Abstract
In 2019, 254 samples were collected from five aquifer systems to evaluate perfluoroalkyl and polyfluoroalkyl substance (PFAS) occurrence in groundwater used as a source of drinking water in the eastern United States. The samples were analyzed for 24 PFAS, major ions, nutrients, trace elements, dissolved organic carbon (DOC), volatile organic compounds (VOCs), pharmaceuticals, and tritium. Fourteen of the 24 PFAS were detected in groundwater, with 60 and 20% of public-supply and domestic wells, respectively, containing at least one PFAS detection. Concentrations of tritium, chloride, sulfate, DOC, and manganese + iron; percent urban land use within 500 m of the wells; and VOC and pharmaceutical detection frequencies were significantly higher in samples containing PFAS detections than in samples with no detections. Boosted regression tree models that consider 57 chemical and land-use variables show that tritium concentration, distance to the nearest fire-training area, percentage of urban land use, and DOC and VOC concentrations are the top five predictors of PFAS detections, consistent with the hydrologic position, geochemistry, and land use being important controls on PFAS occurrence in groundwater. Model results indicate that it may be possible to predict PFAS detections in groundwater using existing data sources.
Keywords: hydrologic position, land use, tritium, pharmaceuticals, modeling, boosted regression tree
Short abstract
PFAS occurrence in groundwater in the eastern United States is variable, but results from this study could help guide future efforts to predict PFAS detections in groundwater.
Introduction
The contamination of groundwater with perfluoroalkyl and polyfluoroalkyl substances (PFAS) is a concern in many countries1−4 because PFAS persist in the environment,5−8 PFAS sources are widespread,5,9,10 and some PFAS are known or suspected to be associated with adverse human-health effects.11−13 Moreover, PFAS in the unsaturated zone can be sources to underlying groundwater systems for decades,7,14 making PFAS in groundwater a long-term public-health concern. The United States does not currently (2022) have nationally enforceable drinking-water standards for PFAS, but in 2016, the U.S. Environmental Protection Agency (EPA) established a drinking-water health advisory level of 70 ng/L for the combined concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA),15 two of the most commonly measured PFAS in groundwater.
Conceptually, the occurrence of PFAS in groundwater should be controlled by land-use (source), hydrologic (transport), and biogeochemical (fate and transport) factors. Several local-scale studies have provided deeper understanding of the source, transport, and fate of PFAS in soils and groundwater by combining PFAS data with data for those factors.7,16,17 A study in Massachusetts used data on groundwater-flow directions and measurements of dissolved oxygen (O2) and boron (B) to help differentiate PFAS in groundwater affected by wastewater effluent from that affected by a fire-training area.7 A study in Japan used measurements of tritium (3H), pharmaceuticals, and personal care products to link PFAS in modern groundwater to sewage sources.18 Measurements of benzene and PFAS and information on enhanced bioremediation activities related to the hydrocarbon contamination improved the understanding of PFAS precursor transformations at a fire-training facility in South Dakota.17 We hypothesize that data for the controlling factors can improve our understanding of the occurrence of PFAS at regional and national scales, such that those data could be used to build large-scale statistical models that predict PFAS occurrence in unmonitored areas, as has been done for arsenic and nitrate.19−21 Such models could be used to guide future sampling efforts and identify areas of high risk for human exposure.
At least one study demonstrated the value of PFAS-source information in predicting PFAS occurrence in drinking-water supplies at the national scale.10 A state-wide study in New Hampshire showed that data on hydrology and PFAS sources can also be predictive of PFAS occurrence in groundwater.22 We are aware of one other study that combined source, hydrologic, and geochemical data to test their power to predict PFAS occurrences in groundwater at a regional scale (state of California).23 Our study expands this type of analysis to a different region of the United States and includes additional geochemical parameters found to be useful in understanding and predicting PFAS in groundwater (e.g., dissolved organic carbon (DOC) and pharmaceuticals).
The purpose of this article is threefold: (1) explore hydrologic, geochemical, and land-use controls on PFAS occurrence in groundwater used as a source of drinking water in the eastern United States, (2) examine chemical co-occurrences with PFAS that could improve understanding of PFAS occurrences, and (3) identify which hydrologic, geochemical, and land-use factors are the strongest predictors of PFAS occurrence using boosted regression tree (BRT) models. Well depth and tritium (3H) provide information on the hydrologic position of samples in the groundwater-flow systems. pH, iron [Fe], B, DOC, pharmaceuticals, VOCs, and other parameters provide information on geochemical conditions, co-occurring chemicals, and potential PFAS sources. Geospatial data provide locations for potential PFAS sources like fire-training facilities, landfills, airports, military bases, and other features.
Materials and Methods
Well Networks
In 2019, 254 wells were sampled in seven networks (Figures 1 and S1; Table S1). The well networks cover 2383 to 164,619 km2 in five principal-aquifer systems: Glacial, Mississippi Embayment, Southeastern Coastal Plain, Stream Valley, and Surficial aquifer systems. The networks were established by the U.S. Geological Survey’s National Water-Quality Assessment (USGS NAWQA) project in 1999 to 2019 and are composed of public-supply (64%), domestic (19%), monitoring (12%), and other (5%) well types (Table S1). A stratified random approach was used to select wells for sampling. Hydrogeologic units that are important sources of water supply were targeted for sampling (stratification step; Table S1). To facilitate random well selection, each hydrogeologic unit was subdivided into 30 to 60 equal-area cells, and one well in each cell was randomly selected to sample from a population of existing wells.24 All wells within the NECBS network are water-table monitoring wells installed in 1999 by randomly selecting one location in each equal-area cell.
Figure 1.
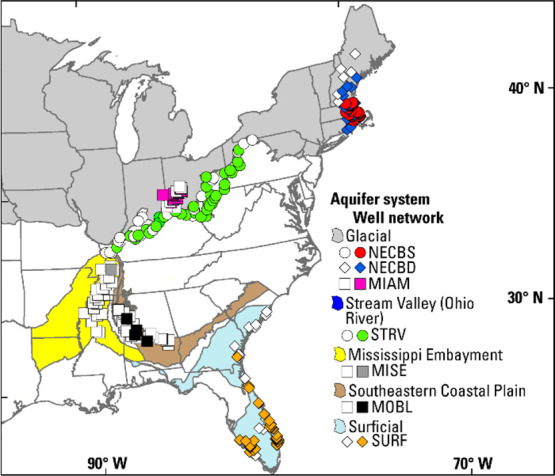
Locations of aquifer systems and well networks. Wells shown with white symbols indicate that PFAS were not detected, and those shown with other colors indicate that PFAS were detected. See Figure S1 for detailed maps of the networks.
In 2012, median percentages of urban land use around the wells ranged from ∼48 (MIAM) to 92% (NECBS) (Table S1).25 Three well networks located in the Glacial aquifer system represent valley-fill aquifers (MIAM) and relatively shallow (NECBS) and deep (NECBD) parts of the stratified-drift aquifer. Metropolitan areas in those networks include Dayton (MIAM) and Boston (NECBS; NECBD). Network MISE represents the Memphis Sand aquifer in the Mississippi Embayment aquifer system. The Memphis metropolitan area is a major user of groundwater from the Memphis Sand aquifer. Network MOBL represents the Black Warrior River aquifer in the Southeastern Coastal Plain aquifer system. Network STRV represents the Ohio River alluvial aquifer in the Stream Valley aquifer system. Metropolitan areas in STRV include Louisville, Cincinnati, and Pittsburgh. Network SURF represents the Surficial aquifer system. Metropolitan areas in SURF include Fort Lauderdale and West Palm Beach. Networks were sampled as a part of the USGS NAWQA, National Hydrologic Monitoring project.
Sample Collection and Laboratory Analysis
Standard USGS protocols were used to collect groundwater samples from the wells prior to any treatment, blending, or pressure tanks.26,27 Samples for major-ion, nutrient, trace-element, and DOC analyses were filtered (0.45 μm Versapor filters) and acidified with nitric acid (major cations and trace elements) or sulfuric acid (DOC), and/or chilled (nutrients, DOC) in the field. Pharmaceutical samples were filtered (0.7 μm glass fiber) and chilled in the field.28 Samples for VOC analysis were unfiltered, acidified with hydrochloric acid, and chilled in the field. 3H samples were unfiltered and unpreserved. Samples for PFAS analysis were unfiltered and chilled in the field. Details of the PFAS sampling protocols are provided in Section S1.
Major-ion, nutrient, trace-element, DOC, pharmaceutical, and VOC samples were analyzed at the USGS National Water-Quality Laboratory in Lakewood, Colorado. Major cations were analyzed by inductively coupled plasma-atomic emission spectroscopy.29 Major anions were analyzed by ion chromatography.29 Trace elements were analyzed by inductively coupled plasma-mass spectroscopy.30 DOC was analyzed by UV-promoted persulfate oxidation and infrared spectrometry.31 Pharmaceuticals were analyzed by liquid chromatography/tandem mass spectrometry using an electrospray ionization source operated in the positive ion mode.32 VOCs were analyzed by purge-and-trap gas chromatography/mass spectrometry.333H was analyzed at the USGS Tritium Laboratory in Menlo Park, California, using electrolytic enrichment and liquid-scintillation counting.34 PFAS were analyzed at the Orlando, Florida SGS Laboratory by liquid chromatography/tandem mass spectrometry with isotope dilution (see Section S2 for details).35 Analyzed compounds include 11 perfluoroalkyl carboxylates (PFCAs), 7 perfluoroalkyl sulfonates (PFSAs), perfluorooctane sulfonamide, 2 perfluorooctane sulfonamidoacetates, and 3 fluorotelomer sulfonates (FTS). Reported concentrations are the sum of linear and branched isomers. The chemical data are listed in Table S2, along with PFAS-specific reporting levels (3.8 to 40 ng/L), and are available in ref (36).
PFAS Quality-Control Data and Analysis
Details about the evaluation of quality-control samples associated with this study are provided in Section S3 and Tables S3–S6. Briefly, field and laboratory blanks were used to examine potential sources of contamination. No PFAS detections were reported for any blank types collected in the field, with the exception of one perfluorobutanoate (PFBA) detection reported for a field blank; detection of PFBA in the associated laboratory method blank indicated that this result was likely affected by contamination at the laboratory. Laboratory-blank results prompted censoring of reported detections for PFBA in 15 groundwater samples in two analytical batches and for a detection of 6:2 FTS in one groundwater sample in a separate batch. Examination of data for routine laboratory reagent spikes and matrix spikes indicated little bias (all median recovery values for PFAS between 85 and 103%); reagent spikes exhibited low variability in recovery. Field replicates and laboratory matrix spike duplicates indicated low variability in PFAS detection and (or) concentration.
Geospatial Data
Geospatial data from publicly available sources and from U.S. Government proprietary sources were used to analyze spatial relations between PFAS detections in groundwater and potential sources of PFAS (Table S7). The potential PFAS sources, data availability, and geospatial analysis are described in Section S4. Five-hundred-meter circular buffers around the wells were used to extract and assign selected land-use data to the wells to examine relations between water chemistry and land use (see Section S4 for more information).
Statistical Methods
Mann–Whitney and Kruskal–Wallis tests, as implemented in the software OriginPro 2018,37 were used on ranked data to test for significant differences in concentrations between selected geochemical groups. Spearman correlation analysis was used to examine relations between concentrations and other variables. An α value of 0.05 was used for each test.
BRT Modeling
BRT models were fit to 57 variables including water-quality parameters, land use, and distance from wells to identified places that are potential sources of PFAS to the environment (Tables S2 and S8). Laboratory results for PFAS were converted to a binary (detect or nondetect), and models were fit using methods like those described in ref (19). Models were developed using the R computing environment (R Team. R: A language and environment for statistical computing version 4.0.3. https://www.R-project.org). The dataset was split into training data (80%) and holdout data (20%) to evaluate model performance (Section S5). The model parameters adjusted during tuning were interaction.depth (the number of levels of trees), n.minobsinnode (the minimum number of observations in terminal nodes), shrinkage (the learning rate), and n.trees (number of trees in the model). Metrics for model performance were calculated using fivefold cross validation and include accuracy, sensitivity, specificity, and the area under the receiver operator characteristics curve (ROC) (Section S5 and Tables S9 and S10). Accuracy is the percentage of total correct predictions, sensitivity is the percentage of successfully predicted detections (true positives), and specificity is the percentage of successfully predicted nondetections (true negatives). The ROC curve is plotted as the true positive divided by the false positive rate for a range of probability thresholds, including the 0.5 threshold chosen. A binary variable indicating whether any of the 24 PFAS were detected was used as the primary dependent variable. However, for comparison, multiple models were constructed with the dependent variable being a binary variable indicating the detection of PFOS, PFOA, PFBS, long chain-length perfluoroalkyl acids (PFAA), short chain-length PFAA, PFCA, or PFSA. Long-chain PFCAs and PFSAs are those with ≥7 and ≥6 perfluorinated carbons, respectively.38
Results and Discussion
PFAS Occurrence in Groundwater
Fourteen of the 24 analyzed PFAS were detected at least once in groundwater samples (Tables S2 and S11). At least one PFAS was detected in 54% of the samples (n = 254), and ≥2 PFAS were detected in 47% of the samples. For drinking-water wells, PFAS were detected in 60% of public-supply wells and 20% of domestic wells. At least two PFAS were detected in 53% of public-supply wells and 10% of domestic wells. The most commonly detected PFAS in our samples include the six measured by the EPA’s Third Unregulated Contaminant Monitoring Rule (UCMR3) program (perfluorobutane sulfonate [PFBS], perfluorohexane sulfonate [PFHxS], PFOS, perfluoroheptanoate [PFHpA], PFOA, and perfluorononanoate [PFNA]),39 plus several other PFSA and PFCA compounds in the 4- to 9-carbon range, most notably PFBA, perfluoropentanoate (PFPeA), and perfluorohexanoate (PFHxA) (Figure 2A). PFOA and PFOS represent two of the three most frequently detected PFAS, and 2.4% (n = 6) of the samples have PFOA+PFOS concentrations greater than the 70 ng/L health advisory level (all are from public-supply wells). Eight of the 10 undetected PFAS are PFAA precursors (n = 5) or PFCA with 12+ carbon atoms (n = 3) (Table S11).
Figure 2.
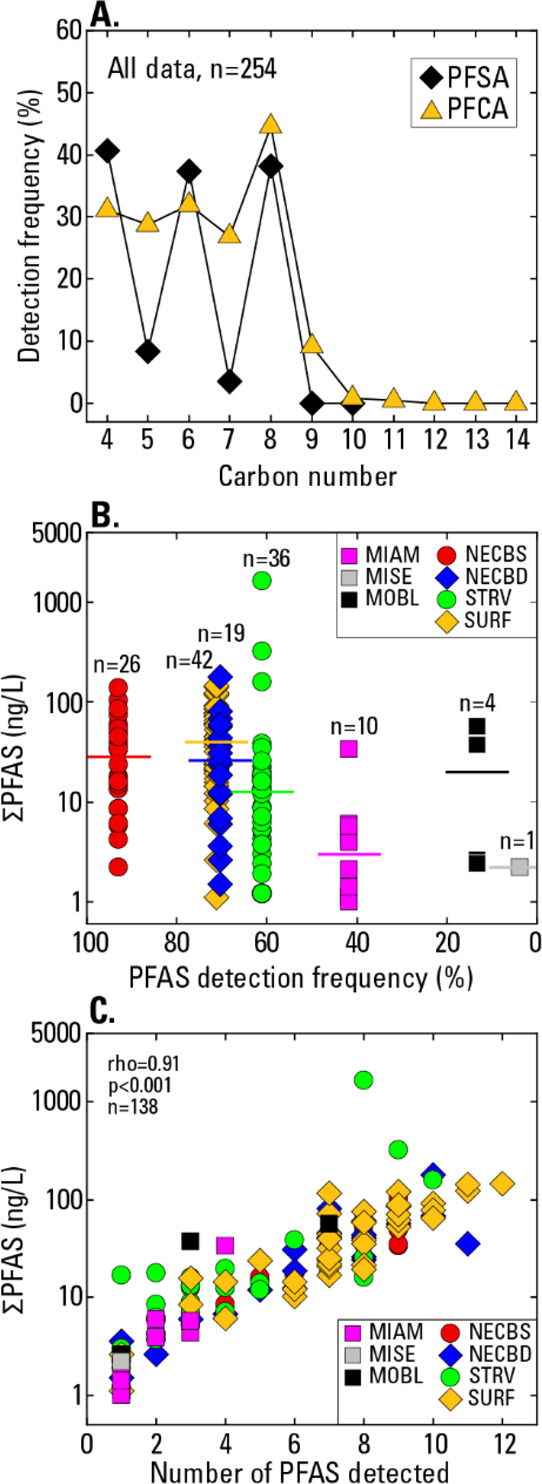
(A) Detection frequency for perfluoroalkyl sulfonates (PFSA) and perfluoroalkyl carboxylates (PFCA) in relation to the carbon number, based on all data, (B) ΣPFAS in relation to PFAS detection frequency by the well network, and (C) ΣPFAS in relation to the number of PFAS detected in samples. In (B), the horizontal lines show median concentrations; n is the number of samples with at least one PFAS detection. In (B and C), ΣPFAS is the summed concentration of detected PFAS.
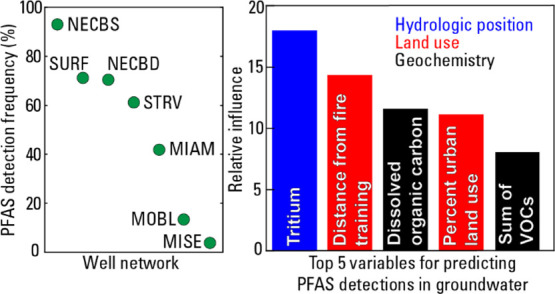
Substantial differences in PFAS detection frequencies and summed concentrations of detected PFAS (ΣPFAS) are observed between the well networks. PFAS detection frequencies range from 3.7 (MISE network) to 92.9% (NECBS) (Figure 2B). Although the wells in NECBS are monitoring wells screened near the water table, not wells that supply drinking water, the data from NECBS are important because they provide information on the quality of recharge that may eventually reach the deeper public-supply wells in NECBD. ΣPFAS medians range from 2.2 (MISE) to 40.0 ng/L (SURF) (Figure 2B). MOBL has a relatively high ΣPFAS median despite its low detection frequency; nevertheless, there is a significant positive correlation (rho = 0.86; p = 0.014) between detection frequency and ΣPFAS medians. The highest ΣPFAS (1645 ng/L) occurs in a public-supply well from STRV.
Relatively little is known about potential effects of complex PFAS mixtures in drinking-water sources on human health,13,40 but better understanding of the composition of those mixtures could help inform toxicity studies. The relatively common occurrence of multiple PFAS in the samples has implications for ΣPFAS and the complexity of PFAS mixtures in groundwater, consistent with previous point-of-use drinking-water studies.41,42 ΣPFAS exhibit a significant positive Spearman correlation with the number of PFAS detected in the samples (rho = 0.91; p < 0.001) (Figure 2C), also consistent with results from the UCMR3 program.3 Networks NECBS, NECBD, and SURF have relatively large fractions of samples containing >6 PFAS (37 to 54%) (Figure S2A), which may be related to the large fractions of urban land use in 500 m buffers around those wells (74 to 92%) (Table S1). Not surprisingly, networks with the largest numbers of co-occurring PFAS (NECBS, NECBD, SURF, and STRV) also have the largest numbers of unique PFAS mixtures relative to the number of samples in the networks (Figure S2B). In NECBS, 68% of the samples contain combinations of two or more PFAS that are unique to that sample. Overall, three PFAS occur in ≥80% of the mixtures (PFOA > PFBS > PFOS), but the dominant PFAS in mixtures vary between networks. Network NECBS has three PFAS that occur in ≥80% of the mixtures (PFOA > PFOS > PFBS), whereas SURF has 7 PFAS that occur in ≥80% of the mixtures (PFOA > PFHxS > PFOS = PFHxA > PFBS = PFPeA = PFHpA) (Figure S2C).
Hydrologic Controls on PFAS Occurrence
Hydrologic characteristics of groundwater systems could influence the occurrence of PFAS in groundwater, yet data describing those characteristics are not commonly examined in regional PFAS studies.22 The position of a groundwater sample in the flow system relative to the land surface and the age of groundwater relative to the age of PFAS sources are important with respect to PFAS occurrence in groundwater because PFAS are derived from modern land-surface sources. Data for well depth and 3H in groundwater indicate that the samples collected for this study represent a broad spectrum of hydrologic positions and age categories.
There are significant differences in well depths between the networks (Figure S3A), with median depths ranging from 7.7 (NECBS) to 88.4 m (MISE). There are also significant differences in 3H concentrations between the well networks (Figure S3B), with median concentrations ranging from 0.1 tritium units (TU) (MISE) to 4.7 TU (STRV). Generally, 3H concentrations less than about 0.1 to 0.5 TU in groundwater collected in 2019 indicate that the groundwater was recharged before the start of above-ground nuclear weapon testing in 1953 (referred to as premodern water in this article).43 More precise estimates of the threshold 3H concentration for premodern water depend on a sample’s location and collection date due to spatial variations in 3H concentrations in precipitation and radioactive decay. Groundwater 3H data are used to assign the samples to one of three age categories using previously developed methodology and datasets for 3H in U.S. precipitation:43,44 premodern (pre-1953 recharge), modern (recharged during or after 1953), or mixed (mixture of premodern and modern recharge). Threshold 3H concentrations (TU) for the premodern and modern age categories are 0.3, 2.8 (NECBS; NECBD); 0.2, 2.3 (MIAM); 0.2, 1.9 (STRV); 0.1, 0.6 (SURF); and 0.1, 1.2 (MISE; MOBL). The cutoff date for premodern water (pre-1953) is close to the date when PFAS started to be widely used (∼1950);9 thus, knowing age categories of the samples could help characterize the risk of groundwater contamination with PFAS. Samples of modern and mixed-age groundwater contain at least some water recharged after the start of widespread PFAS use, whereas samples in the premodern age category were probably recharged prior to the widespread use of PFAS. Moreover, modern samples are from wells with significantly shallower depths than premodern samples (Figure S2C). Only the shallowest network (NECBS), with the highest PFAS detection frequency (Figure 2B), has 100% of its samples assigned to the modern age category (Figure 3A and Table S2). Two other networks (NECBD; MIAM) have >95% of their samples assigned to the modern category. The four remaining networks have samples in all three age categories, with MISE and MOBL having 44 to 54% of their samples in the premodern age category and the lowest PFAS detection frequencies.
Figure 3.
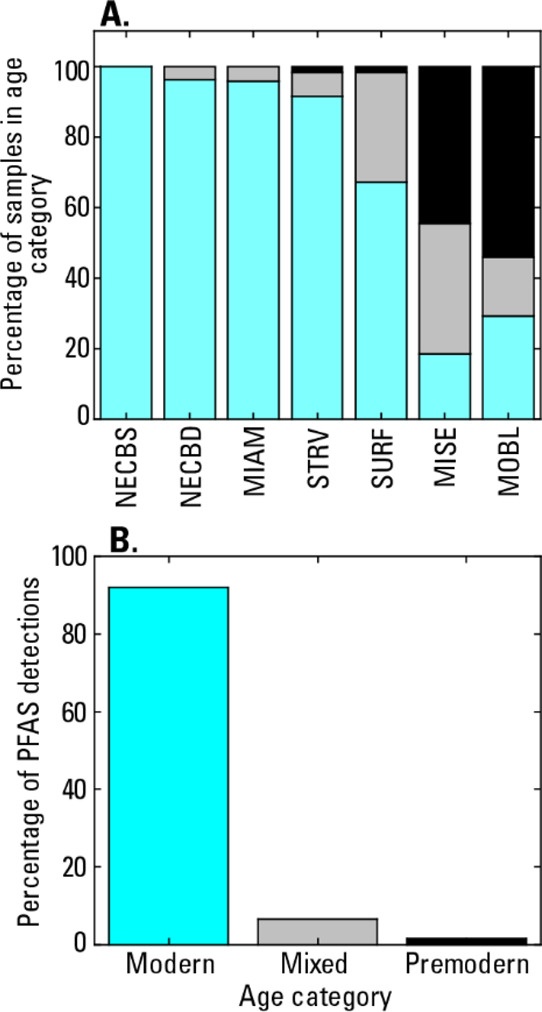
(A) Percentage of samples in each of three groundwater age categories (modern, mixed, and premodern), by well networks and (B) percentage of PFAS detections that occur in each age category. See the text for 3H concentrations used to define age categories in each well network.
The relations between depth, age categories, and onset of widespread PFAS use imply that the likelihood of detecting PFAS in groundwater decreases with increasing well depth. For example, network NECBS is characterized by very shallow wells, high fraction of modern water, and a high PFAS detection frequency, whereas MISE is characterized by deep wells, low fraction of modern water, and a low PFAS detection frequency (Figures 2B, 3A and S3A). Overall, samples that contain a PFAS detection have significantly shallower well depths and higher 3H concentrations than samples with no detections (Table S12), consistent with the hydrologic position and groundwater age being controlling factors for PFAS occurrence in groundwater. In samples that have both 3H data and PFAS detections (n = 136), 92% of the detections occur in modern water and about 99% occur in samples with at least some modern water (modern + mixed) (Figure 3B). The level of protection against PFAS contamination provided by depth and age could decrease over time as shallow groundwater moves deeper into an aquifer system.
Geochemical Controls on PFAS Occurrence
Geochemical characteristics of groundwater like pH and concentrations of DOC, divalent cations, and chloride (Cl) could affect PFAS sorption to aquifer solids. PFAS sorption, for example, can be greater under conditions of low pH,7,45 elevated concentrations of divalent cations,46,47 or lower concentrations of DOC and Cl.47−49 Reductive dissolution of manganese (Mn) and Fe oxides in anoxic groundwater could reduce the sorption capacity of solids or mobilize PFAS already adsorbed to those solids.7 Values of pH and concentrations of selected inorganic constituents and DOC indicate that there are significant differences in geochemical characteristics between the networks (Figure S4). For example, median pH values range from 5.6 (NECBS) to 7.1 (STRV). Median Ca + Mg, DOC, and Mn + Fe concentrations range from 2.88 (MOBL) to 128 (MIAM) mg/L, <0.23 (MISE) to 7.6 (SURF) mg/L, and 0.8 (MISE) to 357 (SURF) μg/L, respectively.
Concentrations of DOC, Cl, SO4, Ca + Mg, and Mn + Fe are significantly higher in samples containing PFAS detections than in samples with no detections; however, pH is not significantly different between the two PFAS groups (Table S12). The similarity in pH between the two PFAS groups is due in part to high PFAS detection frequencies in networks with low (NECBS, NECBD median pH = 5.9) and high (STRV, SURF median pH = 7.0) pH values. In those networks, PFAS-source characteristics may be more dominant controls on PFAS occurrence than pH. Higher DOC concentrations in samples containing PFAS detections are consistent with previous studies indicating that elevated DOC concentrations could promote PFAS mobility through electrostatic or hydrophobic interactions in the dissolved phase or by competing with PFAS for sorption sites.48,49 Competition for sorption sites between negatively charged PFAS and inorganic anions could also help explain the higher Cl and SO4 concentrations in samples containing PFAS detections.47,48 In contrast, previous studies indicate that increasing concentrations of divalent cations could increase PFAS sorption by reducing the negative charge on soil surfaces, thereby reducing the electrostatic repulsion between the surface and negatively charged PFAS.45 The higher Ca + Mg concentrations in samples containing PFAS detections appear to be inconsistent with those results, but the observation could be influenced by Cl and SO4 concentrations, given the competing effects of anions and cations on PFAS sorption. The relation between PFAS detections and Ca + Mg appears to be more consistent with previous studies when Ca + Mg concentrations are normalized to Cl or SO4 concentrations. Ca + Mg/Cl and Ca + Mg/SO4 ratios are significantly lower in samples that contain PFAS detections than in samples with no detections (Table S12). The relation between PFAS detections and Mn + Fe is consistent with reductive dissolution of Mn and Fe oxides in anoxic groundwater that could reduce the sorption capacity of solids or mobilize PFAS already adsorbed to those solids. Concentrations of Mn + Fe, Mn, and Fe are not significantly correlated with urban land use, but there is a significant inverse correlation (p < 0.001) between well depth and Mn (but not Fe). Thus, the PFAS, Mn + Fe relation could also more generally reflect higher pollution loading near the land surface in some settings that results in more reducing conditions and Mn reduction.
Land-Use Controls on PFAS Occurrence
Urban areas could be expected to have a greater density of potential PFAS sources compared to agricultural and undeveloped landscapes, although biosolids are recognized as a potential PFAS source on agricultural lands.50 There is a significantly larger percentage of urban land within 500 m of wells that contain PFAS detections than near wells with no detections (Table S13), whereas there are significantly smaller percentages of agricultural and undeveloped lands within 500 m of wells that contain PFAS detections than near wells with no detections. The relation between PFAS and urbanization is important because it implies that the risk of PFAS contamination of groundwater could increase as urbanization encroaches on the agricultural and undeveloped land.
More specific to PFAS occurrences in groundwater than land use is information on potential PFAS sources near the sampled wells. U.S. Government proprietary databases provided this study with geospatial information for facility categories that could be potential PFAS sources (Table S8). Among the source categories are public-use airports, chemical manufacturing facilities, fire-training facilities, and landfills. These source categories and most others listed in Table S8 are significantly closer to wells that contain PFAS detections than to wells with no detections (Table S13). Moreover, the cumulative number of potential PFAS sources <5 km from the sampled wells is significantly higher for wells containing PFAS detections than for wells with no detections. It is unknown which, if any, of the potential sources listed in Table S8 used or released PFAS to the environment; nevertheless, the results are consistent with the conceptual model of PFAS concentrations in groundwater increasing with decreasing distance to PFAS sources and increasing density of PFAS sources. Similar spatial relations have been observed in other regional studies.10
Differences in the number of unique PFAS mixtures in the well networks could be related to the diversity of PFAS sources in the networks that is not captured in general land-use data. NECBD has a larger fraction of samples with unique PFAS mixtures than SURF (Figure S2B), even though SURF (86%) has a larger fraction of urban land within 500 m of its wells than NECBD (74%). The number of potential PFAS sources within 5 km of NECBD wells is significantly higher than the number within 5 km of SURF wells (Figure S5A). NECBD and SURF appear to have similar numbers of septic systems in 500 m buffers around their wells (Figure S5B), based on estimates of N input to septic systems in the United States,51 which is used here as a proxy for septic-system density. Septic systems are a suspected source of PFAS in groundwater.52 Differences in the number and composition of PFAS mixtures between the networks could also be affected by PFAS degradation and sorption processes. As previously discussed, the differences in geochemical characteristics of groundwater in the networks could result in different amounts of PFAS sorption to aquifer solids in the networks.
PFAS Co-Occurrence with Other Chemicals
Linking the PFAS detections in our samples to specific PFAS sources is beyond the scope of this study, given its regional scale. Nevertheless, PFAS co-occurrences with other chemicals can provide useful information about the chemical complexity of PFAS-contaminated groundwater and, more generally, about PFAS sources. VOC and pharmaceutical detection frequencies are substantially higher in samples that contain PFAS detections (62 and 33%, respectively) than in samples with no detections (21 and 2%, respectively) (Table S12). For samples from public-supply wells that have PFAS detections, 68, 37, and 24% also have at least one detection of a VOC, pharmaceutical, or VOC + pharmaceutical, respectively. ΣVOCs (median = 0.07 μg/L) and Σpharmaceuticals (median = 0.025 μg/L) are low, but their presence indicates that the chemical inventory in groundwater contaminated with PFAS is complex. This finding is important because estimating the toxicity of complex chemical mixtures in water can be challenging.53 The top 10 VOCs co-occurring with PFAS include chlorodifluoromethane (CDFM), the most common co-occurring VOC, and 1,1-dichloroethane (Figure S6A). The co-occurrence of CDFM and 1,1-dichloroethane with PFAS was also noted in the UCMR3 dataset.3 The most common co-occurring pharmaceuticals include carbamazepine > gabapentin > meprobamate (Figure S6B).
The co-occurrence of PFAS with other chemicals does not necessarily indicate that they are from the same source, particularly in urban areas where independent sources could coexist in proximity to each other. PFAS and pharmaceuticals, however, are known to co-occur in sources associated with human-waste disposal (e.g., septic-system/landfill leachate and wastewater treatment plants).10,54−56 Those types of sources could account for some of the PFAS in the 37% of PFAS-contaminated samples from public-supply wells that contain at least one pharmaceutical. In NECBD, 41% of the public-supply wells with PFAS detections also have at least one detection of a pharmaceutical. That network has a relatively high abundance of septic systems within 500 m of the wells (Figure S5B), and nitrate and B concentrations, for the most part, are significantly higher in NECBD samples that contain PFAS or pharmaceutical detections than in samples with no detections (Table S14). Nitrate and B can have elevated concentrations in groundwater due to contamination with septic leachate.55 The overall dataset also appears to be consistent with at least some PFAS in the samples with co-occurring PFAS and pharmaceutical detections being associated with septic/landfill leachate, wastewater effluent, or stormwater runoff.52,54,56,57 Samples with co-occurring detections of PFAS and pharmaceuticals have significantly higher concentrations of B and total N than samples that do not have co-occurring PFAS and pharmaceutical detections (Figure S7). All three sources can have elevated B and total N concentrations,55,58,59 so they could produce similar chemical patterns with respect to PFAS, pharmaceuticals, B, and total N.
VOCs known to be associated with PFAS sources could also be potentially useful PFAS-source indicators in groundwater. Fuel hydrocarbons like benzene co-occur with PFAS in groundwater in some fire-training areas.17 CDFM, the most common co-occurring VOC in our dataset, is used to manufacture monomers like tetrafluoroethene, which is an important building block in fluoropolymer production. Historically, the polymerization process used PFOA and PFOS as emulsifiers.60 Thus, co-occurrences of CDFM, PFOA, and PFOS in groundwater could indicate that some of the PFAS are from fluoropolymer manufacturing. CDFM has also been used as a replacement for chlorofluorocarbons in air conditioning, refrigerants, and aerosols, resulting in increasing atmospheric CDFM concentrations.61 Atmospherically derived CDFM could enter groundwater in recharge that is unrelated to PFAS sources, although CDFM concentrations in air-saturated water (0.0009 μg/L at 20 °C)61 are very low compared to the concentrations measured in this study. In this study, the median detected CDFM concentration is 0.075 μg/L, ∼85 times higher than the concentration in air-saturated water. PFAS detections co-occur in 90% of the samples that contain a CDFM detection, and 96% of the samples with co-occurring CDFM and PFAS also contain PFOA and/or PFOS. In the SURF network, where 70% of the CDFM detections occur, wells with co-occurring CDFM and PFAS detections are significantly closer to chemical manufacturing facilities (median distance = 4.3 km) than wells without co-occurring detections (median = 12.1 km) (Figure S8). We do not know if PFAS and CDFM were used or released to the environment at those facilities, but the chemical and spatial data are consistent with at least some of the PFAS in samples with co-occurring CDFM and PFAS detections being sourced from chemical manufacturing. SURF samples with co-occurring PFAS and CDFM detections and samples with only CDFM detections do not have significantly higher B or total N concentrations than samples without those detections (Figure S9), suggesting that the PFAS + CDFM co-occurrences are not associated with human-waste disposal. The regional analyses of co-occurring PFAS-pharmaceuticals and PFAS-CDFM provide internally consistent hypotheses about some PFAS being sourced from human-waste-disposal activities and chemical manufacturing, respectively, that need to be tested with data from local-scale studies.
BRT Models
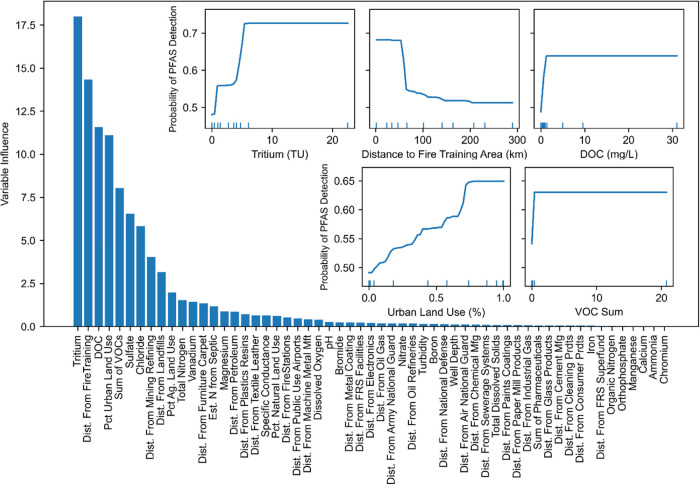
Models were developed to predict PFAS detections using geochemical and geospatial data as predictor variables. The model selected, with the dependent variable being a binary variable indicating whether one or more of the 24 PFAS were detected, was constructed using 1000 trees, an interaction depth of 2, shrinkage (learning rate) of 0.002, and 10 as the minimum number of observations in terminal nodes of a tree. The accuracy, sensitivity, specificity, and ROC were 0.91, 0.93, 0.89, and 0.97 for the training data and 0.84, 0.96, 0.72, and 0.90 for the holdout data, respectively, indicating excellent model performance (Table S9). The relative importance of the model variables (Figure 4) indicates that the top five predictors of PFAS detection are 3H concentration, distance to the nearest fire-training area, DOC concentration, percentage of urban land use, and ΣVOC. Variables potentially related to PFAS sorption (e.g., SO4 and Cl) are also among the top 10 predictors. Overall, the model results are consistent with the hydrologic position, groundwater geochemistry, and land use being important controls on PFAS occurrence in groundwater. 3H is rarely collected in assessments of PFAS contamination of groundwater, but given its apparent predictive power, inclusion of 3H in such efforts seems warranted.
Figure 4.
Relative influence of potential predictor variables sorted from largest to smallest contribution to the model is shown in blue bars. Inset panels are partial dependence plots for tritium, distance to the nearest fire-training area, DOC, the percentage urban land use around each well, and the sum of detected VOC concentrations. Partial dependence plots show the relationship between probability of PFAS detection and the predictor variable. Blue tick marks along the x axis of the inset panels indicate the variable minimum, maximum, and deciles of the model training dataset. Plateaus in the inset graphs are usually in areas with little or no data. FRS, facility registry service.
Partial dependence plots can help visualize the relationship between the probability of detection and the predictor variable.62 Partial dependence plots (Figure 4) indicate increasing probability of PFAS detection with increasing 3H, DOC, and VOC concentrations and increasing urban land-use percentages. Conversely, the probability of PFAS detection decreases with increasing distance from a fire-training area. These partial dependence plots are in line with expectations discussed in this article. Higher 3H concentrations are indicative of more modern water, urban areas have been associated with PFAS occurrence, and fire-training areas are known point sources of PFAS.7,17,63 As discussed above, VOC detection frequencies and DOC concentrations were higher in samples containing PFAS detections: these associations are reflected in the model, which found that DOC and VOCs have large relative influence on the model.
Different models were constructed with the dependent variable being a binary variable indicating the detection of PFOS, PFOA, PFBS, long chain-length PFAA, short chain-length PFAA, PFCA, or PFSA. In all scenarios, 3H, DOC, and VOC concentrations and distance to the nearest fire-training area were in the top 10 most influential predictor variables (Table S10). The percentage of urban land use was also in the top 10 most influential predictor variables for all scenarios except for the model predicting PFOA, where it was number 11. These results indicate robustness of the model.
The model developed here indicates that it may be possible to predict PFAS detections in drinking-water wells using data sources already available, consistent with another recent study that used statistical models to predict susceptibility of private wells to PFAS contamination.22 The USGS National Water Information System (NWIS)64 contains many years of water-quality data, including the chemical parameters used here, from wells across the U.S. Distance metrics could likewise be developed from existing geospatial datasets.
Limitations and Implications
The data represent a conservative estimate of human exposure to PFAS because samples were collected prior to treatment. Nevertheless, many water-treatment processes do not effectively remove PFAS.41,42 Also, treatment is often absent for households that rely on private domestic wells as a source of drinking water. Thus, the PFAS results are likely to be relevant to human exposure at the tap. Although the dataset represents a broad spectrum of hydrogeologic settings in the eastern United States, important aquifer systems like the Northern Atlantic Coastal Plain system and the fracture-flow dominated Floridan carbonate-rock and Piedmont crystalline-rock systems are not represented. Likewise, the 24 PFAS analyzed here may not represent the total PFAS inventory in groundwater, given the large number of PFAS reported in commercial use and the environment.9 Nevertheless, the dataset provides insight into the relations between PFAS in groundwater and large numbers of hydrologic, geochemical, and geospatial variables. Broadly, groundwater affected by modern anthropogenic activity appears to be associated with PFAS, given significant relations between PFAS detections and variables such as 3H, urban land use, VOCs, and pharmaceuticals. BRT modeling indicates that it is possible to predict PFAS occurrence based on the explanatory variables investigated here. Therefore, future sampling related to PFAS may consider adding targeted analyses such as 3H, DOC, SO4, and Cl to build comprehensive datasets that may allow for national prediction of PFAS occurrence.
Acknowledgments
We thank Andrew Lindstrom, Michael Barrette, Drew Pilant, and Nicholas Spalt (U.S. Environmental Protection Agency) for sharing geospatial data on potential PFAS sources and insights regarding PFAS occurrences in the environment. At the time of publication, the geospatial data from the EPA and U.S. Department of Homeland Security were not publicly available owing to proprietary and sensitivity restrictions. This work was funded by the USGS National Water-Quality Assessment (NAWQA) Project. This article was improved by the reviews of Paul Bradley, Robert Kent, and anonymous reviewers for the journal. Any use of trade, firm, or product names is for description purposes only and does not imply endorsement by the U.S. Government.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c04795.
The authors declare no competing financial interest.
Supplementary Material
References
- Gobelius L.; Hedlund J.; Dürig W.; Tröger R.; Lilja K.; Wiberg K.; Ahrens L. Per-and polyfluoroalkyl substances in Swedish groundwater and surface water: Implications for environmental quality standards and drinking water guidelines. Environ. Sci. Technol. 2018, 52, 4340–4349. 10.1021/acs.est.7b05718. [DOI] [PubMed] [Google Scholar]
- Sharma B. M.; Bharat G. K.; Tayal S.; Larssen T.; Bečanová J.; Karásková P.; Whitehead P. G.; Futter M. N.; Butterfield D.; Nizzetto L. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: Emissions and implications for human health. Environ. Pollut. 2016, 208, 704–713. 10.1016/j.envpol.2015.10.050. [DOI] [PubMed] [Google Scholar]
- Guelfo J. L.; Adamson D. T. Evaluation of a national data set for insights into sources, composition, and concentrations of per- and polyfluoroalkyl substances (PFAS) in U.S. drinking water. Environ. Pollut. 2018, 236, 505–513. 10.1016/j.envpol.2018.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M.; Kuroda K.; Sato N.; Fukushi T.; Takizawa S.; Takada H. Groundwater pollution by perfluorinated surfactants in Tokyo. Environ. Sci. Technol. 2009, 43, 3480–3486. 10.1021/es803556w. [DOI] [PubMed] [Google Scholar]
- Prevedouros K.; Cousins I. T.; Buck R. C.; Korzeniowski S. H. Sources, fate, and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Wang N.; Liu J.; Buck R. C.; Korseniowski S. H.; Wolstenholme B. W.; Folsom P. W.; Sulecki L. M. 6:2 fluorotelomer sulfonate aerobic biotransformation in activated sludge of waste water treatment plants. Chemosphere 2011, 82, 853–858. 10.1016/j.chemosphere.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Weber A. K.; Barber L. B.; LeBlanc D. R.; Sunderland E. M.; Vecitis C. D. Geochemical and hydrologic factors controlling subsurface transport of poly- and perfluoroalkyl substances, Cape Cod, Massachusetts. Environ. Sci. Technol. 2017, 51, 4269–4279. 10.1021/acs.est.6b05573. [DOI] [PubMed] [Google Scholar]
- Newell C. J.; Adamson D. T.; Kulkarni P. R.; Nzeribe B. N.; Stroo H. Comparing PFAS to other groundwater contaminants: Implications for remediation. Remediation 2020, 30, 7–26. 10.1002/rem.21645. [DOI] [Google Scholar]
- Buck R. C.; Franklin J.; Berger U.; Conder J. M.; Cousins I. T.; de Voogt P.; Jensen A. A.; Kannan K.; Mabury S. A.; van Leeuwen S. P. J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manage. 2011, 7, 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. C.; Andrews D. Q.; Lindstrom A. B.; Bruton T. A.; Schaider L. A.; Grandjean P.; Lohmann R.; Carigan C. C.; Blum A.; Balan S. A.; Higgins C. P.; Sunderland E. M. Drinking water linked to industrial sites, military fire training areas, and wastewater treatments plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinking Water Health Advisory for Perfluorooctanoic Acid; U.S. Environmental Protection Agency, 2016. https://www.epa.gov/sites/production/files/2016-05/documents/pfoa_health_advisory_final-plain.pdf (accessed November 16, 2020). [Google Scholar]
- Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS); U.S. Environmental Protection Agency, 2016. https://www.epa.gov/sites/production/files/2016-05/documents/pfos_health_advisory_final-plain.pdf (accessed November 16, 2020). [Google Scholar]
- Sunderland E. M.; Hi X. C.; Dassuncao C.; Tokranov A. K.; Wagner C. C.; Allen J. G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFAS) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B.; Zeng J.; Brusseau M. L. A mathematical model for the release, transport, and retention of per- and polyfluoroalkyl substances (PFAS) in the vadose zone. Water Resour. Res. 2019, 56, e2019WR026667 10.1029/2019WR026667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifetime Health Advisories and Health Effects Support Documents for Perfluorooctanoic Acid and Perfluorooctane Sulfonate; U.S. Environmental Protection Agency, 2016, EPA 505-F-17-001. https://www.govinfo.gov/content/pkg/FR-2016-05-25/pdf/2016-12361.pdf (accessed November 16, 2020).
- Davis K. L.; Aucoin M. D.; Larsen B. S.; Kaiser M. A.; Hartten A. S. Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere 2007, 67, 2011–2019. 10.1016/j.chemosphere.2006.11.049. [DOI] [PubMed] [Google Scholar]
- McGuire M. E.; Schaefer C.; Richards T.; Backe W. J.; Field J. A.; Houtz E.; Sedlak D. L.; Guelfo J. L.; Wunsch A.; Higgins C. P. Evidence of remediation-induced alteration of subsurface poly- and perfluoroalkyl substance distribution at a former firefighter training area. Environ. Sci. Technol. 2014, 48, 6644–6652. 10.1021/es5006187. [DOI] [PubMed] [Google Scholar]
- Kuroda K.; Murakami M.; Oguma K.; Takada H.; Takizawa S. Investigating sources and pathways of perfluoroalkyl acids (PFAAs) in aquifers in Tokyo using multiple tracers. Sci. Total Environ. 2014, 488–489, 51–60. 10.1016/j.scitotenv.2014.04.066. [DOI] [PubMed] [Google Scholar]
- Ayotte J. D.; Nolan B. T.; Gronberg J. A. Predicting arsenic in drinking water wells of the Central Valley, California. Environ. Sci. Technol. 2016, 50, 7555–7563. 10.1021/acs.est.6b01914. [DOI] [PubMed] [Google Scholar]
- Nolan B. T.; Hitt K. J. Vulnerability of shallow groundwater and drinking-water wells to nitrate in the United States. Environ. Sci. Technol. 2006, 40, 7834–7840. 10.1021/es060911u. [DOI] [PubMed] [Google Scholar]
- Lombard M. A.; Bryan M. S.; Jones D. K.; Bulka C.; Bradley P. M.; Backer L. C.; Focazio M. J.; Silverman D. T.; Toccalino P.; Argos M.; Gribble M. O.; Ayotte J. D. Machine learning models of arsenic in private wells throughout the conterminous United States as a tool for exposure assessment in human health studies. Environ. Sci. Technol. 2021, 55, 5012–5023. 10.1021/acs.est.0c05239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. C.; Ge B.; Ruyle B. J.; Sun J.; Sunderland E. M. A statistical approach for identifying private wells susceptible to perfluoroalkyl substances (PFAS) contamination. Environ. Sci. Technol. Lett. 2021, 8, 596–602. 10.1021/acs.estlett.1c00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S.; Dixit A. A machine learning approach for prioritizing groundwater testing for per- and polyfluoroalkyl substances (PFAS). J. Environ. Manage. 2021, 295, 113359 10.1016/j.jenvman.2021.113359. [DOI] [PubMed] [Google Scholar]
- Scott J. C.Computerized stratified random site-selection approaches for design of a groundwater-quality sampling network; U.S. Geol. Surv. Water-Resour. Invest. Rep. 90–4101; USGS, 1990. [Google Scholar]
- Falcone J. A.U.S. conterminous wall-to-wall anthropogenic land use trends (NWALT), 1974–2012; U.S. Geological Survey Data Series 948; USGS, 2015. [Google Scholar]
- Wilde F. D.Field measurements; U.S. Geological Survey Techniques of Water-Resources Invest. book 9, chap. A6, with sec. 6.0–6.8; USGS, variously dated. http://pubs.water.usgs.gov/twri9A6/ (accessed December 1, 2020). [Google Scholar]
- U.S. Geological Survey . Processing of water samples; U.S. Geological Survey Techniques of Water-Resources Investigations, book 9, chapter A5; USGS, 2002. 10.3133/twri09A5 (accessed December 1, 2020). [DOI] [Google Scholar]
- Bexfield L. M.; Toccalino P. L.; Belitz K.; Foreman W. T.; Furlong E. T. Hormones and pharmaceuticals in groundwater used as a source of drinking water across the United States. Environ. Sci. Technol. 2019, 53, 2950–2960. 10.1021/acs.est.8b05592. [DOI] [PubMed] [Google Scholar]
- Fishman M. J.Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory--Determination of inorganic and organic constituents in water and fluvial sediments; U.S. Geological Survey Open-File Report 93–125; USGS, 1993. [Google Scholar]
- Garbarino J. R.; Kanagy L. K.; Cree M. E.. Determination of elements in natural-water, biota, sediment, and soil samples using collision/reaction cell inductively coupled plasma–mass spectrometry; U.S. Geological Survey Techniques and Methods, Book 5, Sec. B, Chap. 1; USGS, 2006. [Google Scholar]
- Brenton R. W.; Arnett T. L.. Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory--Determination of dissolved organic carbon by UV-promoted persulfate oxidation and infrared spectrometry; U.S. Geological Survey Open-File Rep. 92–480; USGS, 1993. [Google Scholar]
- Furlong E. T.; Noriega M. C.; Kanagy C. J.; Kanagy L. K.; Coffey L. J.; Burkhardt M. R.. Determination of human-use pharmaceuticals in filtered water by direct aqueous injection–high-performance liquid chromatography/tandem mass spectrometry; U.S. Geological Survey Techniques and Methods, Book 5, Chap. B10; USGS, 2014. [Google Scholar]
- Rose D. L.; Sandstrom M. W.; Murtagh L. K.. Determination of heat purgeable and ambient purgeable volatile organic, compounds in water by gas chromatography/mass spectrometry; U.S. Geological Survey Techniques and Methods, book 5, chap. B12; USGS, 2016. [Google Scholar]
- Thatcher L. L.; Janzer V. J.; Edwards K. W.. Methods for determination of radioactive substances in water and fluvial sediments; U.S. Geological Survey Techniques of Water-Resources Investigations chap. A–5; USGS, 1977. [Google Scholar]
- PFAS Testing; https://www.sgsgroup.us.com/en/environment-health-and-safety/testing-services/product-types/specialty-testing-and-forensics/pfas-testing (accessed December 3, 2020).
- McMahon P. B.; Bexfield L. M.; Tokranov A. K.; Johnson T. D.; Watson E.; Lindsey B. D.. Environmental and quality-control chemical data, and geospatial data, collected by the USGS National Water-Quality Assessment Project for Per- and Polyfluoroalkyl Substances in Groundwater Used as a Source of Drinking Water in the Eastern United States; U.S. Geological Survey Data Release; USGS, 2021. [Google Scholar]
- OriginLab Corporation, 2018 . OriginPro 2018 Software. http://www.OriginLab.com. (accessed April 5, 2021).
- Wang Z.; DeWitt J. C.; Higgins C. P.; Cousins I. T. A never-ending story of per- and polyfluoroalkyl substances (PFASs). Environ. Sci. Technol. 2017, 51, 2508–2518. 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Third Unregulated Contaminant Monitoring Rule. U.S. Environmental Protection Agency, 2016. Third Unregulated Contaminant Monitoring Rule | Monitoring the Occurrence of Unregulated Drinking Water Contaminants; US EPA; (accessed January 11, 2021).
- Goodrum P. E.; Anderson J. K.; Luz A. L.; Ansell G. K. Application of a framework for grouping and mixtures toxicity assessment of PFAS: A closer examination of dose-additivity approaches. Toxicol. Sci. 2020, 179, 262. 10.1093/toxsci/kfaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P. M.; LeBlanc D. R.; Romanok K. M.; Smalling K. L.; Focazio M. J.; Cardon M. C.; Clark J. M.; Conley J. M.; Evans N.; Givens C. E.; Gray J. L.; Gray L. E.; Hartig P. C.; Higgins C. P.; Hladik M. L.; Iwanowicz L. R.; Loftin K. A.; McCleskey R. B.; McDonough C. A.; Medlock-Kakaley E. K.; Weis C. P.; Wilson V. S. Public and private tapwater: Comparative analysis of contaminant exposure and potential risk, Cape Cod, Massachusetts, USA. Environ. Int. 2021, 152, 106487 10.1016/j.envint.2021.106487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone J. S.; Vigo C.; Boone T.; Byrne C.; Ferrario J.; Benson R.; Donohue J.; Simmons J. E.; Kolpin D. W.; Furlong E. T.; Glassmeyer S. T. Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci. Total Environ. 2019, 653, 359–369. 10.1016/j.scitotenv.2018.10.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey B.D.; Jurgens B.C.; Belitz K.. Tritium as an indicator of modern, mixed, and premodern groundwater age; U.S. Geological Survey Scientific Investigations Report 2019–5090; USGS, 2019. [Google Scholar]
- Michel R. L.; Jurgens B. C.; Young M. B.. Tritium deposition in precipitation in the United States, 1953–2012; U.S. Geological Survey Scientific Investigations Report 2018–5086; USGS, 2018. [Google Scholar]
- Oliver D. P.; Li Y.; Orr R.; Nelson P.; Barnes M.; McLaughlin M. J.; Kookana R. S. The role of surface charge and pH changes in tropical soils on sorption behavior of per- and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2019, 673, 197–206. 10.1016/j.scitotenv.2019.04.055. [DOI] [PubMed] [Google Scholar]
- Higgins C. P.; Luthy R. G. Sorption of perfluorinated surfactants on sediments. Environ. Sci. Technol. 2006, 40, 7251–7256. 10.1021/es061000n. [DOI] [PubMed] [Google Scholar]
- Lyu X.; Liu X.; Sun Y.; Ji R.; Gao B.; Wu J. Transport and retention of perfluorooctanoic acid (PFOA) in natural soils: Importance of soil organic matter and mineral contents, and solution ionic strength. J. Contam. Hydrol. 2019, 225, 103477. 10.1016/j.jconhyd.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Du Z.; Deng S.; Bei Y.; Huang Q.; Wang B.; Huang J.; Yu G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents – A review. J. Hazard Mater. 2014, 274, 443–454. 10.1016/j.jhazmat.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Kothawala D. N.; Köhler S. J.; Östlund A.; Wiberg K.; Ahrens L. Influence of dissolved organic matter concentration and composition on the removal efficiency of perfluoroalkyl substances (PFASs) during drinking water treatment. Water Res. 2017, 121, 320–328. 10.1016/j.watres.2017.05.047. [DOI] [PubMed] [Google Scholar]
- Sepulvado J. G.; Blaine A. C.; Hundal L. S.; Higgins C. P. Occurrence and fate of perfluorochemicals in soil following the land application of municipal biosolids. Environ. Sci. Technol. 2011, 45, 8106–8112. 10.1021/es103903d. [DOI] [PubMed] [Google Scholar]
- LaMotte A.E.Estimated nitrogen from septic for the conterminous United States, 2010 (SepN_CONUS_bg_2010); U.S. Geological Survey data release; USGS, 2018. [Google Scholar]
- Schaider L. A.; Ackerman J. M.; Rudel R. A. Septic systems as sources of organic wastewater compounds in domestic drinking water wells in a shallow sand and gravel aquifer. Sci. Total Environ. 2016, 547, 470–481. 10.1016/j.scitotenv.2015.12.081. [DOI] [PubMed] [Google Scholar]
- Rotter S.; Beronius A.; Boobis A. R.; Hanberg A.; van Klaveren J.; Luijten M.; Machera K.; Nikolopoulou D.; van der Voet H.; Zilliacus J.; Solecki R. Overview on legislation and scientific approaches for risk assessment of combined exposure to multiple chemicals: the potential EuroMix contribution. Crit. Rev. Toxicol. 2018, 48, 796–814. 10.1080/10408444.2018.1541964. [DOI] [PubMed] [Google Scholar]
- Benskin J. P.; Li B.; Ikonomou M. G.; Grace J. R.; Li L. Y. Per- and polyfluoroalkyl substances in landfill leachate: Patterns, time trends, and sources. Environ. Sci. Technol. 2012, 46, 11532–11540. 10.1021/es302471n. [DOI] [PubMed] [Google Scholar]
- Schaider L. A.; Rudel R. A.; Ackerman J. M.; Dunagan S. C.; Brody J. G. Pharmaceuticals, perfluorosurfactants, and other organic wastewater compounds in public drinking water wells in a shallow sand and gravel aquifer. Sci. Total Environ. 2014, 468–469, 384–393. 10.1016/j.scitotenv.2013.08.067. [DOI] [PubMed] [Google Scholar]
- Bradley P. M.; Barber L. B.; Clark J. M.; Duris J. W.; Foreman W. T.; Furlong E. T.; Givens C. E.; Hubbard L. E.; Hutchinson K. J.; Journey C. A.; Keefe S. H.; Kolpin D. W. Pre/post-closure assessment of groundwater pharmaceutical fate in a wastewater-facility-impacted stream reach. Sci. Total Environ. 2016, 568, 916–925. 10.1016/j.scitotenv.2016.06.104. [DOI] [PubMed] [Google Scholar]
- Houtz E. F.; Sedlak D. L. Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff. Environ. Sci. Technol. 2012, 46, 9342–9349. 10.1021/es302274g. [DOI] [PubMed] [Google Scholar]
- Cozzarelli I. M.; Böhlke J. K.; Masoner J.; Breit G. N.; Lorah M. M.; Tuttle M. L. W.; Jaeschke J. B. Biogeochemical evolution of a landfill leachate plume, Norman, Oklahoma. Ground Water 2011, 49, 663–687. 10.1111/j.1745-6584.2010.00792.x. [DOI] [PubMed] [Google Scholar]
- Vengosh A.; Heumann K. G.; Juraske S.; Kasher R. Boron isotope application for tracing sources of contamination in groundwater. Environ. Sci. Technol. 1994, 28, 1968–1974. 10.1021/es00060a030. [DOI] [PubMed] [Google Scholar]
- Dams R.; Hintzer K.. Chapter 1. Industrial Aspects of Fluorinated Oligomers and Polymers. Fluorinated Polymers: Vol. 2: Applications; Ameduri B.; Sawada H., Eds.; The Royal Society of Chemistry, 2017, pp 3–31. [Google Scholar]
- Haase K. B.; Busenberg E.; Plummer L. N.; Casile G.; Sanford W. E. Measurements of HFC-134a and HCFC-22 in groundwater and unsaturated-zone air: Implications for HFCs and HCFCs as dating tracers. Chem. Geol. 2014, 385, 117–128. 10.1016/j.chemgeo.2014.07.016. [DOI] [Google Scholar]
- Zhao Q.; Hastie T. Causal Interpretations of Black-Box Models. J. Bus. Econ. Stat. 2021, 39, 272–281. 10.1080/07350015.2019.1624293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiña P.; Leahy P.; Metzeling L.; Stevenson G.; Hinwood A. Emerging and legacy contaminants across land-use gradients and the risk to aquatic ecosystems. Sci. Total Environ. 2019, 695, 133842 10.1016/j.scitotenv.2019.133842. [DOI] [PubMed] [Google Scholar]
- U.S. Geological Survey . USGS Water Data for the Nation; U.S. Geological Survey National Water Information System Database; USGS, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.