Abstract
In the present study, we tested the hypothesis that there are significant sex differences in angiotensin II (Ang II)-induced hypertension and kidney injury using male and female wildtype (WT) and proximal tubule-specific AT1a receptor knockout mice (PT-Agtr1a−/−). Twelve groups (n=8–12 per group) of adult male and female WT and PT-Agtr1a−/− mice were infused with a pressor dose of Ang II via osmotic minipump for 2 weeks (1.5 mg/kg/day, i.p.) and simultaneously treated with or without losartan (20 mg/kg/day, p.o.) to determine the respective roles of AT1a receptors in the proximal tubules versus systemic tissues. Basal systolic, diastolic, and mean arterial pressure were approximately 13 ± 3 mmHg lower (P<0.01), while basal 24-h urinary Na+, K+, and Cl− excretion were significantly higher in both male and female PT-Agtr1a−/− mice than WT controls (P<0.01) without significant sex differences between different strains. Both male and female WT and PT-Agtr1a−/− mice developed hypertension (P<0.01), and the magnitudes of the pressor responses to Ang II were similar between male and female WT and PT-Agtr1a−/− mice (n.s.). Likewise, Ang II-induced hypertension was significantly attenuated in both male and female PT-Agtr1a−/− mice (P<0.01). Furthermore, losartan attenuated the hypertensive responses to Ang II to similar extents in both male and female WT and PT-Agtr1a−/− mice. Finally, Ang II-induced kidney injury was attenuated in PT-Agtr1a−/− mice (P<0.01). In conclusion, the present study demonstrates that deletion of AT1a receptors in the proximal tubules of the kidney attenuates Ang II-induced hypertension and kidney injury without revealing significant sex differences.
Introduction
Hypertension is one of the most prevalent disorders in the world, affecting billions of people, and is widely recognized to be the major risk factor for stroke, cardiovascular disease (CVD), and chronic kidney diseases [1,2]. Recent American Heart Association/American College of Cardiology Guidelines have shown that 46% of adults in the United States are hypertensive, and currently modify their dietary habits and lifestyles, or take at least one of the antihypertensive drugs [1,2]. However, the specific etiology and mechanisms underlying the development of hypertension remain incompletely understood. Although hypertension is a multifactorial cardiovascular disorder involving genetic, neural, hormonal, dietary, and lifestyle factors; the renin–angiotensin system (RAS) still plays one of most important roles in the pathogenesis of hypertension and target organ injury [3-9]. In the kidney, all major components of the RAS are expressed in the renal vasculature, glomeruli, and renal tubules [3,6,7,10,11]. One of the most important sources and targets of the RAS in the kidney is the proximal tubules [3,6,7,10,11]. Indeed, the proximal tubules express abundant precursor angiotensinogen, the key enzymes, the rate-limiting renin and angiotensin-converting enzyme (ACE), and two major classes of G protein-coupled receptors, AT1 (AT1a and AT1b) and AT2 [3,6,7,10,11]. Renin cleaves angiotensinogen to generate angiotensin I (Ang I), whereas ACE converts inactive Ang I into angiotensin II (Ang II), the most potent peptide of the system, which binds and activates AT1 (AT1a and AT1b) and AT2 receptors in the proximal tubules. Physiologically, Ang II acts to stimulate proximal tubule Na+ reabsorption and helps maintain body salt and fluid balance and basal blood pressure homeostasis [3,6,7,10,11]. However, overactivation of the circulating and intratubular Ang II system not only directly induces glomerular and tubulointerstitial injury, but also promotes proximal tubule Na+ reabsorption and elevates blood pressure [3-6,10,12]. The importance of the RAS in hypertension and target organ injury is further emphasized by the widespread use of ACE inhibitors and Ang II receptor blockers to treat hypertension and reduce stroke, cardiovascular and renal disease risks, and prevent target organ damage [1,2]. Nevertheless, the roles or the contributions of intratubular RAS via activation of AT1 (AT1a) receptors in the proximal tubules of the kidney to Ang II-induced hypertension and glomerular and tubulointerstitial injury remain incompletely understood.
Recently, there has been an explosion of interest on the studies on the important roles of gender and/or sex differences in the physiological regulation of cardiovascular and kidney function, blood pressure, and the development of Ang II-induced hypertension [13-18]. The gender- and/or sex-related factors being considered as biological variables have even been mandated in the NIH Policies for Biomedical Research [19-22]. For instance, sex differences have been reported in the blood pressure or tubuloglomerular feedback responses to Ang II [13,16], the renal thiazide-sensitive NaCl cotransporter (NCC) or epithelial sodium channel (ENaC) response to Ang II [23,24], the development of Ang II-induced hypertension [18], Ang II-mediated diabetic nephropathy [25], or Ang II-induced abdominal aortic aneurysms [26]. However, whether the sex differences contribute to the roles of intratubular Ang II and its AT1a receptors in the proximal tubules in the development of hypertension and glomerular and tubulointerstital injury has not been studied previously.
In the present study, we tested the hypothesis that there are significant sex differences in the contribution of AT1a receptors in the proximal tubules to the development of Ang II-induced hypertension and kidney injury. Specifically, AT1a receptors in the proximal tubules of female mice contribute less to Ang II-induced hypertension and kidney injury. Using adult male and female wildtype (WT) and mutant mice with proximal tubule-specific knockout of Ang II AT1a receptors (PT-Agtr1a−/−), we systematically compared the pressor, glomerular, and tubulointerstitial responses to Ang II-induced hypertension to determine whether there are any significant sex differences in these phenotypic responses to Ang II in mice.
Methods and materials
Animals
Mutant mice with proximal tubule-specific deletion of AT1a receptors, PT-Agtr1a−/−, were generated and bred in this laboratory using the iL1-Sglt2-Cre/Agtr1a-floxed approach [27,28]. The iL1-Sglt2-Cre mice were originally provided by Rubera et al. [29], whereas Agtr1a-floxed mice, C57BL/6N-Agtr1atm1Uky/J (Stock No: 016211), were purchased from Jackson Laboratories [30]. To obtain PT-Agtr1a−/− mice, C57BL/6N-Agtr1atm1Uky/J mice were crossed with iL1-Sglt2-Cre mice for the present study. As both iL1-Sglt2-Cre and C57BL/6N-Agtr1atm1Uky/J mice were generated on a C57BL/6J genetic background, WT mice are referred to either littermates from of iL1-Sglt2-Cre/Agtr1a-floxed breeding colony, which were C57BL/6N-Agtr1atm1Uky/J or iL-Sglt2-Cre mice [27,28]. Both male and female (>12–16-week-old) WT and PT-Agtr1a−/− mice, derived from three generations were used in the current study except the kidney pathology studies. The Institutional Animal Care and Use Committees at the University of Mississippi Medical Center and Tulane University School of Medicine approved all experiments reported in the present study.
Anesthesia and euthanasia
Unless specified elsewhere, animals were anesthetized with pentobarbital sodium injection, USP (Nembutal, 50 mg/kg, i.p.) for implantation of osmotic minipump infusion of Ang II to induce hypertension and kidney injury or for measuring glomerular filtration rate (GFR) responses (see below), as we described recently [8,9,27,28]. At the end of the experiments, all animals were euthanized by decapitation, blood samples and kidneys collected for biochemical and molecular assays and processed for light and electron microscopic (EM) analyses [8,9,27,28]. Animal anesthesia by pentobarbital and euthanization by decapitation procedures were approved by the Institutional Animal Care and Use Committees at the University of Mississippi Medical Center and Tulane University School of Medicine, respectively.
Comparisons of basal blood pressure in adult male and female WT and PT-Agtr1a−/− mice
To determine whether proximal tubule-specific deletion of AT1a receptors alters basal blood pressure or reveals any sex differences in basal blood pressure phenotypes, groups (n=12 each group) of adult male and female WT and PT-Agtr1a−/− mice were fed a normal rodent chow and had free access to tap water. Their basal systolic, diastolic and mean arterial blood pressure phenotypes were first confirmed by the telemetry technique (n=6 each group), and then measured and compared using the non-invasive tail-cuff approach (Visitech, NC) [27,28,31]. We have carefully compared basal blood pressure and its responses with Ang II-induced hypertension using these two different techniques, and found no significant differences in basal blood pressure and its responses to either a slow pressor (0.5 mg/kg/day, i.p.) or a high pressor dose (1.5 mg/kg/day, i.p.) of Ang II infusion. Regardless of which technique was used, all mice were first trained daily for blood pressure measurements between 9 and 11 a.m. for a week, and those data were not used. Then basal blood pressure was measured at least twice during the following week to obtain basal blood pressure data. This was followed by implantation of an osmotic minipump to infuse Ang II intraperitoneally (1.5 mg/kg/day, i.e., ~1000 ng/kg/min, or ~26 ng/mouse/min, i.p.) or 2 weeks). The blood pressure responses to Ang II were again measured at days 3, 7, and 14, respectively. However, only a peak and plateau blood pressure response at day 14 was presented in the present study.
Comparisons of basal GFR in adult male and female WT and PT-Agtr1a−/− mice
To determine whether proximal tubule-specific deletion of AT1a receptors alters their whole-kidney GFRs and reveals any sex differences in basal GFR responses, groups (n=8–10 for each group) of adult male and female WT and PT-Agtr1a−/− mice were used to measure GFR using the transdermal GFR monitoring approach with FITC-sinistrin (MediBeacon, Germany), as described [27,28]. Briefly, mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and the left jugular vein was canulated for a bolus injection of FITC-sinistrin, dissolved in sterilized saline, at a dose of 10 mg/kg. A transdermal monitor (~1 × 1.5 cm) powered by an internal battery was attached to a cleanly shaped, left flank of abdominal area (1.5 × 1.5 cm). The fluorescence emission signals from the skin microcirculation were continuously monitored and recorded for 2–3 h. The data were then transferred to the computer and analyzed based on the half-time of the decreasing concentration of FITC-sinistrin in the blood [31-33].
Comparisons of basal urinary sodium, potassium, chloride, and water excretion in adult male and female WT and PT-Agtr1a−/− mice
To determine whether proximal tubule-specific deletion of AT1a receptors alters and reveals any sex differences in basal urinary water and electrolyte excretion, groups (8–12 for each group) of adult male and female WT and PT-Agtr1a−/− mice were used to measure 24-h urinary sodium (Na+), potassium (K+), chloride (Cl−) and urine output using metabolic cage, as we described previously [19-22]. Specifically, all mice were placed in a clean metabolic cage individually and had free access to a standard rodent chow and water. Animals were first trained in a metabolic cage for 24 h urine sample collection before formal urine samples were collected for measurements of 24-h urinary sodium, potassium, and chloride excretion under basal conditions [19-22]. The urinary concentrations of Na+, K+, and Cl− were measured using an Electrolyte Analyzer (Nova Biomedical, MA).
Comparisons of the development of Ang II-induced hypertension in adult male and female WT and PT-Agtr1a−/− mice
To determine whether there are sex differences in the blood pressure responses to Ang II-induced hypertension, three groups (n=8–12 per group) of adult male and female WT and PT-Agtr1a−/− mice were infused with or without a pressor dose of Ang II (1.5 mg/kg/day via an osmotic minipump, Model 2002, 0.5 μl per hour for 14 days, Alzet) for 2 weeks, or treated concurrently with losartan to block AT1 receptors at all tissues (20 mg/kg/day, p.o.), as we described previously [27,28,32,34].
Comparisons of the natriuretic responses to Ang II-induced hypertension in adult male and female WT and PT-Agtr1a−/− mice
To determine whether there are sex differences in the natriuretic responses to Ang II-induced hypertension, groups (n=8–15 for each group) of adult male and female WT and PT-Agtr1a−/− mice were infused with or without Ang II, as described above, and placed in a metabolic cage for urine sample collection to determine 24-h urinary sodium, potassium, chloride, and water excretion, as we described recently [10,27,28,31,32,34]. However, sodium balance responses under basal conditions and in response to Ang II infusion or losartan treatment were not performed in the present study due to the difficulty and uncertainty of accurately and reliably measuring the sodium balance status in a mouse.
Comparisons of basal glomerular and proximal tubule structures in male and female WT and PT-Agtr1a−/− mice
To determine whether proximal tubule-specific deletion of AT1a receptors alters glomerular and proximal tubular structures, one group each (n=8) of adult male WT and PT-Agtr1a−/− mice were studied. Specifically, the kidneys were collected and bisected, with half of one kidney being fixed in 4% buffered formaldehyde, embedded in paraffin, and sectioned for light microscopic imaging. A small piece of the renal cortex (2 × 2 mm thick) was further processed for high-resolution electron microscopic (EM) imaging of the glomerular and proximal tubular histology [28,31]. For light microscopic examination, sections of the kidney were cut 5-μm-thick, placed on glass slides, and stained with Hematoxylin (HE) and Eosin, or Masson’s trichrome to examine glomerular and proximal tubule structures, as well as glomerular and tubulointerstitial collagen deposition. Glomerular or tubulointerstitial injury was stained and analyzed on the intensity and extent of collagen staining independently by Tulane University School of Medicine Pathology Core Laboratory investigators not involved in performing the experiments. Collagen staining in at least 10–20 glomeruli or cortical tubulointerstitial areas from each mouse, 5–8 mice per group, were scored and grouped to obtain averaged injury scores. For EM imaging, the superficial cortex of the kidney was processed and examined independently by the Department of Pathology Electron Microscope Laboratory at the University of Mississippi Medical Center, as we described recently [28,31]. Glomerular mesangial, endothelial, and epithelial cells and podocytes, as well as brush border membranes, microvillar, and mitochondria of proximal tubules were imaged at 6430× for glomeruli and 31900× for the proximal tubules, respectively.
Comparisons of glomerular and tubulointerstitial fibrotic responses to Ang II-induced hypertension in male WT and PT-Agtr1a−/− mice
To determine whether there are differences in glomerular and tubulointerstitial fibrotic responses to Ang II-induced hypertension, two groups of male WT and PT-Agtr1a−/− mice (n=8) were infused with or without Ang II for 2 weeks, as described above. Female WT and PT-Agtr1a−/− mice were not studied in this experimental protocol. Animals were euthanized and the kidneys were collected and processed for light and EM imaging, as described recently [27,28,32,34].
Measurement of blood volume and hematocrit in WT and PT-Agtr1a−/− mice
Whole blood samples were collected via decapitation at the end of the studies for measurements of whole blood volume and hematocrit in control and Ang II-infused male and female WT and PT-Agtr1a−/− mice. This information serves as indirect indices for basal body volumes and their responses to Ang II-induced hypertension.
Measurement of AT1a, AT1b, and AT2 receptor expression in the superficial renal cortex of WT and PT-Agtr1a−/− mice
To determine whether AT1a deletion selectively in the proximal tubules up-regulates or increases AT2 or AT1b receptor expression, we used Droplet Digital PCR (ddPCR) to quantify the mRNA copy numbers for AT2 and AT1b receptor expression in the proximal tubules between male and female WT littermates and PT-Agtr1a−/− mice [35,36]. Unlike a semi-quantitative RT-PCR, ddPCR is a novel technique that is widely used to perform absolute quantification of mRNA copy numbers of a particular target gene [35,36]. The hypothesis to be tested was that deletion of AT1a receptors selectively in the proximal tubules will lead to the up-regulation of AT2 or AT1b receptor expression in the proximal tubules of the kidney. Briefly, total RNA samples were isolated from the superficial renal cortex (~90% are the proximal tubules) of male and female WT littermates and PT-Agtr1a−/− mice (n=10 each strain, 5 males and 5 females) using a commercially available RNA isolation kit (Qiagen). RNA concentrations were quantitated using Nanodrop 2000 (Thermo Scientific). For mouse AT2 receptor expression, we purchased a pre-designed mouse AT2 receptor primer/probe set from Bio-Rad (mAgtr2: dMmuCPE5122376), whereas for AT1b receptor expression, we bought a pre-designed mouse AT1b receptor primer/probe set from Thermo Fisher (mAgtr1b: Mm01701115) [35,36], respectively. All reagents for the One-step RT-ddPCR system were purchased from Bio-Rad to generate cDNA and quantify gene expression. After droplet generation and PCR amplification, droplets were analyzed on the QX200 droplet reader and target cDNA concentration was determined using the QuantaSoft analysis software (Bio-Rad). For each target gene, the amount of total RNA in a PCR was optimized by a pilot ddPCR, using serially diluted total RNA, in which the determined amount is in a linear range [35,36]. Data are expressed as copy numbers of per target gene in 1 ng total RNA.
Statistical analysis
Unless specified elsewhere, all experimental results are presented as mean ± SEM. Specifically, the basal renal functional data and their responses to Ang II or losartan treatment were normalized by the kidney weight for gender comparisons. The differences in all responses between different groups of male and female WT and PT-Agtr1a−/− mice, including basal systolic, diastolic and mean arterial pressure, 24-h urinary Na+ excretion, the hypertensive and natriuretic responses to Ang II or concurrent treatment with losartan were first analyzed using one-way ANOVA. This was followed by Student’s unpaired t test if a significant response between different groups of WT and PT-Agtr1a−/− mice, or between male and female animals in the same strains was detected. The significance of statistical differences between responses were set P<0.05.
Results
AT1a, AT1b, and AT2 receptor mRNA expression in the superficial renal cortex of WT and PT-Agtr1a−/− mice
Table 1 summarizes the expression levels of AT1a, AT1b, and AT2 receptors in the superficial cortex of the kidney in adult male and female WT littermates and PT-Agtr1a−/− mice (n=10 each strain, 5 males and 5 females). In WT littermates, AT1a receptors were expressed at a very high level in the superficial cortex, as expected, and ~90% of AT1a receptor expression was markedly decreased in isolated proximal tubules of male and female PT-Agtr1a−/− mice. By contrast, AT1b and AT2 receptors in the superficial cortex of the kidney were very low in adult male and female WT littermates, and there were no significances in the expression levels of AT1b or AT2 receptors in the superficial cortex of the kidney between WT littermates and PT-Agtr1a−/− mice.
Table 1.
The expression levels of AT1a, AT1b, and AT2 receptors in the superficial cortex of the kidney in male and female WT littermates and PT-Agtr1a−/− mice (n=10 each strain, 5 males and 5 females)
| Parameter | WT littermate | PT-Agtr1a−/− |
|---|---|---|
| AT1a mRNA, copy/ng RNA | 652.9 ± 33.8 | 54.6 ± 4.52 |
| AT1b mRNA, copy/ng RNA | 1.3 ± 0.091 | 0.94 ± 0.051 |
| AT2 mRNA, copy/ng RNA | 0.17 ± 0.011 | 0.15 ± 0.011 |
P<0.01 vs. AT1a.
P<0.01 vs. WT littermate.
Note that AT1a mRNA expression in PT-Agtr1a−/− mice was assayed from freshly isolated proximal tubules, and that AT1b or AT2 expression was not significantly up-regulated or increased in the superficial cortex of the kidney in PT-Agtr1a−/− mice.
Comparisons of general phenotypic responses to basal conditions, during Ang II-hypertension, and treatment with losartan between male and female WT and PT-Agtr1a−/− mice
Under basal conditions, body wt., heart wt., or kidney wt. were smaller proportionally in age-matched WT female than male mice (P<0.01), but the heart or the kidney to body wt. ratios were similar between male and female WT mice (Table 2). The whole blood volume and hematocrit were not different between male and female WT mice. Basal 24 h urine output and urinary Na+, K+, or Cl− concentrations were significantly lower in female WT mice (P<0.01). In PT-Agtr1a−/− mice, there were also no significant differences in the body wt., heart wt., or the heart to body wt. ratios between male and female mice (n.s.), but the kidney wt. and kidney wt. to body wt. ratio were significantly decreased in female mice (P<0.01). Likewise, basal 24 h urine output and urinary Na+, K+, or Cl− concentrations were significantly lower in female PT-Agtr1a−/− mice (P<0.01). There were no significances in most of these basal phenotypes between male WT and PT-Agtr1a−/− mice, except that the heart wt. and heart wt. to body wt. ratio were lower in male PT-Agtr1a−/− mice (P<0.01). There were also no significances in most of these basal phenotypes between female WT and PT-Agtr1a−/− mice, except that the heart wt. or the kidney wt. to body wt. ratios were lower in female PT-Agtr1a−/− mice (P<0.01) (Table 2).
Table 2.
Basal general physiological phenotypes of control adult male and female WT and proximal tubule-specific PT-Agtr1a−/− mice
| Parameter | WT male (n=8) |
PT-Agtr1a−/− male (n=10) |
WT female (n=16) |
PT-Agtr1a−/− female (n=10) |
|---|---|---|---|---|
| Body wt., g | 26 ± 0.5 | 26 ± 0.5 | 21 ± 0.63,4 | 24 ± 0.91 |
| Heart wt., g | 0.18 ± 0.01 | 0.13 ± 0.012 | 0.14 ± 0.013,4 | 0.12 ± 0.01 |
| Heart wt./body wt. ratio | 0.62 ± 0.2 | 0.52 ± 0.012 | 0.66 ± 0.03 | 0.53 ± 0.022 |
| Left kidney wt., g | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.14 ± 0.013,4 | 0.14 ± 0.011,3,4 |
| Left kidney wt./body wt. ratio | 0.68 ± 0.05 | 0.68 ± 0.03 | 0.67 ± 0.02 | 0.61 ± 0.12,3,4 |
| Blood volume, mL. | 0.64 ± 0.03 | 0.66 ± 0.04 | 0.61 ± 0.06 | 0.58 ± 0.033,4 |
| Hematocrit, % | 48.0 ± 0.9 | 47.0 ± 0.5 | 50.0 ± 0.5 | 46.0 ± 0.52 |
| Fluid intake, ml/24 h | 3.0 ± 0.16 | 2.8 ± 0.4 | 3.4 ± 0.2 | 2.2 ± 0.32 |
| Urine volume, ml/24 h | 1.2 ± 0.15 | 1.1 ± 0.05 | 0.74 ± 0.133,4 | 0.77 ± 0.143,4 |
| Urine Na+ conc., mmol/l | 196 ± 30 | 199 ± 12 | 102 ± 123,4 | 107 ± 153,4 |
| Urine K+ conc., mmol/l | 278 ± 26 | 285 ± 25 | 174 ± 203,4 | 151 ± 233,4 |
| Urine Cl− conc., mmol/l | 235 ± 37 | 257 ± 19 | 151 ± 223,4 | 163 ± 223,4 |
P<0.05 or
P<0.01 vs. male or female WT mice.
P<0.05 or
P<0.01 vs. male WT or PT-Agtr1a−/− mice, respectively.
In response to Ang II-induced hypertension, the kidney wt. to body wt. was higher, while hematocrit was lower in female than male WT mice (P<0.01) (Table 3). However, other phenotypic responses to Ang II-induced hypertension were similar between male and female WT mice including 24 h urine excretion (Table 2). In PT-Agtr1a−/− mice, the heart wt. to body wt. ratio was significantly lower in female mice in response to Ang II-induced hypertension, without altering the kidney to body wt. ratio (n.s.). Female PT-Agtr1a−/− mice drank significantly less (P<0.01) and excreted more K+ from the kidney (P<0.05) (Table 3). There was no difference in 24 h urine excretion in response to Ang II between male and female PT-Agtr1a−/− mice (Table 2). Comparisons between Ang II-infused male WT and PT-Agtr1a−/− mice showed significantly lower hematocrit (P<0.05), and higher urinary Na+ concentration in male PT-Agtr1a−/− than WT mice (P<0.01). In Ang II-infused female PT-Agtr1a−/− mice, the heart wt. or kidney wt. to body wt. ratios were significantly lower (P<0.01), blood volume and urinary Na+, K+, and Cl− concentrations were higher than Ang II-infused female WT mice (P<0.01) (Table 3).
Table 3.
General phenotypic responses to Ang II-induced hypertension (1.5 mg/kg/day, i.p.) for 2 weeks in adult male and female WT and proximal-tubule specific PT-Agtr1a−/− mice
| Parameter | WT male + Ang II (n=9) |
PT-Agtr1a−/− male + Ang II (n=9) |
WT female + Ang II (n=8) |
PT-Agtr1a−/−female + Ang II (n=10) |
|---|---|---|---|---|
| Body wt., g | 23.0 ± 0.5 | 26.0 ± 0.6 | 20.0 ± 0.83 | 22.0 ± 0.43 |
| Heart wt., g | 0.17 ± 0.03 | 0.18 ± 0.01 | 0.14 ± 0.013 | 0.14 ± 0.013 |
| Heart wt./body wt. ratio | 0.76 ± 0.04 | 0.74 ± 0.04 | 0.79 ± 0.03 | 0.70 ± 0.023 |
| Left kidney wt., g | 0.15 ± 0.02 | 0.17 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.063 |
| Left kidney wt./body wt. ratio | 0.68 ± 0.03 | 0.70 ± 0.01 | 0.77 ± 0.044 | 0.72 ± 0.04 |
| Blood volume, ml | 0.54 ± 0.04 | 0.56 ± 0.05 | 0.53 ± 0.03 | 0.59 ± 0.041,3 |
| Hematocrit, % | 54 ± 0.7 | 50 ± 1.31 | 50 ± 1.43 | 49 ± 1 |
| Fluid intake, ml/24 h | 4.6 ± 0.3 | 5.7 ± 0.62 | 4.9 ± 0.5 | 4.6 ± 0.24 |
| Urine volume, ml/24 h | 2.3 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 1.5 ± 0.23 |
| Urine Na+ conc., mmol/l | 194 ± 15 | 244 ± 241 | 173 ± 21 | 236 ± 452 |
| Urine K+ conc., mmol/l | 262 ± 28 | 285 ± 25 | 273 ± 25 | 385 ± 752,4 |
| Urine Cl− conc., mmol/l | 298 ± 18 | 294 ± 44 | 237 ± 26 | 319 ± 872 |
P<0.05 or
P<0.01 vs. male or female WT mice.
P<0.05 or
P<0.01 vs. male WT or PT-Agtr1a−/− mice, respectively.
Table 4 summarizes the sex differences in the general phenotypic responses to the AT1 receptor blockade with losartan in Ang II-infused male and female WT and PT-Agtr1a−/− mice. In age-matched WT mice, body wt., heart wt., and kidney wt. were all significantly lower in female than male mice (P<0.01), but the heart wt. or kidney wt. to body wt. ratios were not significantly different between male and female WT mice (n.s.). Furthermore, 24 urine output and urinary Na+ and K+ concentrations were significantly lower in female than male WT mice (P<0.01). Like losartan-treated female WT mice, body wt., heart wt., kidney wt., and the heart wt. to body wt. ratio were significantly lower in female than male PT-Agtr1a−/− mice (P<0.01). Unlike losartan-treated female WT mice, however, urinary Na+, K+, and Cl− concentrations were not different between male and female PT-Agtr1a−/− mice in response to losartan treatment (n.s.) (Table 4). Further comparisons show that the heart wt. and kidney wt. ratios and blood volume were significantly lower in losartan-treated, Ang II-infused male PT-Agtr1a−/− mice (P<0.01), while urinary Na+ and K+ concentrations were higher in losartan-treated, Ang II-infused female PT-Agtr1a−/− mice (P<0.01) (Table 4).
Table 4.
General phenotypic responses to Ang II-induced hypertension (1.5 mg/kg/day, i.p.) treated with the AT1 receptor blocker losartan (20 mg/kg/day, p.o.) for 2 weeks in adult male and female WT and proximal-tubule specific PT-Agtr1a−/− mice
| Parameter | WT male + Ang II + Los (n=9) |
PT-Agtr1a−/− male + Ang II + Los (n=9) |
WT female + Ang II + Los (n=9) |
PT-Agtr1a−/− female + Ang II + Los (n=10) |
|---|---|---|---|---|
| Body wt., g | 27 ± 0.5 | 28 ± 0.7 | 22 ± 0.52,4 | 23 ± 0.44 |
| Heart wt., g | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.15 ± 0.012,4 | 0.13 ± 0.014 |
| Heartwt./body wt. ratio | 0.79 ± 0.01 | 0.71 ± 0.042 | 0.75 ± 0.013 | 0.62 ± 0.032,4 |
| Left kidney wt., g | 0.20 ± 0.03 | 0.18 ± 0.01 | 0.14 ± 0.021,4 | 0.15 ± 0.014 |
| Left kidney wt./body wt. ratio | 0.76 ± 0.03 | 0.70 ± 0.032 | 0.72 ± 0.02 | 0.70 ± 0.01 |
| Blood volume, ml | 0.77 ± 0.05 | 0.68 ± 0.022 | 0.56 ± 0.034 | 0.61 ± 0.021,4 |
| Hematocrit, % | 48.0 ± 0.7 | 48.0 ± 1.1 | 50.0 ± 1.1 | 47.0 ± 0.8 |
| Fluid intake, ml/24 h | 3.0 ± 0.4 | 3.2 ± 0.4 | 3.3 ± 0.3 | 3.4 ± 0.4 |
| Urine volume, ml/24 h | 1.5 ± 0.1 | 1.3 ± 0.1 | 0.68 ± 0.12,4 | 0.80 ± 0.12,4 |
| Urine Na+ conc., mmol/l | 248 ± 24 | 223 ± 23 | 159 ± 142,4 | 203 ± 262 |
| Urine K+ conc., mmol/l | 299 ± 23 | 296 ± 32 | 208 ± 324 | 277 ± 362 |
| Urine Cl− conc., mmol/l | 303 ± 27 | 290 ± 33 | 270 ± 44 | 261 ± 59 |
P<0.05; or
P<0.01 vs. male or female WT mice.
P<0.05 or
P<0.01 vs. male WT or PT-Agtr1a−/− mice, respectively.
Comparisons of glomerular and proximal tubule ultrastructure in male and female WT and PT-Agtr1a−/− mice
Glomerular mesangial cell (MC), capillary (Ca), and glomerular basement membranes (GBMs) and podocytes (POs), as well as brush border membranes, microvillar (MV), and mitochondria (Mito) of proximal tubules were carefully examined and compared in male and female WT and PT-Agtr1a−/− mice by high-resolution EM imaging in Figures 1 and 2, respectively. In the glomerulus (Figure 1), MC, Ca, GBM, PO looked similar in their size, orientations, or thickness between WT male (A) and female mice (C), between male (B) and female PT-Agtr1a−/− mice (D). There were also no clear differences in the overall glomerular ultrastructure between male and female WT and PT-Agtr1a−/− mice. In the proximal tubules of the kidney (Figure 2), the brush border membranes, especially MV, and Mito were similar in male (A) and female WT mice (C). More peroxisomes, i.e., membrane-bound organelles, are clearly visible in the cytoplasm in both male (B) and female PT-Agtr1a−/− mice (D). The microvillar appeared to be more disorganized and shorter in length in male PT-Agtr1a−/− mice (B). Whether it was due to the orientation of tissue section cutting during the preparations is not known.
Figure 1. High resolution EM micrographs comparing the glomerular ultrastructures between male and female WT and proximal tubule-specific AT1 (AT1a) receptor-knockout mice, PT-Agtr1a−/− mice.
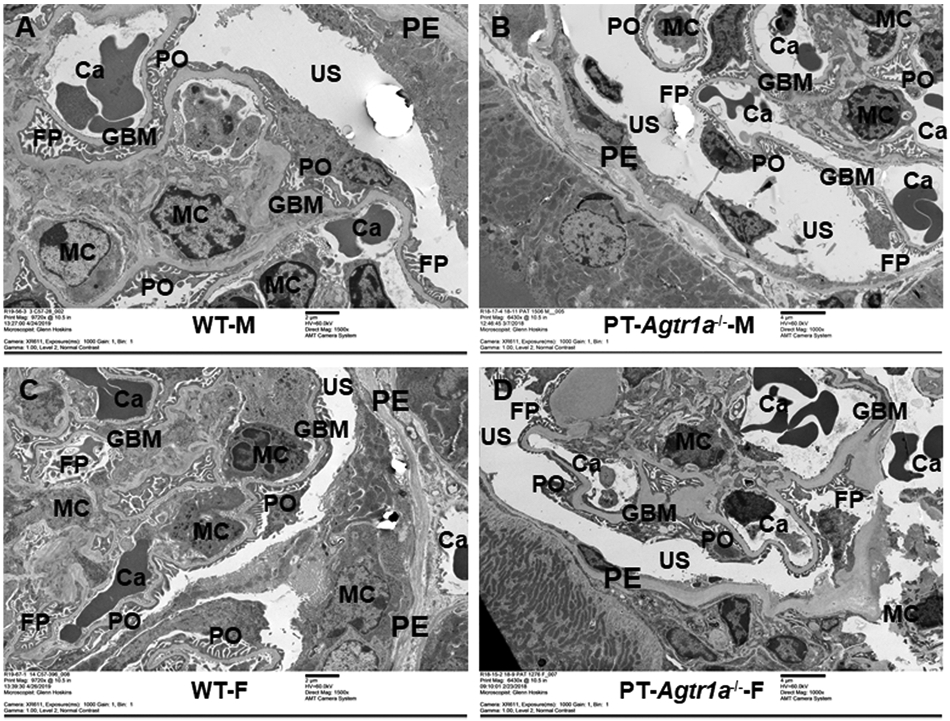
(A,C) High-resolution EM micrographs showing the ultrastructures of representative glomerulus (9720×) in adult male and female WT mice. (B,D) EM micrographs showing the ultrastructures of representative glomerulus (6430 or 9720×) in adult male and female PT-Agtr1a−/− mice, respectively. Glomerular ultrastructures including capillaries (Ca), epithelial (PE), mesangial cells (MCs), and POs were largely similar between male and female WT mice, or between male and female PT-Agtr1a−/− mice. Urinary space (US) of Bowman’s capsule, and foot processes (FP) looks similar between male WT (A) and PT-Agtr1a−/− mice (B), or between female WT (C) and PT-Agtr1a−/− mice (D). Likewise, GBMs were not different between male and female WT and PT-Agtr1a−/− mice. n=8 per group.
Figure 2. High-resolution EM images comparing the proximal tubule ultrastructures between male and female WT and proximal tubule-specific AT1 (AT1a) receptor-knockout mice, PT-Agtr1a−/− mice.
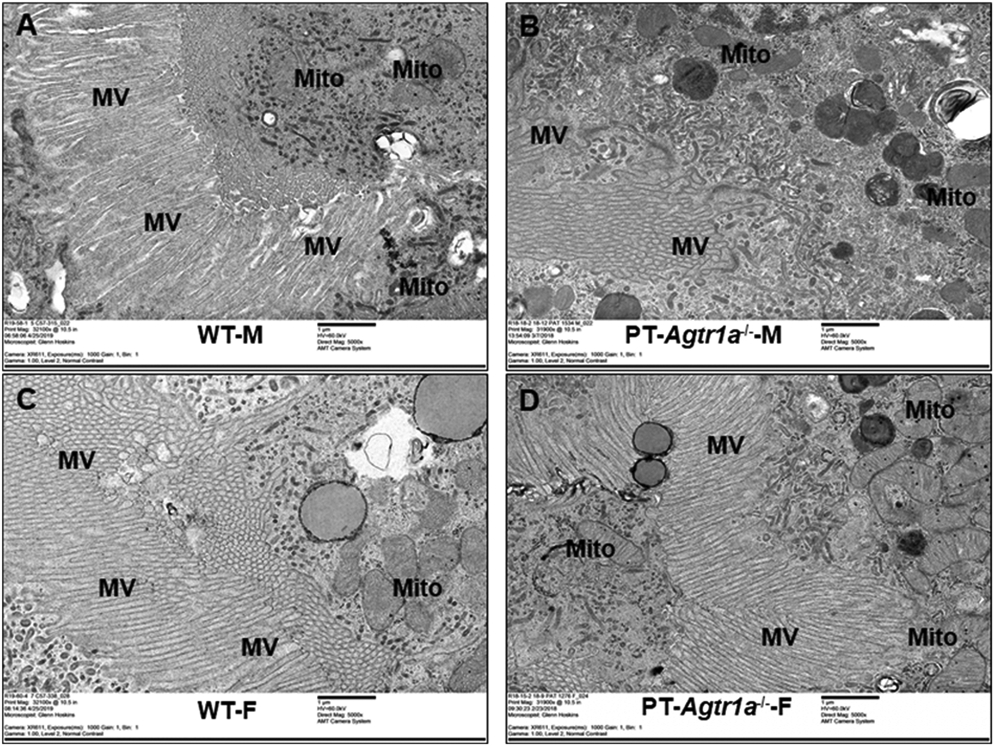
(A,C) High-resolution EM micrographs showing the ultrastructures of representative proximal tubules in adult male and female WT mice (31900 or 32000×). (B,D) EM micrographs showing the ultrastructures of representative proximal tubules in adult male and female Agtr1a−/− mice (31900 or 32000×), respectively. No significant differences were found in the proximal tubule ultrastructures between male (A) and female WT mice (C), or between male (B) and female PT-Agtr1a−/− mice (D). Not sure whether it is due to the orientation of section cutting, the microvillars looks shorter and disorganized in male PT-Agtr1a−/− mice (D). n=8 per group.
Comparisons of basal blood pressure phenotypes and their responses to Ang II and AT1 receptor blockade in adult male and female WT and PT-Agtr1a−/− mice
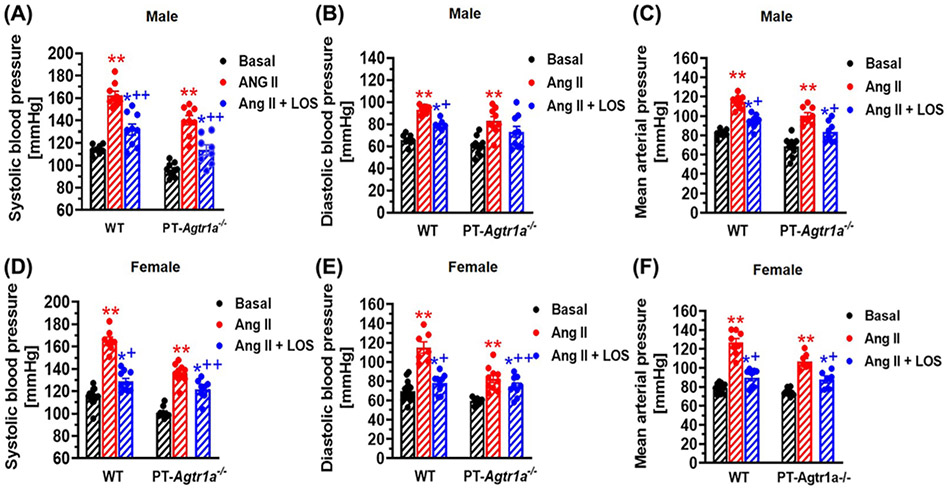
Under basal conditions, systolic, diastolic and mean arterial blood pressure in male and female WT and PT-Agtr1a−/− mice are shown in Figure 3. In WT mice, there were no significant differences in basal systolic (male: 114 ± 2 mmHg vs. female: 115 ± 2 mmHg, n.s.), diastolic and mean arterial blood pressure between male (A) and female mice (D). In response to Ang II infusion, systolic blood pressure increased to 165 ± 3 mmHg inmale (P<0.01) and to 163 ± 4 mmHg in female WT mice (P<0.01), respectively. No difference in the pressor response to Ang II infusion was found between male and female WT mice. Concurrent treatment with losartan attenuated Ang II-induced hypertension to 132 ± 5 mmHg in male (P<0.01), and to 129 ± 3 mmHg in female WT mice (P<0.01), respectively. Both diastolic (B,E) and mean arterial blood pressure responses (C,F) to Ang II with or without concurrent losartan treatment were similar to systolic blood pressure responses, and no significant differences were detected between male and female WT mice. In PT-Agtr1a−/− mice, there were also no significant differences in basal systolic (male: 101 ± 3 mmHg vs. female: 96 ± 2 mmHg, n.s.), diastolic and mean arterial blood pressure between male (A) and female mice (D). In response to Ang II infusion, systolic blood pressure increased to 140 ± 3 mmHg in male (P<0.01) and to 136 ± 4 mmHg in female PT-Agtr1a−/− mice (P<0.01), respectively. Concurrent treatment with losartan attenuated Ang II-induced hypertension to 114 ± 5 mmHg in male (P<0.01), and to 122 ± 3 mmHg in female PT-Agtr1a−/− mice (P<0.01), respectively. Both diastolic (B,E) and mean arterial blood pressure responses (C,F) to Ang II with or without concurrent losartan treatment were similar to systolic blood pressure responses, and no significant differences were detected between male and female PT-Agtr1a−/− mice. However, basal systolic, diastolic and mean arterial blood pressure were all >13 ± 2 mmHg lower in male and female PT-Agtr1a−/− than WT male and female mice (P<0.01), and their responses to Ang II-induced hypertension were significantly attenuated in both male and female PT-Agtr1a−/− than WT mice (P<0.01). Concurrent treatment of Ang II-induced hypertension with losartan significantly, but not completely attenuated the pressor responses to their basal levels in both male and female Ang II-infused PT-Agtr1a−/− mice (P<0.01).
Figure 3. Basal systolic, diastolic, and mean arterial pressure and their responses to Ang II infusion with or without concurrent treatment with losartan in adult male and female wildtype and proximal tubule-specific PT-Agtr1a−/− mice.
Comaprisons of basal systolic (A,D), diastolic (B,E) and mean arterial blood pressure (C,F) and their responses to Ang II infusion with or without AT1 (AT1a) receptor blocker losartan between male and female WT and PT-Agtr1a−/− mice. Proximal tubule-specific deletion of AT1a receptors significantly decreased basal blood pressure by ~13–15 ± 3 mmHg similarly in male and female PT-Agtr1a−/−mice under basal conditions, and significantly atteuated the hypertensive responses to Ang II in both male and female PT-Agtr1a−/−mice. No sex differences were found in basal blood pressure and its responses to Ang II with or without losartan treatment between male and female WT or between male and female PT-Agtr1a−/− mice. *P<0.05 or **P<0.01 vs. control WT or PT-Agtr1a−/− mice; +P<0.05 or ++P<0.01 vs. Ang II-infused male or female WT or PT-Agtr1a−/− mice.
Comparisons of basal GFR and its response to Ang II in adult male and female WT and PT-Agtr1a−/− mice
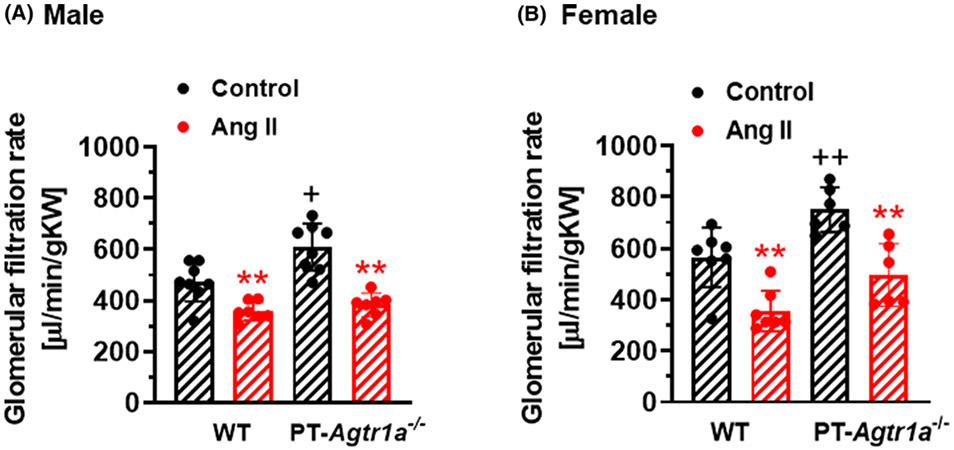
Basal whole-kidney GFRs were slightly, but not significantly, higher in female WT or PT-Agtr1a−/− mice than male counterparts (n.s.). However, basal GFR was significantly higher in male and female PT-Agtr1a−/− mice than WT counterparts (P<0.01) (Figure 4). Interestingly, Ang II infusion significantly decreased GFR in both male and female WT and PT-Agtr1a−/− mice (P<0.01), but no significant differences in the magnitude of the responses were observed between two strains of mice (n.s.).
Figure 4. Basal glomerular filtration rate (GFR) and its response to Ang II-induced hypertension in adult male and efamle wildtype and proximal tubule-specific PT-Agtr1a−/− mice.
Comaprisons of basal GFR and its responses to Ang II-induced hypertension between male (A) and female (B) WT and PT-Agtr1a−/− mice. The data were normalized by the kidney weight for comparisons of gender differences. Under basal conditions, there were no significant sex differences in basal GFR levels between male and female WT mice, or between male and female PT-Agtr1a−/− mice. In response to Ang II-induced hypertension, GFR largely decreased proportionally in male and female WT and PT-Agtr1a−/− mice. **P<0.01 vs. control or untreated WT or PT-Agtr1a−/− mice; +P<0.05 or ++P<0.01 vs. male or female WT controls.
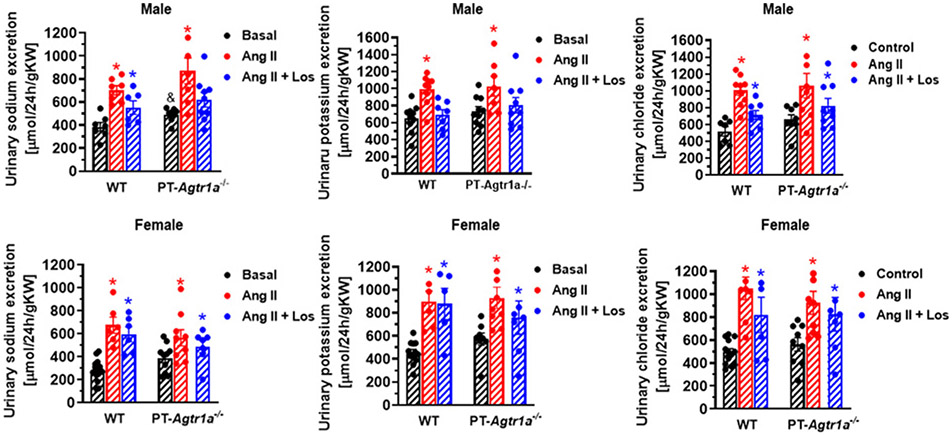
Comparisons of basal urinary sodium, potassium, chloride, and water excretion and their responses to Ang II or losartan in adult male and female WT and PT-Agtr1a−/− mice
In WT mice, basal 24-h urinary Na+ and K+ excretion was significantly lower in female than male mice (P<0.05), without significantly altering urinary Cl− excretion (Figure 5). Ang II infusion induced significant pressure-related natriuretic, kaliuretic, and chloride excretory responses in both male and female WT mice (P<0.05), whereas losartan had no further or attenuated significant effects (n.s.). In PT-Agtr1a−/− mice, basal 24-h urinary Na+, K+ and Cl− excretion were higher in male than WT male mice (P<0.05), but not between female WT and PT-Agtr1a−/− mice (n.s.). Similarly, losartan treatment did not attenuate the urinary Na+, K+ and Cl− excretory responses to Ang II infusion in male and female PT-Agtr1a−/− mice (n.s.).
Figure 5. Comaprisons of basal 24-h urinary Na+, K+, Cl− excretion and their responses to Ang II-induced hypertension treated with or without the AT1 receptor blocker losartan between male and female WT and PT-Agtr1a−/− mice.
The data were normalized by the kidney weight for comparisons of gender differences. Note that basal 24-h urinary Na+ and K+ excretion were significantly higher in male and female PT-Agtr1a−/− mice than their male and female WT mice. However, basal 24 h urinary Na+ and K+ excretion were lower in female than male WT and PT-Agtr1a−/− mice. These natriuretic and kaliuric responses to Ang II-induced hypertension were moderately augmented in male but blunted in female PT-Agtr1a−/− mice. *P<0.05 vs. basal WT or PT-Agtr1a−/− controls. &P<0.05 vs. wildtype basal.
Comparisons of glomerular and tubulointerstitial fibrotic responses to Ang II-induced hypertension in male WT and PT-Agtr1a−/− mice
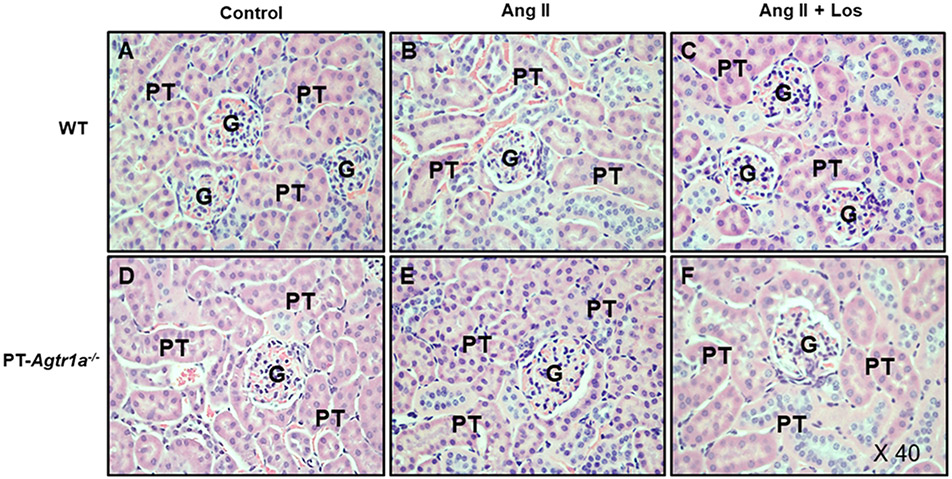
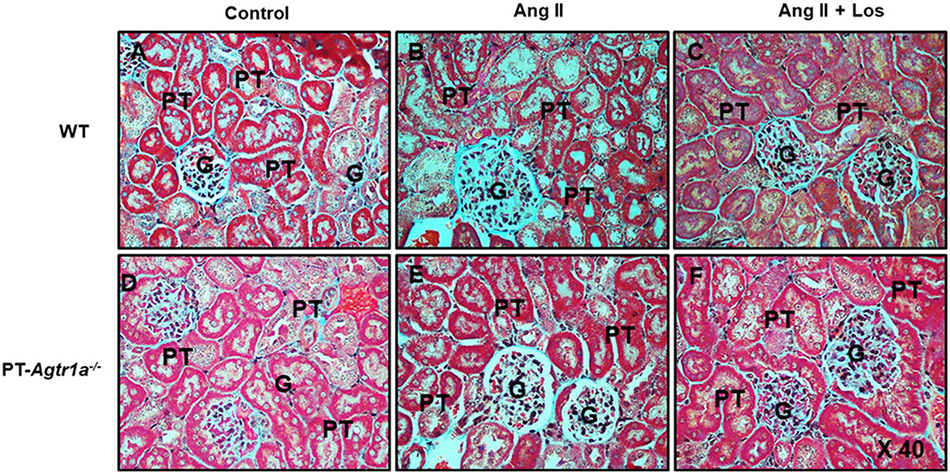
Figures 6 and 7 show the glomerular histology stained with Hematoxylin (HE) and fibrotic responses to Ang II-induced hypertension with or without concurrent losartan treatment in male WT and PT-Agtr1a−/− mice. Light microscopic HE staining revealed no significant differences in the glomerular histology between male WT and PT-Agtr1a−/− mice under basal conditions, during Ang II infusion or losartan treatment (Figure 6). However, although Masson’s trichrome staining showed no significant differences between male WT (Figure 7A) and PT-Agtr1a−/− mice (Figure 7D) under basal conditions, it revealed marked glomerular injury or fibrotic responses during Ang II-induced hypertension in male WT mice (Figures 7B and 10A). The Ang II-induced glomerular fibrotic responses were blocked by concurrent losartan treatment (Figures 7C and 10A). Interestingly Ang II-induced glomerular fibrotic responses were significantly attenuated in male PT-Agtr1a−/− mice (Figures 7E and 10A). Concurrent losartan treatment had no further significant effect in male PT-Agtr1a−/− mice (Figures 7F and 10A).
Figure 6. Comparisons of general glomerular histology as stained by HE and Eosin in male WT and PT-Agtr1a−/− mice under control conditions and during Ang II-induced hypertension treated with or without AT1 receptor blocker losartan.
(A) WT control mice. (B) WT mice with Ang II infusion. (C) WT mice with concurrent Ang II infusion and losartan treatment. (D) PT-Agtr1a−/− control mice. (E) PT-Agtr1a−/− mice with Ang II infusion. (F) PT-Agtr1a−/− mice with concurrent Ang II infusion and losartan treatment. There were no differences found in the glomerular histology between male WT and PT-Agtr1a−/− mice. G, glomerulus; PT, proximal tubule. Magnification: ×40.
Figure 7. Comparisons of glomerular fibrotic responses, as determined by Masson’s trichrome staining, to Ang II-induced hypertension treated with or without losartan between male WT and PT-Agtr1a−/− mice.
(A) WT control mice. (B) WT mice with Ang II infusion. (C) WT mice with concurrent Ang II infusion and losartan treatment. (D) PT-Agtr1a−/− control mice. (E) PT-Agtr1a−/− mice with Ang II infusion. (F) PT-Agtr1a−/− mice with concurrent Ang II infusion and losartan treatment. Note that Ang II induced marked fibrotic response in the glomerulus (B), compared with control WT mice (A). The glomerular fibrotic response was almost completely prevented by concurrent treatment with losartan (C). In PT-Agtr1a−/− mice, however, Ang II-induced glomerular injury was markedly attenuated (E vs. B), compared with control PT-Agtr1a−/− mice (D). Further treatment with losartan revealed no significant further effect in PT-Agtr1a−/− mice (F). G, glomerulus; PT, proximal tubule. Magnification: ×40.
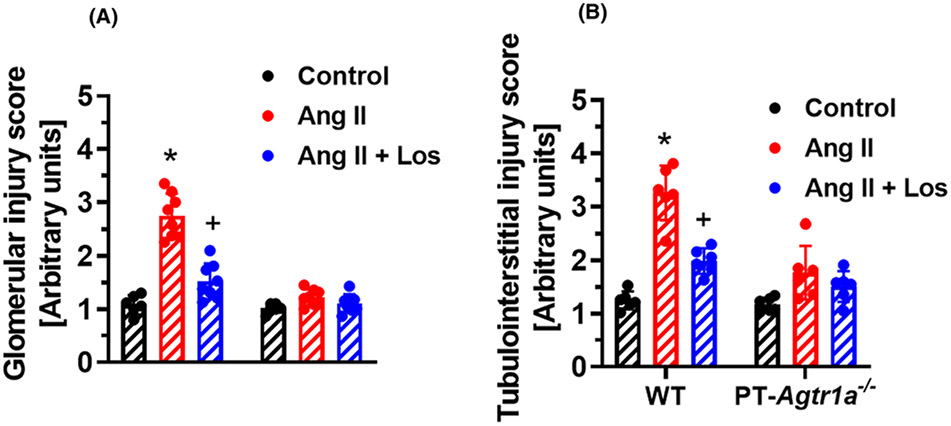
Figure 10. Proximal tubule-specific deletion of AT1a receptors attenuates glomerular and tubulointerstitial injury in Ang II-induced hypertension in adult male PT-Agtr1a−/− mice.
Semi-quantitative data showing that genetic deletion of AT1 (AT1a) receptors selectively in the proximal tubules of the kidney prevents Ang II-induced glomerular (A) and tubulointerstitail injury (B) in male PT-Agtr1a−/− mice. *P<0.05 vs. WT control. +P<0.05 vs. Ang II-infused WT mice. n=8 for each group.
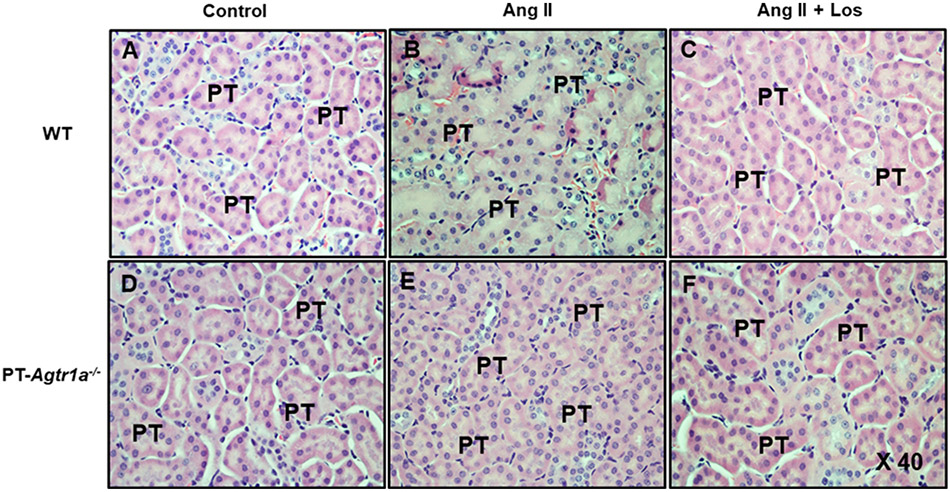
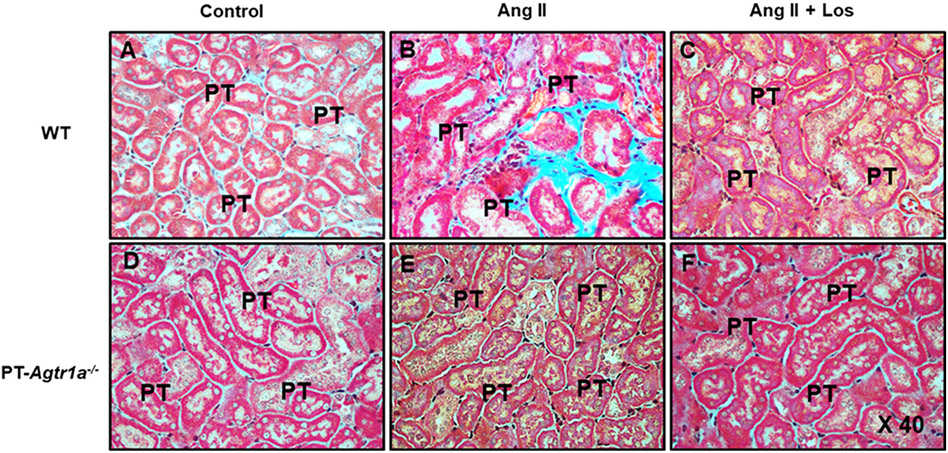
Figures 8 and 9 show the tubulointerstitial histology stained with HE and fibrotic responses stained with Masson’s trichrome to Ang II-induced hypertension with or without concurrent losartan treatment in male WT and PT-Agtr1a−/− mice. Although there were not significant differences in the tubule-interstitial histology between WT and PT-Agtr1a−/− mice under control, during Ang II infusion, or concurrent with losartan treatment (Figure 8), significant tubulointerstitial fibrotic responses were observed in Ang II-infused WT mice (Figures 9B and 10A). Concurrent treatment with losartan completely attenuated Ang II-induced tubulointerstitial fibrotic response in WT mouse kidney (Figure 9C). However, Ang II-induced tubulointerstitial fibrotic response occurred in WT mouse kidney was completely blocked in the kidneys of PT-Agtr1a−/− mice, whereas concurrent losartan treatment was without further effects (Figures 9E and 10B).
Figure 8. Comparisons of general proximal tubule and interstitial histology as stained by HE and Eosin in male WT and PT-Agtr1a−/− mice under control conditions and during Ang II-induced hypertension treated with or without losartan.
(A) WT control mice. (B) WT mice with Ang II infusion. (C) WT mice with concurrent Ang II infusion and losartan treatment. (D) PT-Agtr1a−/− control mice. (E) PT-Agtr1a−/− mice with Ang II infusion. (F) PT-Agtr1a−/− mice with concurrent Ang II infusion and losartan treatment. There were no differences found in the proximal tubule and interstitial histology between male WT and PT-Agtr1a−/− mice. Magnification: ×40.
Figure 9. Comparisons of the proximal tubular and interstitial fibrotic responses, as determined by Masson’s trichrome staining, to Ang II-induced hypertension treated with or without losartan between male WT and PT-Agtr1a−/− mice.
(A) WT control mice. (B) WT mice with Ang II infusion. (C) WT mice with concurrent Ang II infusion and losartan treatment. (D) PT-Agtr1a−/− control mice. (E) PT-Agtr1a−/− mice with Ang II infusion. (F) PT-Agtr1a−/− mice with concurrent Ang II infusion and losartan treatment. Note that Ang II induced marked tubulointerstitial fibrotic response in WT mice (B), compared with control WT mice (A), and Ang II-induced tubulointerstitail injury response was completely blocked by concurrent losartan treatment (C). By contrast, Ang II did not significantly induce significant tubulointerstitial injury or fibrotic response in PT-Agtr1a−/− mice (E vs. D) with or without losartan treatment (F). PT, proximal tubule. Magnification: ×40.
Discussion
Gender or sex differences have been recognized in most, if not all, cardiovascular, blood pressure, and renal responses or homeostasis in animal as well as clinical research. Indeed, this recognition has led to the National Institute of Health (NIH) to mandate the Consideration of Sex as a Biological Variable in all NIH-funded Research in 2014, and an explosion of gender- or sex-related studies recently [19-22]. As expected, sex or gender differences have been reported differently in blood pressure, proinflammatory, and renal excretory or transport responses to Ang II or blockers of the RAS [13-15,17,18,37]. One of major objectives of the present study was to determine whether there are sex differences in the blood pressure, renal excretory, and fibrotic responses to Ang II between male and female WT mice, and between male and female PT-Agtr1a−/− mice. We demonstrated that proximal tubule-specific deletion of AT1a receptors consistently lowered systolic, diastolic, and mean arterial blood pressure by 13–15 mmHg in male and female PT-Agtr1a−/− mice in the absence of AT1a receptors in the proximal tubules. Although the present study found sex differences in some minor phenotypic responses, there were no sex differences in basal blood pressures between male and female wildtype, and between male and female PT-Agtr1a−/− mice. In response to 2-week-infusion of a pressor dose of Ang II, both male and female WT and PT-Agtr1a−/− mice developed severe hypertension, and the magnitudes of the pressor response were similar between WT male and female, and between male and female PT-Agtr1a−/− mice, respectively. The magnitudes of diastolic and mean arterial pressure responses to Ang II were similar to the systolic blood pressure response in both WT and PT-Agtr1a−/− mice. Still, the maximal blood pressure levels remained to be ~20 mmHg lower in male and female PT-Agtr1a−/− than male and female WT mice. Furthermore, we found that concurrent blockade of AT1 receptors with losartan significantly decreased the pressor response to similar extents in male and female WT and PT-Agtr1a−/− mice, respectively. Ang II and losartan induced similarly decreased heart rate response in both male and female WT and PT-Agtr1a−/− mice (not shown). Taken together, the present study does not reveal significant sexual dimorphism or sex differences in the blood pressure response to Ang II or losartan treatment in WT and PT-Agtr1a−/− mice.
So how the findings of the present study on Ang II-induced hypertension in male and female WT and PT-Agtr1a−/− mice compare with previous studies? Veiras et al. recently demonstrated that Ang II provokes similar increases in blood pressure, natriuretic, or diuretic responses in male and female Sprague–Dawley and C57BL/6J mice [15]. In an early study, Xue et al. found no sex differences in basal blood pressure levels in male and female mice [18]. Likewise, Brown et al. also carefully compared basal blood pressure and their responses to Ang II in male and female WT and AT2 receptor knockout mice, and reported no significant sex differences in basal blood pressure between male and female WT or AT2 receptor knockout mice [13]. Our results are consistent with these previous studies and suggest that sex differences may not play a key role in maintaining basal blood pressure homeostasis in mice.
However, some inconsistencies have been reported in the roles of sex differences in Ang II-induced hypertension in both WT and some mutant mouse models [13-18,35]. A number of previous studies have shown that there were sex differences in Ang II-induced hypertension, in which Ang II-induced hypertension was completely attenuated in female mice [13,16-18]. The pressor doses of Ang II to induced Ang II-dependent hypertension ranged from 0.71 mg/kg/day [27], 1.15 mg/kg/day [16,18], to 1.44mg/kg/day [17], respectively. All these doses of Ang II are considered to be pressor doses. Conversely, the routes of Ang II infusion ranged from subcutaneously to osmotic minipump infusion intraperitoneally [13,15-18]. Furthermore, animal models used to study the sex differences in Ang II-induced hypertension included Sprague–Dawley rats [15], C57BL/6J [13,16,18], global AT2 receptor-knockout [13], vascular AT1a receptor-knockout [17], or Rag−/− mice [37], respectively. Thus, it is very difficult to directly compare the present study with above-referenced studies and draw a clear conclusion. Given the same animal model, the same basal blood pressure level, and similar pressor doses of Ang II, it is unlikely that nearly 50 to ~80% of the pressor responses to Ang II would be attenuated in female animals as reported previously [13,18,37].
Sex differences in basal diuretic and natriuretic responses to Ang II in Ang II-dependent hypertension have also recently attracted attention. In the present study, we reasoned that female WT and PT-Agtr1a−/− mice may behave differently in their diuretic and natriuretic responses to Ang II-induced hypertension. However, the results do not support our hypothesis. Although basal 24-h urine output and urinary excretion of Na+, K+, and Cl− were lower in female wildtype and PT-Agtr1a−/− mice than their male counterparts, it is likely due to their lower body wt. and kidney wt. However, both male and female PT-Agtr1a−/− mice excreted more urine, Na+, K+, and Cl− than their WT controls. These differences may be interpreted to be primarily due to the deletion of AT1 (AT1a) receptors selectively in the proximal tubules of the kidney. Indeed, Ang II exerts a stimulatory effect on proximal tubule Na+ reabsorption via activation of AT1 (AT1a) receptors and the Na+/H+ exchanger 3 (NHE3) in the proximal tubules [4,6,11,27,28,38-40]. Our results are consistent with the study of Veiras et al. [15], which showed no significant differences in the hypertensive response, proximal tubular and distal transporter, diuretic or natriuretic responses to Ang II-induced hypertension in male and female Sprague–Dawley rats and C57BL/6J mice. Likewise, Li et al. showed that there were no gender differences in the expression of NHE3 in male and female WT and global AT1a receptor-knockout mice [23]. However, our results on female wildtype and PT-Agtr1a−/− mice are different from those of Tiwari et al. who reported that young female C57BL/6J mice had 0% change in the activity of renal NCC or γ-ENaC activity in response to Ang II infusion [24]. More studies may be necessary to further uncover whether sex differences play significant roles in basal and Ang II-induced pressure–natriuresis responses and the expression of major sodium transporters in male and female animals.
Finally, Ang II is well recognized to induce glomerular and tubulointerstitial injury responses in animals and humans [5,12,34,41-43]. The present study determined whether the deletion of AT1 (AT1a) receptors selectively in the proximal tubules of the kidney may attenuate Ang II-induced glomerular and tubulointerstitial injury in part by attenuating the hypertensive response and improving glomerular filtration during Ang II-induced hypertension. Previous studies have shown that the expression of angiotensinogen and AT1 (AT1a) receptors in the proximal tubules is augmented rather than downregulated in Ang II-induced hypertension [6,8,9,34,39,44]. We reasoned that circulating and intratubular Ang II in the proximal tubules via activation of AT1 (AT1a) receptors may play an important role in the glomerular and tubulointerstitial injury during Ang II-induced hypertension. Thus, deletion of AT1 (AT1a) receptors selectively in the proximal tubules is expected to attenuate Ang II-induced glomerular and tubulointerstitial injury in PT-Agtr1a−/− mice. In the present study, Ang II induced significant glomerular and tubulointerstital injury responses in adult male WT mice, as expected, but Ang II-induced glomerular and tubulointerstitial fibrotic responses were almost completely blocked in PT-Agtr1a−/− mice. These results suggest that AT1 (AT1a) receptors in the proximal tubule play a key role in mediating circulating and intratubular Ang II-induced glomerular and tubulointerstitial injury responses. How Ang II-induced glomerular and tubulointerstitial injury responses are attenuated in PT-Agtr1a−/− mice remained unknown. One potential mechanism may be due to the deletion of AT1 (AT1a) receptors in the proximal tubules that block AT1 (AT1a) receptor-mediated proximal tubule sodium reabsorption[8,10,34,38,40,45]. This response is expected to significantly increase the salt and fluid delivery from the end of the proximal tubules. The significant increases in GFR in male and female PT-Agtr1a−/− mice may be due to the impairment of the tubuloglomerular feedback response, compared with WT mice. Another potential mechanism may include the possibility that deletion of AT1 (AT1a) receptors selectively in the proximal tubules would block AT1 (AT1a) receptor-mediated proinflammatory and fibrotic responses in the renal cortical tubulointerstitium. This interpretation is consistent with previous studies showing that Ang II induced glomerular and tubulointerstitial injury in rats or mice [5,12,41-43]. However, the present study has not studied whether Ang II-induced glomerular and tubulointerstitial injury in female PT-Agtr1a−/− mice. Further studies may be necessary to further determine precise molecular, cellular, and signaling mechanisms underlying renal protective effects of proximal tubule-specific deletion or targeting of AT1 (AT1a) receptors in hypertension.
In summary, we have demonstrated that deletion of AT1 (AT1a) receptor selectively in the proximal tubules of the kidney significantly attenuated Ang II-induced hypertension in both male and female PT-Agtr1a−/− mice, and Ang II-induced glomerular and tubulointerstitial injury. Our results suggest that these protective effects of proximal tubule-specific deletion of AT1 (AT1a) receptors are likely due to the inhibition of proximal tubule sodium reabsorption leading to a greater pressure-natriuresis response and the inhibition of AT1 (AT1a) receptor-mediated glomerular and tubulointerstitial fibrotic responses in the kidney. Since AT2 or AT1b receptor expression in the proximal tubules was very low in WT littermate and not different in PT-Agtr1a−/− mice, the results of the present study were unlikely due to the up-regulation of either AT2 or AT1b receptor expression in the proximal tubules in male and female WT and PT-Agtr1a−/− mice. Thus, the present study does not support the notion that significant sex differences play a key role in Ang II-induced hypertension and kidney injury. However, further studies are necessary to comprehensively determine the molecular, cellular, and signaling mechanisms underlying the protective effects of proximal tubule-specific deletion of AT1 (AT1a) receptors in the kidney from Ang II-induced hypertension and kidney injury.
Data Availability
The authors will make all methods and materials including the protocols for genotyping mutant mice with proximal tubule-specific deletion of AT1a receptors, surviving and non-surviving surgical protocols, experimental protocols, and all supporting raw data available to other researchers.
Clinical perspectives.
In 2014, National Institute of Health (NIH) mandated the Consideration of Sex as a Biological Variable in most, if not all, NIH-funded research.
The present study demonstrated that Ang II induces hypertension similarly in male and female WT mice, and that genetic deletion of AT1 (AT1a) receptor selectively in the proximal tubules attenuated Ang II-induced hypertension similarly in male and female PT-Agtr1a−/− mice.
The results of the present study suggest that sex differences unlikely play a key role in Ang II-induced hypertension.
Acknowledgements
We sincerely thank Drs. Isabelle Rubera and Michel Tauc of Université Côte d’Azur, France, for providing breeding pairs of iL1-sglt2-Cre mice to generate mutant mice with proximal tubule-specific deletion of AT1 (AT1a) receptors in the present study. We also thank Mr. Glenn Hoskins of the University of Mississippi Medical Center for excellent technical work in EM imaging. Finally, we sincerely thank Dr. Ryosuke Sato and Akemi Sato form Tulane Hypertension and Renal Center of Excellence Molecular Core Laboratory for measuring AT2 or AT1b receptor expression in the proximal tubules of male and female WT and PT-Agtr1a−/− mice. The portions of the results were presented previously as conference abstracts in J. Hypertens. 36: e84, 2018; J. Hypertens. 36: e92, 2018; JASN 2018 KIDNEY WEEK: TH-PO118; FASEB J. 33(1_supplement): 867.9, 2019; Kidney Int. Rep., 2019; 4(7): Supplement, S51; Hypertension. 2019; 74:AP2016; FASEB J., 33(S1), 2020; and Hypertension. 2020; 75: PO41, respectively.
Funding
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases [grant numbers 2R01DK067299-06A2, 2R01DK067299-10A1, 1R01DK123144-01, 1R01DK102429-01, 2R01DK102429-03A1 (to Jia L. Zhuo)]; and the Pre-Doctoral Research Support from CAPES, Brazil (to Ana Paula Oliveira Leite).
Abbreviations
- ACE
angiotensin-converting enzyme
- Ang I
angiotensin I
- Ang II
angiotensin II
- AT1 (AT1a)
type 1 (1a) receptor for Ang II
- AT2
type 2 receptor for Ang II
- Ca
glomerular capillary
- ddPCR
Droplet Digital PCR
- EM
electron microscopic
- ENaC
epithelial sodium channel
- GBM
glomerular basement membrane
- GFR
glomerular filtration rate
- HE
Hematoxylin
- i.p.
intraperitoneally
- MC
glomerular mesangial cell
- Mito
mitochondria
- MV
microvillar
- n.s.
not significantly
- NCC
thiazide-sensitive NaCl cotransporter
- NHE3
Na+/H+ exchanger 3
- p.o.
orally by drinking water
- PO
podocyte
- PT-Agtr1a−/−
mutant mice with genetic deletion of AT1 (AT1a) receptors selectively in the proximal tubules of the kidney
- RAS
renin–angiotensin system
- WT
wildtype
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr et al. (2003) National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289, 2560–2572, 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Himmelfarb C et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, e13–e115 [DOI] [PubMed] [Google Scholar]
- 3.Carey RM (2015) The intrarenal renin-angiotensin system in hypertension. Adv. Chronic Kidney Dis 22, 204–210, 10.1053/j.ackd.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 4.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ et al. (2005) Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J. Clin. Invest 115, 1092–1099, 10.1172/JCI23378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J et al. (1992) Renal injury from angiotensin II-mediated hypertension. Hypertension 19, 464–474, 10.1161/01.HYP.19.5.464 [DOI] [PubMed] [Google Scholar]
- 6.Kobori H, Nangaku M, Navar LG and Nishiyama A (2007) The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev 59, 251–287, 10.1124/pr.59.3.3 [DOI] [PubMed] [Google Scholar]
- 7.Navar LG (2004) The intrarenal renin-angiotensin system in hypertension. Kidney Int. 65, 1522–1532, 10.1111/j.1523-1755.2004.00539.x [DOI] [PubMed] [Google Scholar]
- 8.Zhuo JL, Ohishi M and Mendelsohn FA. (1999) Roles of AT1 and AT2 receptors in the hypertensive Ren-2 gene transgenic rat kidney. Hypertension 33, 347–353, 10.1161/01.HYP.33.1.347 [DOI] [PubMed] [Google Scholar]
- 9.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E and Navar LG (2002) Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension 39, 116–121, 10.1161/hy0102.100780 [DOI] [PubMed] [Google Scholar]
- 10.Li XC, Zhu D, Zheng X, Zhang J and Zhuo JL (2018) Intratubular and intracellular renin-angiotensin system in the kidney: a unifying perspective in blood pressure control. Clin. Sci. (Lond.) 132, 1383–1401, 10.1042/CS20180121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhuo JL and Li XC (2013) Proximal nephron. Compr. Physiol 3, 1079–1123, 10.1002/cphy.c110061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK et al. (2009) Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J. Clin. Invest 119, 943–953, 10.1172/JCI34862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RD, Hilliard LM, Head GA, Jones ES, Widdop RE and Denton KM (2012) Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension 59, 129–135, 10.1161/HYPERTENSIONAHA.111.178715 [DOI] [PubMed] [Google Scholar]
- 14.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA et al. (2014) Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64, 573–582, 10.1161/HYPERTENSIONAHA.114.03663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veiras LC, McFarlin BE, Ralph DL, Buncha V, Prescott J, Shirvani BS et al. (2020) Electrolyte and transporter responses to angiotensin II induced hypertension in female and male rats and mice. Acta Physiol. (Oxf.) 229, e13448, 10.1111/apha.13448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venegas-Pont M, Sartori-Valinotti JC, Glover PH, Reckelhoff JF and Ryan MJ (2010) Sexual dimorphism in the blood pressure response to angiotensin II in mice after angiotensin-converting enzyme blockade. Am. J. Hypertens 23, 92–96, 10.1038/ajh.2009.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf E, Diaz EJ, Hollis AN, Hoang TA, Azad HA, Bendt KM et al. (2018) Vascular type 1 angiotensin receptors control blood pressure by augmenting peripheral vascular resistance in female mice. Am. J. Physiol. Renal. Physiol 315, F997–F1005, 10.1152/ajprenal.00639.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue B, Pamidimukkala J and Hay M (2005) Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am. J. Physiol. Heart Circ. Physiol 288, H2177–H2184, 10.1152/ajpheart.00969.2004 [DOI] [PubMed] [Google Scholar]
- 19.Clayton JA and Collins FS (2014) Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283, 10.1038/509282a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galea LAM, Choleris E, Albert AYK, McCarthy MM and Sohrabji F (2020) The promises and pitfalls of sex difference research. Front. Neuroendocrinol 56, 100817, 10.1016/j.yfrne.2019.100817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandberg K, Umans JG and Georgetown Consensus Conference Work Group (2015) Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. FASEB J. 29, 1646–1652, 10.1096/fj.14-269548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallabhajosyula S, Ponamgi SP, Shrivastava S, Sundaragiri PR and Miller VM (2020) Reporting of sex as a variable in cardiovascular studies using cultured cells: a systematic review. FASEB J. 34, 8778–8786, 10.1096/fj.202000122R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Hatano R, Xu S, Wan L, Yang L, Weinstein AM et al. (2017) Gender difference in kidney electrolyte transport. I. Role of AT1a receptor in thiazide-sensitive Na+-Cl− cotransporter activity and expression in male and female mice. Am. J. Physiol. Renal Physiol 313, F505–F513, 10.1152/ajprenal.00087.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiwari S, Li L, Riazi S, Halagappa VK and Ecelbarger CM (2009) Sex and age result in differential regulation of the renal thiazide-sensitive NaCl cotransporter and the epithelial sodium channel in angiotensin II-infused mice. Am. J. Nephrol 30, 554–562, 10.1159/000252776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clotet-Freixas S, Soler MJ, Palau V, Anguiano L, Gimeno J, Konvalinka A et al. (2018) Sex dimorphism in ANG II-mediated crosstalk between ACE2 and ACE in diabetic nephropathy. Lab. Invest 98, 1237–1249, 10.1038/s41374-018-0084-x [DOI] [PubMed] [Google Scholar]
- 26.Alsiraj Y, Thatcher SE, Charnigo R, Chen K, Blalock E, Daugherty A et al. (2017) Female mice with an XY sex chromosome complement develop severe angiotensin II-induced abdominal aortic aneurysms. Circulation 135, 379–391, 10.1161/CIRCULATIONAHA.116.023789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XC, Soleimani M, Zhu D, Rubera I, Tauc M, Zheng X et al. (2018) Proximal tubule-specific deletion of the NHE3 (Na+/H+ exchanger 3) promotes the pressure-natriuresis response and lowers blood pressure in mice. Hypertension 72, 1328–1336, 10.1161/HYPERTENSIONAHA.118.10884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XC, Zhu D, Chen X, Zheng X, Zhao C, Zhang J et al. (2019) Proximal tubule-specific deletion of the NHE3 (Na+/H+ exchanger 3) in the kidney attenuates Ang II (angiotensin II)-induced hypertension in mice. Hypertension 74, 526–535, 10.1161/HYPERTENSIONAHA.119.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubera I, Poujeol C, Bertin G, Hasseine L, Counillon L, Poujeol P et al. (2004) Specific Cre/Lox recombination in the mouse proximal tubule. J. Am. Soc. Nephrol 15, 2050–2056, 10.1097/01.ASN.0000133023.89251.01 [DOI] [PubMed] [Google Scholar]
- 30.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP III, Howatt DA, Subramanian V et al. (2011) Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor−/− mice. Circ. Res 108, 574–581, 10.1161/CIRCRESAHA.110.222844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li XC, Zhou X and Zhuo JL (2020) Evidence for a physiological mitochondrial angiotensin II system in the kidney proximal tubules: novel roles of mitochondrial Ang II/AT1a/O2− and Ang II/AT2/NO signaling. Hypertension 76, 121–132, 10.1161/HYPERTENSIONAHA.119.13942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XC, Shull GE, Miguel-Qin E and Zhuo JL (2015) Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension. Physiol. Genomics 47, 479–487, 10.1152/physiolgenomics.00056.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiber A, Shulhevich Y, Geraci S, Hesser J, Stsepankou D, Neudecker S et al. (2012) Transcutaneous measurement of renal function in conscious mice. Am. J. Physiol. Renal Physiol 303, F783–F788, 10.1152/ajprenal.00279.2012 [DOI] [PubMed] [Google Scholar]
- 34.Li XC and Zhuo JL (2011) Phosphoproteomic analysis of AT1 receptor-mediated signaling responses in proximal tubules of angiotensin II-induced hypertensive rats. Kidney Int. 80, 620–632, 10.1038/ki.2011.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satou R, Cypress MW, Woods TC, Katsurada A, Dugas CM, Fonseca VA et al. (2020) Blockade of sodium-glucose cotransporter 2 suppresses high glucose-induced angiotensinogen augmentation in renal proximal tubular cells. Am. J. Physiol. Renal Physiol 318, F67–F75, 10.1152/ajprenal.00402.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods TC, Satou R, Miyata K, Katsurada A, Dugas CM, Klingenberg NC et al. (2019) Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am. J. Nephrol 49, 331–342, 10.1159/000499597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL et al. (2014) Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64, 384–390, 10.1161/HYPERTENSIONAHA.114.03581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris PJ and Navar LG (1985) Tubular transport responses to angiotensin. Am. J. Physiol 248, F621–F630, 10.1152/ajprenal.1985.248.5.F621 [DOI] [PubMed] [Google Scholar]
- 39.Harrison-Bernard LM, Zhuo JL, Kobori H, Ohishi M and Navar LG (2002) Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. Am. J. Physiol. Renal Physiol 282, F19–F25, 10.1152/ajprenal.0335.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuo JL, Thomas D, Harris PJ and Skinner SL (1992) The role of endogenous angiotensin II in the regulation of renal haemodynamics and proximal fluid reabsorption in the rat. J. Physiol 453, 1–13, 10.1113/jphysiol.1992.sp019214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kagami S, Border WA, Miller DE and Noble NA (1994) Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J. Clin. Invest 93, 2431–2437, 10.1172/JCI117251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Huang XR and Lan HY (2012) Smad3 mediates ANG II-induced hypertensive kidney disease in mice. Am. J. Physiol. Renal Physiol 302, F986–F997, 10.1152/ajprenal.00595.2011 [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Ortega M and Egido J (1997) Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int. 52, 1497–1510, 10.1038/ki.1997.480 [DOI] [PubMed] [Google Scholar]
- 44.Kobori H, Harrison-Bernard LM and Navar LG (2001) Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension 37, 1329–1335, 10.1161/01.HYP.37.5.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cogan MG (1990) Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension 15, 451–458, 10.1161/01.HYP.15.5.451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors will make all methods and materials including the protocols for genotyping mutant mice with proximal tubule-specific deletion of AT1a receptors, surviving and non-surviving surgical protocols, experimental protocols, and all supporting raw data available to other researchers.