Abstract
Mosquitoes are important vectors that transmit pathogens to human and other vertebrates. Each mosquito species has specific ecological requirements and bionomic traits that impact human exposure to mosquito bites, and hence disease transmission and vector control. A study of human biting mosquitoes and their bionomic characteristics was conducted in West Sumba and Southwest Sumba Districts, Nusa Tenggara Timur Province, Indonesia from May 2015 to April 2018. Biweekly human landing catches (HLC) of night biting mosquitoes both indoors and outdoors caught a total of 73,507 mosquito specimens (59.7% non-Anopheles, 40.3% Anopheles). A minimum of 22 Culicinae species belonging to four genera (Aedes, Armigeres, Culex, Mansonia), and 13 Anophelinae species were identified. Culex quinquefasciatus was the dominant Culicinae species, Anopheles aconitus was the principal Anopheles species inland, while An. sundaicus was dominant closer to the coast. The overall human biting rate (HBR) was 10.548 bites per person per night (bpn) indoors and 10.551 bpn outdoors. Mosquitoes biting rates were slightly higher indoors for all genera with the exception of Anopheles, where biting rates were slightly higher outdoors. Diurnal and crepuscular Aedes and Armigeres demonstrated declining biting rates throughout the night while Culex and Anopheles biting rates peaked before midnight and then declined. Both anopheline and non-anopheline populations did not have a significant association with temperature (p = 0.3 and 0.88 respectively), or rainfall (p = 0.13 and 0.57 respectively). The point distribution of HBR and seasonal variables did not have a linear correlation. Data demonstrated similar mosquito–human interactions occurring outdoors and indoors and during early parts of the night implying both indoor and outdoor disease transmission potential in the area–pointing to the need for interventions in both spaces. Integrated vector analysis frameworks may enable better surveillance, monitoring and evaluation strategies for multiple diseases.
Author summary
This study outlines the array of mosquitoes that bite humans at night on the island of Sumba, Indonesia, with data on behavioural traits that impact when and where disease transmission may occur. Biweekly human landing catches (HLCs) were performed in four selected houses in 12 clusters (villages) from sunset to sunrise over a three years period (May 2015 to April 2018). The collection and analysis of 73,507 mosquito specimens revealed the presence of various species of Anopheles, Aedes, Culex, Armigeres and Mansonia, that potentially transmit several diseases including malaria, filaria, dengue and other mosquito borne viral diseases. Even though these data represent only night-time collections, this represents a comprehensive geographic description and inventory of species, bionomics and temporal distribution of mosquitoes on the island of Sumba. Data demonstrate that the high diversity of species with associated diversity in behaviours results in mosquito-human contact occurring throughout the night and both indoors and outdoors–relevant to both disease transmission and intervention applicability. Vector specific behaviours are specifically relevant to intervention strategies for specific diseases. The use of molecular methods to determine and validate morphological identification of specimens resulted in the characterization of multiple novel sequences–indicating the presence of undescribed species, members of cryptic species complexes or species without molecular data. Species identification using molecular methods are essential towards determine vector species compositions–especially in areas where data is absent. Though the correlation between temperature, rainfall and HBR was not statistically significant, the presence of mosquito populations throughout the year allow for perennial transmission of mosquito-borne diseases. Overall, these findings represent baseline and novel data for Sumba and may be utilized to develop disease and vector-specific or integrated strategies that mitigate the transmission of mosquito borne diseases in Indonesia.
Introduction
Mosquitoes (Order: Diptera; Family: Culicidae) are an important group of arthropods that transmit diseases to humans and animals through their blood feeding behaviour [1]. Diseases transmitted by mosquitoes include malaria, dengue, Zika, filariasis, Japanese encephalitis, and chikungunya, all documented in Indonesia, contributing to mortality and morbidity throughout the country with millions of people at risk of infection [1,2].
Globally, there are approximately 3,200 identified mosquito species in three subfamilies: Toxorhynchitinae (Toxorhynchites), Culicinae (Aedes, Culex, Mansonia, Armigeres) and Anophelinae (Anopheles) [3]. Of the many species included in these subfamilies, only a subset have been confirmed as vectors of diseases. Transmitted by Anopheles, malaria is endemic in Sumba Island with higher clinical cases in the rainy season compared to the dry season—attributed to increased mosquito vector populations associated with rain [4]. Kodi Balaghar sub district in southwest Sumba district is also endemic for filariasis [5]. Filariasis is caused by filarial nematode worms Wucheria bancrofti, Brugia malayi and B. timori and are transmitted by mosquito species within multiple genera including Mansonia, Anopheles, Culex and Aedes [6]. Mansonia uniformis and An. nigerrimus have been confirmed as B. malayi vectors while An. barbirostris has been reported as the vector of B. timori, commonly found in East Nusa Tenggara and South Maluku regions in Indonesia. Anopheles sundaicus, An.vagus and An. subpictus are vectors of W. bancrofti in East Nusa Tenggara [7,8]. Dengue transmitted by Aedes species is also prevalent in East Nusa Tenggara Province with incidence rates reported in 2017 as high as 19.5 per 100,000 people [9]. Primary dengue vectors include Ae. albopictus and Ae. aegypti, with the Chikungunya virus also being transmitted by Ae. aegypti [10,11].
Interventions directed at mosquito vectors of disease rely on vector behavioural traits. Efficient and impactful intervention strategies are dependent on vector knowledge that describe their behaviour and ecology in combination with the epidemiology of the disease in humans. Species specific bionomic traits and species specific drivers of transmission, rely on morphological and molecular characterization of the vector species. Misidentification of vectors impacts potential downstream analyses and intervention strategies. This effect was seen in central Vietnam, where An. varuna was mistakenly described as An. minimus as a primary vector. Vector control efforts were hence directed towards described An. minimus population peaks resulting in wasted resources since local An. varuna is highly zoophagic and unlikely to be a malaria vector [12]. The correct identification of any mosquito implicated as a vector is key to successful control or elimination measures. Similarly the comprehension of species specific impacts of interventions allowed for the description of gaps in protection in Kenya [13] and the Solomon Islands [14].
Standard practices towards the identification of mosquito species include morphological and sometimes, molecular identification, combined with parallel ecological and bionomic data used to improve the accuracy of species identification [15]. Morphological identification using regional morphological keys is commonly used as it is less labour intensive and time efficient. However, the specificity and sensitivity of morphological identification may be compromised based on local applicability of the specific morphological identification keys, appropriate training and human error, as well as difficulty associated with identifying sibling or cryptic species, regional morphological variants, and new or novel species. Thus, molecular identification may be used in conjunction with morphological methods along with ecological analysis towards improving accuracy and produce more informative data.
Towards filling this important knowledge gap in West Sumba, Indonesia, this study aimed to catalogue and identify human, night-biting mosquito species, their temporal presence and bionomic characteristics.
Methods
Ethics statement
Ethical review and approval was granted by the Ethics Committee (EC) of the Faculty of Medicine, Universitas Hasanuddin, Indonesia and the University of Notre Dame, USA. Verbal and written informed consent was obtained from local volunteers for landing catches, who were all more than 18 years old and from house owners.
Study site and design
This study was conducted in Southwest and West Sumba Districts, East Nusa Tenggara Province located on the island of Sumba in the eastern part of Indonesia (Fig 1) from May 2015 to April 2018. The climate is tropical, with a dry season from May to November and a wet season from December to April. This dataset was collected as part of a parent, cluster randomized, double-blind, placebo-controlled, clinical trial, that measured the public health impact of a spatial repellent on malaria incidence [4]. Of the 24 clusters included in the parent study, 12 geographically distributed clusters were utilized for entomological follow-up with bi-weekly human landing catches (HLCs). Climatic parameters such as temperature and rainfall were obtained from HOBO weather stations installed in three locations across the study area. Monthly average rainfall was calculated from the daily values of the three weather stations. Rainfall data was obtained from July 2016 to April 2018. Temperature data was obtained from May 2015 to April 2018.
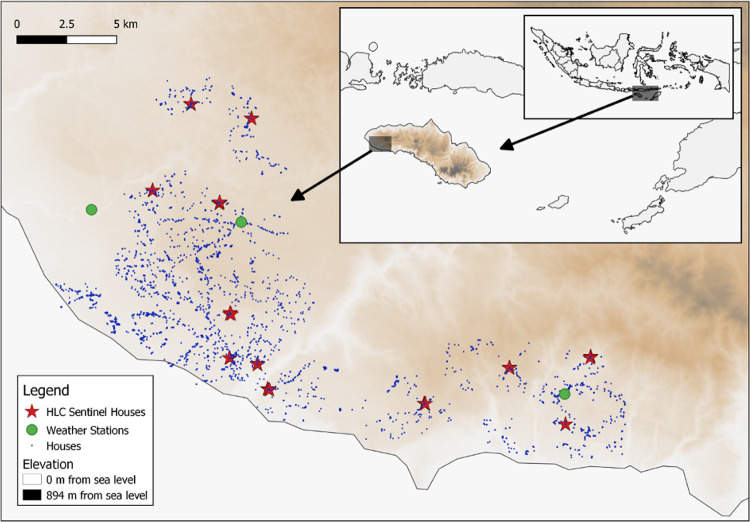
Fig 1. Map of the study site: 4 sentinel houses in each clusters are marked by stars.
A map depicting the location of study site in Southwest and West Sumba Districts, East Nusa Tenggara Province, Indonesia (http://www.naturalearthdata.com/).
Mosquitoes collection
In each cluster, four traditional houses were selected having similar size and design, and with house owners agreeing to participate in the study. These sentinel houses were located close to permanent or semi-permanent Anopheles larval sites. Indoor and outdoor (household veranda) paired HLCs were performed by a two-member team (local volunteers with informed consent) per house, in four sentinel HLC houses in each cluster [4]. Host seeking mosquitoes landing on exposed feet and legs were caught using an aspirator for 50 min each hour from 1800 h to 0600h. Mosquitoes were held in individual paper cups labelled for each hour, location (indoor or outdoor) and house code. Female mosquito specimens were transported to an on-site study laboratory for further processing. In total, with 52 nights of HLCs in 12 clusters, and in 4 houses each (inside and outside) there were 4,992 person nights of HLC collections (2,496 indoors and outdoors each).
Mosquitoes identification
Mosquito specimens were morphologically identified to genera (Culex, Anopheles, Aedes, Armigeres, and ‘other’) using taxonomic keys [16,17]. Samples were stratified by morphological species, cluster, location, and time of capture, and a random subset (representing at least 10% of each morphologically identified species) spanning all strata were sequenced at the internal transcribed spacer2 (ITS2) and/or cytochrome c oxidase subunit I (COI) loci towards species determination [18–24]. A larger subset of Anopheles was analysed based on the parent study focus on Anopheles and malaria.
For molecular species confirmation, DNA was extracted from whole specimens using a Chelex-100 ion exchange (BioRad Laboratories, Hercules, CA, USA). PCR amplicons were sequenced at the Eijkman Institute for Molecular Biology, Indonesia, and the University of Notre Dame, USA. Comparisons were made between morphological and molecular species identifications. Final species confirmation required high sequence identity (thresholds of 97% for ITS2 and 94% for CO1) to sequences in multiple databases. CO1 and ITS2 database comparisons for each sample were paired to determine species when either CO1 or ITS2 alone did not produce significant results to voucher sequences [18,21,23]. Consensus sequences were manually inspected for insertions, deletions, and repeat regions to ensure these sequence differences did not inflate divergence and decrease identity scores.
Analysis
The night-time human biting rate (HBR) was determined as bites per person per night (bpn) or bites per person per hour (bph). The relationship between independent variables (climatic parameters) and dependent variables (HBR) were tested using the Pearson correlation coefficients and scatterplot correlation. Tests were also conducted to determine the relationship between mosquito abundance and rainfall and temperature [25]. Bionomic inferences were made at the genus level for Anopheles, Armigeres, Aedes and Culex, as well as for the five most common Anopheles species. Consensus sequences of each sequence group were compared (BLASTn) to the NCBI nr and BOLD [26] databases to identify species. Species belonging to the An. barbirostris species complex were compared to type specimen sequences [27,28] towards molecular identification.
Results
Mosquitoes composition
During the 52 nights of human landing collections, a total of 73,507 adult female Culicidae mosquitoes were captured. These samples included Culex (40.29%; n = 29,612), Anopheles (40.30%; n = 29,636); Aedes (12.86%, n = 9,451), and Armigeres (6.27%; n = 4,608). Unidentified female mosquitoes and Mansonia species were grouped together as ‘others’ (0.27%; n = 200). Overall, 50.0% (36,759) of the mosquitoes were captured outdoors and 50.0% (36,748) indoors.
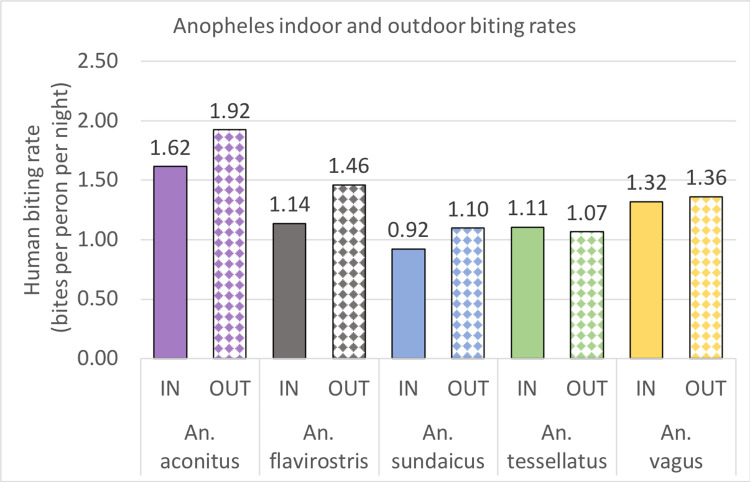
Average daily human biting rates (HBR) for the 35 months of the study were determined with the assumption that landing rates in the HLCs were a proxy for HBRs (Fig 2). Indoor and outdoor biting rates did not vary significantly within for Aedes, Armigeres, or Culex, with slightly higher indoor biting seen. Of the five dominant Anopheles species, four had slightly higher outdoor biting rates (An. aconitus: 1.62 bpn indoor versus 1.92 bpn outdoor; An. flavirostris: 1.14 bpn indoor versus 1.46 bpn outdoor; An. sundaicus: 0.92 bpn indoor versus 1.10 bpn outdoor; An. vagus: 1.32 bpn indoor versus 1.36 bpn outdoor) while An. tesselatus had a slightly higher indoor biting rate (1.11 bpn indoor versus 1.07 bpn outdoor) (Fig 3). Biting rates varied by geography of collection and was usually associated with the availability of larval sites and with agricultural irrigation. Interestingly, the biting rate of An. sundaicus, the historical primary vector in the area, dropped from 8.79 bpn (June 2015-December 2015; with a high of 24.81 bpn in August 2015) to an average of 0.29 bpn post December, 2016. The biting densities of An. sundaicus only recovered slightly between August and October 2017 (a high of 4.14 bpn in September, 2017).
Fig 2. Indoor and outdoor biting rates of Culicidae.
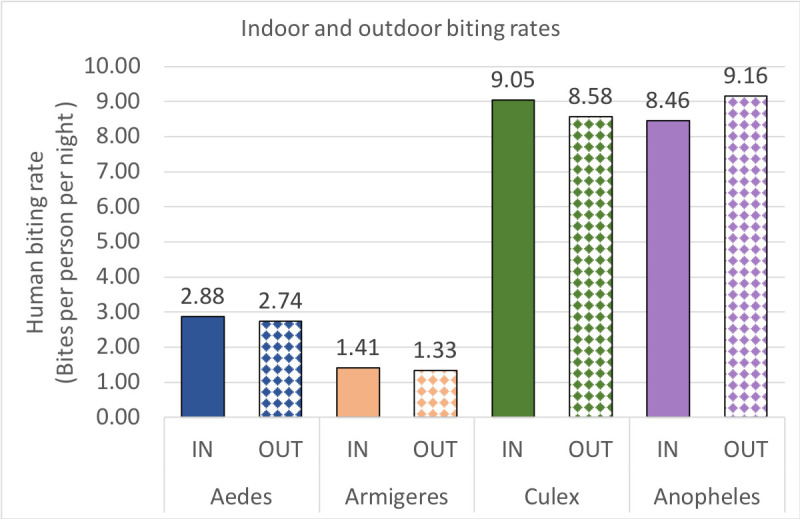
Fig 3. Indoor and outdoor biting rates of Anopheles species.
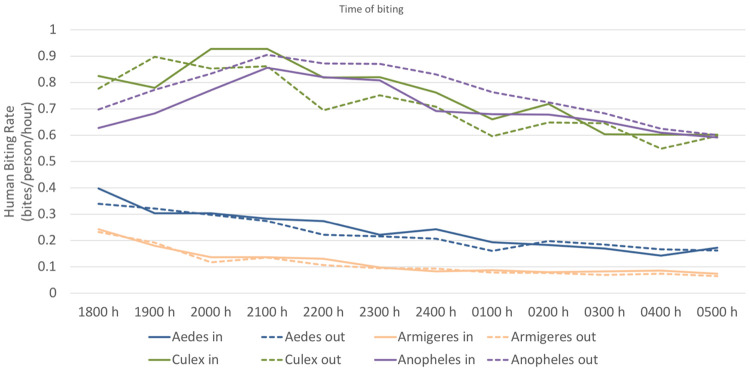
All mosquitos were found to bite throughout the night both indoors and outdoors. Aedes and Armigeres species tended to have their biting rates drop over the course of a night–Aedes: from 0.37 bites per person per hour (bph) between 1800 to 1900h, to 0.16 bph between 0500 to 0600h; Armigeres: from 0.23 bph between 1800h to 1900h, to 0.16 bpn between 0500h to 0600h). Culex biting rates peaked between 1900 h and 2100 h (0.87 bph) while Anopheles bites peaked between 2100h and 2300h (0.85 bpn). Both Culex and Anopheles biting rates dropped to 0.6 and 0.59 bph respectively at the 0500 to 0600h collection timepoint (Fig 4).
Fig 4. Indoor and outdoor biting rates of Aedes, Armigeres, Culex and Anopheles species over the course of a night.
The five dominant Anopheles species had similar biting rates indoors and outdoors over the course of the night. Though HBRs did not vary significantly over the night between species, An. aconitus and An. tesselatus tended to have slightly higher hourly biting rates from 1800 to 2200h (0.17 bph), both An. flavirostris and An. sundaicus had peak biting between 2100 and 0100h (01.3 bph), while An. vagus had a fairly consistent HBR over the night, with a slightly higher biting rate from 0200 to 0600h (0.12 bph).
Morphological species identifications
Morphological species identification was performed on Aedes (n = 589), Armigeres (n = 183), Culex (n = 1,312), Mansonia (n = 44) and Anopheles (n = 29,636) specimens. Morphologically identified Culex species included Cx. quinquefasciatus (the most abundant species), followed by Cx. gelidus, Cx. vishnui, Cx. tritaeniorhynchus, Cx. fuscocephala, Cx. bitaeniorhynchus, Cx. sinensis, Cx. halifaxii, and Cx. whitmorei. Aedes species morphologically identified included Ae. aegypti, Ae. albopictus, Ae. poicilius, Ae. vexans, and Ae. (mucidus) spp. Morphologically identified Armigeres species included Armigeres malayi and Ar. subalbatus, while Mansonia uniformis was identified in the ‘other’ set.
All Anopheles specimens were morphologically identified to species. Of the 13 Anopheles species identified, the five dominant species included An. aconitus (20.07% of Anopheles, n = 5,946), An. vagus (15.2% of Anopheles; n = 4,502); An. flavirostris (14.74% of Anopheles; n = 4,365), An. tesselatus (12.32% of Anopheles; n = 3,651), and An. sundaicus (11.45% of Anopheles; n = 3,392) (Table 1). The remaining eight identified species of Anopheles representing 26.22% of the total Anopheles comprised of An. annularis, An. balabacensis, An. barbirostris, An. indefinitus, An. kochi, An. leucosphyrus, An. maculatus, and An. subpictus. Anopheles aconitus was the predominant species found in the upland interior locations whereas An. sundaicus was dominant in coastal areas. A small number (n = 20) of Anopheles specimens remain unidentified morphologically.
Table 1. Species identifications.
Molecular species identifications (with the number of specimens) are listed with ITS2 and CO1 similarities to sequences in the databases (NCBI and BOLD). Morphological identifications of the molecularly identified specimens are also listed.
| Genera | Molecular species (#) | Sequence Type | Morphological Identifications (#) | |
|---|---|---|---|---|
| (% Identity) | ||||
| ITS2 | CO1 | |||
| Culex | Cx. bitaeniorhynchus (5) | 97.9 | 100 | Cx. vishnui (4), Cx. sinensis (1) |
| Cx. fuscochepala (5) | - | 100 | Cx. tritaeniorhynchus (4), Cx. quinquefasciatus (1) | |
| Cx. gelidus (37) | 99.7 | 100 | Cx. gelidus (33), Cx. tritaeniorhynchus (2), Cx. vishnui (2) | |
| Cx. nigropunctatus (4) | - | 98.7 | Cx. quinquifasciatus (4) | |
| Cx. orientalis (2) | 98.2 | - | Cx. vishnui (1), Cx. sinensis (1) | |
| Cx. pseudovishnui (5) | 98.5 | - | Cx. tritaeniorhynchus (2), Cx. vishnui (2), Ae. vexans (1) | |
| Cx. quinquefasciatus (4) | 99.2 | - | Cx. quinquefasciatus (4) | |
| Cx.tritaeniorhynchus (17) | 99.4 | 99.6 | Cx. tritaeniorhynchus (10), Cx. vishnui (6), Cx. quinquefasciatus (1) | |
| Cx. vishnui (6) | 99.3 | - | Cx. vishnui (3), Cx. tritaeniorhynchus (3) | |
| Culex species 1 (3) | 86 | - | Cx. tritaeniorhynchus (1), Cx. vishnui (1), Cx. quinquefasciatus (1) | |
| Culex species 2 (4) | 78.6 | - | Cx. quinquefasciatus (4) | |
| Aedes | Ae. albopictus (12) | 99.2 | 99.9 | Ae. albopictus (11) |
| 99.4 | Ae. albopictus (1) | |||
| Ae. vexans (7) | 93.7 | 99.9 | Ae. vexans (5), Cx. vishnui (2) | |
| Aedes species 1 (5) | 81.5 | - | Ar. subalbatus (5) | |
| Aedes species 2 (5) | 80 | - | Ar. malayi (2), Ar. subalbatus (2), Cx. tritaeniorhynchus (1) | |
| Aedes species 3 (36) | 89.3 | 93.4 | Ae. albopictus (25), Ae. vexans (11) | |
| Aedes species 4 (4) | 94.4 | - | Ae. albopictus (4) | |
| Aedes species 5 (1) | - | 89 | Ae. poicilius (1) | |
| Armigeres | Ar. malayi (16) | - | 100 | Ar. malayi (14), Ar. subalbatus (2) |
| Ar. subalbatus (27) | 94.3 | 97.3 | Ar. subalbatus (27) | |
| Ar. cf. subalbatus (3) | 91.1 | Ar. subalbatus (2), Cx. quinquefasciatus (1) | ||
| Other | Mansonia uniformis (12) | 100 | - | Ae. poicilius (12) |
| Anopheles | An. aconitus (1,822) | 100 | 99 | An. aconitus (1,644), An. annularis (9), An. flavirostris (134), An. barbirostris (3), An. farauti (1) An. kochi (6), An. maculatus (10), An. tesselatus (6), An. vagus (5), An. sundaicus (1), An. leucosphyrus (1), An. indefinitus (1), An. karwari (1) |
| An. annularis (491) | 99.8 | 95.81 | An. annularis (459), An. aconitus (12), An. barbirostris (3), An. subpictus (1), An. flavirostris (1), An. kochi (1), An. maculatus (2), An. sinensis (2), An. sundaicus (1), An. tesselatus (3), An. vagus (6) | |
| An. balabacensis (34) | 99.9 | - | An. leucosphyrus (30), An. flavirostris (1), An. maculatus (3) | |
| An. barbirostris clade 1 (116) | 99.5 | 100 | An. barbirostris | |
| An. barbirostris clade 2 (74) | 99.5 | - | An. barbirostris | |
| An. sundaicus s.l (296) | 100 | 100 | An. sundaicus (258), An. aconitus (10), An. barbirostris (17), An. flavirostris (5), An. vagus (3), An. annularis (2), An. subpictus (1) | |
| An. flavirostris (1,097) | 100 | 98 | An. flavirostris (955), An. aconitus (102), An. annularis (5), An. barbirostis (7), An. maculatus (6), An. sundaicus (10), An. kochi (3), An. leucosphyrus (2), An. montanus (1), An. subpictus (2), An. tesselatus (2), An. vagus (2) | |
| An. indefinitus (3) | 100 | - | An. aconitus (1), An. sundaicus (2) | |
| An. kochi (758) | 100 | 100 | An. kochi (700), An. aconitus (6), An. barbirostris (9), An. tesselatus (16), An. vagus (12), An. annularis (3), An. flavirostris (3), An. maculatus (2), An. punctulatus (1), An. sinensis (1), An. subpictus (3), An. sundaicus (2) | |
| An. maculatus (335) | 100 | 100 | An. maculatus (309), An. aconitus (10), An. annularis (2), An. vagus (3), An. barbirostris (2), An. flavirostris (5), An. kochi (1), An. sundaicus (1), An. tesselatus (2) | |
| An. subpictus (68) | 99.8 | 94.39 | An. subpictus (31), An. indefinitus (8), An. vagus (23), An. sundaicus (2), An. maculatus (1), An. aconitus (1), An. tesselatus (2) | |
| An. tesselatus (876) | 100 | 99.84 | An.tesselatus (799), An. annularis (4), An. kochi (7), An. maculatus (8), An. aconitus (7), An. barbirostris (6), An. barbumrosus (3), An. indefinitus (2), An. kochi (12), An. parangensis (1), An. sinensis (1), An. subpictus (4), An. flavirostris (7), An. vagus (15) | |
| An. vagus (1,004) | 98.7 | 100 | An. vagus (924), An. annularis (5), An. maculatus (5), An. subpictus (11), An. sundaicus (9), An. kochi (7), An. tesselatus (17), An. indefinitus (6), An. aconitus (7), An. annularis (5), An. barbirostris (2), An. flavirostris (14). | |
Molecular species identifications
There were seven Aedes taxa identified molecularly, two taxa were identified to species while five remained unidentified. Identified species had similarities higher than the thresholds (97% for ITS2, and 94% for CO1) to sequences in the database (NCBI and BOLD) [29–31]. Identified species include Ae. albopictus and Ae. vexans. Two groups of Ae. albopictus CO1 sequences were detected that were 5% different from each other. Unidentified Aedes specimens had sequences with low similarity (below 94%) to Ae. aegypti, Ae. ochraceus, and Ae. geniculatus (Table 1).
Of the three Armigeres species documented molecularly, Ar. malayi and Ar. subalbatus were identified to species, while the third (Ar. cf. subalbatus) set of sequences were only 91.12% similar to Ar. subalbatus CO1 sequences and consequently do not have a species designation due to the identity thresholds applied (Table 1).
Similarly, there were 11 taxa of Culex identified from ITS2 and/or CO1 sequences—including nine known and two unidentified species. The known species, with high sequence similarity in the databases, included Cx. gelidus, Cx. quinquefasciatus, Cx. vishnui, Cx. tritaeniorhynchus, Cx. pseudovishnui, Cx. bitaeniorhynchus, Cx. orientalis, Cx. nigropunctatus, and Cx. fuscochepala. The unidentified species, Culex species 1 and 2, were closest to Cx. dolosus (86.0%) and Cx. palpalis (78.6%)—similarity below conservative thresholds to confirm identity (Table 1). There were 13 molecularly identified Anopheles species including An. aconitus, An. annularis, An. sundaicus s.l, An. balabacensis, An. barbirostris clade 1 (An. barbirostris s.s.), An. barbirostris clade 2 (An. vanderwulpi), An. flavirostris, An. indefinitus, An. kochi, An. maculatus, An. tesselatus, An. subpictus and An. vagus (Table 1). Anopheles barbirostris s.s. and An. vanderwulpi were identified to species based on SNPs and homology to type sequences [28].
Mansonia uniformis was identified in the ‘other’ group, in addition to a single unidentified set of sequences pointing to a Dipteran species that remains unidentified. This unknown Dipteran had low identity (15%) to Culicidae sequences and was left out of the analysis.
Bionomics—Seasonality
Weather stations recorded rainfall throughout the study period. Rainfall was analysed as a driver of seasonal mosquitoes density. The highest daily rainfall occurred in February 2017 (a mean of 24.73 mm), while the lowest was in August 2017 (mean of 0.19 mm). Overall, there were an increasing number of Culicidae specimens caught during the rainy season as compared to the dry season—with the most significant increase being in Culex and Anopheles species (Fig 5). Statistical analysis with a Pearson correlation test demonstrates that rainfall intensity did not have a significant correlation with the number of mosquitoes (p = 0.3 for anopheline, p = 0.88 for non- anopheline).
Fig 5. Seasonality of Culicidae mosquito genera related to rainfall over the 35 months of data collection.
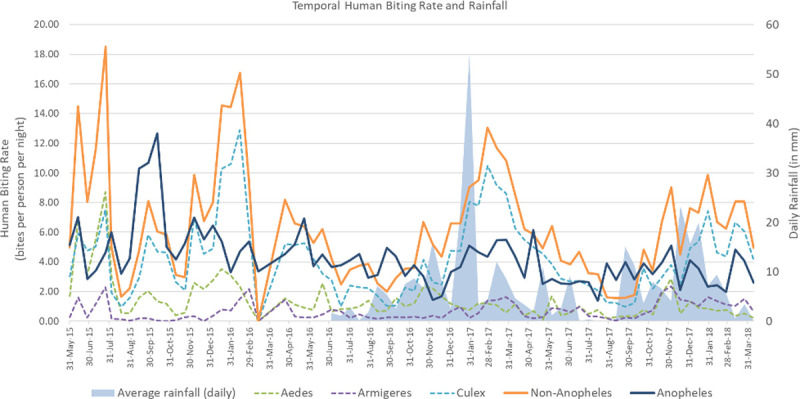
Temperature ranged between lows of 22.6°C and highs of 32.8°C without any significant short-term fluctuations. Statistical analyses (Pearson) demonstrated the lack a relationship between temperature and mosquito density (p = 0.13 for anopheline, p = 0.57 for non-anopheline). Similar results with scatterplot correlations, demonstrated that both anopheline and non-anopheline do not have linear correlations with rainfall or temperature.
Discussion
The parent intervention trial enabled standardized, 35 month collections of night-time human host seeking mosquitos in Sumba, Indonesia. The use of sentinel structures with paired indoor and outdoor HLCs enabled the first time evaluation of species compositions, seasonal variations, population drivers, and human host-seeking behaviours of Aedes, Armigeres, Culex and Anopheles mosquitoes over the night, in Indonesia.
Approximately 73,507 mosquito specimens collected were found to consist of at least 35 molecularly determined species. Culicinae mosquitoes were separated and processed differently than Anopheles, as the parent study was directed at Anopheles and malaria. A smaller set of Culicinae mosquitoes were identified morphologically and molecularly (Aedes: 589 morphologically (6.23%), 126 molecularly (1.33%); Armigeres: 183 morphologically (3.97%), 45 molecularly (0.98%); Culex: 1,312 morphologically (4.43%), 118 molecularly (0.40%); and ‘Other’: 44 morphologically (22.00%), 14 molecularly (7%)). Though the samples were randomly selected to represent all collection times and sites, the lower morphological and molecular identifications for Culicinae mosquitoes indicates that some species may have been missed. The high stringency used for identifying species molecularly [18,21,23] enabled a conservative approach. Several Culicinae species remain unidentified primarily due to a lack of matching sequences in the databases. These may also indicate the presence of new or novel species or members of cryptic species complexes. Though care was taken to match ITS2 to CO1 sequences towards the best possible identity, this was not possible in some cases where PCR amplification failed. This is the first time a comprehensive look at Culicinae mosquitoes has taken place at this site and points to the importance of saving non target species trapped as a by-product of a study. There was a high level of discrepancy between morphology and molecular identifications for Culicinae mosquitoes relative to Anophelinae mosquitoes. This may be attributed to a historically lesser focus on the development and validation of non-Anopheles morphological keys combined less research and consequently less experience on morphological identification related to these species. The presence of species complexes, sibling species and novel species in baseline characterizations (such as this) further complicates morphological identification. This is reflective of fewer studies (and consequent morphological identification experience) on non-anopheline mosquitoes in this region with a lack of ITS2 or CO1 sequences for some species, e.g. sequences for Cx. sinensis and Ae. poicilius are absent in the databases. This study identified Cx. quinquefasciatus as the dominant Culicinae species with highest relative abundance (determined morphologically). The role of Cx. quinquefasciatus as a vector of filaria was reported by other studies in Indonesia [32]. Cx. tritaeniorhynchus, Cx. vishnui, Cx. pseudovishnui and Cx.fuscocephala, identified in this study, tend to be indiscriminate feeders attracted to both human and animals [33]. The presence of the these Culex species combined with the local cultural practices of using domesticated pig as traditional currency point to a possible explanation for the maintenance of Japanese encephalitis transmission in the area [34].
Both Armigeres malayi and Ar. subalbatus were identified with peak biting during the crepuscular period with declining biting rates after, similar to that seen in other reports [35]. Armigeres subalbatus specifically demonstrated peak densities right after sunset, with no activity after 2100h –data supported by other studies that report similar temporal biting peaks [36,37].
Anopheles mosquitoes remain the genera with the highest identities with 100% of them having being morphologically and 24.55% (n = 7,276) being molecularly identified. A previous study [38] trapped similar species with the exception of Group Hyrcanus species not being caught in this study. Furthermore, both An. barbirostris clade I (An. barbirostris s.s.) and An. barbirostris clade 2 (An. vanderwulpi) were confirmed to both be present at these sites along with An. sundaicus s.l. [39].
Temporal density of vectors overall were reliant on the availability of larval habitats driven by rainfall and agricultural irrigation [40]. The availability of multiple larval habitats throughout the year indicates a year round availability of nuisance mosquitoes as well as vectors of disease. Though Culex densities were the most impacted by rainfall, rainfall was a driver of other genera populations as well. Interestingly, the primary malaria vector along the coast, An. sundaicus [38,41,42] all but disappeared by December 2016 with a small recovery in 2017. Though the reasons for this decline is not yet clear, it may be attributed to contamination of larval sites (permanent and semi-permanent brackish water pools [42] by agricultural effluence rendering them hypoxic, or the community wide distribution of long lasting insecticide impregnated bed nets (LLINs) impacting adult populations [13].
Open house construction, typical of traditional Sumba houses, may contribute to the similar indoor and outdoor biting rates determined. Houses being mostly made of bamboo walls, elevated bamboo floors, with thatch roofs and open eaves allow for mosquito entry and exit. Data from Malawi demonstrate that an open house construction (similar to that seen in traditional Sumba houses) had higher (14x) malaria vector entry when compared to more closed houses [43]. Domestic animals, often kept under the elevated floors over the night, may also contribute to mosquitoes being attracted toward and into houses. Zoophagic mosquitoes such as Armigeres [44] may be attracted towards these animals under houses and consequently feed on humans instead.
The HBRs from night biting determined point to the highest biting rates in Anopheles and Culex mosquitoes with lower biting rates for Aedes and Armigeres. Interestingly, Aedes were found to bite, albeit at low levels, throughout the night. This finding is important as it demonstrates the potential of Aedes, based virus transmission in future studies that incorporate HLCs. Studies that characterize the diurnal biting profile of these mosquitoes would enable a 24 hour characterization of bionomic traits [35,36]. Equivalent indoor and outdoor biting rates indicate the need for interventions in both spaces as well as indicate that indoor interventions may also impact outdoor biting mosquitoes that may go indoors to feed [45,46].
This presence of multiple Aedes species indicate the potential transmission of Aedes-borne diseases such as Zika, dengue, chikungunya and Yellow fever; while the presence of Armigeres species point to transmission of Zika, filariasis and Japanese encephalitis [47–49]. Both Anopheles and Culex species had peak biting both indoors and outdoors before midnight reflecting human activity, with declining HBRs after. These periods represent temporal exposure where interventions may be most applicable towards reducing man vector contact. Multiple Culex species with relevant biting periods and rates may support the transmission of multiple Culex-borne diseases such as human filariasis and Japanese encephalitis that have been reported in the area [50]. The five most common Anopheles species reported here have been confirmed as vectors for malaria and filariasis [49]. Mosquitoes in the Mansonia genus were also found in this study, although at lower densities. Mansonia species have been documented in transmitting filariasis in Indonesia [51]. Nocturnal B. malayi is transmitted by An. barbirostris, which lives in paddy fields, and nocturnal sub-periodic B. malayi is transmitted by Mansonia species. In Sumba, two species of filarial parasite in human have been documented, Wucheria bancrofti and B. timori [52].
This study revealed a wide diversity of night-time human host seeking mosquito species in Sumba that potentially transmit multiple vector borne diseases. The presence of night biting vectors of non-malaria diseases warrants the careful application of the HLC technique to ensure the safety of volunteers conducting the mosquito trappings [53,54], or the implementation of other innovative tools for adult mosquito collection, such as the double net trap [55] and Host Decoy Trap [44].
In conclusion, this study describes the array of human biting mosquitoes on the island of Sumba, Indonesia, demonstrating preliminary bionomic traits that impact human mosquito contact, and points to the potential of mosquito borne diseases that may be transmitted. This set of data is important for devising evidence-based vector borne disease mitigation strategies in Sumba and reveals the complexity of the mosquito biome in a single geography.
Acknowledgments
The authors are grateful for the supports of the study volunteers in Southwest and West Sumba Districts, East Nusa Tenggara Province. The authors are grateful for the support of the Eijkman Institute for Molecular Biology (EIMB) Jakarta, Ministry of Health Republic of Indonesia, District health departments of Southwest and West Sumba, and East Nusa Tenggara Province. We appreciate the contribution of the entomology teams, local field workers, data entry clerks, and the numerous local volunteers for their dedication and active participation in this study. We thank Nadha Rizky Pratama, Sylvia Sance Marantina, Jenifer Kiem Aviani, and Annisa Rizkia for their assistance in the EIMB laboratory. This publication is dedicated to the memory of Dr. Michael Bangs who had a special interest in this site and study—where he imparted much experience and knowledge. Mike’s contribution to the fight against malaria and other diseases across the world is prodigious and far reaching. He is greatly missed.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study was funded by an award from the Bill and Melinda Gates Foundation (BMGF) to the University of Notre Dame (Grant# OPP1081737) granted to NLA and NFL, the Government of Indonesia granted to DS, Ministry of Research and Technology/National Research and Innovation Agency through Eijkman Institute for Molecular Biology. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Robert Jr L, Debboun M. Arthropods of Public Health Importance. Ryan ET, Hill DR, Solomon T, Aronson NE, Endy TP, editors2020.
- 2.Indonesia Ministry of Health. Indonesia Health Profile 2019. Data and health information. 2020; 1
- 3.Foster WA, Walker ED. mosquitoes (Culicidae). In medical and veterinary entomology. 2019. Jan 1 (pp. 261–325). Academic press. 10.1016/B978-012510451-7/50014-1 [DOI] [Google Scholar]
- 4.Syafruddin D, Asih PBS, Rozi IE, Permana DH, Nur Hidayati AP, Syahrani L, et al. Efficacy of a Spatial Repellent for Control of Malaria in Indonesia: A Cluster-Randomized Controlled Trial. Am J Trop Med Hyg. 2020;103(1):344–58. Epub 20200514. doi: 10.4269/ajtmh.19-0554 ; PubMed Central PMCID: PMC7356406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Ryu JS. Current Status of Parasite Infections in Indonesia: A Literature Review. Korean J Parasitol. 2019;57(4):329–39. Epub 2019/09/20. doi: 10.3347/kjp.2019.57.4.329 ; PubMed Central PMCID: PMC6753303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra G. Nature limits filarial transmission. Parasites & vectors. 2008. Dec;1(1):1–6. doi: 10.1186/1756-3305-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adnyana NWD, Laumalay HM, Tallan MM. Penentuan Nyamuk Anopheles spp sebagai Vektor Filariasis di Kabupaten Sumba Timur dan Sumba Barat Provinsi Nusa Tenggara Timur. Health Research and Development Media. 2019;(29 (2)):177–88. [Google Scholar]
- 8.Santoso S, Yahya Y, Suryaningtyas NH, Rahayu KS. Deteksi mikrofilaria Brugia malayi pada nyamuk Mansonia spp dengan pembedahan dan metode PCR di Kabupaten Tanjung Jabung Timur. ASPIRATOR-Jurnal Penelitian Penyakit Tular Vektor (Journal of Vector-borne Diseases Studies). 2015;7(1):29–35. [Google Scholar]
- 9.Haryanto B. Indonesia dengue fever: status, vulnerability, and challenges. Current Topics in Tropical Emerging Diseases and Travel Medicine. 2018:81–92. [Google Scholar]
- 10.Harapan H, Michie A, Mudatsir M, Sasmono RT, Imrie A. Epidemiology of dengue hemorrhagic fever in Indonesia: analysis of five decades data from the National Disease Surveillance. BMC Res Notes. 2019;12(1):350. Epub 2019/06/22. doi: 10.1186/s13104-019-4379-9 ; PubMed Central PMCID: PMC6587249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulyatno KC, Kotaki T, Yotopranoto S, Rohmah EA, Churotin S, Sucipto TH, et al. Detection and Serotyping of Dengue Viruses in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) Collected in Surabaya, Indonesia from 2008 to 2015. Jpn J Infect Dis. 2018;71(1):58–61. Epub 2017/11/03. doi: 10.7883/yoken.JJID.2017.117 . [DOI] [PubMed] [Google Scholar]
- 12.Garros C, Van Nguyen C, Trung HD, Van Bortel W, Coosemans M, Manguin S. Distribution of Anopheles in Vietnam, with particular attention to malaria vectors of the Anopheles minimus complex. Malar J. 2008;7:11. Epub 2008/01/15. doi: 10.1186/1475-2875-7-11 ; PubMed Central PMCID: PMC2248199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. Epub 2010/03/02. doi: 10.1186/1475-2875-9-62 ; PubMed Central PMCID: PMC2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:56. Epub 2013/02/08. doi: 10.1186/1475-2875-12-56 ; PubMed Central PMCID: PMC3570334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson JJ. DNA barcodes for insects. Humana Press, Totowa, NJ: 2012. [DOI] [PubMed] [Google Scholar]
- 16.Harrison BA, Scanlon JE. The subgenus Anopheles in Thailand (Diptera: Culicidae). Contr Am Entomol Inst. 1975;12:1–307. [Google Scholar]
- 17.Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE, Panthusiri P. Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J Trop Med Public Health. 2006;37 Suppl 2:1–128. Epub 2007/02/01. . [PubMed] [Google Scholar]
- 18.Davidson JR, Wahid I, Sudirman R, Small ST, Hendershot AL, Baskin RN, et al. Molecular analysis reveals a high diversity of Anopheles species in Karama, West Sulawesi, Indonesia. Parasit Vectors. 2020;13(1):379. Epub 2020/07/31. doi: 10.1186/s13071-020-04252-6 ; PubMed Central PMCID: PMC7392657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3(5):294–9. Epub 1994/10/01. [PubMed] [Google Scholar]
- 20.Kumar NP, Rajavel AR, Natarajan R, Jambulingam P. DNA barcodes can distinguish species of Indian mosquitoes (Diptera: Culicidae). J Med Entomol. 2007;44(1):1–7. Epub 2007/02/14. doi: 10.1603/0022-2585(2007)44[1:dbcdso]2.0.co;2 . [DOI] [PubMed] [Google Scholar]
- 21.Lobo NF, St Laurent B, Sikaala CH, Hamainza B, Chanda J, Chinula D, et al. Unexpected diversity of Anopheles species in Eastern Zambia: implications for evaluating vector behavior and interventions using molecular tools. Sci Rep. 2015;5:17952. Epub 20151209. doi: 10.1038/srep17952 ; PubMed Central PMCID: PMC4673690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murugan K, Vadivalagan C, Karthika P, Panneerselvam C, Paulpandi M, Subramaniam J, et al. DNA barcoding and molecular evolution of mosquito vectors of medical and veterinary importance. Parasitol Res. 2016;115(1):107–21. Epub 2015/09/12. doi: 10.1007/s00436-015-4726-2 . [DOI] [PubMed] [Google Scholar]
- 23.St Laurent B, Cooke M, Krishnankutty SM, Asih P, Mueller JD, Kahindi S, et al. Molecular Characterization Reveals Diverse and Unknown Malaria Vectors in the Western Kenyan Highlands. Am J Trop Med Hyg. 2016;94(2):327–35. Epub 20160119. doi: 10.4269/ajtmh.15-0562 ; PubMed Central PMCID: PMC4751935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toma T, Miyagi I, Crabtree MB, Miller BR. Identification of Culex vishnui subgroup (Diptera: Culicidae) mosquitoes from the Ryukyu Archipelago, Japan: development of a species-diagnostic polymerase chain reaction assay based on sequence variation in ribosomal DNA spacers. J Med Entomol. 2000;37(4):554–8. Epub 2000/08/01. doi: 10.1603/0022-2585-37.4.554 . [DOI] [PubMed] [Google Scholar]
- 25.Roiz D, Ruiz S, Soriguer R, Figuerola J. Climatic effects on mosquito abundance in Mediterranean wetlands. Parasit Vectors. 2014;7:333. Epub 2014/07/18. doi: 10.1186/1756-3305-7-333 ; PubMed Central PMCID: PMC4223583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratnasingham S, Hebert PDN. BOLD: The Barcode of Life Data System: Barcoding. Mol Ecol Note. 2007;7(3):355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilai P, Namgay R, Made Ali RS, Saingamsook J, Saeung A, Junkum A, et al. A Multiplex PCR Based on Mitochondrial COI Sequences for Identification of Members of the Anopheles barbirostris Complex (Diptera: Culicidae) in Thailand and Other Countries in the Region. Insects. 2020;11(7). Epub 2020/07/08. doi: 10.3390/insects11070409 ; PubMed Central PMCID: PMC7412068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brosseau L, Udom C, Sukkanon C, Chareonviriyaphap T, Bangs MJ, Saeung A, et al. A multiplex PCR assay for the identification of five species of the Anopheles barbirostris complex in Thailand. Parasit Vectors. 2019;12(1):223. Epub 2019/05/16. doi: 10.1186/s13071-019-3494-8 ; PubMed Central PMCID: PMC6515612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan A, Chiang LP, Hapuarachchi HC, Tan CH, Pang SC, Lee R, et al. DNA barcoding: complementing morphological identification of mosquito species in Singapore. Parasit Vectors. 2014;7:569. Epub 2014/12/17. doi: 10.1186/s13071-014-0569-4 ; PubMed Central PMCID: PMC4282734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen YY, Chen X, Murphy RW. Assessing DNA barcoding as a tool for species identification and data quality control. PLoS One. 2013;8(2):e57125. Epub 2013/02/23. doi: 10.1371/journal.pone.0057125 ; PubMed Central PMCID: PMC3576373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weeraratne TC, Surendran SN, Parakrama Karunaratne SHP. DNA barcoding of morphologically characterized mosquitoes belonging to the subfamily Culicinae from Sri Lanka. Parasit Vectors. 2018;11(1):266. Epub 2018/04/27. doi: 10.1186/s13071-018-2810-z ; PubMed Central PMCID: PMC5918568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Astuti EP, Ipa M, Wahono T, Ruliansyah A, Hakim L, Dhewantara PW. The Distribution of Culex spp (Diptera: Culicidae) in Selected Endemic Lymphatic Filariasis Villages in Bandung District West Java Indonesia. Aspirator 2017;9(2). [Google Scholar]
- 33.Garjito TA, Anggraeni YM, Alfiah S, Satoto TB, Farchanny A, Samaan G, Afelt A, Manguin S, Frutos R, Aditama TY. Japanese encephalitis in Indonesia: An update on epidemiology and transmission ecology. Acta tropica. 2018. Nov 1;187:240–7. doi: 10.1016/j.actatropica.2018.08.017 [DOI] [PubMed] [Google Scholar]
- 34.Lindahl J, Chirico J, Boqvist S, Thu HTV, Magnusson U. Occurrence of Japanese encephalitis virus mosquito vectors in relation to urban pig holdings. Am J Trop Med Hyg. 2012;87(6):1076–82. Epub 2012/10/04. doi: 10.4269/ajtmh.2012.12-0315 ; PubMed Central PMCID: PMC3516078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor B, Jones MD. The circadian rhythm of flight activity in the mosquito Aedes aegypti (L.). The phase-setting effects of light-on and light-off. J Exp Biol. 1969;51(1):59–70. Epub 1969/08/01. doi: 10.1242/jeb.51.1.59 . [DOI] [PubMed] [Google Scholar]
- 36.Pandian RS. Circadian rhythm in the biting behaviour of a mosquito Armigeres subalbatus (Coquillett). Indian J Exp Biol. 1994;32(4):256–60. Epub 1994/04/01. . [PubMed] [Google Scholar]
- 37.Pandian RS, Chandrashekaran MK. Rhythms in the biting behaviour of a mosquito Armigeres subalbatus. Oecologia. 1980;47(1):89–95. Epub 1980/01/01. doi: 10.1007/BF00541780 . [DOI] [PubMed] [Google Scholar]
- 38.Barbara KA, Sukowati S, Rusmiarto S, Susapto D, Bangs MJ, Kinzer MH. Survey of Anopheles mosquitoes (Diptera:Culicidae) in West Sumba District, Indonesia. Southeast Asian J Trop Med Public Health. 2011;42(1):71–82. Epub 2011/02/18. . [PubMed] [Google Scholar]
- 39.Syafruddin D, Lestari YE, Permana DH, Asih PBS, St Laurent B, Zubaidah S, et al. Anopheles sundaicus complex and the presence of Anopheles epiroticus in Indonesia. PLoS Negl Trop Dis. 2020;14(7):e0008385. Epub 2020/07/03. doi: 10.1371/journal.pntd.0008385 ; PubMed Central PMCID: PMC7363104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenea O, Balkew M, Gebre-Michael T. Environmental factors associated with larval habitats of anopheline mosquitoes (Diptera: Culicidae) in irrigation and major drainage areas in the middle course of the Rift Valley, central Ethiopia. J Vector Borne Dis. 2011;48(2):85–92. Epub 2011/07/01. . [PubMed] [Google Scholar]
- 41.Asih PB, Dewi RM, Tuti S, Sadikin M, Sumarto W, Sinaga B, et al. Efficacy of artemisinin-based combination therapy for treatment of persons with uncomplicated Plasmodium falciparum malaria in West Sumba District, East Nusa Tenggara Province, Indonesia, and genotypic profiles of the parasite. Am J Trop Med Hyg. 2009;80(6):914–8. Epub 2009/05/30. . [PubMed] [Google Scholar]
- 42.Nixon CP, Nixon CE, Arsyad DS, Chand K, Yudhaputri FA, Sumarto W, et al. Distance to Anopheles sundaicus larval habitats dominant among risk factors for parasitemia in meso-endemic Southwest Sumba, Indonesia. Pathog Glob Health. 2014;108(8):369–80. Epub 2014/12/17. doi: 10.1179/2047773214Y.0000000167 ; PubMed Central PMCID: PMC4394670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawada H, Nakazawa S, Shimabukuro K, Ohashi K, Kambewa EA, Foster Pemba D. Effect of Metofluthrin-Impregnated Spatial Repellent Devices Combined with New Long-Lasting Insecticidal Nets on Pyrethroid-Resistant Malaria Vectors and Malaria Prevalence: Field Trial in South-Eastern Malawi. Jpn J Infect Dis. 2020;73(2):124–31. Epub 2019/11/02. doi: 10.7883/yoken.JJID.2019.311 . [DOI] [PubMed] [Google Scholar]
- 44.Abong’o B, Yu X, Donnelly MJ, Geier M, Gibson G, Gimnig J, et al. Host Decoy Trap (HDT) with cattle odour is highly effective for collection of exophagic malaria vectors. Parasit Vectors. 2018;11(1):533. Epub 2018/10/16. doi: 10.1186/s13071-018-3099-7 ; PubMed Central PMCID: PMC6191991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Killeen GF, Govella NJ, Lwetoijera DW, Okumu FO. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar J. 2016;15:225. Epub 2016/04/21. doi: 10.1186/s12936-016-1280-z ; PubMed Central PMCID: PMC4837512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell TL, Beebe NW, Bugoro H, Apairamo A, Chow WK, Cooper RD, et al. Frequent blood feeding enables insecticide-treated nets to reduce transmission by mosquitoes that bite predominately outdoors. Malar J. 2016;15:156. Epub 2016/03/13. doi: 10.1186/s12936-016-1195-8 ; PubMed Central PMCID: PMC4788858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aliota MT, Fuchs JF, Rocheleau TA, Clark AK, Hillyer JF, Chen CC, et al. Mosquito transcriptome profiles and filarial worm susceptibility in Armigeres subalbatus. PLoS Negl Trop Dis. 2010;4(4):e666. Epub 2010/04/28. doi: 10.1371/journal.pntd.0000666 ; PubMed Central PMCID: PMC2857672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira-de-Lima VH, Lima-Camara TN. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: a systematic review. Parasit Vectors. 2018;11(1):77. Epub 2018/02/03. doi: 10.1186/s13071-018-2643-9 ; PubMed Central PMCID: PMC5793400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Liang G. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther Clin Risk Manag. 2015;11:435–48. Epub 2015/04/08. doi: 10.2147/TCRM.S51168 ; PubMed Central PMCID: PMC4373597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gopalakrishnan R, Baruah I, Veer V. Monitoring of malaria, Japanese encephalitis and filariasis vectors. medical journal armed forces india. 2014. Apr 1;70(2):129–33. doi: 10.1016/j.mjafi.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ridha MR. Bionomik Mansonia uniformis dan Mansonia dives sebagai Vektor Filariasis pada Beberapa Wilayah di Kalimantan. Balaba: Jurnal LITBANG Pengendalian Penyakit Bersumber Binatang Banjarnegara. 2018:63–70. [Google Scholar]
- 52.Yunarko R, Patanduk Y. Distribusi Filariasis Brugia Timori dan Wuchereria Bancrofti di Desa Kahale, Kecamatan Kodi Balaghar, Kabupaten Sumba Barat Daya, Nusa Tenggara Timur. Balaba: Jurnal LITBANG Pengendalian Penyakit Bersumber Binatang Banjarnegara. 2016;12 (2):89–98. [Google Scholar]
- 53.Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, et al. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am J Trop Med Hyg. 2013;88(2):301–8. Epub 2012/12/20. doi: 10.4269/ajtmh.2012.12-0209 ; PubMed Central PMCID: PMC3583321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wotodjo AN, Trape JF, Richard V, Doucouré S, Diagne N, Tall A, et al. No difference in the incidence of malaria in human-landing mosquito catch collectors and non-collectors in a Senegalese village with endemic malaria. PLoS One. 2015;10(5):e0126187. Epub 2015/05/13. doi: 10.1371/journal.pone.0126187 ; PubMed Central PMCID: PMC4428811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vantaux A, Riehle MM, Piv E, Farley EJ, Chy S, Kim S, et al. Anopheles ecology, genetics and malaria transmission in northern Cambodia. Sci Rep. 2021;11(1):6458. Epub 2021/03/21. doi: 10.1038/s41598-021-85628-1 ; PubMed Central PMCID: PMC7979810. [DOI] [PMC free article] [PubMed] [Google Scholar]