Abstract
Purpose
We assessed long-term outcomes of dexamethasone 12 mg versus 6 mg given daily for up to 10 days in patients with coronavirus disease 2019 (COVID-19) and severe hypoxaemia.
Methods
We assessed 180-day mortality and health-related quality of life (HRQoL) using EuroQoL (EQ)-5D-5L index values and EQ visual analogue scale (VAS) in the international, stratified, blinded COVID STEROID 2 trial, which randomised 1000 adults with confirmed COVID-19 receiving at least 10 L/min of oxygen or mechanical ventilation in 26 hospitals in Europe and India. In the HRQoL analyses, higher values indicated better outcomes, and deceased patients were given a score of zero.
Results
We obtained vital status at 180 days for 963 of 982 patients (98.1%) in the intention-to-treat population, EQ-5D-5L index value data for 922 (93.9%) and EQ VAS data for 924 (94.1%). At 180 days, 164 of 486 patients (33.7%) had died in the 12 mg group versus 184 of 477 (38.6%) in the 6 mg group [adjusted risk difference − 4.3%; 99% confidence interval (CI) − 11.7–3.0; relative risk 0.89; 0.72–1.09; P = 0.13]. The adjusted mean differences between the 12 mg and the 6 mg groups in EQ-5D-5L index values were 0.06 (99% CI − 0.01 to 0.12; P = 0.10) and in EQ VAS scores 4 (− 3 to 10; P = 0.22).
Conclusion
Among patients with COVID-19 and severe hypoxaemia, dexamethasone 12 mg compared with 6 mg did not result in statistically significant improvements in mortality or HRQoL at 180 days, but the results were most compatible with benefit from the higher dose.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06677-2.
Keywords: COVID-19, Hypoxaemia, Critical illness, Corticosteroids, Quality of life, Mortality
Take-home message
| In patients with COVID-19 and severe hypoxemia, dexamethasone 12 mg compared with 6 mg did not result in statistically significant improvements in mortality or health-related quality of life at 180 days, but the results were most compatible with benefit from the higher dose. |
Introduction
Critical coronavirus disease 2019 (COVID-19) is characterised by severe pulmonary inflammation and high rates of death despite anti-inflammatory treatment [1]. Survivors from critical COVID-19 suffer from reduced health-related quality of life (HRQoL), including physical and mental problems, for months after hospital discharge [2].
Dexamethasone 6 mg daily for up to 10 days is recommended for patients with critical COVID-19 [3] based on the results of a meta-analysis of 7 randomised trials reporting reduced short-term mortality with the use of systemic corticosteroids [1]. Subsequently, the results of the COVID STEROID 2 trial suggested that dexamethasone 12 mg as compared with 6 mg may result in more days alive without life support at 28 days in patients with COVID-19 and severe hypoxaemia [adjusted mean difference 1.3 days (95% confidence interval 0.0–2.6)] [4]. In a pre-planned Bayesian analysis of the COVID STEROID 2 trial, the probability of benefit with 12 mg versus 6 mg was 94% for days alive without life support at 28 days and between 84 and 96% for all secondary outcomes assessed up to 90 days [5].
The effects of dexamethasone dosing on longer-term outcomes, including HRQoL, in patients with COVID-19 and severe hypoxaemia are important for patients and should inform clinicians, guideline committee members and policymakers. Here we present the 180-day mortality and HRQoL results, which were pre-specified secondary outcome measures of the COVID STEROID 2 trial [6].
Methods
Trial design
The COVID STEROID 2 trial was an investigator-initiated, international, parallel-group, stratified, blinded randomised clinical trial. The trial protocol was approved by the relevant medicine agencies and ethics committees [4]. The trial protocol, statistical analysis plan and the primary report have all been published [4, 6] [also presented in Electronic Supplement Material (ESM 1)]. We prepared this report in accordance with the CONSORT checklist (ESM 2).
Trial sites and patients
Patients were enrolled between August 27, 2020 and May 20, 2021 at 31 sites in 26 hospitals in Denmark, India, Sweden, and Switzerland.
Eligible patients were 18 years or older, had confirmed SARS-CoV-2 infection and received (i) supplementary oxygen at a flow of at least 10 L/min independent of delivery system, (ii) non-invasive ventilation or continuous positive airway pressure for hypoxemia, or (iii) invasive mechanical ventilation. We mainly excluded patients for whom consent could not be obtained, who had received systemic corticosteroids for COVID-19 for 5 days or more or received systemic corticosteroids for other indications than COVID-19 in doses higher than 6 mg dexamethasone equivalents, and those with invasive fungal infection or active tuberculosis. The exclusion criteria and trial definitions are presented in the protocol (ESM 1) and in the primary publication [4, 6]. We obtained informed consent from the patients or their legal surrogates according to national regulations before enrolment. If consent was withdrawn or not granted, permission was sought from the patient or relatives to continue collection and use of trial data.
Procedures
Enrolled patients were randomised 1:1 to intravenous dexamethasone 12 mg or 6 mg (as dexamethasone phosphate 14.4 mg or 7.2 mg, respectively, in isotonic saline to a total bolus volume of 5 ml in identical syringes prepared by unblinded trial staff from shelf medication at each hospital) for up to 10 days. Treatment assignments were concealed from patients, their relatives, clinical staff, and the investigators assessing the outcomes.
Outcome measures at 180 days
All-cause mortality and HRQoL at 180 days after randomisation were pre-specified secondary outcome measures in the protocol (ESM 1) [4, 6]. Data were obtained from patient’s medical records and contact to patients or relatives by phone or e-mail. As soon as possible after day 180, surviving patients were interviewed over the phone by blinded, trained and qualified trial staff using the EuroQol (EQ)-5D-5L questionnaire [7]. The EQ-5D-5L consists of a descriptive system and the EQ visual analogue scale (VAS). The descriptive system comprises 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The respondents were asked to tick one of 5 boxes (5 levels) for each domain that best described their health today: no, slight, moderate, severe, or extreme problems [7]. On EQ VAS, the respondents were asked to mark how good or bad their health was today on a scale from 100 (‘the best health you can imagine’) to zero (‘the worst health you can imagine’). If a patient was unable to answer, a relative was approached to do so on behalf of the patient; if so, the version of the questionnaire for proxies was used. Trial sites made several attempts for at least 4 weeks to obtain answers from patients and relatives, a process that was centrally monitored by the coordinating centres in Denmark and India, who supported and encouraged sites to obtain replies. The defined outcome measures were the EQ-5D-5L index value (a summary score based on the 5 domains reflecting health state according to the preference of general population; it ranges for 1.0 (perfect health) to values below zero (health states valued worse than death with zero defined as a state equivalent to death)) and EQ VAS [7]. We used the country specific value sets to calculate the index values for Danish [8], Indian [9] and Swedish [10] patients and the German one [11] for those enrolled in Switzerland because there is no Swiss value set available. We also calculated index values using the Danish value set for all patients (as most patients were enrolled in Denmark) as recommended [12].
Statistical analysis
The analyses were done according to the predefined statistical analysis plan for the 180-day outcomes (ESM 1) in the intention-to-treat population defined as all randomised patients (n = 1000) excluding the 18 patients who withdrew consent for the use of any data resulting in 982 patients to be analysed. We present descriptive baseline data (stratified by intervention group, 180-day mortality and HRQoL-respondent status) and outcome data as medians with interquartile ranges (IQRs; for numeric data) and numbers with percentages (for categorical data).
As per the analysis plan (ESM 1), we performed multiple imputations of the HRQoL data, because more than 5% of the patients had missing data (6.1% non-respondents for EQ-5D-5L index values and 5.9% for EQ VAS scores). We used predictive mean matching with 25 datasets imputed separately in each treatment group. We included all stratification variables, all variables used in the HRQoL analyses, important baseline prognostic variables (age, co-morbidities, use of life supportive measures at baseline), and all outcomes available in the imputation model (ESM 1). We also analysed HRQoL in a dataset with best–worst and worst–best imputation of missing data (using the highest or lowest observed values) and in a complete case dataset.
Analyses were adjusted for the stratification variables (trial site, age below 70 years and use of invasive mechanical ventilation). We analysed landmark mortality at 180 days using G-computation with an adjusted logistic regression model and 50,000 bootstrap resamples with results presented as adjusted relative risks and risk differences (as the planned log-binomial models did not converge) supplemented with unadjusted relative risks and risk differences (using the planned log-binomial models) and Fisher’s exact tests. Time to death was presented as Kaplan–Meier survival curves and compared post hoc using Cox regression with results presented as an unadjusted hazard ratio as in the primary report [4]. The differences in adjusted means of EQ-5D-5L index values and EQ VAS scores were analysed using the Kryger Jensen and Lange test [13] with confidence intervals (CIs) calculated using bootstrapping with 50,000 resamples. The primary analysis included patients who had died at 180 days after randomisation; they were assigned scores of zero corresponding to a health state equivalent to death for EQ-5D-5L index values or the worst possible perceived health state value for EQ VAS. We supplemented the analyses of EQ-5D-5L index values and EQ VAS scores with analyses of adjusted median differences and unadjusted mean and median differences and post hoc adjusted mean differences among 180-day survivors in the multiply imputed datasets.
We performed the analyses using R software, versions 3.6.3 and 4.1.0, presented the results with 99% confidence intervals (CI) and considered P values below 0.01 as statistically significant due to multiple testing (9 outcomes in total, among which the 6 outcomes assessed at 28 or 90 days were reported in the primary publication [4]).
Results
We obtained vital status 180 days after randomisation for 963 (98.1%) of the 982 patients in the intention-to-treat population (Fig. 1). We obtained data for EQ-5D-5L index values for 922 (93.9%) patients and for EQ VAS scores for 924 (94.1%) patients at a median of 187 days (interquartile range 182–201) after randomisation in the 12 mg group and 186 days (182–202) in the 6 mg group. The HRQoL questionnaire was answered by relatives in 36 of 300 (12%) respondents in the 12 mg group and by 37 of 276 (13%) in the 6 mg group.
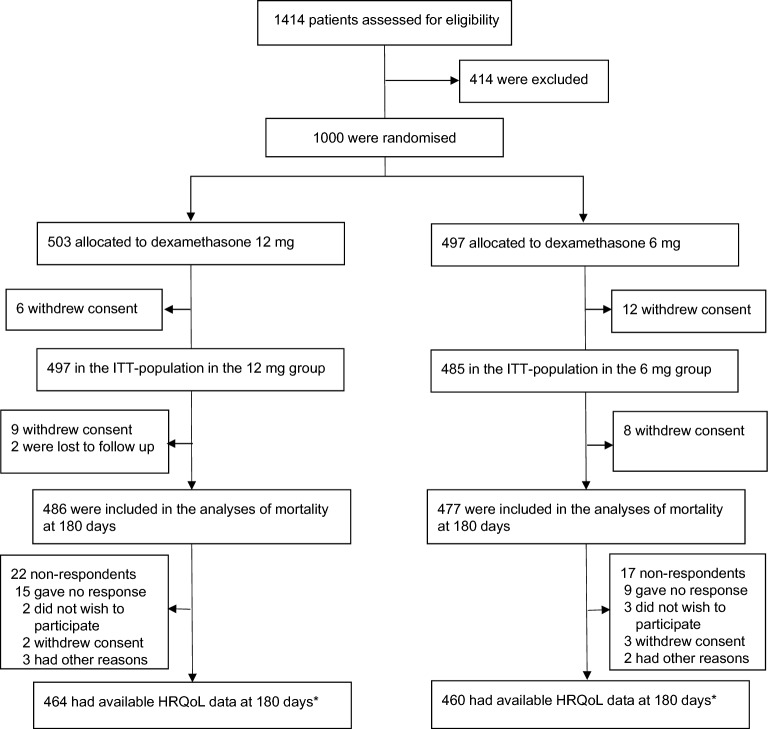
Fig. 1.
Patient flow in the COVID STEROID 2 trial. The details up to 90 days were presented in the primary report [4]. Eighteen patients withdrew consent for the use of any data (12 patients before the first dosing of trial medication and 6 after); the intention-to-treat (ITT) population therefore consisted of 982 patients in total. There were patient withdraws at three levels because of repeated follow-up of patients. *The primary HRQoL analyses were done in the ITT population (n = 982) with deceased patients assigned zero and missing data (n = 60 for EQ-5D-5L index values and n = 58 for EQ VAS scores) multiply imputed
Table 1 reports the baseline characteristics of all patients in the intention-to-treat population by status at 180 days as patients who had died at 180 days, the respondents to HRQoL assessment and those who had missing HRQoL data; there appeared to be no major differences in the characteristics between the 12 mg and 6 mg groups in any of the populations. Overall, the patients received dexamethasone for median 1 day (1–2) before randomisation and 7 days (6–9) after randomisation.
Table 1.
Baseline characteristics in all patients in the intention-to-treat population by status at 180 days and intervention group (12 mg or 6 mg of dexamethasone) in the COVID STEROID 2 trial
| Dead at 180 days | HRQoL respondentsa | Missing HRQoL datab | ||||
|---|---|---|---|---|---|---|
| 12 mg (n = 164) | 6 mg (n = 184) | 12 mg (n = 298) | 6 mg (n = 276) | 12 mg (n = 35) | 6 mg (n = 25) | |
| Country of enrolment, n (%) | ||||||
| Denmark, n = 485 | 66 (40%) | 77 (42%) | 157 (53%) | 142 (51%) | 28 (80%) | 15 (60%) |
| India, n = 369 | 82 (50%) | 87 (47%) | 98 (33%) | 96 (35%) | 2 (6%) | 4 (16%) |
| Sweden, n = 79 | 10 (6%) | 8 (4%) | 26 (9%) | 25 (9%) | 4 (11%) | 6 (24%) |
| Switzerland, n = 49 | 6 (4%) | 12 (7%) | 17 (6%) | 13 (5%) | 1 (3%) | 0 |
| Age, years | 71 (64–76) | 69 (60–75) | 61 (53–69) | 61 (52–70) | 61 (52–69) | 55 (47–71) |
| Weight, kg | 73 (63–88) | 75 (65–90) | 85 (70–100) | 80 (70–96) | 85 (70–101) | 91 (78–97) |
| Co-morbidities, n (%) | ||||||
| Diabetes | 55 (34%) | 65 (35%) | 74 (25%) | 93 (34%) | 6 (17%) | 5 (20%) |
| Ischaemic heart disease | 30 (18%) | 34 (19%) | 34 (11%) | 34 (12%) | 3 (9%) | 1 (4%) |
| Pulmonary disease | 20 (12%) | 28 (15%) | 34 (11%) | 28 (10%) | 3 (9%) | 0 |
| Immunosuppression | 21 (13%) | 29 (16%) | 18 (6%) | 12 (4%) | 1 (3%) | 2 (8%) |
| Co-interventions, n (%) | ||||||
| Chronic corticosteroid use | 6 (4%) | 13 (7%) | 7 (2%) | 2 (1%) | 0 | 1 (4%) |
| Limits of care | 22 (13%) | 19 (10%) | 6 (2%) | 6 (2%) | 2 (6%) | 0 |
| Invasive mechanical ventilation | 46 (28%) | 48 (26%) | 52 (17%) | 47 (17%) | 9 (26%) | 4 (16%) |
| Vasopressor or inotrope | 30 (18%) | 35 (19%) | 43 (14%) | 30 (11%) | 8 (23%) | 3 (12%) |
| Renal replacement therapy | 6 (4%) | 10 (5%) | 4 (1%) | 3 (1%) | 1 (3%) | 1 (4%) |
| Anti-inflammatory drugs | 15 (9%) | 16 (9%) | 40 (13%) | 38 (14%) | 3 (9%) | 3 (12%) |
| IL-6-RA | 13 (8%) | 9 (5%) | 36 (12%) | 35 (13%) | 3 (9%) | 3 (12%) |
Numeric data are presented as medians with interquartile ranges
IL-6-RA interleukin-6-receptor antagonist
aIncluding responses from patients (n = 503) and relatives on behalf of patients (n = 73); 2 patients included here only had complete EQ VAS data and not complete EQ-5D-5L index value data
bIncluding non-respondents (22 in 12 mg group and 17 in the 6 mg group) and those with no HRQoL data available (patients with partially available HRQoL data were included as respondents)
180-day mortality
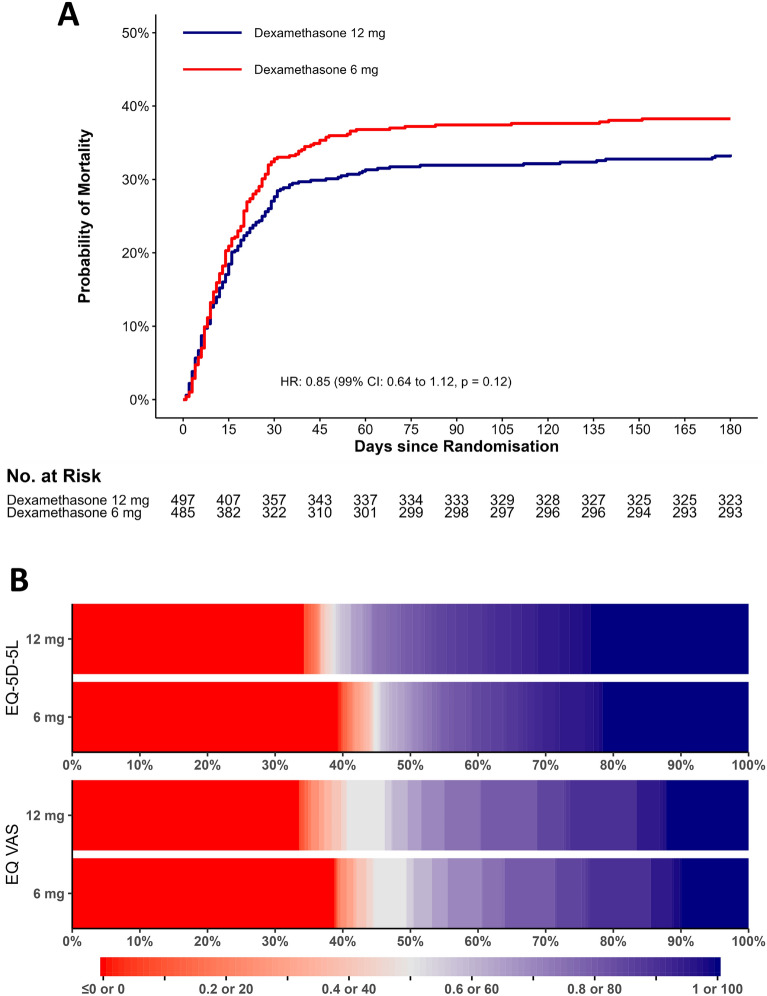
At 180 days after randomisation, 164 of 486 (33.7%) patients in the 12 mg group had died compared to 184 of 477 (38.6%) patients in the 6 mg group (adjusted risk difference − 4.3%; 99% CI − 11.7 to 3 and adjusted relative risk 0.89; 0.72–1.09; P = 0.13) (Table 2 and Table S1, ESM 3). Figure 2A presents the Kaplan–Meier mortality curves up to 180 days in the two intervention groups.
Table 2.
Outcome measures at 180 days
| Dexamethasone 12 mg | Dexamethasone 6 mg | Adjusted risk difference or adjusted mean differences (99% CI) | P valuea | |
|---|---|---|---|---|
| Mortality | ||||
| Death by 180 days no./total no. (%) | 164/486 (33.7) | 184/477 (38.6) | − 4.3 (− 11.7 to 3) | 0.13b |
| Health-Related Quality of Lifec medians (IQRs) | ||||
| EQ-5D-5L index values | 0.80 (0–0.97) | 0.68 (0–0.95) | 0.06 (− 0.01 to 0.12) | 0.10d |
| Survivors onlye | 0.93 (0.81–1) | 0.92 (0.77–1) | 0.02 (− 0.02 to 0.07) | 0.39 |
| EQ VAS | 65 (0–90) | 55 (0–85) | 4 (− 3 to 10) | 0.22f |
| Survivors onlye | 80 (65–95) | 80 (65–90) | 0 (− 4 to 4) | 0.49 |
CI confidence interval; EQ-5D-5L EuroQol-5 domains-5 levels; IQR interquartile range; VAS visual analogue scale
aThe analyses were adjusted for the stratification variables being trial site, age below 70 years and the use of invasive mechanical ventilation at baseline
bP = 0.12 by Fisher’s exact test
cPatients who died within 180 days after randomisation were assigned the value zero corresponding to a health state as bad as being dead for EQ-5D-5L index values and the worst possible value for EQ VAS. Data from non-responders were multiply imputed (n = 60 for the index values and n = 58 for EQ VAS scores); all health-related quality of life data in this table were calculated using the multiply imputed datasets. The data for each domain and the analyses using the Danish value set only and those of the adjusted median differences, the best–worst and worst–best imputed dataset and the complete case dataset (including the descriptive data) are presented in Fig. 3 and in Tables 1 and 2 in ESM 3. Higher values indicate better quality of life
dP = 0.04 based on the analyses of bootstrapped adjusted mean differences because of markedly skewed data
ePost hoc analyses as these were not predefined in the statistical analysis plan. Descriptive data and adjusted analyses in survivors only were calculated using the multiply imputed datasets to match the primary analyses
fP = 0.14 based on the analyses of bootstrapped adjusted mean differences because of markedly skewed data
Fig. 2.
Time to death or censoring and distribution of HRQoL data at 180 days in the two intervention groups. A Mortality curves in the two intervention groups up to 180 days. Patients who withdrew consent for further data registration or were lost to follow-up were censored at the time of the withdrawal or loss to follow-up. The time to death was compared post hoc using Cox regression with results presented as an unadjusted hazard ratio (HR) with 99% confidence interval (CI) and P value. B Distribution of the HRQoL data as horizontally stacked proportions in the two intervention groups. Patients who died within 180 days after randomisation were assigned the value 0, corresponding to a health state equal to being dead for EQ-5D-5L index values and the worst possible value for EQ VAS. Data from non-respondents were multiply imputed (n = 60 for the index values and n = 58 for EQ VAS scores). Red represents worse outcomes, and blue represents better outcomes. For EQ-5D-5L index values, < 1% of the values in each group in the imputed datasets were below 0, corresponding to health states worse than being dead. These values are displayed together with the value zero
Health-related quality of life
In the primary analysis including the patients who had died at 180 days and those with missing data (multiply imputed), median EQ-5D-5L index values for patients who received 12 mg vs. 6 mg were 0.80 vs. 0.67 (adjusted mean difference 0.06 (99% CI − 0.01 to 0.12; P = 0.10), respectively (Table 2 and Fig. 2B). The median EQ VAS scores were 65 vs. 55 (adjusted mean difference 4 (− 3 to 10; P = 0.22), respectively (Table 2 and Fig. 2B). The results did not change noticeably in the sensitivity analyses (Table S1, ESM 3). In 180-day survivors, the EQ-5D-5L index values and EQ VAS scores were similar between the 12 mg and 6 mg groups (Table 2). The data from the 180-day survivors for each of the 5 domains in EQ-5D-5L are presented in Fig. 3 and Table S2 in ESM 3.
Fig. 3.
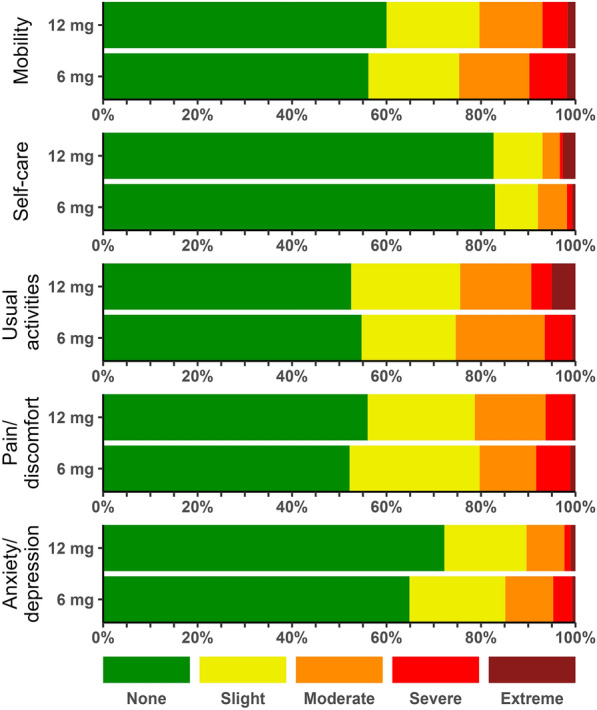
Distribution of single HRQoL domain levels among the 180-day survivors in the two intervention groups. Values are from the responding survivors only (patients (n = 503) and relatives on behalf of patients (n = 73; for these 73 patients, relatives were unable to respond for one patient in the 12 mg group in the usual activities domain and for one patient in the 12 mg group in the anxiety/depression domain)). Patients or relatives answered one of 5 levels (no problems or slight, moderate, severe, or extreme problems) for each of the 5 domains in the EQ-5D-5L survey. The corresponding numeric data are presented in Table S2, ESM 3
Discussion
In this international, randomised clinical trial of patients with COVID-19 and severe hypoxaemia, we observed no statistically significant differences in mortality or HRQoL at 180 days among patients assigned to dexamethasone 12 mg versus 6 mg for up to 10 days. Our estimate of the effect of dexamethasone 12 mg vs 6 mg on mortality at 180 days was consistent with a 12% absolute reduction to a 3% absolute increase. Taken together, the results are in line with those observed at 28- and 90-day follow-up in our trial [4].
At least three trials have assessed the effects of a higher daily dose of dexamethasone versus the recommended 6 mg in patients with severe or critical COVID-19. A higher dose of dexamethasone may improve short-term outcomes among COVID-19 patients receiving oxygen supplementation [14], in those with severe hypoxaemia [4, 5], and those with acute respiratory distress syndrome (ARDS) [15]. In our trial, the survival curves separated between the two intervention groups between days 20 and 60 with no apparent changes before and after that. The reason for this cannot be assessed directly from our data or analyses, but the point estimates of all outcomes assessed in this time period favoured the 12 mg group, including days alive without life support and the occurrence of serious adverse reactions/events [4, 5]. Uncertainty remains because none of these 3 trials observed statistically significantly improvements in patient-important outcomes, e.g. mortality, days alive out of hospital or HRQoL, with higher daily doses of dexamethasone as compared with the 6 mg dose. In addition, there was heterogeneity in settings, disease severity, use of co-interventions, outcome measures and time of follow-up. Importantly, less than 1300 patients were included in the three trials in total.
The HRQoL values observed in the survivors in both intervention groups in our trial were high as compared to those observed in other studies of COVID-19 survivors [2]. In previous studies of COVID-19 patients after hospital discharge in the UK, Norway, Belgium, and Iran, both EQ-5D-5L index values and EQ VAS scores appeared lower than those observed in our patients. In those studies, populations of mixed disease severity were surveyed 4–10 weeks after hospital discharge. A UK study assessed HRQoL in COVID-19 patients, who had been in critical care, 3 to 7 months (median 135 days) after hospital discharge and found both EQ-5D-5L index values and EQ VAS scores that appeared lower than those observed by us [16]. The reasons for these potential differences are less clear, but our HRQoL data were obtained in the context of a clinical trial that had a large sample size, long follow-up and high response rate. Also, we allowed relatives to answer and imputed missing data. Compared to a previous corticosteroid trial (ADRENAL) in patients with septic shock [17], both the EQ-5D-5L index values and EQ VAS scores appeared higher in our trial patients, which may be explained by differences in patient populations. The patterns in impairment among the 5 domains appeared somewhat similar between the two trials (more moderate and severe problems in the usual activities and pain/discomfort domains and less problems in the self-care domain) [17].
There are several strengths to our trial. Our trial is the first large interventional trial to report long-term outcomes in patients with COVID-19. It was investigator-initiated, blinded, enrolled patients in both Europe and India, and 94 to 98% of patients had outcome data reported and analysed. We assessed HRQoL by generic scales, EQ-5D-5L and EQ VAS, which have been widely used in critically ill patients [18]. These factors increase the internal and external validity of our results.
Our results come with some limitations. First, while EQ-5D-5L has been used in COVID-19 survivors [2], it has not been fully validated in this population. Second, as no value set is available for Switzerland, we used the German value set to calculate index values for Swiss patients. Of note, a sensitivity analysis using the Danish value set for all patients did not change the results, indicating consistent effects across countries in the trial. Third, as 6% of patients had missing HRQoL outcome data, we performed multiple imputation and did the final analyses in the imputed datasets supplemented with best–worst, worst–best, and complete case analyses according to the protocol and recommendations for the handling of missing data [6, 19]. Even though 6% missingness is low for studies of HRQoL, this may have affected the results. Fourth, in the primary analyses of HRQoL, we included deceased patients according to the intention-to-treat principle and assigned them the value zero. While zero in EQ-5D-5L index values is valued by the public as a health state equal to being dead, this is not the case for EQ VAS. In general, there is no optimal solution to this problem, but in a trial with potential difference in mortality at HRQoL follow-up analysing HRQoL in survivors only may bias results [20]. We therefore post hoc analysed HRQoL in survivors only to ease interpretation. Fifth, only 10% of our trial patients received interleukin-6-receptor antagonists (IL-6-RA) at baseline, which reduces the generalisability of the results in settings where IL-6-RA are used.
For clinicians who use higher rather than the standard dose of dexamethasone for patients with COVID-19 and severe hypoxaemia, our results should be reassuring because all results were mostly compatible with benefit from 12 mg, and because we could reject at the 99% confidence level an absolute increase in mortality of 3% or more, absolute reductions in EQ-5D-5L index values of 0.01 or more, and EQ VAS score of 3 or more at 180 days with 12 mg versus 6 mg. As described, uncertainty remains, some of which may be reduced with the results of a planned prospective meta-analysis of trials assessing higher versus standard dose dexamethasone in patients with COVID-19 [21] and those of the higher dose dexamethasone domain in the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial [22].
As corticosteroids are cheap, easily available and recommended for patients with COVID-19 and hypoxaemia, even a small difference in mortality or other patient-important outcomes may result in important clinical and health economic benefits at the population level. Whilst our data do not provide unequivocal evidence that dexamethasone 12 mg is superior to 6 mg, the adjusted absolute difference in mortality at 28, 90 and 180 days was consistently 4–5 percentage points lower in the dexamethasone 12 mg group than in the 6 mg group without suggestions of harm (i.e. no increased serious adverse effects or reduced HRQoL) [4]. If this also applies in patients who also receive IL-6-RA is less certain, because of the low use of IL-6-RA in our trial patients.
In conclusion, dexamethasone 12 mg as compared with 6 mg did not result in statistically significant improvements in mortality or HRQoL at 180 days in patients with COVID-19 and severe hypoxaemia, but the results were most compatible with benefit from the higher dose.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank everyone involved in the COVID STEROID 2 trial (patients and relatives, research staff, clinical staff, investigators, funding sources, and regulatory authorities), and everyone involved in the first COVID STEROID trial [23], which served as the foundation for the COVID STEROID 2 trial.
Author contributions
AG and TL conducted all analyses presented in this manuscript, MBNK coordinated the follow-up and AP wrote the first draft, which was critically revised by all authors. MWM was the coordinating investigator of the COVID STEROID 2 trial. All authors contributed to the design and/or conduct of the trial. Detailed author contributions for the complete trial were presented in the primary trial report [4].
Funding
The Novo Nordisk Foundation and the Research Council of Rigshospitalet, Grant nos [0062998, E-22703-06]. The funders had no role in the design, conduct, analyses or reporting of the trial.
Declarations
Conflicts of interest
AG, MBNK, MWM, GKV, TSM, MHM and AP are affiliated with the Dept. of Intensive Care, Rigshospitalet, which has received grants for research from Pfizer, Fresenius Kabi, AM Pharma, and Sygeforsikringen ‘danmark’ outside the submitted work. MH has participated in advisory boards for AstraZeneca, GSK, Gilead, MSD, Roche and Sobi and received speaker’s honoraria from GSK and Gilead. TB reports grants from Novo Nordisk Foundation, grants from Simonsen Foundation, grants and personal fees from GSK, grants and personal fees from Pfizer, personal fees from Astra Zeneca, personal fees from Janssen, personal fees from Boehringer Ingelheim, grants and personal fees from Gilead, personal fees from MSD, grants from Lundbeck Foundation, grants from Kai Hansen Foundation, personal fees from Pentabase ApS, grants from Erik and Susanna Olesen’s Charitable Fund, outside the submitted work. SMJ and LC are affiliated with Inselspital, Bern University Hospital, which has received grants from Edwards Lifesciences Services GmbH, Phagenesis Limited, and Nestlé outside the submitted work. JVD has received personal fees (paid to his institution) from Edwards India outside the submitted work. VJ has received grant funding from GSK, Baxter Healthcare, and Biocon and honoraria from Boehringer Ingelheim, Astra Zeneca, Baxter Healthcare, Bayer, NephroPlus and Zydus Cadilla, under the policy of all monies being paid to the organization.
Ethical approval
The trial protocol was approved by the Ethics Committee of the Capital Region of Denmark with additional national/local approvals as required; additional details on approvals are presented in the protocol (ESM 1) [6].
Consent to participate
Consent was obtained from patients or legal surrogates according to applicable laws and regulations in the participating countries. Enrolment according to an emergency procedure (i.e., consent from a doctor independent of the trial followed by consent from relatives and/or patients) was allowed at many sites; additional details were presented in the primary report [4].
Consent for publication
All authors approved the manuscript for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Juni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Moller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poudel AN, Zhu S, Cooper N, Roderick P, Alwan N, Tarrant C, Ziauddeen N, Yao GL. Impact of COVID-19 on health-related quality of life of patients: a structured review. PLoS ONE. 2021;16:e0259164. doi: 10.1371/journal.pone.0259164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rochwerg B, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, Lytvyn L, Agarwal A, Leo YS, Macdonald H, Zeng L, Amin W, Burhan E, Bausch FJ, Calfee CS, Cecconi M, Chanda D, Du B, Geduld H, Gee P, Harley N, Hashimi M, Hunt B, Kabra SK, Kanda S, Kawano-Dourado L, Kim YJ, Kissoon N, Kwizera A, Mahaka I, Manai H, Mino G, Nsutebu E, Pshenichnaya N, Qadir N, Sabzwari S, Sarin R, Shankar-Hari M, Sharland M, Shen Y, Ranganathan SS, Souza JP, Stegemann M, De Sutter A, Ugarte S, Venkatapuram S, Dat VQ, Vuyiseka D, Wijewickrama A, Maguire B, Zeraatkar D, Bartoszko JJ, Ge L, Brignardello-Petersen R, Owen A, Guyatt G, Diaz J, Jacobs M, Vandvik PO. A living WHO guideline on drugs for COVID-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 4.Munch MW, Myatra SN, Vijayaraghavan BKT, Saseedharan S, Benfield T, Wahlin RR, Rasmussen BS, Andreasen AS, Poulsen LM, Cioccari L, Khan MS, Kapadia F, Divatia JV, Brochner AC, Bestle MH, Helleberg M, Michelsen J, Padmanaban A, Bose N, Moller A, Borawake K, Kristiansen KT, Shukla U, Chew MS, Dixit S, Ulrik CS, Amin PR, Chawla R, Wamberg CA, Shah MS, Darfelt IS, Jorgensen VL, Smitt M, Granholm A, Kjaer MN, Moller MH, Meyhoff TS, Vesterlund GK, Hammond NE, Micallef S, Bassi A, John O, Jha A, Cronhjort M, Jakob SM, Gluud C, Lange T, Kadam V, Marcussen KV, Hollenberg J, Hedman A, Nielsen H, Schjorring OL, Jensen MQ, Leistner JW, Jonassen TB, Kristensen CM, Clapp EC, Hjortso CJS, Jensen TS, Halstad LS, Bak ERB, Zaabalawi R, Metcalf-Clausen M, Abdi S, Hatley EV, Aksnes TS, Gleipner-Andersen E, Alarcon AF, Yamin G, Heymowski A, Berggren A, La Cour K, Weihe S, Pind AH, Engstrom J, Jha V, Venkatesh B, Perner A. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and Severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326:1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granholm A, Munch MW, Myatra SN, Vijayaraghavan BKT, Cronhjort M, Wahlin RR, Jakob SM, Cioccari L, Kjaer MN, Vesterlund GK, Meyhoff TS, Helleberg M, Moller MH, Benfield T, Venkatesh B, Hammond NE, Micallef S, Bassi A, John O, Jha V, Kristiansen KT, Ulrik CS, Jorgensen VL, Smitt M, Bestle MH, Andreasen AS, Poulsen LM, Rasmussen BS, Brochner AC, Strom T, Moller A, Khan MS, Padmanaban A, Divatia JV, Saseedharan S, Borawake K, Kapadia F, Dixit S, Chawla R, Shukla U, Amin P, Chew MS, Wamberg CA, Gluud C, Lange T, Perner A. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022;48:45–55. doi: 10.1007/s00134-021-06573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munch MW, Granholm A, Myatra SN, Kumar Tirupakuzhi Vijayaraghavan B, Cronhjort M, Rubenson Wahlin R, Jakob SM, Cioccari L, Norregaard Kjaer MB, Kingo Vesterlund G, Sylvest Meyhoff T, Helleberg M, Hylander Moller M, Benfield T, Venkatesh B, Hammond N, Micallef S, Bassi A, John O, Jha V, Tjelle Kristiansen K, Suppli Ulrik C, Lind Jorgensen V, Smitt M, Bestle MH, Sofie Andreasen A, Musaeus Poulsen L, Steen Rasmussen B, Craveiro Brochner A, Strom T, Moller A, Saif Khan M, Padmanaban A, Vasishtha Divatia J, Saseedharan S, Borawake K, Kapadia F, Dixit S, Chawla R, Shukla U, Amin P, Chew MS, Gluud C, Lange T, Perner A. Higher vs lower doses of dexamethasone in patients with COVID-19 and severe hypoxia (COVID STEROID 2) trial: protocol and statistical analysis plan. Acta Anaesthesiol Scand. 2021;65:834–845. doi: 10.1111/aas.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen CE, Sorensen SS, Gudex C, Jensen MB, Pedersen KM, Ehlers LH. The Danish EQ-5D-5L value set: a hybrid model using cTTO and DCE data. Appl Health Econ Health Policy. 2021;19:579–591. doi: 10.1007/s40258-021-00639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jyani G, Prinja S, Kar SS, Trivedi M, Patro B, Purba F, Pala S, Raman S, Sharma A, Jain S, Kaur M. Valuing health-related quality of life among the Indian population: a protocol for the development of an EQ-5D value set for India using an extended design (DEVINE) Study. BMJ Open. 2020;10:e039517. doi: 10.1136/bmjopen-2020-039517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burstrom K, Teni FS, Gerdtham UG, Leidl R, Helgesson G, Rolfson O, Henriksson M. Experience-based Swedish TTO and VAS value sets for EQ-5D-5L health states. Pharmacoeconomics. 2020;38:839–856. doi: 10.1007/s40273-020-00905-7. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig K, Graf von der Schulenburg JM, Greiner W. German Value Set for the EQ-5D-5L. Pharmacoeconomics. 2018;36:663–674. doi: 10.1007/s40273-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devlin N, Parkin D, Janssen B. Methods for analysing and reporting EQ-5D data. Cham: Springer; 2020. [PubMed] [Google Scholar]
- 13.Jensen AK, Lange T (2019) A novel high-power test for continuous outcomes truncated by death. arXiv:1910.12267
- 14.Taboada M, Rodriguez N, Varela PM, Rodriguez MT, Abelleira R, Gonzalez A, Casal A, Diaz Peromingo JA, Lama A, Dominguez MJ, Rabade C, Paez EM, Riveiro V, Pernas H, Del Carmen BM, Caruezo V, Naveira A, Carinena A, Cabaleiro T, Estany-Gestal A, Zarra I, Pose A, Valdes L, Alvarez-Escudero J. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 Pneumonia: an open-label, randomised clinical trial. Eur Respir J. 2021 doi: 10.1183/13993003.02518-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maskin LP, Bonelli I, Olarte GL, Palizas F, Jr, Velo AE, Lurbet MF, Lovazzano P, Kotsias S, Attie S, Lopez Saubidet I, Baredes ND, Setten M, Rodriguez PO. High- versus low-dose dexamethasone for the treatment of covid-19-related acute respiratory distress syndrome: a multicenter, randomized open-label clinical trial. J Intensive Care Med. 2021 doi: 10.1177/08850666211066799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McPeake J, Shaw M, MacTavish P, Blyth KG, Devine H, Fleming G, Griffin J, Gemmell L, Grose P, Henderson M, Henderson P, Hogg L, King K, McInnes I, O'Brien P, Puxty K, Rainey C, Sharma V, Sim M, Strachan L, Siebert S, Quasim T. Long-term outcomes following severe COVID-19 infection: a propensity matched cohort study. BMJ Open Respir Res. 2021;8:e001080. doi: 10.1136/bmjresp-2021-001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond NE, Finfer SR, Li Q, Taylor C, Cohen J, Arabi Y, Bellomo R, Billot L, Harward M, Joyce C, McArthur C, Myburgh J, Perner A, Rajbhandari D, Rhodes A, Thompson K, Webb S, Venkatesh B, Investigators AT, the A, New Zealand Intensive Care Society Clinical Trials G Health-related quality of life in survivors of septic shock: 6-month follow-up from the ADRENAL trial. Intensive Care Med. 2020;46:1696–1706. doi: 10.1007/s00134-020-06169-1. [DOI] [PubMed] [Google Scholar]
- 18.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colantuoni E, Li X, Hashem MD, Girard TD, Scharfstein DO, Needham DM. A structured methodology review showed analyses of functional outcomes are frequently limited to "survivors only" in trials enrolling patients at high risk of death. J Clin Epidemiol. 2021;137:126–132. doi: 10.1016/j.jclinepi.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Møller MH, Munch MW, Granholm A, Svobodnik A, Savović J, Rodriguez PO, Khalili H, Maláska J, Stašek J, Kratochvíl M, Muñiz MT, Jha V, Vijayaraghavan BKT, Myatra SN, Villar J, Venkatesh B, Perner A (2021) Higher vs. standard doses of dexamethasone in patients with COVID-19 and hypoxia: a prospective meta-analysis. Open Source Framework, Published May 31, 2021. https://osf.io/fr5sv. Accessed January 28, 2022
- 22.(2021) RECOVERY Trial to investigate whether higher doses of dexamethasone deliver greater benefit for patients with severe COVID-19 Published December 30, 2021. https://www.recoverytrial.net/news/recovery-trial-to-investigate-whether-higher-doses-of-dexamethasone-deliver-greater-benefit-for-patients-with-severe-covid-19. Accessed January 28, 2022
- 23.Munch MW, Meyhoff TS, Helleberg M, Kjaer MN, Granholm A, Hjortso CJS, Jensen TS, Moller MH, Hjortrup PB, Wetterslev M, Vesterlund GK, Russell L, Jorgensen VL, Tjelle Kristiansen K, Benfield T, Ulrik CS, Andreasen AS, Bestle MH, Poulsen LM, Hildebrandt T, Knudsen LS, Moller A, Solling CG, Brochner AC, Rasmussen BS, Nielsen H, Christensen S, Strom T, Cronhjort M, Wahlin RR, Jakob SM, Cioccari L, Venkatesh B, Hammond N, Jha V, Myatra SN, Jensen MQ, Leistner JW, Mikkelsen VS, Svenningsen JS, Laursen SB, Hatley EV, Kristensen CM, Al-Alak A, Clapp E, Jonassen TB, Bjerregaard CL, Osterby NCH, Jespersen MM, Abou-Kassem D, Lassen ML, Zaabalawi R, Daoud MM, Abdi S, Meier N, la Cour K, Derby CB, Damlund BR, Laigaard J, Andersen LL, Mikkelsen J, Jensen JLS, Rasmussen AH, Arnerlov E, Lykke M, Holst-Hansen MZB, Tostesen BW, Schwab J, Madsen EK, Gluud C, Lange T, Perner A. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: the COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol Scand. 2021;65:1421–1430. doi: 10.1111/aas.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.