Abstract
Introduction
Mild cognitive impairment (MCI) is the state between normal cognition and dementia. This study objective was to estimate an average 1‐year rate of conversion from MCI to dementia and explore the associated factors of conversion in a hospital‐based cohort.
Methods
A retrospective cohort study of participants with MCI was conducted in a tertiary care hospital in Thailand. Two hundred fifty participants, 50 years of age or older, were enrolled.
Results
An average 1‐year conversion rate from MCI to dementia was 18.4%. MCI patients who converted to dementia were likely older (P < .001), predominantly female (P = .028), vitamin D deficient (P = .012), and associated with lower Mini–Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores during first assessments (P < .001, P < .001 respectively) and follow‐up assessments (P < .045, P < .001 respectively). We conducted two models of multivariate analysis, using binary logistic regression. In the first model, adjusted for age, sex, education, vitamin D deficiency, and first assessment MMSE scores, we found that underlying vitamin D deficiency (odds ratio [OR] = 3.13, 95% confidence interval [CI] 1.04 to 9.44) and first assessment MMSE scores (OR = 0.83, 95% CI 0.73 to 0.93) were significantly associated with conversion to dementia. In the second model, adjusted for age, sex, education, vitamin D deficiency and first assessment MoCA scores, only first assessment MoCA scores (OR = 0.58, 95% CI 0.45 to 0.76) were significantly associated with conversion to dementia.
Discussion
The 1‐year conversion rate from MCI to dementia was 18.4%. MMSE and MoCA were useful tools to assess baseline cognitive status in MCI patients and predict dementia progression. The association between vitamin D deficiency and risk of conversion from MCI to dementia requires further investigations.
Keywords: conversion, dementia, mild cognitive impairment, predictors, progression, rates, retrospective, risk factor
1. INTRODUCTION
Dementia is the fifth leading cause of death globally and causes a heavy burden to individuals, their families, and society. Recent studies found that dementia attributed to ≈28.8 million disability‐adjusted life‐years (DALYs). 1 , 2 It negatively affects individuals by causing cognitive decline in various domains. Thailand is now becoming an aging society in which 16.7% of total population, or up to 11 million people, are 60 or older. 3 According to the Thai National Health Examination Survey, the prevalence of dementia in Thai elderly people is 8.1%. 4 To prevent or slow the progression of dementia, it is crucial to understand the prognosis and risks, and to identify the earliest signs of dementia.
Mild cognitive impairment (MCI) is a state between normal cognitive aging and dementia. 5 Diagnosis of MCI is usually not sustainable. The condition may remain stable, revert to cognitively intact, or progress to dementia. 6 Because patients with MCI have a higher risk of conversion to dementia compared with cognitively normal people, the diagnosis of MCI carries a predictive value. 6 , 7 Early detection and interventions require knowledge regarding the risk of dementia conversion in MCI and possible associated risk factors.
In the past few decades, several studies have been conducted to identify the conversion rate of MCI to dementia. Most published data are from Europe and America, whereas data in Asian population are very limited. The Alzheimer's Disease Neuroimaging Initiative (ADNI) study reported that the subjects with MCI progressed to dementia in 12 months at a rate of 16.5%. 8 A meta‐analysis of 19 longitudinal studies published between 1991 and 2001 (both hospital‐based and community‐based) estimated a mean annual conversion rate (ACR) of 10.24%. 9 Another meta‐analysis study of 41 robust cohort studies estimated the adjusted ACR from MCI to dementia, which was 9.6% in specialist clinical settings. 10 In 2013, a systematic review summarized conversions over 1 year ranged from 10.2% to 33.6% (five studies, median: 19.0%), and ACRs ranged from 7.5 to 16.5% (seven studies, median: 11.0%) per person‐year for studies recruiting from clinics. 11 Other studies showed a wide range of approximate conversion rate of MCI to dementia in 1 year from 5% to 39%. 5 , 7 , 12 , 13 , 14 , 15 , 16 , 17 Results from previous studies showed substantial variation in prognosis and progression of MCI, which depended on the starting point of decline, the criteria implementation, and the research methodology. Although most previous studies in developed countries are conducted in community settings, 9 , 10 , 11 , 12 , 18 most patients in Thailand are currently being diagnosed and treated by specialists in hospital settings. Nonetheless, there has been no study regarding the conversion rate from MCI to dementia conducted in Thailand. Because of the differences in genetic risks and management settings compared to western countries, data regarding dementia conversion rate and associated factors can be used to support and monitor the status of dementia management in Thailand.
The major risk factors associated with cognitive decline and dementia—for example, older age and lower level of education—have also been repeatedly associated with MCI. 19 , 20 Some studies reported that the presence of vascular risk factors (ie, diabetes mellitus, hypertension, cerebrovascular disease, and hyperlipidemia) and neuropsychiatric symptoms such as depression and anxiety were associated with an increased risk of cognitive impairment and dementia. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 This study aimed to estimate an average 1‐year rate of conversion from MCI to dementia, and to explore associated factors in Thai population 50 years of age or older.
RESEARCH IN CONTEXT
Systematic Review: Dementia is the fifth leading cause of death globally and causes a heavy burden to individuals, their families, and society. Mild cognitive impairment (MCI) is a state between normal cognitive aging and dementia. Diagnosis of MCI is usually not sustainable; it may remain stable, revert to normal cognition, or progress to dementia. Because patients with MCI have a higher risk of conversion to dementia compared with cognitively normal people, the diagnosis of MCI carries a predictive value. In the past few decades, several studies have been conducted to identify the conversion rate of MCI to dementia. Most published data are form Europe and America, whereas data from Asian populations are very limited. This study was a retrospective cohort of newly diagnosed MCI patients, from January 2014 to December 2016, in a tertiary care hospital in Thailand and aimed to estimate the 1‐year rate of conversion and explore associated factors of conversion.
Interpretation: The 1‐year conversion rate from MCI to dementia was 18.4%. Mini–Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) were useful tools to assess baseline cognitive status in MCI patients and predict dementia progression. The association between vitamin D deficiency and risk of conversion from MCI to dementia requires further investigations.
Future Directions: Further studies in larger groups of participants with MCI and longer periods of follow‐up need to be investigated to improve understanding and increase the accuracy of prognoses for dementia.
2. METHODS
2.1. Study design
This study is a hospital‐based retrospective cohort study of Ramathibodi hospital in Bangkok, Thailand. The protocol was approved by the Ethics Committee on Human Experimentation of the Institute.
2.2. Study participants
We reviewed data from medical records of newly diagnosed MCI patients, age 50 years or older, who visited Psychiatry, Neurology, Family Medicine and Geriatric medicine clinics at Ramathibodi hospital in Bangkok, Thailand, from January 2014 to December 2016. Participants were excluded from the study if they were known to have dementia, diagnosed with MCI before enrolled to the study, no follow‐up data or <1‐year follow‐up period, loss of data, or insufficient information.
Five hundred fifty participants with electronic medical record diagnosis of MCI were initially recruited. After exclusion, 250 participants were enrolled. Researchers thoroughly reviewed medical records of participants for conversion to dementia/major neurocognitive disorder (major NCD) over 1‐year follow‐up from their index dates.
2.3. Data collection
Demographic data were recorded including sex, age, level of education, years of education, marital status, family history of NCD, history of alcohol use, history of smoking, underlying diseases, and use of acetylcholinesterase inhibitor (AChEI).
Underlying diseases were recorded including Parkinson disease, vitamin D deficiency, and cardiovascular risk diseases such as diabetes mellitus, hypertension, hyperlipidemia, cardiac diseases (ischemic heart, cardiac arrhythmia, valvular heart disease, heart failure), and stroke. Data regarding underlying diseases included self‐reports, medications, and records of regularly follow‐up and treatment.
Depressive disorders were categorized into underlying depressive disorders and comorbid depression. Underlying depressive disorders defined as had been diagnosed with major depressive disorder (MDD) or other depressive disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) or self‐reported depression in present illness or past history in medical record with no requirement of standard questionnaire; or history of medication with antidepressant drugs before 3 months of MCI diagnosis (index date). Comorbid depression defined as had been diagnosied with depression in medical record within 3 months of MCI diagnosis(index date).
Presenting symptoms or concerning problems were divided into two major groups: cognitive problem and non‐cognitive problem. Cognitive problem included dysfunction of all six cognitive domains (complex attention, executive function, learning and memory, language, perceptual motor, and social cognition). Non‐cognitive problems included mood disturbance (eg, depression, irritable mood, anxiety, and so on); sleep problem; behavior problems (eg, aggression, wandering, impulsive behavior, and so on); psychiatric problems (eg, delusion, hallucination, and so on); and other problems such as fatigue, dizziness, ataxia, or tremor.
2.4. Diagnosis of MCI and dementia
The cognitive stage and clinical assessment findings of the participants were reviewed and determined by researchers using the DSM‐5 criteria. 29 The diagnosis of MCI required (1) evidence of modest cognitive decline from a previous level of performance in one or more cognitive domains, (2) did not interfere with capacity for independence in everyday activities (ie, complex instrumental activities of daily living [IADLs] are preserved, but greater effort, compensatory strategies, or accommodation may be required), (3) not delirium, and (4) not better explained by another mental disorder.
Diagnosis of dementia/major NCD 29 defined according to DSM‐5 criteria: (1) evidence of significant cognitive decline from a previous level of performance in one or more cognitive domains; a substantial impairment in cognitive performance was required; (2) the cognitive deficits interfere with independence in everyday activities; (3) not delirium; (4) not better explained by another mental disorder.
We collected both subjective and objective retrospective data. Subjective history of cognitive decline was collected from concern of the individual, a knowledgeable informant, or the clinician. The objective cognitive assessments that we collected were the Mini–Mental State Examination (MMSE) Thai 2002 version and the Montreal Cognitive Assessment (MoCA) Thai version. Both the MMSE and MoCA scores were recorded at time of MCI diagnosis as first assessment and recorded again after an average 1‐year follow‐up or at the time of conversion as second assessment. Full baseline neuropsychological assessments were performed in 62 of 250 participants, accounting for ≈24.8%. Baseline brain magnetic resonance imaging (MRI) studies were performed in about 50% of participants. No genetic test result was recorded in any participants. Because these three tests were not performed in all participants and the reason for performing the tests were not uniformly recorded, the diagnosis of each case was based on DSM‐5 clinical criteria of NCD.
2.5. Analysis
We performed statistical analysis using SPSS 18.0 for windows. Demographic variables were presented as mean with standard deviation (SD) or median with interquartile range for continuous data, and frequency and percentage for categorical data. Descriptive analysis was done to assess incidence of dementia at 1‐year follow‐up in participants with MCI at baseline. Chi‐square or Fisher's exact test was used to compare categorical data. Paired and independent t‐tests or non‐parametric tests were used to compare continuous data. We conducted univariate analysis to compare participants with MCI who did not convert to dementia in 1 year and who converted to dementia in 1 year. Multivariate analysis was done by binary logistic regression to confirm factors associated with rate of conversion from MCI to dementia. A P value < .05 was considered statistically significant.
3. RESULT
From 550 participants initially recruited, 300 were excluded due to misdiagnosis, missing or insufficient data, and cases that had been diagnosed with MCI prior to evaluation or < 1‐year follow‐up (Figure 1). A total of 250 participants with MCI at baseline were enrolled. Sixty‐four percent were women. Mean age at enrollment was 71 years (range 50 to 90 years). Most patients were married (68.7%) (Table 1). Information about education, family history of NCD, and substance use (alcohol use, smoking) was collected from 208, 144, and 130 cases, respectively. Half of the participants had 12 years of education or more (50.5%). Some participants reported a family history of NCD (27.1%), used alcohol (18.5%), and smoked (16.9%). Almost all participants had cognitive problems (92.4%), and about one‐third had non‐cognitive problems (30.4%). Most patients had underlying hypertension (70%) or hyperlipidemia (73%), whereas vitamin D deficiency was reported in 14%.
FIGURE 1.
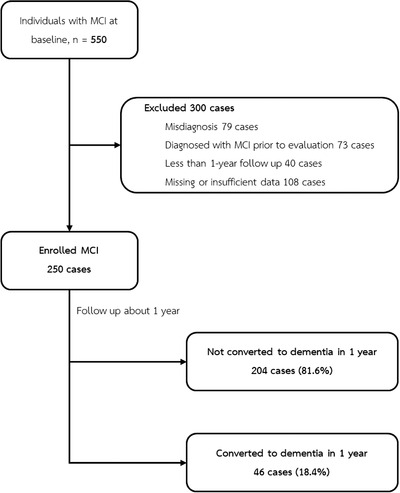
Study flow chart
TABLE 1.
Demographic data of patients with mild cognitive impairment
| Demographic and clinical characteristics | Mean ± standard deviation or number (%) |
|---|---|
| Total N = 250 | |
| Age | 71.28 ± 9.28 |
| Female | 160 (64%) |
| Marital status | |
| Single | 34 (13.8%) |
| Married | 169 (68.7%) |
| Divorced or widowed | 43 (17.5%) |
| Educational years a | |
| Uneducated to 6 years | 64 (30.8%) |
| 7‐12 years | 39 (18.8%) |
| More than 12 years | 105 (50.5%) |
| Family history of NCD b | 39 (27.1%) |
| Only first‐degree relationship b | 28 (19.4%) |
| Substance (current or ever) c | |
| Alcohol use | 24 (18.5%) |
| Smoking | 22 (16.9%) |
| Presenting symptom | |
| Cognitive | 231 (92.4%) |
| Non‐cognitive | 76 (30.4%) |
| Underlying disease and comorbidities | |
| Diabetes mellitus | 72 (28.8%) |
| Hypertension | 175 (70%) |
| Hyperlipidemia | 183 (73.2%) |
| Cardiovascular risk group or stroke | 54 (21.6%) |
| Parkinson disease | 14 (5.6%) |
| Vitamin D deficiency | 35 (14%) |
| Psychiatric disorder | |
| Underlying; Depressive disorders | 23 (9.2%) |
| Comorbid depression | 31 (12.4%) |
| Medication ‐ AChEI | 44 (17.6%) |
Abbreviations: AChEI, acetylcholinesterase inhibitor; MCI, mild cognitive impairment; NCDs, neurocognitive disorders.
Data from 208 cases.
Data from 144 cases.
Data from 130 cases.
Among 250 enrolled participants, 46 converted to dementia within 1 year. Therefore, the 1‐year conversion rate from MCI to dementia in our hospital‐based Thai elderly is 18.4%.
Compared with MCI patients who did not convert to dementia in 1 year, those who converted to dementia were more likely to be older (P < .001), women (P = .028), and have more underlying vitamin D deficiency (P = .012). There was no difference between the marital status, education level, family history of NCD, alcohol use, smoking, presenting symptom, other underlying diseases, and use of AChEI (Table 2).
TABLE 2.
Baseline characteristics between those who progressed from MCI and those who did not
| Median (interquartile range) or number (%) | |||
|---|---|---|---|
| Demographic and clinical characteristics | Not converted to dementia in 1 year (n = 204) | Converted to dementia in 1 year (n = 46) | P‐value |
| Age | 70.33 (13) | 75.50 (12) | <.001* |
| Female | 124 (60.8%) | 36 (78.3%) | .028* |
| Marital status | .878 | ||
| Single | 28 (13.9%) | 6 (13.3%) | |
| Married | 139 (69.2%) | 30 (66.7%) | |
| Divorced or widowed | 34 (16.9%) | 9 (20%) | |
| Educational years | .097 | ||
| Uneducated to 6 years | 46 (27.4%) | 18 (45%) | |
| 7‐12 years | 33 (19.6%) | 6 (15%) | |
| More than 12 years | 89 (53%) | 16 (40%) | |
| Family history of NCD | 31 (78.5%) | 8 (25.8%) | 1 |
| Only first‐degree relationship | 20 (17.7%) | 8 (25.8%) | .442 |
| Substance (current or ever) | |||
| Alcohol use | 21 (20.2%) | 3 (11.5%) | .404 |
| Smoking | 19 (18.3%) | 3 (11.5%) | .563 |
| Concerned problem | |||
| Cognitive | 187 (91.7%) | 44 (95.7%) | .54 |
| Non‐cognitive | 59 (28.9%) | 17 (37%) | .292 |
| Underlying disease and comorbidities | |||
| Diabetes mellitus | 56 (27.5%) | 16 (34.8%) | .368 |
| Hypertension | 142 (69.6%) | 33 (71.7%) | .86 |
| Hyperlipidemia | 150 (73.5%) | 33 (71.7%) | .854 |
| Cardiovascular risk group or stroke | 43 (21.1%) | 11 (23.9%) | .693 |
| Parkinson disease | 13 (6.4%) | 1 (2.2%) | .477 |
| Vitamin D deficiency | 23 (11.3%) | 12 (26.1%) | .012* |
| Psychiatric disorder | |||
| Underlying; depressive disorders | 20 (9.8%) | 3 (6.5%) | .777 |
| Comorbid Depression | 26 (12.7%) | 5 (10.9%) | .811 |
| Medication–AChEI | 32 (15.7%) | 12 (26.1%) | .131 |
Abbreviations: AChEI, acetylcholinesterase inhibitor; MCI, mild cognitive impairment; NCDs, Neurocognitive disorders.
P value < .05 is considered as statistically significant.
The neurocognitive assessments between those who converted to dementia and those who did not were summarized in Table 3. The data of MMSE were not normally distributed, but data of MoCA were. Therefore, we performed a non‐parametric test (Mann‐Whitney U test) for MMSE and independent t‐tests for MoCA. We found that MMSE scores at first assessment in participants with MCI who did not convert to dementia (n = 140) were higher than participants with MCI who converted to dementia (n = 32) (median scores 25 vs 21, P < .001). Similarly, MoCA scores at first assessment in participants with MCI who did not convert to dementia (n = 109) were higher than participants with MCI who converted to dementia (n = 16) (mean scores 21.59 vs 15.81, P < 0.001). Change of scores after an average 1‐year follow‐up or at the time of conversion: MMSE scores of participants with MCI who did not convert to dementia (n = 40) were not changed, whereas scores of participants with MCI who converted to dementia (n = 11) were decreased (median score difference 0 vs 3, P = .018). Likewise, MoCA scores of participants with MCI who did not convert to dementia (n = 37) were increased, whereas scores of participants with MCI who converted to dementia (n = 5) were decreased (mean score difference −1.27 vs 3.00, P = .001).
TABLE 3.
Neurocognitive assessment scores between those who converted to dementia and those who did not
| First assessment | Follow‐up assessment (2nd) | Scores difference between 1st and 2nd assessment | ||||
|---|---|---|---|---|---|---|
| Outcome | Median (IQR) | P value | Median (IQR) | P value | Median (IQR) | P value |
| MMSE | ||||||
| Not converted to dementia in 1 year | 25.07 (5.00) a | <.001 * | 24.84 (5.00) c | .045 * | −‐0.20 (2.75) e | .018 * |
| Converted to dementia in 1 year | 21.16 (6.00) b | 21.25 (12.00) d | 3.18 (6.00) f | |||
| Mean ± SD | P value | Mean ± SD | P value | Mean ± SD | P value | |
|---|---|---|---|---|---|---|
| MoCA | ||||||
| Not converted to dementia in 1 year | 21.59 ± 4.08 g | <.001 * | 22.65 ± 4.75 i | <0.001 * | −1.27 ± 2.50 k | 0.001 * |
| Converted to dementia in 1 year | 15.81 ± 4.15 h | 15.17 ± 5.52 j | 3.00 ± 2.35 l |
Note:
Data from 140 cases.
Data from 32 cases.
Data from 44 cases.
Data from 12 cases.
Data from 40 cases.
Data from 11 cases.
Data from 109 cases.
Data from 16 cases.
Data from 52 cases.
Data from 12 cases.
Data from 37 cases.
Ddata from five cases.
Abbreviations: IQR, interquartile range; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; SD, standard deviation.
P‐value < .05 is considered as statistically significant.
We conducted two models of multivariate analysis (Table 4) using binary logistic regression analysis. In the first model, the data were adjusted for age, sex, education, vitamin D deficiency, and first assessment MMSE scores. We found that having vitamin D deficiency increased the odds of converting to dementia in 1 year afterward by 213% and that the 1‐point increase in MMSE score at first assessment lowered the odds of converting to dementia in the following year by 17%. In the second model, the data were adjusted for age, sex, education, vitamin D deficiency, and first assessment MoCA scores. We found that a 1‐point increase in MoCA score at first assessment lowered the odds of converting to dementia in the following year by 42%.
TABLE 4.
Association between baseline factors and MCI conversion to dementia by logistic regression analysis
| Model 1 (n = 155) | Model 2 (n = 109) | |||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Age | 1.03 (0.98‐1.01) | .191 | 0.95 (0.88‐1.03) | .231 |
| Female | 1.58 (0.50‐4.97) | .439 | 1.47 (0.34‐6.39) | .605 |
| Educational years | ||||
| Uneducated to 6 years | 1 | – | 1 | – |
| 6‐12 years | 0.67 (0.17‐2.65) | .569 | 0.97 (0.10‐9.8) | .978 |
| More than 12 years | 1.19 (0.39‐3.57) | .763 | 9.05 (0.82‐99.76) | .072 |
| Underlying disease | ||||
| Vitamin D deficiency | 3.13 (1.04‐9.44) | .042* | 3.79 (0.48‐29.85) | .206 |
| Neurocognitive test | ||||
| MMSE first assessment | 0.83 (0.73‐0.93) | .002* | – | – |
| MoCA first assessment | – | – | 0.58 (0.45‐0.76) | <.001* |
Abbreviations: MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; OR, odds ratio; SD, standard deviation.
P‐value < .05 is considered as statistically significant.
4. DISCUSSION
An average 1‐year rate of conversion from MCI to dementia was 18.4%. MCI patients who converted to dementia were likely older, predominantly female, vitamin D deficient, and associated with lower MMSE and MoCA scores during first and follow‐up assessments. Multivariate analysis, using logistic regression, found that vitamin D deficiency and low MMSE and MoCA baseline scores were associated with dementia conversion. The population in this study was predominantly female and highly educated. Most of them presented with cognitive symptoms and had comorbidities, especially dyslipidemia and hypertension. They did not have significant substance/alcohol use disorders or family history of NCD. Approximately 10% of participants had underlying depression or comorbid depression. Less than 20% of participants were on AchEI.
Our study showed that 18.4% of participants with MCI at baseline had progressed to dementia over 1‐year of follow‐up, which is comparable with a previous systematic review. 11 Compared with the 16.5%, 1‐year conversion rate reported in the ADNI study, this study reported a slightly higher 1‐year conversion rate of MCI to dementia. Both ADNI 8 and our study were similar in clinical characteristic of urbanized participants with highly education level; a previous study 22 was found to have better prognosis of converting to dementia. In many longitudinal studies, the conversion rate was reported from the overall follow‐up period and then the ACR was calculated to estimate the progression of MCI in 1 year, but our study reported only 1‐year rate of conversion. Because some MCI patients may revert to normal especially during the 1 year after diagnosis, 30 and the rate of conversion may progress over time, the average ACR may be the better marker to represent the overall MCI prediction.
There were few epidemiological and prognostic studies of MCI conversion to dementia in Asia. From previous studies, 13 , 14 , 15 , 16 , 17 the results were varied in a wide range. In one systematic review, 9 the 1‐year conversion rate from hospital‐based reports was higher (range from 10.9 to 31.1) than community‐based reports (range from 5.6 to 23.1). Community‐based interventions 31 were found to have a positive impact on some MCI outcomes, and the community‐based report may have a lower conversion rate due to early detection and screening of less severe cases. Our study represented MCI patients in a hospital‐based cohort, which may have higher conversion rate due to more severe cases and delay in diagnosis. Therefore, patients with MCI, especially in hospital settings, should be closely monitored for conversion to dementia by using appropriate tools for early detection. Early intervention can also be useful for patients with MCI and their families.
Association between a low level of vitamin D and risk of conversion to dementia from previous studies was still inconsistent. Olsson et al. 32 reported that there was no association between baseline vitamin D status and long‐term risk of dementia or cognitive impairment over an 18‐year period. However, systematic review and meta‐analysis 33 showed an increased risk of cognitive impairment in those with low vitamin D compared with normal vitamin D. Our result was consistent with the latter study. In our study, the diagnosis of vitamin D deficiency was determined by self‐reports, laboratory results, history of medications related with diseases, and records of regularly followed up and treated for the diseases, but we did not collect the detail of treatment (eg, medication, dosage, and duration of treatment) and a time‐cause relationship would be difficult to determine. The explanation that vitamin D deficiency was found to be associated with conversion to dementia in Model 1 but not Model 2 is likely due to limitation of a retrospective study. In Model 2, we included fewer participants who had both MoCA and vitamin D results, which may have resulted in under statistical power. 34 Therefore, when more participants who had both MMSE and vitamin D results were included in Model 1, we found a statistically significant result.
In this study, we investigated neurocognitive assessments, MMSE and MoCA tests, focusing on baseline scores and change of scores during the follow‐up period of both tests. MCI participants with lower MMSE and MoCA total scores at first assessment were more likely to convert to dementia, which was congruent with other studies. 8 , 21 , 35 Our study investigated both baseline scores and change of scores because the MMSE had low sensitivity that could cause a false‐negative diagnosis or “ceiling effect” in very mild disease. 36 Another assessment was the MoCA, which was better at detecting MCI than MMSE. 37 , 38 , 39 , 40 A previous retrospective cohort study, including 165 individuals with MCI, examined the predictive nature of MoCA cognitive domains. The results showed that individuals with MCI with a low MoCA total score at the time of diagnosis were at risk of conversion to Alzheimer's disease, but the rate of decline in the MoCA total score was not significantly different between the converter and non‐converter group. 40 Of interest, our study found that both baseline MoCA score at first assessment and change in score during the follow‐up period were significantly associated with the likelihood of converting to dementia. Nevertheless, it should be noted that the score differences between two assessments in the converter group was from only five cases, since many clinicians performed only MMSE to make a diagnosis of dementia. In addition, baseline MMSE and MoCA scores in the converter group of our study was initially lower than the cut‐off scores suggested from the ADNI study (MMSE from 24 or over and MoCA from 17 or over). 41 This may represent a bias in our hospital‐based cohort with more severe cases and a higher rate of dementia conversion. Our study found that 1‐point increase in MMSE and MoCA at baseline assessment lowered the odds of conversion to dementia in the following year by 17% and 42%, respectively; therefore patients with a clinical diagnosis of MCI who had low score on MMSE and MoCA, especially lower than the ADNI cut‐off, should be closely monitored for potential rapid dementia conversion.
Surprisingly, our study did not find neuropsychiatric symptoms, especially depression, to be associated with dementia conversion like many previous studies. 23 , 24 , 25 , 26 , 27 , 28 It should be noted that the prevalence of comorbid depression was only about 10%. This is likely due to the retrospective nature of our study in which data regarding comorbid depression would be gathered only from medical records. The conclusion on this issue may not be sufficient to make from our data.
Patients with MCI should be assessed and monitored regularly due to a risk for conversion to dementia. Detection of MCI was useful in terms of prognosis and plan of treatment to prevent or slow the progression. Both MMSE and MoCA are useful tools for detecting and tracking changes of cognitive impairment. Clinicians should use these tools to evaluate both baseline status and change in scores over time, and to assist clinical evaluation including history taking and examination, but not to substitute.
4.1. Limitations
There are several limitations in this study. First, this was a retrospective study. Some important data were not available for us to conclude an association with dementia progression. Because the dementia diagnosis was based on clinical diagnosis by DSM‐5 criteria of NCD, only some patients had performed full neuropsychological tests or MRI to confirm their diagnosis. Data were extracted retrospectively; therefore, we could not distinguish subtypes of dementia explicitly. Numerous participants were excluded due to insufficient data despite our effort to review various sources of information (ie, electronic medical record program, laboratory program, and imaging program of Ramathibodi hospital). Second, data were collected from various clinics, including psychiatry, neurology, family medicine, and the geriatric clinic. There were minor differences in chart record form. However, including heterogeneous participants that could represent individuals with MCI in real a situation is better than collecting data from only one clinic. Finally, this was a hospital‐based study (predominantly higher education and potentially more complicated cases); therefore, generalization of the results to other settings (eg, community setting or individuals with low level of education but less complicated) should be done with caution.
5. CONCLUSION
Our retrospective cohort study showed that the 1‐year conversion rate from MCI to dementia was 18.4%. MMSE and MoCA were useful tools to assess baseline cognitive status in MCI patients and predict dementia progression. The association between vitamin D deficiency and risk of conversion from MCI to dementia requires further investigation. Larger studies with longer follow‐up periods are needed to improve the knowledge regarding risk factors and accuracy of dementia prediction.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE/PUBLICATION
The study protocol was approved by the research ethics committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University.
CONFLICT OF INTEREST
All authors declare no conflict of interest for this study.
ACKNOWLEDGMENTS
Thank you Dr. Iyavut Thaipisuttikul for checking the language and editing the manuscript. Special thanks to assistant professor Dr. Thanavadee Prachason and associate professor Dr. Sasivimol Rattanasiri for statistical consulting.
Thaipisuttikul P, Jaikla K, Satthong S, Wisajun P. Rate of conversion from mild cognitive impairment to dementia in a Thai hospital‐based population: A retrospective cohort. Alzheimer's Dement. 2022;8:e12272. 10.1002/trc2.12272
REFERENCES
- 1. Nichols E, Szoeke CE, Vollset SE, et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Launer LI. Statistics on the burden of dementia: need for stronger data. Lancet Neurol. 2019;18(1):25‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Stiatistics Organization. Report on the 2017 survey of the older persons in Thailand. Ministry of Digital Economy and Society. National Stiatistics Organization; 2021. [Google Scholar]
- 4. Aekplakorn W. Thai National Health Examination Survey. NHES V. Bangkok: Health Systems Research Institute; 2016. [Google Scholar]
- 5. Petersen RC. Mild cognitive impairment. Continuum (Minneap Minn). 2016;22(2 Dementia):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts RO, Knopman DS, Mielke MM, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82(4):317‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petersen RC, Aisen P, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr. 2004;16(2):129‐140. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell AJ, Shiri‐Feshki M. Rate of progression of mild cognitive impairment to dementia–meta‐analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119(4):252‐265. [DOI] [PubMed] [Google Scholar]
- 11. Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer's disease to Alzheimer's dementia: a systematic review of the literature. Dement Geriatr Cogn Disord. 2013;3(1):320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pandya SY, Clem MA, Silva LM, Woon FL. Does mild cognitive impairment always lead to dementia? A review. J Neurol Sci. 2016;369:57‐62. [DOI] [PubMed] [Google Scholar]
- 13. Ding D, Zhao Q, Guo Q, et al. Progression and predictors of mild cognitive impairment in Chinese elderly: a prospective follow‐up in the Shanghai Aging Study. Alzheimers Dement. 2016;4:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tokuchi R, Hishikawa N, Kurata T, et al. Clinical and demographic predictors of mild cognitive impairment for converting to Alzheimer's disease and reverting to normal cognition. J Neurol Sci. 2014;346(1‐2):288‐292. [DOI] [PubMed] [Google Scholar]
- 15. Ishikawa T, Ikeda M. Mild cognitive impairment in a population‐based epidemiological study. Psychogeriatrics. 2007;7(3):104‐108. [Google Scholar]
- 16. Han JW, Kim TH, Lee SB, et al. Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimers Dement. 2012;8(6):553‐559. [DOI] [PubMed] [Google Scholar]
- 17. Afgin AE, Massarwa M, Schechtman E, et al. High prevalence of mild cognitive impairment and Alzheimer's disease in Arabic villages in northern Israel: impact of gender and education. J Alzheimers Dis. 2012;29(2):431‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Natale G, Clouston S. Incidence of mild cognitive impairment, conversion to probable dementia, and mortality. Am J Alzheimers Dis Other Dement. 2021;36:15333175211012235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275(3):214‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perquin M, Schuller AM, Vaillant M, et al. The epidemiology of mild cognitive impairment (MCI) and Alzheimer's disease (AD) in community‐living seniors: protocol of the MemoVie cohort study, Luxembourg. BMC Public Health. 2012;12:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campbell NL, Unverzagt F, LaMantia MA, Khan BA, Boustani MA. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med. 2013;29(4):873‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Göthlin M, Eckerström M, Rolstad S, Kettunen P, Wallin A. Better prognostic accuracy in younger mild cognitive impairment patients with more years of education. Alzheimers Dement. 2018;10:402‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan WC, Lam LC, Tam CW, et al. Neuropsychiatric symptoms are associated with increased risks of progression to dementia: a 2‐year prospective study of 321 Chinese older persons with mild cognitive impairment. Age Ageing. 2011;40(1):30‐35. [DOI] [PubMed] [Google Scholar]
- 24. Palmer K, Berger A, Monastero R, Winblad B, Bäckman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68(19):1596‐1602. [DOI] [PubMed] [Google Scholar]
- 25. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population‐based perspective. Alzheimers Dement. 2015;11(6):718‐726. [DOI] [PubMed] [Google Scholar]
- 26. Reijmer YD, van den Berg E, Dekker JM, et al. Development of vascular risk factors over 15 years in relation to cognition: the Hoorn Study. J Am Geriatr Soc. 2012;60(8):1426‐1433. [DOI] [PubMed] [Google Scholar]
- 27. Masters MC, Morris JC, Roe CM. Noncognitive” symptoms of early Alzheimer disease: a longitudinal analysis. Neurology. 2015;84(6):617‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013;21(7):685‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition DSM‐V. American Psychiatric Association; 2013. [Google Scholar]
- 30. Qarni T, Salardini A. A multifactor approach to mild cognitive impairment. Semin Neurol. 2019;39(2):179‐187. [DOI] [PubMed] [Google Scholar]
- 31. Olsson E, Byberg L, Karlström B, et al. Vitamin D is not associated with incident dementia or cognitive impairment: an 18‐y follow‐up study in community‐living old men. Am J Clin Nutr. 2017;105(4):936‐943. [DOI] [PubMed] [Google Scholar]
- 32. Backhouse A, Ukoumunne OC, Richards DA, McCabe R, Watkins R, Dickens C. The effectiveness of community‐based coordinating interventions in dementia care: a meta‐analysis and subgroup analysis of intervention components. BMC Health Serv Res. 2017;17(1):717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Etgen T, Sander D, Bickel H, Sander K, Förstl H. Vitamin D deficiency, cognitive impairment and dementia: a systematic review and meta‐analysis. Dement Geriatr Cogn Disord. 2012;33(5):297‐305. [DOI] [PubMed] [Google Scholar]
- 34. Chen PH, Cheng SJ, Lin HC, Lee CY, Chou CH. Risk factors for the progression of mild cognitive impairment in different types of neurodegenerative disorders. Behav Neurol. 2018;2018:6929732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Astivia OLO, Gadermann A, Guhn M. The relationship between statistical power and predictor distribution in multilevel logistic regression: a simulated‐based approach. BMC Med Res Methodol. 2019;19:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitchell AJ. A meta‐analysis of the accuracy of the mini‐mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411‐431. [DOI] [PubMed] [Google Scholar]
- 37. Simard M. The Mini‐Mental State Examination: strengths and weaknesses of a clinical instrument. Can Alzheimer Dis Rev. 1998;12:10‐12. [Google Scholar]
- 38. Ciesielska N, Sokolowski R, Mazur E, Podhorecka M, Polak‐Szabela A, Kedziora‐Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini‐Mental State Examination (MMSE) in Mild Cognitive Impairment (MCI) detection among people aged over 60? Meta‐analysis. Psychiatr Pol. 2016;50(5):1039‐1052. [DOI] [PubMed] [Google Scholar]
- 39. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 40. Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551‐2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS. Montreal Cognitive Assessment Memory Index Score (MoCA‐MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer's disease. J Am Geriatr Soc. 2014;62(4):679‐684. [DOI] [PubMed] [Google Scholar]
- 42. Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ. Alzheimer's Disease Neuroimaging Initiative . Relationship between the montreal cognitive assessment and Mini‐Mental Status Examination for assessments of mild cognitive impairments in older adults. BMC Geriatr. 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]


