Abstract
The β-lactamases from 403 Moraxella (Branhamella) catarrhalis clinical isolates obtained during 1994–1995 and 1997–1998 U.S. multicenter surveillance studies were characterized by isoelectric focusing. The overall prevalences of the BRO-1 and BRO-2 enzymes among β-lactamase-positive isolates were estimated to be 97.5 and 2.5%, respectively. The minimum inhibitory concentrations (MICs) of ampicillin for all BRO-2-producing isolates were ≤1 μg/ml; however, numerous β-lactamase-positive isolates for which the ampicillin MICs were ≤1 μg/ml produced the BRO-1 enzyme (88.1%).
Isoelectric focusing (IEF) of β-lactamases extracted from Moraxella (Branhamella) catarrhalis typically reveals one of two different banding patterns; these have been referred to as Ravasio (BRO-1) and 1908 (BRO-2) (7, 15). The BRO-1 pattern has been the most prevalent, accounting for 87 to 95% of M. catarrhalis β-lactamases characterized in the United States, France, Denmark, Sweden, England, and Scotland, with the remaining 5 to 13% identified as BRO-2 (2, 3, 5, 6, 8, 11, 13).
Although substrate hydrolysis rates of BRO-1 and BRO-2 enzymes are similar, BRO-1-producing isolates are more resistant to penicillins than BRO-2 strains (8, 10, 15). The higher β-lactam minimum inhibitory concentrations (MICs) associated with BRO-1 strains have been attributed to larger amounts of enzyme produced (15).
The purpose of this study was to characterize the β-lactamases of M. catarrhalis isolates collected during 1994–1995 and 1997–1998 U.S. multicenter surveillance studies. Enzyme type was determined by IEF, and the relationship to ampicillin susceptibility was examined.
Collection and identification of isolates.
Between 1 November 1994 and 30 April 1995, 606 clinical isolates of M. catarrhalis were obtained from 24 U.S. health care institutions. Between 1 November 1997 and 30 April 1998, 513 additional clinical isolates of M. catarrhalis were obtained from the same 24 centers. Stock cultures of all isolates were prepared by using an absorbent bead system (Prolab Diagnostics, Austin, Tex.), and organisms were stored at −70°C until further use. The identity of the organisms was confirmed using conventional criteria (9). The details of these two studies and antimicrobial susceptibility patterns of the isolates collected have been described previously (4, 14).
β-Lactamase production and susceptibility testing.
β-Lactamase production was assessed with a nitrocefin disk assay (Cefinase; Becton-Dickinson Microbiology Systems, Cockeysville, Md.). MICs of ampicillin were determined by a broth microdilution procedure (total volume per well, 100 μl; final inoculum concentration, ca. 5 × 105 CFU/ml) in cation-adjusted Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) according to the National Committee for Clinical Laboratory Standards (NCCLS) guidelines (12). Microdilution trays were incubated at 35°C in ambient air for 22 to 24 h prior to the determination of the results. Ampicillin was tested at 12 different concentrations (from 0.015 to 32 μg/ml) to limit the number of off-scale results. Staphylococcus aureus ATCC 29213 and Escherichia coli ATCC 25922 were used as controls. The lowest concentration of antimicrobial tested that resulted in no visible growth was defined as the MIC.
Selection of strains for IEF.
A total of 403 isolates were selected for enzyme extraction and IEF on the basis of β-lactamase production and ampicillin MIC (202 from the 1994–1995 collection and 201 from the 1997–1998 collection). All of the β-lactamase-positive isolates in these collections for which ampicillin MICs were ≤1 μg/ml and ≥32 μg/ml were focused. Among isolates for which MICs were 2 μg/ml, 48 of 113 isolates from the 1994–1995 study and 53 of 83 strains from the 1997–1998 study were analyzed by IEF. For each of the remaining MIC categories (4 μg/ml, 8 μg/ml, and 16 μg/ml), 10 or 11 strains from each collection were analyzed.
β-Lactamase extraction.
β-Lactamase extraction was accomplished by a modification of the acetone method as described by Nash and colleagues (11). The isolates were subcultured twice on 5% sheep blood agar, suspended in 0.05 M phosphate-buffered saline, and centrifuged for 15 min at 2,000 × g. The pellets were washed three times in 10 ml of cold acetone and dried for 24 h at 4°C. The pellets were resuspended in 0.5 ml of 1% glycine with 0.025% sodium azide and held for 4 to 5 days at 4°C. Samples were centrifuged for 20 min, and the supernatant was immediately focused or stored at −70°C for up to 1 week prior to IEF analysis.
IEF.
IEF of the enzymes was performed using commercially prepared polyacrylamide gels with a pH range of 5.5 to 8.5 according to the manufacturer's instructions (Ampholine PAGplate; Pharmacia Biotech, Uppsala, Sweden). β-Lactamase staining was accomplished by flooding the gels with nitrocefin (500 μg/ml in 0.05 M phosphate-buffered saline). Control β-lactamases (TEM-4, SHV-1, and SHV-3) with known isoelectric points (pIs) and extracts from control strains of M. catarrhalis known to produce the BRO-1 and BRO-2 β-lactamases were included on each gel.
Among the 606 M. catarrhalis isolates collected during the 1994–1995 surveillance study, 576 (95%) were β-lactamase positive (4). The same prevalence of β-lactamase production was observed with isolates from the 1997–1998 survey (i.e., 487 of 513 isolates were β-lactamase positive) (14). A total of 202 β-lactamase-positive isolates from the 1994–1995 study and 201 β-lactamase-positive isolates from the 1997–1998 study were chosen for characterization by IEF (Table 1). These included all isolates from both collections for which the MICs were ≤1 μg/ml, 42 to 64% of isolates for which the MICs was 2 μg/ml, 10 or 11 isolates each from organisms for which the MIC was 4, 8, or 16 μg/ml, and all isolates for which the MICs were ≥32 μg/ml.
TABLE 1.
Characterization of β-lactamases of M. catarrhalis isolates obtained during multicenter U.S. national surveillance studies conducted from 1994 to 1995 and from 1997 to 1998
| Study time and ampicillin MIC (μg/ml) | Total no. (%) of β-lactamase-positive isolates in study | No. of isolates characterized by IEF | No. (%) of BRO-1-producing isolatesa | No. (%) of BRO-2-producing isolatesa |
|---|---|---|---|---|
| 1994 to 1995 | ||||
| 0.06 | 2 (0.3) | 2 | 1 (50.0) | 1 (50.0) |
| 0.12 | 6 (1.0) | 6 | 1 (16.7) | 5 (83.3) |
| 0.25 | 21 (3.6) | 21 | 19 (90.5) | 2 (9.5) |
| 0.5 | 27 (4.7) | 27 | 24 (88.9) | 3 (11.1) |
| 1 | 60 (10.4) | 60 | 59 (98.3) | 1 (1.7) |
| 2 | 113 (19.6) | 48 | 48 (100) | |
| 4 | 162 (28.1) | 10 | 10 (100) | |
| 8 | 118 (20.5) | 10 | 10 (100) | |
| 16 | 59 (10.2) | 10 | 10 (100) | |
| 32 | 7 (1.2) | 7 | 7 (100) | |
| >32 | 1 (0.2) | 1 | 1 (100) | |
| Total | 576 | 202 | 190 | 12 |
| 1997 to 1998 | ||||
| 0.06 | 5 (1.0) | 5 | 5 (100) | |
| 0.12 | 8 (1.6) | 8 | 2 (25.0) | 6 (75.0) |
| 0.25 | 9 (1.8) | 9 | 8 (88.9) | 1 (11.1) |
| 0.5 | 42 (8.6) | 42 | 41 (97.6) | 1 (2.4) |
| 1 | 46 (9.4) | 46 | 44 (95.7) | 2 (4.3) |
| 2 | 83 (17.0) | 53 | 53 (100) | |
| 4 | 156 (32.0) | 10 | 10 (100) | |
| 8 | 94 (19.3) | 11 | 11 (100) | |
| 16 | 37 (7.6) | 10 | 10 (100) | |
| 32 | 7 (1.4) | 7 | 7 (100) | |
| >32 | ||||
| Total | 487 | 201 | 186 | 15 |
Isolates characterized by IEF which revealed the BRO-1 or BRO-2 profile.
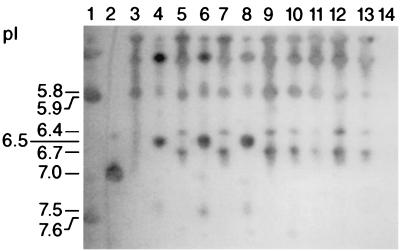
The banding patterns observed are illustrated in Fig. 1. The BRO-1 β-lactamase was identified by unique major bands with pIs of 6.4 and 6.7. The BRO-2 enzyme demonstrated unique bands at pIs of 6.5 (major) and 7.5 (minor). Many common bands at pIs of 5.8 and less were seen with both BRO-1- and BRO-2-producing organisms, as has been previously reported for reference strains (5, 11). All of the β-lactamase-positive isolates focused revealed either the BRO-1 or BRO-2 banding pattern. No bands were seen in the lanes of the three β-lactamase-negative isolates focused.
FIG. 1.
Lane 1, TEM-4 (pI = 5.9) and SHV-1 (pI = 7.6) β-lactamases; lane 2, SHV-3 (pI = 7.0); lanes 3 and 4, Ravasio (BRO-1) and 1908 (BRO-2) control strains, respectively; lanes 5 and 7 and 9 to 13, clinical isolates showing BRO-1 banding patterns; lanes 6 and 8, clinical strains showing BRO-2 banding patterns; lane 14, a β-lactamase-negative isolate.
As can be seen in Table 1, the ampicillin MICs for large numbers of β-lactamase-positive isolates of M. catarrhalis from both 1994–1995 and 1997–1998 were very low. Indeed, the ampicillin MICs for 116 of 576 (20.1%) 1994–1995 isolates and 110 of 487 (22.6%) isolates from the 1997–1998 study were ≤1 μg/ml; these isolates would thus have been classified as being susceptible to ampicillin according to MIC interpretive criteria presently advocated for use by the NCCLS with Haemophilus influenzae (nota bene: presently, there are no NCCLS MIC interpretive criteria for tests with M. catarrhalis).
The relative frequency of production of the BRO-1 and BRO-2 enzymes together with the relationship between ampicillin MICs and the production of a specific enzyme is depicted in Table 1. It is apparent from Table 1 that all tested isolates from both collections for which ampicillin MICs were ≥2 μg/ml produced the BRO-1 β-lactamase. The ampicillin MICs for BRO-2-producing strains were always ≤1 μg/ml; however, most isolates for which the MICs were this low (i.e., 104 of 116 [89.7%] isolates from the 1994–1995 study and 95 of 110 [86.4%] isolates from the 1997–1998 study) produced the BRO-1 enzyme. Among the isolates for which ampicillin MICs were low, the likelihood of an organism producing the BRO-2 enzyme was greatest as ampicillin MICs dropped to 0.12 and 0.06 μg/ml.
The only prior U.S. study of this type identified 8% of 146 M. catarrhalis β-lactamases from 1982–1983 as BRO-2 (11). Based on the results of this study, we estimate that 2.1 and 3.1% of β-lactamase-producing isolates of M. catarrhalis from the United States in 1994–1995 and 1997–1998, respectively, produced the BRO-2 β-lactamase. Despite the fact that not all β-lactamase-positive isolates were characterized in this study, it is likely that the remaining strains produced the BRO-1 enzyme.
Our study showed the same general trend of higher ampicillin resistance among BRO-1-producing strains reported previously (8, 10, 15); however, the wide range of ampicillin MICs observed for BRO-1 strains was unexpected and suggests that factors other than enzyme type determine antibiotic susceptibility.
Bootsma et al. reported a 21-bp deletion in the promoter region of the M. catarrhalis β-lactamase gene (bla) from a BRO-2-producing strain as a possible cause of the lower level of enzyme production (1). Sequencing the bla gene and regulatory sequences from a greater number of BRO-1- and BRO-2-producing isolates showing a range of ampicillin resistance may reveal additional mutations or deletions to explain the variation in antibiotic susceptibility observed in our study.
Acknowledgments
We are indebted to the following individuals for provision of clinical isolates: Carla Clausen, Children's Hospital (Seattle, Wash.); Susan Rossmann, Texas Children's Hospital (Houston); Paul Southern, University of Texas Southwestern Medical Center (Dallas); Michael Wilson, Denver General Hospital (Denver, Colo.); Michael Saubolle, Good Samaritan Medical Center (Phoenix, Ariz.); John Washington, Cleveland Clinic (Cleveland, Ohio); Michael Dunne, Henry Ford Hospital (Detroit, Mich.); Gerald Denys, Methodist Hospital (Indianapolis, Ind.); Melodie Beard, Rush-Presbyterian St. Luke's Medical Center (Chicago, Ill.); Richard Thompson, Evanston Hospital (Evanston, Ill.); Susan Kehl, Children's Hospital (Milwaukee, Wis.); Franklin Cockerill, Mayo Clinic (Rochester, Minn.); Patrick Murray, Barnes-Jewish Hospital (St. Louis, Mo.); Ann Robinson, Hartford Hospital (Hartford, Conn.); Betty Forbes, SUNY Health Sciences Center (Syracuse, N.Y.); Dwight Hardy, University of Rochester Medical Center (Rochester, N.Y.); Phyllis Della-Latta, Presbyterian Hospital (New York, N.Y.); Allan Truant, Temple University Hospital (Philadelphia, Pa.); Joseph Campos, National Children's Hospital (Washington, D.C.); Paul Bourbeau, Geisenger Medical Center (Danville, Pa.); Peter Gilligan, University of North Carolina Hospital (Chapel Hill); Robert Jerris, Dekalb General Hospital (Decatur, Ga.); Kim Chapin-Robertson, University of South Alabama Medical Center (Mobile); and Susan Sharp, Mount Sinai Medical Center (Miami Beach, Fla.).
REFERENCES
- 1.Bootsma H J, van Duk H, Verhoef J, Fleer A, Mooi F R. Molecular characterization of the BRO β-lactamase of Moraxella (Branhamella) catarrhalis. Antimicrob Agents Chemother. 1996;40:966–972. doi: 10.1128/aac.40.4.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chäibi E B, Mugnier P, Kitzis M D, Goldstein F W, Acar J F. β-lactamases de Branhamella catarrhalis et leurs implications phénotypiques. Res Microbiol. 1995;146:761–771. doi: 10.1016/0923-2508(96)81072-2. [DOI] [PubMed] [Google Scholar]
- 3.Christensen J J, Keiding J, Bruun B. Antimicrobial susceptibility and β-Lactamase characterization of Branhamella catarrhalis isolates from 1983/1984 and 1988. APMIS. 1990;98:1039–1044. [PubMed] [Google Scholar]
- 4.Doern G V, Brueggemann A B, Pierce G, Hogan T, Holley H P, Rauch A. Prevalence of antimicrobial resistance among 723 outpatient clinical isolates of Moraxella catarrhalis in the United States in 1994 and 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:2884–2886. doi: 10.1128/aac.40.12.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ejlertsen T J, Skov R. The β-lactamases of Moraxella (Branhamella) catarrhalis from Danish children. APMIS. 1996;104:557–562. doi: 10.1111/j.1699-0463.1996.tb04911.x. [DOI] [PubMed] [Google Scholar]
- 6.Eliasson I, Kamme C, Vang M, Waley S G. Characterization of cell bound papain-soluble beta-lactamases in BRO-1 and BRO-2 producing strains of Moraxella (Branhamella) catarrhalis and Moraxella nonliquefaciens. Eur J Clin Microbiol Infect Dis. 1992;11:313–321. doi: 10.1007/BF01962070. [DOI] [PubMed] [Google Scholar]
- 7.Farmer T, Reading C. β-lactamases of Branhamella catarrhalis and their inhibition by clavulanic acid. Antimicrob Agents Chemother. 1982;21:506–508. doi: 10.1128/aac.21.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung C P, Yeo S, Livermore D M. Susceptibility of Moraxella catarrhalis isolates to β-lactam antibiotics in relation to β-lactamase pattern. J Antimicrob Chemother. 1994;33:215–222. doi: 10.1093/jac/33.2.215. [DOI] [PubMed] [Google Scholar]
- 9.Knapp J S, Rice R J. Neisseria and Branhamella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: ASM Press; 1995. pp. 324–340. [Google Scholar]
- 10.Luman I, Wilson R W, Wallace R J, Jr, Nash D R. Disk diffusion susceptibility of Branhamella catarrhalis and relationship to β-lactamase production. Antimicrob Agents Chemother. 1986;30:774–776. doi: 10.1128/aac.30.5.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nash D R, Wallace R J, Steingrube V A, Shurin P A. Isoelectric focusing of β-lactamases from sputum and middle ear isolates of Branhamella catarrhalis recovered in the United States. Drugs. 1986;31(Suppl. 3):48–54. doi: 10.2165/00003495-198600313-00012. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Philippon A, Riou J Y, Guibourdenche M, Sotolongo F. Detection, distribution, and inhibition of Branhamella catarrhalis β-lactamases. Drugs. 1986;31(Suppl. 3):64–69. doi: 10.2165/00003495-198600313-00014. [DOI] [PubMed] [Google Scholar]
- 14.Richter S S, Brueggemann A B, Huynh H K, Rhomberg P R, Wingert E M, Flamm R, Doern G V. A 1997–1998 national surveillance study: Moraxella catarrhalis and Haemophilus influenzae antimicrobial resistance in 34 U.S. institutions. Int J Antimicrob Agents. 1999;13:99–107. doi: 10.1016/s0924-8579(99)00112-0. [DOI] [PubMed] [Google Scholar]
- 15.Wallace R J, Steingrube V A, Nash D R, Hollis D G, Flanagan C, Brown B A, Labidi A, Weaver R E. BRO β-lactamases of Branhamella catarrhalis and Moraxella subgenus Moraxella, including evidence for chromosomal β-lactamase transfer by conjugation in B. catarrhalis, M. nonliquefaciens, and M. lacunata. Antimicrob Agents Chemother. 1989;33:1845–1854. doi: 10.1128/aac.33.11.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]