Abstract
Ornithine decarboxylase (ODC; EC 4.1.1.17) catalyzes the conversion of ornithine to putrescine, the rate-limiting first step for de novo polyamine biosynthesis. Previously, we reported that genetic knockdown of xanthine dehydrogenase 1 (XDH1) - a gene encoding the enzyme involved in the last two steps of uric acid synthesis - causes an increase of ODC transcript levels in fat body of blood-fed Aedes aegypti mosquitoes, suggesting a crosstalk at molecular level between XDH1 and ODC during nitrogen disposal. To further investigate the role of ODC in nitrogen metabolism, we conducted several biochemical and genetic analyses in sugar- and blood-fed Ae. aegypti females. Distinct ODC gene and protein expression patterns were observed in mosquito tissues dissected during the first gonotrophic cycle. Both pharmacological and RNA interference-mediated knockdown of ODC negatively impacted mosquito survival, disrupted nitrogen waste disposal, delayed oviposition onset, and decreased fecundity in vitellogenic blood-fed females. A lag in the expression of two major digestive serine proteases, a reduction of blood meal digestion in the midgut, and a decrease of vitellogenin yolk protein uptake in ovarian follicles were observed by western blots in ODC-deficient females. Moreover, genetic silencing of ODC showed a broad transcriptional modulation of genes encoding proteins involved in multiple metabolic pathways in mosquito fat body, midgut and Malpighian tubules prior to and after blood feeding. All together, these data demonstrate that ODC plays an essential role in mosquito metabolism, and that ODC cross-talks with multiple genes and proteins to prevent deadly nitrogen perturbations in Ae. aegypti females.
Keywords: ammonia metabolism, glucose metabolism, survival, polyamines, oxidative stress
1. INTRODUCTION
Polyamines are aliphatic polycations present in all eukaryotes and most prokaryotes. They interact with negatively charged macromolecules such as DNA, RNA and proteins (1), and participate in a wide variety of cellular processes including gene expression, protein synthesis, cellular oxidative homeostasis, cell growth and proliferation (2–5). In aging and several diseases, such as cancer and neurodegenerative disorders, variations in polyamine concentrations have been exploited for therapeutic interventions (6–8). Alterations in polyamine levels have also been investigated as potential targets for drug discovery in viruses, bacteria and protozoan parasites associated with human diseases (9–13).
Ornithine decarboxylase (ODC; EC 4.1.1.17) catalyzes the conversion of ornithine to putrescine, the rate-limiting first step for de novo polyamine biosynthesis. In mammalian cells, ODC is tightly regulated at multiple levels, which include transcription, translation, post-translational modification and protein degradation (14). A family of polyamine-induced proteins called antizymes and their inhibitors are also important regulators of ODC activity (14, 15).
ODC and polyamines have been implicated in different physiological processes including vitellogenesis, lipid metabolism, locomotor function and aging in fruit flies (16–21); oviposition behavior in crickets (22); olfactory function in diamondback moths (23); and cell cycle progression in cultured silkworm cells (24). It was also reported that chemical inhibition of ODC disrupted polyamine synthesis, as evidenced by the decreased conversion of radiolabeled ornithine into putrescine, spermidine and spermine in Aedes aegypti mosquitoes following DL-α difluoromethylornithine (DFMO) treatment (25). Inhibition of ODC activity by DFMO also reduced trypsin activity and impaired the synthesis of DNA, RNA and vitellogenin (Vg) in mosquitoes (25). More recently, a link between ODC and nitrogen waste disposal was discovered in blood-fed Ae. aegypti females. Specifically, ODC mRNA level was found to be up-regulated in mosquito fat body in response to the genetic silencing of xanthine dehydrogenase-1 (XDH1), an enzyme that catalyzes the last two steps of uric acid synthesis (26).
In this study, ODC expression patterns at mRNA and protein levels were first determined in Ae. aegypti tissues prior to and after blood feeding. Then, the effects of the pharmacological inhibition of ODC with its inhibitor (DFMO) were evaluated on survival, nitrogen waste disposal, oviposition onset and fecundity in Ae. aegypti females. ODC function was also examined in Ae. aegypti females injected with double-stranded RNA (dsRNA) against ODC. Disruption of ODC by either DFMO or RNA interference (RNAi) impaired survival, nitrogen metabolism and reproduction in Ae. aegypti mosquitoes. Alterations in the protein expression patterns of midgut digestive serine proteases and Vg, in addition to changes in transcript levels of multiple genes, were observed in several ODC-deficient mosquito tissues. Our data provide evidence that ODC is required to maintain nitrogen homeostasis in Ae. aegypti females.
2. METHODS
2.1. Chemicals, reagents and antibodies
Oligo-(dT)20 primer and reverse transcriptase were purchased from Promega (Madison, WI, USA). TRIzol® Reagent was obtained from Thermo Fisher Scientific Inc. (Waltham, MA, USA) and the reagents for quantitative real-time PCR (qPCR) were purchased from Quanta Biosciences (Gaithersburg, MD, USA). Bovine blood was acquired from Pel-Freeze Biologicals (Rogers, AR, USA). A uric acid kit was obtained from Pointe Scientific (Canton, MI, USA). DFMO and other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Custom-made rabbit polyclonal antibodies against Ae. aegypti ODC (AaODC antigen sequence: YPQPRLIGQQSGKT) and Vg yolk protein (AaVg antigen sequence: YSYNMPSDKKNYVR) were obtained from GenScript USA Inc. (Piscataway, NJ, USA). Previously custom-synthesized rabbit polyclonal antibodies against two Ae. aegypti midgut serine proteases, Aa5G1 (antigen sequence: APVKLPQKDAPVNEGT by Proteintech Group Inc, Chicago, IL, USA) and AaLT (antigen sequence: GAVDFEDTTNDGRV by GenScript) were also used (27). The α-tubulin monoclonal antibody (12G10) was acquired from Developmental Studies Hybridoma Bank (The University of Iowa, Iowa City, IA, USA). Secondary antibodies, IRDye 800CW goat anti-rabbit and IRDye 680RD donkey anti-mouse, were purchased from LI-COR Biosciences (Lincoln, NE, USA).
2.2. Mosquito rearing and feeding
Ae. aegypti (National Institutes of Health-Rockefeller strain) colony was reared and maintained under standard laboratory conditions as previously described (28, 29). Mosquitoes were kept in Percival Intellus I-41VL incubators (Percival Scientific, Inc., Perry, IA, USA) and in a CARON 6015 Insect Growth Chamber connected to a CARON CRSY 102 condensate recirculating system (Caron Products and Services, Inc., Marietta, OH, USA) at 28°C and 75% relative humidity and with a photoperiod of 16:8 h light-dark cycle. Adult mosquitoes were maintained on 3% sucrose-soaked cotton balls. Unless otherwise stated, four-day old females were provided with bovine blood supplemented with 5 mM ATP (Blood meal, BM) or BM supplemented with 100 mM DFMO for 15 min via artificial membrane feeders connected to a 37°C water bath. Only fully engorged females were used for experiments. These females were kept in groups or individually placed into 20 ml polyethylene scintillation vials covered with nylon mesh and secured with rubber bands. All mosquitoes were allowed to feed on 3% sucrose-soaked cotton balls throughout the experiments. For each experiment, at least three replicates were performed with three separate biological cohorts of mosquitoes.
2.3. Microinjection of dsRNA and qPCR assays
A gene encoding ODC was previously identified by bioinformatics analysis in Ae. aegypti (26). The dsRNAs targeting AaODC gene or firefly luciferase (FL) gene (control) were designed and synthesized as previously described for other genes (30). Oligonucleotide primer sequences for dsRNA synthesis are shown in Table 1. Briefly, the 23 base T7 RNA polymerase promoter sequence (TAATACGACTCACTATAGGGAGA) was linked to the 5’ end of each dsRNA primer (Table 1), and PCR was performed using Taq 2X Master Mix (New England Biolabs, Ipswich, MA). In vitro dsRNA synthesis was performed using the HiScribe™ T7 RNA Synthesis kit (New England Biolabs, Ipswich, MA) in an overnight reaction at 37°C following the manufacturer’s instructions. The purified dsRNA was resuspended in nuclease-free water at 7.5 μg/μl and stored at −80°C. Ae. aegypti females were microinjected twice with 2.0 μg dsRNA using a Nanoject II microinjector (Drummond Scientific Company, Broomall, PA, USA). The first injection was done within 4 h of female eclosion, whereas the second injection was completed at 4 days post-eclosion. Injected mosquitoes were provided with BM at 7 days post-eclosion and maintained on 3% sucrose-soaked cotton balls throughout the experiments.
Table 1.
Gene-specific primers used for RNAi and qPCR
| Genes | Accession No./ | Primer sequence (5’ to 3’) | PCR size | |
|---|---|---|---|---|
| Vectorbase ID | ||||
| Gene-specific primers used for RNAi | ||||
| Firefly Luciferase, FL | U47295 | Forward | AGCACTCTGATTGACAAATACGA | 548 bp |
| pGL3-Basic Vector | Reverse | AGTTCACCGGCGTCATCGTC | ||
| Ornithine decarboxylase, ODC | AAEL007880 | Forward | ACCGAGCTGGTTCAGGTCGT | 580 bp |
| Reverse | AGCAACCTGATCCTACGTG | |||
| Gene-specific primers used for qPCR | ||||
| Alanine aminotransferase 1, ALT1 | AAEL009875 | Forward | CAACTGCCGGAGAAGGCAAT | 140 bp |
| Reverse | TGATAGGTTCCATCCTTCTGACC | |||
| Alanine aminotransferase 2, ALT2 | AAEL009872 | Forward | TTCTATGCATTCCAGCTGTTAGAGCA | 148 bp |
| Reverse | ACGCCCGGAACATGTCGAG | |||
| Rhesus 50 glycoprotein, Rh50–1 | AAEL008046 | Forward | CTGAGCGAGGAAGATATGCACGA | 130 bp |
| Reverse | CTGTGAAGAGACCGCCTACGAT | |||
| Rhesus 50 glycoprotein, Rh50–2 | AAEL023409 | Forward | CCTGCGATGAAACCGAACAC | 131 bp |
| Reverse | GCGATAACGACCGTGAGCAAGAT | |||
| Arginase | AAEL002675 | Forward | AAGGAATTGGCGGATTACTGGC | 143 bp |
| Reverse | GCGTGGATACCGAACTTCTCAAT | |||
| Catalase 1, CAT1 | AAEL013407 | Forward | GCTGCTCGTGAGCGCATGAT | 116 bp |
| Reverse | CGGCGTCCAAAGTCAGCATC | |||
| Catalase 2, CAT2 | AAEL013407 | Forward | GATGAGGCTGCTCGCAAGCG | 119 bp |
| Reverse | CGTCCAAAGTCAGCATCGAC | |||
| Glucose-6-phosphate dehydrogenase, G6PDH | AAEL009507 | Forward | TCACAGATGCACTTTGTCCGAGC | 125 bp |
| Reverse | GGTCCACGAGAACCGTGCA | |||
| Glutamate dehydrogenase, GDH | AAEL010464 | Forward | GGCGAGAACCTGATGTACGA | 300 bp |
| Reverse | GAGCAGGTGGTAGTTGGACT | |||
| Glutamate synthase, GltS | AAEL014768 | Forward | CTCCTACAATACGGCATTCCAAC | 276 bp |
| Reverse | CGTGATCCGAGTAACTTCTTCTG | |||
| Glutamine synthetase 1, GS1 | AAEL001887 | Forward | CCTCTATGCTGGAGTTGACT | 237 bp |
| Reverse | CGCATCTGCTTGGTGGAGA | |||
| Glutamine synthetase 2, GS2 | AAEL013458 | Forward | GAGGAGTTTGGCATCGTTG | 181 bp |
| Reverse | GGTCGTAGGCTCGGATATG | |||
| Ornithine aminotransferase, OAT | AAEL05289 | Forward | GAGTTCGTCACCGACCTGTT | 126 bp |
| Reverse | GTTCTCCGGCACCTTCTTCAC | |||
| Ornithine decarboxylase, ODC | AAEL007880 | Forward | CGCAGCATGAACCTAGACGT | 119 bp |
| Reverse | TGCTTGGCGTAGTCGAACAG | |||
| Proline dehydrogenase, PDH | AAEL013431 | Forward | TGCAGAAGATCAAGAAGGAGAAAC | 111 bp |
| Reverse | CAGCTTAGATCGGTACGTAGT | |||
| Pyruvate kinase, PK | AAEL014913 | Forward | CTGGTGGTTGACAGTATTAGCGG | 132 bp |
| Reverse | CTTATCCTTTTCGGAGACGGC | |||
| Ribosomal protein S7, RPS7 | AAEL009496 | Forward | ACCGCCGTCTACGATGCCA | 131 bp |
| Reverse | ATGGTGGTCTGCTGGTTCTT | |||
| Superoxide dismutase 1, SOD1 | AAEL000274 | Forward | AGCGGAGTCGCCAAGGTCG | 108 bp |
| Reverse | GCCCAAATCATCAGGATCGG | |||
| Superoxide dismutase 2, SOD2 | AAEL006271 | Forward | GGAAATGCTGGAGGCAGACT | 113 bp |
| Reverse | CCTAGCACAACATGAATGGGTCT | |||
| Thioredoxin, TRX | AAEL010777 | Forward | GACGAGTGCGAAGATCTGGC | 120 bp |
| Reverse | CATCTCCAGCTTCTGGTCGTT | |||
| Urate oxidase, UO | AAEL002194 | Forward | CAGTCGGCGTTCGTGAACTT | 135 bp |
| Reverse | CCAGCAGAAATCGAAATCCAC | |||
| Xanthine dehydrogenase 1, XDH1 | AAEL002683 | Forward | GCGATTGACATTGGACAGATCGA | 146 bp |
| Reverse | CCAGGAATGTCGGCGAAACC | |||
| Xanthine dehydrogenase 2, XDH2 | AAEL014493 | Forward | GTTATGGACATTGGCTCTAGCCT | 140 bp |
| Reverse | CCTGGACCTCTCGATAGCAGTGT |
T7 promoter sequence (5’ TAATACGACTCACTATAGGGAGA 3’) was added at 5’ of each RNAi primer.
Ae. aegypti fat body, midgut, Malpighian tubules (MTs), ovaries and thorax without appendages, were dissected from sugar- and blood-fed females at 3, 6, 12, 18, 24, 36, 48, 72, and 96 h post blood meal (PBM). Total RNA was isolated from mosquito tissues using TRIzol® Reagent according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized using oligo-(dT)20 and reverse transcriptase, and qPCR was performed as previously described for other genes (28). Ribosomal protein S7 mRNA level was used as an internal control for normalization of transcript levels in all samples. Transcript levels of genes encoding ODC, arginase, ornithine aminotransferase (OAT), proline dehydrogenase (PDH), glutamine synthetase (GS1 and GS2), glutamate synthase (GltS), glutamate dehydrogenase (GDH), pyruvate kinase (PK), glucose-6-phosphate dehydrogenase (G6PDH), xanthine dehydrogenase (XDH1 and XDH2), urate oxidase (UO), ammonia transporter Rhesus 50 glycoprotein (Rh50–1 and Rh50–2), catalase (CAT1 and CAT2), superoxide dismutase (SOD1 and SOD2) and thioredoxin (TRX) in response to ODC knockdown were determined in fat body, midgut and MTs dissected from sugar- and blood-fed females at 24 h PBM. Primer sequences for qPCR are shown in Table 1.
2.4. Western blotting procedures
Mosquito tissues were dissected in 1X phosphate-buffer saline (PBS, pH 7.4) from sugar- and blood-fed females at 3, 6, 12, 18, 24, 36, 48, and 72 h PBM. Total proteins were extracted from 5 mosquito tissues (fat body, midgut, ovaries, MTs and thorax without legs and wings), separated on 12% or 4–15% gradient polyacrylamide gels and subjected to western blotting as previously described (28). The resolved proteins were electrophoretically transferred onto a nitrocellulose membrane (LI-COR Biosciences, Lincoln, Nebraska). The membranes were blocked with 4% nonfat dry milk dissolved in PBS for 1 h at room temperature and then incubated with each primary antibody in 4% nonfat dry milk in PBS containing 0.1% Tween 20 (PBST) or StartingBlock™ T20 (TBST) blocking buffer (Thermo Fisher Scientific) overnight at 4°C. The dilutions of the primary antibodies were as follows: AaODC (1:2,000), Aa5G1 (1:1,000), AaLT (1:1,000), AaVg (1:5,000), and α-tubulin antibody (1:2,000). The membranes were washed 10 times with PBST or TBST and incubated with the secondary antibodies, IRDye 800CW goat anti-rabbit (1:10,000) and IRDye 680 RD donkey anti-mouse (1:10,000) for 1 h in the dark at room temperature. The membranes were washed 10 times with PBST, and the immunoreactive protein bands were visualized with an Odyssey infrared imaging system (LI-COR Biosciences) or ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA). Grayscale TIFF images of western blots were subjected to a densitometry analysis using the ImageJ software (31).
2.5. Survival, nitrogen waste and reproduction assays
Mosquito mortality was recorded daily for each female until the last mosquito died. Excreta accumulated from individual females were collected for various time intervals (between 0 and 24, 24 and 36, 36 and 48, and 48 and 72 h PBM), and uric acid and heme concentrations in these excreta were determined as previously described (26, 29). Onset of oviposition and fecundity were examined in individual females as previously reported (29). Briefly, blood-fed females were individually transferred into 20 ml polyethylene scintillation vials and provided with an oviposition paper and 5 ml of water at 48 h PBM. Oviposition activity was recorded daily by visual observation, and after 7 days PBM, the oviposition paper was removed. Eggs laid by each female were counted under a Nikon SMZ-10A light microscope (Melville, NY USA). To analyze ovarian follicle development, ovaries were dissected at 72 h PBM and representative images captured using a Leica EZ4 HD microscope (Buffalo Grove, IL, USA).
2.6. Statistical analysis
Statistical analyses were carried out using GraphPad Prism version 9.0 for Mac OS X (GraphPad Software, La Jolla, CA, USA). One-way ANOVA was used to examine the difference in ODC mRNA relative abundance during the first gonotrophic cycle, and to analyze uric acid and heme data in mosquito excreta. Survival analysis was performed by the Kaplan-Meier method, and differences were assessed by the Log-rank (Mantel-Cox) test. Densitometry values, oviposition and other relative transcript levels were analyzed by an unpaired Student’s t-test. A value of p < 0.05 was considered significant.
3. RESULTS
3.1. Blood feeding induces ODC gene and protein expression in mosquito tissues
Ae. aegypti ODC gene (VectorBase: AAEL007880) encodes a protein of 433 amino acid residues and shares 79 and 46% amino acid identity to Anopheles gambiae (AGAP011805) and Drosophila melanogaster (P40807) orthologs, respectively. Three putative ODCs, AGAP011806, AGAP011807 and AGAP011808 were identified in An. gambiae (32). Our bioinformatics analysis found an additional ODC in An. gambiae, AGAP011805, which is located downstream of AGAP011806 and AGAP011807. Both AGAP011806 and AGAP011807 are closely related to each other (91% identical at amino acid level) and present in the nearby genomic loci, suggesting that they arise via recent gene duplication in Anopheles lineage, and both genes are distantly related to AGAP011808. Thus, An. gambiae has four ODCs, while Ae. aegypti has one ODC gene. The Ae. aegypti ODC gene is orthologous to AGAP011805 in An. gambiae.
ODC mRNA expression profiles were examined in five tissues of sugar- and blood-fed vitellogenic Ae. aegypti mosquitoes by qPCR-based methods. We found that ODC transcript is differentially expressed in a temporal- and tissue-specific manner during the first gonotrophic cycle (Figure 1 A–E). In fat body, the mRNA level significantly increased between 6 and 36 h PBM and returned to that of sugar-fed mosquitoes at 48 to 96 h PBM. The relative abundance of ODC mRNA in midgut, MTs and thorax significantly increased between 12 and 36 h after blood feeding and then returned to that of sugar-fed females, except in the midgut where higher levels of ODC transcript were maintained until 48 h PBM. In ovaries, ODC expression changes were only observed toward the end of ovarian follicle maturation with a peak of expression at 96 h PBM (Figure 1 A–E). Overall, our data suggest that ODC transcript levels are relatively more abundant in fat body and midgut compared to MTs, thorax and ovaries.
FIGURE 1. Ornithine decarboxylase (ODC) gene and protein expression patterns in Aedes aegypti tissues.
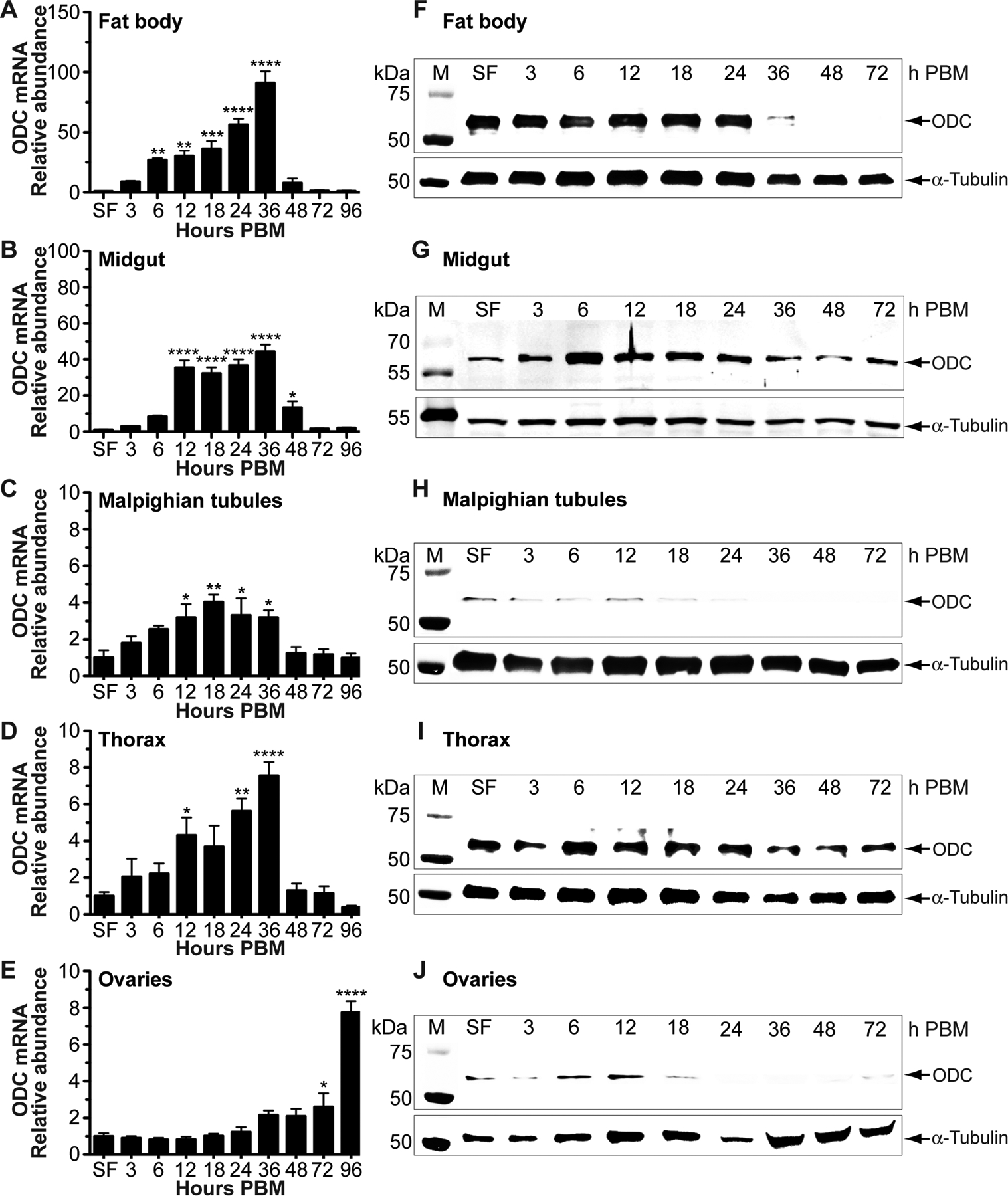
Four-day old sucrose-fed (SF) mosquitoes were fed only on 3% sucrose after adult eclosion. Tissues were dissected from SF and blood-fed mosquitoes at intervals after feeding. Four-day old females were allowed to feed on blood. A-E) ODC mRNA levels obtained by qPCR were normalized to mRNA levels of the ribosomal protein S7. Data are presented as mean ± SEM of 3 independent samples. Each cDNA replicate was prepared from a pool of 10 tissues. N = 300. *P < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 when compared to SF. F-J) ODC protein levels were analyzed using a custom-made polyclonal antibody against Ae. aegypti ODC. For fat body, midgut, and ovary, each lane contains 1 tissue equivalent of the protein extracts. For thorax and Malpighian tubules, each lane contains 0.2 and 2 tissue equivalent of the protein extracts, respectively. Anti-α-tubulin antibody was used as an internal control for protein loading. Western blots are representative of 3 biological replicates. Each protein extract replicate was prepared from a pool of 10 mosquito tissues. N = 270. M = protein markers, PBM = post blood meal.
Western blot analysis using a custom-synthesized AaODC antibody revealed a distinct protein expression of ODC in tissues dissected during the first gonotrophic cycle (Figure 1 F–J). The ODC protein expression in the fat body (Figure 1F) was highly and constitutively expressed until 24 h PBM, decreased at 36 h PBM, and was not detected at 48–72 h PBM. In the midgut (Figure 1G), ODC protein expression increased after blood feeding and reached a peak at 6 h PBM. It was maintained at high levels until 36 h PBM and then returned to that of sugar-fed females until the end of the first gonotrophic cycle (timeframe of 72 h). In the thorax (Figure 1I), the ODC protein relative abundance was highly and constitutively expressed during the time course analyzed (72 h). In MTs and ovaries (Figure 1 H and J), a similar ODC protein profile was observed. The ODC protein relative abundance in these tissues increased after blood feeding reaching the highest level of expression at around 12 h PBM and decreased gradually thereafter (Figure 1 H and J). It is not surprising that the correlation between ODC transcript and protein abundance is variable within and across mosquito tissues. This reflects the complex regulation of ODC protein expression.
3.2. Pharmacological inhibition of ODC impairs mosquito survival, nitrogen waste excretion oviposition and fecundity
To assess the effect of DFMO on mosquito survival, female mosquitoes were fed a BM supplemented with 100 mM DFMO, as indicated above. Mosquitoes fed on a BM alone served as a control group. As shown in Figure 2A, there was a significant difference in survival between DFMO-treated and control mosquitoes. Mosquitoes exposed to DFMO had a shorter lifespan than control mosquitoes (Figure 2A).
FIGURE 2. Effect of DL-α-difluoromethylornithine (DFMO) on Aedes aegypti survival, excretion and egg production.
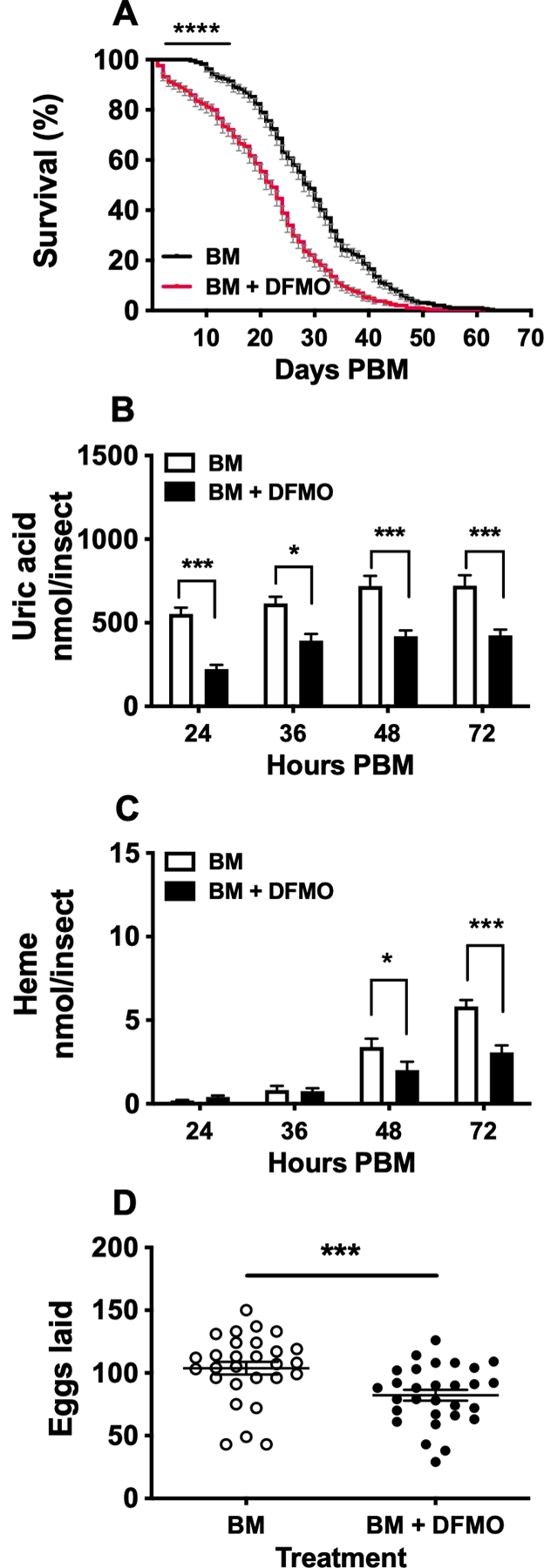
Mosquitoes were fed a blood meal (BM) supplemented with 0 or 100 mM DFMO. A) Survival analysis. Mortality was recorded daily throughout the experimental period. Data are expressed as mean percentages ± SEM of 3 independent experiments. In each treatment, 300 females were monitored. N = 600. B) Uric acid concentration in excreta. C) Heme concentration in excreta. Nitrogen waste data (B and C) are expressed as mean ± SEM of 10 individual mosquitoes. In each treatment, 10 individual females were used for the time course analyzed. N = 20. D) Numbers of eggs laid by individual females. Data are expressed as mean ± SEM of 30 individual mosquitoes. In each treatment, 30 individual females were used. N = 60. *P < 0.05, ***p < 0.001, ****p < 0.0001 when compared to control.
To evaluate the effect of ODC inhibition by DFMO on nitrogen waste excretion, we measured uric acid and heme concentrations in mosquito excreta at 24, 36, 48, and 72 h PBM. The amount of uric acid significantly decreased in mosquitoes fed BM supplemented with DFMO compared to control at all time points studied (Figure 2B). The heme concentrations were also found to be lower in DFMO-fed mosquitoes at 48 and 72 h PBM when compared to controls (Figure 2C).
To determine the effect of ODC chemical inhibition on mosquito reproduction, the timing of oviposition was compared between mosquitoes fed a BM only and mosquitoes fed BM supplemented with 100 mM DFMO. The administration of DFMO led to a delay in oviposition behavior and disrupted egg-laying in mosquitoes. Only 26% of DFMO-treated mosquitoes laid eggs by 3 days PBM compared to 86% controls. The percentage of females that laid eggs by 4 days PBM was 66% in the DFMO-treated mosquitoes compared to 14% controls. Only 4% of DFMO-treated mosquitoes laid eggs beyond 4 days PBM whereas 4% did not lay eggs. Total egg production was significantly reduced in the DFMO-treated mosquitoes compared to the mosquitoes fed BM only. Overall, the supplementation of the BM with DFMO led to a 21% reduction in the mean number of eggs laid by blood-fed females (Figure 2 D).
3.3. Genetic silencing of ODC by RNAi disrupts mosquito survival, uric acid and heme disposal
RNAi knockdown efficiency of ODC in each individual dsRNA-microinjected female was first assessed by gene-specific ODC and Ribosomal protein S7 qPCR primers (Table 1). As shown in Figure 3 A–F, ODC transcript levels significantly decreased in fat body, midgut and MTs dissected from individual sugar- and blood-fed dsRNA-ODC injected females when compared to dsRNA-FL injected control mosquitoes. We observed that two dsRNA microinjections prior to BM had a significant effect on RNAi knockdown efficiency. More than 95% effective RNAi knockdown was detected in fat body and midgut tissues, while nearly 80% of mRNA encoding ODC was reduced in MTs. Subsequently, western blots probed with an AaODC specific primary antibody confirmed a decrease or depletion of ODC protein expression in tissues dissected prior to blood-feeding and 24 h PBM from dsRNA-ODC injected females, relative to dsRNA-FL injected control mosquitoes (Figure 3 G–I).
FIGURE 3. Efficiency of ornithine decarboxylase (ODC) knockdown on Aedes aegypti tissues.
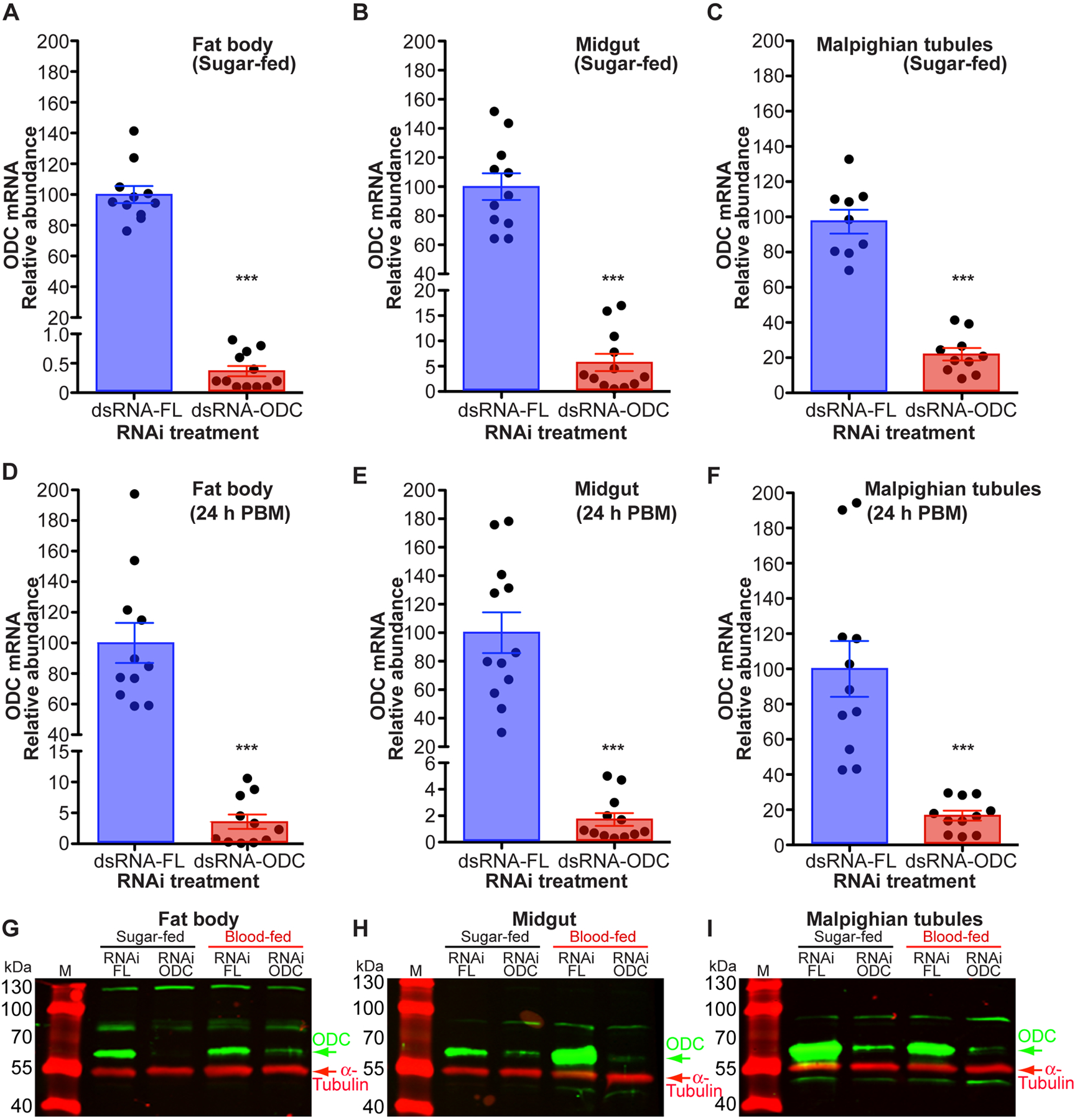
Ae. aegypti females were microinjected with dsRNA-firefly luciferase (dsRNA-FL) or dsRNA-ODC. In each treatment, fat body, midgut and Malpighian tubules were dissected from sugar- and blood-fed females at 24 h PBM. A-F) ODC gene expression in individual mosquitoes. Data are expressed as mean ± SEM of 9–12 individual mosquitoes. N = 133. ***P < 0.001 when compared to control (dsRNA-FL). G-I) ODC protein expression. ODC protein levels were analyzed using a custom-made polyclonal antibody against Ae. aegypti ODC. For fat body, each lane contains 0.5 tissue equivalent of the protein extracts. For midgut and Malpighian tubules, each lane contains 2.0 tissue equivalent of the protein extracts. Anti-α-tubulin antibody was used as an internal control for protein loading. Western blots are representative of 3 biological replicates. Each protein extract replicate was prepared from a pool of 5 mosquito tissues. N = 60. M = protein markers, PBM = post blood meal.
We further evaluated the effect of ODC disruption by RNAi-knockdown on sugar- and blood-fed mosquito survival (Figure 4 A–B). In the first experiment, the dsRNA microinjected mosquitoes were allowed only to feed on 3% sucrose throughout the study period (Figure 4A). Inhibition of ODC by RNAi significantly increased mortality of female mosquitoes when compared to control mosquitoes (Figure 4 A). The median survival time was 40 days in control mosquitoes, compared to 26.5 days in ODC knockdown mosquitoes. The last mosquito from each of the RNAi-FL and RNAi-ODC groups died 57 and 47 days after the first dsRNA microinjection, respectively (Figure 4 A). In the second experiment, the dsRNA microinjected mosquitoes were fed a BM 3 days after the second dsRNA microinjection and maintained on 3% sucrose thereafter. Mosquito adult survival was significantly reduced in females injected with dsRNA-ODC compared to the females injected with dsRNA-FL (Figure 4B). The median survival times for control and ODC knockdown mosquitoes were 41 and 25.5 days, respectively. The last mosquito from RNAi-ODC groups died 17 days sooner than the last RNAi-FL survivor.
FIGURE 4. Effect of ornithine decarboxylase (ODC) knockdown on Aedes aegypti survival and excretion.
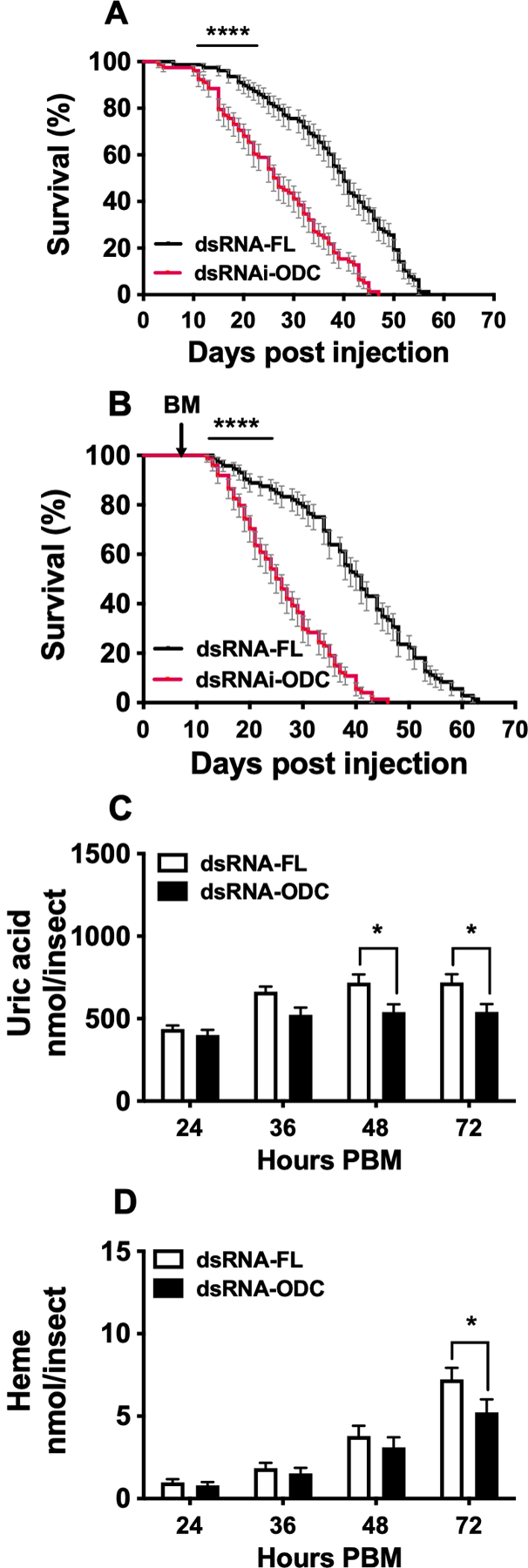
Ae. aegypti females were microinjected with dsRNA-firefly luciferase (dsRNA-FL) or dsRNA-ODC, fed only on 3% sucrose or a blood meal and maintained on 3% sucrose. A) Survival analysis of sugar-fed dsRNA-injected females. Mortality was recorded daily throughout the experiment period. Data are expressed as mean percentages ± SEM of 3 independent experiments. In each treatment, 78 females were monitored. N = 156. B) Survival analysis of blood-fed dsRNA-injected females. Data are expressed as mean percentages ± SEM of 3 independent experiments. C) Uric acid concentration in excreta. D) Heme concentration in excreta. Nitrogen waste data (C and D) are expressed as mean ± SEM of 10 individual mosquitoes. In each treatment, 10 individual females were used for the time course analyzed. N = 20. *P < 0.05, ****p < 0.0001 when compared to control. BM = blood meal, PBM = post blood meal.
We observed that ODC silencing by RNAi also impaired nitrogen waste disposal. The effect on uric acid excretion was significant at 48 and 72 h PBM, whereas heme excretion significantly decreased at 72 h PBM relative to FL control mosquitoes (Figure 4 B–C).
3.4. RNAi-mediated knockdown of ODC delays serine proteases expression and midgut proteolysis
To determine whether the reduction of nitrogen waste disposal is associated with impairment in BM protein catabolism, we monitored late trypsin (LT) and 5G1 trypsin expression in midguts from dsRNA-ODC and dsRNA-FL mosquitoes at different times PBM by using AaLT and Aa5G1 antibodies (Figure 5 A–D), densitometry analysis (Figure 5 E–F), and representative dissected midgut images (Figure 5 G). As shown in Figure 5 A–D, the expression of LT and 5G1 serine proteases was delayed in the midguts of ODC-deficient females relative to control. In midguts of control females, LT was expressed at low levels at 12 h PBM, although its maximal expression is also observed at 24–36 h PBM, whereas 5G1 is more highly expressed than LT at 12 h PBM, and its maximal expression is observed at 24–36 h PBM (Figure 5 A–D) as previously reported (27). Protein expression of both proteases is delayed in the midguts of ODC-deficient females (Figure 5 A–F). Midguts of RNAi-ODC females dissected at 24, 36, 48 and 60 h PBM exhibited differences in the amount of blood degraded relative to midguts of RNAi-FL females. Midguts from both looked empty by 72 h PBM (Figure 5 G).
FIGURE 5. Effect of ornithine decarboxylase (ODC) knockdown on Aedes aegypti blood protein digestion.
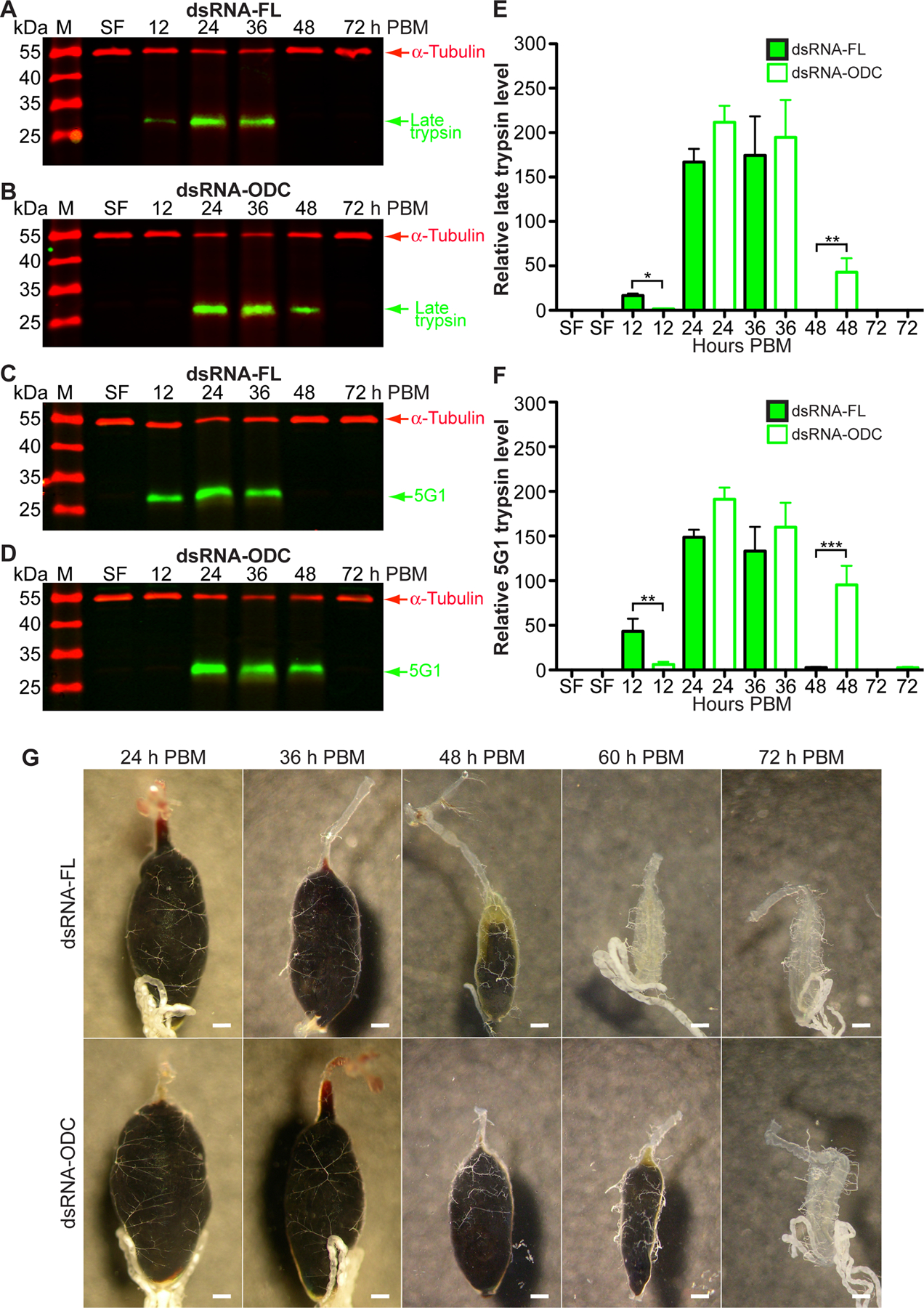
Ae. aegypti females were microinjected with dsRNA-firefly luciferase (dsRNA-FL) or dsRNA-ODC. Sucrose-fed (SF) mosquitoes were fed only on 3% sucrose. A-D) Western blot analysis of two major midgut serine proteases in response to RNAi treatment using custom-made polyclonal antibodies against Ae. aegypti late trypsin and 5G1. Each lane contains 0.5 tissue equivalent of the midgut protein extracts. Anti-α-tubulin antibody was used as an internal control for protein loading. Western blots are representative of 3 biological replicates. Each protein extract replicate was prepared from a pool of 10 midguts. N = 360. E-F) Densitometry analysis of western blots by ImageJ software. Data are expressed as means ± SEM of 3 biological replicates. *P < 0.05, **p < 0.01, ***p < 0.001 when compared to control. G) Midgut images at 24, 36, 48, 60 and 72 h PBM. Each midgut is presented as anterior to posterior orientation. Images shown are representative of 5 mosquitoes from each time course. N = 50. Each scale bar represents 200 μm. M = protein markers, PBM = post blood meal.
3.5. Disruption of ODC by RNAi delays the rate of vitellogenin uptake in oocytes, reduces ovarian follicle development and fecundity
To elucidate whether the knockdown of ODC by RNAi affects vitellogenesis and reproduction, we monitored Vg protein uptake in oocytes by western blotting, ovary development and oviposition in individual dsRNA-FL and dsRNA-ODC females. We used an AaVg specific antibody to determine Vg protein levels in dissected ovaries. Vg uptake by ovaries of dsRNA-ODC females was significantly reduced at 12, 48, and 72 h PBM compared to dsRNA-FL females, as shown by immunoblot and densitometry analyses (Figure 6 A–C), demonstrating that the amount of Vg synthesized in fat body is affected in ODC-deficient blood-fed females. The delay of Vg uptake in RNAi-ODC females is likely due to the fact that BM digestion in midgut lumen was significantly delayed (Figure 5). RNAi-driven silencing of ODC also had an impact on overall fecundity. RNAi-ODC females exhibited impairment in ovary development at 72 h PBM and laid fewer eggs than RNAi-FL control females (Figure 6 D–E). The mean values of eggs laid by RNAi-FL and -ODC females were 94 and 75, respectively (Figure 6 E). Only 23% of RNAi-ODC females oviposited eggs by 3 days PBM compared to 93% of RNAi-FL females. The rest of RNAi-FL females (7%) deposited eggs by 4 days PBM. The peak oviposition activity (60%) by RNAi- ODC females was observed by 4 days PBM, whereas the rest of the RNAi-ODC females (17%) oviposited eggs by 5 days PBM. Most of the eggs laid by both RNAi groups hatched as normal.
FIGURE 6. Effect of ornithine decarboxylase (ODC) knockdown on Aedes aegypti reproduction.
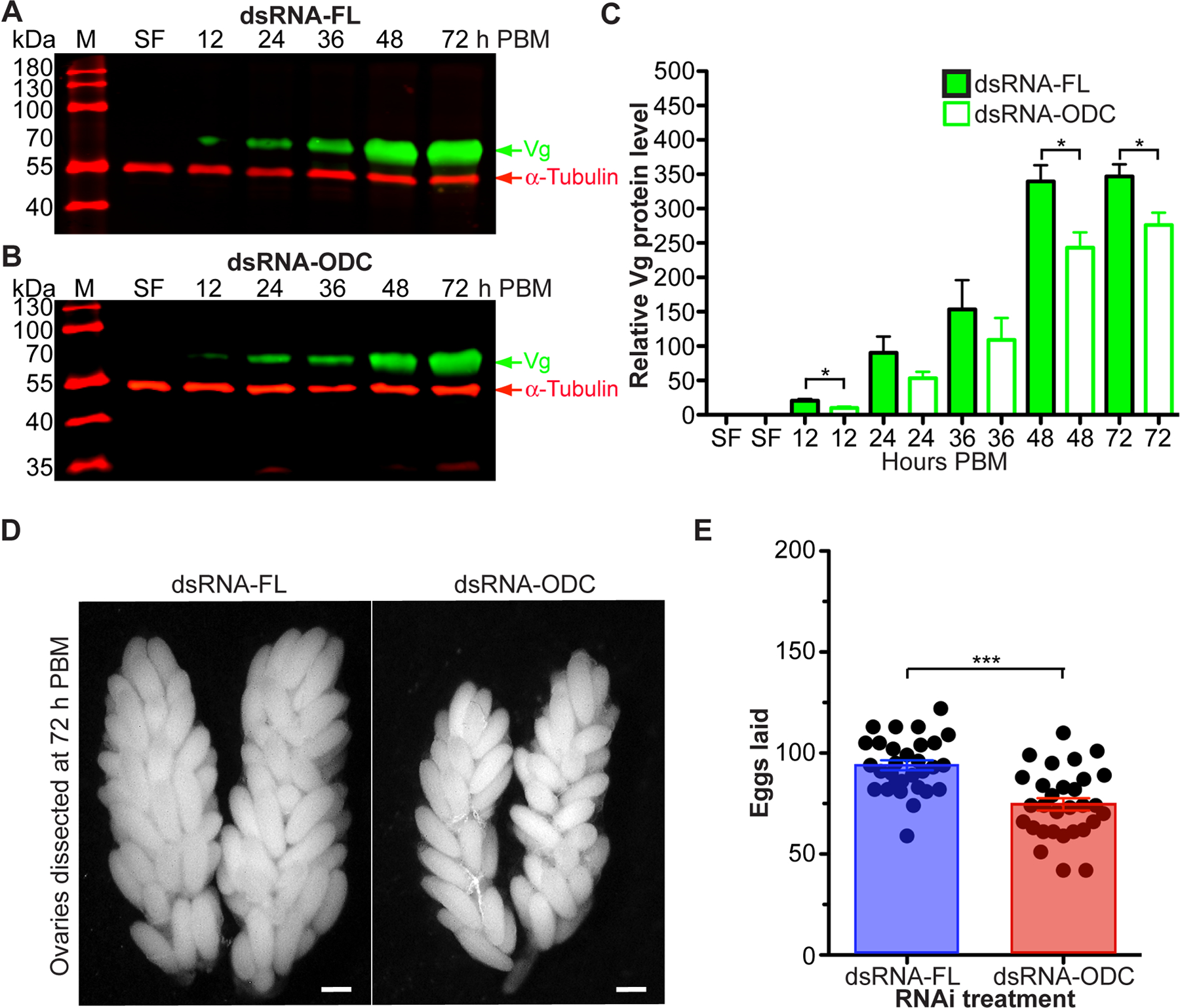
Ae. aegypti females were microinjected with dsRNA-firefly luciferase (dsRNA-FL) or dsRNA-ODC. Sucrose-fed (SF) mosquitoes were fed only on 3% sucrose. A-B) Vitellogenin (Vg) protein expression in response to RNAi treatment. Relative abundance of Vg was analyzed using a custom-made polyclonal antibody against Ae. aegypti Vg. Each lane contains 0.5 tissue equivalent of the protein extracts. Anti-α-tubulin antibody was used as an internal control for protein loading. Western blots are representative of 3 biological replicates. Each protein extract replicate was prepared from a pool of 10 ovaries. N = 360. C) Densitometry analysis. Data are expressed as means ± SEM of 3 replicates. D) Ovary images at 72 h PBM. Images shown are representative of 3 mosquitoes from each treatment. N = 6. Each scale bar represents 200 μm. E) Numbers of eggs laid by individual females in the first gonotrophic cycle. Data are expressed as mean ± SEM of 30 individual mosquitoes. In each treatment, 30 individual females were used. N = 60. *P < 0.05, ***p < 0.001 when compared to control. M = protein markers, PBM = post blood meal.
3.6. ODC deficiency in mosquitoes induces differential transcription of genes encoding proteins involved in multiple metabolic pathways
To elucidate whether ODC disruption impacts the transcriptional regulation of specific genes in female mosquitoes, we evaluated the relative abundance of selected mRNA transcripts known to be involved in argininolysis, ammonia and glucose metabolism, ammonia transporters, and oxidative stress response by using qPCR in individual fat body, midgut and MTs dissected from sugar- and blood-fed dsRNA-injected females (Figure 7). Since mosquitoes may respond differently to RNAi, and the inclusion of individuals that have a decreased RNAi effect into pooled samples may lead to ambiguous results, females were pre-screened for high levels of ODC knockdown prior to downstream qPCR analysis.
FIGURE 7. Effect of ornithine decarboxylase (ODC) knockdown on transcripts levels of multiple genes in Aedes aegypti tissues.
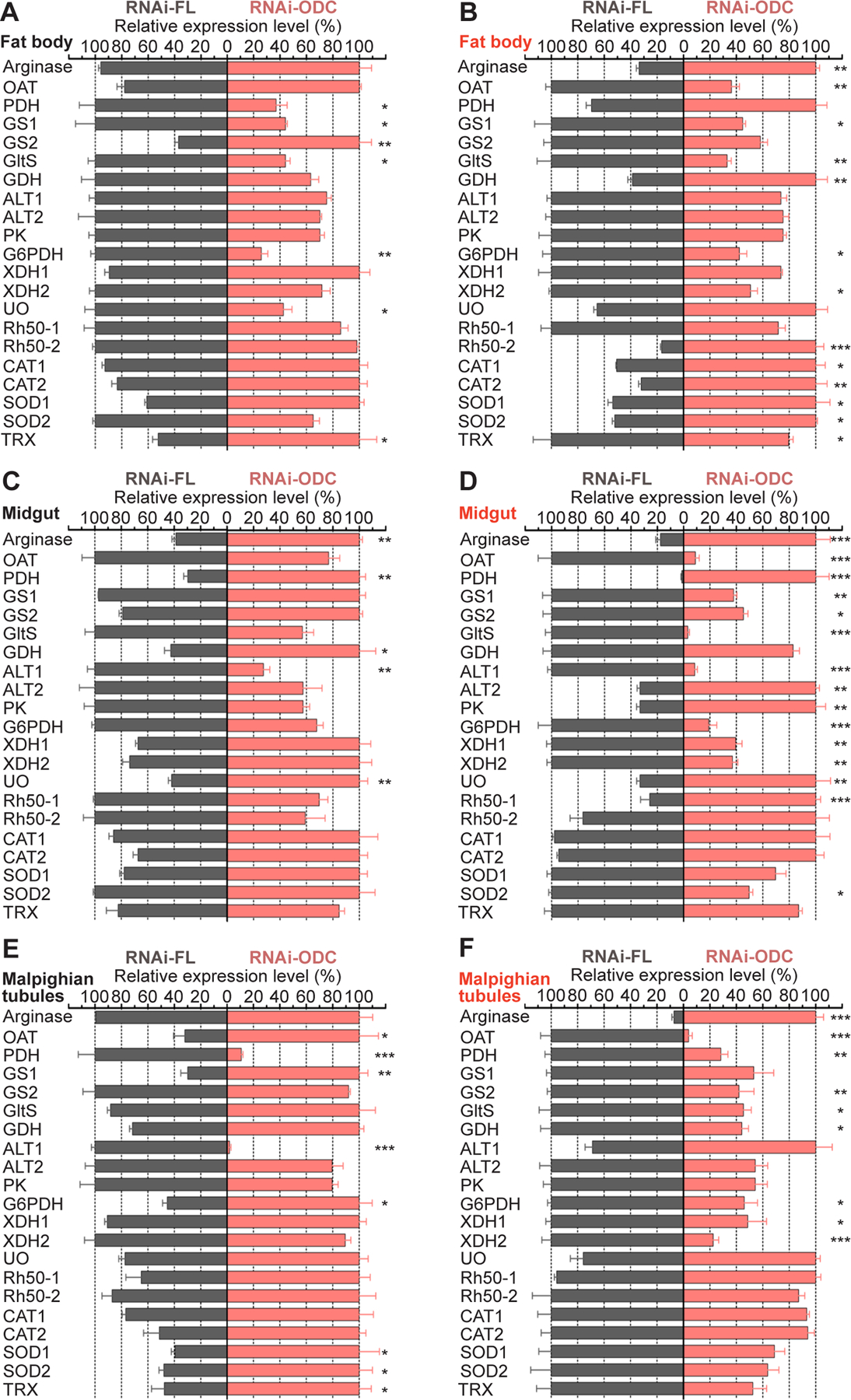
Ae. aegypti females were microinjected with dsRNA-firefly luciferase (dsRNA-FL) or dsRNA-ODC. A, C, E) Relative abundance of transcripts in fat body, midgut and Malpighian tubules dissected from sugar-fed mosquitoes. B, D, F) Relative abundance of transcripts in fat body, midgut and Malpighian tubules dissected at 24 h after blood feeding. Transcript levels were normalized to mRNA levels of the ribosomal protein S7. Data are expressed as mean ± SEM of 10 individual tissues. Each cDNA replicate was prepared from 10 individual tissues. N = 120. *P < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 when compared to control. ALT, alanine aminotransferase; CAT, catalase; G6PDH, glucose-6-phosphate dehydrogenase; GDH, glutamate dehydrogenase; GltS, glutamate synthase; GS, glutamine synthetase; OAT, ornithine aminotransferase; ODC, ornithine decarboxylase; PK, pyruvate kinase; PDH, proline dehydrogenase; Rh50, Rhesus 50 glycoprotein; SOD, superoxide dismutase; TRX, thioredoxin; UO, urate oxidase and XDH, xanthine dehydrogenase.
Arginase transcript did not change significantly in fat body and MTs of sugar-fed dsRNA-ODC females (Figure 7 A and E), while it was up-regulated in midguts of sugar-fed dsRNA-ODC females (Figure 7 C) and in all tissues of blood-fed dsRNA-ODC females, when compared to dsRNA-FL female controls (Figure 7 B, D and F).
OAT mRNA level did not show significant differences in fat body and midguts of sugar-fed ODC deficient females, while it was up-regulated in MTs of sugar-fed dsRNA-ODC females (Figure 7 A, C and E). In contrast, OAT transcript was down-regulated in all the tissues of blood-fed dsRNA-ODC females (Figure 7 B, D and F).
In tissues dissected from sugar-fed dsRNA-ODC mosquitoes, PDH transcript decreased in fat body and MTs, but it increased in midguts (Figure 7 A, C and E). Conversely, in tissues dissected from blood-fed dsRNA-ODC mosquitoes, PDH mRNA level did not change in fat body, increased in midguts, and decreased in MTs (Figure 7 B, D and F).
GS1 and GltS transcripts decreased, GS2 transcript increased and GDH transcript was not significantly modified in fat body of sugar-fed dsRNA-ODC mosquitoes (Figure 7 A). In midguts of sugar-fed dsRNA-ODC females, GS1, GS2 and GltS transcripts remained stable, but GDH transcript increased (Figure 7 C). In MTs dissected from sugar-fed dsRNA-ODC females, GS1 mRNA level increased, whereas GS2, GltS and GDH mRNA levels did not change (Figure 7 E). In fat body of blood-fed dsRNA-ODC females, the GS1 and GltS transcripts decreased, the GS2 gene expression was not modified, whereas GDH gene expression increased (Figure 7 B). GS1, GS2 and GltS transcripts decreased but GDH transcript did not change in midguts of blood-fed dsRNA-ODC females (Figure 7 D). In MTs of blood-fed dsRNA-ODC females, GS1 mRNA level was not modified, whereas GS2, GltS and GDH mRNA levels decreased significantly (Figure 7 F).
ALT1, ALT2 and PK transcripts remained stable, but G6PDH transcript decreased in fat body of sugar- and blood-fed ODC deficient females (Figure 7 A and B). In midguts of sugar-fed dsRNA-ODC females, ALT1 transcript decreased, but ALT2, PK and G6PDH transcripts did not change (Figure 7 C). Interesting, ALT1 and G6PDH mRNA levels were down-regulated but ALT2 and PK mRNA levels were up-regulated in midguts of blood-fed dsRNA-ODC mosquitoes (Figure 7 D). In MTs of sugar-fed dsRNA-ODC females, ALT1 transcript decreased, G6PDH transcript increased, but ALT2 and PK transcripts did not change (Figure 7 E). On the other hand, in MTs of blood-fed dsRNA-ODC females, the G6PDH transcript was down-regulated, whereas ALT1, ALT2 and PK transcripts did not change (Figure 7 F).
XDH1 and XDH2 expression profiles remained unchanged in tissues dissected from sugar-fed mosquitoes with ODC reduced by RNAi. (Figure 7 A, C and E). In fat body of blood-fed females with ODC deficiency, XDH1 transcript did not change but XDH2 transcript decreased, whereas both XDH1 and XDH2 transcripts decreased in midguts and MTs of blood-fed females with ODC deficiency (Figure 7 B, D and F).
UO mRNA relative abundance decreased in fat body of sugar-fed dsRNA-ODC females but it increased in midguts of both sugar- and blood-fed dsRNA-ODC females (Figure 7 A, C and D). UO transcript did not change in MTs of sugar-fed females (Figure 7 E), and in the fat body and MTs of blood-fed females with ODC deficiency (Figure 7 B and F).
The gene expression of ammonia transporters Rh050–1 and Rh050–2 did not change in sugar-fed dsRNA-ODC females (Figure 7 A, C, and E). In fat body of blood-fed dsRNA-ODC females, Rh050–1 transcript was not modified but Rh050–2 transcript was up-regulated (Figure 7 B). By contrast, in midguts of blood-fed dsRNA-ODC females, Rh050–1 transcript was up-regulated and Rh050–2 transcript remained unchanged (Figure 7 D). In MTs of blood-fed dsRNA-ODC mosquitoes, both Rh050–1 and Rh050–2 transcripts were not modified when compared to controls (Figure 7 F).
In fat body of sugar-fed ODC-deficient females, CAT1, CAT2, SOD1, SOD2 transcripts did not change significantly, while TRX transcript was up-regulated (Figure 7 A). In fat body of blood-fed ODC-deficient females, CAT1, CAT2 and SOD1, SOD2 transcripts were up-regulated, whereas TRX transcript decreased (Figure 7 B). In midguts of sugar-fed dsRNA-ODC females and in MTs of blood-fed ODC-deficient females, CAT1, CAT2, SOD1, SOD2 and TRX transcripts did not change (Figure 7 C and F). Interesting, SOD2 transcript was down-regulated in midguts of blood-fed ODC-deficient females (Figure 7 D), whereas SOD1, SOD2 and TRX mRNA levels were all up-regulated in MTs of sugar-fed dsRNA-ODC females when compared to controls (Figure 7 E).
4. DISCUSSION
It was previously reported that blood-fed Ae. aegypti females metabolize and excrete excess nitrogen through the combined action of multiple pathways (26, 28–30, 33–35). It was also found that glucose catabolism contributes to ammonia detoxification and nitrogen waste disposal in female adult mosquitoes (36–38). Although uric acid is the major nitrogen waste that blood-fed Ae. aegypti females synthesize and excrete (26, 29, 39), mosquitoes have evolutionarily adapted to produce urea through uricolysis (30) and argininolysis (28, 39), which is independent of the urea cycle (30 and Figure 8). The discovery of a cross-talk regulatory mechanism between uricolysis and argininolysis has contributed to partially reveal the complexity of nitrogen waste metabolism in Ae. aegypti females (28). Arginine can be obtained from diet or protein turnover, utilized by different metabolic pathways or directly excreted and contribute to eliminate excess nitrogen in blood-fed Ae. aegypti (28, 39). Ornithine - one of the products of argininolysis - can be decarboxylated by ODC into putrescine in the first biochemical step of the polyamine pathway (Figure 8). It was reported that chemical inhibition of ODC by DFMO disrupts several physiological processes including trypsin activity in midguts and synthesis of DNA, RNA, Vg and polyamines in fat body of Ae. aegypti females (25). More recently, we reported that ODC transcript level is up-regulated in the fat body of XDH1-deficient mosquitoes, suggesting that ODC is actively involved in nitrogen waste clearance and free radical detoxification (26). In the present study, we further explored the ODC functional roles in mosquito metabolism by pharmacological and RNAi-mediated genetic silencing of ODC. We found that ODC plays a critical role in mosquito nitrogen metabolism, reproduction and survival. We also discovered that ODC prevents detrimental perturbations in mosquito nitrogen metabolism by transcriptionally regulating genes encoding proteins involved in multiple metabolic pathways.
FIGURE 8. Schematic representation of metabolic interactions between glucose, ammonia, arginine and polyamine pathways in blood-fed Aedes aegypti mosquitoes.
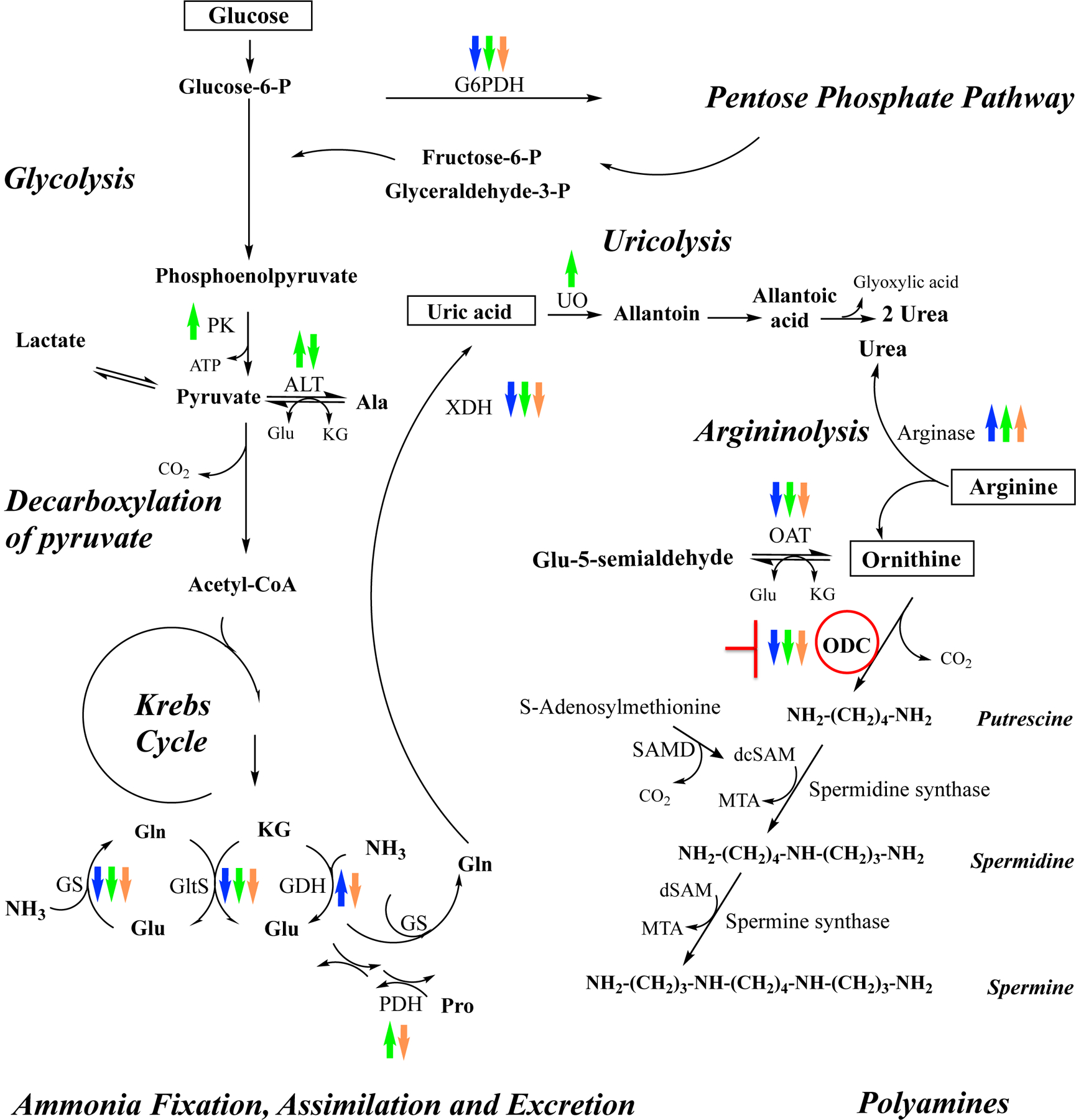
Transcriptional changes of multiple genes in tissues dissected from blood-fed ODC-deficient females at 24 h PBM are shown with up and down arrows, which indicate up-regulation and down-regulation respectively. Blue arrows indicate changes in fat body, green arrows indicate changes in midgut, and orange arrows indicate changes in Malpighian tubules. ALT, alanine aminotransferase; dsSAM: decarboxylated 5-adenosylmethionine; G6PDH, glucose-6-phosphate dehydrogenase; GDH, glutamate dehydrogenase; GltS, glutamate synthase; GS, glutamine synthetase; KG, ketoglutarate; MTA: methylthioadenosine; OAT, ornithine aminotransferase; ODC, ornithine decarboxylase; PK, pyruvate kinase; PDH, proline dehydrogenase; SAMD: S-adenosylmethionine decarboxylase; UO, urate oxidase, and XDH, xanthine dehydrogenase. This scheme is adapted from Scaraffia et al., 2006, 2008 and Horvath et al., 2018 (30, 34, 36). Copyright permission obtained. Copyright (2006) Elsevier. Copyright (2008) National Academy of Sciences, U.S.A. Copyright (2018) John Wiley and Sons.
ODC transcript exhibited a distinct expression pattern in sugar- and blood-fed Ae. aegypti tissues. Changes in three ODC transcripts were also reported in An. gambiae after blood feeding (32). We also observed that ODC protein abundance was differentially expressed in Ae. aegypti tissues during the first gonotrophic cycle. In blood-fed Ae. aegypti fat body extracts analyzed through the period of Vg synthesis, variations of putrescine, spermidine and spermine concentrations were also well correlated with changes of ODC and S-adenosylmethionine decarboxylase activities (25).
Chemical and genetic knockdown of ODC by DFMO and RNAi significantly compromised Ae. aegypti survival relative to control mosquitoes. Notably, ODC silencing by RNAi caused high mortality in both sugar- and blood-fed mosquitoes, underlying the critical role of ODC in mosquito metabolism. It was previously shown that polyamine levels decline with age in fruit flies (19) and that treatment with polyamines, such as spermidine, can restore polyamine levels (19) and extend lifespan in several organisms including insects (6, 20). More recently, it was shown that fruit fly homozygous ODC mutants are lethal, but heterozygotes are viable to adulthood (18). Our mosquito survival data are in agreement with the findings reported in fruit flies. Taken together, these data indicate that polyamine homeostasis is critical for survival of several organisms, including mosquitoes.
The RNAi-driven depletion of ODC also strongly impaired nitrogen metabolism and reproduction in blood-fed Ae. aegypti females. It was previously reported that the administration of DFMO negatively impacts insect reproduction (16, 17, 22, 25). Oocyte development was inhibited in Ae. aegypti females fed an artificial BM supplemented with 75 mM DFMO (25). A reduction in midgut trypsin activity and a decrease of DNA, RNA, polyamine and Vg synthesis were also observed in blood-fed Ae. aegypti females treated with 50 to 100 mM DFMO (25). Digestive serine proteases such as LT and 5G1 trypsins play a major role during BM protein digestion in Ae. aegypti (27). In correlation with the findings reported by Kogan and Hagerdon (25), we observed a delayed LT and 5G1 protein expression in midguts of ODC-deficient mosquitoes. This delay correlated with a decrease in BM proteolysis and a reduction in the synthesis and excretion of uric acid and heme. Furthermore, ODC deficiency also adversely influenced primary follicle development in the first gonotrophic cycle as shown by the reduced amount of overall Vg proteins in ovaries, resulting in fewer oocytes becoming fully mature than control mosquitoes and a reduction of the numbers of eggs laid by ODC-deficient females. These biochemical and developmental phenotypes indicate that vitellogenic blood-fed mosquitoes cope with ODC deficiency by decreasing and adjusting BM protein catabolism, nitrogen waste disposal and vitellogenesis. These biochemical strategies have also been previously reported when genes encoding central proteins in nitrogen metabolism were silenced by RNAi in Ae. aegypti females (26, 28, 29).
Comparative transcriptional analysis of multiple genes in sugar- and blood-fed mosquitoes injected with dsRNA against ODC reveals that a wide variety of crosstalk mechanisms exists between ODC and genes encoding proteins involved in glucose, ammonia, arginine and free-radical detoxification pathways. Notably, most of the transcriptional changes are seen in fat body and midguts of blood-fed Ae. aegypti females with ODC deficiency.
Our data indicate that the arginase gene, which encodes the enzyme that catalyzes the conversion of arginine into ornithine and urea, is up-regulated, whereas OAT, which encodes the enzyme that catalyzes the reversible transamination of ornithine and ketoglutarate to glutamyl-5-semialdehyde and glutamate, is down-regulated in fat body, midguts and MTs of blood-fed ODC-deficient females. The down-regulation of OAT could also cause a reduction of glutamate and ultimately a decrease in proline synthesis. Notably, an up-regulation of PDH gene is observed in midguts of sugar- and blood-fed dsRNA-ODC females, suggesting an increase of proline catabolism in this tissue.
We have previously reported that a delay and/or disruption in BM digestion and excretion caused by silencing genes involved in nitrogen and carbon metabolism led to a reduction of transcript levels of genes encoding enzymes involved in ammonia fixation, assimilation and excretion pathways (26, 28, 29), and a decrease of glucose catabolism in blood-fed Ae. aegypti tissues (37). Here, ODC gene silencing triggered by RNAi also resulted in a down-regulation of several genes involved in nitrogen and carbon metabolism (such as GS, GltS, XDH and G6PDH) in fat body, midguts and MTs of blood-fed ODC deficient Ae. aegypti females at 24 h PBM. Interestingly, GDH is up-regulated in fat body but down-regulated in MTs of blood-fed females with ODC deficiency. We also observed a distinct ammonia transporter transcriptional response in fat body and midguts of blood-fed dsRNA-ODC females. We previously reported that fat body and midgut tissues differentially detoxify ammonia (35). Variations in GS and GltS mRNA levels were detected in midgut of blood-fed Ae. aegypti during the first gonotrophic cycle but GltS activity was not detected in Ae. aegypti midgut (33). Additional stable-isotope tracing analysis coupled with specific GS and GltS inhibitors indicated that the GS/GltS pathway is functional in fat body but not in the midgut (35). It was also shown that ammonia detoxification in fat body mainly occurs through glutamine and proline synthesis by the GS/GltS pathway and enzymes involved in proline synthesis, whereas ammonia detoxification in midgut mainly occurs through the synthesis of glutamine and alanine by GS, GDH and ALT (35). As expected, the disruption of nitrogen metabolism caused by ODC silencing led to a down-regulation of GS and GltS transcripts in fat body, and a down-regulation of GS and ALT1 in midguts of blood-fed females. The decrease of GltS transcript abundance observed in midguts of blood-fed ODC-deficient females was unexpected and could be not functionally relevant. Remarkably, the loss of ODC in blood-fed female mosquitoes also induced an Rh50–1 transcriptional up-regulation in midgut but an Rh50–2 transcriptional down-regulation in fat body indicating that the two putative ammonia transporters may act differently in midgut tissue during BM digestion. Moreover, unlike fat body and MTs, an up-regulation of PK, ALT2 and UO was observed in midgut of blood-fed dsRNA-ODC Ae. aegypti, suggesting that ODC knockdown could indirectly enhance glycolysis and uricolysis in this tissue.
A genetic silencing of ODC in Ae. aegypti females is also expected to cause a significant reduction in polyamine synthesis in the downstream pathway - as reported by a chemical ODC inhibition in mosquitoes and silkworms (24, 25) - and a decrease in free radical scavengers (40). In mutant fruit flies, the loss of spermine synthase which catalyzes the conversion of spermidine to spermine (Figure 8), caused survival defects, excessive spermidine catabolism and oxidative stress (41). Consequently, the transcriptional up-regulation of genes encoding certain antioxidant proteins such as CAT, SOD and TRX in specific tissues of Ae. aegypti mosquitoes in response to ODC suppression by RNAi could be an acute strategy to reduce oxidative stress.
In summary, we provide molecular and biochemical evidence that ODC plays an essential role in Ae. aegypti mosquitoes, and that ODC cross-talks with multiple key factors to maintain nitrogen homeostasis. Our findings contribute to advance our knowledge of the mosquito-metabolic networks, provide a foundation to further better understand mosquito-host-pathogen interactions, and ultimately lead to the discovery of novel targets for selectively controlling mosquito populations.
ACKNOWLEDGMENTS
This work was financially supported by the Corine Adams Baines Professorship Award, Carol Lavin Bernick Faculty Grants, and U.S., National Institutes of Health, National Institute of Allergy and Infectious Diseases Grant R01AI146199 (to PYS). The authors thank Dr. Stacy Mazzalupo for critical reading of the manuscript.
Abbreviations
- ALT
alanine aminotransferase
- BM
bovine blood supplemented with ATP
- CAT
catalase
- DFMO
DL-α-difluoromethylornithine
- dsRNA
double-stranded RNA
- FL
firefly luciferase
- G6PDH
glucose-6-phosphate dehydrogenase
- GDH
glutamate dehydrogenase
- GltS
glutamate synthase
- GS
glutamine synthetase
- MTs
Malpighian tubules
- LT
late trypsin
- OAT
ornithine aminotransferase
- ODC
ornithine decarboxylase
- PBS
phosphate-buffer saline
- PBM
post blood meal
- PDH
proline dehydrogenase
- PK
pyruvate kinase
- qPCR
quantitative real-time PCR
- Rh50
Rhesus 50 glycoprotein
- RNAi
RNA interference
- SOD
superoxide dismutase
- TRX
thioredoxin
- UO
urate oxidase
- Vg
vitellogenin
- XDH
xanthine dehydrogenase
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the methods.
REFERENCES
- 1.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY) 2011;3:716–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura K, Okudaira H, Ochiai E, et al. Identification of proteins whose synthesis is preferentially enhanced by polyamines at the level of translation in mammalian cells. Int J Biochem Cell Biol 2009;41:2251–2261. [DOI] [PubMed] [Google Scholar]
- 3.Rider JE, Hacker A, Mackintosh CA, et al. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007;33:231–240. [DOI] [PubMed] [Google Scholar]
- 4.Murray Stewart T, Dunston TT, Woster PM, Casero RA Jr. Polyamine catabolism and oxidative damage. J Biol Chem 2018;293:18736–18745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae DH, Lane DJR, Jansson PJ, Richardson DR. The old and new biochemistry of polyamines. Biochim Biophys Acta Gen Subj 2018;1862:2053–2068. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 2009;11:1305–1314. [DOI] [PubMed] [Google Scholar]
- 7.Inoue K, Tsutsui H, Akatsu H, et al. Metabolic profiling of Alzheimer’s disease brains. Sci Rep 2013;3:2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmann AS, Geerts D. Polyamine synthesis as a target of MYC oncogenes. J Biol Chem 2018;293:18757–18769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mounce BC, Olsen ME, Vignuzzi M, Connor JH. Polyamines and their role in virus infection. Microbiol Mol Biol Rev 2017;81:e00029–00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firpo MR, Mounce BC. Diverse functions of polyamines in virus infection. Biomolecules 2020;10:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips MA. Polyamines in protozoan pathogens. J Biol Chem 2018;293:18746–18756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M, Zhang W, Chen H, Zeng J. Targeting polyamine metabolism for control of human viral diseases. Infect Drug Resist 2020;13:4335–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanduri B, Swiatlo E. The expansive effects of polyamines on the metabolism and virulence of Streptococcus pneumoniae. Pneumonia (Nathan) 2021;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem 2006;281:14529–14532. [DOI] [PubMed] [Google Scholar]
- 15.Kahana C. The antizyme family for regulating polyamines. J Biol Chem 2018;293:18730–18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso VV, Moreira JC, Oliveira AK. Effect of alpha-difluormethylornithine on Anastrepha fraterculus (Diptera, Tephritidae) ovary size. Braz J Biol 2009;69:149–152. [DOI] [PubMed] [Google Scholar]
- 17.Birnbaum MJ, Gilbert LI. Juvenile hormone stimulation of ornithine decarboxylase activity during vitellogenesis in Drosophila melanogaster. J Comp Physiol B 1990;160:145–151. [DOI] [PubMed] [Google Scholar]
- 18.Leon KE, Fruin AM, Nowotarski SL, DiAngelo JR. The regulation of triglyceride storage by ornithine decarboxylase (Odc1) in Drosophila. Biochem Biophys Res Commun 2020;523:429–433. [DOI] [PubMed] [Google Scholar]
- 19.Gupta VK, Scheunemann L, Eisenberg T, et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci 2013;16:1453–1460. [DOI] [PubMed] [Google Scholar]
- 20.Tain LS, Jain C, Nespital T, et al. Longevity in response to lowered insulin signaling requires glycine N-methyltransferase-dependent spermidine production. Aging Cell 2020;19:e13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coni S, Falconio FA, Marzullo M, et al. Translational control of polyamine metabolism by CNBP is required for Drosophila locomotor function. Elife 2021;10:e69269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cayre M, Strambi C, Charpin P, et al. Inhibition of polyamine biosynthesis alters oviposition behavior in female crickets. Behav Neurosci 1996;110:1117–1125. [PubMed] [Google Scholar]
- 23.Zhang Z, Wang M, Li H, Zhang G. Effects of polyamines and polyamine synthesis inhibitor on antennal electrophysiological responses of diamondback moths, Plutella xylostella. Entomol Exp Appl 2008;129:18–25. [Google Scholar]
- 24.Chang L, Li Z, Guo H, et al. Function of polyamines in regulating cell cycle progression of cultured silkworm cells. Insects 2021;12:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kogan PH, Hagedorn HH. Polyamines, and effects from reducing their synthesis during egg development in the yellow fever mosquito, Aedes aegypti. J Insect Physiol 2000;46:1079–1095. [DOI] [PubMed] [Google Scholar]
- 26.Isoe J, Petchampai N, Isoe YE, et al. Xanthine dehydrogenase-1 silencing in Aedes aegypti mosquitoes promotes a blood feeding-induced adulticidal activity. Faseb j 2017; 10.1096/fj.201601185R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isoe J, Rascón AA Jr., Kunz S, Miesfeld RL. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem Mol Biol 2009;39:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isoe J, Scaraffia PY. Urea synthesis and excretion in Aedes aegypti mosquitoes are regulated by a unique cross-talk mechanism. PLoS One 2013;8:e65393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzalupo S, Isoe J, Belloni V, Scaraffia PY. Effective disposal of nitrogen waste in blood-fed Aedes aegypti mosquitoes requires alanine aminotransferase. Faseb j 2016;30:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scaraffia PY, Tan G, Isoe J, et al. Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 2008;105:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vannini L, Augustine Dunn W, Reed TW, Willis JH. Changes in transcript abundance for cuticular proteins and other genes three hours after a blood meal in Anopheles gambiae. Insect Biochem Mol Biol 2014;44:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scaraffia PY, Isoe J, Murillo A, Wells MA. Ammonia metabolism in Aedes aegypti. Insect Biochem Mol Biol 2005;35:491–503. [DOI] [PubMed] [Google Scholar]
- 34.Scaraffia PY, Zhang Q, Wysocki VH, Isoe J, Wells MA. Analysis of whole body ammonia metabolism in Aedes aegypti using [15N]-labeled compounds and mass spectrometry. Insect Biochem Mol Biol 2006;36:614–622. [DOI] [PubMed] [Google Scholar]
- 35.Scaraffia PY, Zhang Q, Thorson K, Wysocki VH, Miesfeld RL. Differential ammonia metabolism in Aedes aegypti fat body and midgut tissues. J Insect Physiol 2010;56:1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horvath TD, Dagan S, Lorenzi PL, Hawke DH, Scaraffia PY. Positional stable isotope tracer analysis reveals carbon routes during ammonia metabolism of Aedes aegypti mosquitoes. FASEB J 2018;32:466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petchampai N, Isoe J, Horvath TD, et al. Mass spectrometry-based stable-isotope tracing uncovers metabolic alterations in pyruvate kinase-deficient Aedes aegypti mosquitoes. Insect Biochem Mol Biol 2020;121:103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horvath TD, Dagan S, Scaraffia PY. Unraveling mosquito metabolism with mass spectrometry-based metabolomics. Trends Parasitol 2021;37:747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Dungern P, Briegel H. Enzymatic analysis of uricotelic protein catabolism in the mosquito Aedes aegypti. J Insect Physiol 2001;47:73–82. [DOI] [PubMed] [Google Scholar]
- 40.Ha HC, Sirisoma NS, Kuppusamy P, et al. The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci U S A 1998;95:11140–11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Brazill JM, Liu S, et al. Spermine synthase deficiency causes lysosomal dysfunction and oxidative stress in models of Snyder-Robinson syndrome. Nat Commun 2017;8:1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in the methods.


