Abstract
Post-translational modifications alter the biophysical properties of proteins and thereby influence cellular physiology. One emerging manner by which such modifications regulate protein function is through their ability to perturb protein stability. Despite rising interest in this phenomenon, there are few methods that enable global interrogation of the biophysical effects of post-translational modifications on the proteome. Here, we describe an unbiased proteome-wide approach to explore the influence of protein modifications on the thermodynamic stability of thousands of proteins in parallel. We apply this profiling strategy to study the effects of O-linked N-acetylglucosamine (O-GlcNAc), an abundant modification found on hundreds of proteins in mammals that has been shown in select cases to stabilize proteins. Using this thermal proteomic profiling strategy, we identify a set of 72 proteins displaying O-GlcNAc-dependant thermostability and validate this approach using orthogonal methods targeting specific proteins. These collective observations reveal that the majority of proteins influenced by O-GlcNAc are, surprisingly, destabilized by O-GlcNAc and cluster into distinct macromolecular complexes. These results establish O-GlcNAc as a bi-directional regulator of protein stability and provide a blueprint for exploring the impact of any protein modification on the meltome of, in principle, any organism.
Keywords: Post-translational modification, mass spectrometry, proteomics, O-GlcNAc, thermostability, protein stability, proteostasis, aggregation, thermal proteome profiling, meltome, heat shock response, heat shock factor I
Graphical Abstract
Introduction:
There are more than 300 known post-translational modifications (PTMs) found on proteins1 and they have diverse roles in regulating the structure and biochemical functions of proteins. Among these PTMs, some are present on relatively few proteins whereas others are widespread. The widespread distribution of a specific PTM is generally perceived as suggesting it serves important roles in cell function. One emerging role for such widely distributed PTMs is to alter the thermodynamic stability of proteins by modulating various intra- and intermolecular interactions2. Indeed, some PTMs have been shown to alter the conformational free energy landscape of proteins impacting, for example, the stability of their native states3, intermediates along folding pathways4, their unfolded states5, or higher order quaternary complexes6. From the perspective of cellular function, tuning the thermodynamic stability of proteins can serve as a means to control the levels of their properly folded forms, which in turn may impact the delicate balance between their synthesis and degradation – a process of central importance for cellular health known as protein homeostasis or ‘proteostasis’7–8.
Despite their emerging importance as regulators of proteostasis, however, global methods to assess the role of PTMs in regulating the thermal stability of proteins are lacking. In recent years, rapid advances in high-throughput mass spectrometry (MS)-based proteomics have enabled interrogation of the thermal unfolding of thousands of proteins in parallel using thermal proteome profiling (TPP)9–12. This strategy was originally developed to identify protein targets of small molecule ligands within cells10–11, where even a small number of interactions can often significantly thermostabilize proteins13. Recently, TPP has revealed that the thermostability of individual proteins varies across the phases of the cell cycle11, and for certain proteins this variation is synchronized with changes in phosphorylation levels during the mitotic transition. These observations suggest that PTMs, similar to small molecule ligands, may be physiological regulators of protein thermodynamic stability within the cell through their ability to form stabilizing interactions11.
Recently, a method called HotSpot thermal profiling was developed that enables studying the proteome wide impact of phosphorylation on protein thermostability14. Following on this pioneering study, subsequent phosphosite specific methods were developed that build upon this proof-of-concept work to improve technical aspects14–17. This method enables simultaneous mapping and determination of thermal melt profiles for thousands of phosphopeptides in parallel. Though powerful, this method would be difficult to implement on labile PTMs, such as O-GlcNAc, that are refractory to MS/MS mapping. Furthermore, as the method relies on mapping individual phosphopeptides, it does not report on the thermostability of the complete ensemble of proteoforms of a given protein. This is relevant because PTMs are typically present in substoichiometric levels, often on a small overall fraction of protein sites18. Therefore, inspired by the high interest in this area and the needs of the field, we set out to develop a method for O-GlcNAc that reports on the global ensemble of proteoforms of a given protein. Notably, such a method could in principle be applied to any PTM and serve a complementary use to HotSpot profiling.
Toward this goal, we envisioned a strategy that could leverage TPP to assess the effect of one type of PTM on the thermostability of proteins by applying it to samples in which global levels of a specific PTM were altered, an approach we call posttranslational modification-thermal proteome profiling (PTM-TPP). Ideally, such a method could enable determining the influence of one specific type of PTM on the stability of the population averaged ensemble of peptides from a given protein. Such a method would avoid the analytically demanding requirement of mapping specific PTMs, which would in turn enable greater depth of coverage of the overall proteome.
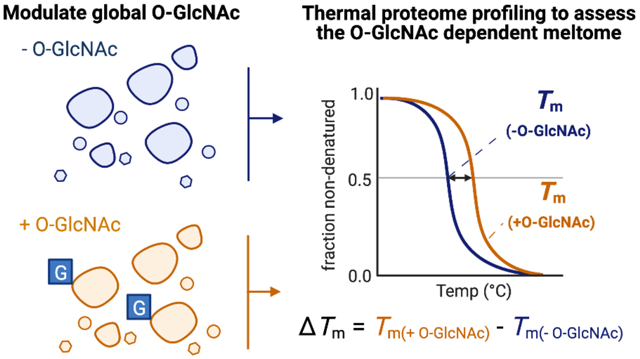
To develop such a strategy, we decided to explore as a model PTM the modification of select hydroxyl groups of serine and threonine residues of proteins with O-linked N-acetylglucosamine (O-GlcNAc)19. We selected this PTM because it is of high interest owing to its emerging roles in a range of physiological processes and because manipulation of O-GlcNAc has emerged as a therapeutic strategy with compounds having entered into the clinic20. In addition, O-GlcNAc is found within multicellular eukaryotes on over a thousand nuclear, cytoplasmic, and mitochondrial proteins. We were particularly intrigued by O-GlcNAc because it is known to be coordinated with the heat shock response21 and confers resistance to heat during development in ectotherms22. Additionally, genetic studies in Caenorhabditis elegans have illuminated O-GlcNAc-dependant stability differences for several cellular proteins22–24. Furthermore, several proteins are known to be stabilized against aggregation by O-GlcNAc, including polyhomeotic (PH-P), TGF-β-activated kinase 1 binding protein 1 (TAB-1), nucleotide-binding oligomerization domain receptors 1 and 2 (NOD1 and NOD2), tau (MAPT), and α-synuclein (SNCA)25–30. Recently, O-GlcNAc has been shown to reduce phase-separation and enhance condensate dynamics for the RNA-binding protein EWS (EWSR1)31, providing a striking example of how O-GlcNAc can regulate intermolecular interactions of its target proteins. We also noted that other forms of glycosylation, such as N-linked glycosylation, have been shown to alter the thermodynamic stability of specific proteins32–33. Given these lines of reasoning, we hypothesized that O-GlcNAc could be a general regulator of the thermodynamic stability of a subset of proteins (Figure 1B) and we accordingly set out to harness TPP to explore this possibility. Furthermore, O-GlcNAc is notoriously challenging to map by MS/MS34, and thus, provides an ideal model for demonstrating the utility of our method that does not rely on site-mapping.
Figure 1. Exploring the O-GlcNAc-dependant meltome.
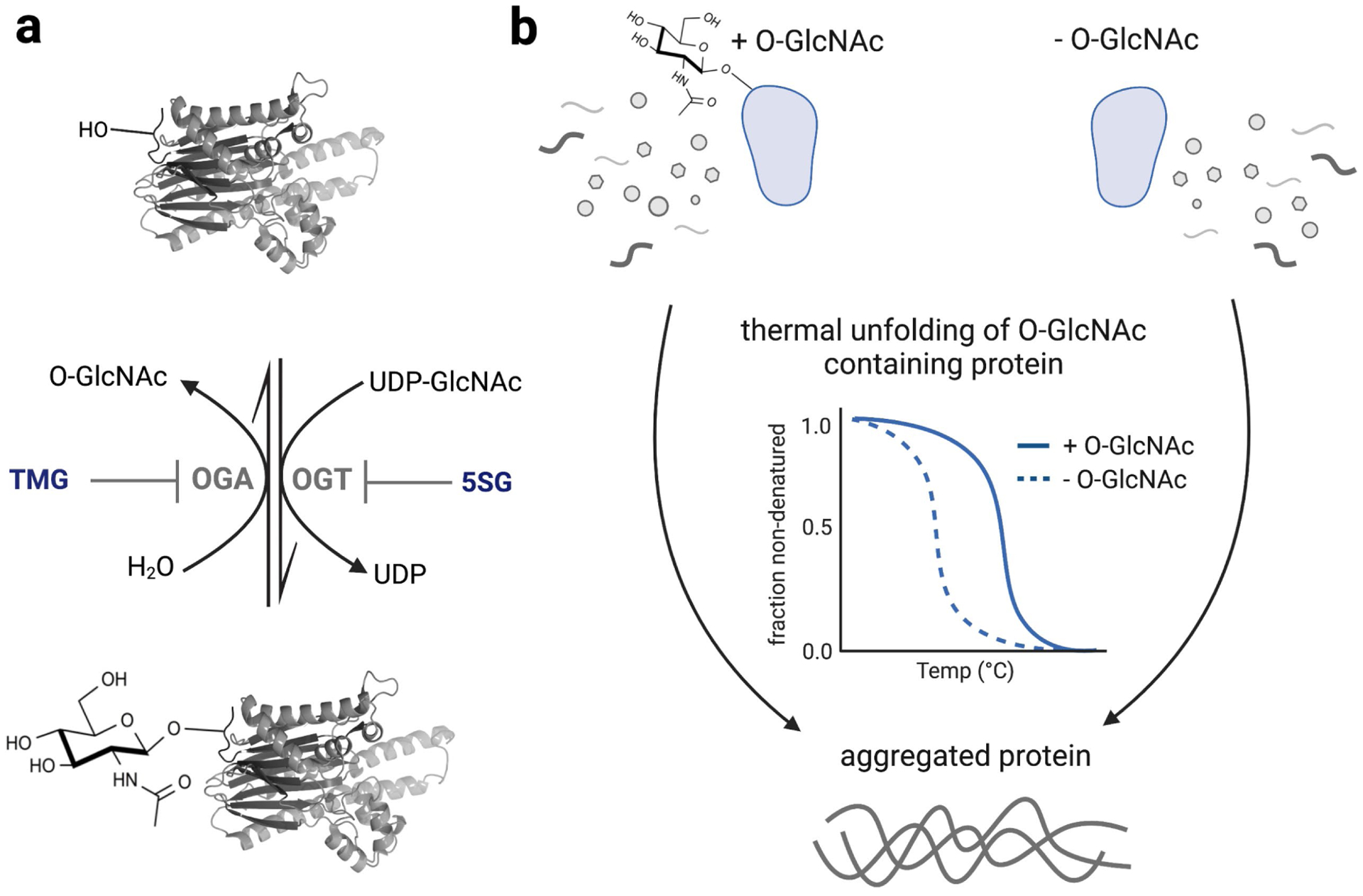
a) Installation of O-GlcNAc on proteins by OGT and its removal by OGA can each be antagonized within cells using the small molecule inhibitors 5SG and TMG, respectively. b) Hypothesis that O-GlcNAc stabilizes proteins against thermal unfolding. Abbreviations: 5SG; 2-acetamido-2-deoxy-5-thio-α-D-glucopyranose, TMG; Thiamet-G, OGA; O-GlcNAc hydrolase, OGT; O-GlcNAc transferase.
Levels of protein O-GlcNAc are regulated by just two enzymes that act antagonistically. O-GlcNAc transferase (OGT, CAZy35 glycosyltransferase family GT41) installs O-GlcNAc on target proteins and O-GlcNAc hydrolase (OGA, CAZy glycoside hydrolase family GH84) removes this modification. Regulation by just these two enzymes should enable facile control over its global levels on proteins (Figure 1A). We reasoned that maximizing the difference in global levels of O-GlcNAc between samples would facilitate successful application of TPP to study this modification. To vary levels of O-GlcNAc in vitro, we expected we could simply add an exogenous O-GlcNAc hydrolase to remove the modification. Alternatively, to alter levels of O-GlcNAc within cells one can use small molecule inhibitors to vary endogenous enzyme activity or expression levels36. Because altering O-GlcNAc levels within cells induces a heat shock response37, and heat shock conversely increases O-GlcNAc levels38, we expected that an in vitro approach using cell lysates would be both more simple and more direct.
Results:
Given these various considerations, we first set out to assess the effects of O-GlcNAc on protein stability by treating cell lysates with a recombinant OGA homolog to selectively remove O-GlcNAc. In this strategy, which we term enzymatic removal (erPTM-TPP, Figure 2A), we exploited the Bacteroides thetaiotaomicron BtGH8439 glycoside hydrolase that is known to efficiently remove O-GlcNAc and digested cellular lysate obtained from human embryonic kidney cells HEK293T (Figure S1A–D). Following hydrolysis, BtGH84 was removed using magnetic beads (Figure 2B) to generate the O-GlcNAc-deficient (−O-GlcNAc) sample. As a negative control, we used the same concentration of a catalytically dead variant of BtGH8440 in which a key catalytic residue is mutated (D242A-BtGH84) to generate the O-GlcNAc-enriched (+O-GlcNAc) sample (Figure 2B and Figure S1E–F). We confirmed that O-GlcNAc levels in both the +O-GlcNAc and −O-GlcNAc samples were altered as expected by immunoblot using a pan-specific anti-O-GlcNAc antibody (Figure 2B). Subsequently, we also confirmed that the WT and D242A BtGH84 enzymes were successfully removed from these samples (Figure 2B). The resulting pair of quadruplicate samples were then subjected to the subsequent steps of the TPP workflow (Figure 2A). One potential complicating factor of our method was the possibility that D242A BtGH84 may remove O-GlcNAcylated proteins from our lysate samples. This seemed possible since a different bacterial OGA homolog, rendered inactive by site directed mutagenesis of a key active site residue, has been used to enrich O-GlcNAc modified proteins41. To rule out this possibility, we showed that overall protein abundances remain consistent between both enzymatic treatments (Figure S1G). Furthermore, we observed no significant difference in relative abundance of known O-GlcNAcyated proteins when comparing WT and D242A BtGH84 treatments in our erPTM-TPP dataset (Figure S2). The data from this erPTM-TPP experiment allowed us to investigate the O-GlcNAc-dependant thermostability of a proteome comprising 3213 proteins. A histogram of the resulting Tm values revealed an almost identical mean global Tm for the O-GlcNAc modified and unmodified conditions (Figure 2C and Figure S1H), suggesting O-GlcNAc has minimal overall impact on the global stability of the proteome.
Figure 2. erPTM-TPP enables global identification of proteins having O-GlcNAc-dependant thermostability.
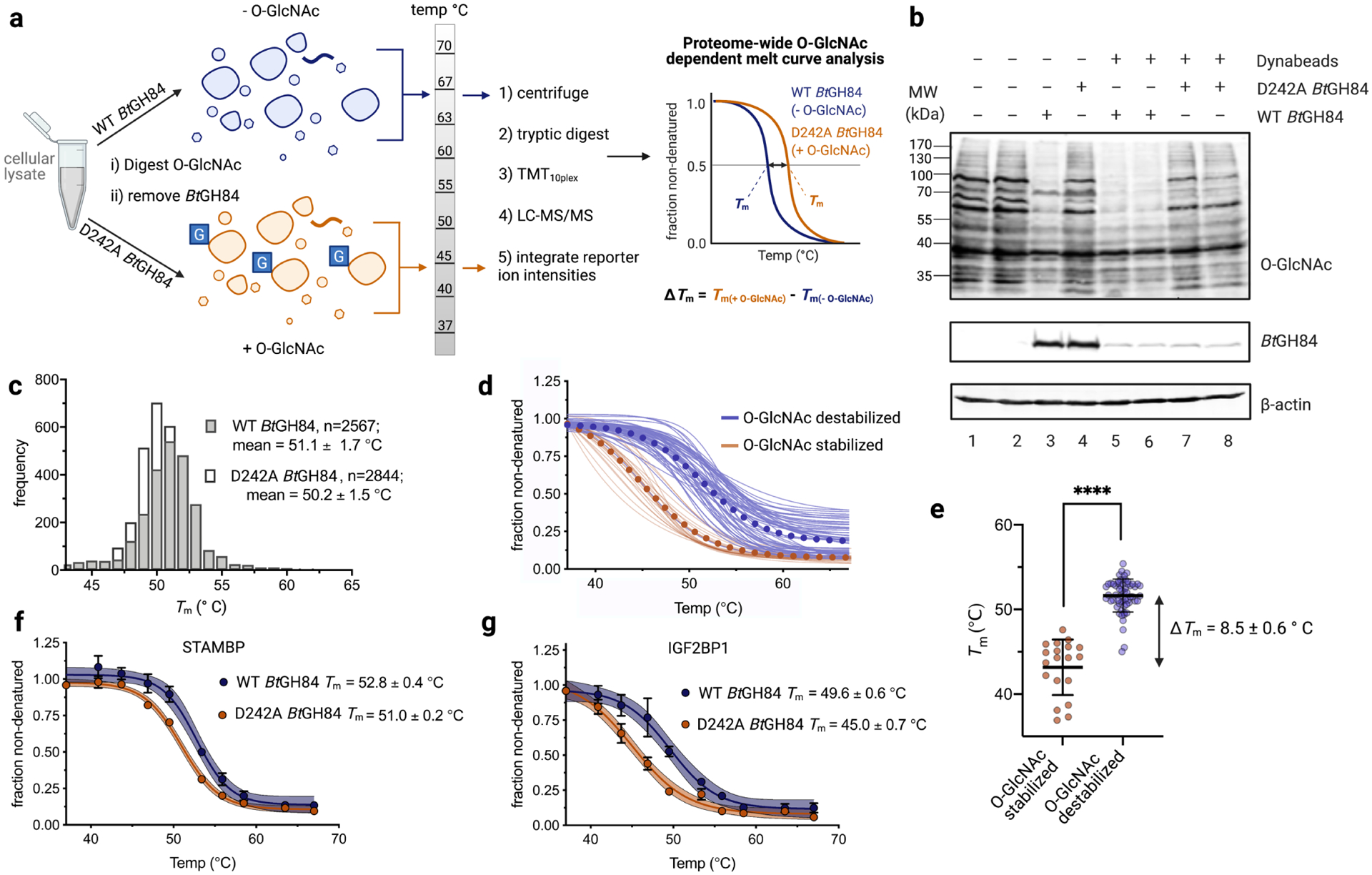
a, Schematic outlining the erPTM-TPP method. b, Immunoblot following treatment of cellular lysates with WT or D242A BtGH84. c, Histogram of Tm values for all proteins that could be curve-fit in the WT and D242A BtGH84 treated samples. d, Overlay of melt curves for O-GlcNAc stabilized and O-GlcNAc destabilized proteins. Curves for both WT and D242A BtGH84 conditions are shown. e, Scatter plot comparing Tm values for O-GlcNAc stabilized and destabilized proteins from d, with P values: two tailed student’s t-test assuming unequal variance; ****P < 0.0001. f-g, Melt curves for select proteins comparing WT and D242A BtGH84 conditions. Error bars correspond to SE from four experimental replicates. See also Figures S1–S4, and Tables S1–S2.
To identify candidate O-GlcNAc modulated proteins, we used the following set of four stringent criteria. First, to be included for analysis of potential O-GlcNAc dependent stability differences, each protein needed to be identified in at least two experimental replicates from each experimental condition of + and − O-GlcNAc (see Table S5). This criterion enabled assessing error for each identified thermal melt curve as done previously in general TPP experiments to avoid false positives42–43. A total of 2482 of the 3213 identified proteins met this first criterion. Second, for any given protein the change in its average Tm (|ΔTm(erPTM-TPP) = Tm(D242A BtGH84) − Tm(WT BtGH84)|) needed to be greater than 1.0 °C, a shift that is commonly used as a selection criterion in TPP11, 44. Third, the average ΔTm value needed to be greater than its standard error. These three criteria were applied to the panel by fitting melt curves to each experimental replicate separately as is typical in TPP10–11. This strategy enabled productively focusing our panel of candidates without inadvertently excluding potential hits and led, in the erPTM-TPP experiment, to selection of 1392 proteins that met these three initial common TPP criteria. A histogram of all |ΔTm| values for the complete panel of proteins analyzed revealed that numerous proteins displayed O-GlcNAc dependant thermostability differences (Figure S3).
However, we found that individual melt curves were often skewed by poor curve-fits or missing data for certain experimental replicates. Therefore, we found that in selecting our final panel of candidates it was valuable to introduce a fourth and final criterion that involved plotting the averaged data from all replicates for a given experimental condition and fitting this to a Boltzmann sigmoidal. We then leveraged these fitted data to test for our fourth criterion, which required that the 95% confidence intervals between the two experimental conditions did not overlap at the inflection point (Figures S4–S6). We judged this was an important stringent criterion that enabled detecting statistically robust stability effects. Notably, this rigorous analysis pipeline revealed 40 proteins in total that displayed clear O-GlcNAc dependant thermostability differences within the erPTM-TPP dataset (Figure S4–S6, Tables S1–S2).
Using this stringent data analysis pipeline, ten proteins from erPTM-TPP met our strict criteria for O- GlcNAc dependant thermostabilization (ΔTm(erPTM-TPP); 1.7 to 6.8 °C, Figure S4 and Table S1) and, remarkably, 30 displayed O-GlcNAc dependant destabilization (ΔTm(erPTM-TPP); −1.2 to −7.0 °C, Figure 2F–G, Figures S5–6, and Table S2). Among the 40 O-GlcNAc modulated proteins, only 17 have been identified as being O-GlcNAc modified (Tables S1 and S2). Notably, O-GlcNAc stabilized proteins had a significantly lower mean Tm as compared to O-GlcNAc destabilized proteins (ΔTm = Tm(destabilized) − Tm(stabilized) = 8.5 ± 0.6 °C, Figure 2D–E). The large majority of O-GlcNAc modulated proteins identified using erPTM-TPP displayed no significant difference in protein abundance between experimental conditions (Tables S1, S2, and S6), indicating that differences in protein abundances was not a significant factor impacting our data. These data showed that erPTM-TPP allows interrogation of a large fraction of the proteome and enabled identification of candidate proteins which may have their stability regulated by O-GlcNAc.
Having identified a panel of O-GlcNAc modulated proteins using erPTM-TPP, we next aimed to validate this set using a different approach to alter global PTM levels using chemical-modulators (cmPTM-TPP). We envisioned that this strategy could be applied in live cell culture and might also identify a unique subset of O-GlcNAc regulated proteins since the melting of proteins would happen within cells in the presence of a range of potential binding partners. To increase global O-GlcNAc levels, we turned to the potent and selective OGA inhibitor Thiamet-G (TMG)45. Modification levels were decreased using an established metabolic inhibitor of OGT, 2-acetamido-2-deoxy-5-thio-α-D-glucopyranose (5SG)46 (Figure 1A, Figure 3A). Both inhibitors are frequently used to modulate O-GlcNAc levels within cells46–47. We recognized, however, that a complicating consequence of increasing O-GlcNAc levels in cellulo would be induction of the heat shock response (HSR) via activation of the master transcriptional regulator heat shock factor 1 (HSF1)37, 48. The resulting increase in chaperone expression could confoundingly enhance O-GlcNAc dependant thermostabilization of proteins and/or mask destabilization. To circumvent this issue, we turned to a chemically inducible cell line that stably expresses a potent dominant negative form of HSF1 (dn-cHSF1) that suppresses cytoplasmic heat shock response in a doxycycline-dependant fashion49–51. We confirmed by immunoblot that dn-cHSF1 was robustly induced in this cell line upon addition of doxycycline and that its induction supressed HSP70 and HSP40 expression even after exposure to various stressors (Figure S7A). Importantly, we also showed that when dn-cHSF1 is induced HSP levels were similar in all samples treated with vehicle, TMG, or 5SG (Figure S7B). Notably, both OGA and OGT interact with cellular protein partners52–54. Recently, Savitski and colleagues showed that proteins that are part of a complex tend to co-melt, and that small molecules can impact the overall stability of these protein complexes55. Therefore, to avoid potential false positives, we diluted residual OGA and OGT inhibitors following cell culture to avoid potential impact on the thermostability of OGT and OGA and their protein partners in cmPTM-TPP experiments (Figure S8). These data show this cell system and probes are suitable for interrogating the effects of altering O-GlcNAc on protein stability directly within cells.
Figure 3. cmPTM-TPP validates a panel of proteins as being thermo-destabilized by O-GlcNAc.
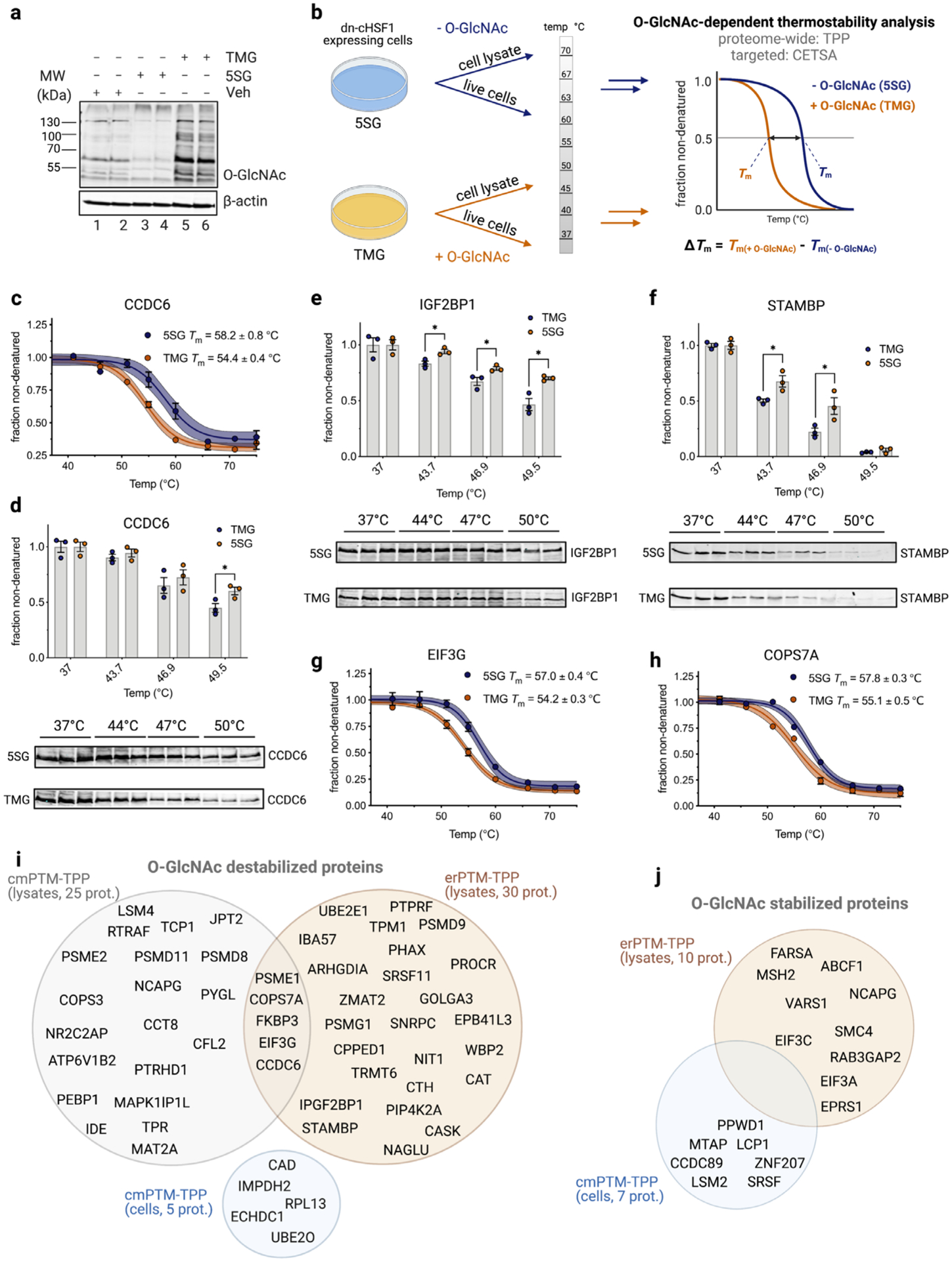
a, Immunoblot analysis of O-GlcNAc modified proteins following treatment of dn-cHSF1-HEK293T-REx cells with TMG (70 nM), 5SG (250 μM) or vehicle control. b, Schematic diagram outlining the cmPTM-TPP approach. c, g-h, cmPTM-TPP melt curve profiles for select proteins in lysates. Error bars are SE from two experimental replicates. d, e-f, Immunoblots and densitometry analysis for select proteins following cmPTM-CETSA on live cells. Error bars are SE from three experimental replicates, with P values: two tailed student’s t-test assuming unequal variance; *P < 0.05. i-j, Venn diagrams for proteins displaying O-GlcNAc-dependant destabilization and stabilization identified using different PTM-TPP methods.
We next applied our TMG and 5SG treated cells to the cmPTM-TPP workflow (Figure 3B), enabling us to assess O-GlcNAc dependant thermostability [ΔTm(cmPTM-TPP) = Tm(TMG) − Tm(5SG)] of 2473 proteins in samples where temperature treatments were applied to lysates derived from these cells as well as for 2393 proteins where the temperature treatments were instead applied directly to live cells. Global average melt curves for 5SG and TMG treatments were similar when comparing results for live cells and lysates (Figure S9), indicating there was no significant thermostability effect on the bulk proteome, which was consistent with our erPTM-TPP experiments using cell lysates. Detailed analysis of the melting curves using the same criteria as we defined for erPTM-TPP, however, revealed 25 proteins displaying O-GlcNAc-dependant destabilization within lysates and five within live cells (Tables S3 and S4, Figures S10–S12). All O-GlcNAc modulated candidates identified using the cmPTM-TPP experiment displayed no significant difference in protein abundance at the lowest temperature point between the TMG and 5SG conditions (Tables S3, S4, and S6), indicating that protein abundance was not a major factor influencing our data. Notably, five of these O-GlcNAc modulated proteins were destabilized in both erPTM-TPP and cmPTM-TPP experiments in lysates including coiled-coil domain containing protein 6 (CCDC6; ΔTm(erPTM-TPP) = −2.0 ± 0.4 °C, ΔTm(cmPTM-TPP) = −3.8 ± 0.9 °C), Cop9 signalosome 7A (COPS7A; ΔTm(erPTM-TPP) = −2.3 ± 0.5 °C, ΔTm(cmPTM-TPP) = −2.7 ± 0.6 °C), proteosome activator subunit 1 (PSME1; ΔTm(erPTM-TPP) = −1.4 ± 0.4 °C, ΔTm(cmPTM-TPP) = −5.5 ± 1.0 °C), elongation initiation factor 3G (EIF3G; ΔTm(erPTM-TPP) = −1.2 ± 0.5 °C, ΔTm(cmPTM-TPP) = −2.8 ± 0.5 °C), and peptidyl-prolyl cis trans isomerase 3 (FKBP3; ΔTm(erPTM-TPP) = −1.7 ± 0.3 °C, ΔTm(cmPTM-TPP) = −2.8 ± 0.9 °C) (Figure 3C, G–J, Figures S10, and Table S3). Using commercially available antibodies that we assessed as being of high quality, we performed targeted cellular thermal shift assay (CETSA) using cells treated with chemical modifiers (cmPTM-CETSA). In this way we validated three proteins as having their stability influenced within cells by O-GlcNAc including, STAM binding protein (STAMBP) and insulin-like growth factor 2 mRNA binding protein 1 (IGF2BP1), both of which were also destabilized by O-GlcNAc in erPTM-TPP (Figure 3E–F, I), as well as CCDC6 (Figure 3C–D, I), which was destabilized by O-GlcNAc in all experiments. Of these seven proteins, only EIF3G, PSME1, and CCDC6 are known to be directly O-GlcNAc modified38, 56. We leveraged the O-GlcNAc database to identify known O-GlcNAcylated proteins from within our complete list of candidates from all three PTM-TPP experiments. This analysis revealed a total of 34 known O-GlcNAcylated proteins from within our panel of 72 O-GlcNAc modulated proteins (Tables S1–S4).
Among our observations there were two aspects we found particularly surprising. One is that many proteins appear to be destabilized by O-GlcNAc and the second is that the majority of O-GlcNAc modulated proteins identified were not known to be O-GlcNAcylated. These observations suggested to us that some of the effects of O-GlcNAc on thermostability likely arise through perturbation of protein-protein interactions. We therefore assessed whether members from our panel of proteins interact with one another. We inputted all 72 O-GlcNAc modulated proteins into the STRING server to build a protein interaction network using a combination of experimental and computational sources to score interactions as described previously57. This analysis uncovered 34 high confidence interactors, several of which form complexes (Figure 4). To assess whether O-GlcNAc broadly affected protein complexes, we input the full list of proteins identified in our erPTM-TPP experiment using our first selection criterion into the STRING server to build a comprehensive protein interaction network. This analysis revealed a total of 984 different high-confidence protein complexes, associated with different biological gene ontology terms, that were identified as not being modulated by O-GlcNAc (Table S7). This analysis supports the specificity of the effect of O-GlcNAc modulation we observe on the set of seven protein complexes identified using this high-throughput proteomic analysis.
Figure 4. Proteins displaying O-GlcNAc dependant thermostability cluster into distinct protein complexes.
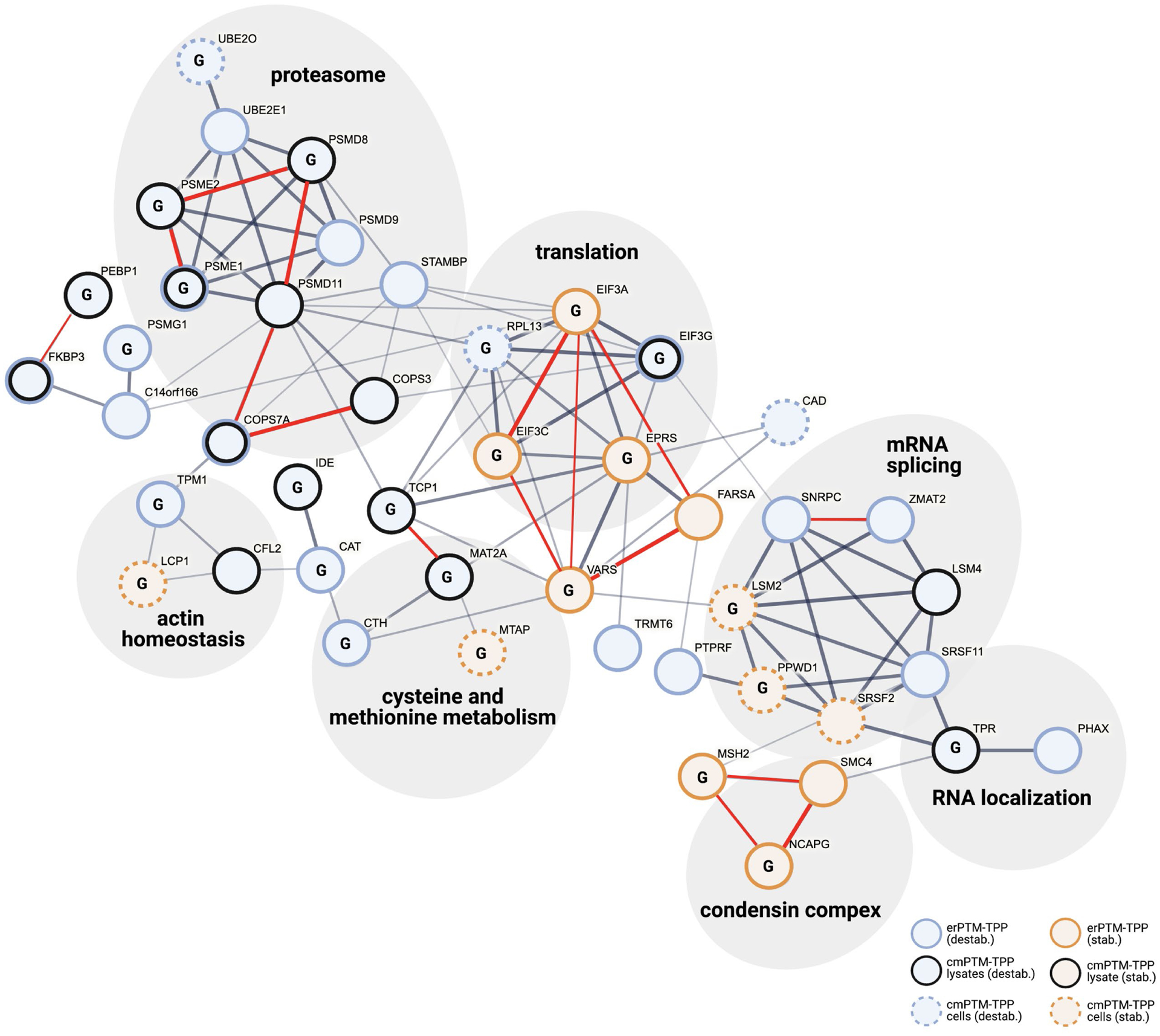
STRING protein interaction network of proteins displaying O-GlcNAc dependant thermostability. Nodes represent individual proteins coded by stability difference and PTM-TPP method(s). The network was constructed using the STRINGv11 server57. Line thickness connecting nodes reflects the interaction score as outlined in methods, and proteins are grouped into cellular components (gene ontology, false discovery rate < 0.001). Proteins displaying evidence for direct interaction in TPCA analysis (P < 0.1) have a red line connecting nodes (see methods). Known O-GlcNAcylated proteins are labelled (G).
Interestingly, members from several complexes were identified within different PTM-TPP experiments. Notably, several complexes are parts of macromolecular machines including the proteasome, translation initiation machinery, and the spliceosome – all of which have been reported to have at least one node that is O-GlcNAcylated and to also be functionally regulated by O-GlcNAc58–60 (Tables S1–S4, Figure 4). Strikingly, all proteasomal proteins displayed matched O-GlcNAc-dependant destabilization (Figure 4). In contrast, other complexes such as the translation initiation machinery and spliceosome had a mixture of stabilized and destabilized nodes. Closer inspection of these mixed-stability complexes revealed that subsets of connected nodes identified within the same experiment generally had matched stability shifts (Figure 4), a finding that is unlikely to occur simply by chance and supports their joint regulation by O-GlcNAc. Recently, a method called Thermal Proteome Co-Aggregation (TPCA) was developed to detect protein-protein interactions within TPP datasets55. This method relies on the premise that when two proteins interact, they tend to co-melt. Applying this TPCA method to our data revealed that several protein partners within our protein interaction network display clear evidence for co-aggregation (Figures 4 and S13, Tables S8 and S9), supporting our proposed network. Importantly, each identified protein interaction cluster contained at least one protein that is known to be O-GlcNAcylated61 (Figure 4). Taken together, these data suggest that perturbations in O-GlcNAc likely impact the stability of protein complexes.
Conclusion:
Despite the crucial role of PTMs in regulating protein structure and stability, high-throughput methods to interrogate their effect on the thermodynamic stability of the proteome are lacking. Here, we advance a simple thermal profiling approach, PTM-TPP, to interrogate the effect of O-GlcNAc on thermostability of the population averaged ensemble of proteoforms of thousands of proteins within the human proteome. We leverage two strategies, enzymatic removal and chemical modulation, to alter global O-GlcNAc levels and illustrate how these orthogonal approaches can complement and cross-validate one another. A recent report elegantly adapted TPP to study the thermostability effect of protein phosphorylation by a method called Hotspot Thermal Profiling14. In contrast to our method, HTP involves prior enrichment of phosphorylated peptides to permit simultaneous phosphosite mapping and thermal melt determination for proteoforms modified with phosphorylation in the same experiment. Accordingly, HTP focuses on PTM modified peptides which are generally substoichiometric14. By comparison, our PTM-TPP method is complementary to HTP in that it elucidates the effect of PTMs on the thermodynamic stability of the total endogenous protein pool rather than substoichiometric pools of modified proteoforms. In addition, PTM-TPP has high accuracy in its relative quantitation due to the ability to average reporter ion intensities across multiple identified peptides within a given protein. Importantly, because PTM-TPP examined effects on the global proteome, we believe it allows for detection of robust effects that are likely to mediate cellular processes. These features accordingly enable robust ranking of proteins for downstream study and detection of indirect effects such as those occurring through PTM-regulated protein-protein interactions. Moreover, though here we use direct chemical inhibitors of the PTM-processing enzymes, it is equally feasible to use alternative strategies. For example, transient genetic perturbations can be used to target such enzymes in cellulo, or alternatively, PTM levels can be varied by altering flux through biosynthetic pathways responsible for generating a key precursor62, or chemical mimetics of PTMs could be used63. In addition, PTM-TPP is simple in that it does not require purification of PTM modified peptides, and therefore is particularly useful for certain PTMs for which no enrichment strategies exist. As such, we envision that PTM-TPP can be conveniently pursued in a variety of contexts and will prove to be a useful complement to HTP. Thus, PTM-TPP will be valuable for exploring the effects of any PTM on the thermodynamic stability of proteomes.
Our application of PTM-TPP uncovered 72 proteins with O-GlcNAc regulated stability, seven of which were observed over more than one dataset. Our various PTM-TPP methods use different approaches to modulate global O-GlcNAc levels, and thus, are expected to differentially affect certain O-GlcNAc sites on different proteins. This could lead to variation in O-GlcNAc-dependant stability between these varied methods for certain proteins as was observed for NCAPG (Tables S1 and S3). Nevertheless, the panel of seven proteins that were congruent across experiments validate our approach and provide key leads for follow-up by the field. We note that the remaining proteins that were uniquely identified by a single independent method, though potentially important, should be further validated prior to conducting detailed follow up studies. Nevertheless, our complete panel of identified proteins demonstrates that endogenous O-GlcNAcylation, though typically substoichiometric on proteins64, can substantially impact the overall thermodynamic stability of a subset of the proteome. Notably, O-GlcNAc-thermostabilized proteins had a significantly lower average Tm compared to the meltome average, consistent with O-GlcNAc conferring physiologically relevant stabilization on certain proteins. This observation is in keeping with previous work, whereby O-GlcNAc was also found to hinder aggregation of various proteins including polyhomeotic (Ph), tau, and α-synuclein25–27. Unexpectedly however, despite being generally viewed as a stabilizing modification25–30, our unbiased method revealed O-GlcNAc as having a predominantly destabilizing effect on this panel of proteins. Notably, the majority of O-GlcNAc modulated proteins identified here are not known to be glycosylated, raising the possibility of indirect regulation. Following on this line of reasoning, several of these O-GlcNAc modulated proteins interact with one another as part of large macromolecular assemblies, members of which can be modified by O-GlcNAc. These observations suggest O-GlcNAc may mediate these destabilising effects by perturbing protein-protein interactions, as seen for PKM2 where its tetramerization was hindered by O-GlcNAc65. This analysis provides a valuable starting point to explore the thermostability effects of O-GlcNAc on protein complexes. However, targeted downstream studies are needed to further validate and explore the detailed molecular effects of O-GlcNAc on these complexes. Our results accordingly indicate that O-GlcNAc can act as a bi-directional regulator of protein thermostability, an attribute that is in keeping with its emerging role as a regulator of proteostasis that can act on protein complexes58–60, 66. We believe these observations should open new lines of inquiry into how O-GlcNAc may both stabilize and destabilize proteins and protein complexes perhaps, in the latter case for example, by impairing protein-protein interactions. More generally, these findings highlight the potential for PTMs to regulate macromolecular complex thermostability in a similar fashion to small-molecule effectors55. Notably, this new PTM-TPP method enables detecting both direct and indirect effects of PTMs on thermostability. Future studies combining this approach with HTP should enable pinpointing those modification sites having the greatest effects on the stability of proteins and protein complexes. We also envision that combining PTM-TPP with TPCA55, which allows identifying protein-protein interaction partners on the proteome-wide scale, could help identify protein complexes regulated by PTMs. Finally, we anticipate that adaptation and application of PTM-TPP to other PTMs should yield new insights into how PTMs regulate the stability of both proteins and protein complexes – illuminating how the diverse set of protein modifications influences cell function in a proteome-wide manner.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to the Canadian Institutes of Health Research (CIHR; PJT-148732, PJT-156202), and the Natural Sciences and Engineering Council of Canada (Discovery-RGPIN298406) for supporting this research. D.J.V. thanks the Canada Research Chairs program for support as a Tier I Canada Research Chair in Chemical Biology. This work was also supported by an American Cancer Society Research Scholar Award and the U.S.A. National Institutes of Health (5R35GM136354) to M.D.S and the Canada Foundation for Innovation, the British Columbia Knowledge Development Fund, Genome Canada/Genome BC (264PRO) to L.J.F. D.T.K. is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR) and a Trainee Award from the Michael Smith Foundation for Health Research (MSFHR). C.L.M. was supported by a U.S.A. National Science Foundation Graduate Research Fellowship. The authors wish to acknowledge the UVic-Genome BC Proteomics Centre, Victoria, Canada for performing mass spectrometry experiments. Finally, we are grateful to Prof. Samy Cecioni, Kyung-Mee Moon, and Dr. Daniela Salas for providing expert input.
Footnotes
Supporting Information
The supporting Information is available free of charge on the ACS Publications website.
PDF file containing all methods, supplementary figures and tables.
Four .xls files with source data for erPTM-TPP and cmPTM-TPP MS-proteomics experiments.
The authors declare no competing interests.
REFERENCES
- 1.Bagwan N; El Ali HH; Lundby A, Proteome-wide profiling and mapping of post translational modifications in human hearts. Sci Rep 2021, 11 (1), 2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A; Narayanan V; Sekhar A, Characterizing Post-Translational Modifications and Their Effects on Protein Conformation Using NMR Spectroscopy. Biochemistry 2020, 59 (1), 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bah A; Vernon RM; Siddiqui Z; Krzeminski M; Muhandiram R; Zhao C; Sonenberg N; Kay LE; Forman-Kay JD, Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature 2015, 519 (7541), 106–9. [DOI] [PubMed] [Google Scholar]
- 4.Bachman AB; Keramisanou D; Xu W; Beebe K; Moses MA; Vasantha Kumar MV; Gray G; Noor RE; van der Vaart A; Neckers L; Gelis I, Phosphorylation induced cochaperone unfolding promotes kinase recruitment and client class-specific Hsp90 phosphorylation. Nat Commun 2018, 9 (1), 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shental-Bechor D; Levy Y, Effect of glycosylation on protein folding: a close look at thermodynamic stabilization. Proc Natl Acad Sci U S A 2008, 105 (24), 8256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan G; Walther D, The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput Biol 2015, 11 (2), e1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balch WE; Morimoto RI; Dillin A; Kelly JW, Adapting Proteostasis for Disease Intervention. Science 2008, 319, 916–919. [DOI] [PubMed] [Google Scholar]
- 8.Sebastian RM; Shoulders MD, Chemical Biology Framework to Illuminate Proteostasis. Annu Rev Biochem 2020, 89, 529–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mateus A; Kurzawa N; Becher I; Sridharan S; Helm D; Stein F; Typas A; Savitski MM, Thermal proteome profiling for interrogating protein interactions. Mol Syst Biol 2020, 16 (3), e9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franken H; Mathieson T; Childs D; Sweetman GM; Werner T; Togel I; Doce C; Gade S; Bantscheff M; Drewes G; Reinhard FB; Huber W; Savitski MM, Thermal proteome profiling for unbiased identification of direct and indirect drug targets using multiplexed quantitative mass spectrometry. Nat Protoc 2015, 10 (10), 1567–93. [DOI] [PubMed] [Google Scholar]
- 11.Savitski MM; Reinhard FB; Franken H; Werner T; Savitski MF; Eberhard D; Martinez Molina D; Jafari R; Dovega RB; Klaeger S; Kuster B; Nordlund P; Bantscheff M; Drewes G, Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 2014, 346 (6205), 1255784. [DOI] [PubMed] [Google Scholar]
- 12.Becher I; Andres-Pons A; Romanov N; Stein F; Schramm M; Baudin F; Helm D; Kurzawa N; Mateus A; Mackmull MT; Typas A; Muller CW; Bork P; Beck M; Savitski MM, Pervasive Protein Thermal Stability Variation during the Cell Cycle. Cell 2018, 173 (6), 1495–1507 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niesen FH; Berglund H; Vedadi M, The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2007, 2 (9), 2212–21. [DOI] [PubMed] [Google Scholar]
- 14.Huang JX; Lee G; Cavanaugh KE; Chang JW; Gardel ML; Moellering RE, High throughput discovery of functional protein modifications by Hotspot Thermal Profiling. Nat Methods 2019, 16 (9), 894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith IR; Hess KN; Bakhtina AA; Valente AS; Rodriguez-Mias RA; Villen J, Identification of phosphosites that alter protein thermal stability. Nat Methods 2021, 18 (7), 760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potel CM; Kurzawa N; Becher I; Typas A; Mateus A; Savitski MM, Impact of phosphorylation on thermal stability of proteins. bioRxiv 2020. [DOI] [PubMed] [Google Scholar]
- 17.Stein BD; Huang JX; Wu D; Cantley LC; Moellering RE, Diverse Hotspot Thermal Profiling Methods Detect Phosphorylation-Dependent Changes in Protein Stability. bioRxiv 2021. [Google Scholar]
- 18.Larsen MR; Thingholm TE; Jensen ON; Roepstorff P; Jorgensen TJ, Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics 2005, 4 (7), 873–86. [DOI] [PubMed] [Google Scholar]
- 19.Alteen MG; Tan HY; Vocadlo DJ, Monitoring and modulating O-GlcNAcylation: assays and inhibitors of O-GlcNAc processing enzymes. Curr Opin Struct Biol 2021, 68, 157–165. [DOI] [PubMed] [Google Scholar]
- 20.Selnick HG; Hess JF; Tang C; Liu K; Schachter JB; Ballard JE; Marcus J; Klein DJ; Wang X; Pearson M; Savage MJ; Kaul R; Li TS; Vocadlo DJ; Zhou Y; Zhu Y; Mu C; Wang Y; Wei Z; Bai C; Duffy JL; McEachern EJ, Discovery of MK-8719, a Potent O-GlcNAcase Inhibitor as a Potential Treatment for Tauopathies. J Med Chem 2019, 62 (22), 10062–10097. [DOI] [PubMed] [Google Scholar]
- 21.Zachara NE; O’Donnell N; Cheung WD; Mercer JJ; Marth JD; Hart GW, Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 2004, 279 (29), 30133–42. [DOI] [PubMed] [Google Scholar]
- 22.Radermacher PT; Myachina F; Bosshardt F; Pandey R; Mariappa D; Muller HA; Lehner CF, O-GlcNAc reports ambient temperature and confers heat resistance on ectotherm development. Proc Natl Acad Sci U S A 2014, 111 (15), 5592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love DC; Ghosh S; Mondoux MA; Fukushige T; Wang P; Wilson MA; Iser WB; Wolkow CA; Krause MW; Hanover JA, Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proc Natl Acad Sci U S A 2010, 107 (16), 7413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang P; Lazarus BD; Forsythe ME; Love DC; Krause MW; Hanover JA, O-GlcNAc cycling mutants modulate proteotoxicity in Caenorhabditis elegans models of human neurodegenerative diseases. Proc Natl Acad Sci U S A 2012, 109 (43), 17669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuzwa SA; Shan X; Macauley MS; Clark T; Skorobogatko Y; Vosseller K; Vocadlo DJ, Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat Chem Biol 2012, 8 (4), 393–9. [DOI] [PubMed] [Google Scholar]
- 26.Levine PM; Galesic A; Balana AT; Mahul-Mellier AL; Navarro MX; De Leon CA; Lashuel HA; Pratt MR, alpha-Synuclein O-GlcNAcylation alters aggregation and toxicity, revealing certain residues as potential inhibitors of Parkinson’s disease. Proc Natl Acad Sci U S A 2019, 116 (5), 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marotta NP; Lin YH; Lewis YE; Ambroso MR; Zaro BW; Roth MT; Arnold DB; Langen R; Pratt MR, O-GlcNAc modification blocks the aggregation and toxicity of the protein alpha-synuclein associated with Parkinson’s disease. Nat Chem 2015, 7 (11), 913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gambetta MC; Muller J, O-GlcNAcylation prevents aggregation of the Polycomb group repressor polyhomeotic. Dev Cell 2014, 31 (5), 629–39. [DOI] [PubMed] [Google Scholar]
- 29.Drake WR; Hou CW; Zachara NE; Grimes CL, New use for CETSA: monitoring innate immune receptor stability via post-translational modification by OGT. J Bioenerg Biomembr 2018, 50 (3), 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim KH; Chang HI, O-linked N-acetylglucosamine suppresses thermal aggregation of Sp1. FEBS Lett 2006, 580 (19), 4645–52. [DOI] [PubMed] [Google Scholar]
- 31.Nosella ML; Tereshchenko M; Pritisanac I; Chong PA; Toretsky JA; Lee HO; Forman-Kay JD, O-Linked-N-Acetylglucosaminylation of the RNA-Binding Protein EWS N-Terminal Low Complexity Region Reduces Phase Separation and Enhances Condensate Dynamics. J Am Chem Soc 2021, 143 (30), 11520–11534. [DOI] [PubMed] [Google Scholar]
- 32.Culyba EJ; J.L. P; Hanson SR; Dhar A; Wong C; Gruebele M; Powers ET; Kelly JW, Protein Native-State Stabilization by Placing Aromatic Side Chains in N-Glycosylated Reverse Turns. Science 2011, 331, 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MM; Bartlett AI; Nerenberg PS; Friel CT; Hackenberger CP; Stultz CM; Radford SE; Imperiali B, Perturbing the folding energy landscape of the bacterial immunity protein Im7 by site-specific N-linked glycosylation. Proc Natl Acad Sci U S A 2010, 107 (52), 22528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson JW; Griffin ME; Hsieh-Wilson LC, Methods for the Detection, Study, and Dynamic Profiling of O-GlcNAc Glycosylation. Methods Enzymol 2018, 598, 101–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombard V; Golaconda Ramulu H; Drula E; Coutinho PM; Henrissat B, The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 2014, 42 (Database issue), D490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang A; Cho K; Park HS, Chemical biology approaches for studying posttranslational modifications. RNA Biol 2018, 15 (4–5), 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazemi Z; Chang H; Haserodt S; McKen C; Zachara NE, O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J Biol Chem 2010, 285 (50), 39096–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez MR; Dias TB; Natov PS; Zachara NE, Stress-induced O-GlcNAcylation: an adaptive process of injured cells. Biochem Soc Trans 2017, 45 (1), 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen DL; Gloster TM; Yuzwa SA; Vocadlo DJ, Insights into O-linked N-acetylglucosamine ([0–9]O-GlcNAc) processing and dynamics through kinetic analysis of O-GlcNAc transferase and O-GlcNAcase activity on protein substrates. J Biol Chem 2012, 287 (19), 15395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennis RJ; Taylor EJ; Macauley MS; Stubbs KA; Turkenburg JP; Hart SJ; Black GN; Vocadlo DJ; Davies GJ, Structure and mechanism of a bacterial beta-glucosaminidase having O-GlcNAcase activity. Nat Struct Mol Biol 2006, 13 (4), 365–71. [DOI] [PubMed] [Google Scholar]
- 41.Selvan N; Williamson R; Mariappa D; Campbell DG; Gourlay R; Ferenbach AT; Aristotelous T; Hopkins-Navratilova I; Trost M; van Aalten DMF, A mutant O-GlcNAcase enriches Drosophila developmental regulators. Nat Chem Biol 2017, 13 (8), 882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peck Justice SA; McCracken NA; Victorino JF; Qi GD; Wijeratne AB; Mosley AL, Boosting Detection of Low-Abundance Proteins in Thermal Proteome Profiling Experiments by Addition of an Isobaric Trigger Channel to TMT Multiplexes. Anal Chem 2021, 93 (18), 7000–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrin J; Werner T; Kurzawa N; Rutkowska A; Childs DD; Kalxdorf M; Poeckel D; Stonehouse E; Strohmer K; Heller B; Thomson DW; Krause J; Becher I; Eberl HC; Vappiani J; Sevin DC; Rau CE; Franken H; Huber W; Faelth-Savitski M; Savitski MM; Bantscheff M; Bergamini G, Identifying drug targets in tissues and whole blood with thermal-shift profiling. Nat Biotechnol 2020, 38 (3), 303–308. [DOI] [PubMed] [Google Scholar]
- 44.Saei AA; Beusch CM; Sabatier P; Wells JA; Gharibi H; Meng Z; Chernobrovkin A; Rodin S; Nareoja K; Thorsell AG; Karlberg T; Cheng Q; Lundstrom SL; Gaetani M; Vegvari A; Arner ESJ; Schuler H; Zubarev RA, System-wide identification and prioritization of enzyme substrates by thermal analysis. Nat Commun 2021, 12 (1), 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuzwa SA; Macauley MS; Heinonen JE; Shan X; Dennis RJ; He Y; Whitworth GE; Stubbs KA; McEachern EJ; Davies GJ; Vocadlo DJ, A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol 2008, 4 (8), 483–90. [DOI] [PubMed] [Google Scholar]
- 46.Liu TW; Zandberg WF; Gloster TM; Deng L; Murray KD; Shan X; Vocadlo DJ, Metabolic Inhibitors of O-GlcNAc Transferase That Act In Vivo Implicate Decreased O-GlcNAc Levels in Leptin-Mediated Nutrient Sensing. Angew Chem Int Ed Engl 2018, 57 (26), 7644–7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macauley MS; Vocadlo DJ, Increasing O-GlcNAc levels: An overview of small-molecule inhibitors of O-GlcNAcase. Biochim Biophys Acta 2010, 1800 (2), 107–21. [DOI] [PubMed] [Google Scholar]
- 48.Hamiel CR; Pinto S; Hau A; Wischmeyer PE, Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am J Physiol Cell Physiol 2009, 297 (6), C1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore CL; Dewal MB; Nekongo EE; Santiago S; Lu NB; Levine SS; Shoulders MD, Transportable, Chemical Genetic Methodology for the Small Molecule-Mediated Inhibition of Heat Shock Factor 1. ACS Chem Biol 2016, 11 (1), 200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips AM; Ponomarenko AI; Chen K; Ashenberg O; Miao J; McHugh SM; Butty VL; Whittaker CA; Moore CL; Bloom JD; Lin YS; Shoulders MD, Destabilized adaptive influenza variants critical for innate immune system escape are potentiated by host chaperones. PLoS Biol 2018, 16 (9), e3000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liebelt F; Sebastian RM; Moore CL; Mulder MPC; Ovaa H; Shoulders MD; Vertegaal ACO, SUMOylation and the HSF1-Regulated Chaperone Network Converge to Promote Proteostasis in Response to Heat Shock. Cell Rep 2019, 26 (1), 236–249 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma J; Hou C; Li Y; Chen S; Wu C, OGT Protein Interaction Network (OGT-PIN): A Curated Database of Experimentally Identified Interaction Proteins of OGT. Int J Mol Sci 2021, 22 (17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez M; Renuse S; Kreimer S; O’Meally R; Natov P; Madugundu AK; Nirujogi RS; Tahir R; Cole R; Pandey A; Zachara NE, Quantitative Proteomics Reveals that the OGT Interactome Is Remodeled in Response to Oxidative Stress. Mol Cell Proteomics 2021, 20, 100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groves JA; Maduka AO; O’Meally RN; Cole RN; Zachara NE, Fatty acid synthase inhibits the O-GlcNAcase during oxidative stress. J Biol Chem 2017, 292 (16), 6493–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan CSH; Go KD; Bisteau X; Dai L; Yong CH; Prebhu N; Ozturk MB; Lim YT; Sreekumar L; Lengqvist J; Tergaonkar V; Kaldis P; Sobota RM; Nordlund P, Thermal proximity coaggregation for system-wide profiling of protein complex dynamics in cells. Science 2018, 359, 1170–1177. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W; Liu T; Dong H; Bai H; Tian F; Shi Z; Chen M; Wang J; Qin W; Qian X, Synthesis of a Highly Azide-Reactive and Thermosensitive Biofunctional Reagent for Efficient Enrichment and Large-Scale Identification of O-GlcNAc Proteins by Mass Spectrometry. Anal Chem 2017, 89 (11), 5810–5817. [DOI] [PubMed] [Google Scholar]
- 57.Szklarczyk D; Gable AL; Lyon D; Junge A; Wyder S; Huerta-Cepas J; Simonovic M; Doncheva NT; Morris JH; Bork P; Jensen LJ; Mering CV, STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019, 47 (D1), D607–D613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang F; Su K; Yang X; Bowe DB; Paterson AJ; Kudlow JE, O-GlcNAc Modification Is an Endogenous Inhibitor of the Proteasome. Cell 2003, 115, 715–725. [DOI] [PubMed] [Google Scholar]
- 59.Li X; Zhu Q; Shi X; Cheng Y; Li X; Xu H; Duan X; Hsieh-Wilson LC; Chu J; Pelletier J; Ni M; Zheng Z; Li S; Yi W, O-GlcNAcylation of core components of the translation initiation machinery regulates protein synthesis. Proc Natl Acad Sci U S A 2019, 116 (16), 7857–7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan ZW; Fei G; Paulo JA; Bellaousov S; Martin SES; Duveau DY; Thomas CJ; Gygi SP; Boutz PL; Walker S, O-GlcNAc regulates gene expression by controlling detained intron splicing. Nucleic Acids Res 2020, 48 (10), 5656–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wulff-Fuentes E; Berendt RR; Massman L; Danner L; Malard F; Vora J; Kahsay R; Olivier-Van Stichelen S, The human O-GlcNAcome database and meta-analysis. Sci Data 2021, 8 (1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gloster TM; Zandberg WF; Heinonen JE; Shen DL; Deng L; Vocadlo DJ, Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat Chem Biol 2011, 7 (3), 174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chuh KN; Batt AR; Pratt MR, Chemical Methods for Encoding and Decoding of Posttranslational Modifications. Cell Chem Biol 2016, 23 (1), 86–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darabedian N; Thompson JW; Chuh KN; Hsieh-Wilson LC; Pratt MR, Optimization of Chemoenzymatic Mass Tagging by Strain-Promoted Cycloaddition (SPAAC) for the Determination of O-GlcNAc Stoichiometry by Western Blotting. Biochemistry 2018, 57 (40), 5769–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y; Liu J; Jin X; Zhang D; Li D; Hao F; Feng Y; Gu S; Meng F; Tian M; Zheng Y; Xin L; Zhang X; Han X; Aravind L; Wei M, O-GlcNAcylation destabilizes the active tetrameric PKM2 to promote the Warburg effect. Proc Natl Acad Sci U S A 2017, 114 (52), 13732–13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo B; Liang Q; Li L; Hu Z; Wu F; Zhang P; Ma Y; Zhao B; Kovacs AL; Zhang Z; Feng D; Chen S; Zhang H, O-GlcNAc-modification of SNAP-29 regulates autophagosome maturation. Nat Cell Biol 2014, 16 (12), 1215–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


