Abstract
Receptor protein tyrosine phosphatase rho (RPTPρ, gene symbol PTPRT) is a transmembrane protein expressed at high levels in the developing hippocampus, olfactory bulb, cortex, and cerebellum. It has an extracellular domain that interacts with other cell adhesion molecules, and it has two intracellular phosphatase domains, one of which is catalytically active. In a recent genome-wide association study, PTPRT was identified as a potential candidate gene for autism spectrum disorder (ASD) susceptibility. Mutation of a critical aspartate to alanine (D1046A) in the PTPRT catalytic domain inactivates phosphatase function but retains substrate binding. We have generated a knockin mouse line carrying the PTPRT D1046A mutation. The D1046A mutation in homozygous knockin mice did not significantly change locomotor activities or anxiety-related behaviors. In contrast, male homozygous mice had significantly higher social approach scores than wild-type animals. Our results suggest that PTPRT phosphatase function is important in modulating neural pathways involved in mouse social behaviors relevant to the symptoms in human ASD patients.
Keywords: PTPRT, RPTPρ, social interaction, animal model
Introduction
Receptor protein tyrosine phosphatase rho (RPTPρ; gene symbol PTPRT) is a transmembrane molecule expressed at high levels in the mouse brain during development [McAndrew et al., 1998a, 1998b]. Over the past decade, PTPRT gene structure has been characterized [Besco et al., 2001, 2004], its expression pattern in developing mouse brain determined [McAndrew et al., 1998a, 1998b], and several substrates identified [Besco et al., 2006;Park et al., 2012; Zhang et al., 2007; Zhao et al., 2010]. The PTPRT gene was mapped to human chromosome 20q12-13.1 and to a syntenic region on mouse chromosome 2 [McAndrew et al., 1998b]. It is classified as a receptor protein tyrosine phosphatase (RPTP) of the type 2B subfamily, together with RPTPκ, RPTPμ, and PCP-2 [Gebbink et al., 1991; Tonks, 2006; Wang et al., 1996; Yang et al., 1997]. The RPTPρ protein contains an extracellular segment, featuring a meprin/A5-protein/PTPmu (MAM) domain, an immunoglobulin-like domain, four fibronectin type II repeats, a transmembrane domain, and an intracellular segment. The intracellular segment contains two highly conserved phosphatase domains, only the first of which is catalytically active [Fischer et al., 1992]. Motifs in the extracellular ectodomain interact with identical conserved segments on other molecules, whereas the intracellular segment facilitates signal transduction through protein dephosphorylation via the catalytic phosphatase domain [Besco et al., 2006; Brady-Kalnay, Flint, & Tonks, 1993; Johnson & Van Vactor, 2003].
A recent analysis of a large, extended autism pedigree identified PTPRT/RPTPρ as a potential candidate for autism susceptibility [Allen-Brady et al., 2009]. Autism spectrum disorder (ASD) is a complex, heterogeneous disorder with variations in multiple genes likely contributing to its etiology [Kumar & Christian, 2009]. Numerous genes identified as risk factors for ASD are involved in maintaining synaptic function, indicating that altered synaptic homeostasis is an important underlying factor in ASD pathogenesis [Betancur, Sakurai, & Buxbaum, 2009; Huguet, Ey, & Bourgeron, 2013]. In vitro studies in hippocampal neurons have shown that altering PTPRT expression affects dendritic arborization [Park et al., 2012] and the number of spines and synapses [Lim et al., 2009]. It is possible that PTPRT may regulate neuronal pathways involved in animal behaviors relevant to ASD by modulating proteins important in synapse formation and synaptic transmission. In order to test this, we generated a knockin mouse model of the PTPRT gene. Zhang et al. previously demonstrated that a mutation in the phosphatase domain of PTPRT inactivates the phosphatase activity of the protein but retains substrate binding [Zhang et al., 2007]. The mutated protein has also been used as a substrate trap to identify substrates of PTPRT [Zhao et al., 2010]. We introduced the same mutation in mouse PTPRT in our animal model. We have conducted behavioral analyses of these mice to determine if inactivation of PTPRT phosphatase function has an impact on mouse behaviors relevant to behavioral symptoms seen in ASD patients.
Materials and Methods
Generation of the RPTPρ Knockin Mouse
A ptprtD1046A knockin mouse line was generated using procedures similar to those employed previously in our laboratories [Chen, Han, & Gu, 2005; Chen et al., 2006a, 2006b; Gu, Wu, & Han, 2006; Wu & Gu, 2003]. Briefly, a targeting construct was assembled by joining the long and short homology arms, a positive selection marker (neomycin resistant gene) flanked by two loxP sites, and a negative selection marker (thymidine kinase gene) (Fig. 1). The homology arms were polymerase chain reaction (PCR) amplified using a bacterial artificial chromosome (BAC) clone of C57BL6J mouse genomic DNA as the template (RP23-466K24, Children’s Hospital, Oakland). The short arm was 1.3 kb, and the long arm was a 5.5 kb fragment of the PTPRT gene containing exons 21 and 22. Three point mutations were introduced in Exon 22 (ENSMUSE00000551815), one introduced the D1046A mutation, and the other two were synonymous introducing a Spe1 restriction site nearby for construct assembly and diagnosis. The linearized targeting construct was electroporated into mouse embryonic stem (ES) cells (129 SvJ). G418-resistant ES cell clones were analyzed by PCR. A clone displaying the desired homologous recombination was microinjected into blastocysts of C57BL6J mice and implanted into pseudopregnant C57BL6J foster female mice at the Ohio State University (OSU) Genetically Engineered Mouse Modeling Core. Chimeric mice were bred with C57BL6J mice, and germ line-transmitting mice were identified by PCR.
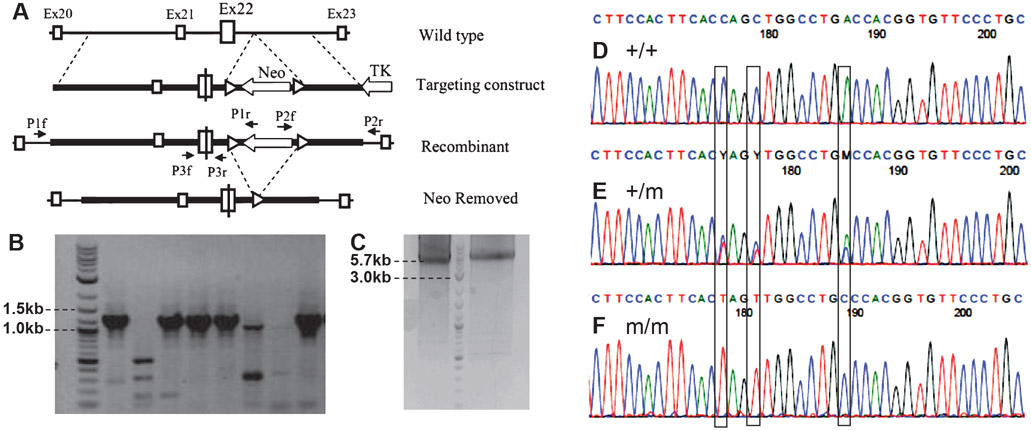
Figure 1.
Targeting strategy for generating PTPRT knockin mouse and confirmation of correct homologous recombination. (A) Targeting strategy. Thin lines represent wild-type mouse genomic DNA between exons 20 and 23; thick lines represent sequences included in the targeting construct. Open boxes represent exons; the large box represents exon 22, which contains a line representing the mutation in exon 22. Open arrows represent the positive selection marker (Neo cassette) and the negative selection marker (thymidine kinase gene). Triangles represent LoxP sites. Small arrows represent polymerase chain reaction (PCR) primers. (B) Agarose gel image of PCR screening for mice with correct homologous recombination using primers P2f annealing to the Neo gene and P2r annealing to the cell genomic sequence just outside of the short arm (see panel A). The presence of a 1.35-kb band indicates positive mice. (C) Image of a preparative agarose gel of PCR using primers P1r annealing to the Neo gene and P1f annealing to the mouse genomic sequence just outside of the long arm. The presence of a 5.7-kb band confirms that the two mice have correct recombination at the long arm side. (D, E, F) portions of chromatograms of the sequencing results of the PTPRT mRNAs (cDNAs) from wild-type (+/+), heterozygous (+/m), and homozygous (m/m) knockin mice. The three mutation sites are highlighted.
Wild-type (+/+), heterozygous (+/m), and homozygous (m/m) knockin mice used in the experiments were genotyped by PCR using primers that amplify the regions around the Neo cassette insertion site. Tissue from the cerebellum (from each genotype) was dissected and disrupted using a polytron tissue homogenizer and mRNA was isolated using an mRNA isolation kit (Qiagen Corp., Valencia, CA, USA). The cDNAs were synthesized, and the mutated region in the PTPRT cDNA was PCR amplified for all three genotypes. The PCR products were sequenced by the OSU Comprehensive Cancer Center Nucleic Acid Shared Resource.
Behavioral Assays
Wild-type (+/+), heterozygous (+/m), and homozygous (m/m) PTPRT knockin mice were used in the following behavioral experiments. Data from heterozygotes were, in general, similar to that of wild-type and is not shown.
Open field locomotion.
Spontaneous locomotor activity was measured in a 40 × 40 cm acrylic box placed in a sound-attenuating chamber for 20 min [Brielmaier et al., 2012; Fonken et al., 2011]. Total distance travelled was measured using a video tracking software (ANYmaze, Stoelting Co., Wood Dale, IL, USA).
Elevated plus maze.
The elevated plus maze apparatus consisted of an elevated cross, placed 24 cm above the floor, with two open arms (35 × 6 cm) and two closed arms (35 × 6 × 22 cm), at right angles to each other. Mice were placed at the intersection of the two arms and allowed to explore the maze for 5 min [Holmes & Rodgers, 2003]. Time spent in the open vs. closed arms and the number of entries into each arm was recorded and analyzed using the ANYmaze video tracking system. Arm entry was defined as the placement of all fours limbs into the arm.
Social interaction task.
Social approach was measured in a three-chambered apparatus using a procedure as previously described [Thirtamara Rajamani et al., 2013] and similar to the procedure developed by Crawley et al. [Crawley, 2004]. Briefly, the test was divided into three 10-min stages. During the first 10 min, mice were allowed to explore the apparatus. This was followed by another 10-min stage, wherein an unfamiliar mouse (stranger 1) was placed under a wire cage in one of the side chambers, along with an empty wire cage in the opposing chamber. The third stage involved the introduction of a second unfamiliar mouse (stranger 2), which was placed under wire cage in the opposite side chamber. Movement was recorded with the video tracking system; time spent in each chamber during all three stages was measured. Cylindrical wire cages (Galaxy Cup, Spectrum Diversified Designs, Streetsboro, OH, USA) were used to hold the stranger mice. The placement of the stranger mice in the side chambers was counterbalanced among test animals to minimize any initial preference of the side chambers. Age- and sex-matched C57BL/6J mice were used as unfamiliar (stranger) mice and were habituated to the wire cages for 15 min/day for 3 days prior to the test.
Results
Confirmation of RPTPρ Knockin Mouse
The D1046A mutation was introduced into the mouse RPTPρ gene by standard homologous recombination in mouse ES cells. The general targeting strategy is described in Figure 1. Briefly, after G418 selection, ES cell colonies were picked in areas of culture dishes having very sparse colonies to avoid the possibility that the picked colonies are from two clones fused together. PCR were performed using pairs of primers that anneal to the Neo cassette and to the RPTPρ gene sequence outside of the targeting short arm (P2f/P2r, Fig. 1A). Only the ES cell clones with correct recombination of the targeting construct will produce a PCR band of correct size. The correct ES cell clones were further confirmed with PCR using pairs of primers that anneal to the Neo cassette and to the PRPRT gene sequence outside of the long arm of the targeting construct (P1f/P1r, Fig. 1A). The results (data not shown) indicate that the Neo cassette and the mutations were inserted correctly into the PTPRT gene in the ES cells as designed. We then generated the knockin mice by micro-injection of the ES cells into blastocysts of C57BL6J mice and implanting into pseudopregnant C57BL6J foster female mice. Chimeric mice were bred with C57BL6J mice, and germ line-transmitting mice were identified by PCR using primers P2f + P2r and with mouse genomic DNA as template. Figure 1B shows that five of the eight tested mice had a strong 1.35-kb band indicating the presence of the mutated allele. The correct recombination on the long arm side was also verified by PCR using primers P1f and P1r (Fig. 1A). Figure 1C shows the image of a preparation gel of the long arm PCR producing a 5.7-kb band from two mice. The DNA bands were purified and sequenced, which confirms the correct mutations in mouse genomic DNA. To further confirm the correct targeting and desired mutations, mRNAs were isolated from the cerebellum of adult wild-type, heterozygous, and homozygous knockin mice, and cDNA was synthesized. A region of the RPTPρ cDNA containing exon 22 was amplified by PCR and sequenced. As expected, the sequence of the mutation region from wild-type mice matches perfectly with the known wild-type reference sequence (Fig. 1D). The sequence from the heterozygous mice also matches the reference sequence except at the three mutated sites. At these three sites, double peaks were observed in the sequencing chromatogram reflecting the presence of both the wild-type and the mutant alleles (Fig. 1E). The double peaks have comparable heights with differences within the variations of sequencing peaks. This is consistent with the expected 1:1 ratio in heterozygous mice. The sequencing was repeated with another heterozygous mouse, and the results were confirmed. These results indicate that the mutations and presence of the Neo cassette in the intron between exons 22 and 23 in the mutant allele did not change the mutant RPTPρ mRNA expression level; therefore, we did not go further as planned to remove the Neo cassette (Fig. 1A). Finally, the mRNA sequence from the homozygous mice has single peaks at all three mutation sites matching the expected mutated sequence (Fig. 1F). This is solid evidence that the targeting construct was introduced into the mouse genome by correct homologous recombination because the wild-type RPTPρ sequence will be detected in mice if the targeting construct was inserted at random.
Open Field Locomotor Activity
Wild-type (+/+) and homozygous (m/m) mutant PTPRT KI mice were placed in an open field box, and their locomotor activity was recorded. Total distance traveled by male and female mice, and the distance traveled in 5-min bins throughout the 20 min were measured. A one-way analysis of variance (ANOVA) revealed no differences in total distance traveled in male (Fig. 2A, F1,18 = 2.354, P = 0.143; +/+ n = 8, m/m n = 11) and female (Fig. 2C, F1,29 = 0.036, P = 0.85; +/+ n = 13, m/m n = 17) PTPRT knockin mice. A repeated-measures ANOVA revealed a significant decrease in distance traveled over time for both genotypes, suggesting habituation to the open field apparatus (males, Fig. 2B, F3,18 = 6.534, P < 0.05; females, Fig. 2D, F3,29 = 35.114, P < 0.05). Male m/m knockin mice were slightly, but not significantly, less active than wild-type controls at each 5-min data point throughout the 20-min period.
Figure 2.
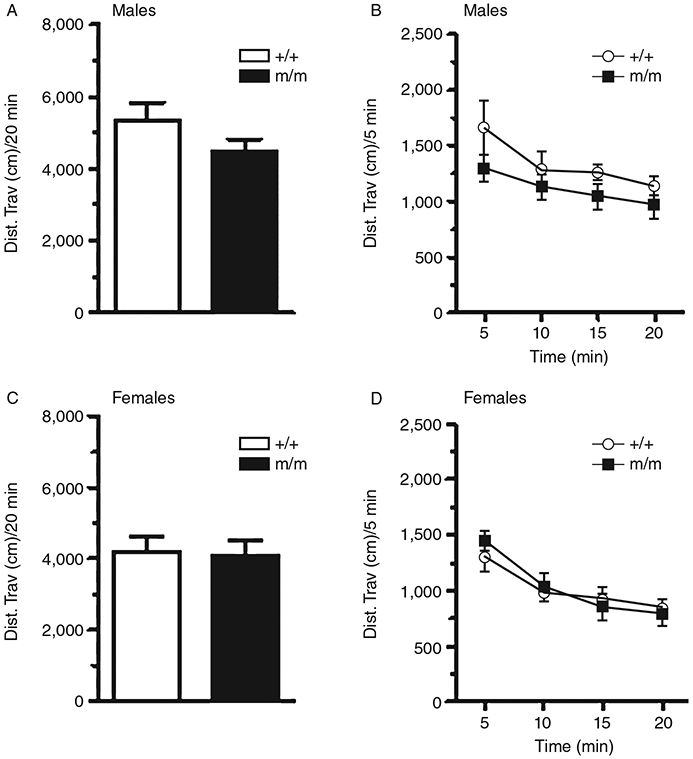
Open field Locomotor activity. The total distance traveled in 20 min by male and female wild-type (+/+) and homozygous PTPRT mutant mice (m/m) are shown. No significant difference in the total locomotor activity was observed between +/+ and m/m mice (Fig. 2A and C). Distance traveled over time is shown for males (Fig. 2B) and females (Fig. 2D); a significant effect of time but no effect of genotype was observed. Data are presented as mean ± standard error of the mean (SEM).
Elevated Plus Maze
Animals were placed in the center of an elevated plus maze with two open arms and two closed arms. Mice were monitored for 5 min, and the number of entries and time spent on the open arm of the maze was measured. No statistically significant difference between the two genotypes was observed in the number of entries into the open arms in males (Fig. 3A, Kruskal–Wallis test, P = 0.706, unequal variances observed using Levene’s statistic, P = 0.027) or females (Fig. 3C, One-way ANOVA, F1,29 = 0.833, P = 0.369). This result suggests that exploratory behavior does not differ between the two groups. Time spent in the open arms of the maze also did not differ significantly between +/+ and m/m mice in both males (Fig. 3B, F1,18 = 0.053, P = 0.822) and females (Fig. 3D, F1,29 = 0.006, P = 0.938), suggesting that homozygous knockin mice did not display an anxiogenic phenotype.
Figure 3.

Elevated plus maze. The number of entries into the open arms are shown for male (Fig. 3A) and female (Fig. 3C) wild-type (+/+) and homozygous PTPRT mutant mice (m/m). Time spent on the open arms for males and females is shown in Figure 3B and 3D. Data are presented as mean ± standard error of the mean (SEM).
Social Interaction Tests
Social interaction was tested using a three-chambered apparatus. In the first 10-min period of this three-part test, mice were allowed to explore the apparatus with empty cages in both side chambers; each group showed no preference for either of the two side chambers (data not shown).
Social Approach
In the second period, a stranger mouse (stranger 1) was placed under a cage in one of the side chambers; the alternate side chamber contained an empty wire cage. Test mice were allowed to explore for 10 min. Both male (Fig. 4A) and female (Fig. 4B) PTPRT +/+ and m/m mice spent more time in the chamber containing stranger 1 than with the empty wire cage (male +/+, one-way ANOVA, F1,44 = 12.818, P < 0.05; male m/m, F1,44 = 37.867, P < 0.05; female +/+, F1,44 = 6.658, P < 0.05; female m/m, F1,44 = 6.282, P < 0.05), demonstrating the tendency for social interaction. Social approach scores, defined as the difference in time spent between the chamber containing stranger 1 and the chamber containing the empty wire cage, were also analyzed. Male PTPRT m/m (Fig. 4C, n = 8) mice had a significantly higher social approach scores than +/+ (n = 16) mice (Student’s t-test, t = 2.157, P < 0.05), suggesting that male PTPRT m/m mice are significantly more socially inclined than their wild-type counterparts. However, no difference in social approach scores was observed between female +/+ (n = 13) and m/m (n = 11) PTPRT mice (Fig. 4D) (Student’s t-test, t = 0.07025, P = 0.9446).
Figure 4.
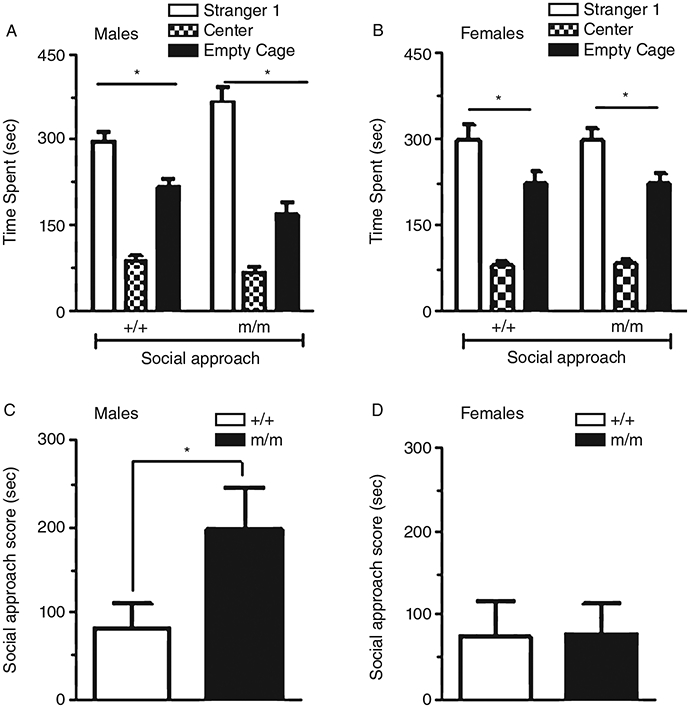
Social approach. Time spent in the center chamber, the side chamber containing an unfamiliar mouse (Sstranger 1), or the side chamber containing an empty wire cage for male (Fig. 4A) and female (Fig. 4B) wild-type (+/+) and homozygous PTPRT mutant mice (m/m). In males, both genotypes spent significantly more time in the chamber containing stranger 1 than the chamber containing the wire cage (*P < 0.05). Social approach scores (Fig. 4C) were significantly higher in male m/m mice (n = 8) compared wth +/+ (n = 16) mice (*P < 0.05). Both +/+ and m/m female mice showed preference for the chamber containing stranger 1 over wire cage (*P < 0.05). No differences in social approach scores were observed between female +/+ (n = 13) and m/m (n = 11) mice (Fig. 4D). Data are presented as mean ± standard error of the mean (SEM).
Social Novelty
In the third stage of the social interaction test, social novelty, a novel stranger mouse (stranger 2) was placed under the previously empty wire cage in the side chamber, while the stranger 1 mouse remained in the alternate compartment. Test mice were allowed to explore for 10 min, and time spent in each of the three chambers was recorded. In general, male (Fig. 5A) and female (Fig. 5B) mice of both genotypes preferred to spend more time in the chamber containing stranger 2 than with stranger 1 (male +/+, one-way ANOVA, F1,44 = 18.287, P < 0.05; male m/m, one-way F1,44 = 12.220, P < 0.05; female +/+, F1,44 = 12.43, P < 0.05; female m/m, F1,44 = 0.693, P = 0.41). The social novelty scores (defined as difference between times spent in the chambers containing stranger 2 and stranger 1, respectively) were also measured. The male wild-type and m/m mice had similar social novelty scores (Fig. 5C; F1,23 = 0.085, P = 0.773), while female m/m mice showed a lower social novelty score than the wild-type group. However, the difference was not significant (Fig. 5D; F1,23 = 1.653, P = 0.212).
Figure 5.
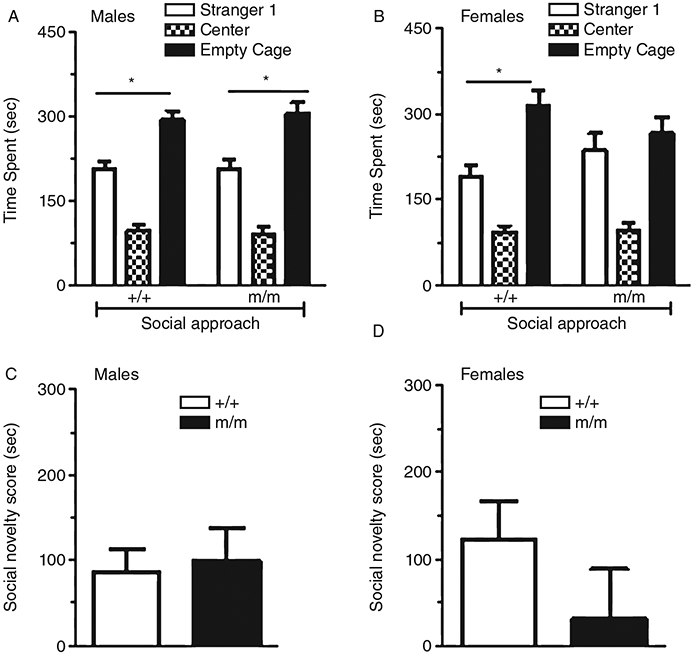
Social novelty. Time spent with stranger 1 and 2 for male (Fig. 5A) and female (Fig. 5B) wild-type (+/+) and homozygous PTPRT mutant mice (m/m). In males, both +/+ and m/m mutant mice spent significantly more time with stranger 2 than stranger 1 (*P < 0.05). In females, only +/+ and not m/m mutant mice showed a significant preference for the chamber containing stranger 2 over stranger 1 (*P < 0.05). Figure 5C and 5D show the difference in time spent with stranger 2 vs. stranger 1 (social novelty score) by (C) male and (D) female +/+ and m/m mice. Data are presented as mean ± standard error of the mean (SEM).
Discussion
Protein tyrosine phosphatase rho (RPTPρ/PTPRT) is a transmembrane protein expressed predominantly in the central nervous system [CNS; Paul & Lombroso, 2003], where it plays a role in signal transduction [Xu & Fisher, 2012], cell adhesion [Sallee, Wittchen, & Burridge, 2006], and synaptogenesis [Lim et al., 2009]. Improper synapse formation can contribute to neurodevelopmental disorders, and many genes identified in the pathogenesis of ASD are associated with synaptogenesis [Zoghbi & Bear, 2012]. We have generated a knockin mouse line carrying a mutated PTPRT with the phosphatase function inactivated. We speculate that this could lead to increased phosphorylation states of PTPRT substrate proteins, thus affecting neural pathways mediating certain animal behaviors. We have found that inactivating the phosphatase function of PTPRT in the homozygous knockin mice significantly increased the social approach score compared with the wild-type mice. The performances of homozygous knockin mice on locomotor tests and anxiety-related tests were generally unchanged compared with wild-type mice, ruling out the possibility that altered locomotor activity could significantly impact the outcome of the social interaction tasks.
Although PTPRT has been shown to play a critical role as a colorectal tumor suppressor [Wang et al., 2004], its functional role in the CNS is only now emerging. Unlike other RPTPs, PTPRT gene expression is almost exclusively restricted to the CNS. High levels of PTPRT expression have been reported during early embryonic development in the olfactory bulb and cortex. Gene expression was also observed in hippocampal formation, anterior cerebellar cortex, and spinal cord [McAndrew et al., 1998a, 1998b]. A similar expression profile of the PTPRT gene was also shown in the Allen Mouse Brain Atlas [Hawrylycz et al., 2012; Allen Institute for Brain Science, Allen Mouse Brain Atlas]. Recent in vitro studies in hippocampal neurons have shown that upregulation of PTPRT expression results in increased dendritic spine density, and number of excitatory and inhibitory synapses [Lim et al., 2009]. When PTPRT was knocked down, dendritic arborization and number of spines were reduced [Park et al., 2012]. Recent evidence also suggests PTPRT may regulate proteins involved in process of synaptic vesicle fusion. Syntaxin-binding protein 1, a component of the protein complex involved in synaptic vesicle fusion, was identified as an endogenous target of PTPRT. It has been demonstrated that PTPRT dephosphorylates tyrosine 145 of Syntaxin-binding protein 1, thereby regulating its interaction with Syntaxin-1 [Lim et al., 2013], a member of the SNARE complex, which is necessary for fusion of synaptic vesicles with the presynaptic membrane [Jahn & Sudhof, 1999]. RPTPρ is also known to interact with neuroligins and protein tyrosine kinase, Fyn, to regulate synapse formation [Lim et al., 2009]. Mutations in both neurogulins (NL-3 and NL-4) and neurexin-1 have been reported in a subset of patients with ASD [Kim et al., 2008;Yan et al., 2005]. Together, the above studies strongly suggest that PTPRT may play a regulatory role in synaptogenesis.
ASDs are complex polygenic disorders. Genome-wide analysis studies have identified multiple genes associated with ASD, many of which are involved in synapse formation and synaptic transmission [Durand et al., 2007; Jamain et al., 2003]. Using linkage analysis, genetic variants within chromosomal region 20q11.21-q13.12 were identified in a large pedigree of autistic individuals [Allen-Brady et al., 2009]. These variants are single-nucleotide polymorphisms (SNPs) in the chromosomal loci containing the PTPRT gene, and they exhibit significantly high linkage to ASD. However, it is not known which of these SNPs are functional variants that may change PTPRT expression or function. In this study, we introduced a loss-of-function missense mutation in the catalytically active phosphatase domain of PTPRT gene. This mutation inactivates the phosphatase while retaining the ability of the protein to bind to other substrate proteins [Zhang et al., 2007]. Interestingly, a number of functional SNPs have been identified in the phosphatase domain of the PTPRT gene in colorectal and lung cancer populations [Wang et al., 2004]. The three core diagnostic symptoms of ASD [impaired sociability, deficits in social communication and repetitive, and restrictive behaviors; American Psychiatric Association, 2013] have been modeled in animals [Crawley, 2007; Ricceri, Moles, & Crawley, 2007]. We tested PTPRT mutant mice for deficits in social behaviors using the three-chambered social interaction test. This test has been extensively studied and employed for screening social deficits in various mouse models of autism [McFarlane et al., 2008; Moy et al., 2007, 2008; Silverman et al., 2011]. Animal models of autism have been reported with deficits in social approach [DeLorey et al., 2008;Jamain et al., 2008; Molina et al., 2008; Moy et al., 2009; Nakatani et al., 2009]. We hypothesized that inactivating phosphatase function of PTPRT might shift the balance of phosphorylation/dephosphorylation states of proteins critical for synapse formation resulting in altered neural pathways and animal behaviors relevant to ASD. Indeed, male homozygous PTPRT m/m knockin mice initiated more contact with the stranger 1 (social approach) mice than their wild-type controls, suggesting increased, rather than decreased, social interaction. Interestingly, increases in social behaviors have been reported in two other mutant mouse strains. Animals with targeted mutation in the Mecp2 gene are considered a mouse model for Rett syndrome [Pearson et al., 2012]. The other mutant mouse line has a deficient phosphodiesterase 10A [Sano, Nagai, Miyakawa, Shigemoto, & Yokoi, 2008]. In either model, the mechanism by which the mutation promotes social behaviors is not fully understood, although it has been hypothesized that an increase in cAMP signaling within striatal medium spiny neurons may facilitate social interaction in the PDE10A2-deficient mice.
Although the precise neuronal pathways underlying these findings are unknown, it is well understood that tyrosine phosphatases serve as signal-transducing agents for proteins involved in synaptic development [Dabrowski & Umemori, 2011]. Reduced PTPRT phosphatase function is likely to result in higher phosphorylation states of substrate proteins, disrupting the balance of phosphorylation/dephosphorylation in critical pathways and potentially modifying behaviors. Our results indicate a possible role for PTPRT/RPTPρ in shaping neural pathways important for specific social behaviors. Further studies are required to better understand RPTPρ’s involvement in mouse behaviors relevant to ASD.
Acknowledgments
The authors would like to acknowledge Pauline Chen and Michael Chee for help with mouse colony maintenance.
Grant sponsor:
National Institute on Drug Abuse; Grant number: DA014610
Contributor Information
Keerthi Thirtamara Rajamani, Department of Pharmacology, The Ohio State University, Columbus, Ohio; Neuroscience Graduate Studies Program, The Ohio State University, Columbus, Ohio.
Brian O’Neill, Department of Pharmacology, The Ohio State University, Columbus, Ohio; Neuroscience Graduate Studies Program, The Ohio State University, Columbus, Ohio.
Dawn D. Han, Department of Pharmacology, The Ohio State University, Columbus, Ohio
Adrienne Frostholm, Department of Pharmacology, The Ohio State University, Columbus, Ohio.
Andrej Rotter, Department of Pharmacology, The Ohio State University, Columbus, Ohio.
Howard H. Gu, Department of Pharmacology, The Ohio State University, Columbus, Ohio
References
- Allen-Brady K, Miller J, Matsunami N, Stevens J, Block H, et al. (2009). A high-density SNP genome-wide linkage scan in a large autism extended pedigree. Molecular Psychiatry, 14, 590–600. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. 10.1176/appi.books.9780890425596.910646. [DOI] [Google Scholar]
- Besco J, Popesco MC, Davuluri RV, Frostholm A, & Rotter A (2004). Genomic structure and alternative splicing of murine R2B receptor protein tyrosine phosphatases (PTPkappa, mu, rho and PCP-2). BMC Genomics, 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besco JA, Frostholm A, Popesco MC, Burghes AH, & Rotter A (2001). Genomic organization and alternative splicing of the human and mouse RPTPrho genes: Correction. BMC Genomics, 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besco JA, Hooft van Huijsduijnen R, Frostholm A, & Rotter A (2006). Intracellular substrates of brain-enriched receptor protein tyrosine phosphatase rho (RPTPrho/PTPRT). Brain Research, 1116, 50–57. [DOI] [PubMed] [Google Scholar]
- Betancur C, Sakurai T, & Buxbaum JD (2009). The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends in Neurosciences, 32, 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Flint AJ, & Tonks NK (1993). Homophilic binding of PTP mu, a receptor-type protein tyrosine phosphatase, can mediate cell-cell aggregation. The Journal of Cell Biology, 122, 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, et al. (2012). Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS ONE, 7, e40914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Han DD, & Gu HH (2005). A triple mutation in the second transmembrane domain of mouse dopamine transporter markedly decreases sensitivity to cocaine and methylphenidate. Journal of Neurochemistry, 94, 352–359. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, et al. (2006a). Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proceedings of the National Academy of Sciences of the United States of America, 103, 9333–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Wu X, Wei H, Han DD, & Gu HH (2006b). Molecular cloning and functional characterization of the dopamine transporter from Eloria noyesi, a caterpillar pest of cocaine-rich coca plants. Gene, 366, 152–160. [DOI] [PubMed] [Google Scholar]
- Crawley JN (2004). Designing mouse behavioral tasks relevant to autistic-like behaviors. Mental Retardation and Developmental Disabilities Research Reviews, 10, 248–258. [DOI] [PubMed] [Google Scholar]
- Crawley JN (2007). Mouse behavioral assays relevant to the symptoms of autism. Brain Pathology (Zurich, Switzerland), 17, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowski A, & Umemori H (2011). Orchestrating the synaptic network by tyrosine phosphorylation signalling. Journal of Biochemistry, 149, 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, & Clark JD (2008). Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: A potential model of autism spectrum disorder. Behavioural Brain Research, 187, 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, et al. (2007). Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nature Genetics, 39, 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer EH, Charbonneau H, Cool DE, & Tonks NK (1992). Tyrosine phosphatases and their possible interplay with tyrosine kinases. Ciba Foundation Symposium, 164, 132–140, discussion 140–144. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, & Nelson RJ (2011). Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Molecular Psychiatry, 16, 987–995, 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink MF, van Etten I, Hateboer G, Suijkerbuijk R, Beijersbergen RL, Geurts van Kessel A, & Moolenaar WH (1991). Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Letters, 290, 123–130. [DOI] [PubMed] [Google Scholar]
- Gu HH, Wu X, & Han DD (2006). Conserved serine residues in serotonin transporter contribute to high-affinity cocaine binding. Biochemical and Biophysical Research Communications, 343, 1179–1185. [DOI] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, et al. (2012). An anatomically comprehensive atlas of the adult human brain transcriptome. Nature, 489, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, & Rodgers RJ (2003). Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol, 459, 221–230. [DOI] [PubMed] [Google Scholar]
- Huguet G, Ey E, & Bourgeron T (2013). The genetic land-scapes of autism spectrum disorders. Annual Review of Genomics and Human Genetics, 14, 191–213. [DOI] [PubMed] [Google Scholar]
- Jahn R, & Sudhof TC (1999). Membrane fusion and exocytosis. Annual Review of Biochemistry, 68, 863–911. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, et al. (2003). Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nature Genetics, 34, 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, et al. (2008). Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences of the United States of America, 105, 1710–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KG, & Van Vactor D (2003). Receptor protein tyrosine phosphatases in nervous system development. Physiological Reviews, 83, 1–24. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, et al. (2008). Disruption of neurexin 1 associated with autism spectrum disorder. American Journal of Human Genetics, 82, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RA, & Christian SL (2009). Genetics of autism spectrum disorders. Current Neurology and Neuroscience Reports, 9, 188–197. [DOI] [PubMed] [Google Scholar]
- Lim SH, Kwon SK, Lee MK, Moon J, Jeong DG, et al. (2009). Synapse formation regulated by protein tyrosine phosphatase receptor T through interaction with cell adhesion molecules and Fyn. The EMBO Journal, 28, 3564–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SH, Moon J, Lee M, & Lee JR (2013). PTPRT regulates the interaction of Syntaxin-binding protein 1 with Syntaxin 1 through dephosphorylation of specific tyrosine residue. Biochemical and Biophysical Research Communications, 439, 40–46. [DOI] [PubMed] [Google Scholar]
- McAndrew PE, Frostholm A, Evans JE, Zdilar D, Goldowitz D, et al. (1998a). Novel receptor protein tyrosine phosphatase (RPTPrho) and acidic fibroblast growth factor (FGF-1) transcripts delineate a rostrocaudal boundary in the granule cell layer of the murine cerebellar cortex. The Journal of Comparative Neurology, 391, 444–455. [DOI] [PubMed] [Google Scholar]
- McAndrew PE, Frostholm A, White RA, Rotter A, & Burghes AH (1998b). Identification and characterization of RPTP rho, a novel RPTP mu/kappa-like receptor protein tyrosine phosphatase whose expression is restricted to the central nervous system. Brain Research. Molecular Brain Research, 56, 9–21. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, & Crawley JN (2008). Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, Brain, and Behavior, 7, 152–163. [DOI] [PubMed] [Google Scholar]
- Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, … Walz K (2008). Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Human Molecular Genetics, 17, 2486–2495. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, et al. (2009). Social approach in genetically engineered mouse lines relevant to autism. Genes, Brain, and Behavior, 8, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, et al. (2008). Social approach and repetitive behavior in eleven inbred mouse strains. Behavioural Brain Research, 191, 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, et al. (2007). Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behavioural Brain Research, 176, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, et al. (2009). Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell, 137, 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park AR, Oh D, Lim SH, Choi J, Moon J, et al. (2012). Regulation of dendritic arborization by BCR Rac1 GTPase-activating protein, a substrate of PTPRT. Journal of Cell Science, 125, 4518–4531. [DOI] [PubMed] [Google Scholar]
- Paul S, & Lombroso PJ. (2003). Receptor and nonreceptor protein tyrosine phosphatases in the nervous system. Cellular and Molecular Life Sciences, 60, 2465–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Defensor EB, Pobbe RL, Yamamoto LH, Bolivar VJ, et al. (2012). Mecp2 truncation in male mice promotes affiliative social behavior. Behavior Genetics, 42, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, Moles A, & Crawley J (2007). Behavioral phenotyping of mouse models of neurodevelopmental disorders: Relevant social behavior patterns across the life span. Behavioural Brain Research, 176, 40–52. [DOI] [PubMed] [Google Scholar]
- Sallee JL, Wittchen ES, & Burridge K (2006). Regulation of cell adhesion by protein-tyrosine phosphatases: II. Cell-cell adhesion. The Journal of Biological Chemistry, 281, 16189–16192. [DOI] [PubMed] [Google Scholar]
- Sano H, Nagai Y, Miyakawa T, Shigemoto R, & Yokoi M (2008). Increased social interaction in mice deficient of the striatal medium spiny neuron-specific phosphodiesterase 10A2. Journal of Neurochemistry, 105, 546–556. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, et al. (2011). Sociability and motor functions in Shank1 mutant mice. Brain Research, 1380, 120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirtamara Rajamani K, Doherty-Lyons S, Bolden C, Willis D, Hoffman C, et al. (2013). Prenatal and early-life exposure to high-level diesel exhaust particles leads to increased locomotor activity and repetitive behaviors in mice. Autism Research: Official Journal of the International Society for Autism Research, 6, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks NK (2006). Protein tyrosine phosphatases: From genes, to function, to disease. Nature Reviews. Molecular Cell Biology, 7, 833–846. [DOI] [PubMed] [Google Scholar]
- Wang H, Lian Z, Lerch MM, Chen Z, Xie W, & Ullrich A (1996). Characterization of PCP-2, a novel receptor protein tyrosine phosphatase of the MAM domain family. Oncogene, 12, 2555–2562. [PubMed] [Google Scholar]
- Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, et al. (2004). Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science, 304, 1164–1166. [DOI] [PubMed] [Google Scholar]
- Wu X, & Gu HH (2003). Cocaine affinity decreased by mutations of aromatic residue phenylalanine 105 in the transmembrane domain 2 of dopamine transporter. Molecular Pharmacology, 63, 653–658. [DOI] [PubMed] [Google Scholar]
- Xu Y, & Fisher GJ (2012). Receptor type protein tyrosine phosphatases (RPTPs)—Roles in signal transduction and human disease. Journal of Cell Communication and Signaling, 6, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Oliveira G, Coutinho A, Yang C, Feng J, et al. (2005). Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Molecular Psychiatry, 10, 329–332. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gil MC, Choi EY, Park SH, Pyun KH, & Ha H (1997). Molecular cloning and chromosomal localization of a human gene homologous to the murine R-PTP-kappa, a receptor-type protein tyrosine phosphatase. Gene, 186, 77–82. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Guo AL, Yu JS, Possemato A, Chen YT, et al. (2007). Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proceedings of the National Academy of Sciences of the United States of America, 104, 4060–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YQ, Zhang XD, Guda K, Lawrence E, Sun Q, et al. (2010). Identification and functional characterization of paxillin as a target of protein tyrosine phosphatase receptor T. Proceedings of the National Academy of Sciences of the United States of America, 107, 2592–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, & Bear MF (2012). Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harbor Perspectives in Biology, 4, a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]