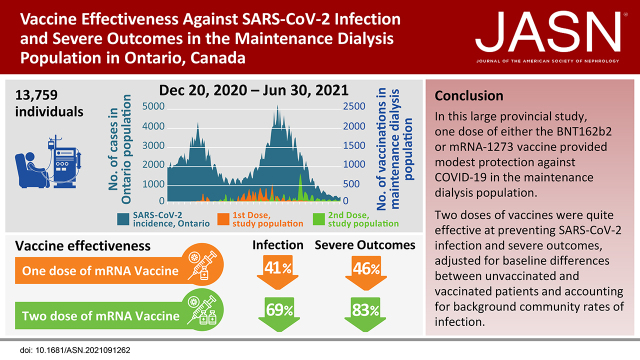
Significance Statement
Serology studies of coronavirus disease 2019 vaccines in the maintenance dialysis population have found weak antibody responses to one dose of vaccine and lower responses to two doses than among healthy controls. However, it is undetermined how these serologic responses correlate with the prevention of infection, hospitalization, and mortality in this immunosuppressed population. We identified 13,759 individuals receiving maintenance dialysis in Ontario, Canada. One dose of vaccine reduced the risk of infection and severe outcomes by 41% and 46%, respectively, compared with unvaccinated patients. Two doses of vaccine reduced the risk of infection and severe outcomes by 69% and 83%, respectively. The study confirms that coronavirus disease 2019 vaccination is effective in the maintenance dialysis population.
Keywords: chronic dialysis, hospitalization, mortality, COVID-19, SARS-CoV-2, Ontario, maintenance
Visual Abstract
Abstract
Background
Vaccination studies in the hemodialysis population have demonstrated decreased antibody response compared with healthy controls, but vaccine effectiveness for preventing SARS-CoV-2 infection and severe disease is undetermined.
Methods
We conducted a retrospective cohort study in the province of Ontario, Canada, between December 21, 2020, and June 30, 2021. Receipt of vaccine, SARS-CoV-2 infection, and related severe outcomes (hospitalization or death) were determined from provincial health administrative data. Receipt of one and two doses of vaccine were modeled in a time-varying cause-specific Cox proportional hazards model, adjusting for baseline characteristics, background community infection rates, and censoring for non-COVID death, recovered kidney function, transfer out of province, solid organ transplant, and withdrawal from dialysis.
Results
Among 13,759 individuals receiving maintenance dialysis, 2403 (17%) were unvaccinated and 11,356 (83%) had received at least one dose by June 30, 2021. Vaccine types were BNT162b2 (n=8455, 74%) and mRNA-1273 (n=2901, 26%); median time between the first and second dose was 36 days (IQR 28–51). The adjusted hazard ratio (HR) for SARS-CoV-2 infection and severe outcomes for one dose compared with unvaccinated was 0.59 (95% CI, 0.46 to 0.76) and 0.54 (95% CI, 0.37 to 0.77), respectively, and for two doses compared with unvaccinated was 0.31 (95% CI, 0.22 to 0.42) and 0.17 (95% CI, 0.1 to 0.3), respectively. There were no significant differences in vaccine effectiveness among age groups, dialysis modality, or vaccine type.
Conclusions
COVID-19 vaccination is effective in the dialysis population to prevent SARS-CoV-2 infection and severe outcomes, despite concerns about suboptimal antibody responses.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the maintenance dialysis population has led to high rates of hospitalization and significant mortality. In the first two waves of the coronavirus disease 2019 (COVID-19) pandemic, two thirds of individuals receiving dialysis were hospitalized, and one in four patients on dialysis died within 30 days of infection.1–4 Due to this higher risk, patients on dialysis have been prioritized for COVID-19 vaccination in many countries.5 Antibody response to the receptor-binding domain of the spike protein after receiving the first dose of the BNT162b2 (Pfizer) or mRNA-1273 (Moderna) is only 35%–47%.6 The response rate to the second dose is stronger, ranging from 76%–96%, but the level of antibody is only 7%–66% of that in healthy controls.7–11 The low response rate to one dose of vaccine is concerning because many regions, including Ontario, Canada, delayed the administration of the second dose due to the initial lack of vaccine supply. T cell–mediated immunity may also be an important measure of protection, with relative response rates ranging from 62% to 68%, but this has been less well studied.10,12
To date, few studies have estimated the vaccine effectiveness (VE), measured by the prevention of SARS-CoV-2 infection and associated hospitalization or mortality, in the dialysis population. Sibbel et al. reported a 31%–79% reduced risk of infection after receipt of the BNT162b2 vaccine and a 49%–73% reduced risk of infection after receipt of the mRNA-1273 vaccine.13
The seminal randomized controlled studies of the BNT162b2 and mRNA-1273 reported vaccine efficacy of 94%–95%, respectively, including protection against severe COVID-19 in the general population.14,15 Effectiveness was maintained among individuals with comorbid conditions measured by the Charlson Comorbidity Index (CCI), but only 0.7% were reported to have moderate to advanced CKD. Nonrandomized studies from Ontario, Canada, found a VE of 92% after two doses of vaccine (BNT162b2 or mRNA-1273) among individuals with comorbidities, which included CKD, but the group with CKD was not reported separately.16
The primary objective of this study was to determine the VE of COVID-19 vaccination in the maintenance dialysis population to prevent infection and severe outcomes. A secondary objective was to examine VE in important subgroups.
Methods
Study Population
This retrospective cohort study included all patients on maintenance dialysis in the province of Ontario, Canada, as of December 21, 2020, and those who initiated dialysis therapy between December 21, 2020, and June 30, 2021. Individuals were identified using provincial health administrative databases, including the Ontario Renal Reporting System, which is a mandatory provincial reporting system for regional renal programs administered by the Ontario Renal Network.17 Individuals receiving acute dialysis were excluded. Individuals required a valid Ontario Health Insurance Plan (OHIP) number to permit linking across provincial databases. OHIP is a single-payer insurance plan for all residents of Ontario or individuals with another valid status (landed immigrant, refugee, or valid work permit). Individuals were excluded if they had a positive SARS-CoV-2 infection confirmed by RT-PCR reported in the Ontario Laboratories Information System (OLIS) before December 21, 2020, or within 14 days of study entry. Patients on incident dialysis were excluded if they were vaccinated before starting dialysis.
Primary Exposure
The primary exposure was the receipt of either the BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) vaccine. Those who received the ChAdOx1 nCoV-19 (Oxford–AstraZeneca) vaccine were excluded (<1% of vaccines administered during the study period). The primary source of vaccine data was Ontario’s provincial COVAX database.16 In Ontario, vaccination was prioritized on December 15, 2020, for individuals living in congregate settings, including retirement homes and long-term care facilities, staff in those facilities, individuals receiving home care services, Indigenous adults, individuals aged ≥80 years, and health care workers. Some regional renal programs began administering vaccines to the general dialysis population in January 2021, but the dialysis population was not officially prioritized by the government for their first dose until April 6, 2021. The second dose was delayed due to limited vaccine supply and a policy of extended interval vaccination. However, on May 10, 2021, this decision was changed, and patients on dialysis became eligible for an accelerated second dose.
Individuals were observed from December 21, 2020, to June 30, 2021, and were able to change their vaccination status (unvaccinated, single dose, or double dose) during the follow-up period using a time-varying exposure. If different types of vaccines were received, the individual was grouped by the type they received for the first dose.
Outcomes
Individuals were followed up for outcomes to June 30, 2021. The primary outcome was the first laboratory-confirmed SARS-CoV-2 infection defined as a positive RT-PCR reported in the OLIS or as an infection reported by regional renal programs to the Ontario Renal Network. Data on symptoms at presentation were not available. A secondary outcome was first SARS-CoV-2-related severe outcomes defined as either hospitalization or death with a recent positive RT-PCR test. Hospitalizations were identified using the Canadian Institute for Health Information’s Discharge Abstract Database/Same-Day Surgery. For hospitalization to be attributed to COVID-19, we required a positive test to have occurred within 14 days before or 3 days after admission or for an International Classification of Disease-10 diagnosis of COVID-19 to be listed as a contributing cause of hospitalization on the discharge summary (the positive test could have occurred any time during the hospitalization). Deaths were identified using the Registered Persons Database, and we required a positive test to have occurred within 30 days before death. The date of the severe outcomes was the earliest of the test or clinical event. Individuals were followed from study entry to the first positive SARS-CoV-2 test (for the primary outcome analysis) or first severe outcome (for the secondary outcome analysis), discontinuation of dialysis (due to non-COVID death, recovered kidney function, transfer out of province, solid organ transplant, or withdrawal from dialysis), or end of the study period. Individuals who were hospitalized for COVID
-19 were still followed for 30 days after the infection date for the mortality outcome. The number of SARS-CoV-2 infections in the general population was based on data reported by Public Health Ontario as of July 17, 2021.18
Confounders
Demographic confounders included age, sex, and ethnicity. Ethnicity information is reported within the Ontario Renal Reporting System by the data lead in each regional renal program at the time of patient registration, on the basis of charting by clinical staff who could ask patients to self-identify ethnicity if necessary but are not mandated to do so. Treatment-specific confounders included dialysis modality (home dialysis versus in-center hemodialysis) and duration of dialysis therapy (vintage). We also included a history of diabetes, cardiac disease (myocardial infarction or congestive heart failure), and cancer (including skin cancer) along with the CCI using International Classification of Disease-10 codes in the OHIP database or the Canadian Institute for Health Information’s Discharge Abstract Database (Supplemental Table 1). All individuals were given a base score of 2 in the CCI for advanced CKD. The lookback period for baseline characteristics was 5 years from the study entry date. The number of RT-PCR tests before study entry was determined from the OLIS database. We determined residency in long-term care from physicians’ billing codes in the OHIP database that indicated long-term care services (“W” fee codes) in the 6-month period before study entry. We linked postal codes to 2011 Statistic Canada Census data or LHIN Crosswalk to determine neighborhood income quintiles, public health unit region, economic dependency index, and geographic location.19 Monthly community SARS-CoV-2 infection rates were obtained from Public Health Ontario and treated as a time-varying confounder. We reported neighborhood income quintiles and public health unit regions but did not include them in our final model because they were collinear with other confounders.
Statistical Analyses
Vaccination doses over time were plotted as a histogram, along with background cases of COVID-19 in the Ontario population. Individuals were grouped as never vaccinated or having received at least one dose as of June 30, 2021, to compare baseline characteristics. Baseline characteristics were summarized using standard statistical methods for qualitative and quantitative data. Differences in baseline groups were compared with standardized differences, with a value >0.1 being significant.20,21
For the VE analysis, all individuals began the study as unvaccinated, and their vaccine status was updated if and when they received their first and second doses of vaccine. One individual could therefore contribute follow-up time to the unvaccinated, one-dose, and two-dose exposure groups similar to methods described by Shrotri et al.22 We considered the first dose to be effective after 14 days and the second dose to be effective after 7 days, similar to prior randomized controlled studies.14,15,23 The follow-up time by vaccine exposure time, number of infections, severe outcomes, and rates per 100,000 patient-days were calculated.
Extended time-varying cause-specific Cox proportional hazards (PH) models using the counting process extension were used to determine unadjusted and adjusted VE, with the vaccine status model as a time-varying exposure. The timescale for the model was calendar time to control for the changes in waves during the study period.24,25 The adjusted VE accounted for differences in baseline characteristics (age, sex, ethnicity, dialysis modality, CCI, economic dependency quintiles, long-term care residences, vintage, and number of RT-PCR tests before December 21, 2020) and monthly public health unit region SARS-CoV-2 infection rates.22,26 Models were tested to confirm PH assumption, examined for influential observations and model fit. If variables violated the assumption, then time-stratified models were used to create a piecewise constant function within relevant time strata. A sensitivity analysis was performed with no lag period examining the vaccine effect immediately after vaccination.
Individuals receiving maintenance dialysis are susceptible to competing risks, which are treated as censored in our primary analysis. We also examine the effect of vaccine status for each of the competing risk events (i.e., non-COVID death, recovered kidney function, and solid organ transplant) through additional cause-specific models. In the primary analysis, individuals were followed until a SARS-CoV-2 infection or a competing event. In the severe outcome analysis, individuals who had a SARS-CoV-2 infection were followed for 30 days to determine if they had a severe outcome (hospitalization or death). If an individual had a SARS-CoV-2 infection but did not have a severe outcome, they were censored 30 days after the infection in the severe outcome analysis. VE was calculated as (1–HR)×100%.16
The adjusted VE after two doses of vaccine was determined for the subgroups of vaccine type, age groups (18–64 versus 65+), and type of dialysis (home versus in-center). Interaction terms were tested for significance to examine if the VE differed between subgroups.
All statistical analyses were conducted using R v3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria), and an a priori level of statistical significance was set at 0.05. Confidence interval widths were not adjusted for multiple testing. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies (Supplemental Table 2).
Ethical Considerations
Ontario Renal Network, part of Ontario Health, is a provincial agency that funds and manages services for patients with CKD. The data collection and analysis were in accordance with Ontario Health’s legislative authority under the Ontario Personal Health Information Protection Act, 2004.
Results
From December 21, 2020, to June 30, 2021, 14,626 individuals received maintenance dialysis in Ontario, Canada. Exclusions included individuals <18 years old (n=8), those without OHIP coverage (n=149), those with previous SARS-CoV-2 infection (n=418), incident patients who were vaccinated before starting dialysis (n=163), and those receiving the ChAdOx1 nCoV-19-vaccine (n=129), leaving 13,759 individuals in the study, of whom 2403 (17%) were never vaccinated and 11,356 (83%) received at least one dose of vaccine by June 30, 2021. The vaccine types were BNT162b2 (n=8455; 74%) and mRNA-1273 (n=2901; 26%).
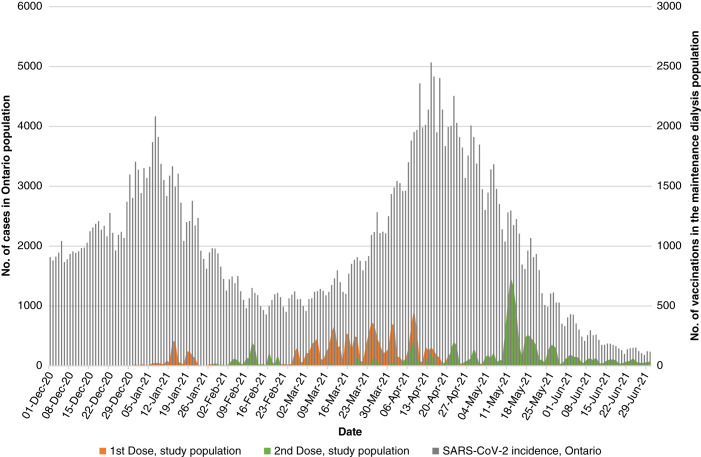
Vaccination over time and COVID-19 cases in the community are presented in Figure 1. The median time between doses was 36 days (interquartile range 28–51); 809 (8%) received the second dose within 21 days.
Figure 1.
Receipt of the first and second dose of the coronavirus disease 2019 (COVID-19) vaccines in the maintenance dialysis population over time plotted against a background of COVID-19 cases in the general population in Ontario, Canada. First and second doses are plotted in yellow and green, respectively. Cases in the general population are plotted in gray. The majority of vaccination occurred during the third wave of the pandemic in Ontario, Canada. The population was followed until June 30, 2021.
Baseline differences by never vaccinated or at least one dose are presented in Table 1. Missing data were <0.5% for all variables except ethnicity, which was coded as unknown in 4% of individuals. Never vaccinated patients were younger, more likely to be of Black ethnicity, and had a slightly higher CCI. Other characteristics were similar. The frequency of RT-PCR tests before study entry was high, with 33%–35% of the two groups having received three or more tests. The median follow-up time was 105 days for the unvaccinated group and 193 days (maximum follow-up time) for both the one- and two-dose groups. The never vaccinated group was much more likely to leave the study due to non-COVID-19 death (20%), withdrawal of dialysis (6%), recovered kidney function (6%), or solid organ transplant (5%; Supplemental Table 3).
Table 1.
Baseline characteristics of the maintenance dialysis population by vaccine exposure
| Characteristics | Never Vaccinated | Received at least One Dose | Standardized Differencee |
|---|---|---|---|
| Individuals, n | 2403 | 11,356 | |
| Age group (years), n (%) | |||
| 18–39 | 209 (9) | 606 (5) | 0.13 |
| 40–69 | 1282 (53) | 5438 (48) | 0.11 |
| ≥70 | 912 (38) | 5312 (47) | 0.18 |
| Age (years), median (IQR) | 64 (53–74) | 68 (58–77) | |
| Women, n % | 953 (40) | 4484 (40) | 0 |
| Ethnicity, n (%) | |||
| Asian | 161 (7) | 933 (8) | 0.06 |
| Black | 269 (11) | 686 (6) | 0.18 |
| White | 1346 (56) | 6758 (60) | 0.07 |
| Indian Subcontinenta | 189 (8) | 1102 (10) | 0.06 |
| Other non-White | 340 (14) | 1502 (13) | 0.03 |
| Unknown | 98 (4) | 375 (3) | 0.04 |
| Comorbidity, n (%) | |||
| Diabetes | 1253 (52) | 5750 (51) | 0.03 |
| Cardiac disease | 824 (34) | 3513 (31) | 0.07 |
| Cancer | 193 (8) | 879 (8) | 0.01 |
| CCI, mean (SD)b | 4.3 (2.2) | 4.1 (2) | 0.1 |
| Years on dialysis, median (IQR) | 1.8 (0.3–4.4) | 2 (0.6–4.4) | 0.01 |
| Home dialysis,c n (%) | 589 (25) | 2848 (25) | 0.01 |
| Long-term care, n (%) | 99 (4) | 436 (4) | 0.01 |
| Public health unit regions, n (%) | |||
| Central East | 150 (6) | 817 (7) | 0.04 |
| Central West | 423 (18) | 1979 (17) | 0.01 |
| Durham | 96 (4) | 582 (5) | 0.05 |
| Eastern | 157 (7) | 765 (7) | 0.01 |
| North | 203 (8) | 904 (8) | 0.02 |
| Ottawa | 118 (5) | 650 (6) | 0.04 |
| Peel | 333 (14) | 1223 (11) | 0.09 |
| Southwest | 260 (11) | 1073 (9) | 0.05 |
| Toronto | 536 (22) | 2578 (23) | 0.01 |
| York | 122 (5) | 773 (7) | 0.07 |
| Missing | 5 | 12 | |
| Income quintiles, n (%) | |||
| 1 (lowest) | 766 (32) | 3158 (28) | 0.09 |
| 2 | 515 (21) | 2517 (22) | 0.02 |
| 3 | 463 (19) | 2120 (19) | 0.02 |
| 4 | 345 (14) | 1969 (17) | 0.08 |
| 5 (highest) | 304 (13) | 1548 (14) | 0.03 |
| Missing | 10 | 44 | |
| Economic dependency index,d n (%) | |||
| 1 (least marginalized) | 375 (16) | 1983 (18) | 0.05 |
| 2 | 466 (19) | 2003 (18) | 0.05 |
| 3 | 421 (18) | 2146 (19) | 0.04 |
| 4 | 541 (23) | 2372 (21) | 0.04 |
| 5 (most marginalized) | 578 (24) | 2780 (25) | 0.01 |
| Missing | 22 | 72 | |
| Previous PCR tests, n (%) | |||
| 0 | 483 (20) | 2555 (22) | 0.06 |
| 1 | 627 (26) | 2953 (26) | 0 |
| 2 | 455 (19) | 2136 (19) | 0 |
| ≥3 | 838 (35) | 3712 (33) | 0.05 |
Vaccine status was defined as of June 30, 2021. CCI, Charlson Comorbidity Index; IQR, interquartile range.
Includes Pacific Islander, Aboriginal, Middle Eastern/Arabian, Latin American, and other/multiracial. These categories are based on the classification scheme of the Canadian Organ Replacement Registry and the Ontario Renal Reporting System.
CCI includes include chronic respiratory diseases, chronic heart diseases, hypertension, diabetes, immunocompromising conditions due to underlying diseases or therapy, autoimmune diseases, CKD, advanced liver disease, dementia/frailty, and history of stroke or transient ischemic attack.
Home hemodialysis and home peritoneal dialysis.
Economic dependency is one of four indexes in the Canadian Index of Multiple Deprivation and relates to reliance on the workforce, or a dependence on sources of income other than employment income. https://www150.statcan.gc.ca/n1/en/catalogue/45200001.
Bold indicates differences between groups.
The unadjusted rates of SARS-CoV-2 infection and of severe outcomes for patient time spent in each vaccine status are presented in Table 2. The rates of SARS-CoV-2 infection were 34.9, 26.4, and 11.2 during unvaccinated time, after one and two doses of vaccine, respectively (all rates are per 100,000 patient-days). The corresponding rates of severe SARS-CoV-2 outcomes were 18.5, 12, and 3.4, respectively.
Table 2.
Unadjusted rates of SARS-CoV-2 infection and severe outcome by vaccine group
| Outcome | Unvaccinated | One Dosea | Two Dosesb |
|---|---|---|---|
| Follow-up for infection, daysc | 1,398,544 | 424,427 | 563,396 |
| SARS-CoV-2 infections, n | 488 | 112 | 63 |
| Infection rate per 100,000 days | 34.9 | 26.4 | 11.2 |
| Follow-up for severe outcome, days | 1,403,562 | 426,643 | 564,927 |
| Hospitalizations, n (% of cases) | 253 (52) | 51 (46) | 19 (30) |
| Hospitalization rate per 100,000 days | 17.6 | 11.6 | 3.4 |
| Deaths, n (% of cases) | 77 (16) | 11 (9.8) | 6 (9.5) |
| Mortality rate | 5.3 | 2.5 | 1.1 |
| Severe outcome,d n (% of cases) | 260 (53) | 51 (46) | 19 (30) |
| Severe outcome rate | 18.5 | 12 | 3.4 |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Includes SARS-CoV-2 infections that occurred ≥14 days after the first dose of vaccine.
Includes SARS-CoV-2 infections that occurred ≥7 days after the second dose of vaccine.
Follow-up days are for the SARS-CoV-2 infection outcome. For the infection outcome, follow-up ends at the date of the infection. For the severe outcomes, follow-up ends at the date of the hospitalization or death. Individuals with SARS-CoV-2 infection who did not have a severe outcome were censored 30 days after the infection in the severe outcome analysis.
Includes hospitalization or death related to a SARS-CoV-2 infections. The counts are not mutually exclusive.
The PH assumption for the extended Cox model was satisfied for one- and two-dose exposure. There were no significant outliers or influential observations in the model. Background infection rates by public health unit region were correlated with the public health unit region variable; so, only the former was included in the model. The adjusted hazard ratio (HR) for SARS-CoV-2 infection and severe outcomes after one dose of vaccine compared with the unvaccinated time was 0.59 (95% confidence interval [CI], 0.46 to 0.76) and 0.54 (95% CI, 0.37 to 0.77), respectively (Table 3; includes unadjusted HR). The adjusted HRs for SARS-CoV-2 infection and severe outcomes after two doses of vaccine compared with the unvaccinated time were 0.31 (95% CI, 0.22 to 0.42) and 0.17 (95% CI, 0.11 to 0.3), respectively. Individuals were at higher risk for SARS-CoV-2 infection if they were non-White, on dialysis for a shorter time, lived in a long-term care facility, or resided in a region with a higher community rate of infection (Supplemental Table 4).
Table 3.
Adjusted HRs of SARS-CoV-2 infection and severe outcomes by person-time according to vaccine status
| Outcome | Unvaccinated | One Dosea | Two Dosesb | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| SARS-CoV-2 infections | 1 (ref.) | 0.68 (052 to 0.87) | 0.59 (0.46 to 0.76) | 0.49 (0.36 to 0.68) | 0.31 (0.22 to 0.42) |
| Severe outcomesc | 1 (ref.) | 0.67 (0.46 to 0.97) | 0.54 (0.37 to 0.77) | 0.3 (0.18 to 0.53) | 0.17 (0.1 to 0.3) |
| Hospitalizations | 1 (ref.) | 0.6 (0.42 to 0.87) | 0.49 (0.34 to 0.71) | 0.3 (0.17 to 0.51) | 0.18 (0.1 to 0.31) |
| Mortality | 1 (ref.) | 0.4 (0.19 to 0.84) | 0.29 (0.14 to 0.60) | 0.28 (0.11 to 0.72) | 0.15 (0.05 to 0.41) |
| Sensitivity analyses | |||||
| SARS-CoV-2 infections no lag periodd | 1 (ref.) | 0.67 (0.53 to 0.85) | 0.63 (0.5 to 0.79) | 0.56 (0.41 to 0.75) | 0.35 (0.26 to 0.48) |
Data are shown as HRs (95% CI). HRs were adjusted for age, sex, ethnicity, dialysis modality, Charlson Comorbidity Index, economic dependency quintiles, long-term care residences, vintage, number of RT-PCR tests prior December 21, 2020, and monthly public health unit region SARS-CoV-2 infection rate. HR, hazard ratio; CI, confidence interval.
Includes SARS-CoV-2 infections that occurred ≥14 days after the first dose of vaccine.
Includes SARS-CoV-2 infections that occurred ≥7 days after the second dose of vaccine.
Includes hospitalization or death related to a SARS-CoV-2 infection.
Includes SARS-CoV-2 infections that occurred immediately following the first dose or second dose of the vaccine (no lag period for the vaccine to become effective).
For the SARS-CoV-2 infection analysis, the competing risk events of non-COVID-19 death and recovered kidney function were associated with vaccine status. The corresponding adjusted HRs for non-COVID-19 death were 0.7 (95% CI, 0.57 to 0.85) and 0.36 (95% CI, 0.29 to 0.85), respectively (Supplemental Table 3). We did not find an association between solid organ transplant and vaccine status.
Analyzing the vaccine effect on the date of administration rather than allowing a lag period for 14 days for the first dose and 7 days for the second dose reduced VE, which likely reflected active infections either just before or after vaccination. The adjusted HRs were 0.63 (95% CI, 0.5 to 0.79) for one dose and 0.35 (95% CI, 0.26 to 0.48) for two doses.
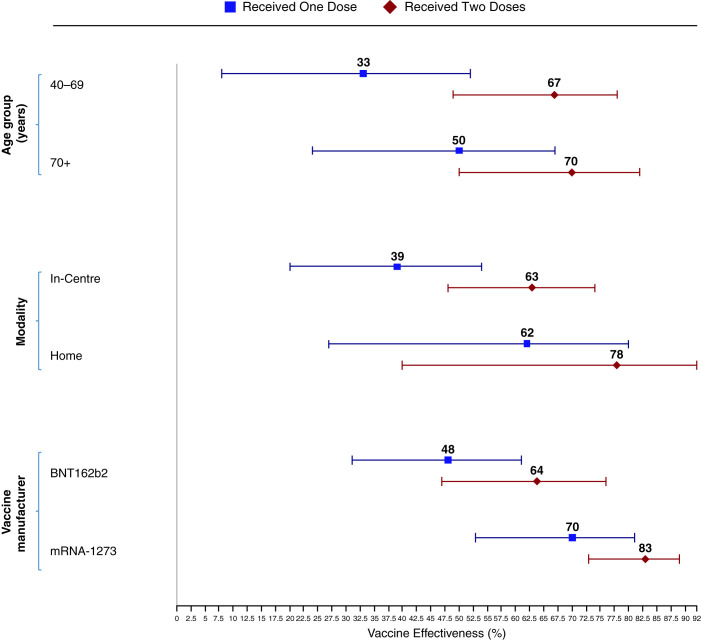
The subgroup analysis of VE is presented in Figure 2. There was no significant difference in the VE for any subgroup. The point estimates for VE were less for one dose compared with two doses in all three subgroups; the point estimate for VE was greater for younger individuals, those receiving home dialysis, and those receiving the BNT162b2 vaccine, but CIs overlapped. However, overlapping CIs should not be interpreted because the two groups are not statistically different.27
Figure 2.
Vaccine effectiveness by age group, dialysis modality, and vaccine type. Vaccine effectiveness was adjusted for age, sex, ethnicity, location, comorbidities, income quintiles, residence in a long-term care facility, years on dialysis, number of RT-PCR tests before December 21, 2020, and background community infection rates.
Discussion
This study found that one dose of COVID-19 mRNA vaccines was moderately effective in preventing SARS-CoV-2 infection, and two doses were very effective in preventing infection and associated severe outcomes in the maintenance dialysis population. Receipt of the vaccine was associated with older age, race, and comorbidity. Unvaccinated individuals were more likely to leave dialysis for non-COVID-19-related reasons, and the majority of the second vaccine doses occurred when community SARS-CoV-2 infection cases were dropping. Our analysis adjusted for this complexity to produce robust VE estimates that were consistent across subgroups.
The VE observed in this study is consistent with the serologic response rate observed in the maintenance dialysis population. Antibody responses, defined by thresholds, range from 82% to 96% in smaller studies in dialysis without prior COVID-19.8,28,29 In a larger study of 1140 individuals receiving maintenance hemodialysis in the United States, Anand et al. found a response rate of only 77%, 97% of whom received the BNT162b2 or mRNA-1273 vaccine.30 Although these response rates are reasonable, antibody levels are substantially lower, ranging from 7% to 66% of the levels achieved in healthy controls.7–11 These antibody levels, although suboptimal, have not been correlated with clinical outcomes.
VE—defined by clinical outcomes, specifically the prevention of SARS-CoV-2 infection and related severe outcomes—has only been reported by Sibbel et al. in the maintenance dialysis population. Using a propensity score matched design, they estimated the effectiveness of COVID-19 vaccination within 21 days, 22–42 days, and ≥ 43 days after the first dose of BNT162b2 or mRNA-1 vaccine. The median time to second dose was 21 days and 28 days for each vaccine, respectively. VE was estimated to be 79% and 73% for the BNT162b2 and the mRNA-1273 vaccine, respectively, more than 43 days after first dose of vaccine, which is similar to a VE of 69% in this study measured 7 days after the second dose. Our study estimated VE on the basis of timing of both the first and second dose and used lag periods similar to other VE studies.14–16,22 We also studied an entire population within a province, not one restricted to one dialysis organization, and included patients on home dialysis, which improves the generalizability of the study.
The initial randomized controlled trials of the BNT162b2 vaccine in the general population reported a VE of 95% for preventing symptomatic infection and 89% for severe infection.14 Similarly, Baden et al. reported a VE of 94% for symptomatic COVID-19 illness, and no severe cases were reported in the vaccinated group.15 Chung et al. used a test-negative design in the general Ontario population and reported a VE of 91% against symptomatic COVID-19 and 98% against severe outcomes.16 In contrast, the lower VE of 69% in this study is similar to other higher-risk groups such as individuals with cirrhosis and inflammatory bowel disease in whom a VE of 79% and 80%, respectively, have been reported.
Apart from the muted antibody response, another reason for the lower VE compared with studies in the general population may be that the primary outcome of this study was a laboratory-confirmed infection, not symptomatic infection. We did not have access to symptom data; however, assessing symptoms in the chronic dialysis population is also challenging. A province-wide point prevalence study that we conducted found 6% of patients could not reliably answer a symptom questionnaire due to language or cognitive issues, and 17% reported symptoms, often atypical, that could suggest COVID-19, even when RT-PCR testing was negative. Our results show RT-PCR testing was frequent in this population because these in-center hemodialysis patients answer screening questions three times per week, often have chronic symptoms, and may be tested during outbreaks, even when asymptomatic.31 Even in the general population, studies in Ontario found 83% of individuals did not have symptoms recorded or had symptoms inconsistent with COVID-19 recorded in public health databases when tested for SAR-CoV-2. Nonetheless, examining VE for symptomatic infection in this population using methods such as test-negative designs should still be explored in the future to compare with our results.16
This study demonstrates the methodologic challenges for estimating VE in this population. We chose a cohort design, which allowed the inclusion of the whole dialysis population, and modeled both vaccine exposure and background infection rates as a time-dependent covariate in an extended cause-specific Cox PH model using a calendar timescale.24,25 This approach has been used in other studies of COVID-19 and non-COVID-19 vaccines.22,32 We included the background infection rates to reduce bias because the majority of second doses of vaccine occurred when the community rates were falling in the third wave of the pandemic in Ontario. Patients on dialysis also frequently experience censoring events. Unvaccinated patients were more likely to exit the study for non-COVID-19-related reasons, including non-COVID-19 death, and recovered kidney function because they did not remain on dialysis long enough to have the opportunity to receive a vaccine. Residual confounding is still possible in that risky health behaviors, which were not measured, in the unvaccinated population could lead to a higher risk of non-COVID-19 death. It is less likely that early non-COVID-19 death is misclassified because all deaths within 30 days of a SARS-CoV-2 infection were considered COVID-19 related, which is quite inclusive. However, deaths after 30 days from the sequelae of infection could be misclassified. We did not use the Fine and Gray model to account for competing risks because of the methodologic limitations of using this approach with time-varying covariates, which may explain this result.33–36
A strength of this study was the inclusion of the entire maintenance dialysis population in Canada's most populous province, including a substantial home dialysis population. We had access to province-wide data sources that reliably captured the receipt of vaccines and RT-PCR testing for SARS-CoV-2 infections. These sources provide an accurate assessment of vaccine coverage and the risk of COVID-19 in a generalizable dialysis population.16 A limitation is that we did not have access to variant data during this study. However, the B.1.1.7 (Alpha) variant was responsible for most cases in the community, with the B.1.617.2 (Delta) variant being responsible for <10%.37 This study preceded the use of third doses of mRNA vaccine and the more recent appearance of the Omicron variant of the virus, which will likely require VE studies to be repeated in vulnerable populations such as those on maintenance dialysis. The study also had limited power to detect differences in important dialysis subgroups.
In summary, our results demonstrate that COVID-19 vaccines are effective in the maintenance dialysis population to prevent both SARS-CoV-2 infection and severe outcomes, despite concerns about suboptimal antibody responses. One dose of vaccine provided weak protection, justifying the prioritization of patients on dialysis for timely and complete series of vaccinations. We recommend conducting further studies of varying designs to examine and refine our understanding of VE in this very high-risk population, including developing protocols to assess the durability of vaccine protection in the face of evolving variants of the virus.
Disclosures
S. Balamchi, J. Ip, D. Thomas, and A. Yeung are salaried employees of Ontario Health. P.G. Blake is a contracted medical lead at Ontario Renal Network, Ontario Health. He has received honoraria from Baxter Global for speaking engagements. He also reports being on the editorial board of the American Journal of Nephrology. A.X. Garg reports research funding from Astellas and Baxter; is currently on the editorial boards of Kidney International and the American Journal of Kidney Disease; and serves on the data safety and monitoring board for an anemia trial program funded by Glaxo Smith Kline and is a medical lead to improve access to kidney transplantation and living kidney donation for the Ontario Renal Network (government funded agency located within Ontario Health). M.A. Hladunewich reports consultancy for Alnylam Pharmaceuticals; research funding from Calliditas Therapeutics, Chemocentryx, Ionis, Pfizer, and Roche; an advisory or leadership role for Kidney International and UpToDate; and other interests or relationships as the medical lead for the Glomerular Disease Ontario Renal Network. M. Krajden has received contracts/grants paid to his institution from Abcellera, Hologic, Roche, and Siemens unrelated to this work. A. Levin reports consultancy for the Bayer, Chinook Therapeutics, the National Institutes of Health, and REATA; research funding from Astra Zeneca, Boehringer Ingelheim, the Canadian Institute of Health Research (CIHR), and the Kidney Foundation of Canada; honoraria from AstraZeneca, Bayer, Fresenius, Janssen, and the National Institutes of Health; an advisory or leadership role for AstraZeneca, Boehringer Ingelheim, and NIDDK; and is on the DSMB for Chinook Therapeutics, GSK, the International Society of Nephrology Research Committee, Kidney Precision Medicine, KRESCENT (Kidney Scientist Education Research National Training Program), the NIDDK, REATA, and the University of Washington Kidney Research Institute Scientific Advisory Committee; and has other interests or relationships with the ALIGN trial Steering Committee, the Canadian Society of Nephrology, the CREDENCE National Coordinator from Janssen directed to academic team, the DSMB Chair RESOLVE Trial (Australian Clinical Trial Network), the International Society of Nephrology, the Kidney Foundation of Canada, and the NIDDK CURE Chair Steering Committee. M.J. Oliver is a contracted medical lead at Ontario Renal Network, Ontario Health. He is the owner of Oliver Medical Management, Inc., which licenses Dialysis Management Analysis and Reporting System software. He has received honoraria for speaking from Baxter Healthcare and participated on advisory boards for Amgen and Janssen. He also reports patents or royalties from Oliver Medical Management, Inc., and is co-owner of a Canadian patent for DMAR systems. J. Perl reports grants from the Agency for Healthcare Research and Quality during the conduct of the study; personal fees from AstraZeneca Canada, Baxter Healthcare, DaVita Healthcare Partners, DCI, Fresenium Medical Care, LiberDi, Otsuka, and US Renal Care; research funding and salary support from Arbor Research Collaborative for Health and Agency for Health Research and Quality; participates in a speakers’ bureau for Baxter Healthcare and Fresenium Medical Care; and is on the advisory board for Liberdi, outside of the submitted work. J. Singer reports royalties for the IBDQ questionnaire from McMaster University Industrial Liaison Office.
Funding
This project was funded by the COVID-19 Immunity Task Force (grant number: 2122-HQ-000071).
Supplementary Material
Acknowledgments
The authors acknowledge that data used in this publication were obtained through the Ontario Renal Reporting System and the ORN COVID-19 Data tracker, collected and provided by the Ontario Renal Network, a part of Ontario Health. This study was supported by the ICES, which is funded by an annual grant from the Ontario Ministry of Health. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by Institute for Clinical Evaluative Sciences or the Ontario Ministry of Health is intended or should be inferred. Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information. However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors, and not necessarily those of Canadian Institute for Health Information. Income quintile analysis adapted from Statistics Canada Postal CodeOM Conversion File and/or Postal CodesOM by Federal Ridings File and/or Postal CodeOM Conversion File Plus (November 2018), which is based on data licensed from Canada Post Corporation. The authors thank the Ontario Regional Renal Programs and all individuals submitting data each week for their efforts to serve those with kidney disease.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
M. Atiquzzaman, S. Balamchi, P.G. Blake, S.N. Dixon, J. Ip, J. Kwong, E. McArthur, K. Naylor, M.J. Oliver, J. Perl, J. Singer, and D. Thomas were responsible for methodology; M. Atiquzzaman, P.G. Blake, S.N. Dixon, A.X. Garg, M.A. Hladunewich, M. Krajden, J. Kwong, J.A. Leis, A. Levin, E. McArthur, K. Naylor, J. Perl, J. Singer, K. Yau, and A. Yeung reviewed and edited the manuscript; S. Balamchi, P.G. Blake, J. Ip, M.J. Oliver, and D. Thomas were responsible for conceptualization; S. Balamchi and D. Thomas were responsible for formal analysis; P.G. Blake was responsible for supervision; and M.J. Oliver wrote the original draft of the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021091262/-/DCSupplemental.
Supplemental Table 1. Diagnostic codes.
Supplemental Table 2. STROBE Statement—checklist of items that should be included in reports of observational studies.
Supplemental Table 3. Non-COVID-19-related reasons for study exit by vaccine group in severe acute respiratory syndrome coronavirus 2 infection analysis.
Supplemental Table 4. Adjusted hazard ratios for risk factors for severe acute respiratory syndrome coronavirus 2 infection, including two doses of vaccine.
References
- 1.Taji L, Thomas D, Oliver MJ, Ip J, Tang Y, Yeung A, et al. : COVID-19 in patients undergoing long-term dialysis in Ontario. Can Med Assoc J 193: E278–E284, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu CM, Weiner DE, Aweh G, Miskulin DC, Manley HJ, Stewart C, et al. : COVID-19 among US dialysis patients: Risk factors and outcomes from a national dialysis provider. Am J Kidney Dis 77: 748–756.e1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, et al. : Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 98: 1540–1548, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, et al. ; French REIN registry : Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int 98: 1519–1529, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake PG, Hladunewich MA, Oliver MJ: COVID-19 vaccination imperatives in people on maintenance dialysis: An international perspective. Clin J Am Soc Nephrol 16: 1746–1748, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goupil R, Benlarbi M, Beaubien-Souligny W, Nadeau-Fredette AC, Chatterjee D, Goyette G, et al. ; Réseau Rénal Québécois (Quebec Renal Network) COVID-19 Study Investigators : Short-term antibody response after 1 dose of BNT162b2 vaccine in patients receiving hemodialysis. CMAJ 193: E793–E800, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yau K, Abe KT, Naimark D, Oliver MJ, Perl J, Leis JA, et al. : Evaluation of the SARS-CoV-2 antibody response to the BNT162b2 vaccine in patients undergoing hemodialysis. JAMA Netw Open 4: e2123622, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupper A, Sharon N, Finn T, Cohen R, Israel M, Agbaria A, et al. : Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 16: 1037–1042, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon B, Rubey H, Treipl A, Gromann M, Hemedi B, Zehetmayer S, et al. : Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant 36: 1709–1716, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrezenmeier E, Bergfeld L, Hillus D, Lippert JD, Weber U, Tober-Lau P, et al. : Immunogenicity of COVID-19 Tozinameran vaccination in patients on chronic dialysis. Front Immunol 12: 690698, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanay NB, Freiman S, Shapira M, Wishahi S, Hamze M, Elhaj M, et al. : Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int 99: 1496–1498, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broseta JJ, Rodríguez-Espinosa D, Rodríguez N, Mosquera MDM, Marcos MÁ, Egri N, et al. : Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am J Kidney Dis 78: 571–581, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibbel S, McKeon K, Luo J, Wendt K, Walker AG, Kelley T, et al. : Real-world effectiveness and immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in patients on hemodialysis. J Am Soc Nephrol 33: 49–57, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. ; C4591001 Clinical Trial Group : Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383: 2603–2615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. ; COVE Study Group : Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403–416, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung H, He S, Nasreen S, Sundaram ME, Buchan SA, Wilson SE, et al. ; Canadian Immunization Research Network (CIRN) Provincial Collaborative Network (PCN) Investigators : Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: Test negative design study. BMJ 374: n1943, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ontario Renal Network OH : Ontario Renal Reporting System (ORRS) Release 8 (R8) Data Dictionary. Available at: https://ext.cancercare.on.ca/ext/databook/db1920/documents/Appendix/ORRS_Release_8_(R8)_Data_Dictionary_V3.pdf. Accessed September 1, 2021
- 18.www.publichealthontario.ca : All Ontario: Case numbers and spread. Available at: https://covid-19.ontario.ca/data/case-numbers-and-spread. Accessed September 1, 2021
- 19.Statistics Canada: Canadian Index of Multiple Deprivation: Dataset and User Guide. Available at: https://www150.statcan.gc.ca/n1/en/catalogue/45200001. Accessed September 1, 2021
- 20.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 21.Yang D, Dalton JE: A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum. Stat Data Anal paper 335-2012, 1–6, 2012 [Google Scholar]
- 22.Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, et al. : Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): A prospective cohort study. Lancet Infect Dis 21: 1529–1538, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan N, Mahmud N: Effectiveness of SARS-CoV-2 vaccination in a Veterans Affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology 161: 827–836, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. : Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 398: 1407–1416, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolkewitz M, Cooper BS, Palomar-Martinez M, Alvarez-Lerma F, Olaechea-Astigarraga P, Barnett AG, et al. : Multiple time scales in modeling the incidence of infections acquired in intensive care units. BMC Med Res Methodol 16: 116, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, et al. : Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med 385: 320–329, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC, Hux JE: A brief note on overlapping confidence intervals. J Vasc Surg 36: 194–195, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Attias P, Sakhi H, Rieu P, Soorkia A, Assayag D, Bouhroum S, et al. : Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int 99: 1490–1492, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agur T, Ben-Dor N, Goldman S, Lichtenberg S, Herman-Edelstein M, Yahav D, et al. : Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients—A prospectivecohort study [published online ahead of print April 11, 2021]. Nephrol Dial Transplant [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand S, Montez-Rath ME, Han J, Garcia P, Cadden L, Hunsader P, et al. : Antibody response to COVID-19 vaccination in patients receiving dialysis. J Am Soc Nephrol 32: 2435–2438, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yau K, Muller MP, Lin M, Siddiqui N, Neskovic S, Shokar G, et al. : COVID-19 outbreak in an urban hemodialysis unit. Am J Kidney Dis 76: 690–695.e1, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camerino M, Jackson S, Chinnakotla S, Verghese P: Effects of the influenza vaccine on pediatric kidney transplant outcomes. Pediatr Transplant 23: e13354, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Austin PC, Latouche A, Fine JP: A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat Med 39: 103–113, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau B, Cole SR, Gange SJ: Competing risk regression models for epidemiologic data. Am J Epidemiol 170: 244–256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poguntke I, Schumacher M, Beyersmann J, Wolkewitz M: Simulation shows undesirable results for competing risks analysis with time-dependent covariates for clinical outcomes. BMC Med Res Methodol 18: 79, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beyersmann J, Schumacher M: Time-dependent covariates in the proportional subdistribution hazards model for competing risks. Biostatistics 9: 765–776, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Public Health Ontario : COVID-19 variants of concern in Ontario: December 1, 2020 to May 9, 2021. 2021. Available at: https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-variant-epi-summary.pdf. Accessed July 17, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.