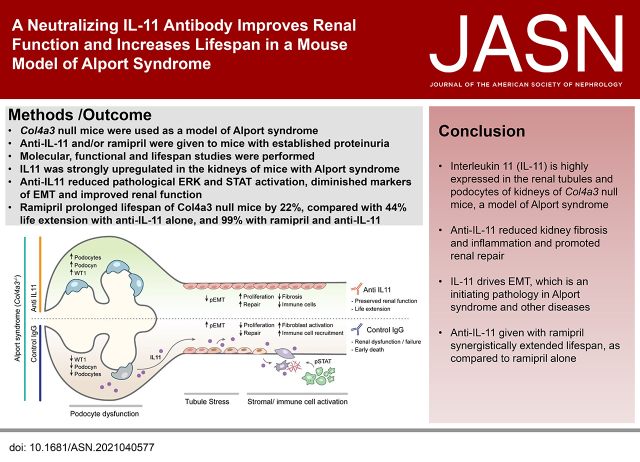
Significance Statement
Alport syndrome (AS), a genetic disorder of the glomerular basement membrane, frequently leads to end stage renal failure. In an animal model of AS—mice lacking the Col4a3 gene, —angiotensin-converting enzyme inhibition is protective. The authors show that IL-11 is upregulated in the renal tubular epithelia of Col4a3−/− mice; the IL-11 receptor (IL11RA1), expressed on podocytes and tubule cells, is upregulated in the diseased kidneys of Col4a3−/− mice. Giving 6-week-old Col4a3−/− mice a neutralizing IL-11 antibody (X203) reduced pathologic ERK and STAT3 activation and limited epithelial-to-mesenchymal transition; reduced kidney fibrosis, inflammation, and tubule damage; and improved kidney function. The median lifespan of Col4a3−/− mice was prolonged 22% by ramipril alone, 44% with X203 alone, and 99% with ramipril+X203. These data suggest that anti-IL-11 therapies hold promise for treating kidney disease in AS.
Keywords: Alport syndrome, fibrosis, interleukin 11, podocyte, therapy, glomerular disease, glomerulosclerosis, chronic kidney disease
Visual Abstract
Abstract
Background
Alport syndrome is a genetic disorder characterized by a defective glomerular basement membrane, tubulointerstitial fibrosis, inflammation, and progressive renal failure. IL-11 was recently implicated in fibrotic kidney disease, but its role in Alport syndrome is unknown.
Methods
We determined IL-11 expression by molecular analyses and in an Alport syndrome mouse model. We assessed the effects of a neutralizing IL-11 antibody (×203) versus an IgG control in Col4a3−/− mice (lacking the gene encoding a type IV collagen component) on renal tubule damage, function, fibrosis, and inflammation. Effects of ×203, the IgG control, an angiotensin-converting enzyme (ACE) inhibitor (ramipril), or ramipril+X203 on lifespan were also studied.
Results
In Col4a3−/− mice, as kidney failure advanced, renal IL-11 levels increased, and IL-11 expression localized to tubular epithelial cells. The IL-11 receptor (IL-11RA1) is expressed in tubular epithelial cells and podocytes and is upregulated in tubular epithelial cells of Col4a3−/− mice. Administration of ×203 reduced albuminuria, improved renal function, and preserved podocyte numbers and levels of key podocyte proteins that are reduced in Col4a3−/− mice; these effects were accompanied by reduced fibrosis and inflammation, attenuation of epithelial-to-mesenchymal transition, and increased expression of regenerative markers. X203 attenuated pathogenic ERK and STAT3 pathways, which were activated in Col4a3−/− mice. The median lifespan of Col4a3−/− mice was prolonged 22% by ramipril, 44% with ×203, and 99% with ramipril+X203.
Conclusions
In an Alport syndrome mouse model, renal IL-11 is upregulated, and neutralization of IL-11 reduces epithelial-to-mesenchymal transition, fibrosis, and inflammation while improving renal function. Anti-IL-11 combined with ACE inhibition synergistically extends lifespan. This suggests that a therapeutic approach targeting IL-11 holds promise for progressive kidney disease in Alport syndrome.
Alport syndrome (AS) is caused by mutation in the A3/4/5 genes that encode chains of type IV collagen.1,2 These mutations lead to abnormalities in glomerular basement membrane (GBM) collagen composition, integrin-mediated podocyte dysfunction, glomerular hypertension, and ultrafiltration.3,4 AS affects up to 60,000 people in the United States and is associated with hearing loss, ocular abnormalities, and CKD.
In the commonest form of disease due to X-linked mutation of COL4A5, 90% of affected men develop ESKD by the age of 40.5 Early disease can manifest as hematuria, microalbuminuria, or proteinuria, and although there are no specific therapies, affected children are commonly treated with an angiotensin converting enzyme inhibitor (ACEi), on the basis in part of extrapolation of studies conducted in Col4a3−/− mice6 and supported by more recent data from clinical trials.7,8
The Col4a3−/− mouse strain is widely viewed as one of the best animal models of progressive AS. In seminal studies, treatment of 4-week-old Col4a3−/− mice with an ACEi (ramipril), before the onset of proteinuria and tubulointerstitial fibrosis, attenuated kidney dysfunction and prolonged lifespan.6 However, if ramipril treatment of Col4a3−/− mice was delayed until 7 weeks of age, after proteinuria was established, there was limited beneficial effect.6,9 There are no specific or second-line medical therapies for AS, and renal transplantation is the preferred treatment for progressive CKD in AS.10
Kidney dysfunction in AS is initiated in the glomerulus, related to altered GBM mechanics and podocyte dysfunction. However, as in other primary glomerular diseases, a major determinant of progressive kidney failure is in the associated tubulointerstitial disease.4 Indeed, similar to other forms of CKD, kidney function in AS patients correlates most strongly with the degree of tubulointerstitial fibrosis rather than glomerular pathology.11 Disease pathogenesis in AS is complex, involving the renin-angiotensin system (RAS) and TGF-β activation, inflammation, partial epithelial-to-mesenchymal transition (pEMT) of tubular epithelial cells (TECs)/podocytes and fibrosis, among other factors.9,12,13 Increasingly the role of pEMT, a failed-repair proximal tubule cell (FR-PTC) state,14 is viewed as an initiating factor for renal fibrosis, inflammation, and failure, particularly because it prevents TEC proliferation and renal repair.15–20
Here, we investigated whether (1) IL-11, recently implicated as important for tubulointerstitial fibrosis and renal dysfunction,21 is involved in the kidney pathology of AS and (2) a neutralizing IL-11 antibody given to Col4a3−/− mice with established renal disease and proteinuria could improve molecular pathology, renal structure, and function, and delay onset of death due to kidney failure.
Methods
Antibodies
Cyclin D1 (55506; Cell Signaling Technology, Danvers, MA), E-cadherin (3195; Cell Signaling Technology), p-ERK1/2 (4370; Cell Signaling Technology), ERK1/2 (4695; Cell Signaling Technology), GAPDH (2118; Cell Signaling T), green fluorescent protein (GFP; ab6673; Abcam, Cambridge, UK), IgG (11E10; Aldevron, Fargo, ND), neutralizing anti-IL-11 (X203; Aldevron), anti-IL-11RA (X209; Aldevron), NHPS2/podocin (ab181143; Abcam), PCNA (13110; Cell Signaling Technology), α-smooth muscle actin (19245; Cell Signaling Technology, Western blot [WB]), SNAI1 (3879; Cell Signaling Technology, WB), p-STAT3 (4113; Cell Signaling Technology), STAT3 (4904; Cell Signaling Technology), TGF-β (3711; Cell Signaling Technology), Wilms’ tumor 1 (WT1; ab89901; Abcam, immunofluorescence [IF] and immunohistochemistry [IHC]), WT1 (ab267377; Abcam, WB), anti-goat Alexa Fluor 488 (ab150129; Abcam), anti-rabbit Alexa Fluor 647 (ab150067; Abcam), anti-rabbit horseradish peroxidase (HRP; 7074; Cell Signaling Technology), and anti-mouse HRP (7076; Cell Signaling Technology).
Ethics Statements
Animal studies were carried out in compliance with the recommendations in the Guidelines on the Care and Use of Animals for Scientific Purposes of the National Advisory Committee for Laboratory Animal Research. All experimental procedures were approved (SHS/2019/1482) and conducted in accordance with the SingHealth Institutional Animal Care and Use Committee.
Mouse Model of Alport
Col4a3−/− (Col4a3tm1Dec) mice were purchased from the Jackson Laboratory (https://www.jax.org/strain/002908). Mice were housed at temperatures of 21°C–24°C with 40%–70% humidity on a 12-hour/12-hour light/dark cycle and provided with food and water ad libitum. For the treatment study, Col4a3−/− were administered 20 mg/kg of anti-I-L11 (×203) or IgG isotype control (11E10) by intraperitoneal injection, starting from 6 weeks of age twice a week for 2.5 weeks; wild-type (WT) littermates were used as controls. Mice were euthanized for blood and kidney collection when they were 8.5 weeks old. For the lifespan study, mice were intraperitoneally administered either ×203 (twice a week, 20 mg/kg) or 11E10 (twice a week, 20 mg/kg) alone or in combination with ramipril (10 mg/kg; 6 days/week) starting from 6 weeks of age until death ensued.
Col4a3−/−-Il-11:EGFP
Col4a3−/− mice were crossed to transgenic mice with EGFP constitutively knocked in to the Il-11 gene22 to generate hybrid cross of Col4a3−/−-Il-11:EGFP+/−. Age-matched Col4a3+/+-Il-11:EGFP+/− littermates were used as controls. Mice were euthanized at 7.5 weeks of age; kidneys were excised and OCT embedded for IF staining.
Western Blot (WB)
WB was carried out on total protein extracts from mouse kidney tissues. Kidneys were lysed in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA), followed by centrifugation to clear the lysate. Protein concentrations were determined by Bradford assay (Bio-Rad, Hercules, CA). Protein lysates were separated by SDS-PAGE, transferred to a polyvinylidene fluoride membrane, and subjected to immunoblot analysis for various antibodies (1:1000 in Tris-buffered saline with Tween 20) as outlined in the main text, figures, and/or figure legends. Proteins were visualized using the enhanced chemiluminescence detection system (Pierce, Rockford, IL) with the appropriate secondary antibodies: anti-rabbit HRP or anti-mouse HRP (1:2000 in Tris-buffered saline with Tween 20).
Quantitative PCR
Total RNA was extracted from snap-frozen kidney tissues using Trizol (Invitrogen, Carlsbad, CA) followed by RNeasy column (Qiagen, Hilden, Germany) purification. cDNAs were synthesized with iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s instructions. Gene expression analysis was performed on duplicate samples with either TaqMan (Applied Biosystems, Foster City, CA) or fast SYBR green (Qiagen) technology using StepOnePlus (Applied Biosystem) over 40 cycles. Expression data were normalized to GAPDH mRNA expression, and fold change was calculated using the 2–ΔΔCt method. The sequences of specific TaqMan probes and SYBR green primers are available upon request.
Colorimetric Assays
The levels of BUN and creatinine (Cr) in mouse serum were measured using a Urea Assay Kit (ab83362; Abcam) and Creatinine Assay Kit (ab65340; Abcam), respectively. Urine albumin and Cr levels were measured using a Mouse Albumin ELISA kit (ab108792; Abcam) and Creatinine Assay Kit (ab204537; Abcam), respectively. All ELISA and colorimetric assays were performed according to the manufacturer’s protocol.
Histology
Kidney tissues were fixed for 48 hours at room temperature in 10% neutral-buffered formalin, dehydrated, embedded in paraffin, and sectioned at 7 µm. Transverse kidney sections were then stained with periodic acid–Schiff (PAS) and Masson’s trichrome according to standard protocol. Images of the sections were captured by light microscopy, and blue-stained fibrotic areas were semi-quantitatively determined with ImageJ software (color deconvolution-Masson’s Trichrome; National Institutes of Health, Bethesda, MD) from the whole kidney area (×100 field, n=4 kidneys/group). Kidney sections (n=4–7/group) were independently scored for tubulointerstitial fibrosis (from Masson’s trichrome–stained kidney sections) and for glomerulosclerosis and tubular atrophy (from PAS-stained kidney sections) by a renal pathologist in a blinded fashion with the following criteria:
-
•
Interstitium (0, no fibrosis; 1, <25% fibrosis; 2, 25%–50% fibrosis; 3, >50% fibrosis).
-
•
Glomeruli (0, no sclerosis; 1, <25% sclerosis; 2, 25%–50% sclerosis; 3, >50% sclerosis).
-
•
Tubules (0, no atrophy; 1, <25% atrophy; 2, 25%–50% atrophy; 3, >50% atrophy).
-
•
Total score is the sum of interstitial fibrosis score, glomeruli score, and tubule atrophy score.
Treatment and genotypes were not disclosed to investigators performing the histology and generating semi-quantitative readouts.
IHC
Kidneys were fixed in 10% neutral-buffered formalin, paraffinized, cut into 7-µm sections, incubated with primary antibodies overnight, and visualized using the appropriate ImmPRESS HRP IgG polymer detection kit: anti-rabbit (MP-7401; Vector Laboratories, Burlingame, CA), anti-mouse (MP-7402; Vector Laboratories) with ImmPACT DAB Peroxidase Substrate (SK-4105; Vector Laboratories). Quantification of WT+ve cells were performed in a blinded fashion from four images (×200 field)/kidney (n=3–4 kidneys/group).
IF
Kidneys were rinsed in cold PBS, patted dry with lint-free paper, and cryo-molded in OCT compound (4583; Tissue-Tek). After the OCT compound was frozen, kidney specimens were wrapped in aluminum foil and stored in –80°C. Cryo-embedded kidneys were cryosectioned (–20°C) at a thickness of 7 µm and allowed to dry on the slides for 1 hour at room temperature. Kidney sections were fixed in cold acetone for 15 minutes before brief PBS washes, permeabilized with 0.1% TritonX-100 (T8787; Sigma–Aldrich, St. Louis, MO), and blocked with 2.5% normal horse serum (S-2012; Vector Laboratories) for 1 hour at room temperature. Kidney sections were incubated with GFP and WT1 (1:500 in PBS containing 0.1% Tween 20) primary antibodies overnight at 4°C, followed by incubation with the appropriate Alexa Fluor 488/647 secondary antibodies (1:250) for 1 hour at room temperature. DAPI was used to stain the nuclei before imaging by fluorescence microscope (Leica, Wetzlar, Germany).
Statistical Analyses
Statistical analyses were performed using GraphPad Prism v8 (GraphPad Software, La Jolla, CA). Statistical significance between control and experimental groups was analyzed by two-sided Student’s t tests or by one-way ANOVA as indicated in the figure legends. P values were corrected for multiple testing according to Tukey when several conditions were compared with each other within one experiment. Comparison analysis for two parameters from two different groups was performed by two-way ANOVA. The criterion for statistical significance was P<0.05.
Results
IL-11 Is Upregulated in the Kidneys of Col4a3−/− Mice
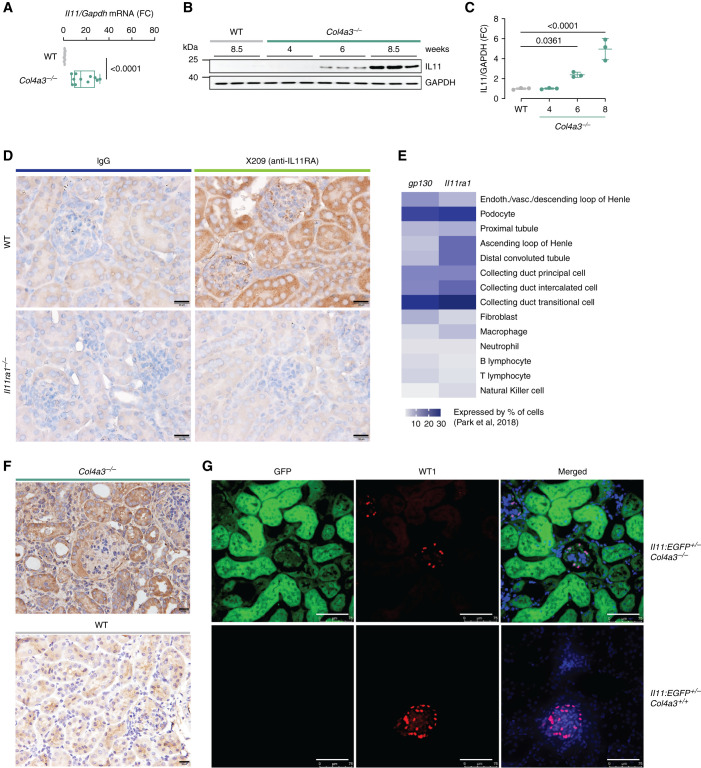
IL-11 is not expressed in normal healthy tissues, but its induction is commonly seen in fibroinflammatory diseases.23 We profiled the Il-11 mRNA expression in kidneys of Col4a3−/− mice and found it to be upregulated (17.8-fold; P<0.001) compared with WT littermate controls (Figure 1A). We then assessed renal IL-11 expression at the protein level in Col4a3−/− mice. IL-11 was not detectable at 4 weeks of age (or in 8.5-week-old WT mice), but it was upregulated in the kidneys of 6-week-old mice (2.4-fold; P=0.04) and highly expressed by 8.5 weeks of age (5.9-fold; P<0.001; Figure 1, B and C).
Figure 1.
IL-11 is upregulated in the kidneys of Col4a3−/− mice, and IL-11RA is expressed in podocytes and renal tubular epithelial cells. (A–C) Renal (A) Il-11 RNA (n=8–11/group) and (B–C) IL-11 protein expression (n=3/group) in WT and Col4a3−/− mice. (D) IHC staining of IL-11RA with anti-IL-11RA (×209) or IgG (11E10) as control on the kidneys of WT and Il-11ra1−/− mice (scale bars, 20 µm; representative of n=3 datasets/group). (E) Comparison of Il-11ra1 and gp130 expression in mouse kidney cells on the basis of single-cell transcriptomic analysis by Park et al.24 (F) IHC staining of IL-11RA with ×209 on the kidneys of WT and Col4a3−/− mice (scale bars, 20 µm; representative of n=3 datasets/group). (G) IF images (scale bars, 75 µm; representative of n=3 datasets/group) of EGFP and WT1 expression in the kidneys of Col4a3+/+-Il-11:EGFP+/− and Col4a3−/−-Il-11:EGFP+/−mice. (A) Data are shown as box and whisker with median (middle line), 25th–75th percentiles (box), and minimum–maximum values (whiskers); two-tailed Student’s t test. (C) Data are shown as mean±SD; one-way ANOVA with Dunnett’s correction. FC, fold change.
We sought to determine the target cells in the kidney that express the IL-11 receptor (IL-11RA1) by both IHC and mining publicly available single-cell RNA sequencing data.24 In WT mice, IL-11RA1 expression was easily seen in tubules and in the glomerulus, whereas no staining was seen in sections from Il-11ra1−/− mice, confirming specificity of detection (Figure 1D). In single-cell RNA sequencing data from WT mice,24 we observed that Il-11ra1 and its partner receptor (gp130) were most highly expressed in podocytes, collecting ducts with lesser expression in tubule cells across the nephron, and fibroblasts (Figure 1E). IL-11RA1 expression, like IL-11, may be dynamically regulated in disease, and we determined its expression in kidneys from both WT and Col4a3−/− mice and found it to be upregulated in diseased kidneys, notably in the tubules (Figure 1F).
IL-11 is secreted by epithelial cells exposed to infective and toxic factors and in response to cytokines, chemokines, and other signaling molecules.23,25 IL-11 is also secreted from activated stromal cells across tissues. To define the source of IL-11 better in the kidneys of Col4a3−/− mice, we crossed this strain with mice with EGFP knocked into the Il-11 locus—the Il-11:EGFP mouse.22 Kidneys of Col4a3−/−-Il-11:EGFP+/− mice exhibited large upregulation of EGFP in the renal tubules and also showed EGFP expression in the glomeruli, which co-localized with WT1 in podocytes (Figure 1G).
Antibody Neutralization of IL-11 Reduces Molecular Pathology in Col4a3−/− Mice
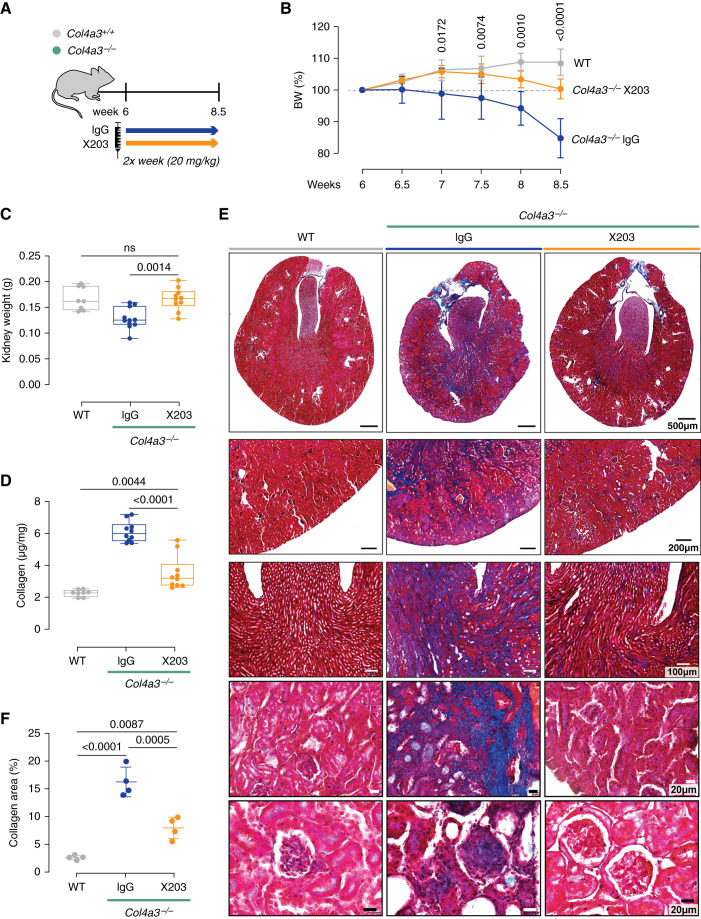
Over recent years, we have developed and characterized antibodies that inhibit IL-11 signaling in murine and human cells.23,26 We administered one of these neutralizing IL-11 antibodies (X20326,27) or an IgG control antibody to 6-week-old Col4a3−/− mice—a time point when initiation of ramipril likely has limited effect.6 We examined renal pathologies in these mice 2.5 weeks after starting treatment, when they are close to death due to renal failure, and compared data with WT controls (Figure 2A).
Figure 2.
In Col4a3−/− mice, a neutralizing IL-11 antibody (×203) preserves kidney mass and reduces renal fibrosis. (A) Schematic showing therapeutic dosing of Col4a3−/− mice for experimental data shown in B-I. Six-week-old Col4a3−/− mice were administered IgG/X203 (20 mg/kg, twice a week) for 2.5 weeks; WT littermates were used as controls (n=8–11/group). (B) Body weight (shown as a percentage of initial body weight). (C) Kidney weight. (D) Total renal collagen content per milligram of kidney weight as measured by quantitative colorimetric determination of hydroxyproline residues obtained by acid hydrolysis of collagen (see Methods for more details). (E) Representative and (F) quantification (from 100× field images) of Masson’s trichrome staining (representative datasets from n=4/group). (B and F) Data are shown as mean±SD. (C and D) Data are shown as box and whisker with median (middle line), 25th–75th percentiles (box), and minimum–maximum values (whiskers). (B) Two-way ANOVA with Tukey’s correction. (C, D, and F) One-way ANOVA with Tukey’s correction.
At the end of the study period, total body weight loss, measured as a percentage of their starting body weight, was significantly attenuated in Col4a3−/− mice receiving ×203, compared with those administered IgG (IgG: 30%; X203:17%; P=0.0003; Figure 2B). As compared with Col4a3−/− mice receiving IgG, X203-treated mice exhibited preserved kidney mass (Figure 2C) and had significantly less kidney fibrosis by both biochemical and histologic assessments (Figure 2, D–F).
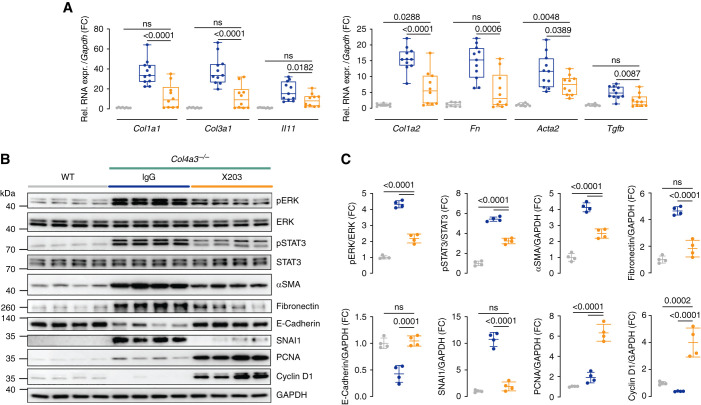
Gene expression analyses showed renal levels of extracellular matrix genes (Col1a1, Col1a2, Col3a1, and Fn), the myofibroblast marker Acta2, and pro-fibrotic factors (Il-11 and Tgfb1) were all reduced by ×203 compared with IgG (Figure 3A). The effect seen on transcript expression was confirmed at the protein level for α-smooth muscle actin and fibronectin (Figure 3B).
Figure 3.
X203 reduces renal ERK and STAT3 activation, fibrosis, and a signature of epithelial-to-mesenchymal transition in mice with AS. (A) Relative renal mRNA expression of profibrotic markers (Col1a1, Col3a1, Il-11, Col1a2, Fn, Acta2, and Tgf-β; (n=8–11/group). (B) WB and (C) densitometry analysis of p-ERK, ERK, p-STAT3, STAT3, α-smooth muscle actin, fibronectin, E-cadherin, SNAI1, PCNA, cyclin D1, and GAPDH (n=4/group). (A) Data are shown as box and whisker with median (middle line), 25th–75th percentiles (box), and minimum–maximum values (whiskers). (C) Data are shown as mean±SD. (A and C) One-way ANOVA with Tukey’s correction.
At the signaling level, IL-11 is known to activate ERK across cell types, and this pathway has been mechanistically linked with IL-11-driven fibrosis.26,28,29 IL-11 inhibition in vivo can also be associated with reduced STAT3 activation, which is thought to be largely a secondary phenomenon reflecting lesser stromal-driven inflammation.26,30 Compared with WT mice, kidneys from Col4a3−/− mice treated with IgG exhibited elevated ERK and STAT3 activation. In contrast, ERK and STAT3 phosphorylation was largely diminished in the kidneys of ×203-treated Col4a3−/− mice (Figure 3, B and C). These data are consistent with ×203 target engagement in the kidney, reduced ERK activation, and diminished inflammation.
In many kidney diseases, it is thought that damaged TECs transition to a pEMT/FR-PCT state, which is central to the subsequent development of tubulointerstitial fibrosis and CKD.15–18 TEC pEMT/FR-PCT14 is characterized by increased SNAI1 expression and reciprocal downregulation of E-cadherin that is regulated, in part, by TGF-β.15,16 Compared with WT controls, Col4a3−/− mice receiving IgG exhibited a strong molecular signature of EMT with increased SNAI1 and decreased E-cadherin expression (Figure 3, B and C). In contrast, SNAI1 and E-cadherin levels in Col4a3−/− mice receiving ×203 were similar to those seen in WT mice. Thus, anti-IL-11 reduces TEC pEMT in the kidneys of Col4a3−/− mice.
A specific feature of injured TECs that enter a pEMT/FR-PCT state is their inability to replicate—a process that relates to SNAI1 repression of cyclins D1/2 that blocks G1/S transitions.19,20,31 We profiled levels of cyclin D1 and those of PCNA, a marker of G1/S, in the kidneys of Col4a3−/− mice treated with either IgG or ×203 and also in WT controls. Col4a3−/− mice receiving ×203 showed marked upregulation of cyclin D1 and PCNA compared with WT mice and Col4a3−/− mice receiving IgG (Figure 3, B and C). This suggests anti-IL-11 inhibits pEMT/FR-PCT transitions in the kidneys of Col4a3−/− mice and releases TECs to re-enter G1/S, to replicate, and to repair damaged tubules.
Podocyte Preservation and Lesser Renal Inflammation Is Associated with Inhibition of IL-11 Signaling in Col4a3−/− Mice
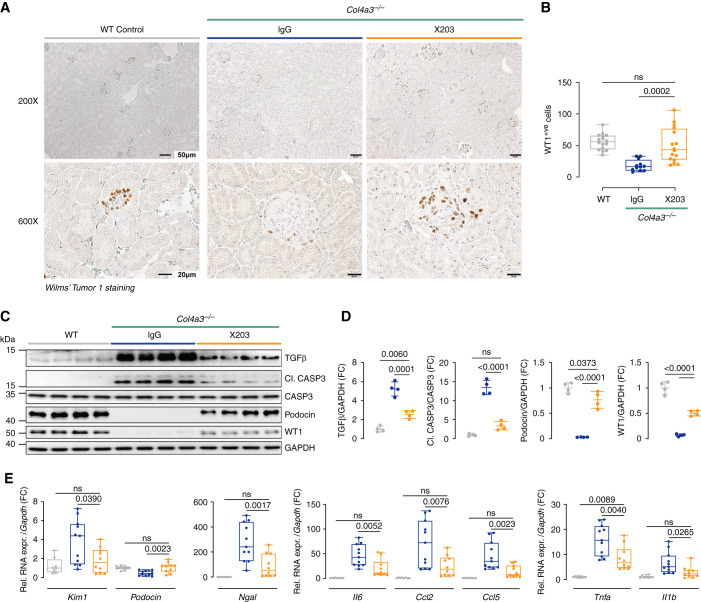
AS affects GBM composition, leading to podocyte dysfunction/loss that relates to TGF-β activity and pEMT processes in both podocytes and TECs.9,18 IHC analysis of the podocyte marker WT1 revealed greater staining in WT mice and ×203-treated Col4a3−/− mice compared with IgG-treated Col4a3−/− mice (Figure 4A). Quantification of the number of WT1-positive cells (podocytes) was carried out in a blinded fashion and confirmed significant (P<0.001) preservation of podocyte integrity in Col4a3−/− mice receiving ×203 compared with Col4a3−/− mice receiving IgG (Figure 4B). Preservation of podocytes in ×203-treated Col4a3−/− mice was further ascertained by immunoblotting, and findings were extended to podocin, a second podocyte marker (Figure 4, C and D).
Figure 4.
Inhibition of IL-11 signaling with a neutralizing IL-11 antibody preserves podocytes and reduces renal inflammation and tubule damage in Col4a3−/− mice. (A–E) Data for experiments shown in schematic Figure 2A. (A) Representative images (representative datasets from n=3/group) and (B) quantification (from 200× field images) of WT1 staining. (C) WB and (D) densitometry analysis of TGF-β, cleaved caspase 3, caspase 3, podocin, WT1, and GAPDH (n=4/group). (E) Relative renal mRNA expression of kidney injury markers (Kim1 and Ngal), podocyte marker (podocin), and proinflammation markers (Il-6, Ccl2, Ccl5, Tnf-α, and Il1-β; n=8–11/group). (B and E) Data are shown as box and whisker with median (middle line), 25th–75th percentiles (box), and minimum–maximum values (whiskers). (D) Data are shown as mean±SD; one-way ANOVA with Tukey’s correction.
TGF-β upregulation in podocytes and tubular cells, which coincides with the onset of proteinuria in the Col4a3−/− mouse,9,32 is thought to be of importance for disease pathogenesis in AS. We thus examined TGF-β levels and observed that ×203, but not IgG, significantly reduced the degree of TGF-β upregulation in the kidneys of Col4a3−/− mice (Figure 4, C and D). Apoptosis of podocytes and tubule cells is implicated in AS, and caspase activity is reduced in Col4a3−/− mice given Olmesartan.32 We observed caspase 3 activation in the IgG-treated Col4a3−/− mice that was reduced by ×203 administration (Figure 4, C and D).
Tnf-α expression in podocytes is of particular importance in AS and leads to podocyte apoptosis and glomerulosclerosis.13 It was therefore notable that ×203 reduced Tnf-α expression in Col4a3−/− mice compared with IgG-treated controls (Figure 4E). Markers of tubule damage and inflammation were also assessed. Compared with WT mice, control Col4a3−/− mice had elevated indicators of tubule damage (Kim1 and Ngal), which were restored by ×203 administration toward the levels seen in WT mice (Figure 4E). Proinflammatory interleukins (Il-6 and Il-1b) and CC chemokines (Ccl2 and Ccl5) were also elevated in Col4a3−/− mice receiving IgG and were equally diminished by administration of ×203 (Figure 4E).
Inhibition of IL-11 Signaling Improves Kidney Histopathology and Function
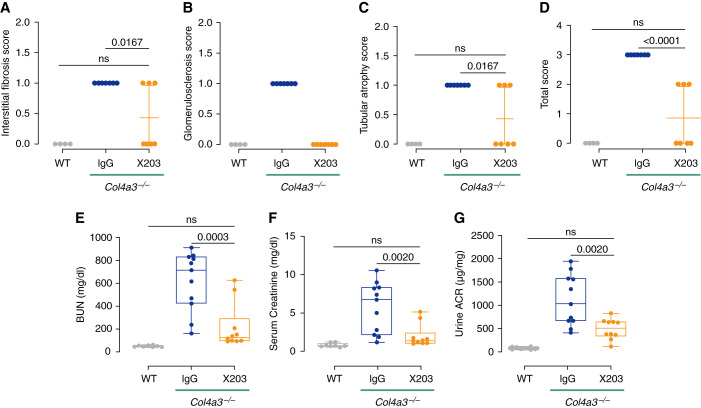
Next, we tested whether inhibition of IL-11 signaling, which mitigated intermediate phenotypes of kidney pathology in Col4a3−/− mice, also improved renal structure and function. To determine the effect of anti-IL-11 therapy on kidney pathology, kidney sections stained with PAS and Masson’s trichrome were evaluated by a renal pathologist who was blinded to treatments, and the severity of pathologic features, including fibrosis, glomerulosclerosis, and tubular atrophy, was assigned a composite score (see Methods for scoring details). Compared with IgG-treated mice, X203 treatment was associated with diminished levels of tubular atrophy and interstitial fibrosis, consistent with our internal analysis (Figure 2F), whereas glomerulosclerosis was completely abrogated, and the overall damage score was significantly reduced (Figure 5, A–D).
Figure 5.
Inhibition of IL-11 signaling in Col4a3−/− mice improves renal histology and function. (A–G) Data for experiments shown in schematic Figure 2A. (A) Interstitial fibrosis, (B) glomerulosclerosis, (C) tubular atrophy, and (D) total histology composite scores (n=4–7/group). (E) BUN, (F) serum Cr, and (G) urinary albumin-creatinine ratios (n=8–11/group). (A–D) Data are shown as mean±SD. (E–G) Data are shown as box and whisker with median (middle line), 25th–75th percentiles (box), and minimum–maximum values (whiskers); one-way ANOVA with Tukey’s correction.
To assess renal function, we measured BUN, serum Cr, and urinary albumin-creatinine ratios at the end of the anti-IL-11 monotherapy study (at 8.5 weeks of age). Compared with WT mice, IgG-treated Col4a3−/− mice had elevated BUN, Cr, and urinary albumin-creatinine ratio levels (fold elevation compared with WT: 12.4, 7.3, and 13.6, respectively), whereas administration of ×203 from week 6 lowered BUN, Cr, and urinary albumin-creatinine ratio to levels seen in WT mice, consistent with a significant overall improvement in renal function (Figure 5, E–G).
Anti-IL-11 Extends Lifespan in Col4a3−/− Mice
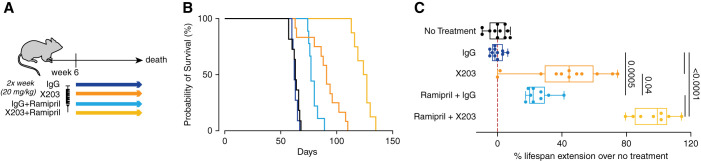
Death from progressive renal failure typically occurs in Col4a3−/− mice starting from 8.5 weeks of age, and mean survival times are reproducibly reported at around 10 weeks (e.g., 71 days6; 69 days33). Untreated Col4a3−/− mice used for the studies described here had a mean survival of 62.7±1.9 days. Previous studies have shown that administration of ramipril from 4 weeks of age, before the onset of proteinuria and before IL-11 is expressed (Figure 1B), extends lifespan in Col4a3−/− mice, whereas initiation of ramipril from 7 weeks of age, when disease is established, does not.6 We sought to determine if anti-IL-11 could extend lifespan when initiated at 6 weeks of age, after proteinuria is present and IL-11 is upregulated in the kidneys, and compared its effects with ramipril alone or ramipril combined with anti-IL-11, also administered from 6 weeks of age (Figure 6A).
Figure 6.
Therapeutic targeting of IL-11 extends lifespan when given alone or in combination with ramipril. (A) Schematic showing therapeutic dosing of Col4a3−/− mice in lifespan study for data shown in (B) and (C) (n=8–12/group). Col4a3−/− mice were administered IgG/X203 (20 mg/kg, twice a week, intraperitoneally) alone or in combination with ramipril (10 mg/kg per day, 6 days/week, intraperitoneally) starting from 6 weeks of age until death ensued. (B) Survival curves and (C) percentage of lifespan extension (over untreated Col4a3−/− mice) of mice treated with IgG, X203, ramipiril+IgG, or ramipril+X203. (C) Data are shown as box and whisker with median (middle line), 25th–75th percentiles (box), and minimum–maximum values (whiskers); one-way ANOVA with Tukey’s correction.
Compared with untreated controls, administration of IgG had no effect on survival (Figure 6, B and C). In contrast, ramipril significantly extended median lifespan by 22% (14 days), whereas anti-IL-11 alone increased median lifespan by 44% (29 days; Figure 6, B and C). Notably, anti-IL-11 combined with ramipril acted synergistically to extend the median lifespan of Col4a3−/− mice further by 99% (62 days; Figure 6, B and C). This suggests that anti-IL-11 and ramipril inhibit different pathologic processes in the diseased kidneys of Col4a3−/− mice.
Discussion
Blockade of the RAS is a mainstay of therapy for patients with AS and other forms of CKD, but unfortunately progression to end stage renal failure is typical in individuals with aggressive AS.5,10 This shortcoming likely reflects the complex renal pathology of progressive AS, involving GBM-specific initiating factors and generic tubulointerstitial disease mechanisms that cannot be completely ameliorated by RAS blockade alone. Here, we identify IL-11 as a novel cause of kidney injury in AS and show that inhibition of IL-11 has independent and additive therapeutic benefits relative to ACE inhibition in Col4a3−/− mice.
IL-11 is a misunderstood cytokine22,23 that is secreted from a variety of stromal and epithelial cells in response to cellular injury to act in an autocrine and paracrine manner, causing epithelial cell dysfunction, stromal cell activation, and inflammation.23 In the kidney parenchyma, IL-11RA is expressed on TECs throughout the nephron and in podocytes—two key cell lineages that can be affected by pEMT, an initiating factor for kidney fibrosis, inflammation, and failure in a range of different kidney diseases.15–18 IL-11RA is also expressed on stromal fibroblasts and vascular smooth-muscle cells34 and is important for myofibroblast transformation.23
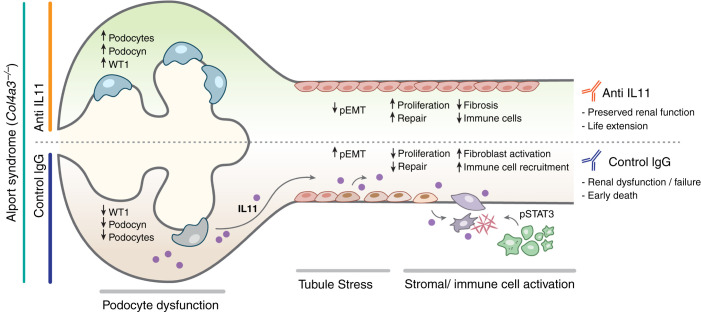
One mechanism for kidney protection by inhibition of IL-11 signaling in Col4a3−/− mice could be through inhibition of pEMT. In support of this, X203 reduced SNA1 expression, central to EMT/pEMT,15,16,35 and restored E-cadherin levels31 while preserving podocyte numbers and expression of podocyte-specific proteins17 (Figure 7). Furthermore, X203 dosing of Col4a3−/− mice induced substantial upregulation of renal cyclin D1 and PCNA expression associated with SNAI1 inhibition, suggesting escape of TECs from pEMT/FR-PCT, restoration of TEC proliferation, and kidney repair.19,20,31This effect could be related to suppression of TGF-β, a determinant of pEMT in the kidney,15,16,18 as anti-IL-11 lowered TGF-β expression. However, although inhibition of TGF-β signaling is proinflammatory,36 we show here, as we have in other tissues, that inhibition of IL-11 reduces inflammation.26,34 Thus, pEMT/FR-PCT in the kidney may be driven by IL-11 itself, similar to reports of its effects in fibrotic lung disease and cancer.37,38
Figure 7.
Proposed mechanism for IL-11-induced renal failure in AS. Glomerular basement membrane disruption due to Col4A3 mutation causes podocyte dysfunction and consequent tubular stress. Injured TECs, and podocytes, upregulate IL-11, leading to autocrine pEMT (SNAI1 upregulation and E-cadherin downregulation) of TECs and podocytes and paracrine activation of stromal myofibroblasts, which themselves secrete IL-11 that amplifies the fibrotic response and stimulates the recruitment/activation of immune cells. Neutralizing antibodies against IL-11 reduce renal dysfunction and extend life in Col4a3-deleted mice.
Although pEMT of damaged podocytes and TECs may initiate renal pathology, the consequent activation of stromal and inflammatory cells is needed for disease progression. Indeed, deletion of Il-11ra1 in fibroblasts diminishes pathogenic ERK signaling and protects against kidney dysfunction in folic acid nephropathy.21 It is therefore likely that some of the effects of ×203 in Col4a3−/− mice are mediated through inhibition of IL-11-dependent myofibroblast activation (Figure 7), in addition to STAT3 phosphorylation, and IL-6 levels. IL-6 is linked with a range of kidney diseases, and although not a therapeutic target itself, IL-6 may serve as a biomarker for latent IL-11 activity.39 Interestingly, urinary IL-11 levels correlate with proteinuria in IgA nephropathy and lupus nephritis, and perhaps might be useful for patient stratification.40 This also suggests IL-11 may be important in other diseases of the renal glomerulus, which remains to be explored.
The Col4a3−/− mouse line has been a useful model of progressive AS, and death from kidney failure typically occurs between 63 (this study) and 71 days.6,33 A previous study showed that ACEi extended the lifespan of Col4a3−/− mice by 79 days (110%) when initiated at 4 weeks of age, before renal IL-11 is upregulated. However, there was no survival benefit if ACEi was initiated at 7 weeks of age, after kidney injury was established. Here, we find that ACEi therapy started in 6-week-old Col4a3−/− mice modestly extended lifespan by 14 days (22%). In contrast, anti-IL-11 monotherapy begun at 6 weeks of age was more effective than ramipril alone, increasing lifespan by 29 days (44%). Most notably, the combination of anti-IL-11 and ramipril prolonged survival of Col4a3−/− mice by 62 days (99%), which is substantially longer than ACEi alone—the current standard of care in AS patients.
Except for limited and incompletely penetrant developmental defects of teeth and skull sutures, humans with loss-of-function of IL-11RA appear well, with normal immune function. A similar phenotype is seen in Il-11ra1 null mice. Interestingly, two recently and separately developed Il-11 null mice appear normal, with no obvious bony deficits,41,42 suggesting that inhibiting IL-11 might have advantages in side-effect profile over targeting IL-11RA. Taken together, the mild phenotypes of humans and mice lacking IL-11RA or IL-11, along with absence of untoward effects with lengthy anti-IL-11RA and anti-IL-11 treatment in mice,26 provide an encouraging safety signal for long-term inhibition of IL-11 signaling in chronic diseases such as AS.23,25
We end by suggesting that inhibition of IL-11 signaling may be considered as a novel therapeutic approach for patients with AS and perhaps other progressive forms of CKD. Anti-IL-11 therapy combined with RAS blockade may be of particular interest, given the near ubiquitous use of ACEi/ARB in CKD and the synergistic interaction between anti-IL-11 and ACEi therapy shown here. With anti-IL-11/anti-IL-11RA drugs nearing the clinic, it will be interesting to see if a therapeutic approach for AS discovered in the Col4a3−/− mouse translates to patients for a second time.6,7
Disclosures
S.A. Cook is a co-inventor of the patent applications WO/2017/103108 (Treatment of Fibrosis), WO/2018/109174 (IL-11 Antibodies), and WO/2018/109170 (IL-11RA Antibodies). S.A. Cook and A.A. Widjaja are co-inventors of the patent application US US2020/0270340A1 (Treatment of Kidney Injury) and GB2009292.0 (Treatment and Prevention of Disease Caused by Type IV Collagen Dysfunction). S.A. Cook is a co-founder and shareholder of Enleofen Bio PTE Ltd., a company that made anti-IL-11 therapeutics, which were acquired for further development by Boehringer Ingelheim in 2019. T. Coffman reports being on the editorial boards of Cell Metabolism and JCI and on the board of directors for Singapore Health Services, the Singapore Eye Research Institute, and the Kidney Research Institute University of Washington. P.H. Tan reports honoraria for delivering a talk on prostate cancer from AstraZeneca. A.A. Widjaja reports patents and inventions with Boehringer Ingelheim. S.A. Cook reports research funding from Boehringer Ingelheim. All remaining authors have nothing to disclose.
Funding
This research was supported by the National Medical Research Council (NMRC), Singapore STaR awards (NMRC/STaR/0029/2017), NMRC Centre Grant to the NHCS, MOH‐CIRG18nov‐0002, MRC-LMS (UK), Tanoto Foundation (to S.A. Cook). A.A. Widjaja is supported by the NMRC (NMRC/OFYIRG/0053/2017). N. Hübner and S.A. Cook are supported by a grant from the Leducq Foundation (16CVD03). N. Hübner is a recipient of an ERC advanced grant under the European Union Horizon 2020 Research and Innovation Program (AdG788970).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
E. Adami was responsible for visualization; T. Coffman, S.A. Cook, S.G. Shekeran, and A.A. Widjaja were responsible for the methodology; T. Coffman, S.A. Cook, and A.A. Widjaja reviewed and edited the manuscript; S.A. Cook and N. Hübner were responsible for funding acquisition; S.A. Cook, P.H. Tan, and A.A. Widjaja conducted the formal analysis; S.A. Cook and A.A. Widjaja conceptualized the study, were responsible for supervision, and wrote the original draft of the manuscript; J.W.T. Goh, S.Y. Lim, S.G. Shekeran, J. Tan, P.H. Tan, S. Viswanathan, and A.A. Widjaja were responsible for the investigation; and S.G. Shekeran was responsible for validation.
References
- 1.Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 268: 26033–26036, 1993 [PubMed] [Google Scholar]
- 3.Savige J: Alport syndrome: its effects on the glomerular filtration barrier and implications for future treatment. J Physiol 592: 4013–4023, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funk SD, Lin M-H, Miner JH: Alport syndrome and Pierson syndrome: Diseases of the glomerular basement membrane. Matrix Biol 71–72: 250–261, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jais JP, Knebelmann B, Giatras I, Marchi M, Rizzoni G, Renieri A, et al. : X-linked Alport syndrome: Natural history in 195 families and genotype–phenotype correlations in males. J Am Soc Nephrol 11: 649–657, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Gross O, Beirowski B, Koepke M-L, Kuck J, Reiner M, Addicks K, et al. : Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int 63: 438–446, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Gross O, Tönshoff B, Weber LT, Pape L, Latta K, Fehrenbach H, et al. ; German Pediatric Nephrology (GPN) Study Group and EARLY PRO-TECT Alport Investigators : A multicenter, randomized, placebo-controlled, double-blind phase 3 trial with open-arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport’s syndrome. Kidney Int 97: 1275–1286, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, et al. ; Study Group Members of the Gesellschaft für Pädiatrische Nephrologie : Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 81: 494–501, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Sayers R, Kalluri R, Rodgers KD, Shield CF, Meehan DT, Cosgrove D: Role for transforming growth factor-beta1 in Alport renal disease progression. Kidney Int 56: 1662–1673, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Kashtan CE: Renal transplantation in patients with Alport syndrome: Patient selection, outcomes, and donor evaluation. Int J Nephrol Renovasc Dis 11: 267–270, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hood JC, Dowling J, Bertram JF, Young RJ, Huxtable C, Robinson W, et al. : Correlation of histopathological features and renal impairment in autosomal dominant Alport syndrome in Bull terriers. Nephrol Dial Transplant 17: 1897–1908, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Qi R, Yang C: Renal tubular epithelial cells: The neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis 9: 1126, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryu M, Mulay SR, Miosge N, Gross O, Anders H-J: Tumour necrosis factor-α drives Alport glomerulosclerosis in mice by promoting podocyte apoptosis. J Pathol 226: 120–131, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD: Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A 117: 15874–15883, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grande MT, Sánchez-Laorden B, López-Blau C, De Frutos CA, Boutet A, Arévalo M, et al. : Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21: 989–997, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, et al. : Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21: 998–1009, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying Q, Wu G: Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: An update. Ren Fail 39: 474–483, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y: New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang-Panesso M, Humphreys BD: Cellular plasticity in kidney injury and repair. Nat Rev Nephrol 13: 39–46, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Vega S, Morales AV, Ocaña OH, Valdés F, Fabregat I, Nieto MA: Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 18: 1131–1143, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schafer S, Viswanathan S, Widjaja AA, Lim W-W, Moreno-Moral A, DeLaughter DM, et al. : IL-11 is a crucial determinant of cardiovascular fibrosis. Nature 552: 110–115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widjaja AA, Dong J, Adami E, Viswanathan S, Ng B, Pakkiri LS, et al. : Redefining IL11 as a regeneration-limiting hepatotoxin and therapeutic target in acetaminophen-induced liver injury. Sci Transl Med 13: eaba8146, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Cook SA, Schafer S: Hiding in plain sight: Interleukin-11 emerges as a master regulator of fibrosis, tissue integrity, and stromal inflammation. Annu Rev Med 71: 263–276, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, et al. : Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widjaja AA, Chothani SP, Cook SA: Different roles of interleukin 6 and interleukin 11 in the liver: Implications for therapy. Hum Vaccin Immunother 16: 2357–2362, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widjaja AA, Singh BK, Adami E, Viswanathan S, Dong J, D’Agostino GA, et al. : Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology 157: 777–792.e14, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Ng B, Dong J, D’Agostino G, Viswanathan S, Widjaja AA, Lim W-W, et al. : Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med 11: eaaw1237, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Adami E, Viswanathan S, Widjaja AA, Ng B, Chothani S, Zhihao N, et al. : IL11 is elevated in systemic sclerosis and IL11-dependent ERK signalling underlies TGFβ-mediated activation of dermal fibroblasts. Rheumatology (Oxford) 60: 5820–5826, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widjaja AA, Viswanathan S, Jinrui D, Singh BK, Tan J, Wei Ting JG, et al. : Molecular dissection of pro-fibrotic IL11 signaling in cardiac and pulmonary fibroblasts. Front Mol Biosci 8: 740650, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong J, Viswanathan S, Adami E, Singh BK, Chothani SP, Ng B, et al. : Hepatocyte-specific IL11 cis-signaling drives lipotoxicity and underlies the transition from NAFLD to NASH. Nat Commun 12: 66, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. : The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Suh SH, Choi HS, Kim CS, Kim IJ, Ma SK, Scholey JW, et al. : Olmesartan attenuates kidney fibrosis in a murine model of Alport syndrome by suppressing tubular expression of TGFβ. Int J Mol Sci 20: E3843, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ninichuk V, Gross O, Reichel C, Khandoga A, Pawar RD, Ciubar R, et al. : Delayed chemokine receptor 1 blockade prolongs survival in collagen 4A3-deficient mice with Alport disease. J Am Soc Nephrol 16: 977–985, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Lim W-W, Corden B, Ng B, Vanezis K, D’Agostino G, Widjaja AA, et al. : Interleukin-11 is important for vascular smooth muscle phenotypic switching and aortic inflammation, fibrosis and remodeling in mouse models. Sci Rep 10: 17853, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon-Tillaux N, Hertig A: Snail and kidney fibrosis. Nephrol Dial Transplant 32: 224–233, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. : Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359: 693–699, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strikoudis A, Cieślak A, Loffredo L, Chen Y-W, Patel N, Saqi A, et al. : Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep 27: 3709–3723.e5, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su J, Morgani SM, David CJ, Wang Q, Er EE, Huang Y-H, et al. : TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 577: 566–571, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su H, Lei C-T, Zhang C: Interleukin-6 signaling pathway and its role in kidney disease: An update. Front Immunol 8: 405, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chien J-W, Chen W-L, Tsui Y-G, Lee M-C, Lin A-Y, Lin C-Y: Daily urinary interleukin-11 excretion correlated with proteinuria in IgA nephropathy and lupus nephritis. Pediatr Nephrol 21: 490–496, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Nishina T, Deguchi Y, Ohshima D, Takeda W, Ohtsuka M, Shichino S, et al. : Interleukin-11-expressing fibroblasts have a unique gene signature correlated with poor prognosis of colorectal cancer. Nat Commun 12: 2281, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng B, Widjaja AA, Viswanathan S, Dong J, Chothani SP, Lim S, et al. : Similarities and differences between IL11 and IL11RA1 knockout mice for lung fibro-inflammation, fertility and craniosynostosis. Sci Rep 11: 14088, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]