Abstract
Overcoming the radiosensitivity of chondrosarcoma (CS), the second most common primary bone tumor, is needed. Radioresistance is attributed to cancer stem cells (CSCs) in many malignancies. Disulfiram (DSF), an FDA-approved anti-alcoholism drug, complexed with Cu (DSF/Cu) can radiosensitize epithelial CSCs. This prompted us to investigate the radiosensitizing effect of DSF/Cu on CS CSCs (CCSCs). The radiosensitizing effects of DSF/Cu on CCSCs were investigated in vitro using cell lines SW1353 and CS-1. Stemness was identified independently by flow cytometry for CCSCs (ALDH+CD133+), sphere-forming ability, and Western blot analysis of stemness gene protein expression. The radiosensitizing effect of DSF/Cu was studied in an orthotopic CS xenograft mouse model by analyzing xenograft growth and residual xenografts for stemness. CCSCs were found to be resistant to single-dose (IR) and fractionated irradiation (FIR). IR and FIR increased CS stemness. Combined with DSF/Cu in vitro and in vivo, IR and FIR eliminated CS stemness. RT + DSF/Cu was safer and more effective than either RT ± DSF in inhibiting growth of orthotopic CS xenografts. In conclusion, DSF/Cu radiosensitizes CCSCs. These results can be translated into clinical trials for CS patients requiring RT for improved outcomes.
Keywords: Stemness, NF-κB, CCSCs, Radioresistance, Radiosensitizing
1. Introduction
Chondrosarcoma (CS) is the second most common primary bone tumor in adults [1,2] and characteristically produces cartilage matrix from neoplastic tissue devoid of osteoid. Radiotherapy (RT) is standard care for cases of incompletely resected or inoperable tumors [3,4]. However, RT for such tumors is not associated with improved survival, and only patients receiving both high-dose and advanced RT had improved survival compared to patients who did not receive RT [4].
Improving CS radiosensitivity is clinically important. However, an effective approach to overcome CS radioresistance is currently lacking as its basis remains unclear. Only a few studies have attempted to understand radioresistance mechanisms and restore CS radiosensitivity. The photosensitizer acridine orange (AO) was shown to be excited by X-rays to release cytotoxic singlet oxygen and the AO/low-dose X-Ray (1–5Gy) combination was found to be more effective in killing mouse osteosarcoma cells in vitro and in vivo [5]. Later, it was revealed that silencing the antiapoptotic genes BCL-2, BCL-xL or XIAP enhanced human CS cell radiosensitivity in vitro [6]. It has also been shown that p16 plays a role in radioresistance, since p16-deficient human CS cells were intrinsically more radiation-resistant than normal chondrocytes. Restoring p16 activity restored radiosensitivity in vitro [7]. More recently, mutated defective Rb pathway was found in highly radioresistant CS compared to an intact Rb pathway present in less radioresistant CS [8]. Additionally, it was shown that the poly ADP-ribose polymerase (PARP) inhibitor olaparib sensitized a human CS cell line in vitro to conventional photon, as well as proton and carbon ion irradiation [9]. However, all the above in vitro and the single in vivo studies demonstrated only modest radiosensitizing effects. Clearly, studies identifying radioresistance mechanisms and effective means to overcome it using in vitro and in vivo human CS-derived models and involving single-dose or fractionated irradiation (FIR) are needed.
To date, no study has focused on the role of cancer stem cells (CSCs) in CS radioresistance. There is convincing evidence, mainly from studies in hematopoietic and epithelial tumors, that CSCs are crucial in resistance to cancer therapies, including radiation [10–13]. CSCs are a distinct tumor subpopulation and share several properties with normal stem cells (SC), namely, self-renewal ability and multi-lineage differentiation, that drive tumor formation, progression, and metastasis [10, 14,15]. Considerable evidence indicates that cancer treatment resistance and recurrence are due to CSCs in many types of tumors [11–13]. Consequently, in vitro and in vivo experiments have shown that targeting CSC populations successfully rendered RT-resistant cancer cells as sensitive to radiation as non-CSCs [12,13,16].
High intracellular aldehyde dehydrogenase (ALDH) activity and cell surface CD133 (prominin 1) expression are frequently used markers for CSC isolation/identification in many tumor types, including CS [17–20]. CS CSCs (CCSCs) have been identified as CD133+ cells in primary tumors [20] or ALDH+ cells in a primary tumor-derived CS cell line [21]. However, concordant ALDH/CD133 expression identifies tumor cell populations more enriched for the CSC phenotype than either marker alone, i.e., as few as 11 ALDH+CD133+ cells isolated directly from human ovarian tumors were sufficient to initiate tumors in mice [22].
Recent convincing evidence shows that CSCs and relatively differentiated nonstem cancer cells coexist in dynamic equilibrium and are subject to bidirectional conversion [23]. Thus, any successful therapeutic strategy needs to both target preexisting CSCs and block the formation of therapy-induced CSCs (iCSCs) from nonstem cancer cells [13]. Previously, we reported that by targeting the NF-κB (nuclear factor kappa B)-stemness gene pathway, disulfiram (DSF) and copper (Cu2+) can block radiation induced breast cancer and some pancreatic ductal adenocarcinoma (PDAC) stemness [12,13]. DSF is an ALDH inhibitor and a Federal Drug Administration (FDA)-approved drug for treating alcoholism. Its toxicity against CSCs, however, does not directly involve ALDH inhibition, as initially assumed. Instead, DSF binds to Cu2+ to form a DSF-Cu complex (DSF/Cu), which is a potent proteasome inhibitor and inhibits NF-κB activation [13]. Based on these findings, the effect of DSF/Cu as a novel radiosensitizer for CS cells was investigated in in vitro- and in vivo-based experiments by comparing DSF/Cu + RT to RT alone in its ability to target CS stemness, which was defined as alterations in the levels of i) ALDH+CD133+ CSCSs, ii) sphere-forming cells, iii) stemness transcriptional factor expression in in vitro-based assays, and iv) CS cell growth and stemness inhibition in an orthotopic xenograft mouse model.
2. Materials and methods
2.1. Cell culture
The human CS cell line SW1353, derived from a primary grade II CS of the right humerus removed from a 72-year-old female Caucasian patient, was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), while the human CS cell line, CS-1, was established from a high grade and metastatic CS removed from a 62-year-old male patient [24]. SW1353 and CS-1 cell lines were cultured in a complete medium (CM) consisting of RPMI 1640 medium (Corning Incorporated, Corning, NY, USA) supplemented with 10% fetal bovine serum (Foundation FBS, Gemini Bio-Products, CA, USA) at 37 °C in a 5% CO2 humidified atmosphere.
2.2. Chemical reagents and antibodies
Tetraethylthiuram disulfide (disulfiram, DSF), copper (II) d-gluconate (Cu), NF-κB inhibitor (NF-κBi) IMD 0354, and reactive oxygen species (ROS) inhibitor (ROSi) N-Acetyl-l-cysteine (NAC) were purchased from Sigma-Aldrich (St. Louis, MO). DSF, IMD 0354, and NAC were reconstituted in DMSO for all in vitro experiments. DSF was reconstituted in olive oil for in vivo experiments. Cu was reconstituted in distilled water.
The monoclonal antibodies (mAbs) used for flow cytometry CD133 staining were human CD133/2 mouse mAb (clone: 293C3) and the isotype control mouse IgG2b mAb (clone: IS6-11E5.11) purchased from Miltenyi Biotec (Somerville, MA, USA). ALDEFLUOR™ (STEMCELL Technologies, Cambridge, MA, USA) was used to identify ALDH enzymatic activity in cells by flow cytometric analysis. Primary and secondary antibodies (Cell Signaling Technology, Danvers, MA, USA) used for Western blot analyses were: NF-κB p65 (D14E12, #8242, 1:1000), anti-HER2/ErbB2 (29D8, #2165, 1:1000), anti-c-MYC (D84C12, #5605, 1:1000), anti-SLUG (C19G7, #9585, 1:1000), β-Actin (13E5, #4970, 1:1000) and goat anti-rabbit IgG, HRP-linked antibody (#7074, 1:2000). All primary and secondary antibodies were diluted in Tris Buffered Saline with 0.1% Tween® 20 (TBST) containing 5% nonfat dry milk plus 2% bovine serum albumin (BSA) and prepared immediately before use [25].
2.3. Animals
Female nude or NSG mice, 6–8 weeks old, were obtained from the Massachusetts General Hospital Cox 7 animal facility. The Institutional Animal Care and Use Committee approved all animal studies (Protocol #2019N000025).
2.4. Irradiation (IR) treatment
In vitro IR, whether consisting of FIR (2Gy daily for 5 consecutive days) or single-doses of IR (0–12Gy) was performed on cells seeded in 6-well plates at the indicated density/well in 2 mL CM. In vivo, a single 10Gy dose was delivered locally to each mouse tumor, while the remaining body was protected from IR with lead shields. The X-RAD 320 Biological Irradiator (Precision X-ray, North Branford, CT, USA) was used for all IR experiments in this study.
2.5. Clonogenic assay
In order to obtain enough colonies for evaluation after responding to treatments with DSF/Cu and/or IR, a gradually increased number of cells/well (the number/well for both cell lines was equal under the same condition) was seeded in 6-well plates as indicated below. Next day, cells were treated for 24 h (h) with Cu (1 μM) and DSF(μM) at 0 (200 cells), 0.06 (400 cells), 0.13 (600 cells), 0.25 (800 cells) and 0.5 (1,000 cells) or a fixed dose of DSF/Cu (0.05/1 μM) and X-ray irradiation at 0 Gy (200 cells), 2Gy (400 cells), 4Gy (600 cells), or 6Gy (800 cells). Subsequently, DSF/Cu was removed and plates were washed twice with PBS, and treated cells were cultured in CM. After 10 days, the medium was removed from wells and colonies washed with PBS twice before being stained with 0.5% crystal violet. The plating efficiency (PE) and the surviving fraction (SF) were determined as described previously [12].
2.6. Fluorescence-activated cell sorting (FACS)
To sort the CSCs subpopulations from CS SW1353 and CS-1 cell lines, 6 × 106 cells were used at a time. Cells were incubated with ALDEFLUOR™, with or without the ALDH inhibitor N,N-diethylaminobenzaldehyde (DEAB) according to the manufacturer’s instructions. Following incubation, cells were collected by centrifugation and resuspended in ALDEFLUOR™ Assay Buffer and stained for CD133/2 mAb or mouse IgG2b isotype control for 10 min at 4 °C. Dead cells were excluded by propidium iodide staining (ThermoFisher Scientific, Waltham, MA, USA). Stained cells were sorted for 4 cell populations by BD Biosciences FACS Aria II cell sorter at the HSCI-CRM Flow Cytometry Core Facility, Massachusetts General Hospital, or analyzed by BD Accuri™ C6 Flow Cytometer (BD Biosciences, San Jose, CA, USA) with Flowjo V10 software.
2.7. Sphere formation assay
Sphere formation was performed by seeding 600–1000 cells/well in a 24-well ultra-low adherent plate (Corning Incorporated, Corning, NY, USA) in 500 μL of mixed medium containing 32% MethoCult medium, 20% MammoCult basal human medium with a final concentration of 2% MammoCult proliferation supplements (STEMCELL Technologies) and 48% DMEM supplemented with final concentrations of 100 pg/mL EGF, 50 ng/mL bFGF, 5 ng/mL stem cell factor, 1 × 10−6 M hydrocortisone, and 5 μg/mL insulin. The cells were cultured at 37 °C in a 1% O2 and 5% CO2 humidified atmosphere for 18 days (SW1353 cells) and 11 days (CS-1 cells). Photographs were taken and spheres were quantified by counting sphere numbers/well.
2.8. Western blot analysis
Cells were plated in 6-well plates at a density of 2 × 105 cells/well in 2 mL CM and treated as indicated. Cells were collected and lysed in lysis buffer (10 mM Tris - HCl [pH 8.2], 1% NP40, 1 mM EDTA, 0.1% BSA, 150 mM NaCl) containing 1/50 (vol/vol) protease inhibitor cocktail (Calbiochem, San Diego, CA, USA) or proteins were extracted from mouse tumors by homogenization in the presence of 500 μ,L of lysis buffer. Western blot quantitative analysis for band density of each signaling-related and stemness gene encoded proteins were performed with ImageJ (National Institutes of Health, Bethesda, MD, USA) as previously described [13,25].
2.9. Orthotopic CS xenograft mouse models
Human CS cells SW1353 (2 × 106 cells/mouse) were injected orthotopically in the right tibia bone marrow cavity of nude or NSG mice. Body weight and tumor volume were measured weekly. Radiographs of tibia tumors were obtained by a Faxitron Portable X-Ray cabinet (Hewlett Packard, McMinnville, OR, USA), with imaging film (Portal Pack PPL-2, Merry X-Ray Corporation, San Diego, CA). The output voltage was 90 kVp and the exposure time was 7s [26]. Once the tumor became palpable, tumor sizes were measured by digital calipers and calculated by tumor volume = ½ × length × width2. Treatments were initiated when tumors were detected by X-Ray post-CS cell inoculation and had approximate diameters of 5 mm. Xenograft CS bearing mice were divided randomly into 4 groups of 4 or 5 mice each; there were no significant differences in body weights between each group (p > 0.05). Mice in group 1 were left untreated. Mice in group 2 were treated once with IR (10Gy) and olive oil (vehicle control, 200 μL) orally once a day for 7 days. Mice in group 3 were treated once with IR (10Gy) and DSF (50 mg/kg/day, mornings) dissolved in olive oil orally for 7 days. Mice in group 4 were treated with IR and DSF as those in group 3 and exogenous Cu via intraperitoneal (i.p.) injection (0.03 mg/kg/day, afternoons) for 7 days. Oral administration was given by oral gavage using an 18# gauge plastic feeding tube (Instech Laboratories, Plymouth Meeting, PA, USA). When tumor diameters from the untreated mice reached 2 cm (endpoint), all mice were sacrificed, and their primary tumors were collected for further analysis. In a separate mouse survival experiment, a mouse was sacrificed when it reached the endpoint.
2.10. Samples preparations from primary xenograft tumors
For flow cytometry and sphere formation assays and Western blot analyses, non-necrotic primary xenograft tumor tissue specimens from mice of each group were collected at the time of sacrifice, minced into 3 × 3 mm pieces and digested by Collagenase IV (1 mg/mL) (Worthington Biochemical Corp. Lakewood, NJ, USA) at 37 °C for 1 h. The single-cell suspension was obtained by filtering the digested tissue through a 40 μm cell strainer.
2.11. Statistical analysis
In vitro data shown represent the mean ± standard deviation (SD) of the results obtained in at least three independent experiments, whereas the in vivo data shown represent the mean ± SEM of the results obtained in each group of mice. For the in vitro data, comparisons among groups were performed with one-way ANOVA followed by post-hoc Tukey’s test. For the in vivo data, comparisons among groups were performed using repeated-measures two-way ANOVA followed by post-hoc Tukey’s test. Sphericity was not assumed. Survival time was defined as the interval between the date of tumor cell inoculation and the date of death/sacrifice. Survival curves were plotted using the Kaplan-Meier method. Differences in survival among groups were analyzed by the log-rank test. All tests were two-tailed. A p value of <0.05 was considered statistically significant. Statistical analyses and graph generation were performed with GraphPad Prism, version 8.0 for Windows (GraphPad Software, La Jolla CA, USA).
3. Results
3.1. Identification and isolation of ALDH+CD133+ CCSCs from CS cell lines
ALDH and CD133 have been independently reported as markers for CCSCs [19,20]. However, since the combination of ALDH and CD133 was considered better than either marker alone to identify ovarian CSCs [22], the initial step was to establish that the ALDH+CD133+ subpopulation of CS cells was more enriched for cells with CCSC properties than CS cells identified by only one of these two markers. Therefore, ALDH+CD133+, ALDH+CD133−, ALDH−CD133+ and ALDH−CD133− cell populations were sorted from SW1353 and CS-1 cell lines by FACS (Fig. 1A). Each population was compared for in vitro tumorigenicity using sphere formation assays (Fig. 1B). Sorted SW1353 ALDH−CD133− cells produced the lowest number of spheres/well (2.33 ± 1.53) and thus were considered as non-CSCs. In contrast, sorted SW1353 ALDH+CD133+ cells yielded the highest number of spheres/well (87.33 ± 6.11) followed by ALDH+CD133− cells (59.00 ± 4.58) and ALDH−CD133+ cells (28.67 ± 3.05). A similar pattern was observed in CS-1 cells. Sorted ALDH−CD133− CS-1 cells produced the lowest number of spheres/well (2.00 ± 1.00) and were considered as non-CCSCs. In contrast, sorted CS-1 ALDH+CD133+ cells produced the highest number of spheres/well (81.33 ± 5.03) followed by ALDH+CD133− (54.00 ± 2.00) and ALDH−CD133+ (28.00 ± 2.00). The data showed that for both cell lines, the ALDH marker on its own is superior to CD133 for identifying CCSCs (SW1353: 2.05-fold increase, p < 0.001; CS-1: 1.92-fold increase, p < 0.001), but the combination of ALDH+CD133+ was the best marker for identifying CCSCs as it was significantly better than ALDH+ (SW1353: p < 0.001; CS-1: p < 0.001) (Fig. 1C). Each cell population was sorted by FACS and then analyzed for expression levels of stemness gene erb-b2 receptor tyrosine kinase 2 (ERBB2), MYC proto-oncogene, bHLH transcription factor (c-myc), snail family transcriptional repressor 2 (SNAI2) encoded proteins, HER2, c-MYC and SLUG, respectively, by Western blot (Fig. 1D). Consistently, ALDH+CD133+ cell populations in each cell line expressed the highest stemness gene encoded protein levels compared to all other 3 cell populations (SW1353: p < 0.001; CS-1: p < 0.001) (Fig. 1E).
Fig. 1. The ALDH+ CD133+ CS phenotype is superior to other phenotypes in identifying CCSCs.
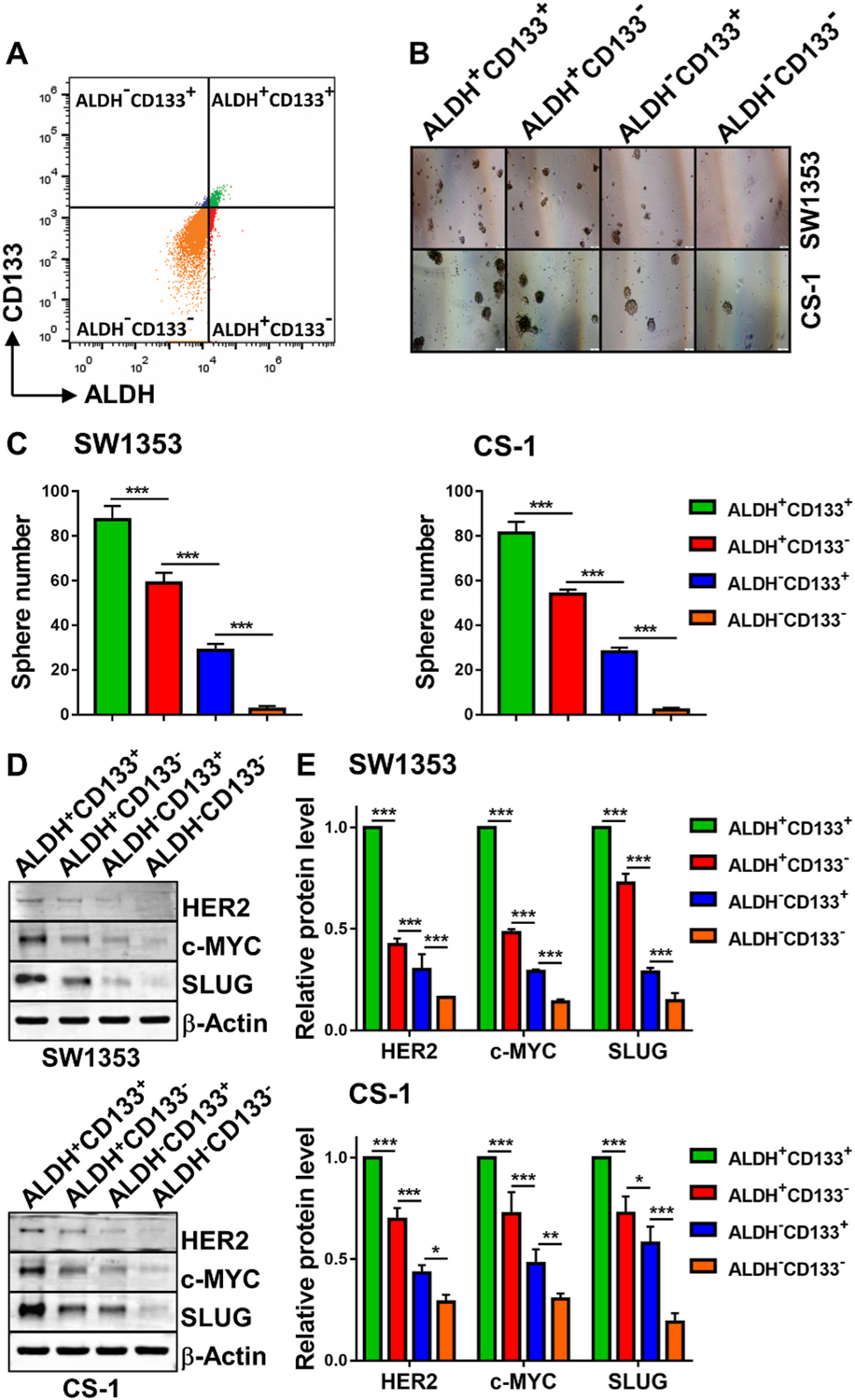
Human CS cells (6 × 106) were stained for intracellular ALDH activity and cell surface CD133 expression and then FACS sorted for the indicated subpopulations (A). Sorted cells were seeded (600 cells/well) in 24-well ultra-low attachment surface plates and cultured for sphere formation. Pictures of spheres were taken on day 18 (SW1353) or 11 (CS-1) (B). The mean ± SD of spheres/well are shown (C). Sorted cells were lysed and analyzed by Western blot for expression levels of stemness gene encoded proteins HER2, c-MYC and SLUG (D). The mean ± SD of band density of each protein from 3 independent blots (while the protein expression level of ALDH+CD133+ cells was standardized to a value of 1) are shown (E). * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
3.2. ALDH+CD133+ CCSCs are IR resistant and radiation induces CCSCs in CS cell lines in a dose dependent manner
A single IR dose (8, 10 or 12Gy) inhibited the cell growth in both CS cell lines from 1.17-fold to 1.55-fold compared to untreated cells (p < 0.01). Conversely, following IR at all three tested doses, percentages of ALDH+CD133+ CCSCs increased in the SW1353 cell line from 4.1% to 7.1–12.4% (p < 0.01) and in the CS-1 cell line from 3.2% to 7.8–10.9% (p < 0.001) in a radiation-dose dependent manner (Fig. 2A). The increased percentages of CCSCs were caused by an increase in the absolute number of CCSCs accompanied by 8.3–23.7% decrease in total cell number in irradiated cells vs. untreated cells. Consequently, the absolute numbers of ALDH+CD133+ CCSCs increased in SW1353 (1.36–2.31-fold, p < 0.01) and CS-1 cells (1.85–2.65-fold, p < 0.001) compared to untreated cells, respectively (Fig. 2B). This finding indicated that ALDH+CD133+ CCSCs are resistant to IR and moreover, IR induced the formation of new CCSCs or iCCSCs. Critically important to this was that the increases in ALDH+ CD133+ CCSCs in both cell lines were found to be IR dose dependent (p < 0.05) (Fig. 2B). The increased ALDH+CD133+ CCSCs were persistently detected from 24 to 72 h after a single IR dose 10Gy (Fig. 2C), and the post-IR increased absolute numbers of ALDH+CD133+ CCSCs remained from 24 to 72 h in SW1353 (1.68–1.92 fold, p < 0.001) and CS-1 cells (1.99–2.20-fold, p < 0.001) compared to non-irradiated cells (Fig. 2D).
Fig. 2. ALDH+ CD133+ CCSCs are IR resistant and IR induces CCSCs in a dose dependent manner.
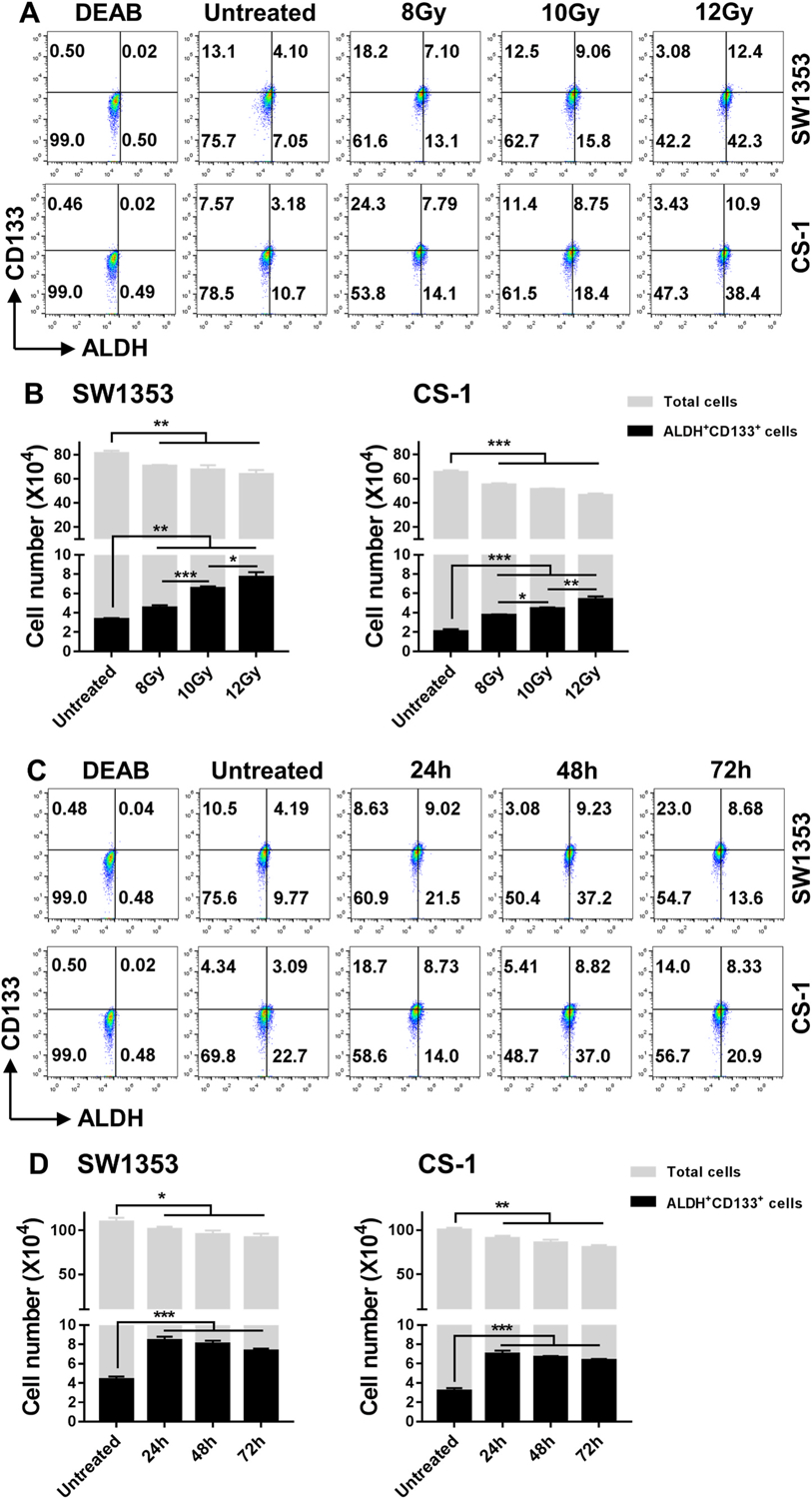
Cells were seeded (1 × 105 cells/2 mL culture medium/well) in 6-well plates and incubated for 24 h, followed by IR with 8Gy, 10Gy, 12Gy, and either cultured for an additional 24 h or IR with 10Gy and cultured for an additional 24 h, 48 h, or 72 h. The treated cells were harvested and analyzed for ALDH+CD133+ cells. The total number of cells was counted using the Trypan blue exclusion method. ALDH+CD133+ cells were calculated based on the total cell number and percentage of ALDH+CD133+ cells detected by flow cytometry (A, C). The mean ± SD of absolute cell numbers of total cells and ALDH+CD133+ cells are shown (B, D). * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
3.3. DSF/Cu is cytotoxic to CS cells and a radiosensitizer of CS cells
Previously we reported that DSF/Cu induces apoptosis of breast and PDAC CSCs and total cancer cells [12,13,16]. However, the effect of DSF/Cu on CS cells had yet to be investigated. Thus, initially, the effect of DSF/Cu on CS cell viability was investigated using MTT assays and the half maximal inhibitory concentration (IC50) of DSF in the presence of Cu (1 μM) was determined to be 0.14 μM (24 h); 0.13 μM (48 h) for SW1353 cells and 0.12 μM (24 h); 0.13 μM (48 h) for CS-1 cells (Fig. 3A). Next, the cytotoxic effect of DSF/Cu on colony formation ability of CS cells was studied. Consistent with the MTT data, DSF/Cu effectively inhibited colony formation in a dose dependent manner; at a concentration of 0.5/1 μM, colony formation was eliminated in both SW1353 and CS-1 cells (Fig. 3B and C). Finally, DSF/Cu as a radiosensitizer for the two CS cell lines was investigated following their treatment with IR + DSF/Cu (0.05/1 μM) using clonogenic survival assays. The dose of DSF/Cu used was chosen based on pilot titrations to allow for colony formation of the cells irradiated up to 6Gy. Clonogenic survival was significantly reduced with DSF/Cu as a radiosensitizer for both SW1353 (p < 0.01) and CS-1 cells (p < 0.01). Although the ability of colony formation of CS-1 cells was lessthan SW1353 cells when the same number of cells seeded at 0Gy, both cell lines showed similar sensitivity to DSF/Cu (Fig. 3D and E).
Fig. 3. DSF/Cu is cytotoxic to CS cells and a radiosensitizer of CS cells.
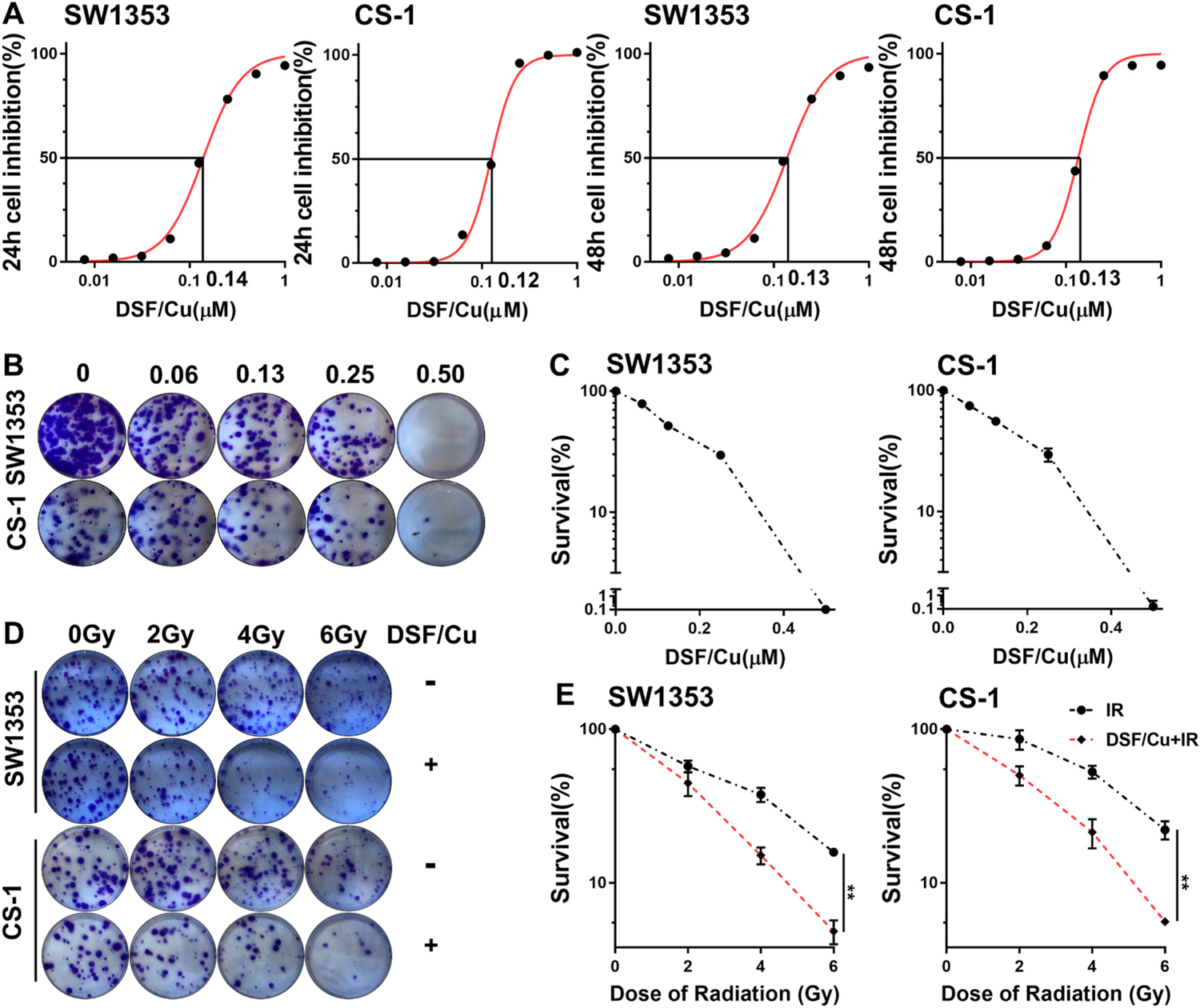
Cell growth inhibition in response to DSF/Cu treatment in SW1353 and CS-1 cell lines at indicated doses of DSF (μM) and a fixed dose of copper gluconate (Cu 1 μM) was evaluated by MTT assays. The IC50 values of DSF in the presence of Cu for each CS cell line at different time points are shown (A). Clonogenic survival of CS cells after treatment with DSF/Cu for 24 h. Colonies were counted 10 days after treatment (B). The surviving fraction of cells after DSF/Cu treatment was calculated using the number of untreated cell colonies as 100% (C). Clonogenic survival of CS cells treated with DSF/Cu (0.05/1 μM) for 24 h after being irradiated once at indicated doses. Colonies were counted 10 days after treatment (D). The surviving fraction of cells after IR alone vs. IR + DSF/Cu is shown (E). ** indicates p < 0.01.
3.4. DSF/Cu decreases ALDH+CD133+ CCSCs and total CS cells in FIR treated CS cells
The ability of DSF/Cu to target preexisting CCSCs and iCCSCs of CS cell lines in a clinically relevant RT [27] setting was investigated using FIR (2 Gy/d × 5 d) with DSF/Cu at a dose chosen based on pilot titration experiments. FIR increased CCSCs from 4.25% (untreated, preexisting CCSCs) to 10.1% (p < 0.001) in SW1353 cells and from 3.24% (untreated) to 10.5% in CS-1 cells (p < 0.001) (Fig. 4A). Again, the increased percentage of CCSCs was caused by an increase in the absolute number of CCSCs accompanied by a 1.15-fold decrease in the total cell number in irradiated cells vs. untreated cells: the absolute numbers of ALDH+CD133+ CCSCs were increased by FIR in SW1353 (2.24-fold, p < 0.001) and CS-1 (2.57-fold, p < 0.001) cell lines compared to untreated cells (Fig. 4B). In contrast, DSF/Cu preferentially reduced the numbers of ALDH+CD133+ CCSCs in SW1353 (3.69-fold, p < 0.001) and CS-1 (3.32-fold, p < 0.001) compared to its inhibitory effect on total cells of SW1353 (1.73-fold, p < 0.001) and CS-1 (1.52-fold, p < 0.001). Strikingly, the combination of DSF/Cu and FIR preferentially and profoundly reduced the numbers of ALDH+CD133+ CCSCs in SW1353 (16.68-fold, p < 0.001) and CS-1 (10.58-fold, p < 0.001) cell lines and reduced the total CS cells in SW1353 (3.63-fold, p < 0.001) and CS-1 (2.46-fold, p < 0.001) cell lines, as well (Fig. 4B). The underlying mechanism for this synergetic effect on targeting CCSCs could be attributed to the ability of DSF/Cu to render CCSCs as sensitive as non-CCSCs to radiation induced cell death and block formation of FIR-induced CCSCs via NF-κB-stemness pathway [16].
Fig. 4. The combination of FIR and DSF/Cu is more effective than either treatment alone in targeting ALDH+ CD133+ CCSCs and non-CCSCs.
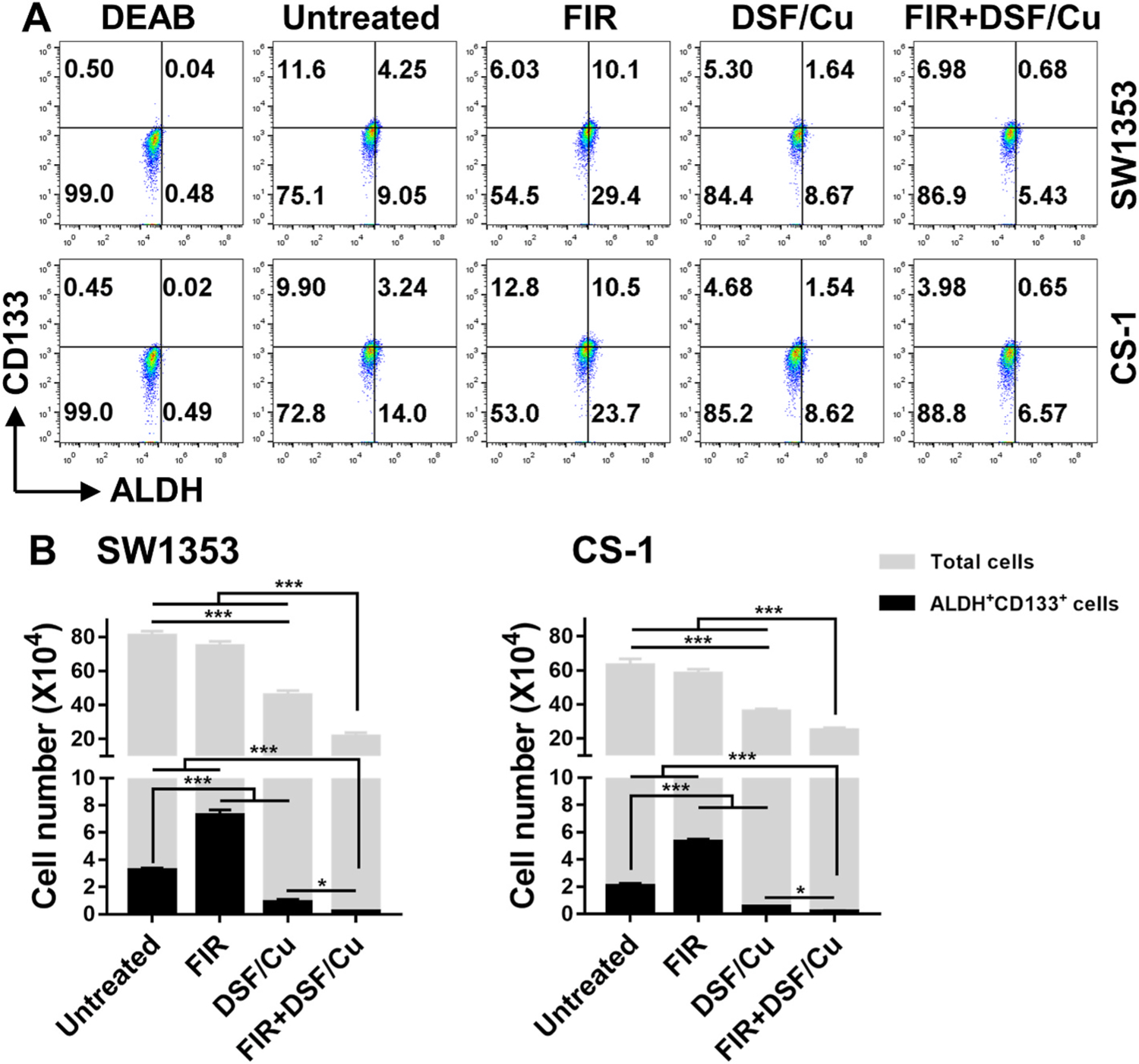
Cells were seeded (1 × 105 cells/2 mL culture medium/well) in 6-well plates and incubated for 24 h. FIR (2 Gy/day × 5) were delivered to the cultured cells. Next day, the irradiated cells were harvested, seeded and incubated as described above, followed by treatment with DSF/Cu (0.15/1 μM) for an additional 24 h. The treated cells were harvested and analyzed for ALDH+CD133+ cells. The total number of cells were counted using the Trypan blue exclusion method, ALDH+CD133+ cells were calculated based on the total cell number and % ALDH+CD133+ cells detected by flow cytometry (A). The mean ± SD of absolute cell numbers of total cells and ALDH+CD133+ cells are shown (B). * indicates p < 0.05, *** indicates p < 0.001.
3.5. Involvement of the NF-κB-stemness gene pathway in DSF/Cu + IR-mediated targeting of stemness of CS cells
DSF/Cu targets breast and PDAC CSCs by inhibiting, in part, the NF-κB-stemness gene pathway [12,13]. NF-κB plays a major role in regulating the expression of the stemness genes, ERBB2, c-myc, SNAI2, which, respectively, encode the stemness transcriptional factors of HER2, c-MYC, SLUG [28]. To determine if NF-κB is also involved in the targeting of CCSCs by DSF/Cu + IR, the effect of DSF/Cu and IR on NF-κB p65 and its regulated downstream stemness transcriptional factors in CS cells were investigated. Significant decreases were detected of NF-κB p65 by DSF/Cu alone (SW1353 2.19-fold, p < 0.01; CS-1 2.26-fold, p < 0.05) and even more pronounced decreases with IR + DSF/Cu treatment (SW1353 4.72-fold, p < 0.001; CS-1 9.15-fold, p < 0.001) as calculated using ImageJ (Fig. 5A and B). As expected, IR increased expression of the stemness transcriptional factors HER2 (SW1353 2.1-fold, p < 0.001; CS-1 1.4-fold, p < 0.001) and SLUG (SW1353 1.4-fold, p < 0.05; CS-1 1.3-fold, p < 0.05) while c-MYC was reduced (Fig. 5C and D).
Fig. 5. DSF/Cu and IR downregulate expression of stemness gene encoded proteins in CS cells.
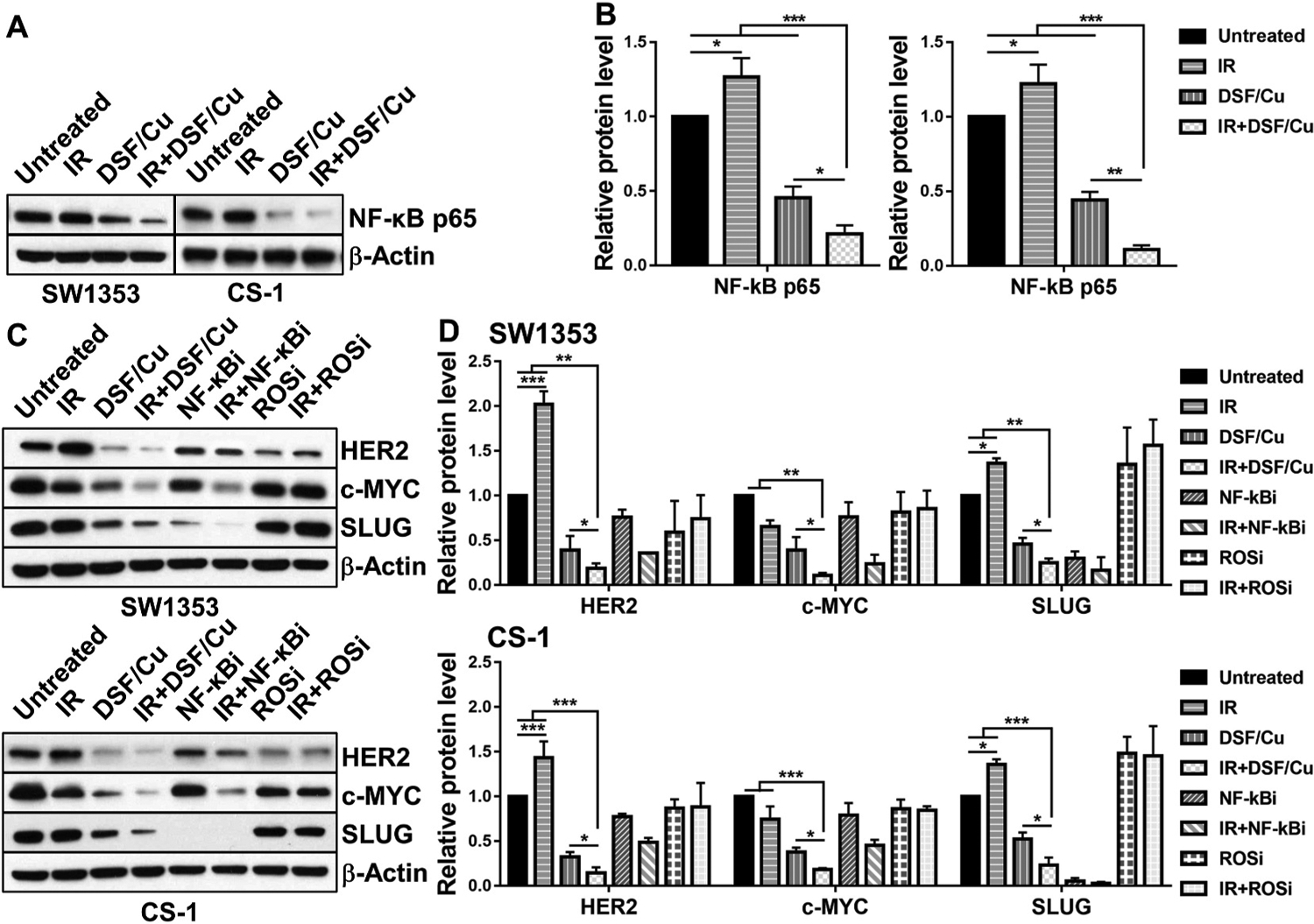
SW1353 and CS-1 cells were irradiated (10Gy) and cultured for 24 h, followed by treatment with DSF/Cu (0.15/1 μM) for an additional 24 h. Treated cells were lysed and analyzed by Western blot for expression levels of NF-κB p65 (A, B) and of NF-κB regulated downstream-stemness gene encoded proteins HER2, c-MYC and SLUG (C, D). NF-κBi (IMD 0354, 1 μM) and ROSi (NAC, 10 μM) were used on CS cells simultaneously but separately from DSF/Cu. The mean ± SD of band density of each protein from 3 independent blots (while the protein expression level of untreated cells was standardized to a value of 1) are shown (B, D). * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
In vitro treatment of irradiated CS cells with DSF/Cu significantly reduced the expression of HER2 (SW1353 5.3-fold, p < 0.01; CS-1 6.8-fold, p < 0.001), c-MYC (SW1353 8.7-fold, p < 0.01; CS-1 5.5-fold, p < 0.001), SLUG (SW1353 4.0-fold, p < 0.01; CS-1 4.3-fold, p < 0.001) compared to untreated CS cells (Fig. 5C and D). Additionally, NF-κBi (IMD 0354) displayed similar downregulation of stemness transcriptional factors on its own or in combination with IR, however, it was less effective than DSF/Cu as a single agent or combined with IR in reducing stemness gene protein expression in the CS cell lines except SLUG (Fig. 5C and D). Consistent with the data obtained from breast cancer cells [13], treatment with the ROSi (NAC) with or without IR, did not have any effect on stemness gene protein expression. Similar results were also obtained in CS cell lines with the CSC functional property assay, namely, in vitro sphere formation. Compared to untreated cells, IR increased sphere formation of SW1353 cells by 77.9% (p < 0.01) and of CS-1 cells by 84.0% (p < 0.01), while IR + DSF/Cu significantly inhibited sphere formation by 95% in both cell lines (p < 0.05 compared to any other treatment) (Fig. 6A and B). Likewise, compared to untreated cells, IR + NF-κBi inhibited sphere formation by 52.2% (p < 0.01) in SW1353 and 39.5% (p < 0.05) in CS-1 (Fig. 6C and D). In contrast, treatment with the ROSi did not show any effect on sphere formation (p > 0.05), leading to the conclusion that ROS does not seem to play a role in modulating the stemness gene pathway in CS cell lines (Fig. 6C and D). These data indicate that inhibition of the NF-κB-stemness gene pathway is partially responsible for DSF/Cu-mediated targeting of stemness of CS cells. Furthermore, both DSF and Cu are needed in combination with IR to inhibit sphere formation, as DSF/Cu worked significantly better than either agent alone (p < 0.05) (Fig. 6A and B). Compared to untreated cells, sphere formation was persistently increased by 77.9% (24 h), 72.6% (48 h), and 70.8% (72 h) (SW1353, p < 0.01); 84.0% (24 h), 79.0% (48 h), and 74.1% (72 h) (CS-1, p < 0.01) after a single IR dose 10Gy. However, DSF/Cu was very effective, when it was given either 24 h or 48 h or 72 h post-IR, in inhibition of IR-increased sphere formation (Fig. 6E and F).
Fig. 6. DSF/Cu and IR deplete CCSCs as measured by sphere formation ability.
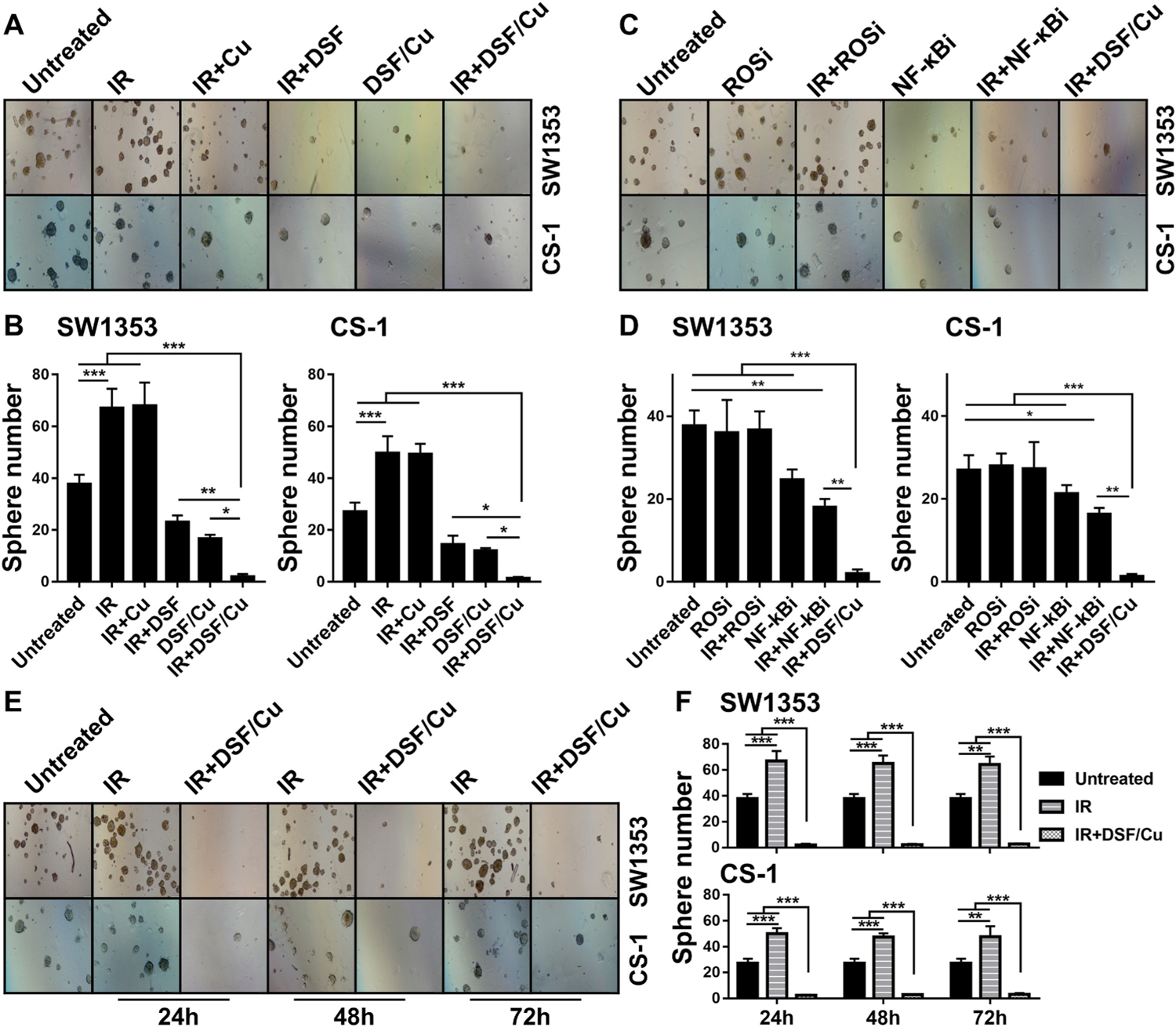
SW1353 and CS-1 cells were seeded (1 × 105 cells/2 mL culture medium/well) in 6-well plates and incubated for 24 h. Then the cells were irradiated (10Gy) and cultured for 24 h, followed by treatment with DSF/Cu (0.15/1 μM) or NF-κBi (IMD 0354, 1 μM) or ROSi (NAC, 10 μM) for an additional 24 h. Then the cells were harvested and seeded (1000 cells/well) in 24-well Ultra-low attachment surface plates and cultured for sphere formation. Pictures of spheres were taken on day 18 (SW1353) and 11 (CS-1) (A, C) and quantified by counting sphere numbers per well (B, D). The 10Gy irradiated cells were cultured for 24 h or 48 h or 72 h, followed by treatment with DSF/Cu (0.15/1 μM) for an additional 24 h before the sphere formation assay was performed. Pictures of spheres (E) and mean ± SD of sphere number per well (F) at different time points are shown. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
3.6. DSF/Cu and IR are more effective than IR alone in inhibiting growth and stemness of CS in an orthotopic xenograft nude mouse model
The efficacy of combining IR + DSF/Cu to target CCSCs cells in vivo was investigated using orthotopic CS xenografts bearing nude mice. The combination of IR + DSF/Cu (95.6%) was found to be more effective in inhibiting primary CS xenograft growth in the mice than IR + DSF (82.8%) or IR + vehicle (37.6%) vs untreated mice (p < 0.001), (Fig. 7A, B, C). It is also worth noting that DSF/Cu or DSF-associated toxicity was not observed in the mice using body weight as an indicator. Among all groups, the mice left untreated had the most significant weight loss (p < 0.001), which correlated with tumor burden/advanced disease associated cachexia (Fig. 7D). In contrast, DSF/Cu significantly reduced IR-induced in vivo toxicity while enhancing its efficacy, implicating DSF/Cu could expand the therapeutic index of RT (Fig. 7B and C). Consistent with in vitro findings, the anti-tumor effect of IR + DSF/Cu compared to IR + DSF, IR alone or no treatment was associated in the mice with a marked reduction in primary tumors of i) percentage of ALDH+CD133+ CCSCs (Fig. 7E and F), ii) sphere formation ability (Fig. 7G and H) and iii) expression level of stemness gene encoded proteins (Fig. 7I and J).
Fig. 7. DSF/Cu + IR is effective in controlling growth of orthotopic CS xenografts in nude mice.
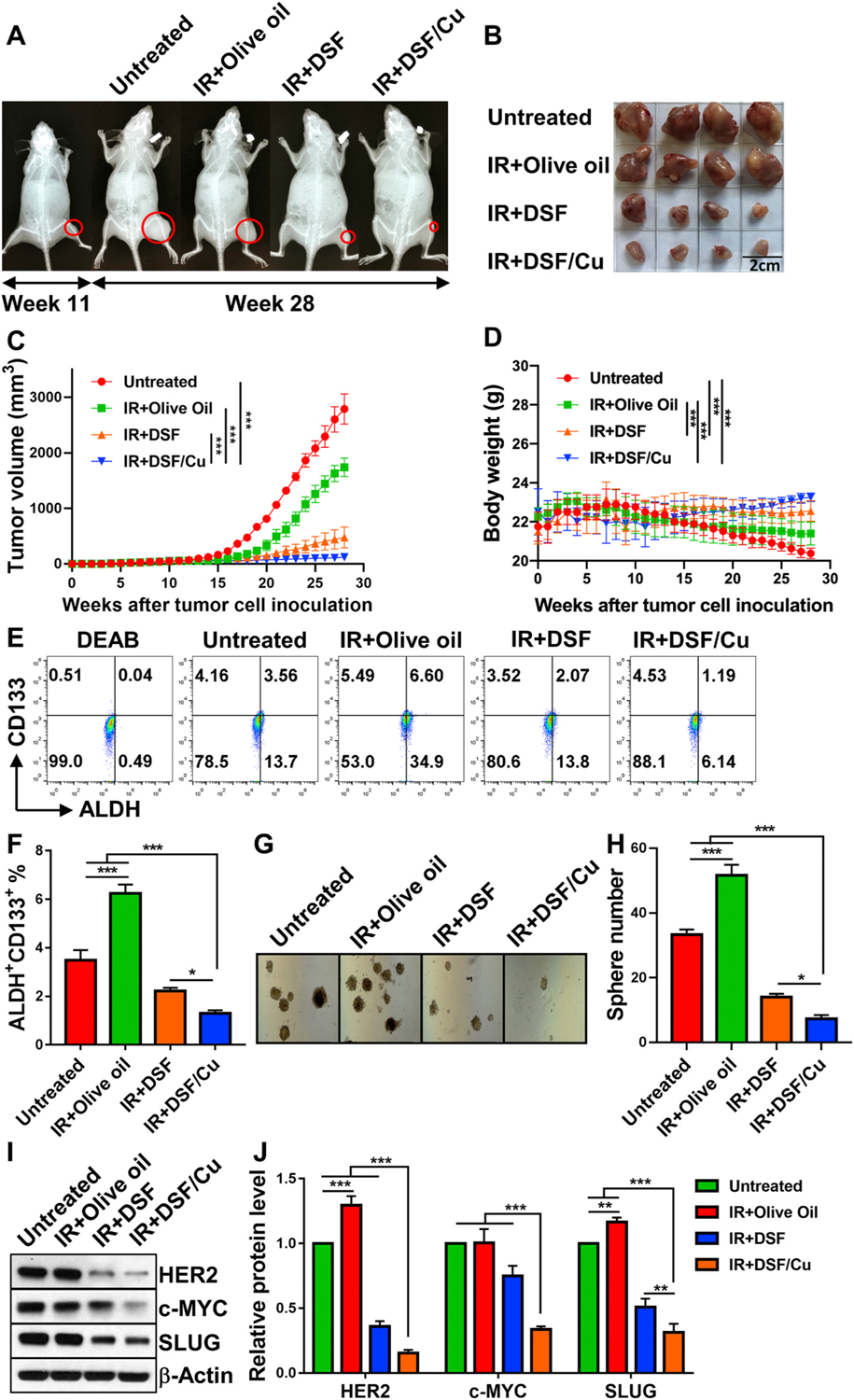
Eleven weeks after SW1353 cell orthotopic inoculation, tumors were detected by X-Ray. Mice were divided randomly into 4 groups (n = 4) and the treatments were initiated as indicated. Radiographs of tibia tumors before and after treatment are shown (A). Mean tumor volumes of each group ± SEM and p values for comparison between groups are shown (B, C). To monitor toxicity of each treatment, mouse body weight was measured once weekly, the mean ± SEM of each group at different weeks after treatment are shown (D). When tumor diameters from the untreated mice reached 2 cm, all mice were sacrificed (week 28). All isolated primary tumors were collected for further analysis: A single cell suspension from 3 pooled tumor tissue samples of each group (tumors from 4 mice of the same group were pooled into 3 pools: two pools from a single different mouse and one pool from the other 2-mouse tumor tissue samples) was analyzed for ALDH+ CD133+ CCSCs by flow cytometry (E) and mean ± SD (%) of ALDH+CD133+ CCSCs are shown (F); sphere formation on day 18 after seeding cells obtained freshly from CS xenograft tumors (G) and mean ± SD of number of spheres per well (H) are shown. Lysates of tumor tissues pooled from all 4 mice per group were used for Western blot to detect expression levels of stemness gene encoded proteins HER2, c-MYC and SLUG (I). And the mean ± SD of band density of each protein from 3 independent blots (while the protein expression level of untreated tumors was standardized to a value of 1) are shown(J). * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
3.7. DSF/Cu and IR are more effective than IR alone in prolonging survival of orthotopic CS xenografts bearing NSG mice
Based on the promising in vivo anti-tumor results (Fig. 7), the efficacy of combining IR + DSF/Cu in improving survival was determined using orthotopic CS xenografts bearing NSG mice. As expected, the combination of IR + DSF/Cu was found to be more effective in prolonging survival of CS bearing mice compared to IR alone (p < 0.01), or IR + DSF (p < 0.05). Post-CS cell inoculation, all untreated and IR alone (with-vehicle)-treated mice died by day 89 (Untreated vs IR, p > 0.05), while 3/5 IR + DSF/Cu- and 1/5 IR + DSF- treated mice remained tumor-free at day 200 (Fig. 8).
Fig. 8. DSF/Cu + IR is effective in increasing survival of orthotopic CS xenografts bearing NSG mice.
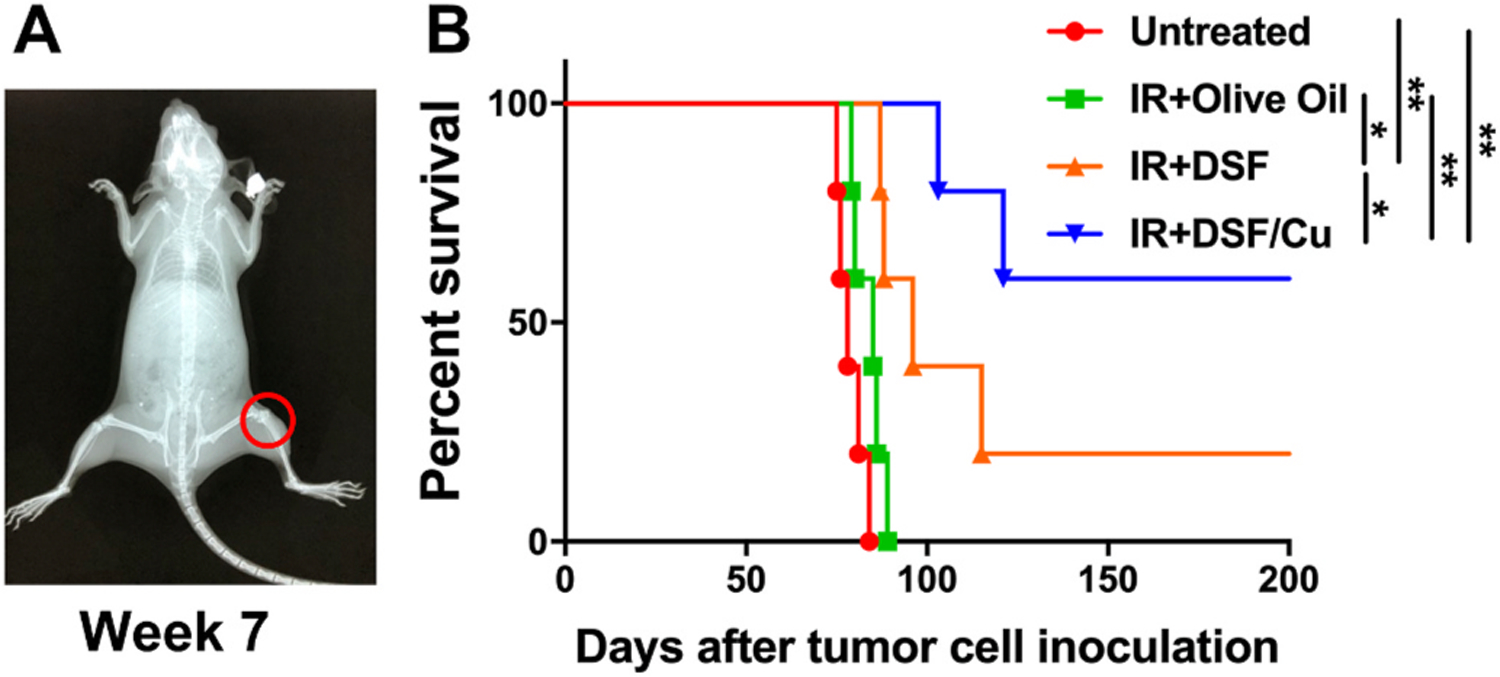
Seven weeks after SW1353 cell orthotopic inoculation, tumors were detected by X-Ray (A). Mice were divided randomly into 4 groups (n = 5) and the treatments were initiated as indicated. Survival curves of each group of mice are shown (B). * indicates p < 0.05, ** indicates p < 0.01.
4. Discussion
A single marker CD133+ or ALDH+ was used to identify CCSCs previously [20,21]. This study determined that double-marker identified ALDH+CD133+ cells rather than single-marker identifiedALDH+ or CD133+ cells were more definitive in identifying CCSCs (Fig. 1) in CS cell lines. Other markers also have been used to identify mesenchymal stem cells (MSC) in chondrosarcoma, including but not limited to CD44, CD271, CD49bhigh/CD10low/CD221 (multipotent MSC), and CD49blow/CD10high/CD221low (a fibroblastic lineage) [29]. Therefore, markers ALDH+CD133+ should be compared side-by-side to other available MSC markers to assess if they somewhat overlap in identifying CCSCs or in some combination define CCSC more definitively. Consistent with previous findings of the radioresistant nature of CSCs in multiple types of epithelial carcinomas [11–13], the results reported here establish that CSCs in CS, a cancer of mesenchymal origin, are also radioresistant and contribute to the CS radioresistance. Furthermore, it was demonstrated that the absolute number of CCSCs in CS cell lines increased in response to radiation regardless of whether single-dose radiation or the clinical setting FIR was used in vitro and in vivo. In other words, radiation induced new CCSCs from non-CCSCs. This radiation induced stemness of CS was confirmed by increased levels of stemness transcriptional factors HER2 and SLUG as well as sphere formation in the two CS cell lines analyzed (Figs. 5 and 6). These findings are consistent with the previous reports involving irradiated breast cancer, PDAC, and hepatocellular carcinoma cells [12,13,30,31], and emphasize the importance of targeting preexisting radioresistant CCSCs and blocking formation of new radiation induced CCSCs. Additionally, the results imply that radiation may induce CSCs in other types of mesenchymal tumors, such as soft tissue and other bone sarcomas, some of which are frequently treated by radiation [32,33]. Thus, research in targeting preexisting CSCs and blocking radiation-induced CSCs is urgently needed to provide insights and guidance on the rational design of more effective radiation-based therapies for tumors of mesenchymal origin.
The repurposing of DSF in combination with Cu2+ as a radiosensitizer of cancer has two significant advantages over other approaches. DSF has been FDA-approved for decades and used safely in the treatment of alcoholism at 250 mg/daily for months to years [34,35], resulting in a serum concentration of ≤2 μM [36]. Cu2+ plasma concentration of 1 μM is within normal limits [37] and can be achieved by 1–2 mg of oral copper gluconate as a dietary supplement. DSF/Cu as monotherapy was shown to be cytotoxic to CS cells at an IC50 of <0.15 (DSF)/1(Cu)μM. When combined with single-dose radiation, DSF/Cu, at the very low dose of 0.05/1 μM, significantly radiosensitized the two tested CS cell lines and, particularly CCSCs (Fig. 3). The combination of DSF/Cu (0.15/1 μM) and FIR preferentially and significantly decreased ALDH+CD133+ CCSCs in vitro (~11–17-fold) while reducing total cells to a lesser extent (< 4-fold). Conversely, FIR increased ALDH+CD133+ CCSCs (> 2-fold) in CS cells compared to untreated CS cells (Fig. 4). Additionally, the combination of DSF/Cu and single-dose radiation eliminated almost all sphere forming cells in vitro (Fig. 6).
In in vivo orthotopic CS xenograft nude mouse model studies, IR + DSF/Cu was significantly more effective in inhibiting primary CS xenograft growth in mice than either IR + DSF or IR + vehicle, in the absence of toxicity, a clear indication of its potential to enhance the therapeutic index of RT (Fig. 7). Providing a low amount of exogenous Cu2+ to mice was necessary in this study as the efficacy of DSF with exogenous Cu2+ was significantly better than in mice not receiving exogenous Cu2+. However, in the future, the dose of exogenous Cu2+ needs to be chosen carefully in order to avoid excess Cu, as tumors usually have elevated Cu2+ levels, which can promote cancer [38,39]. The anti-tumor efficacy of IR + DSF/Cu correlated inversely with stemness present in the treated established CS cell lines and primary xenografts, as indicated by significant decrease in the expression of NF-κB pathway-regulated transcriptional factors HER2, c-MYC and SLUG. These results were similar to the use of NF-κBi which down-regulated IR-induced HER2, c-MYC, and SLUG expression (Fig. 5) [12, 13,28,40]. Moreover, IR + DSF/Cu was significantly more effective in prolonging survival of CS bearing mice compared to either IR alone or IR + DSF (Fig. 8). It is noteworthy that it took a much shorter time to form tumor post-SW1353 cell inoculation in NSG (7 weeks) than in nude mice (11 weeks); and that the survival time was shorter for untreated CS bearing NSG (13 weeks) than nude (28 weeks) mice. This may indicate that the immune system plays an important role in controlling CS formation and growth as NSG mice are more immunodeficient than nude mice [41]. It is also notable that CS-1 cells (2 × 106/mouse) failed to form subcutaneous (s.c.) tumor in nude or NSG mice. This finding contradicted what was found before when CS-1 cells (1 × 106/mouse) formed s. c. tumor in nude mice shortly after cell inoculation [24]. This may have been caused by multiple in vitro cell passages.
Currently, there is great interest in repurposing DSF for cancer therapy. The recent epidemiological study by Sadhukha et al. spanning 13 years, reported the exciting result that DSF (250mg/daily) reduced mortality in cancer patients by 34% compared to patients who did not use DSF after their cancer diagnosis [42]. The authors also discovered that the main metabolite of DSF, diethyldithiocarbamate (DTC) formed complexes with Cu, and that its anti-cancer effect was due to NPL4-p97 pathway targeting and blocking a step upstream of the proteasome and multiple regulatory and stress-response pathways in cells. Our recent independent study also found that DSF/Cu induces endoplasmic reticulum stress by activating the IRE1α-XBP1 pathway, which is partially responsible for induction of autophagy-dependent apoptosis of cancer cells [25]. Therefore, research that focuses on stress responses to cancer therapy of CSCs vs non-CSCs is needed to shed light on the mechanism(s) of DSF/Cu when combined with radiation, chemotherapy and chemoradiation.
Radiation resistance compromises clinical outcomes of incompletely resected or inoperable CS. Currently there are at least 9 clinical trials that are actively recruiting CS patients (NCT04340843, NCT04278781, NCT02821507, NCT04040205, NCT04521686, NCT02389244, NCT03277924, NCT03173976 and NCT01182753) [43], most of which are targeted therapy, epigenetic therapy or immunotherapy with or without chemotherapy. Among these trials, the only one involving RT, is a Phase III trial of proton versus carbon ion RT in patients with CS of the skull base (NCT01182753). The in vitro and in vivo data of the present study firmly established the concept of repurposing DSF and Cu as a radiosensitizer for CS because it effectively targets radioresistance and IR-induced stemness in CS cells. Furthermore, the results presented herein can be readily translated into clinical trials for CS patients who require RT for improvement of their clinical outcomes.
In conclusion, CCSCs are radioresistant and radiation induces stemness in CS cell lines. DSF and Cu2+ are both readily available and affordable and were shown in these preclinical studies to be effective CCSCs radiosensitizers. Their application resulted in profound anti-tumor activity in orthotopic CS xenografts bearing mice, as measured by tumor volume and survival. Additionally, DSF/Cu significantly reduced IR-induced in vivo toxicity while enhancing its efficacy, implying that DSF/Cu could expand the therapeutic index of RT. These results provide a solid foundation for clinical trials for CS patients requiring RT.
Funding
This work was supported by the National Institutes of Health (grant R01CA226981-01A1, X.W.) and the China Scholarship Council (CSC No. 201906370234, K.W.).
Abbreviations
- ALDH
aldehyde dehydrogenase
- ATCC
American Type Culture Collection
- AO
acridine orange
- BSA
bovine serum albumin
- CM
complete medium
- CS
chondrosarcoma
- CSC
cancer stem cell
- CCSC
chondrosarcoma cancer stem cells
- DEAB
N,N-diethylaminobenzaldehyde
- DSF
disulfiram
- DTC
diethyldithiocarbamate
- ER
endoplasmic reticulum
- FACS
fluorescence-activated cell sorting
- FDA
Federal Drug Administration
- FIR
fractionated irradiation
- iCSC
induced cancer stem cell
- IC50
half maximal inhibitory concentration
- i.p.
intraperitoneal
- mAb
monoclonal antibody
- MSC
mesenchymal stem cells
- NAC
N-Acetyl-l-cysteine
- NF-κB
nuclear factor kappa B
- PARP
Poly ADP-ribose polymerase
- PE
plating efficiency
- PDAC
pancreatic ductal adenocarcinoma
- ROS
reactive oxygen species
- ROSi
reactive oxygen species inhibitor
- RT
radiotherapy
- SC
stem cells
- SD
standard deviation
- SEM
standard error of the mean
- SF
surviving fraction
- TBST
Tris Buffered Saline with 0.1% Tween® 20
Footnotes
Credit author statement
Kun Wang: Methodology, Data curation, Formal analysis, Writing – original draft. Theodoros Michelakos: Formal analysis, Writing – review & editing. Bing Wang: Resources. Zikun Shang: Methodology, Validation. Albert B. DeLeo: Writing – review & editing. Zhenfeng Duan: Resources. Francis J. Hornicek: Resources. Joseph H. Schwab: Conceptualization, Resources, Writing – review & editing. Xinhui Wang: Conceptualization, Investigation, Visualization, Supervision, Resources, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Mery B, Espenel S, Guy JB, Rancoule C, Vallard A, Aloy MT, Rodriguez-Lafrasse C, Magne N, Biological aspects of chondrosarcoma: leaps and hurdles, Crit. Rev. Oncol. Hematol 126 (2018) 32–36. [DOI] [PubMed] [Google Scholar]
- [2].Chow WA, Chondrosarcoma: biology, genetics, and epigenetics F1000Res (2018) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].van Maldegem AM, Gelderblom H, Palmerini E, Dijkstra SD, Gambarotti M, Ruggieri P, Nout RA, van de Sande MA, Ferrari C, Ferrari S, Bovee JV, Picci P, Outcome of advanced, unresectable conventional central chondrosarcoma, Cancer 120 (2014) 3159–3164. [DOI] [PubMed] [Google Scholar]
- [4].Catanzano AA, Kerr DL, Lazarides AL, Dial BL, Lane WO, Blazer DG, Larrier NA, Kirsch DG, Brigman BE, Eward WC, Revisiting the Role of Radiation Therapy in Chondrosarcoma: A National Cancer Database Study, vol. 2019, 2019, 4878512. Sarcoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hashiguchi S, Kusuzaki K, Murata H, Takeshita H, Hashiba M, Nishimura T, Ashihara T, Hirasawa Y, Acridine orange excited by low-dose radiation has a strong cytocidal effect on mouse osteosarcoma, Oncology 62 (2002) 85–93. [DOI] [PubMed] [Google Scholar]
- [6].Kim DW, Seo SW, Cho SK, Chang SS, Lee HW, Lee SE, Block JA, Hei TK, Lee FY, Targeting of cell survival genes using small interfering RNAs (siRNAs) enhances radiosensitivity of Grade II chondrosarcoma cells, J. Orthop. Res 25 (2007) 820–828. [DOI] [PubMed] [Google Scholar]
- [7].Moussavi-Harami F, Mollano A, Martin JA, Ayoob A, Domann FE, Gitelis S, Buckwalter JA, Intrinsic radiation resistance in human chondrosarcoma cells, Biochem. Biophys. Res. Commun 346 (2006) 379–385. [DOI] [PubMed] [Google Scholar]
- [8].de Jong Y, Ingola M, Briaire-de Bruijn IH, Kruisselbrink AB, Venneker S, Palubeckaite I, Heijs B, Cleton-Jansen AM, Haas RLM, Bovee J, Radiotherapy resistance in chondrosarcoma cells; a possible correlation with alterations in cell cycle related genes, Clin. Sarcoma Res 9 (2019) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cesaire M, Ghosh U, Austry JB, Muller E, Cammarata FP, Guillamin M, Caruso M, Castera L, Petringa G, Cirrone GAP, Chevalier F, Sensitization of chondrosarcoma cells with PARP inhibitor and high-LET radiation, J Bone Oncol 17 (2019), 100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Batlle E, Clevers H, Cancer stem cells revisited, Nat. Med 23 (2017) 1124–1134. [DOI] [PubMed] [Google Scholar]
- [11].Arnold CR, Mangesius J, Skvortsova II, Ganswindt U, The role of cancer stem cells in radiation resistance, Front Oncol 10 (2020) 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cong J, Wang Y, Zhang X, Zhang N, Liu L, Soukup K, Michelakos T, Hong T, DeLeo A, Cai L, Sabbatino F, Ferrone S, Lee H, Levina V, Fuchs B, Tanabe K, Lillemoe K, Ferrone C, Wang X, A novel chemoradiation targeting stem and nonstem pancreatic cancer cells by repurposing disulfiram, Canc. Lett 409 (2017) 9–19. [DOI] [PubMed] [Google Scholar]
- [13].Wang Y, Li W, Patel SS, Cong J, Zhang N, Sabbatino F, Liu X, Qi Y, Huang P, Lee H, Taghian A, Li JJ, DeLeo AB, Ferrone S, Epperly MW, Ferrone CR, Ly A, Brachtel EF, Wang X, Blocking the formation of radiation-induced breast cancer stem cells, Oncotarget 5 (2014) 3743–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chang JC, Cancer stem cells: role in tumor growth, recurrence, metastasis, and treatment resistance, Medicine (Baltim.) 95 (2016) S20–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ajani JA, Song S, Hochster HS, Steinberg IB, Cancer stem cells: the promise and the potential, Semin. Oncol 42 (Suppl 1) (2015) S3–S17. [DOI] [PubMed] [Google Scholar]
- [16].Sun T, Yang W, Toprani SM, Guo W, He L, DeLeo AB, Ferrone S, Zhang G, Wang E, Lin Z, Hu P, Wang X, Induction of immunogenic cell death in radiation-resistant breast cancer stem cells by repurposing anti-alcoholism drug disulfiram, Cell Commun. Signal 18 (2020) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mallard BW, Tiralongo J, Cancer stem cell marker glycosylation: nature, function and significance, Glycoconj. J 34 (2017) 441–452. [DOI] [PubMed] [Google Scholar]
- [18].Lohberger B, Rinner B, Stuendl N, Absenger M, Liegl-Atzwanger B, Walzer SM, Windhager R, Leithner A, Aldehyde dehydrogenase 1, a potential marker for cancer stem cells in human sarcoma, PloS One 7 (2012), e43664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hoyt AK, Moran A, Granger C, Sedani A, Saigh S, Brown J, Galoian KA, PRP1 significantly decreases the ALDHhigh cancer stem cell population and regulates the aberrant Wnt/betacatenin pathway in human chondrosarcoma JJ012 cells, Oncol. Rep 42 (2019) 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, Pirozzi G, Papaccio G, Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo, Faseb. J 25 (2011) 2022–2030. [DOI] [PubMed] [Google Scholar]
- [21].Rey V, Menendez ST, Estupinan O, Rodriguez A, Santos L, Tornin J, Martinez-Cruzado L, Castillo D, Ordonez GR, Costilla S, Alvarez-Fernandez C, Astudillo A, Brana A, Rodriguez R, New chondrosarcoma cell lines with preserved stem cell properties to study the genomic drift during in vitro/in vivo growth, J. Clin. Med 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds RK, Wicha MS, Buckanovich RJ, Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival, Canc. Res 71 (2011) 3991–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Y, Laterra J, Cancer stem cells: distinct entities or dynamically regulated phenotypes? Canc. Res 72 (2012) 576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morioka H, Weissbach L, Vogel T, Nielsen GP, Faircloth GT, Shao L, Hornicek FJ, Antiangiogenesis treatment combined with chemotherapy produces chondrosarcoma necrosis, Clin. Canc. Res 9 (2003) 1211–1217. [PubMed] [Google Scholar]
- [25].Zhang X, Hu P, Ding SY, Sun T, Liu L, Han S, DeLeo AB, Sadagopan A, Guo W, Wang X, Induction of autophagy-dependent apoptosis in cancer cells through activation of ER stress: an uncovered anti-cancer mechanism by anti-alcoholism drug disulfiram, Am J Cancer Res 9 (2019) 1266–1281. [PMC free article] [PubMed] [Google Scholar]
- [26].Fan Y, Xiao Y, Sabuhi WA, Leape CP, Gil D, Grindy S, Muratoglu OK, Bedair H, Collins JE, Randolph M, Oral E, Longitudinal model of periprosthetic joint infection in the rat, J. Orthop. Res 38 (2020) 1101–1112. [DOI] [PubMed] [Google Scholar]
- [27].Uhl M, Mattke M, Welzel T, Oelmann J, Habl G, Jensen AD, Ellerbrock M, Haberer T, Herfarth KK, Debus J, High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results, Cancer 120 (2014) 1579–1585. [DOI] [PubMed] [Google Scholar]
- [28].Rinkenbaugh AL, Baldwin AS, The NF-kappaB pathway and cancer stem cells, Cells (2016) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Boehme KA, Schleicher SB, Traub F, Rolauffs B, Chondrosarcoma: a rare misfortune in aging human cartilage? The role of stem and progenitor cells in proliferation, malignant degeneration and therapeutic resistance, Int. J. Mol. Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F, Radiation-induced reprogramming of breast cancer cells, Stem Cell. 30 (2012) 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ghisolfi L, Keates AC, Hu X, Lee DK, Li CJ, Ionizing radiation induces stemness in cancer cells, PloS One 7 (2012), e43628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kaushal A, Citrin D, The role of radiation therapy in the management of sarcomas, Surg. Clin 88 (2008) 629–646, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS, Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation, Mol. Canc 16 (2017) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Johansson B, A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites, Acta Psychiatr. Scand. Suppl 369 (1992) 15–26. [DOI] [PubMed] [Google Scholar]
- [35].Krampe H, Stawicki S, Wagner T, Bartels C, Aust C, Ruther E, Poser W, Ehrenreich H, Follow-up of 180 alcoholic patients for up to 7 years after outpatient treatment: impact of alcohol deterrents on outcome, Alcohol Clin. Exp. Res 30 (2006) 86–95. [DOI] [PubMed] [Google Scholar]
- [36].Rae C, Tesson M, Babich JW, Boyd M, Sorensen A, Mairs RJ, The role of copper in disulfiram-induced toxicity and radiosensitization of cancer cells, J. Nucl. Med 54 (2013) 953–960. [DOI] [PubMed] [Google Scholar]
- [37].Rükgauer M, Klein J, Kruse-Jarres JD, Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults, J. Trace Elem. Med. Biol 11 (1997) 92–98. [DOI] [PubMed] [Google Scholar]
- [38].Wang F, Jiao P, Qi M, Frezza M, Dou QP, Yan B, Turning tumor-promoting copper into an anti-cancer weapon via high-throughput chemistry, Curr. Med. Chem 17 (2010) 2685–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ishida S, Andreux P, Poitry-Yamate C, Auwerx J, Hanahan D, Bioavailable copper modulates oxidative phosphorylation and growth of tumors, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 19507–19512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Storci G, Sansone P, Mari S, D’Uva G, Tavolari S, Guarnieri T, Taffurelli M, Ceccarelli C, Santini D, Chieco P, Marcu KB, Bonafe M, TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype, J. Cell. Physiol 225 (2010) 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shultz LD, Goodwin N, Ishikawa F, Hosur V, Lyons BL, Greiner DL, Human cancer growth and therapy in immunodeficient mouse models, Cold Spring Harb. Protoc (2014) 694–708, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Skrott Z, Mistrik M, Andersen KK, Friis S, Majera D, Gursky J, Ozdian T, Bartkova J, Turi Z, Moudry P, Kraus M, Michalova M, Vaclavkova J, Dzubak P, Vrobel I, Pouckova P, Sedlacek J, Miklovicova A, Kutt A, Li J, Mattova J, Driessen C, Dou QP, Olsen J, Hajduch M, Cvek B, Deshaies RJ, Bartek J, Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4, Nature 552 (2017) 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].ClinicalTrials.gov, Available from: URL: https://www.clinicaltrials.gov/ct2/results?cond=Chondrosarcoma&term=&cntry=&state=&city=&dist=. (Accessed 11 December 2020).


