Abstract
Enhanced cerebellar oscillations have recently been identified in essential tremor (ET) patients as a key pathophysiological change. Since ET is considered a heterogeneous group of diseases, we investigated whether cerebellar oscillations differ in ET subtypes (familial vs. sporadic). This study aims to determine cerebellar physiology in familial and sporadic ET. Using surface electroencephalogram, we studied cerebellar physiology in 40 ET cases (n = 22 familial and n = 18 sporadic) and 20 age-matched controls. Both familial and sporadic ET cases had an increase in the intensity of cerebellar oscillations when compared to controls. Interestingly, cerebellar oscillations correlated with tremor severity in familial ET but not in sporadic ET. Our study demonstrated that ET cases have enhanced cerebellar oscillations, and the different relationships between cerebellar oscillations and tremor severity in familial and sporadic ET suggest diverse cerebellar pathophysiology.
Keywords: Essential tremor, Cerebellum, Oscillation, Physiology, Electroencephalogram, Tremor
Introduction
Essential tremor (ET) is the most common adult movement disorder [1, 2], and the cerebellar origin of ET has been long postulated based on the evidence of neuroimaging [3], neuropathology [4], and clinical observation [5]. Several neuropathological features in the cerebellum in subjects with ET have been identified, including dendritic loss and axonal pathologies in Purkinje cells [6–8], alterations of astrocytic protein expression [9, 10], and abnormal climbing fiber synaptic organization [11–13]. Among these pathological findings, climbing fiber synaptic pathology seems to be of particular interest because it correlates with tremor severity [12–14], and it is distinctive from pathological findings in ataxic disorders [15]. Climbing fibers originate from the inferior olivary nucleus, and the olivocerebellar circuit intrinsically oscillates at the ET frequency range [16, 17]. Dysfunction of the olivocerebellar circuit may lead to augmented neuronal oscillations within the circuit, causing tremor [18, 19].
To study the relationship between climbing fiber synaptic pathology and tremor, we recently developed a mouse model with ET-like climbing fiber synaptic pathology. This mouse model develops age-related kinetic tremor that is responsive to primidone and propranolol [20]. Interestingly, we found that this mouse model has strong oscillations in the cerebellar local field potentials associated with the kinetic tremor [20]. We also found similar cerebellar oscillations also exist in ET patients, recorded using surface electroencephalogram [20], supporting a role of climbing fiber pathology and cerebellar oscillations in tremor.
However, ET is considered to encompass a heterogeneous group of disorders, including sporadic and familiar forms [21]. The presentation of familial and sporadic ET differs in some clinical aspects, such as the age of onset and the rate of tremor progression [22, 23]. Although our previous study with postmortem cerebellar histology only identified marginal differences in familial and sporadic ET cases [24], it is possible that they have distinct underlying cerebellar physiology. Therefore, we investigated the cerebellar oscillations in familial and sporadic ET cases to further our understanding of the heterogeneity of ET pathophysiology.
Material and Methods
Study Subjects
We recruited ET cases from the Center for Parkinson’s Disease and Other Movement Disorders at Columbia University. Control subjects were recruited from ET patients’ friends or spouses who were tremor-free. All ET cases were first diagnosed by their treating neurologists; their diagnoses were subsequently confirmed by a movement disorders specialist (SHK), based on the Essential Tremor Centralized Brain Repository criteria [25], free of rigidity, bradykinesia, or dystonic “features”. All the participants signed the informed consent approved by the institutional review board of Columbia University. We performed a power calculation based on our previous study of cerebellar oscillations in ET [20]. With a sample size of 19 in each group, we were powered at 90% to detect differences of the magnitude. Because 43–63% of ET patients reported a family history of tremor [26–28], we enrolled 20 controls and 40 ET cases, with an expectation that approximately half of the ET cases would have a family history of tremor, to compare familial ET and sporadic ET cases.
In this study, we defined familial ET by patients’ self-reports according to conservative and liberal criteria [24]. Using conservative criteria, familial ET was defined by the presence of at least one first-degree relative with ET (n = 19). Using liberal criteria, familial ET was defined by the presence of at least one first- or second-degree relative with ET (n = 22). Sporadic ET was defined as the absence of any first- or second-degree relatives with reported ET (n = 18). The tremor severity was determined clinically with the total tremor score (range = 0 to 46) by rating the severity of postural tremor (arms extended) and kinetic tremor (pouring, drinking, using a spoon, drawing spirals, and performing finger-nose-finger). A higher total tremor score indicates a more severe tremor [12].
Cerebellar Electroencephalography
Physiological recording was performed with Cervello 32 (Blackrock Microsystem Inc, Salt Lake City, United States) using Ag/AgCl electrodes at a sampling rate of 1024 Hz [20]. To obtain bipolar electroencephalography montage, we selected a pair of electrodes, one located below the inion, presumably at the cerebellar hemisphere ipsilateral to the dominant hand and the other at the cerebellar vermis area contralateral to the dominant hand (Fig. 1A, B) [20]. During the recording, participants were instructed to perform repetitive extension and flexion movements of the forearms, similar to the finger-nose-finger test, which induces tremor in ET.
Fig. 1.
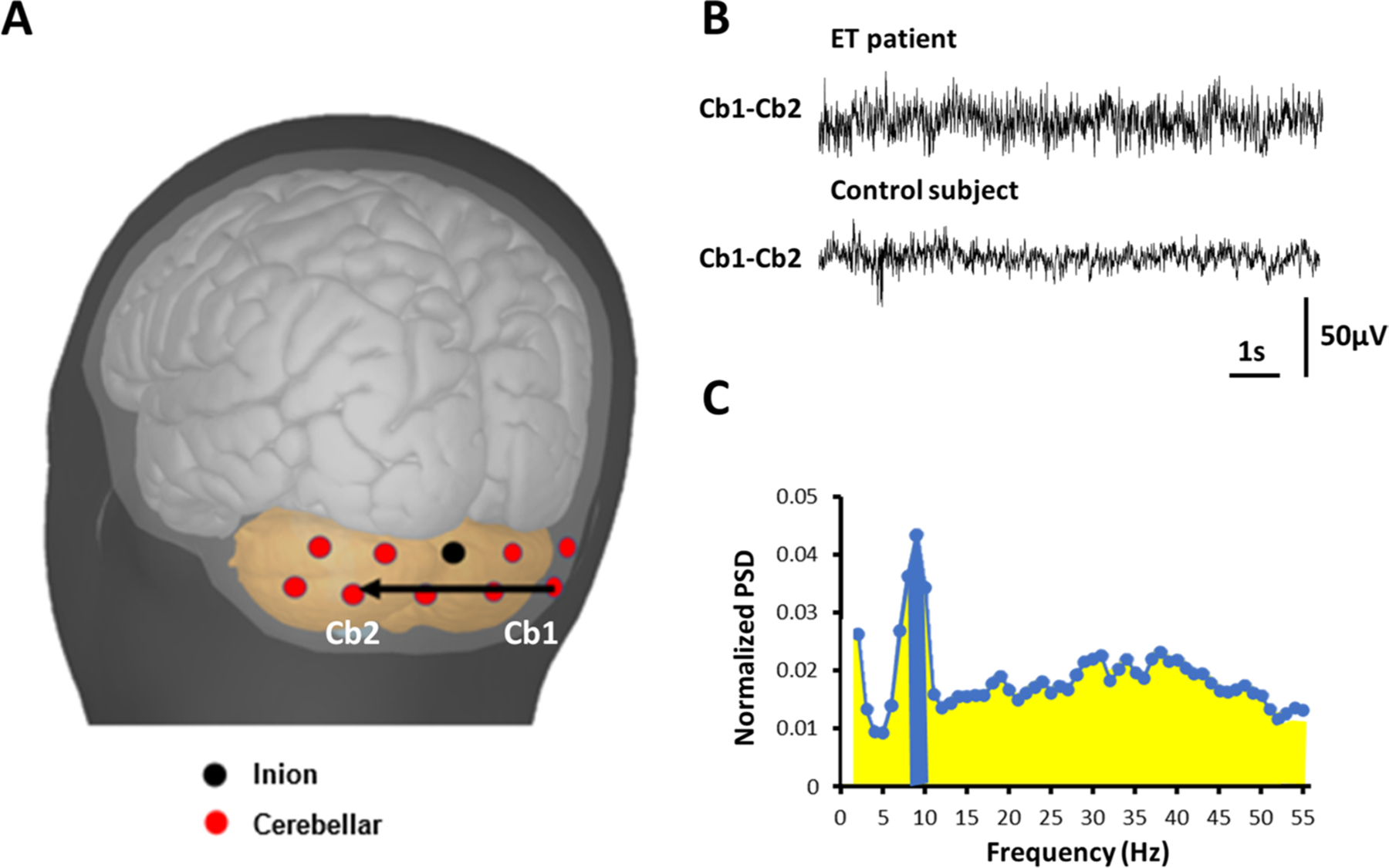
A Schematic of the surface cerebellar electroencephalogram. Cerebellar electrodes (red) were placed below the inion (black dot), over the surface of the cerebellar region. B Representative raw traces of cerebellar electroencephalogram. C After transformation, the power spectrum density (PSD) of each frequency was calculated. We summed up the PSD of the peak and the two adjacent frequencies (± 1 Hz to the peak, blue), divided the sum by the total PSD between 2 and 55 Hz (yellow, only up to 30 Hz was shown), and scaled by 100 to calculate the cerebellar oscillatory index
The cerebellar electroencephalogram has been validated previously [20]. Using the above protocol, we made certain that cerebellar oscillatory activity is neither contaminated by neck muscle artifact nor the alpha rhythm generated in the neighboring occipital cortex [20]. Furthermore, previous source localization analysis revealed that the oscillatory activity obtained using cerebellar electroencephalogram originates in the brain region below the inion, confirming a cerebellar origin [20].
Data Analysis
Digitized cerebellar bipolar electroencephalography data were processed offline using in-house MATLAB (the Math-works, Natick, MA) scripts developed for frequency analyses via power spectrum density (PSD) function. Each PSD data points across different frequencies were constructed from a 10-s window, and we summed up PSD data of 2-min epochs. The PSD was normalized to the sum of the PSDs between 2 and 55 Hz. The peak frequency was then identified between 4 and 20 Hz. The cerebellar oscillatory index (COI) was the sum of the normalized PSDs between the peak frequency ± 1 Hz multiplied by 100 (Fig. 1C) [20]. If no peak could be determined between 4 and 20 Hz, the normalized PSDs between 8 and 10 Hz were chosen to compute the COI because the mean PSD in ET cases and controls peaks around 9 Hz [20].
Statistical Analysis
Statistical analyses were performed using SPSS version 19.0 statistical package for Windows (IBM, Chicago, IL, USA) and the SciPy Python package. The variables were tested for normal distribution using Kolmogorov–Smirnov tests. We used Student’s t tests and Mann–Whitney U tests for two group comparisons of normally distributed and non-normally distributed variables, respectively. We used one-way analysis of variance test with Tukey post hoc analyses and Kruskal–Wallis with Dunn post hoc analyses for three group comparisons of normally distributed and non-normally distributed variables, respectively. Spearman’s correlation analysis was used to test the correlation between total tremor scores and COIs since at least one of the variables was not normally distributed. Two-tailed p values of less than 0.05 were considered statistically significant.
Results
The age and gender distributions between ET and controls were similar. Age of tremor onset was 44.4 ± 19.8 years with a disease duration at the time of enrolment of 22.0 ± 15.4 years (Table 1). Sporadic and familial ET cases, both by liberal or conservative criteria, had similar age, age of tremor onset, disease duration, and tremor severity (Table 2). ET cases had a 71.4% increase in COI compared with controls (12.4 ± 6.6 in ET cases vs. 6.8 ± 2.9 in controls, p < 0.001), indicating that ET cases have enhanced cerebellar oscillations. Among all 40 ET cases, 13 of them (32.5%) had head tremor. The COI in cases with and without head tremor were 12.2 ± 5.1 and 12.5 ± 7.4, respectively (p = 0.886, independent t test). The similar COI in cases with and without head tremor indicated that COI was not contaminated by patients’ head movements.
Table 1.
Basic demographics and cerebellar oscillations for controls and essential tremor cases
| Control | Essential tremor | p value | |
|---|---|---|---|
| n | 20 | 40 | |
| Age | 67.1 ± 9.3 | 66.8 ± 14.8 | 0.940a |
| Female gender | 50% (10) | 40% (16) | 0.461b |
| Family history of tremor* | 0% (0) | 55% (22) | < 0.001b |
| Tremor onset age | NA | 44.4 ± 19.8 | — |
| Tremor duration (y) | NA | 22.0 ± 15.4 | — |
| Total tremor score | 0.1 ± 0.2 Median = 0.0 | 20.2 ± 9.0 Median = 18.5 | < 0.001c |
| Cerebellar oscillation index | 6.8 ± 2.9 Median = 6.8 | 12.4 ± 6.6 Median = 10.3 | < 0.001c |
Independent t test
Chi-squared test
Mann–Whitney test
With liberal criteria
The median is reported if one or more of the variables are not normally distributed
Table 2.
Clinical and cerebellar oscillations of essential tremor cases grouped by family history
| Control | Sporadic ETa | Familial ET (liberal criteriab) | Familial ET (conservative criteriac) | p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liberal criteriab | Conservative criteriac | |||||||||||
| Omnibus | Sporadic ET vs. familial ET | Sporadic ET vs. control | Familial ET vs. control | Omnibus | Sporadic ET vs. familial ET | Sporadic ET vs. control | Familial ET vs. control | |||||
| n | 20 | 18 | 22 | 19 | ||||||||
| Age | 67.1 ± 9.3 | 68.2 ± 17.2 | 65.7 ± 12.8 | 65.5 ± 13.1 | 0.802d | — | — | — | 0.829d | — | — | — |
| Female gender | 50.0% (10) | 22.2% (4) | 54.5% (12) | 52.6% (10) | — | 0.038f | 0.076f | 0.768f | — | 0.057f | 0.076f | 0.869f |
| Tremor onset age | NA | 46.2 ± 21.2 | 43.0 ± 19.1 | 45.9 ± 18.2 | — | 0.611g | — | — | — | 0.966g | — | — |
| Tremor duration (y) | NA | 21.4 ± 15.3 | 22.5 ± 15.7 | 19.3 ± 14.4 | — | 0.816g | — | — | — | 0.674g | — | — |
| Total tremor score | 0.1 ± 0.2 Median = 0.0 | 17.4 ± 8.3 Median = 17.5 | 22.5 ± 9.1 Median = 22.0 | 21.6 ± 8.8 Median = 21.5 | <0.001e | 0.238e | <0.001e | <0.001e | <0.001e | 0.354e | <0.001e | <0.001e |
| Cerebellar oscillation index | 6.8 ± 2.9 Median = 6.8 | 11.2 ± 4.5 Median = 10.5 | 13.4 ± 7.8 Median = 10.2 | 12.8 ± 4.5 Median = 10.4 | <0.001e | 0.765e | <0.001e | <0.001e | <0.001e | 0.814e | 0.002e | <0.001e |
Abbreviation: ET, essential tremor
Sporadic ET requires the absence of either first- or second-degree relative with reported ET
Liberal criteria require at least one first- or second-degree relative with reported ET
Conservative criteria require at least one first-degree relative with reported ET
Analysis of variance with post hoc Tukey analysis
Kruskal–Wallis with post hoc Dunn analysis
Chi-squared test
Independent t test
The median is reported if one or more of the variables are not normally distributed
Next, we examined whether the strength of cerebellar oscillations differs between familial and sporadic ET patients. COI was higher in both sporadic and familial ET cases compared to controls (6.8 ± 2.9 in controls vs. 11.2 ± 4.5 in sporadic ET vs. 13.4 ± 7.8 in familial ET cases by liberal criteria vs. 12.8 ± 4.5 in familial ET cases by conservative criteria) (Fig. 2A and Table 2). COI in sporadic ET cases was similar to COI of familial ET cases, either by conservative criteria (p = 0.814) or liberal criteria (p = 0.765), indicating similar strength of cerebellar oscillations across ET subgroups.
Fig. 2.
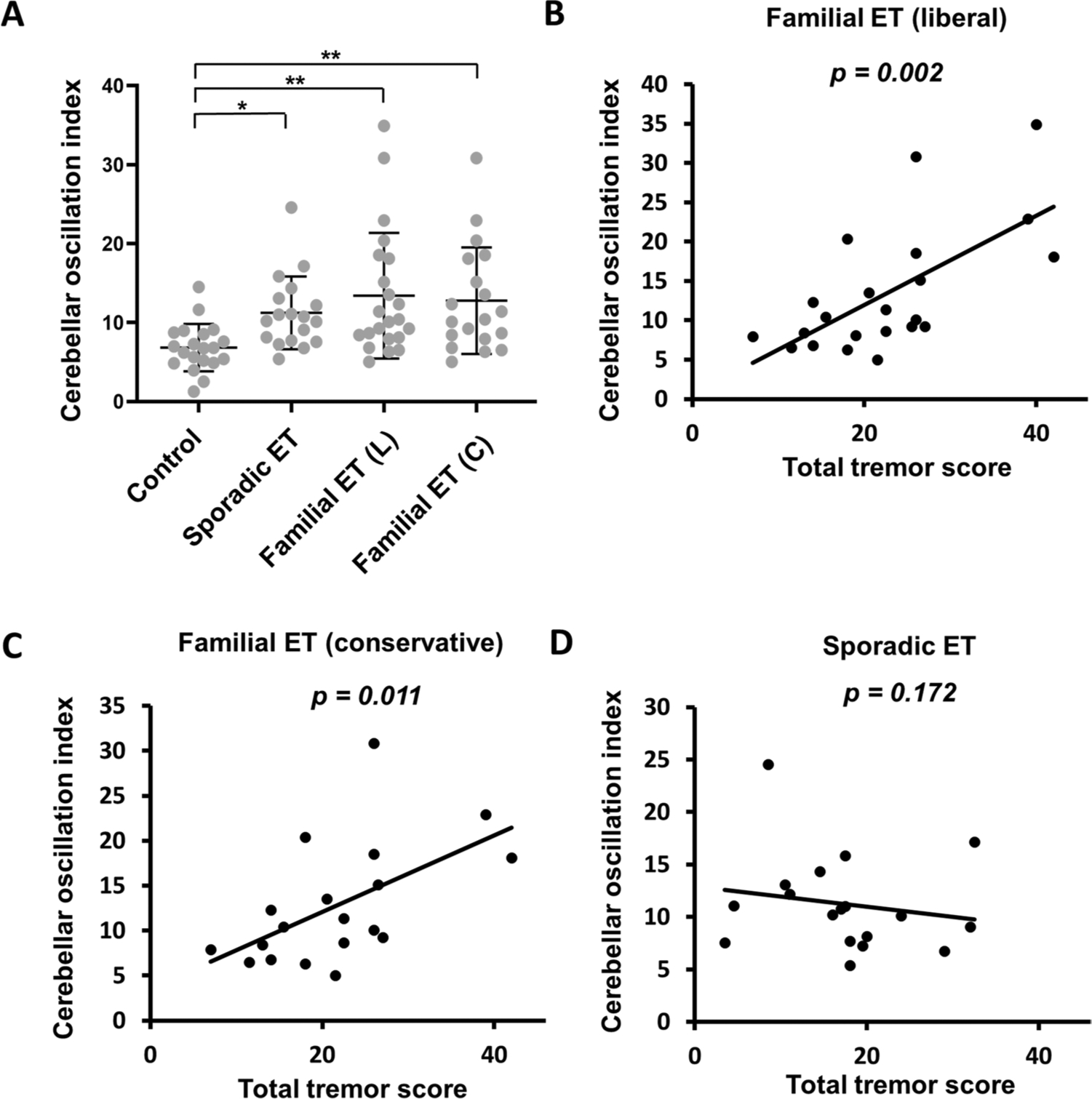
A The comparison between the cerebellar oscillatory index (COI) between familial (liberal and conservative criteria), sporadic ET, and controls. * p < 0.05; ** p < 0.01. B The correlation between COI and tremor severity in familial ET (liberal) cases. C The correlation between COI and tremor severity in familial ET (conservative) cases. D The correlations between COI and tremor severity in sporadic ET cases
We further explored the relationship between COI and tremor severity in familial and sporadic ET cases. In familial ET cases, COI correlated with tremor severity both by liberal criteria (Spearman’s correlation coefficient = 0.617, p = 0.002) (Fig. 2B) and by conservative criteria (Spearman’s correlation coefficient = 0.568, p = 0.011) (Fig. 2C). However, there was no correlation between COI and tremor severity in sporadic ET patients (Spearman’s correlation coefficient = − 0.337, p = 0.172) (Fig. 2D). These data suggested that cerebellar oscillation may play different roles in familial and sporadic ET pathophysiology.
Discussion
The present study demonstrated that both familial ET and sporadic ET patients have enhanced cerebellar oscillation measured by cerebellar electroencephalogram activity as compared to healthy controls, consistent with our previous report that familial ET and sporadic ET share similar cerebellar pathology [24]. Interestingly, cerebellar oscillatory activity correlated with tremor severity in familial ET but not in sporadic ET, suggesting that additional regulatory components downstream to the cerebellum may determine tremor severity in sporadic ET. Our study helps elucidate the clinical heterogeneity of ET, and cerebellar electroencephalogram could be a useful physiological tool to probe such heterogeneity.
Where does the cerebellar oscillatory activity come from? The olivocerebellar system (i.e., climbing fiber connections to Purkinje cells) is the main oscillator center in the central nervous system, for which climbing fiber activity regulates Purkinje cell firing rhythm. Interestingly, abnormal climbing fiber synapses extending into parallel fiber synaptic territory of Purkinje cell dendrites have been identified in the ET cerebellum [11–13] that can alter the cerebellar physiology and generate enhanced oscillatory activity. Therefore, therapies targeting either climbing fiber synaptic pathology and/or cerebellar oscillations could be tested for future clinical trials.
Using cerebellar electroencephalogram, our previous study validated that patients with ET had excessive cerebellar oscillations than control subjects [20]. The cerebellar electroencephalogram is a new technique, and it is crucial to exclude potential artifacts, and signal sources possibly affect the electroencephalogram recording. The muscle signal recorded from nearby capitis muscles had distinct spectral distribution and characteristics compared with the cerebellar electroencephalogram recorded simultaneously [20]. The cerebellar electroencephalogram power at the tremor frequency was also five times larger than the nearby muscle signals which made cerebellar electroencephalogram not easily contaminated by nearby muscle signals [20]. In addition, the frequencies of cerebellar oscillation were different from occipital alpha rhythm in the same patient which exclude the possibility that cerebellar oscillations were only volume reduction from occipital alpha rhythm [20]. In this study, those ET cases with and without head tremor had similar COI, indicating that head movements would not affect the oscillations recorded from cerebellar electroencephalogram. Our study further validated the oscillations recorded from cerebellar encephalogram as the physiological marker of tremor; and in future studies, the cerebellar electroencephalogram technique could be applied to other patient groups with cerebellar pathologies in order to expand its clinical applications.
This study has several strengths. First, we extended our initial observation of cerebellar oscillations in ET to a larger sample size, confirming this physiological feature in the ET population [20]. Second, we probe the presence of cerebellar oscillations in both familial and sporadic ET, which were previously thought to be ET subtypes with distinct brain circuitry and/or disease mechanisms [29]. The main limitation of our study is the modest sample size, and future studies with larger sample sizes are needed to assess the clinicophysiological heterogeneity of ET. We also excluded patients with dystonia and parkinsonian features, so it remains unknown whether ET cases with subtle dystonia or parkinsonism (i.e., ET-plus) also have the cerebellar oscillations. Another unexplored issue is whether increased cerebellar oscillations are specific to ET or they also show in other tremor disorders. For non-rhythmic cerebellar disorders such as cerebellar ataxia, how the cerebellar oscillations behave remains to be explored.
In conclusion, using cerebellar electroencephalography, we found that cerebellar oscillations are conserved in both familial and sporadic ET cases. Identification of additional modulatory components of such oscillations in different subtypes of ET will improve our understanding of the heterogeneity of ET.
Funding
Dr. Kuo has received funding from the National Institutes of Health, NINDS #R01 NS104423 (principal investigator), #R01 NS118179 (principal investigator), NINDS #R03 NS114871 (principal investigator), the National Ataxia Foundation, and the International Essential Tremor Foundation. Dr. Pan has received funding from the Ministry of Science and Technology in Taiwan, grants MOST 109-2326-B-002-013-MY4 (principal investigator), MOST 107-2321-B-002-020 (principal investigator), MOST 108-2321-B-002-011 (principal investigator), and MOST 108-2321-002-059-MY2 (principal investigator), National Taiwan University College of Medicine #110C101-011 (principal investigator) and National Taiwan University Hospital #NSC-145-11 (principle investigator).
Financial Disclosures
All funding sources are declared in the Funding section. The authors confirmed no additional funding from any other institution, including personal relationships, interests, grants, employment, affiliations, patents, inventions, honoraria, consultancies, royalties, stock options/ownership, or expert testimony for the last 12 months.
Footnotes
Conflict of Interest The authors declare no competing interests.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–41. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13:5–10. [DOI] [PubMed] [Google Scholar]
- 3.Cerasa A, Quattrone A. Linking essential tremor to the cerebellum-neuroimaging evidence. Cerebellum. 2016;15:263–75. [DOI] [PubMed] [Google Scholar]
- 4.Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9:613–22. [DOI] [PubMed] [Google Scholar]
- 5.Dupuis MJ-M, Evrard FLA, Jacquerye PG, Picard GR, Lermen OG. Disappearance of essential tremor after stroke. Mov Disord. 2010;25:2884–7. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Faust PL, Vonsattel JPG, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–307. [DOI] [PubMed] [Google Scholar]
- 7.Babij R, Lee M, Cortes E, Vonsattel JPG, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis ED, Lee M, Babij R, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain. 2014;137:3142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Kelly GC, Tate WJ, et al. Excitatory amino acid transporter expression in the essential tremor dentate nucleus and cerebellar cortex: a postmortem study. Parkinsonism Relat Disord. 2016;32:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee M, Cheng MM, Lin CY, Louis ED, Faust PL, Kuo SH. Decreased EAAT2 protein expression in the essential tremor cerebellar cortex. Acta Neuropathol Commun. 2014;2:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo SH, Lin CY, Wang J, et al. Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol. 2017;133:121–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis RJ, Lin CY, Faust PL, Koeppen AH, Kuo S-H. Climbing fiber synaptic changes correlate with clinical features in essential tremor. Neurology. 2015;84:2284–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JPG, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137:3149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo SH, Lin CY, Wang J, et al. Deep brain stimulation and climbing fiber synaptic pathology in essential tremor. Ann Neurol. 2016;80:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis ED, Kerridge CA, Chatterjee D, et al. Contextualizing the pathology in the essential tremor cerebellar cortex: a pathologomics approach. Acta Neuropathol. 2019;138:859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang EJ, Sugihara I, Llinas R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–75. [DOI] [PubMed] [Google Scholar]
- 17.de Montigny C, Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 1973;53:81–95. [DOI] [PubMed] [Google Scholar]
- 18.Llinás RR. The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits. 2013;7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamarre Y, Mercier LA. Neurophysiological studies of harmaline-induced tremor in the cat. Can J Physiol Pharmacol. 1971;49:1049–58. [DOI] [PubMed] [Google Scholar]
- 20.Pan MK, Li YS, Wong S-B, et al. Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci Transl Med. 2020;12:eaay1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis ED, Ottman R. How familial is familial tremor? The genetic epidemiology of essential tremor. Neurology. 1996;46:1200–1200. [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Hernandez N, Rabinowitz D, Ottman R, Clark LN. Predicting age of onset in familial essential tremor: how much does age of onset run in families? Neuroepidemiology. 2013;40:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis ED, Hernandez N, Ionita-Laza I, Ottman R, Clark LN. Does rate of progression run in essential tremor families? Slower vs. faster progressors. Parkinsonism Relat Disord. 2013;19:363–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis ED, Kuo SH, Wang J, et al. Cerebellar pathology in familial vs. sporadic essential tremor. Cerebellum. 2017;16:786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis ED, Borden S, Moskowitz CB. Essential tremor centralized brain repository: diagnostic validity and clinical characteristics of a highly selected group of essential tremor cases. Mov Disord. 2005;20:1361–5. [DOI] [PubMed] [Google Scholar]
- 26.Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology. 1991;41:234–8. [DOI] [PubMed] [Google Scholar]
- 27.Martinelli P, Gabellini AS, Gulli MR, Lugaresi E. Different clinical features of essential tremor: a 200-patient study. Acta Neurol Scand. 1987;75:106–11. [DOI] [PubMed] [Google Scholar]
- 28.Aiyesimoju AB, Osuntokun BO, Bademosi O, Adeuja AO. Hereditary neurodegenerative disorders in Nigerian Africans. Neurology. 1984;34:361–2. [DOI] [PubMed] [Google Scholar]
- 29.Louis ED, Clark LN, Ottman R. Familial versus sporadic essential tremor: what patterns can one decipher in age of onset? Neuroepidemiology. 2015;44:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


