action and to inhibit a motor response following errors.
Altered functional networks responding to P(Stop)
The ventral attention network
IGD showed attenuated activity for Bayesian learning in the ventral attention network. The VAN responds to changes in events and reorients attention to the stop signal (Congdon et al., 2010; Zhang & Li, 2012). Previous studies have implicated many areas in the VAN in updating the probability of stop signal to adjust behavior and compromised activities in these areas in addiction (Hu, Ide, Zhang, Sinha, et al., 2015; Shi et al., 2019). For example, occasional stimulant users demonstrated subtle deficits in anticipation of a stop signal in the inferior frontal gyrus (Harle et al., 2014). Schlosser et al. (2009) reported that methylphenidate attenuated inferior parietal lobule response to uncertainty when making decisions according to sensory information. Thus, the disruption of the VAN suggests lower efficiency of bottom-up signal filtering, resulting in difficulties to adapt behavioral responses to environmental needs (Collantoni et al., 2016).
The anterior default mode network
The anterior default mode network showed greater disengagement during stop signal anticipation. The aDMN was deactivated, consistent with earlier findings of downregulation during a cognitively demanding task (Greicius, Krasnow, Reiss, & Menon, 2003). However, aDMN is also involved in bottom-up cue-elicited attentional processes during cognitive control (Bonini et al., 2014), particularly in response to transiently presented stimuli and stimulus complexity (McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003), and self-processing (Molnar-Szakacs & Uddin, 2013). As a critical area of the aDMN, the medial frontal cortices, showed increasing activation to stop signal likelihood (Hu, Ide, Zhang, & Li, 2015; Vink et al., 2005).
Altered intra-aDMN functional connectivity may be associated with impaired awareness (Blumenfeld et al., 2003), and diminished self-referential thinking may hinder behavioral regulation after unexpected feedback (Patterson & Newman, 1993). In addition, altered activity and functional connectivity of regions within this network (e.g. the ventromedial prefrontal cortex) were also found during error processing and resting-state in addictive individuals (Sutherland, McHugh, Pariyadath, & Stein, 2012; Worhunsky et al., 2013; Zhang et al., 2014). Therefore, the greater disengagement of aDMN found in the present study may reflect less attentional and monitoring/reflectivity processing to unexpected cues and, as a result, suboptimal readiness to withhold response, in IGD.
General discussion
The current findings are in accordance with a previous review of the SST, which implicated the fronto-parietal network in action withholding/cancellation and the ventral attention network in interference resolution (Zhang et al., 2017). Our findings were also consistent with the previous findings that substance dependent participants showed failure to use con-textual information to update the probability of the stop signal (Harle et al., 2014; Ide et al., 2015) and to adjust behavior after errors (Hester et al., 2007; Li et al., 2009). It may suggest that these impairments might be general across different types of addiction.
IGD in this study reported higher levels of anxiety and alcohol use than HC, though none met criteria for an anxiety or alcohol use disorder as assessed with the Chinese version of the MINI International Neuropsychiatric Interview (Si et al., 2009). We controlled anxiety score and drink state in data analyses; however, the potential influence of anxiety and alcohol use on the present findings cannot be ruled out entirely. Based on problem behavior theory and previous studies, comorbidities of anxiety and alcohol use are common in IGD (Demirci, Akgönül, & Akpinar, 2015; Ho et al., 2014; Ko et al., 2008; Tonioni et al., 2012). We hope the current findings would reflect the broad populations of IGD.
The results of conventional GLM did not survive the corrected threshold. However, the main regions revealed with voxel-wise p < 0.005 (uncorrected) were consistent with the ICA findings (Supplementary Information Table S2). ICA identifies regional signals that contribute to functional networks, whereas GLM examines the aggregate signal in a particular region. It is worth noting that different functional networks may share the same brain regions, suggesting that individual brain regions may serve concurrent but different or even opposite functional roles when engaged as part of different functional networks (Xu et al., 2013). Thus, ICA is able to identify alterations in task-related, concurrent, but opposite changes in time courses in the same brain regions that may not be detected by GLM-based analyses (Xu et al., 2015). Furthermore, this highlights the complexity of regional activations and the utility of ICA in considering regional interactions to provide nuanced understanding of the function of a brain region.
Limitations of the study
Some limitations need to be considered for the current study. Firstly, we recruited only male participants in the study, which limits the generalizability of the current findings to female IGD. Secondly, IGD participants in the present study represented a sample of students enrolled in Chinese universities and thus the findings may not be generalized to a broader clinical population. Thirdly, although the results showed acceptable effect size and post-hoc power, the current findings remain to be verified in the future, considering the limited sample size and relatively small trial number of each participant. Finally, this is a cross-sectional study, and whether the alterations in functional network engagement represent a consequence of IGD or a risk factor of IGD remains to be determined.
Conclusions
The current study investigated functional network activities for behavioral adjustment and Bayesian learning from the changing contexts in IGD. We found decreased engagement in functional networks for post-error response restraint and behavioral adjustment; and weaker activity in functional networks for attentional monitoring and sensory salience evaluation during Bayesian learning for behavioral adjustment in IGD. Together, IGD showed not only deficits in trial-by-trial behavioral adjustments, but also a broader impairment to use contextual information to expect and update the adjustment demand. It remains to be seen whether these findings would be shared across drug and behavioral addictions.
Funding sources
This study was supported by the National Natural Science Foundation of China (No. 31871122, 31170990, and 31700966) and Open Project grant from the State Key Laboratory of Cognitive Neuroscience and Learning (to Sheng, Zhang).
Authors’ contribution
JTZ, and XYF were responsible for the study concept and design. SSM, LL, and YWY contributed to the data acquisition. SSM, PW, and SZ assisted with data analysis and interpretation of findings. SSM drafted the manuscript. JTZ, CSRL, NZ, and XYF provided critical revision of the manuscript. All authors critically reviewed the content and approved final version for publication.
Conflict of interest
The authors have no conflict of interest.
Supplementary Material
Contributor Information
Jin-Tao Zhang, Email: zhangjintao@bnu.edu.cn.
Xiao-Yi Fang, Email: fangxy@bnu.edu.cn.
References
- Allen, E. A., Erhardt, E. B., Damaraju, E., Gruner, W., Segall, J. M., Silva, R. F., et al. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience, 5, 2. 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Angela, J. Y., & Cohen, J. D. (2009). Sequential effects: Superstition or rational behavior? Paper presented at the advances in neural information processing systems. [PMC free article] [PubMed] [Google Scholar]
- Argyriou, E., Davison, C. B., & Lee, T. T. C. (2017). Response inhibition and internet gaming disorder: A meta-analysis. Addictive Behaviors, 71, 54–60. 10.1016/j.addbeh.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Beck, A. T., Brown, G., Epstein, N., & Steer, R. A. (1988). An inventory for measuring clinical anxiety – Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. 10.1037/0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck, A. T., Erbaugh, J., Ward, C. H., Mock, J., & Mendelsohn, M. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4(6), 561-&. [DOI] [PubMed] [Google Scholar]
- Blumenfeld, H., Westerveld, M., Ostroff, R. B., Vanderhill, S. D., Freeman, J., Necochea, A., et al. (2003). Selective frontal, parietal, and temporal networks in generalized seizures. Neuroimage, 19(4), 1556–1566. 10.1016/S1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- Bombeke, K., Schouppe, N., Duthoo, W., & Notebaert, W. (2013). The effect of alcohol and placebo on post-error adjustments. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini, F., Burle, B., Liegeois-Chauvel, C., Regis, J., Chauvel, P., & Vidal, F. (2014). Action monitoring and medial frontal cortex: Leading role of supplementary motor area. Science, 343(6173), 888–891. 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- Chen, C. Y., Huang, M. F., Yen, J. Y., Chen, C. S., Liu, G. C., Yen, C. F., et al. (2015). Brain correlates of response inhibition in Internet gaming disorder. Psychiatry and Clinical Neurosciences, 69(4), 201–209. 10.1111/pcn.12224. [DOI] [PubMed] [Google Scholar]
- Chevrier, A. D., Noseworthy, M. D., & Schachar, R. (2007). Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Human Brain Mapping, 28(12), 1347–1358. 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe, J., Jimura, K., Hirose, S., Yamashita, K., Miyashita, Y., & Konishi, S. (2009). Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. Journal of Neuroscience, 29(50), 15870–15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H., Kwon, M., Choi, J.-H., Lee, S.-K., Choi, J. S., Choi, S.-W., et al. (2014). Development of the Internet addiction scale based on the Internet Gaming Disorder criteria suggested in DSM-5. Addictive Behaviors, 39(9), 1361–1366. [DOI] [PubMed] [Google Scholar]
- Collantoni, E., Michelon, S., Tenconi, E., Degortes, D., Titton, F., Manara, R., et al. (2016). Functional connectivity correlates of response inhibition impairment in anorexia nervosa. Psychiatry Research-Neuroimaging, 247, 9–16. [DOI] [PubMed] [Google Scholar]
- Congdon, E., Mumford, J. A., Cohen, J. R., Galvan, A., Aron, A. R., Xue, G., et al. (2010). Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage, 53(2), 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw, N. D., & Doya, K. (2006). The computational neurobiology of learning and reward. Current Opinion in Neurobiology, 16(2), 199–204. 10.1016/j.conb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Demirci, K., Akgönül, M., & Akpinar, A. (2015). Relationship of smartphone use severity with sleep quality, depression, and anxiety in university students. Journal of Behavioral Addictions, 4(2), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton, A., Young, J., Smitherman, S., Gross, R. E., Mletzko, T., & Kilts, C. D. (2014). Neural network activation during a stop-signal task discriminates cocaine-dependent from non-drug-abusing men. Addiction Biology, 19(3), 427–438. 10.1111/adb.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258. 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harle, K. M., Shenoy, P., Stewart, J. L., Tapert, S. F., Yu, A. J., & Paulus, M. P. (2014). Altered neural processing of the need to stop in young adults at risk for stimulant dependence. Journal of Neuroscience, 34(13), 4567–4580. 10.1523/Jneurosci.2297-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester, R., Simoes-Franklin, C., & Garavan, H. (2007). Post-error behavior in active cocaine users: Poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology, 32(9), 1974–1984. 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Himberg, J., Hyvärinen, A., & Esposito, F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage, 22(3), 1214–1222. [DOI] [PubMed] [Google Scholar]
- Ho, R. C., Zhang, M. W. B., Tsang, T. Y., Toh, A. H., Pan, F., Lu, Y. X., et al. (2014). The association between internet addiction and psychiatric co-morbidity: A meta-analysis. BMC Psychiatry, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S., Ide, J. S., Zhang, S., & Li, C. S. R. (2015). Anticipating conflict: Neural correlates of a Bayesian belief and its motor consequence. Neuroimage, 119, 286–295. 10.1016/j.neuroimage.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S., Ide, J. S., Zhang, S., & Li, C. R. (2016). The right superior frontal gyrus and individual variation in proactive control of impulsive response. Journal of Neuroscience, 36(50), 12688–12696. 10.1523/JNEUROSCI.1175-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S., Ide, J. S., Zhang, S., Sinha, R., & Li, C. S. R. (2015). Conflict anticipation in alcohol dependence – A model-based fMRI study of stop signal task. Neuroimage-Clinical, 8, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide, J. S., Hu, S. E., Zhang, S., Yu, A. J., & Li, C. S. R. (2015). Impaired Bayesian learning for cognitive control in cocaine dependence. Drug and Alcohol Dependence, 151, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide, J. S., Shenoy, P., Yu, A. J., & Li, C. S. (2013). Bayesian prediction and evaluation in the anterior cingulate cortex. Journal of Neuroscience, 33(5), 2039–2047. 10.1523/JNEUROSCI.2201-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka, S. R., Scott, G., Ham, T., Pickering, A., Bonnelle, V., Braga, R. M., et al. (2014). Damage to the salience network and interactions with the default mode network. Journal of Neuroscience, 34(33), 10798–10807. 10.1523/JNEUROSCI.0518-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, C. H., Yen, J. Y., Chen, S. H., Wang, P. W., Chen, C. S., & Yen, C. F. (2014). Evaluation of the diagnostic criteria of Internet gaming disorder in the DSM-5 among young adults in Taiwan. Journal of Psychiatric Research, 53, 103–110. 10.1016/j.jpsychires.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Ko, C.-H., Yen, J.-Y., Chen, S.-H., Yang, M.-J., Lin, H.-C., & Yen, C.-F. (2009). Proposed diagnostic criteria and the screening and diagnosing tool of Internet addiction in college students. Comprehensive Psychiatry, 50(4), 378–384. [DOI] [PubMed] [Google Scholar]
- Ko, C. H., Yen, J. Y., Yen, C. F., Chen, C. S., Weng, C. C., & Chen, C. C. (2008). The association between internet addiction and problematic alcohol use in adolescents: The problem behavior model. Cyberpsychology and Behavior, 11(5), 571–576. [DOI] [PubMed] [Google Scholar]
- Laird, A. R., Fox, P. M., Eickhoff, S. B., Turner, J. A., Ray, K. L., McKay, D. R., et al. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, A. J., Luty, J., Bogdan, N. A., Sahakian, B. J., & Clark, L. (2009). Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology, 207(1), 163–172. 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. O., Adali, T., & Calhoun, V. D. (2007). Estimating the number of independent components for functional magnetic resonance Imaging data. Human Brain Mapping, 28(11), 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. S. R., Huang, C., Yan, P. S., Paliwal, P., Constable, R. T., & Sinha, R. (2008). Neural correlates of post-error slowing during a stop signal task: A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience, 20(6), 1021–1029. 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C.-S. R., Luo, X., Yan, P., Bergquist, K., & Sinha, R. (2009). Altered impulse control in alcohol dependence: Neural measures of stop signal performance. Alcoholism: Clinical and Experimental Research, 33(4), 740–750. 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. S., Milivojevic, V., Kemp, K., Hong, K., & Sinha, R. (2006). Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug and Alcohol Dependence, 85(3), 205–212. 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Liao, D. L., Huang, C. Y., Hu, S., Fang, S. C., Wu, C. S., Chen, W. T., et al. (2014). Cognitive control in opioid dependence and methadone maintenance treatment. PloS One, 9(4), e94589. 10.1371/journal.pone.0094589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks, T. L., Simpson, G. V., Dale, C. L., & Hough, M. G.(2007). Preparatory allocation of attention and adjustments in conflict processing. Neuroimage, 35(2), 949–958. 10.1016/j.neuroimage.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan, K. A., Kaufman, J. N., Kucera-Thompson, J., & Binder, J. R. (2003). A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience, 15(3), 394–408. 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs, I., & Uddin, L. Q. (2013). Self-processing and the default mode network: Interactions with the mirror neuron system. Frontiers in Human Neuroscience, 7, 571. 10.3389/Fnhum.2013.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, C. M., & Newman, J. P. (1993). Reflectivity and learning from aversive events – Toward a psychological mechanism for the syndromes of disinhibition. Psychological Review, 100(4), 716–736. 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Reed, P., Vile, R., Osborne, L. A., Romano, M., & Truzoli, R. (2015). Problematic internet usage and immune function. PloS One, 10(8), e0134538. 10.1371/journal.pone.0134538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof, K. R., van den Wildenberg, W. P. M., Segalowitz, S. J., & Carter, C. S. (2004). Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56(2), 129–140. 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rushworth, M. F. S., Paus, T., & Sipila, P. K.(2001). Attention systems and the organization of the human parietal cortex. Journal of Neuroscience, 21(14), 5262–5271. 10.1523/Jneurosci.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar, R., Logan, G. D., Robaey, P., Chen, S., Ickowicz, A., & Barr, C. (2007). Restraint and cancellation: Multiple inhibition deficits in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology, 35(2), 229–238. 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Schlosser, R. G. M., Nenadic, I., Wagner, G., Zysset, S., Koch, K., & Sauer, H. (2009). Dopaminergic modulation of brain systems subserving decision making under uncertainty: A study with fMRI and methylphenidate challenge. Synapse, 63(5), 429–442. [DOI] [PubMed] [Google Scholar]
- Schonberg, T., Daw, N. D., Joel, D., & O’Doherty, J. P. (2007). Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. Journal of Neuroscience, 27(47), 12860–12867. 10.1523/Jneurosci.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy, P., & Yu, A. J. (2011). Rational decision-making in inhibitory control. Frontiers in Human Neuroscience, 5, 48. 10.3389/Fnhum.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L., Lou, W. T., Wong, A., Zhang, F., Abrigo, J., Chu, W. C. W., et al. (2019). Neural evidence for long-term marriage shaping the functional brain network organization between couples. Neuroimage, 199, 87–92. 10.1016/j.neuroimage.2019.05.058. [DOI] [PubMed] [Google Scholar]
- Si, T.-M., Shu, L., Dang, W.-M., Se, Y.-A., Chen, J.-X., Dong, W.-T., et al. (2009). Evaluation of the reliability and validity of Chinese version of the Mini-International Neuropsychiatric Interview in patients with mental disorders. Chinese Mental Health Journal, 23(7), 493–503. [Google Scholar]
- Smith, S. M., Fox, P. T., Miller, K. L., Glahn, D. C., Fox, P. M., Mackay, C. E., et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M. C., Kiehl, K. A., Pearlson, G. D., & Calhoun, V. D. (2009). Brain network dynamics during error commission. Human Brain Mapping, 30(1), 24–37. 10.1002/hbm.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, M. T., McHugh, M. J., Pariyadath, V., & Stein, E. A. (2012). Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage, 62(4), 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonioni, F., D’Alessandris, L., Lai, C., Martinelli, D., Corvino, S., Vasale, M., et al. (2012). Internet addiction: Hours spent online, behaviors and psychological symptoms. General Hospital Psychiatry, 34(1), 80–87. [DOI] [PubMed] [Google Scholar]
- Verbruggen, F., Stevens, T., & Chambers, C. D. (2014). Proactive and reactive stopping when distracted: An attentional account. Journal of Experimental Psychology: Human Perception and Performance, 40(4), 1295–1300. 10.1037/a0036542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink, M., Kahn, R. S., Raemaekers, M., van den Heuvel, M., Boersma, M., & Ramsey, N. F. (2005). Function of striatum beyond inhibition and execution of motor responses. Human Brain Mapping, 25(3), 336–344. 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Hu, S., Ide, J. S., Zhornitsky, S., Zhang, S., Yu, A. J., et al. (2018). Motor preparation disrupts proactive control in the stop signal task. Frontiers in Human Neuroscience, 12, 151. 10.3389/fnhum.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer, G. E., Daw, N. D., & Shohamy, D. (2012). Generalization of value in reinforcement learning by humans. European Journal of Neuroscience, 35(7), 1092–1104. 10.1111/j.1460-9568.2012.08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky, P. D., Stevens, M. C., Carroll, K. M., Rounsaville, B. J., Calhoun, V. D., Pearlson, G. D., et al. (2013). Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychology of Addictive Behaviors, 27(2), 477–488. 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2020). ICD-11 for mortality and morbidity statistics (ICD-11 MMS). [Google Scholar]
- Xu, J. S., Calhoun, V. D., Pearlson, G. D., & Potenza, M. N. (2014). Opposite modulation of brain functional networks implicated at low vs. high demand of attention and working memory. PloS One, 9(1), e87078. 10.1371/journal.pone.0087078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. S., Calhoun, V. D., & Potenza, M. N. (2015). The absence of task-related increases in BOLD signal does not equate to absence of task-related brain activation. Journal of Neuroscience Methods, 240, 125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. S., Zhang, S., Calhoun, V. D., Monterosso, J., Li, C. S. R., Worhunsky, P. D., et al. (2013). Task-related concurrent but opposite modulations of overlapping functional networks as revealed by spatial IA. Neuroimage, 79, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. G., Wang, X. D., Zuo, X. N., & Zang, Y. F. (2016). DPABI: Data processing & analysis for (Resting-State) brain imaging. Neuroinformatics, 14(3), 339–351. [DOI] [PubMed] [Google Scholar]
- Young, K. (2016). Internet addiction test (IAT): Stoelting. [Google Scholar]
- Zhang, R. B., Geng, X. J., & Lee, T. M. C. (2017). Large-scale functional neural network correlates of response inhibition: An fMRI meta-analysis. Brain Structure and Function, 222(9), 3973–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Hu, S., Bednarski, S. R., Erdman, E., & Li, C. S. R. (2014). Error-related functional connectivity of the thalamus in cocaine dependence. Neuroimage-Clinical, 4, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., & Li, C. S. R. (2012). Functional networks for cognitive control in a stop signal task: Independent component analysis. Human Brain Mapping, 33(1), 89–104. 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]