Significance Statement
Vaccination against SARS-CoV-2 is critically important for patients on maintenance dialysis, who are at high risk for COVID-19–related morbidity and mortality. Previous research has demonstrated that mRNA-based vaccines are highly effective in patients on dialysis. Because specific vaccines may be differentially available to patients, it is important to understand the comparative effectiveness of individual vaccines, including the adenovirus vector–based vaccine Ad26.COV2.S. In this retrospective study, the authors compared the real-world effectiveness of Ad26.COV2.S with that of an mRNA vaccine, BNT162b2, in a population of patients on dialysis. Their findings showed no difference in the effectiveness of these vaccines over the first 6 months postvaccination, and support the continued use of either in the population of patients on dialysis.
Keywords: COVID-19, SARS-CoV-2, vaccine, dialysis, comparative effectiveness, BNT162 vaccine
Abstract
Background
Studies have demonstrated that mRNA-based SARS-CoV-2 vaccines are highly effective among patients on dialysis. Because individual vaccines may be differentially available or acceptable to patients, it is important to understand comparative effectiveness relative to other vaccines, such those on the basis of adenovirus technologies.
Methods
In this retrospective study, we compared the clinical effectiveness of adenovirus vector–based Ad26.COV2.S (Janssen/Johnson & Johnson) to mRNA-based BNT162b2 (Pfizer/BioNTech) in a contemporary cohort of patients on dialysis. Patients who received a first BNT162b2 dose were matched 1:1 to Ad26.COV2.S recipients on the basis of date of first vaccine receipt, US state of residence, site of dialysis care (in-center versus home), history of COVID-19, and propensity score. The primary outcome was the comparative rate of COVID-19 diagnoses starting in the 7th week postvaccination. In a subset of consented patients who received Ad26.COV2.S, blood samples were collected ≥28 days after vaccination and anti–SARS-CoV-2 immunoglobulin G antibodies were measured.
Results
A total of 2572 matched pairs of patients qualified for analysis. Cumulative incidence rates of COVID-19 did not differ for BNT162b2 versus Ad26.COV2.S. No differences were observed in peri–COVID-19 hospitalizations and deaths among patients receiving BNT162b2 versus Ad26.COV2.S, who were diagnosed with COVID-19 during the at-risk period. Results were similar when excluding patients with a history of COVID-19, in subgroup analyses restricted to patients who completed the two-dose BNT162b2 regimen, and in patients receiving in-center hemodialysis. SARS-CoV-2 antibodies were detected in 59.4% of 244 patients who received Ad26.COV2.S.
Conclusions
In a large real-world cohort of patients on dialysis, no difference was detected in clinical effectiveness of BNT162b2 and Ad26.COV2.S over the first 6 months postvaccination, despite an inconsistent antibody response to the latter.
The population of patients receiving maintenance dialysis for ESKD is enriched for risk factors for—and has borne a disproportionate burden of—infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and severe coronavirus disease 2019 (COVID-19) manifestation.1–4 As such, efforts to vaccinate these patients and understand the effectiveness of vaccinations in this cohort are critical. At present, three vaccines have been approved or granted emergency use authorization by the US Food and Drug Administration: the mRNA-based BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna), and the adenovirus-based Ad26.COV2.S (Janssen/Johnson & Johnson). To date, there are no comparative data on the effectiveness of these vaccines on clinical outcomes.
Dialysis providers enrolled as vaccine providers to increase access to SARS-CoV-2 vaccines5; vaccines were received via an allocation from the US federal government (BNT162b2) and from state governments (any vaccine, including Ad26.COV2.S). Typically, at any point of care, individual patients had access to only one vaccine (i.e., providers could not route a particular vaccine to a particular patient on the basis of perceived need or patient preference). Because of the parallel nature of the federal and state allocations, at times, patients living in the same geographic area received different vaccine types (i.e., BNT162b2 and Ad26.COV2.S), creating a natural experiment.
Overall vaccine uptake in the US dialysis population has been robust, with the majority of patients having received BNT162b2 or mRNA-1273, both of which have been demonstrated to be highly effective in preventing COVID-19.6 As vaccination efforts proceed, a meaningful number of patients on dialysis have indicated hesitancy to receive either of these vaccines because they are mRNA based; however, such patients do express a willingness to receive the adenovirus-based Ad26.COV2.S. Recent studies have suggested a lackluster serologic response to Ad26.COV2.S among patients on dialysis, calling into question its effectiveness in this population.7–9 Thus, it is important to understand the effectiveness of Ad26.COV2.S to inform both ongoing vaccination efforts and clinical decisions for the care of patients on dialysis who have already received this vaccine.
Therefore, we leveraged the natural experiment established by vaccine allocation programs to test the comparative effectiveness of Ad26.COV2.S versus one of the mRNA-based vaccines (BNT162b2) in preventing patients with clinical COVID-19 in a large, representative cohort of US patients on dialysis. Given real-world effectiveness is affected by patient adherence (i.e., to the second of a two-dose regimen), we began by considering all matched BNT162b2: Ad26.COV2.S pairs, regardless of whether the former was followed with a second dose. Recognizing there is interest in effectiveness when regimens are followed as intended, we performed subgroup analysis in which matched pairs were considered only if the BNT162b2 patient completed a two-dose regimen (“completer pairs”). Furthermore, for each comparison, we performed separate analyses, including and not including patients with a history of COVID-19.
Methods
Comparative Effectiveness Analyses
This retrospective study was designed to compare two potential vaccination regimens: “use BNT162b2” versus “use Ad26.COV2.S.” BNT162b2 was chosen as the comparator messenger RNA vaccine (as opposed to mRNA-1273) because it had better geotemporal overlap with Ad26.COV2.S in our population. The study included patients on dialysis who were aged ≥18 years and who received a first dose of BNT162b2 or a dose of Ad26.COV2.S between February 27 and May 18, 2021; the date of this dose was set as the index date. During the study period, approximately one-third of patient vaccinations occurred in dialysis facilities. For those patients who did not receive a vaccine in-clinic, vaccine information, including the vaccine type and date of dosing, was ascertained through verbal attestation and review of vaccine cards. To account for geotemporal variability in the intensity of the epidemic, patients receiving BNT162b2 were exactly matched to Ad26.COV2.S patients on US state of residence and index date (±1 day). Furthermore, to promote balance on susceptibility factors, patients receiving BNT162b2 and Ad26.COV2.S were also matched on the site of dialysis care (in-center versus home), history of COVID-19, and propensity score (caliper width ±0.01). Propensity score was estimated using a logistic model in which vaccine type was the dependent variable and predicted on the basis of age (including linear and quadratic terms), sex, race/ethnicity, dialysis vintage, etiology of ESKD, diabetes, Charlson Comorbidity Index, history of renal transplant, serum albumin, index date, US state, site of dialysis care (in-center versus home), and history of COVID-19. Covariate balance between matched patients was assessed using descriptive statistics (means, SDs, counts, and proportions) and quantified in terms of standardized differences; standardized differences exceeding ±10% indicate substantial imbalance.10
The primary outcome was a PCR–confirmed clinical diagnosis of COVID-19. Clinical surveillance protocols at the dialysis organization have been described elsewhere.6,11,12 To summarize, at the time of each clinic visit, all patients undergo a standardized COVID-19 screening. Patients who screen positive for COVID-19 symptoms, or who indicate a recent contact with COVID-19–infected individuals, immediately receive PCR testing. Additional surveillance procedures are in place to identify COVID-19 diagnoses made at other sites of care (e.g., emergency departments, hospitals) and to confirm and document PCR test results from these sites.
The primary analytical cohort consisted of matched pairs in which both members remained alive and COVID-19 free until the start of at-risk time (Figure 1). Time at-risk began the 43rd day (i.e., the first day after 6 weeks had elapsed) postindex to account for the time to a second dose (for BNT162b2) and biologic latency in immune response. Patients remained at risk until the earliest of development of COVID-19, death or loss to follow-up, or study end (September 28, 2021). Comparative effectiveness was estimated using a mixed-effects Poisson model with a random-effects indicator for pair assignment, and expressed as incidence rate differences (IRD) and 95% confidence intervals (95% CIs). As standard, IRDs for which 95% CIs exclude zero were considered statistically significant. In addition, because limited statistical power increases the likelihood of type 2 errors, we further consider a “statistical worst-case scenario” for Ad26.COV2.S on the basis of the upper confidence bound, which is a conservative approach because statistical imprecision implicitly “counts against” Ad26.COV2.S under this approach.
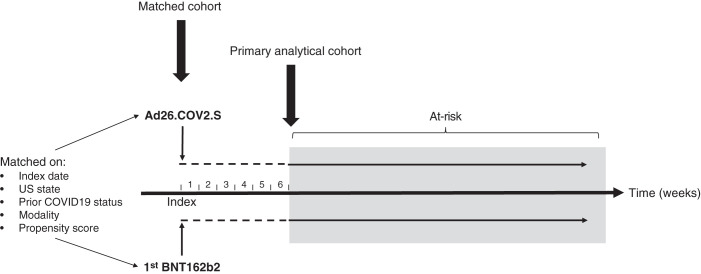
Figure 1.
Study schema. Eligible patients were those receiving an Ad26.COV2.S shot or a first BNT162b2 shot, who were pair matched on the basis of index date (±1 day), US state, prior COVID-19 status, site of dialysis care (in-center versus home), and propensity score (matched cohort). The primary analytical cohort consisted of matched pairs in which both members remained alive and COVID-19 free through the start of the at-risk period. At-risk time began on the 43rd day (i.e., the first day after 6 weeks had elapsed) postindex, and continued until the earliest of development of COVID-19, death, or loss to follow-up.
We also compared hospitalization and mortality among patients receiving BNT162b2 and Ad26.COV2.S who were diagnosed with COVID-19 during the at-risk period, using similar methods to those we have previously reported.6 Peri–COVID-19 hospitalizations were defined as admissions of any cause that occurred during the 7 days before and 21 days after a COVID-19 diagnosis. This window was chosen to allow the capture of scenarios such as those in which: (1) a symptomatic patient was diagnosed with COVID-19 and hospitalized soon thereafter; (2) a patient was diagnosed with COVID-19 and hospitalized later due to worsening of initially mild/moderate disease; (3) a patient was hospitalized for COVID-19–related symptoms, but an official COVID-19 diagnosis occurred after an initial false negative test; (4) a patient was hospitalized for unrelated reasons and COVID-19 was incidentally detected during the admission. Peri–COVID-19 deaths were defined as those that occurred after a COVID-19 diagnosis without a documented recovery from COVID-19, as defined by Centers for Disease Control and Prevention guidelines.13 Peri–COVID-19 hospitalizations and deaths were compared between patients receiving BNT162b2 and Ad26.COV2.S with breakthrough infections using chi-squared tests.
Separate analyses were performed in parallel among four nested populations: (1) all qualifying BNT162b2: Ad26.COV2.S matched pairs; (2) qualifying pairs excluding those with a history of COVID-19; (3) qualifying pairs in whom patients receiving BNT162b2 were given a second dose (completer pairs); and (4) completer pairs excluding those with a history of COVID-19. Within each of these populations, subgroup analyses considering only patients treated with in-center hemodialysis were performed. A planned secondary analysis was conducted that compared BNT162b2 and Ad26.COV2.S effectiveness for the weeks 4–6 postvaccination among matched pairs, in which both members remained alive and COVID-19 free through the end of week 3 postindex.
According to 45 Code of Federal Regulations part 46 from the US Department of Health and Human Services, this study was deemed exempt from institutional review board or ethics committee approval. We adhered to the Declaration of Helsinki, and informed consent was not required. Data were derived from electronic health records. Socioeconomic indicators were derived from the 2020 US census and linked by zip code.14 All analyses were performed using Stata 10.0/MP (College Station, TX).
Antibody Analysis
Elsewhere, we have reported high rates of seroresponse (98.1%) in patients on dialysis completing the two-dose regimen of BNT162b2.6 To estimate vaccine antibody response to Ad26.COV2.S, we conducted a prospective study among a subset of patients who received Ad26.COV2.S between February 27 and March 25, 2021. This study was reviewed and approved by the Advarra institutional review board (Protocol DCR 21-S-0016–00; approved on March 19, 2021). After patients provided written informed consent, a blood sample was collected 28–56 days after vaccine receipt to measure anti–SARS-CoV-2 antibodies. Blood samples were collected before a dialysis treatment in a 5 ml serum separation tube, clotted for 30 minutes, centrifuged, and refrigerated before shipment. All samples were processed at a centralized, accredited laboratory (DaVita Labs). IgG was measured using an indirect chemiluminescence immunoassay for anti–SARS-CoV-2 IgG antibodies (Diazyme Laboratories, Inc.), which detects antibodies against the SARS-CoV-2 spike and nucleocapsid proteins. Per the manufacturer’s recommendation, samples were scored IgG positive if the corresponding test reading was >1 arbitrary unit/ml, and negative otherwise. Notably, the assay only reports a composite score for antispike and antinucleocapsid IgG. We previously used this assay for research purposes, due to its selectivity for SARS-CoV-2 antibodies and low levels of crossreactivity to other coronaviruses or influenza viruses.6,11,12 IgM levels were not measured on the basis of pilot data, demonstrating that measured IgM response was implausibly low in this population (<20% positive even in the 28-day period after documented SARS-CoV-2 infection).
Results
We identified a total of 2732 qualifying matched pairs of patients receiving BNT162b2 and Ad26.COV2.S. The characteristics of patients in the total matched sample were well balanced (Supplemental Table 1). Of these, 48 (1.8%), 39 (1.4%), and 73 (2.7%) pairs were excluded because at least one member developed COVID-19, died, or was lost to follow-up before the start of at-risk time, respectively. The primary analytical cohort consisted of the remaining 2572 pairs of patients receiving BNT162b2 and Ad26.COV2.S.
Comparative Effectiveness in the Primary Analytical Cohort
The cohort was well balanced on demographic, clinical, and socioeconomic factors at baseline (Table 1). There was a slightly lower rate of unemployment among Ad26.COV2.S versus BNT162b2 groups (6.1% versus 6.5%; standardized mean difference −12.3%). As a further validation of the matching process, we compared COVID-19 rates for the first 14 days postindex date (when there should be no vaccine-derived immunity): 11 (0.4%) of BNT162b2 and 15 (0.5%) of Ad26.COV2.S (P difference=0.43). During the at-risk period, 4.3% of patients receiving BNT162b2 and 4.9% receiving Ad26.COV2.S were lost to follow-up. There was a total of 158 patients with COVID-19 observed during the at-risk period; affected individuals were similar to the overall analytical cohort in most respects, but were somewhat more likely to be female, White, and have diabetes (including those with diabetes as the cause of ESKD); most notably, none of these 158 patients had a history of COVID-19 (Supplemental Table 2).
Table 1.
Baseline comparison of matched patients receiving Ad26.COV2.S and BNT162b2 in the primary analytical cohorta,b
| Characteristic | BNT162b2 (n=2572) | Ad26.COV2.S (n=2572) | Standardized Mean Difference |
|---|---|---|---|
| Site of dialysis, %c,d | +0.0% | ||
| In-center HD | 87.3 | 87.3 | |
| Homee | 12.7 | 12.7 | |
| Agec, yr | 61.2±13.1 | 61.7±13.4 | +3.8% |
| Female, %c | 40.4 | 40.7 | +0.6% |
| Race/ethnicity, %c | +2.0% | ||
| White | 31.4 | 33.2 | |
| Black | 34.1 | 33.0 | |
| LatinX | 22.7 | 21.6 | |
| Asian | 3.4 | 4.0 | |
| Other/unknown | 8.4 | 8.2 | |
| Dialysis vintage, yr,c | 2.7 (1.0, 5.2) | 2.6 (1.0, 5.2) | −2.7% |
| Etiology of ESKD, %c | −1.5% | ||
| Diabetes | 35.7 | 35.2 | |
| Hypertension | 25.3 | 25.0 | |
| Other | 39.0 | 39.8 | |
| Diabetesc | 67.6 | 67.2 | −0.9% |
| CCIc | 4.9±1.6 | 4.9±1.5 | +0.0% |
| Prior transplant, %c | 4.7 | 4.2 | −2.4% |
| Prior COVID-19, %c,d,f | 10.4 | 10.4 | +0.0% |
| Albumin, g/dl,c | 4.1±0.4 | 4.1±0.4 | +0.0% |
| Creatinine, mg/dl | 8.6±3.3 | 8.4±3.3 | +0.0% |
| Hemoglobin, g/dl | 10.7±1.4 | 10.7±1.4 | +0.0% |
| Ferritin, ng/ml | 695 (424, 964) | 701 (439, 959) | +1.5% |
| TSAT, % | 27.4±12.9 | 27.0±13.1 | −3.1% |
| Phosphate, mg/dl | 5.7±1.7 | 5.6±1.7 | −5.9% |
| PTH, pg, ml | 387 (250, 587) | 381 (241, 579) | −3.0% |
| Household poverty,%,g | 15.8±9.4 | 15.2±8.4 | −6.7% |
| Housing vacancy, %g | 9.9±6.5 | 10.2±6.4 | 4.7% |
| Unemployment,%g | 6.5±3.4 | 6.1±3.1 | −12.3% |
| 18–24 year olds without a high school diploma %,g | 13.9±7.7 | 13.9±7.6 | +0.0% |
| Index datec,d | Mar 21 (Mar 12, Mar 31) | Mar 21 (12 Mar, Apr 1) | +0.0% |
| State, %c,d | +0.0% | ||
| AL | 2.4 | 2.4 | |
| AR | 1.6 | 1.6 | |
| AZ | 2.8 | 2.8 | |
| CA | 17.2 | 17.2 | |
| CO | 0.1 | 0.1 | |
| CT | 0.2 | 0.2 | |
| FL | 5.7 | 5.7 | |
| GA | 3.7 | 3.7 | |
| IA | 0.3 | 0.3 | |
| ID | <0.1 | <0.1 | |
| IL | 11.7 | 11.7 | |
| IN | 0.1 | 0.1 | |
| KS | 1.7 | 1.7 | |
| KY | 1.6 | 1.6 | |
| LA | 0.4 | 0.4 | |
| MA | <0.1 | <0.1 | |
| MD | 4.7 | 4.7 | |
| ME | 0.2 | 0.2 | |
| MI | 2.5 | 2.5 | |
| MN | 0.1 | 0.1 | |
| MO | 1.0 | 1.0 | |
| NC | 0.7 | 0.7 | |
| NE | 0.2 | 0.2 | |
| NH | 0.2 | 0.2 | |
| NJ | 5.6 | 5.6 | |
| NM | 0.2 | 0.2 | |
| NV | 1.2 | 1.2 | |
| NY | 2.8 | 2.8 | |
| OH | 2.6 | 2.6 | |
| OK | 0.5 | 0.5 | |
| OR | 1.8 | 1.8 | |
| PA | 2.8 | 2.8 | |
| SC | 2.0 | 2.0 | |
| TN | 0.5 | 0.5 | |
| TX | 14.5 | 14.5 | |
| UT | 0.1 | 0.1 | |
| VA | 0.2 | 0.2 | |
| WA | 3.8 | 3.8 | |
| WI | 2.4 | 2.4 | |
| WV | 0.2 | 0.2 |
HD, hemodialyis; CCI, Charlson comorbidity index; TSAT, transferrin saturation; PTH, parathyroid hormone.
Descriptions at index date.
Estimates presented as mean±SD, median (p25, p75), or percentage.
Included in propensity score model.
Matching factor.
Home includes both peritoneal dialysis and home hemodialysis.
Refers to COVID-19 before the index date (date of first vaccination).
On the basis of census-level data linked through zip code.
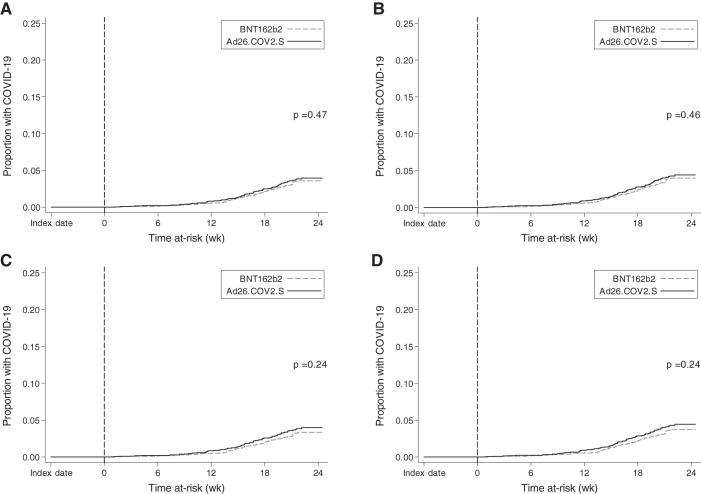
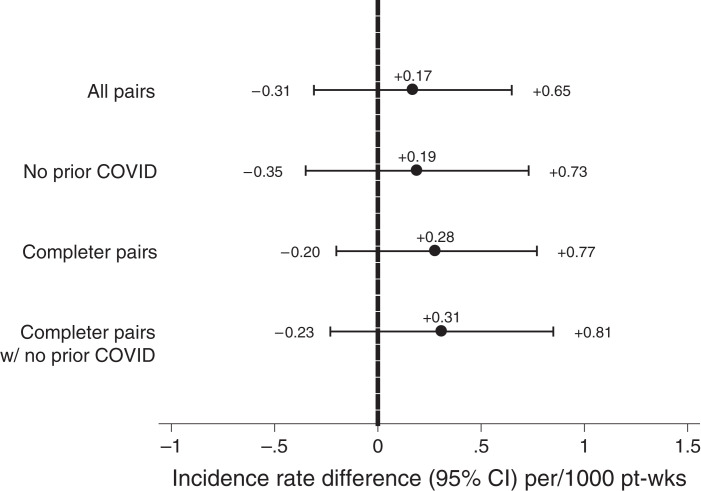
In the primary analytical cohort, cumulative incidence of COVID-19 during the at-risk period was no different for patients receiving BNT162b2 and Ad26.COV2.S (Figure 2A). Table 2 displays the at-risk time, COVID-19 diagnoses, and incidence rates for all patients receiving BNT162b2 and Ad26.COV2.S. The corresponding IRD was +0.17 (95% CI, −0.31 to 0.65), indicating no statistically significant difference in COVID-19 incident rates during the at-risk period (Figure 3). On the basis of the confidence limits, with 97.5% certainty, the number of additional COVID-19 diagnoses per week for each 1000 patients adopting a “use Ad26.COV2.S” (vs a “use BNT162b2”) would not be expected to exceed 0.65. Among patients who received a COVID-19 diagnosis during the at-risk period, rates of peri–COVID-19 hospitalization and death were not statistically different between BNT162b2 and Ad26.COV2.S groups (Table 3).
Figure 2.
Kaplan–Meier cumulative incidence curves for COVID-19 during the at-risk period. In each panel, time at-risk is indicated along the x axis. As described in the text, analyses consider only pairs for which both members remained alive and COVID-19-free between index date (date of first shot) and the start of at-risk time (which began on the 43rd day postindex; vertical dashed line). (A) All matched pairs in the primary analytical cohort (n=2572 pairs); (B) matched pairs from the primary analytical cohort with no prior history of COVID-19 (n=2304 pairs); (C) matched pairs from the primary analytical cohort for which the BNT162b2 member completed the two-dose series (n=2477 pairs); (D) matched pairs from the primary analytical cohort with no history of COVID-19 and in which the BNT162b2 member completed the two-dose series (n=2225 pairs). P values represent the log-rank P value for the difference between Ad26.COV2.S and BNT162b2 groups.
Table 2.
COVID-19 incidence in the primary analytical cohort
| COLUMN ONE | BNT162b2 | Ad26.COV2.S | ||||
|---|---|---|---|---|---|---|
| At-risk Time (pt-weeks) | COVID-19 Diagnoses | Incidence Rate (95% CI) | At-risk Time (pt-weeks) | COVID-19 Diagnoses | Incidence Rate (95% CI) | |
| All pairs (n=2,572 pairs) | 51,339 | 75 | 1.46 (1.17 to 1.83) | 50,916 | 83 | 1.63 (1.31 to 2.02) |
| No prior COVID-19 (n=2304 pairs)a | 45,942 | 75 | 1.63 (1.30 to 2.05) | 45,522 | 83 | 1.82 (1.47 to 2.26) |
| Completer pairs (n=2477 pairs)b | 49,807 | 68 | 1.37 (1.08 to 1.73) | 49,181 | 81 | 1.65 (1.32 to 2.05) |
| Completer pairs with no prior COVID-19 (n=2225 pairs) | 44,632 | 68 | 1.52 (1.20 to 1.93) | 44,078 | 81 | 1.84 (1.48 to 2.28) |
Refers to COVID-19 diagnosis before index date (date of first vaccination).
Matched pairs for which the BNT162b2 member completed a two-dose series.
Figure 3.
IRDs for Ad26.COV2.S versus BNT162b2. Rates are presented for all matched pairs in the primary analytical cohort, those pairs with no history of COVID-19 (i.e., preceding index date), completer pairs (pairs for which the patient receiving BNT162b2 completed the two-dose series), and completer pairs with no history of COVID-19. Rates are presented per 1000 pt-weeks at risk. Confidence intervals crossing the null (0, vertical dashed line) indicate no statistically significant difference between Ad26.COV2.S and BNT162b2.
Table 3.
Peri-COVID-19 hospitalizations and deaths during at-risk time in the primary analytical cohort and subgroup analyses
| COLUMN ONE | BNT162b2 | Ad26.COV2.S | P |
|---|---|---|---|
| All pairs | |||
| COVID-19 diagnoses, n | 75 | 83 | |
| Hospitalizations, n (%)a | 36 (48.0%) | 44 (53.0%) | 0.53 |
| Deaths, n (%)b | 5 (6.7%) | 3 (3.6%) | 0.38 |
| Pairs with no prior COVID-19c | |||
| COVID-19 diagnoses, n | 75 | 83 | |
| Hospitalizations, n (%) | 36 (48.0%) | 44 (53.0%) | 0.53 |
| Deaths, n (%) | 5 (6.7%) | 3 (3.6%) | 0.38 |
| Completer pairsd | |||
| COVID-19 diagnoses, n | 68 | 81 | |
| Hospitalizations, n (%) | 29 (42.7%) | 44 (54.3%) | 0.16 |
| Deaths, n (%) | 5 (7.4%) | 3 (3.7%) | 0.33 |
| Completer pairs with no prior COVID-19c,d | |||
| COVID-19 diagnoses, n | 68 | 81 | |
| Hospitalizations, n (%) | 29 (42.7%) | 44 (54.3%) | 0.16 |
| Deaths, n (%) | 5 (7.4%) | 3 (3.7%) | 0.33 |
Peri–COVID-19 hospitalizations defined as any hospitalization occurring between 7 days before and 21 days after COVID-19 diagnosis; percentages represent the percent of patients with a hospitalization event.
Peri–COVID-19 death defined as any death occurring between COVID-19 diagnosis and documented recovery; percentages represent the percent of patients who died.
Refers to COVID-19 before index date (date of first vaccination).
Matched pairs in which the BNT162b2 member completed a two-dose series.
The IRD for COVID-19 during the at-risk period was nearly identical among the subgroup of the 2245 of these matched pairs treating with in-center hemodialysis: IRD 0.11, 95% CI, −0.40 to 0.62.
Comparative Effectiveness among Patients with No COVID-19 History
Of qualifying matched pairs, 2304 had no history of COVID-19. The at-risk time, COVID-19 diagnoses, incidence rates, and cumulative incidence curves are provided in Table 2 and Figure 2B. There was no statistically significant difference between groups in rates of COVID-19 during the at-risk period (IRD, 0.19; 95% CI, −0.35 to 0.73) (Figure 3). Among patients developing COVID-19 during the at-risk period, rates of peri–COVID-19 hospitalization and death were not statistically different between the BNT162b2 and Ad26.COV2.S groups (Table 3).
The IRD for COVID-19 during the at-risk period was similar among the subgroup of the 1986 of these matched pairs treating with in-center hemodialysis: IRD, 0.12; 95% CI, −0.45 to 0.70.
Comparative Effectiveness among Vaccine Dose Regimen Completers
Of the 2572 patients receiving BNT162b2 in the primary analytical cohort, 2477 (96.3%) completed a two-dose series. The median time to second dose was 21 (interquartile range, 21–22) days. For these patients receiving BNT162b2 and their matched comparators (“completer pairs”), at-risk time, COVID-19 diagnoses, incidence rates, and cumulative incidence curves are provided in Table 2 and Figure 2C. There was no statistically significant difference between groups in rates of COVID-19 during the at-risk period: IRD, 0.28; 95% CI, −0.20 to 0.77 (Figure 3). Among patients developing COVID-19 during the at-risk period, rates of peri–COVID-19 hospitalization and death were not statistically different between the BNT162b2 and Ad26.COV2.S groups (Table 3).
The IRD for COVID-19 during the at-risk period was nearly identical among the subgroup of the 2161 of these matched pairs treating with in-center hemodialysis: IRD 0.30; 95% CI, −0.45 to 0.72.
When consideration was limited to the subset of completer pairs with no history of COVID-19 (n=2255 pairs), results were nearly identical (Figures 2D and 3; Tables 2 and 3).
Secondary Analysis
In a secondary analysis, we considered COVID-19 for 4–6 weeks postindex date (i.e., the period between presumed onset of immunity and achievement of complete immunity). This analysis considered the 2646 matched pairs for which both members remained alive and COVID-19 free for the first 21 days postindex. In the 4–6 weeks postvaccination, patients receiving BNT162b2 contributed 7874 patient-weeks (pt-weeks), during which there were seven patients with COVID-19: incident rate, 0.89; 95% CI, 0.43 to 1.86 patients per 1,000 pt-weeks. Patients receiving Ad26.COV2.S contributed 7877 pt-weeks, during which there were five patients with COVID-19: incident rate 0.63; 95% CI, 0.26 to 1.53 patients per 1000 pt-weeks (P difference=0.56).
Antibody Response to Ad26.COV2.S
We measured antibodies in 244 patients who received Ad26.COV2.S. the median time from Ad26.COV2.S receipt to blood sampling was 36.5 (interquartile range, 34–41) days. IgG antibodies were detected in 59.4% (95% CI, 53.0% to 65.5%) of patients who were vaccinated with Ad26.COV2.S.
Discussion
In this comparative effectiveness study of patients on maintenance dialysis, we observed no difference in clinical effectiveness between Ad26.COV2.S and BNT162b2. Results were similar when we only considered patients without a history of COVID-19, in whom vaccine effectiveness should therefore be minimally affected by naturally acquired immunity. Although this study of Ad26.COV2.S is large by dialysis standards, statistical power was nonetheless finite, and could have contributed to a type II error. Therefore, another way to interpret these data is as a statistical worst-case scenario on the basis of the upper limits of the confidence interval. Viewed that way, the number of additional patients per week would not be expected to exceed (with 97.5% statistical confidence) 0.65 for every 1000 patients treated with Ad26.COV2.S versus BNT162b2; this figure is 0.73 if consideration is limited to patients who were COVID-19 naïve. These results contribute important information to guide clinical practice for patients on dialysis, at a time when there is some speculation that Ad26.COV2.S may be substantially inferior to the mRNA SARS-CoV-2 vaccines.
In clinical trials, the efficacy of Ad26.COV2.S against symptomatic COVID-19 was estimated to be 67%, whereas the mRNA vaccines had reported efficacies against symptomatic COVID-19 of 94%–95%.15–17 However, these trials did not include patients on dialysis. Moreover, comparing clinical efficacy estimates across clinical trials may not be valid, because of differences in the patient populations included in each trial, background rates of SARS-CoV-2 transmission, and surveillance procedures. Finally, efficacy results from clinical trials may not accurately reflect effectiveness in the real-world setting due to patient selection and study oversight, the latter particularly of concern when an opportunity for differential adherence exists (i.e., the need for a second vaccination with mRNA vaccines). Overall, real-world estimates of vaccine effectiveness among patients on dialysis (73%–78% protective) were quite different from those reported from clinical trials in patients who are not on dialysis.6
In the dialysis population, concerns about the effectiveness of Ad26.COV2.S are compounded by recent evidence that antibody responses to Ad26.COV2.S are less robust and consistent than other SARS-CoV-2 vaccines.7–9 In this study, we observed that approximately 60% of patients on dialysis vaccinated with Ad26.COV2.S had detectable SARS-CoV-2 antibodies. This is comparatively lower than in our previous report in patients on dialysis who received mRNA vaccines (96%–98%).6 The reason why the serologic data do not mirror the clinical effectiveness of this vaccine remains unknown. Antibody titers and cellular immune responses have been shown to be positively correlated for mRNA vaccines.9 In contrast, there is some evidence that Ad26.COV2.S can affect humoral and cellular immunity differently. Compared with its effect on the original SARS-CoV-2 strain, Ad26.COV2.S induced significantly lower binding for neutralizing, antispike, and receptor-binding domain antibodies in the presence of viral variants of concern. However, Ad26.COV2.S-induced T cell responses to the same variants of concern were preserved.18 An additional possible explanation is that, although the antibody response induced by A26.COV2.S is blunted, antibodies may still reach sufficient levels to activate an immune “switch,” stimulating protective humoral and cellular responses.19
Because of these uncertainties, it has been unclear how best to approach vaccination among patients on dialysis who, either by circumstance (e.g. vaccine availability) cannot, or, by personal choice, will not, receive an mRNA-based vaccine. Moreover, the appropriate course of action for patients who have received Ad26.COV2.S remains to be determined. In this regard, our results should be seen as reassuring.
Our primary analysis included all patients who began a vaccine regimen, regardless of whether individual patients receiving BNT162b2 completed the two-dose series; this deliberate choice was made to reflect the reality that some patients do not follow through with a second dose, with real-world consequences on effectiveness. Nevertheless, follow-through rates were very high, and Ad26.COV2.S remained noninferior when analysis was restricted to patients who received both BNT162b2 doses. It is worth noting that, although not formally statistically significant, rates of peri–COVID-19 hospitalization were numerically higher for Ad26.COV2.S than for BNT162b2 (54.3% versus 42.7%; P=0.16). Further study of comparative COVID-19–related morbidity is warranted.
A recent study identified several predictors of immunogenicity to SARS-CoV-2 mRNA vaccines in dialysis patients, including COVID-19 history, use of immunosuppressive drugs, albumin levels, lymphocyte counts, diabetes and dialysis vintage.20 The results of our study also suggest a relationship between COVID-19 history and SARS-CoV-2 vaccine effectiveness in patients on dialysis. Specifically, we did not observe any COVID-19 diagnoses among the approximately 10% of our cohort who had a COVID-19 history. Moreover, we observed a greater proportion of females, those of White race, and patients with diabetes among those diagnosed with COVID-19 compared with the overall cohort. Interestingly, age, dialysis vintage, albumin, or history of transplant were not different between patients who were vaccinated who did, or did not, develop COVID-19 in our study.
The strengths of this study include a large sample size, a representative patient population, and a robust statistical matching procedure to account for the geotemporal nature of the COVID-19 pandemic and vaccine rollout in the United States (i.e., each pair of patients was considered contemporaneously (±1 day) throughout follow-up and were in the same US state). The study should be viewed in light of the following limitations. As with any observational study, there is the possibility of residual confounding and bias (e.g., misclassification of exposure status for patients reporting vaccinations outside of the clinic). Data limitations circumscribed our ability to assess vaccines effectiveness on disease severity (i.e., necessitating that we consider peri–COVID-19 hospitalization/death as opposed to COVID-19–related hospitalization/death) and to study the side effects of each vaccine. The assay used to quantify anti–SARS-CoV-2 IgG is not quantitative. Therefore, we could not assess the association of vaccine-induced antibody levels with future risk of being diagnosed with COVID-19. Also, the antibody assay used does not differentiate between antispike and antinucleocapsid antibodies. Because PCR testing was performed only in those who screened positive for COVID-19 symptoms or exposure, comparative effectiveness against asymptomatic infections could not be estimated. The durability of protection could not be estimated beyond 6 months postvaccination. Moreover, given the era of the study, we cannot estimate the effect of booster doses, nor the effects on emerging SARS-CoV-2 variants.
In summary, our results demonstrate no difference in the clinical effectiveness of BNT162b2 and Ad26.COV2.S over the first 6 months postvaccination in patients on dialysis.
Disclosures
A.G. Walker reports having an ownership interest in DaVita. A.G. Walker, A. Young, F. Tentori, G. Marlowe, J.J. Connaire, S.M. Brunelli, S. Karpinski, S. Sibbel, and T. Kelley report being employees of DaVita Clinical Research. A. Young reports having an ownership interest in DaVita; and reports other interests or relationships as a Board Member of the Kidney Health Initiative. D. Van Wyck, J. Giullian, M.L. Zywno, and R. Lazar report being employees of DaVita, Inc. D. Van Wyck reports having an ownership interest in DaVita Kidney Care. F. Tentori reports having an advisory or leadership role with Ardelyx Medical Advisory Board. J. Connaire reports employment with InterMed Consultants; reports being a consultant for Diality, Dynavax, GlaxoSmithKline (GSK), Relypsa, Inc., and Sanifit; reports receiving research funding from Akebia, Ardelyx, AstraZeneca, Chinook, Goldfinch Bio, GSK, Merck, Otsuka, Sanifit, Sera Trials, and Travere; and reports having an advisory or leadership role with GSK and Sanifit. J. Giullian reports having an ownership interest in DaVita, Inc.; and reports having an advisory or leadership role on the Board of Directors, Nephrology News, Insights, Editorial Board, and at Nephrosant, Inc. T. Kelley reports having an ownership interest DaVita.
Funding
None.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and teammates of DaVita, Inc., without whom this research would not have been possible.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
S. Brunelli, J. Guillian, S. Karpinski, S. Sibbel, and D. Van Wyck designed the study; S. Brunelli, J. Connaire, T. Kelley, F. Tentori, and A. Young were responsible for clinical/logistical leadership and oversight; R. Lazar, G. Marlowe, and M. Zywno prepared the analytic data files; S. Brunelli performed the analysis; S. Brunelli, J. Guillian, S. Karpinski, S. Sibbel, F. Tentori, D. Van Wyck, and A. Walker interpreted the findings; S. Brunelli, F. Tentori, and A. Walker wrote the original draft; and all authors reviewed and approved the final manuscript.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021101395/-/DCSupplemental.
Supplemental Table 1. Baseline comparison of all matched patients who received Ad26.COV2.S and BNT162b2.
Supplemental Table 2. Characteristics of patients who were diagnosed with COVID-19 during the at-risk time.
References
- 1.Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. : Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 69: 458–464, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC: Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 324: 782–793, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Hsu CM, Weiner DE, Aweh G, Miskulin DC, Manley HJ, Stewart C, et al. : COVID-19 among US dialysis patients: Risk factors and outcomes from a national dialysis provider. Am J Kidney Dis 77: 748–756.e1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Medicare and Medicaid Services : Preliminary Medicare COVID-19 data snapshot. Available at: https://www.cms.gov/files/document/medicare-covid-19-data-snapshot-fact-sheet-september2020.pdf. Accessed August 1, 2021
- 5.Centers for Disease Control and Prevention : COVID-19 vaccine: What public health jurisdictions and dialysis partners need to know. Available at: https://www.cdc.gov/vaccines/covid-19/planning/dialysis-partners-jurisdictions.html. Accessed June 17, 2021
- 6.Sibbel S, McKeon K, Luo J, Wendt K, Walker AG, Kelley T, et al. : Real-world effectiveness and immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV2 vaccines in patients on hemodialysis. J Am Soc Nephrol 33: 49–57, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand S, Montez-Rath ME, Han J, Garcia P, Cadden L, Hunsader P, et al. : Antibody response to COVID-19 vaccination in patients receiving dialysis. J Am Soc Nephrol 32: 2435–2438, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulhern JG, Fadia A, Patel R, Ficociello LH, Willetts J, Dahne-Steuber IA, et al. : Humoral response to mRNA versus an adenovirus vector-based sars-CoV-2 vaccine in dialysis patients. Clin J Am Soc Nephrol 16: 1720–1722, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia P, Anand S, Han J, Montez-Rath ME, Sun S, Shang T, et al. : COVID-19 vaccine type and humoral immune response in patients receiving dialysis. J Am Soc Nephrol 33: 33–37, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin PC: An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46: 399–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker AG, Sibbel S, Wade C, Moulton N, Marlowe G, Young A, et al. : SARS-CoV-2 antibody seroprevalence among maintenance dialysis patients in the United States. Kidney Med 3: 216–222.e1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen DE, Sibbel S, Marlowe G, Bludorn K, Miller D, Kelley T, et al. : Antibody status, disease history, and incidence of SARS-CoV-2 infection among patients on chronic dialysis. J Am Soc Nephrol 32: 1880–1886, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention : Ending isolation and precautions for people with COVID-19: Interim guidance. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed September 15, 2021
- 14.United States Census Bureau : Explore Census Data. Available at: http://data.census.gov. Accessed August 1, 2021
- 15.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. ; COVE Study Group : Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403–416, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. ; C4591001 Clinical Trial Group : Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383: 2603–2615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. ; ENSEMBLE Study Group : Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 384: 2187–2201, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alter G, Yu J, Liu J, Chandrashekar A, Borducchi EN, Tostanoski LH, et al. : Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 596: 268–272, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartsch YC, Fischinger S, Siddiqui SM, Chen Z, Yu J, Gebre M, et al. : Discrete SARS-CoV-2 antibody titers track with functional humoral stability. Nat Commun 12: 1018, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Praet J, Reynders M, De Bacquer D, Viaene L, Schoutteten MK, Caluwé R, et al. : Predictors and dynamics of the humoral and cellular immune response to SARS-CoV-2 mRNA vaccines in hemodialysis patients: A multicenter observational study. J Am Soc Nephrol 32: 3208, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.