Abstract
Background
There is a paucity of prospective cohort studies evaluating neighborhood walkability in relation to the risk of death.
Methods
We geocoded baseline residential addresses of 13,832 women in the New York University Women’s Health Study (NYUWHS) and estimated the Built Environment and Health Neighborhood Walkability Index (BEH-NWI) for each participant circa 1990. The participants were recruited from 1985 to 1991 in New York City and followed for an average of 27 years. We conducted survival analyses using Cox proportional hazards models to assess the association between neighborhood walkability and risk of death from any cause, obesity-related diseases, cardiometabolic diseases, and obesity-related cancers.
Results
Residing in a neighborhood with a higher neighborhood walkability score was associated with a lower mortality rate. Comparing women in the top versus the lowest walkability tertile, the hazards ratios (and 95% CIs) were 0.96 (0.93–0.99) for all-cause, 0.91 (0.86–0.97) for obesity-related disease, and 0.72 (0.62–0.85) for obesity-related cancer mortality, respectively, adjusting for potential confounders at both the individual and neighborhood level. We found no association between neighborhood walkability and risk of death from cardiometabolic diseases. Results were similar in analyses censoring participants who moved during follow-up, using multiple imputation for missing covariates, and using propensity scores matching women with high and low neighborhood walkability on potential confounders. Exploratory analyses indicate that outdoor walking and average BMI mediated the association between neighborhood walkability and mortality.
Conclusion
Our findings are consistent with a protective role of neighborhood walkability in obesity-related mortality in women, particularly obesity-related cancer mortality.
Keywords: Neighborhood walkability, urban health, all-cause mortality, obesity-related mortality, obesity-related cancer mortality, women’s health
Introduction
The prevalence of obesity in the U.S. has been increasing steadily, affecting nearly 40% of adults by 2018.1 Obesity predisposes individuals to a higher risk for a wide array of chronic diseases, including cardiovascular disease (CVD), diabetes, and at least 13 cancers.2–5 Women may be more susceptible to obesity-related disease. For instance, among women, obesity-related cancers constitute 55% of all cancers diagnosed, a much higher percentage than the corresponding 24% among men.6,7 Modifiable lifestyle behaviors, such as engaging in physical activity, or even low-intensity physical activity such as walking, have been associated with a reduced risk of death from any cause, CVD, and some obesity-related cancers.8,9 However, individual-level interventions to increase physical activity are costly and may only have short-term effects,10 suggesting that interventions on a broader scale should be considered.
The built environment is defined as all the physical constituents of places where people live and work, such as buildings, streets, and open spaces. Evidence indicates that neighborhood walkability, which refers to a combination of urban characteristics that support pedestrian activity,11,12 can have an impact on outdoor physical activity, walking, and obesity.13,14 However, research on the extent to which neighborhood walkability reduces risk of death is inconsistent. While some studies have shown protective effects,15,16 these were either ecologic in design or had short follow-up periods. Most of these studies lacked data on confounding factors at the individual level, and did not consider effect modifiers or mediators.15,17–21 In addition, some studies measured neighborhood walkability relying solely on a land use measure,16 or considered broader measures of neighborhood characteristics that encompassed neighborhood-level socioeconomic status (SES) and environmental factors in addition to, or in lieu of neighborhood walkability,22,23 making the association difficult to attribute to neighborhood walkability. In this prospective cohort of women, we investigated the association between neighborhood walkability and risk of death, using a validated measure of neighborhood walkability at the individual level.
Methods
Study Population
The New York University Women’s Health Study (NYUWHS) is a prospective cohort of 14,274 women between the ages of 34 and 65 recruited at a mammography screening center in NYC between 1985 and 1991.24 Women were ineligible for enrollment if they had used hormonal medications or had been pregnant or lactating in the previous 6 months. Subjects completed a baseline and up to six follow-up questionnaires capturing socio-demographic, lifestyle, and health status. Addresses for active participants were updated during the process of active follow-up either through self-report or by accessing the National Change of Address (NCOA) database of the USPS. Lost participants were located using telephone directories, the National Death Index (NDI), and telephone calls to relatives. As of 31 December 2016, participants in the cohort had an average follow-up of approximately 27 years. This study was approved by the New York University School of Medicine and the Columbia Presbyterian Medical Center Institutional Review Boards.
Outcomes
We obtained causes of death in the cohort by record linkages to the NDI, Social Security Death Index searches, as well as next-of-kin contact via telephone calls. Availability of Social Security number for 97% of the cohort facilitated the linkages with NDI. Causes of death were determined using the criteria denoted by the International Statistical Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10). The endpoints for our study were death from any cause, death from obesity-related disease, death from cardiometabolic disease, and death from obesity-related cancer (eTable 1–3, eAppendix).
Exposure: The Built Environment and Health Neighborhood Walkability Index (BEH-NWI)
A previously published report described the construction of the BEH-NWI in the NYUWHS and its validation.25 Briefly, addresses at enrollment were geo-coded to the street address level with residential neighborhood defined as a circle of 1-km radial buffer around the home. All census variables were derived for each buffer using areal weighting interpolation. The source geographies for the 1990 census variables were block groups. The score was composed of the following four items based on urban planning Active Design literature. Residential density was measured using Decennial Census and American Community Survey (ACS) data in 1990. Destination accessibility was captured using the National Establishment Time Series (NETS) data containing the Dun & Bradstreet (D&B) listing from 1990. Intersection density was measured for the NYC Tri-State Area using the 2007 release of the Esri StreetMap Detailed Streets (2003 data ground date). Lastly, density of public transit was estimated using the Center for Transit-Oriented Development (CTOD) data for all active 1990 rail transit station stops within 1-km of each NYUWHS participant’s residential address. The values of the four measures were z-score-transformed across neighborhoods. The final BEH-NWI score was then calculated for each woman by summing the four 1990 z-scored components corresponding to her neighborhood.
We excluded participants with missing information on baseline BMI (n = 110), baseline residential address (n = 80), or unavailable GIS geocoding or the lack of NETS data coverage for some states (n = 252). As a result, only addresses in NY and NJ had geocoded 1-km radial buffers covered by NETS business establishments. Our final dataset included 13,832 participants, which constitutes 97% of the initial NYUWHS cohort.
Additional Variables
We collected information on self-reported outdoor walking and occupational physical activity at recruitment and converted to metabolic equivalent task (MET)-hours per week. BMI was calculated using self-reported weight and height reported at baseline and follow-up. History of diabetes was defined by self-reported use of diabetes medications or diagnosis of diabetes at either baseline or follow-up. We conducted an assessment of medical records in a subset of self-reported cases and found that 94% of self-reported cases were validated. Information on dietary intake was collected at baseline using a validated, semi-quantitative modified Block Food Frequency Questionnaire.26 We derived healthy dietary index DASH (Dietary Approaches to Stop Hypertension) developed from healthy dietary guidelines using the Fung et al. method.27 Neighborhood poverty rate (percent of population with a ratio of income to federal poverty level below 1), median household income, and percent of black population living in neighborhood from 1990 census data were used to describe neighborhood SES.
Statistical Analyses
We computed person–time for each participant from date of enrollment until either date of death or 31 December 2016, date of our last linkage to NDI. We used Cox proportional hazards (PH) models to compute hazard ratios (HR) describing the association between neighborhood walkability and risk for death from any cause, obesity-related disease, cardiometabolic disease, and obesity-related cancers. We used marginal Cox models for multivariate survival events with robust sandwich covariance matrix estimators to account for potential correlations within counties. The extent of the correlation of the risk of death at county level was evaluated using frailty models, which indicated a weak within-county correlation with mortality (eTable 4, eAppendix). The p-values of the cross-product term between neighborhood walkability index and the log function of survival time were 0.44–0.92 for the outcomes of interest, indicating that the PH assumption was not violated. We modeled neighborhood walkability using tertiles, a dichotomized variable with median as the cut point, and a continuous variable that was scaled using the standard deviation (SD = 3.11) of the neighborhood walkability measure.
Potential confounders adjusted for included age at enrollment (years), educational attainment (high school or less, college or vocational school, and graduate school), race–ethnicity (non-Hispanic white, non-Hispanic African-American, Hispanic, or other), smoking (ever vs. never smoker), average daily alcohol intake dichotomized into above or below the Recommended Dietary Allowance (RDA: 14g/day), menopausal status (yes/no), parity (yes/no), neighborhood poverty rate (continuous variable), and percent of black population living in neighborhood at baseline (continuous variable). We included individuals with missing information on alcohol consumption (15.8%), smoking status (9.5%), education level (18.5%), and race–ethnicity (12.2%) as a recoded category for unknowns in our models. Sensitivity analyses included 1) models additionally adjusted for DASH score (n = 13,567) and occupational physical activity (n = 10,467), including participants with available data only; 2) models adjusted additionally for neighborhood median household income; 3) analyses with multiple imputation of missing data on the covariates; 4) analyses accounting for competing risks from different death events to estimate the marginal probability of an event in the presence of other competing events; and 5) Generalized Estimating Equations (GEE) with logit link (for binomial distribution) and autoregressive correlation matrix. In order to assess whether the association was influenced by history of moving during follow-up, we conducted additional sensitivity analyses, first restricting the analyses to women who never moved (n = 6,440); and secondly, censoring women when they moved (n = 7,392), refused to continue participation (n = 1,804), or were lost to follow-up (n = 283) in full cohort analyses. We further evaluated the main associations using analyses employing propensity scores matching women with higher neighborhood walkability to women with lower neighborhood walkability (determined by the median) for the above-mentioned potential confounding factors and history of moving during the follow-up period.
We conducted stratified analyses by potential effect modifiers including age, obesity (BMI ≥ 30), menopausal status, DASH score (dichotomized at the median healthy diet adherence), smoking status, parity, and neighborhood median household income. We tested for multiplicative interaction using the p-value for the cross-product between dichotomized walkability and each of the potential effect modifiers. We also explored whether the association between dichotomous walkability and risk of death was mediated by outdoor walking, average BMI (mean BMI across baseline and all follow-up questionnaires), and/or history of diabetes using 1) the difference method developed by Spiegelman et al.28 accommodating for multiple mediators using clustered Cox PH models, and 2) the product method using accelerated failure time models (AFT) as it does not require a rare-outcome assumption and accommodates for exposure–mediator interactions. All analyses were implemented using SAS (version 9.4; SAS Institute Inc., Cary, NC).
Results
Participants were on average 50.9 years of age at enrollment. The majority of women in the cohort were white (79%), educated beyond high school (68%), parous at the time of enrollment (69%), and resided in the NYC area (85%) (Table 1). Baseline BMI was the lowest at the highest walkability tertile. Women were followed up for 27.4 years on average, accumulating a total person–time of 378,617 years in the cohort (eTable 5, eAppendix). Person–years associated with baseline address accounted on average for 68% of the NYUWHS follow-up time, although by the end of 2016, 53% of the women had moved from their reported baseline address. A total of 31% women had died (n = 4,338) by the end of 2016. The most common causes of death were cardiovascular disease (n = 1,481, 34%), cancer (n = 1,460, 34%) and neurodegenerative diseases (n = 377, 8.7%) (eTable 1, eAppendix). A total of 3,102 (72%) deaths were due to obesity-related diseases, 1,509 (35%) were due to cardiometabolic diseases, and 793 (18%) were due to obesity-related cancers. The most common obesity-related cancer deaths were from breast (n = 240, 5.5% of all deaths) and pancreatic cancers (n = 137, 3.2%).
Table 1.
Baseline Characteristics by Tertiles of Neighborhood Walkability (N=13,832).
| Neighborhood Walkability Tertiles | ||||
|---|---|---|---|---|
|
|
||||
| Baseline Characteristics | NYUWHS Total (n=13,832) | Q1 [−6.19, −1.52] (n=4,610) | Q2 [−1.52, 0.63] (n=4,608) | Q3 [0.63, 11.31] (n=4,614) |
|
| ||||
| Age at Enrollment (in years), N (%) | ||||
| ≤40 | 1,842 (13) | 505 (11) | 561 (12) | 776 (17) |
| 40–50 | 4,673 (34) | 1,415 (31) | 1,403 (31) | 1,855 (40) |
| 50–60 | 4,638 (34) | 1,736 (38) | 1,595 (35) | 1,307 (28) |
| >60 | 2,679 (19) | 954 (21) | 1,049 (23) | 676 (15) |
| Mean (SD) | 50.9 (8.7) | 51.8 (8.5) | 51.7 (8.8) | 49.1 (8.6) |
| BMI (kg/m2), N (%) a | ||||
| <18.5 | 251 (1.8) | 50 (1.1) | 60 (1.3) | 141 (3.1) |
| 18.5–25 | 8,024 (58) | 2,603 (57) | 2,392 (52) | 3,029 (66) |
| 25–30 | 3,786 (27) | 1,376 (30) | 1,420 (31) | 990 (22) |
| >30 | 1,771 (13) | 581 (13) | 736 (16) | 454 (9.8) |
| Mean (SD) | 24.9 (4.6) | 25.2 (4.4) | 25.6 (4.7) | 24.0 (4.5) |
| MET-hours per Week, mean (SD) a | ||||
| Outdoor Walking | 6.8 (8.0) | 5.7 (6.7) | 6.4 (8.8) | 8.2 (8.3) |
| Occupational Physical Activity | 59.5 (44) | 54.8 (41) | 57.2 (44) | 66.7 (45) |
| Education, N (%) a | ||||
| High School or Less | 3,557 (32) | 1,369 (36) | 1,497 (41) | 691 (18) |
| College/Vocational/Technical School/Other | 4,571 (41) | 1,547 (40) | 1,427 (39) | 1,597 (42) |
| Graduate School | 3,146 (28) | 922 (24) | 737 (20) | 1,487 (39) |
| Race/Ethnicity, N (%) a | ||||
| Non-Hispanic White | 9,545 (79) | 3,483 (85) | 2,846 (72) | 3,216 (79) |
| Non-Hispanic African-American | 1,436 (12) | 343 (8.3) | 685 (17) | 408 (10) |
| Hispanic | 755 (6.2) | 183 (4.4) | 279 (7.0) | 293 (7.2) |
| Other | 413 (3.4) | 109 (2.7) | 172 (4.3) | 132 (3.3) |
| Menopausal Status, N (%) | ||||
| Premenopausal | 7,017 (51) | 2,128 (46) | 2,145 (47) | 2,744 (60) |
| Postmenopausal | 6,815 (49) | 2,482 (54) | 2,463 (54) | 1,870 (41) |
| Smoking Status, N (%) a | ||||
| Never Smoker | 5,898 (47) | 2,036 (48) | 2,152 (53) | 1,710 (41) |
| Ever Smoker | 6,625 (53) | 2,214 (52) | 1,933 (47) | 2,478 (59) |
| Parity at Enrollment, N (%) | ||||
| No | 4,363 (32) | 874 (19) | 1,167 (25) | 2,322 (50) |
| Yes | 9,469 (69) | 3,736 (81) | 3,441 (75) | 2,292 (50) |
| NYC Resident, N (%) | ||||
| No | 2,046 (15) | 1,862 (40) | 182 (4.0) | 2 (0.0) |
| Yes | 11,786 (85) | 2,748 (60) | 4,426 (96) | 4,612 (100) |
| Neighborhood Poverty Rate (% Below 1.00 FPL), mean (SD) b,c | 12.4 (8.9) | 7.0 (6.0) | 14.7 (8.3) | 15.3 (9.4) |
| Neighborhood Black Population Percent, mean (SD) c | 17.2 (24) | 13.4 (21) | 23.8 (30) | 14.5 (19) |
| Neighborhood White Population Percent, mean (SD) c | 69.1 (26) | 77.6 (22) | 60.4 (29) | 69.3 (24) |
| Neighborhood Household Median Income, mean (SD) c,d | 38.7 (14) | 47.2 (15) | 32.1 (7.9) | 36.9 (12) |
| Population Count, mean (SD) c | 46.6 (33) | 15.8 (11) | 42.6 (17) | 81.4 (25) |
| Population Density, mean (SD) c | 16.2 (12) | 5.4 (3.6) | 14.0 (5.4) | 29.3 (9.4) |
| Alcohol Intake, N (%) a,e | ||||
| Below RDA (≤14 g/day) | 10,353 (89) | 3,714 (93) | 3,506 (93) | 3,133 (81) |
| Above RDA (>14 g/day) | 1,298 (11) | 302 (7.5) | 253 (6.7) | 743 (19) |
| DASH Score, N (%) f | ||||
| Below Median (≤24) | 7,391 (55) | 2,521 (56) | 2,510 (56) | 2,360 (52) |
| Above Median (>24) | 6,176 (46) | 2,018 (45) | 2,003 (44) | 2,155 (48) |
Daily alcohol intake was missing in 2,181; race/ethnicity was missing in 1,683; education level was missing in 2,558; smoking was missing in 1,309; MET-hours for walking was missing in 2,817; MET (Metabolic Equivalent of Task)-hours for occupational physical activity was missing in 3,365; and MET-hours for total physical activity (strenuous, moderate, or mild) was missing in 3,143 women. Q1 (Quantile 1 or Tertile 1); Q2 (Quantile 2 or Tertile 2); Q3 (Quantile 3 or Tertile 3). BMI (Body Mass Index).
Percent of population residing in neighborhood in 1989 with a ratio of income to federal poverty level (FPL) below 1.
Census block groups aggregated to 1-km radial buffers. Population count and density are represented in thousands.
1989 median household income in thousands of US dollars.
Recommended Dietary Allowance (RDA)
DASH (Dietary Approaches to Stop Hypertension) score was missing in 265 women
Baseline neighborhood walkability score was inversely associated with the risk of death from any cause, obesity-related disease, and obesity-related cancer (Table 2, Model 2). Compared to women in the bottom tertile of neighborhood walkability, those residing in the highest tertile had a 4% lower all-cause mortality rate [HR=0.96 (95% CI 0.93–0.99)], a 9% lower obesity-related mortality rate [HR=0.91 (95% CI 0.86–0.97)], and a 28% lower obesity-related cancer mortality rate [HR= 0.72 (95% CI 0.62–0.85)]. The inverse association was stronger among women who never moved from the baseline address. For instance, women who never moved and lived in the highest walkability tertile compared to the reference walkability group had a 17% lower all-cause mortality rate [HR=0.83 (95% CI 0.80–0.86)], a 23% lower obesity-related disease mortality rate [HR=0.77 (95% CI 0.71–0.84)], and a 39% lower obesity-related cancer mortality rate [HR=0.61 (95% CI 0.50–0.74)] (Table 2, Model 3). The pattern of effect estimates with censoring at moving time, at withdrawal from active follow-up, or at lost to follow-up, was similar (Table 2, Model 4). We found similar associations in models considering neighborhood walkability as a continuous and as a dichotomized variable. In contrast, we estimated no protective effect for the risk of death from cardiometabolic disease in any of the models.
Table 2.
Hazard Ratios (HR) for the Risk of Death from Any Cause, Obesity-related Disease, Cardiometabolic Disease, and Obesity-related Cancer by Neighborhood Walkability (NW) (N=13,832)
| Cause-specific Mortality | n | Deaths (n) | NW >Median (vs. ≤ Median) HR (95% CI) | NW Tertile 1 Ref. | NW Tertile 2 HR (95% CI) | NW Tertile 3 HR (95% CI) | Per SD a (Continuous NW) HR (95% CI) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| All-Cause | |||||||
| Model 1 b | 13,832 | 4,338 | 0.85 (0.75–0.96) | ref. | 1.09 (0.97–1.22) | 0.82 (0.73–0.92) | 0.91 (0.85–0.98) |
| Model 2 c | 13,832 | 4,338 | 0.94 (0.91–0.97) | ref. | 1.01 (0.94–1.09) | 0.96 (0.93–0.99) | 0.98 (0.96–1.00) |
| Model 3 in Non-Movers d | 6,440 | 2,304 | 0.87 (0.81–0.94) | ref. | 0.95 (0.90–1.00) | 0.83 (0.80–0.86) | 0.94 (0.92–0.96) |
| Model 4 Censoring at LTF, Moving, Withdrawal e | 13,832 | 1,878 | 0.92 (0.87–0.96) | ref. | 0.99 (0.91–1.07) | 0.90 (0.83–0.97) | 0.97 (0.94–1.00) |
| Obesity-related Disease | |||||||
| Model 1 b | 13,832 | 3,102 | 0.83 (0.71–0.96) | ref. | 1.06 (0.93–1.20) | 0.77 (0.68–0.87) | 0.89 (0.82–0.96) |
| Model 2 c | 13,832 | 3,102 | 0.92 (0.87–0.97) | ref. | 0.97 (0.90–1.04) | 0.91 (0.86–0.97) | 0.96 (0.93–0.99) |
| Model 3 in Non-Movers d | 6,440 | 1,644 | 0.84 (0.74–0.95) | ref. | 0.90 (0.85–0.96) | 0.77 (0.71–0.84) | 0.91 (0.87–0.95) |
| Model 4 Censoring at LTF, Moving, Withdrawal e | 13,832 | 1,316 | 0.88 (0.82–0.94) | ref. | 0.93 (0.84–1.03) | 0.84 (0.79–0.89) | 0.95 (0.91–0.98) |
| Cardiometabolic Disease | |||||||
| Model 1 b | 13,832 | 1,509 | 0.85 (0.68–1.05) | ref. | 1.14 (0.95–1.37) | 0.76 (0.63–0.91) | 0.87 (0.78–0.99) |
| Model 2 c | 13,832 | 1,509 | 0.97 (0.82–1.14) | ref. | 1.02 (0.92–1.14) | 0.94 (0.81–1.08) | 0.96 (0.89–1.04) |
| Model 3 in Non-Movers d | 6,440 | 817 | 0.89 (0.67–1.18) | ref. | 1.04 (0.92–1.18) | 0.80 (0.63–1.03) | 0.90 (0.81–1.01) |
| Model 4 Censoring at LTF, Moving, Withdrawal e | 13,832 | 654 | 0.89 (0.70–1.13) | ref. | 1.09 (0.91–1.29) | 0.84 (0.66–1.07) | 0.93 (0.83–1.04) |
| Obesity-related Cancer | |||||||
| Model 1 b | 13,832 | 793 | 0.78 (0.70–0.87) | ref. | 0.89 (0.78–1.03) | 0.75 (0.65–0.85) | 0.89 (0.84–0.94) |
| Model 2 c | 13,832 | 793 | 0.76 (0.70–0.82) | ref. | 0.78 (0.67–0.92) | 0.72 (0.62–0.85) | 0.90 (0.81–0.99) |
| Model 3 in Non-Movers d | 6,440 | 483 | 0.67 (0.61–0.73) | ref. | 0.74 (0.61–0.89) | 0.61 (0.50–0.74) | 0.86 (0.74–0.99) |
| Model 4 Censoring at LTF, Moving, Withdrawal e | 13,832 | 417 | 0.77 (0.72–0.83) | ref. | 0.74 (0.61–0.90) | 0.71 (0.59–0.85) | 0.92 (0.81–1.05) |
Continuous neighborhood walkability variable scaled to the standard deviation (SD = 3.11).
Model 1 did not adjust for covariates (univariate model).
Model 2 adjusted for age, race/ethnicity, education level, smoking status, alcohol intake, menopausal status, parity, percent of black population in neighborhood, and percent below the poverty level living in neighborhood at baseline.
Model 3 adjusted for all model 2 covariates in a subset of participants who stayed at the baseline address throughout all the follow-up.
Model 4 adjusted for all model 2 covariates and censored participants at lost to follow-up time, withdrawal date, or moving date.
The estimated protective effect for obesity-related mortality and obesity-related cancer mortality remained similar in models accounting for competing risks (eTable 6, eAppendix, Model 1), in GEE models (eTable 6, eAppendix, Model 2), in analyses adjusted additionally for median household income in neighborhood, in models adjusted for DASH score or occupational physical activity (eTable 7, eAppendix,), and when we used multiple imputation for missing covariates (eTable 6, eAppendix,, Model 2–4). The distribution of demographic and lifestyle variables in women who moved during follow-up was similar with that in women who did not move (eTable 8, eAppendix,). In analyses using propensity score matching to balance covariate distributions (including moving during the follow-up) among women with high and low level of neighborhood walkability, inverse associations between neighborhood walkability and risk of death were also consistent (eTable 10, eAppendix,). In exploratory analyses, we further evaluated the association of neighborhood walkability with the risk of death from specific obesity-related cancer types. The protective effects of neighborhood walkability on risk of death due to ovarian cancer, pancreatic cancer, and multiple myeloma were apparent, though the sample size was limited for each group (eTable 11, eAppendix,).
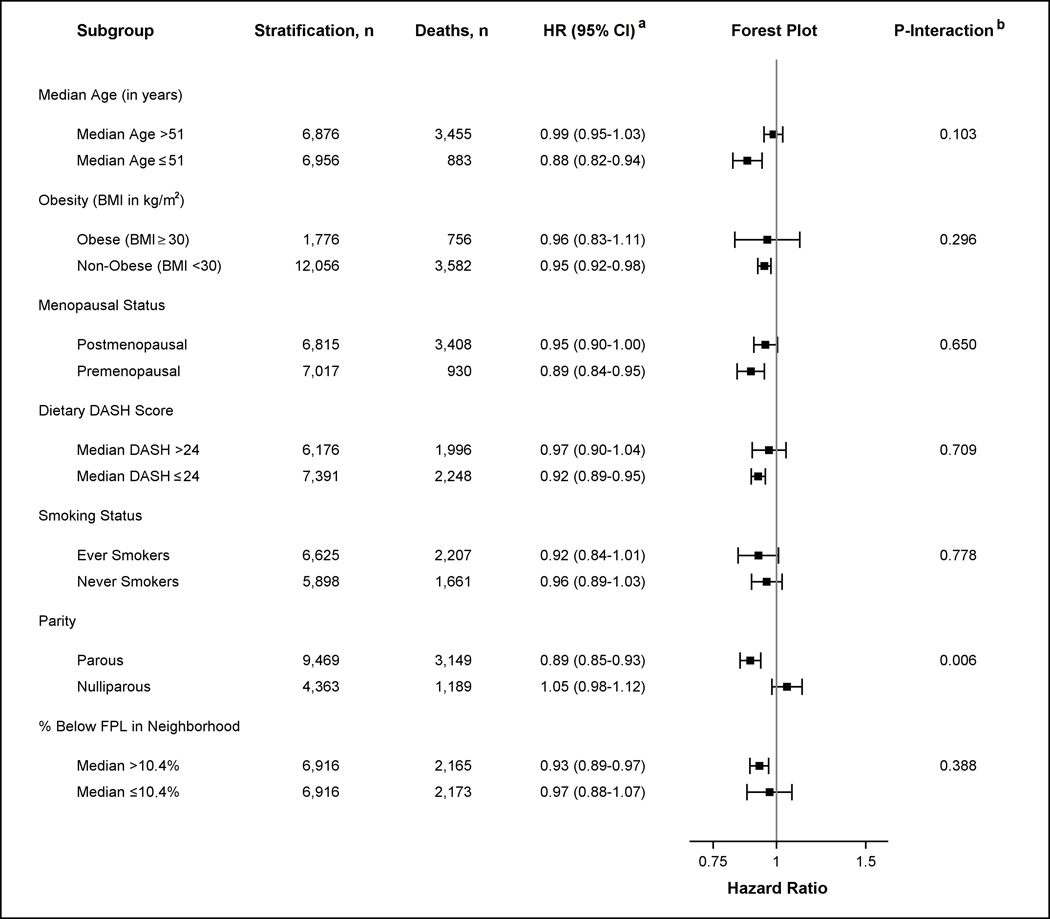
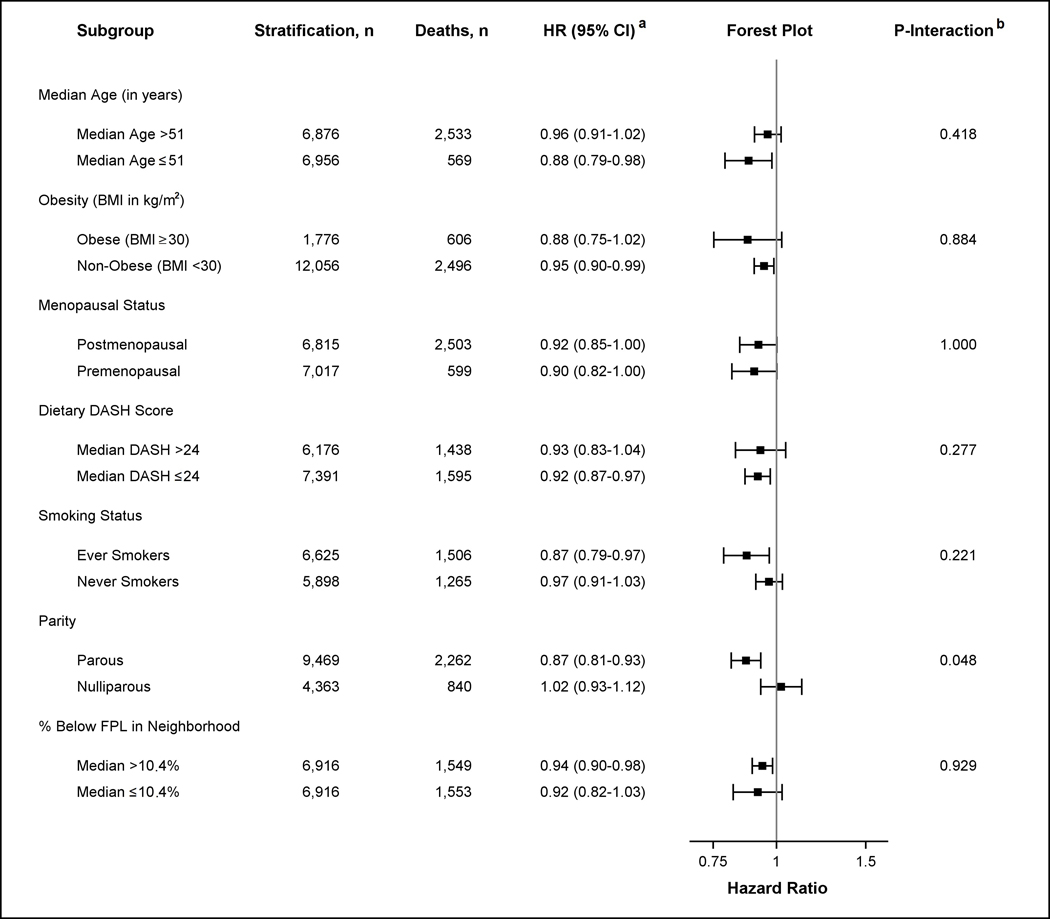
The inverse association between higher neighborhood walkability level and risk of death from any cause did not differ by age, obesity, menopausal status, DASH score, or smoking status (Figure 1). The association between neighborhood walkability and risk of death from any cause in parous women, with a hazard ratio of 0.89 (95% CI 0.85–0.93), differed from that in nulliparous women, with a hazard ratio of 1.05 (95% CI 0.98–1.12) (p-for interaction = 0.01, Figure 1). Similarly, the association between neighborhood walkability and risk of death from obesity-related diseases was stronger in parous women, with a hazard ratio of 0.87 (95% CI 0.81–0.93), compared to a hazard ratio of 1.02 (95% CI 0.93–1.12) in nulliparous women for obesity-related mortality (p-for interaction = 0.05, Figure 2). In contrast, the inverse association between neighborhood walkability and risk of death from obesity-related cancer did not differ by parity status (eFigure 1, eAppendix).
Figure 1.
Hazard Ratios (HR) for Risk of Death from Any Cause comparing High and Low Neighborhood Walkability (NW) by Age, Obesity, Menopausal Status, DASH Score, Smoking Status, Parity, and Poverty Level.
a Stratified models were conducted to assess the association between dichotomized NW (as measured by high and low median level) and risk of death from any cause by potential effect modifiers. Models adjusted for all covariates (age, race–ethnicity, education level, smoking status, alcohol intake, menopausal status, parity, percent of black population in neighborhood, and percent below the poverty level living in neighborhood at baseline). Note that stratified analyses did not adjust for the stratified effect modifier. The x-axis in the forest plot shows the untransformed hazard ratios on the log-scale.
b P-value of the coefficient for the cross-product of dichotomized NW and the effect modifier is shown. Interaction models were computed treating effect modifiers as dichotomized variables. All interactions had 13,832 observations except for analyses including dietary DASH score (n = 13,567) and smoking status (n = 12,523) which were restricted to non-missing values.
Figure 2.
Hazard Ratios (HR) for Risk of Death from Obesity-related Disease comparing High and Low Neighborhood Walkability (NW) by Age, Obesity, Menopausal Status, DASH Score, Smoking Status, Parity, and Poverty Level.
a Stratified models were conducted to assess the association between dichotomized NW (as measured by high and low median level) and risk of death from obesity-related disease by potential effect modifiers. Models adjusted for all covariates (age, race–ethnicity, education level, smoking status, alcohol intake, menopausal status, parity, percent of black population in neighborhood, and percent below the poverty level living in neighborhood at baseline). Note that stratified analyses did not adjust for the stratified effect modifier. The x-axis in the forest plot shows the untransformed hazard ratios on the log-scale.
b P-value of the coefficient for the cross-product of dichotomized NW and the effect modifier is shown. Interaction models were computed treating effect modifiers as dichotomized variables. All interactions had 13,832 observations except for analyses including dietary DASH score (n = 13,567) and smoking status (n = 12,523) which were restricted to non-missing values.
We explored whether the association of neighborhood walkability and the risk of death was mediated by outdoor walking, diabetes, and BMI (eTable 12, eAppendix,). We identified mediation by average BMI, outdoor walking, and diabetes for the inverse association between neighborhood walkability and all-cause and obesity-related disease mortality. In mediation analyses for obesity-related cancer mortality, we identified mediation by both BMI and outdoor walking but not diabetes. These findings were consistent in analyses using accelerated failure time models (eTable 13, eAppendix,). Using this method, we additionally estimated mediation effects in the associations between neighborhood walkability and mean survival ratios under exposure mediator interactions. For instance, while accounting for exposure–mediator interaction in obesity-related cancer mortality, the natural indirect effect of average BMI and outdoor walking was apparent, consistent with a hypothesized mediation effect of these factors.
Discussion
In this prospective cohort study, we found that residing in a neighborhood with higher walkability level, as measured by the BEH-NWI, was associated with a lower risk of death from any cause, obesity-related illness, and in particular, obesity-related cancers. We also observed that the inverse association for the risk of death from any cause and obesity-related diseases was modifiable by parity status. Exploratory mediation analyses suggested that the associations were partly explained by BMI and outdoor walking.
Several previous studies have evaluated neighborhood walkability in relation to all-cause mortality and CVD mortality endpoints. Neighborhood walkability, as measured by WalkScore, was inversely related to CVD mortality in ecologic studies, particularly in women in one study,20 but only in models unadjusted for lifestyle, demographic or socio-economic status covariates.20,21 Unlike WalkScore and other walkability measures, our measure captured neighborhood walkability prior to the mid-2000s, allowing us to assess its association with mortality in a cohort followed up since 1990. In another recent ecologic study using 4-year aggregate mortality data, neighborhood walkability was associated with CVD mortality but only in those living in medium or higher SES communities.19 Two prospective cohort studies of short follow-up time (5 years or less) found that urban residential environments, such as green and walkable spaces, were associated with reaching longevity.17,18 In a prospective study adjusting for socio-demographic variables, land use mix was related to a lower all-cause mortality with 5 years of follow-up but only in individuals ≥ 85 years of age.16 Overall, most of the studies conducted previously on neighborhood walkability and mortality were either ecologic studies, or were prospective studies with short follow-up time. The majority of these studies used a broad neighborhood measure that is not specific to neighborhood walkability and failed to adjust for potential confounders at both the individual and neighborhood level. In comparison, the present study addressed these concerns.
The strongest associations found with neighborhood walkability in the NYUWHS cohort were for obesity-related cancer mortality. Our study is the first prospective cohort study that reports this inverse association. Mounting evidence supports the hypothesis that walking is protective against developing obesity-related cancers.29–31 Consistent with this premise, sitting for long periods of time has been linked to higher risk of cancer at any site in women, including multiple myeloma, and ovarian cancers.32 In our exploratory analyses, we found an inverse association between neighborhood walkability and risk of death from pancreatic cancer, ovarian cancer, and multiple myeloma, but sample size for these cancer cases was small. Interestingly, one ecologic study found that NYC neighborhoods with higher walkability were correlated with lower multiple myeloma incidence and mortality.15 While mortality data for highly fatal cancers, such as pancreatic cancer, would reflect cancer incidence quite accurately, for cancers that are associated with long-term survival, the most useful endpoint to study etiology would be incidence. Our data warrant the need for future large studies using incidence as endpoint.
Cardiometabolic diseases and obesity-related cancers share both obesity and physical activity as risk factors. However, several studies that assessed neighborhood walkability in relation to cardiometabolic disease led to inconsistent findings.13,33–36 We did not observe an association between neighborhood walkability and risk of death from cardiometabolic disease. It is possible that the effect of medications on long-term survival could mask the association between neighborhood walkability and cardiometabolic incidence when mortality data are used. For instance, glucose control drugs such as metformin, have been shown to decrease risk of mortality in diabetes patients,37 and the use of beta-blockers or blood thinners38 can help prevent secondary cardiovascular events. In addition, there may be inaccuracy and uncertainty using data from death certificates to ascertain deaths due to CVDs,39 leading to non-differential misclassification of the outcome and a bias towards the null.
Possible mechanisms that underlie the association of neighborhood walkability with obesity and obesity-related mortality include the potential effects of obesity and walking on hormone levels, chronic inflammation, or metabolic changes such as insulin resistance.40–44 In mediation analyses, we found that BMI and outdoor walking partly mediated the association between neighborhood walkability and risk of death. Neighborhood walkability has been consistently inversely associated with BMI.13,45,46 Obesity was linked to increased risk of premature death from heart disease and cancer in women.47,48 Furthermore, walking has also been related to reduced risk of death from any cause in both healthy and unhealthy individuals.49,50 In our study, the association between neighborhood walkability and risk of death remained after controlling for these mediators. It is possible that while our data provided evidence for mediation by walking and obesity, there may be other effects of neighborhood walkability on mortality that were not captured such as strenuous outdoor physical activity via biking or running.
We observed consistent associations of neighborhood walkability and risk of death across all potential effect modifiers. Interestingly, we found that the inverse association between neighborhood walkability and risk of death from any cause and obesity-related disease was apparent only in parous women. It is not clear why the effects of neighborhood walkability were observed only in this group. Neighborhood walkability may have a stronger influence on walking in parous women than that in nulliparous women due to differences in lifestyle.51,52 In our study, we observed that the positive association between neighborhood walkability and self-reported outdoor walking was stronger in parous women compared to that in nulliparous women (eTable 14, eAppendix,). Further research should investigate other factors that can explain effect modification by parity status.
There were several limitations in the study. We did not incorporate neighborhood walkability changes in residential addresses over time. However, baseline address captured long-term effects of neighborhood walkability, accounting for a substantial follow-up time in the cohort (18.3 years; 68% of follow-up time). We observed consistent associations in non-movers and when we censored women when they moved. We acknowledge that similarly to all observational studies, our study might be susceptible to unmeasured confounding, including factors that may be related to choice of residential neighborhoods, and we did not have additional measures of socioeconomic status beyond educational attainment. However, analyses adjusting for additional potential confounders (median household income, healthy diet, and occupational physical activity) indicated congruent results. Other potential confounders not accounted for in the study, such as air pollution or crime, may have driven results towards the null as negative confounders of the association between walkable areas and mortality. We also note that we used data from 2003 instead of 1990 to characterize baseline intersection density. The spatial accuracy and network connectivity data of older street network shape files created before 2003 are often poor. We have previously described that the higher spatial and network connectivity accuracy of the more recent street network shape file may provide more accurate estimates of intersection density for an earlier time period.25
We acknowledge that these findings may not be generalizable to men or non-metropolitan areas. In the US, 84% of the population lives in Metropolitan Areas53 and worldwide, 3.6 billion people live in urban spaces.54 These data underscore the relevance of conducting studies in urban areas where a sizeable proportion of the population walks on a daily basis. The wide range of neighborhood walkability allowed us to assess dose–response relationships, which could be obscured in studies focusing on populations with a limited range of neighborhood walkability. Potential measurement error in the self-reported mediator outdoor walking would underestimate the mediated effect. Similarly, the use of a 1km egocentric buffer may lead to uncertain geographic context problem but as a result of measurement error we expect it to be non-differential by outcome driving the results towards the null. Last, we acknowledge the limitation that baseline BMI and diabetes status might have been influenced by neighborhood walkability in residences prior to the baseline location, though our data indicated that participants likely resided at the baseline location for a long period of time. Future studies with longitudinal measurements of mediators and walkability should be conducted.
Our study has several strengths. The NYUWHS is a prospective cohort of large sample size and long follow-up. We were also able to adjust for a variety of confounders at both neighborhood and individual level. We conducted a number of sensitivity analyses to confirm the observed association. Our neighborhood walkability measure was validated and constructed using residential address at the individual level for each participant. The proportion of African–Americans (12%) in the present study is close to that of the national average, though future studies should be conducted including men in addition to using a more diverse population. Last, the focus on women with more homogeneous risk factors should enhance the internal validity of the study.
Conclusion
In conclusion, our results are consistent with the hypothesis that neighborhood walkability is protective against risk of all-cause mortality, obesity-related disease mortality, and particularly obesity-related cancer mortality in women. Our findings suggest possible avenues of inquiry for research into urban health interventions to lessen obesity morbidity and mortality.
Supplementary Material
Acknowledgments
Source of Funding: the results reported herein correspond to specific aims of grant UM1CA182934-01A1 to investigator Dr. Zeleniuch-Jacquotte and Dr. Yu Chen from NIH. This work was also supported by grants UM1CA182934-05-S1, P30CA016087, and P30ES000260 from NIH.
Footnotes
Data Availability: due to confidentiality agreement with participants, data are only available upon request from the corresponding author and with IRB approval. Code used to perform main models is publicly available on a GitHub repository (https://github.com/sia34/neighborhoodwalkabilityandmortality).
Conflicts of Interest: none declared.
References
- 1.Hales CMCM, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics. 2020. https://www.cdc.gov/nchs/products/databriefs/db360.htm. [PubMed] [Google Scholar]
- 2.Haslam DW, James WPT. Obesity. The Lancet 2005;366(9492):1197–1209. [DOI] [PubMed] [Google Scholar]
- 3.Basen-Engquist K, Chang MJCOR. Obesity and Cancer Risk: Recent Review and Evidence. 2011;13(1):71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer — Viewpoint of the IARC Working Group. N Engl J Med 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organisation for Economic Co-operation and Development (OECD). Obesity Update 2017. https://www.oecd.org/health/health-systems/Obesity-Update-2017.pdf.
- 6.Massetti GM, Dietz WH, Richardson LC. Excessive Weight Gain, Obesity, and Cancer: Opportunities for Clinical Intervention. JAMA 2017;318(20):1975–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steele CB TC, Henley SJ, et al. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity — United States, 2005–2014.. MMWR Morb Mortal Wkly Rep 2017;66:1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith TC, Wingard DL, Smith B, Kritz-Silverstein D, Barrett-Connor E. Walking decreased risk of cardiovascular disease mortality in older adults with diabetes. Journal of clinical epidemiology 2007;60(3):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Shaper AG, Walker M. Physical Activity and Mortality in Older Men With Diagnosed Coronary Heart Disease. Circulation 2000;102(12):1358–1363. [DOI] [PubMed] [Google Scholar]
- 10.Sjöwall D, Hertz M, Klingberg T. No Long-Term Effect of Physical Activity Intervention on Working Memory or Arithmetic in Preadolescents. Front Psychol 2017;8(1342). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank LD, Engelke PO. The Built Environment and Human Activity Patterns: Exploring the Impacts of Urban Form on Public Health. 2001;16(2):202–218. [Google Scholar]
- 12.Freeman L, Neckerman K, Schwartz-Soicher O, et al. Neighborhood Walkability and Active Travel (Walking and Cycling) in New York City. J Urban Health 2013;90(4):575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creatore MI, Glazier RH, Moineddin R, et al. Association of Neighborhood Walkability With Change in Overweight, Obesity, and Diabetes. JAMA 2016;315(20):2211–2220. [DOI] [PubMed] [Google Scholar]
- 14.Frank LD, Andresen MA, Schmid TL. Obesity relationships with community design, physical activity, and time spent in cars. American Journal of Preventive Medicine 2004;27(2):87–96. [DOI] [PubMed] [Google Scholar]
- 15.Kamath GR, Renteria AS, Jagannath S, et al. Where you live can impact your cancer risk: a look at multiple myeloma in New York City. Annals of Epidemiology 2020;48:43–50.e4. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y-T, Prina AM, Jones A, et al. Land use mix and five-year mortality in later life: Results from the Cognitive Function and Ageing Study. Health & place 2016;38:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takano T, Nakamura K, Watanabe M. Urban residential environments and senior citizens’ longevity in megacity areas: the importance of walkable green spaces. J Epidemiol Community Health 2002;56(12):913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhardwaj R, Amiri S, Buchwald D, Amram O. Environmental Correlates of Reaching a Centenarian Age: Analysis of 144,665 Deaths in Washington State for 2011–2015. International journal of environmental research and public health 2020;17(8):2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koohsari MJ, Nakaya T, Hanibuchi T, et al. Local-Area Walkability and Socioeconomic Disparities of Cardiovascular Disease Mortality in Japan. Journal of the American Heart Association 2020;9(12):e016152-e016152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Villegas P, Cabrera-León A, Gil García E . [Association between neighborhood walkability and mortality due to different causes in Andalusia (Spain)]. Gaceta Sanitaria 2021;35(3):260–263. [DOI] [PubMed] [Google Scholar]
- 21.Gaglioti AH, Xu J, Rollins L, et al. Neighborhood Environmental Health and Premature Death From Cardiovascular Disease. Prev Chronic Dis 2018;15:170220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awuor L, Melles S. The influence of environmental and health indicators on premature mortality: An empirical analysis of the City of Toronto’s 140 neighborhoods. Health & Place 2019;58:102155. [DOI] [PubMed] [Google Scholar]
- 23.Nelson K, Taylor L, Lurie N, et al. Neighborhood environment and health status and mortality among veterans. Journal of general internal medicine 2011;26(8):862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toniolo PG, Pasternack BS, Shore RE, et al. Endogenous hormones and breast cancer: A prospective cohort study. Breast Cancer Res Treat 1991;18(1):S23–S26. [DOI] [PubMed] [Google Scholar]
- 25.Rundle AG, Chen Y, Quinn JW, et al. Development of a Neighborhood Walkability Index for Studying Neighborhood Physical Activity Contexts in Communities across the U.S. over the Past Three Decades. Journal of Urban Health 2019;96(4):583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riboli E, Toniolo P, Kaaks R, et al. Reproducibility of a food frequency questionnaire used in the New York University Women’s Health Study: Effect of self-selection by study subjects. European Journal of Clinical Nutrition 1997;51(7):437–442. [DOI] [PubMed] [Google Scholar]
- 27.Fung TT CS, McCulloguh ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 28.Nevo D, Liao X, Spiegelman D. Estimation and Inference for the Mediation Proportion. The international journal of biostatistics 2017;13(2):/j/ijb.2017.13.issue-2/ijb-2017–0006/ijb-2017–0006.xml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ukawa S, Tamakoshi A, Wakai K, Kurozawa Y. Associations of daily walking and television viewing time with liver cancer mortality: findings from the Japan Collaborative Cohort Study. Cancer Causes Control 2014;25(7):787–93. [DOI] [PubMed] [Google Scholar]
- 30.Williams PT. Reduced risk of incident kidney cancer from walking and running. Med Sci Sports Exerc 2014;46(2):312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi H, Kuriyama S, Tsubono Y, et al. Time spent walking and risk of colorectal cancer in Japan: The Miyagi Cohort Study. European Journal of Cancer Prevention 2007;16(5). [DOI] [PubMed] [Google Scholar]
- 32.Patel AV, Hildebrand JS, Campbell PT, et al. Leisure-Time Spent Sitting and Site-Specific Cancer Incidence in a Large U.S. Cohort. Cancer Epidemiol Biomarkers Prev 2015;24(9):1350–9. [DOI] [PubMed] [Google Scholar]
- 33.Méline J, Chaix B, Pannier B, et al. Neighborhood walk score and selected Cardiometabolic factors in the French RECORD cohort study. BMC Public Health 2017;17(1):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kartschmit N, Sutcliffe R, Sheldon MP, et al. Walkability and its association with prevalent and incident diabetes among adults in different regions of Germany: results of pooled data from five German cohorts. BMC endocrine disorders 2020;20(1):7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.den Braver NR, Lakerveld J, Rutters F, et al. Built environmental characteristics and diabetes: a systematic review and meta-analysis. BMC Med 2018;16(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howell NA, Tu JV, Moineddin R, Chu A, Booth GL. Association Between Neighborhood Walkability and Predicted 10-Year Cardiovascular Disease Risk: The CANHEART (Cardiovascular Health in Ambulatory Care Research Team) Cohort. J Am Heart Assoc 2019;8(21):e013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hippisley-Cox J, Coupland C. Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: cohort study in primary care. BMJ 2016;354:i3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hennekens CH, Dyken ML, Fuster V. Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1997;96(8):2751–3. [DOI] [PubMed] [Google Scholar]
- 39.Pagidipati NJ, Gaziano TA. Estimating Deaths From Cardiovascular Disease: A Review of Global Methodologies of Mortality Measurement. Circulation 2013;127(6):749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444(7121):875–880. [DOI] [PubMed] [Google Scholar]
- 41.Hopkins BD, Goncalves MD, Cantley LC. Obesity and Cancer Mechanisms: Cancer Metabolism. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34(35):4277–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wertheim BC, Martínez ME, Ashbeck EL, et al. Physical activity as a determinant of fecal bile acid levels. Cancer Epidemiol Biomarkers Prev 2009;18(5):1591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winzer BM, Whiteman DC, Reeves MM, Paratz JD. Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control 2011;22(6):811–26. [DOI] [PubMed] [Google Scholar]
- 44.National Cancer Institute (NCI). Physical activity and cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/physical-activity-fact-sheet#r2.
- 45.Nichani V, Turley L, Vena JE, McCormack GR. Associations between the neighbourhood characteristics and body mass index, waist circumference, and waist-to-hip ratio: Findings from Alberta’s Tomorrow Project. Health Place 2020;64:102357. [DOI] [PubMed] [Google Scholar]
- 46.Tarlov E, Silva A, Wing C, et al. Neighborhood Walkability and BMI Change: A National Study of Veterans in Large Urban Areas. Obesity (Silver Spring) 2020;28(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Rexrode KM, Dam RMv, Li TY, Hu FB. Abdominal Obesity and the Risk of All-Cause, Cardiovascular, and Cancer Mortality. 2008;117(13):1658–1667. [DOI] [PubMed] [Google Scholar]
- 48.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med 2000;160(14):2117–28. [DOI] [PubMed] [Google Scholar]
- 49.Patel AV, Hildebrand JS, Leach CR, et al. Walking in Relation to Mortality in a Large Prospective Cohort of Older U.S. Adults. American Journal of Preventive Medicine 2018;54(1):10–19. [DOI] [PubMed] [Google Scholar]
- 50.Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KMV. Relationship of Walking to Mortality Among US Adults With Diabetes. Archives of Internal Medicine 2003;163(12):1440–1447. [DOI] [PubMed] [Google Scholar]
- 51.Jaffe DH, Neumark YD, Eisenbach Z, Manor O. Parity-related mortality: shape of association among middle-aged and elderly men and women. Eur J Epidemiol 2009;24(1):9–16. [DOI] [PubMed] [Google Scholar]
- 52.Grundy E, Kravdal Ø. Reproductive history and mortality in late middle age among Norwegian men and women. Am J Epidemiol 2008;167(3):271–9. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Census Bureau. Paterns of Metropolitan and Micropolitan Population Change: 2000 to 2010. 2010 Census Special Reports Washington DC: US Census Bureau, 2012. [Google Scholar]
- 54.Rydin Y, Bleahu A, Davies M, et al. Shaping cities for health: complexity and the planning of urban environments in the 21st century. Lancet 2012;379(9831):2079–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.