Abstract
The in vitro activity of BMS-207147 against 80 clinical isolates of Aspergillus was compared with that of itraconazole and amphotericin B, using a validated microtiter method. Geometric mean MICs (in μg/ml) were as follows: 1.71 for BMS-207147, 0.67 for itraconazole, and 0.63 for amphotericin B. The range of concentrations of each drug was 0.125 to >16 μg/ml. Aspergillus fumigatus was significantly more susceptible to BMS-207147 (P < 0.05) than A. terreus and A. flavus. No BMS-207147-resistant A. fumigatus isolates were identified, though eight itraconazole-resistant (MIC, >8 μg/ml) isolates were. BMS-207147 is active against Aspergillus spp. at slightly high concentrations compared with itraconazole and amphotericin B.
Aspergillus infections are the most prevalent non-Candida fungal infections, causing 70% of such infections in bone marrow transplantation patients in a recent study (10). Estimates of prevalence in different immunocompromised patient groups range from 25 to 40% in patients with chronic granulomatous disease, from 20 to 25% in lung transplant and high risk leukemia patients, and as low as 1% in patients with systemic lupus erythematosus (2). Other patient risk groups include cancer patients, solid organ transplant recipients, burn patients, and AIDS patients. The intravenous treatment of choice is amphotericin B, although the use of this drug is problematic, with many toxicity problems and a high failure rate (4). However, newer, less-toxic formulations of this drug are now available. Itraconazole therapy is also problematic. The drug can be difficult to absorb, although the oral solution and possibly the new intravenous formulation may help address this issue. There is therefore a need to develop new, safe, effective antifungal agents.
BMS-207147 was originally discovered by Easai Co in Tsukuba, Japan, and called ER-30346 (6, 7). Structurally, it has more in common with fluconazole and voriconazole than the other two azoles active against Aspergillus available for clinical use, itraconazole and SCH 56592 (http://www.aspergillus.man.ac.uk/secure/images/antifungaldrugs.htm). It has potent activity against Candida albicans (5, 7, 14) and non-albicans Candida spp. although it is somewhat less active against Candida glabrata (5, 14) and Candida tropicalis (5). It has good activity against Cryptococcus neoforms (5, 6). Activity against Aspergillus has been reported in vitro (5; A. Espinell-Ingroff, A. Palacio, and A. Carillo-Munoz, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-154, 1998) as well as in vivo (K. Shock, S. Marino, T. Baumgartner, and V. Andriole, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-54, 1998).
In this study, we directly compared the in vitro antifungal activity of BMS-207147 with that of itraconazole and amphotericin B against four different species of Aspergillus. We selected a population of isolates with a disproportionate number resistant to itraconazole. In addition, we examined the fungicidal activity of BMS-207147 and compared it directly with that of itraconazole and amphotericin B.
A total of 80 clinical Aspergillus isolates comprising 50 Aspergillus fumigatus isolates and 10 isolates each of Aspergillus terreus, Aspergillus flavus, and Aspergillus niger were used in this study. Almost all the isolates were from the United States and the United Kingdom, with the greater proportion of itraconazole-resistant isolates coming from the United Kingdom. Included in the test panel were isolates AF65, AF91, AF210, and AF294, for which the MICs of amphotericin B and itraconazole are known (3, 8, 12). All cultures were cultivated from frozen stock on Sabouraud dextrose agar (Oxoid, Basingstoke, United Kingdom) for 3 to 4 days at 30°C.
BMS-207147 (Bristol-Myers Squibb Company, Princeton, New Jersey) and itraconazole (Janssen Pharmaceuticals, Beerse, Belgium) were both provided in pure powder form by their respective manufacturers. Amphotericin B with desoxycholate was obtained from E. R. Squibb & Sons Limited, Middlesex, United Kingdom.
BMS-207147 was dissolved in dimethyl sulfoxide, with the weight adjusted to allow for the potency of the drug, to produce a stock solution of 3,200 μg/ml. Itraconazole was suspended in 1:1 acetone and 0.2 M HCl to produce a final concentration of 3,200 μg/ml. Amphotericin B with desoxycholate was dissolved in sterile distilled water to produce a stock solution of 3,200 μg/ml, after adjusting for potency. All drugs were then dispensed into aliquots and stored in glass vials, protected from the light, at −20°C until required.
Testing for MICs was performed by a microtiter method with RPMI 1640 medium (Sigma, Poole, United Kingdom) supplemented with 2% glucose and buffered to a pH of 7.0 with morpholinepropanesulfonic acid (MOPS; Sigma). The entire study, including reproducibility, was performed using the same batch of RPMI 1640 medium. Drug concentrations ranged from 0.03 to 16 μg/ml for all antifungal agents.
The inoculum was prepared by suspending Aspergillus conidia in phosphate-buffered saline containing 0.05% Tween 80. The conidia were counted using a hemocytometer and then diluted into the growth medium to a concentration of 106 conidia/ml. The final inoculum was 5 × 105 conidia/ml (as has been previously validated) (3, 12). A positive control (drug free) was included for each isolate. Microtiter trays were incubated in moist chambers at 37°C for 48 h. The MICs were read visually and were defined using a no-growth end point. Prior animal model work has demonstrated itraconazole MICs of ≥16 μg/ml correlate with failure of itraconazole in vivo (3, 12). A correlation with outcome was not demonstrable for amphotericin B MICs (8), although A. terreus isolates are usually resistant in vitro and may be so in vivo (8).
Minimum fungicidal concentrations (MFCs) of all drugs were also determined. A 100-μl volume was removed from every well containing no growth and transferred to a blood agar plate. The sample was allowed to soak into the agar and, when dry, was streaked to separate any conidia present and to remove them from the drug source. The plates were incubated at 37°C for 48 h. The MFC was defined as the lowest drug concentration that allowed the growth of five or fewer colonies (≥99.99% killed). MICs or MFCs of >16 μg/ml for itraconazole were assumed to be 32 μg/ml for the purpose of analysis. Some of the isolates (16 of 80 isolates [20%]) were randomly selected and retested to assess the reproducibility of the susceptibility tests.
The MIC results for all 80 isolates for all three drugs are summarized in Table 1. The MIC ranges of all the drugs were the same, 0.125 to >16 μg/ml. Ten isolates were resistant to itraconazole (MIC, ≥16 μg/ml), and these were slightly less susceptible to BMS-207147 over a wide range of concentrations (0.125 to 32 μg/ml). The geometric mean (GM) MICs for all isolates and the MICs at which 90% of the isolates tested were inhibited were, respectively, as follows: BMS-207147, 1.71 and 4 μg/ml; itraconazole, 0.67 and >16 μg/ml; and amphotericin B, 0.63 and 4 μg/ml.
TABLE 1.
GM MICs and MIC ranges of BMS 207147, itraconazole, and amphotericin B for each species
| Species or isolate type (no. of isolates tested) | GM (range) MICa
|
||
|---|---|---|---|
| BMS-207147 | Itraconazole | Amphotericin B | |
| A. fumigatus (50) | 1.27 (0.13–8) | 0.77 (0.13–>16) | 0.38 (0.13–0.5) |
| A. terreus (10) | 3.03 (2–4) | 0.33 (0.25–0.5) | 4.0 (2–8) |
| A. flavus (10) | 2.83 (1–32) | 0.5 (0.25–>16) | 2.46 (0.5–32) |
| A. niger (10) | 2.64 (1–8) | 0.93 (0.5–4) | 0.29 (0.13–0.5) |
| Itraconazole resistant (10) | 5.9 (0.13–32) | >16 | 2.3 (0.25–32) |
| All | 1.71 (0.13–32) | 0.67 (0.13–>16) | 0.63 (0.13–32) |
Values are in micrograms per milliliter.
Isolates of A. fumigatus (n = 50) were found to be significantly more susceptible to BMS-207147 than isolates of A. terreus (n = 10) and A. flavus (n = 10) (P < 0.05). Isolates of A. niger (n = 10) were also found to be significantly more susceptible to BMS-207147 than isolates of A. flavus (P < 0.05). The activity of each drug against each species is shown in Table 1. Only one isolate was resistant to BMS-207147, an A. flavus isolate for which the MICs of all three drugs were >16 μg/ml. This isolate has a trailing end point.
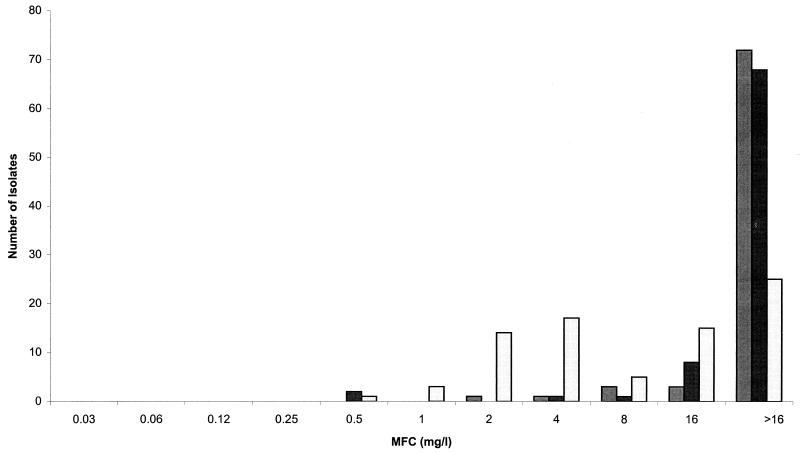
The MFCs are depicted in Fig. 1. The GM-MFCs (range of MFCs) of each drug were as follows (in μg/ml): BMS-207147, 27.9 (2 to >16); itraconazole, 25.8 (0.5 to >16); and amphotericin B, 8.5 (0.5 to >16). For all isolates, BMS-207147 was fungicidal against 10% of the isolates, itraconazole was fungicidal against 15% of the isolates, and amphotericin B was fungicidal against 69% of the isolates at concentrations of ≤16 μg/ml, using the stringent cutoff of 99.99% fungicidality.
FIG. 1.
MFC values for BMS-207147 (light grey bars) compared with those for itraconazole (dark grey bars) and amphotericin B (unshaded bars).
Reproducibility studies showed that for BMS-207147 and itraconazole, 15 of 16 (94%) isolates retested, and for amphotericin B, 14 of 16 (88%) isolates retested, produced either identical MIC results or yielded a result that differed by only one twofold dilution.
Industry studies with BMS-207147 have shown good activity against Aspergillus, with MICs for 16 isolates varying from 0.06 to 2 μg/ml (5) and MICs for 3 isolates varying from 0.2 to 0.39 μg/ml (6). Our data from a much larger collection of isolates from four pathogenic species confirms activity, but at concentrations slightly higher than those observed with itraconazole. Although the data sets are not exactly the same, the GM MICs for SCH 56592 (11), itraconazole, voriconazole (13), and BMS-207147 were 0.09, 0.25, 0.4, and 1.71 μg/ml, respectively. We identified a degree of cross-resistance in A. fumigatus between itraconazole and SCH 56592 (confirmed in an animal model [11]) but not between itraconazole and either BMS-207147 or voriconazole in vitro. This is potentially important, as the incidence of itraconazole resistance in A. fumigatus in the United Kingdom is approximately 5% (unpublished data). The lack of cross-resistance of BMS-207147 with itraconazole probably reflects the substantial structural differences between the two molecules.
In aspergillosis, many factors determine therapeutic outcome. Aside from speed of diagnosis, pace and extent of disease at diagnosis, and underlying patient factors, the relative activity of the antifungal drug and drug exposure is probably important. With respect to the azoles, limited experimental data are in support of there being a pharmacodynamic relationship which determines outcome for azole therapy (1, 9, 11). BMS-207147 is remarkable amongst the azoles available or in development in that it has a long half-life in volunteers, 83 to 145 h (Investigator's Brochure, BMS-207147, Bristol-Myers Squibb, Princeton, N.J.). This compares with approximately 32 to 96 h for itraconazole (http://www.aspergillus.man.ac.uk/secure/treatment/antifungaldrugs/itraconazole.htm), 19 to 31 h for SCH 56592 (M. Laughlin, S. Pai, S. Menon, A. Nomeir, R. Colucci, M. Affrime, and T. Kosoglou, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-87, 1997), and 6 to 20 h for voriconazole (T. F. Patterson, http://www.aspergillus.man.ac.uk). The in vitro activity of BMS-207147 which is slightly lower than that of the other azoles with activity against Aspergillus spp. could well be compensated for by a better pharmacodynamic profile. Careful animal model work could address this, as could clinical studies in more chronic types of Aspergillus infection.
REFERENCES
- 1.Berenger J, Ali N M, Allende M C, Lee J, Garrett K, Battaglia S, Piscitelli S C, Rinaldi M G, Pizzo P A, Walsh T J. Itraconazole for experimental pulmonary aspergillosis—comparison with amphotericin-B, interaction with cyclosporin-A, and correlation between therapeutic response and itraconazole concentrations in plasma. Antimicrob Agents Chemother. 1994;38:1303–1308. doi: 10.1128/aac.38.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denning D W. Invasive aspergillosis in immunocompromised patients. Curr Opin Infect Dis. 1994;7:456–462. [Google Scholar]
- 3.Denning D W, Radford S A, Oakley K, Hall L, Johnson E M, Warnock D W. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome for Aspergillus fumigatus infection. J Antimicrob Chemother. 1997;40:401–414. doi: 10.1093/jac/40.3.401. [DOI] [PubMed] [Google Scholar]
- 4.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 5.Fung-Tomc J C, Huczko E, Minassian B, Bonner D P. In vitro activity of a new oral triazole, BMS-207147 (ER-30346) Antimicrob Agents Chemother. 1998;42:313–318. doi: 10.1128/aac.42.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hata K, Kimura J, Miki H, Toyosawa T, Moriyama M, Katsu K. Efficacy of ER-30346, a novel oral triazole antifungal agent, in experimental models of aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1996;40:2243–2247. doi: 10.1128/aac.40.10.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hata K, Kimura J, Miki H, Toyosawa T, Nakamura T, Katsu K. In vitro and in vivo antifungal activities of ER-30346, a novel oral triazole with a broad antifungal spectrum. Antimicrob Agents Chemother. 1996;40:2237–2242. doi: 10.1128/aac.40.10.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, E., K. L. Oakley, S. Radford, C. B. Moore, P. Warn, D. W. Warnock, and D. W. Denning. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J. Antimicrob. Chemother., in press. [DOI] [PubMed]
- 9.Martin M V, Yates J, Hitchcock C A. Comparison of voriconazole (UK-109,496) and itraconazole in prevention and treatment of Aspergillus fumigatus endocarditis in guinea pigs. Antimicrob Agents Chemother. 1997;41:13–16. doi: 10.1128/aac.41.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison V A, Haake R J, Weisdorf D J. Non-Candida fungal infections after bone marrow transplantation: risk factors and outcome. Am J Med. 1994;96:497–503. doi: 10.1016/0002-9343(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 11.Oakley K L, Moore C B, Denning D W. In vitro activity of SCH-56592 and comparison with amphotericin B and itraconazole against Aspergillus spp. Antimicrob Agents Chemother. 1997;41:1124–1126. doi: 10.1128/aac.41.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oakley K L, Moore C B, Denning D W. The in vitro activity of voriconazole against Aspergillus spp. and comparison with amphotericin B and itraconazole. J Antimicrob Chemother. 1998;42:91–94. doi: 10.1093/jac/42.1.91. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller M A, Messer D A, Hollis R J, Lones R N, Doern G V, Brandt M E, Hajjeh R A. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-207147, SCH 56592, and voriconazole. Antimicrob Agents Chemother. 1998;42:3242–3244. doi: 10.1128/aac.42.12.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]