Abstract
Purpose:
GEP-NENs are rare malignancies with increasing incidence. Their molecular characteristics are still undefined. We explored the underlying biology of GEP-NENs and the differences between gastrointestinal (GI) and pancreatic (PNET), high grade (HG) and low grade (LG) tumors.
Experimental Design:
GEP-NENs were analyzed using next-generation sequencing (NGS; MiSeq on 47 genes, NextSeq on 592 genes), immunohistochemistry, and in-situ hybridization. Tumor mutational burden (TMB) was calculated based on somatic nonsynonymous missense mutations, and microsatellite instability (MSI) was evaluated by NGS of known MSI loci.
Results:
In total, 724 GEP-NENs were examined: GI (N=469), PNEN (N=255), HG (N=135), and LG (N=335). Forty-nine% were female, median age was 59. Among LG tumors, the most frequently mutated genes were ATRX (13%), ARID1A (10%) and MEN1 (10%). HG tumors showed TP53 (51%), KRAS (30%), APC (27%), ARID1A (23%). Immune-related biomarkers yielded a lower prevalence in LG tumors compared to HG: MSI-H 0% vs 4% (P=.04), PD-L1 overexpression 1% vs 6% (P=.03) TMB-high 1% vs 7% (P=.05). Compared to LG, HG NETs showed a higher mutation rate in BRAF (5.4% v 0%, P<.0001), KRAS (29.4% v 2.6%, P<.0001) and PI3KCA (7% v 0.3%, P<.0001). When compared to GI, PNEN carried higher frequency of MEN1 (25.9% v 0.0%, P<.0001), FOXO3 (8.6% v 0.8%, P=.005), ATRX (20.6% v 2.0%, P=.007), and TSC2 (6.3% vs 0.0%, P=.007), but lower frequency of mutations in APC (1.0% v 13.8%, P<.0001).
Conclusions:
Significant molecular differences were observed in GEP-NENs by tumor location and grade, indicating differences in carcinogenic pathways and biology.
Keywords: neuroendocrine, gastrointestinal, pancreatic, tumor mutational burden, microsatellite instability
INTRODUCTION
Neuroendocrine neoplasms (NENs) comprise a heterogeneous group of tumors [1]. Pancreatic and gastrointestinal tract (GEP)-NENs are rare diseases but the incidence and prevalence are increasing, likely owing to improvements in detection and diagnosis [2]. Molecular data which may explain their clinical heterogeneity, from indolent to highly aggressive, and divergent treatment responses, are lacking [3].
In the era of precision medicine where elucidating the molecular pathways to carcinogenesis could guide targeted therapies development [4], the pathogenesis of GEP-NENs is largely unknown and only a few studies have attempted to characterize the molecular features of this group of diseases [5]. In addition, they may be part of the spectrum of some hereditary syndromes such as MEN1, Familial Adenomatous Polyposis (FAP) or Lynch Syndrome. Previous reports on molecular characterization of GEP-NENs have been conducted on cohorts with small sample sizes (N<160) [6–11]. However, a recent study has demonstrated that small intestinal neuroendocrine tumors (NETs) can be classified into three groups based on molecular profiling, with different survival outcomes after resection of the primary tumor [12]. This suggests that novel molecular profiling may be useful in the clinical setting to facilitate personalized management and improve prognostic classification for patients [13–15].
Immune checkpoint inhibitors have dramatically changed the standard of care in many types of cancers and PD-L1 expression has proven to be a positive predictive biomarker of response in many different tumor types [16]. In metastatic GEP-NENs the expression of PD-L1 is associated with higher WHO tumor grade (G3), and has both predictive and prognostic value for survival of patients [17]. These data deserve further validation in order to better understand if patients with GEP-NENs may benefit from this treatment strategy and whether MSI-H status predicts Lynch Syndrome.
Compelling evidences suggests that tumor mutational burden (TMB) may be a useful biomarker to select patients who could respond to immunotherapy, independently from the microsatellite instability (MSI) status of the tumor. However, little is known about the prevalence of TMB in GEP-NENs.
The incorporation of signaling, metabolic and molecular information to improve the classification of GEP-NENs might enable the rational design of clinical trials in order to exploit the efficacy of specific agents, according to the precision medicine paradigm. Additionally, molecular profiling is anticipated to improve prognostication and treatment selection, inform patient follow up, and enhance patient outcomes [18]. Moreover, these data may have direct implications for germline testing: the diagnosis of hereditary syndromes is crucial to prevent second tumors in patients and also because specific surveillance programs may also prevent cancer deaths in their relatives.
As recently shown for pancreatic neuroendocrine tumors (PanNENs) [19], a comprehensive molecular analysis can identify several novel candidate carcinogenetic mechanisms which may be used to develop new biomarkers and targeted therapies.
Based on these data, we can hypothesize that a deep molecular profiling of GEP-NENs will provide new insights into biology of these rare diseases, which may lay the bases to a new molecular-based classification of these tumors, biomarker-driven clinical trial design and novel targeted agents development.
Methods
A cohort of 724 GEP-NENs that underwent comprehensive genomic profiling by Caris Life Sciences (Phoenix, AZ) were identified from a retrospective database from February 2013 to December 2017. All samples were analyzed as part of standard of care (SOC). Molecular characteristics, microsatellite instability (MSI) status, TMB, as well as protein expression by immunohistochemistry (IHC) were analyzed for differences based on the tumor’s primary location (GI vs pancreas) and grade (HG vs LG). Tumors were selected from our database based on their pathology report: the histologic diagnosis and accompanying diagnostic immunohistochemical workup performed at the referring pathology laboratories were reviewed in all cases by a board-certified pathologist at Caris Life Sciences. Specimens included were taken from any biopsy site including both local lesions or metastatic deposits. Tumors were included if the primary location was noted to be from the GI tract (including esophageal/gastroesophageal junction [GEJ], gastric, duodenum, small intestine, large intestine, colon, pancreas, or biliary tract; unknown primary site cases were excluded). In order to limit samples with a mixed histology, cases that had terms that would suggest a mixed histology such as “adenocarinoma” or “carcinoma, NOS” were excluded. Grading was determined based on terminologies included in the pathology notes that accompanied each specimen upon arrival at Caris Life Sciences: a) high-grade cohort included samples with any of the following terms found in the available clinical information (in either the diagnosis or histology fields): poorly differentiated, grade 3, G3, high-grade, small cell carcinoma, large cell carcinoma; while B) the low grade cohort included the following terms: well differentiated, moderately differentiated, moderately well differentiated, Grade 1, Grade 2, intermediate grade, low grade.
Immunohistochemistry (IHC)
IHC was performed on FFPE sections. Protein staining was scored for intensity (0 = no staining; 1+ = weak staining; 2+ = moderate staining; 3+ = strong staining) and staining percentage (0–100%) by pathologists. PD-L1 testing was performed using the SP142 anti-PD-L1 clone (Ventana, Tucson, AZ).
Next-generation sequencing (NGS)
NGS was performed in a CAP/CLIA/ISO-certified commercial laboratory on genomic DNA isolated from FFPE tumor samples using the NextSeq platform (Illumina, Inc., San Diego, CA.). A custom-designed SureSelect XT assay was used to enrich 592 whole-gene targets or 44-gene oncogenic hot-spot targets (Agilent Technologies). All variants were detected with >99% confidence based on allele frequency and amplicon coverage, with an average sequencing depth of coverage of 750 and an analytic sensitivity of 5%. Prior to molecular testing, tumor enrichment was achieved by harvesting targeted tissue using manual microdissection techniques. Genetic variants identified were interpreted by board-certified molecular geneticists and categorized as “pathogenic,” “presumed pathogenic,” “variant of unknown significance,” “presumed benign,” or “benign,” according to the American College of Medical Genetics and Genomics (ACMG) standards. When assessing mutation frequencies of individual genes, “pathogenic,” and “presumed pathogenic” were counted as mutations, whereas “benign”, “presumed benign” variants, and “variants of unknown significance” were excluded.
Microsatellite instability (MSI)
MSI was examined by counting number of microsatellite loci that were altered by somatic insertion or deletion for each sample. The threshold to determine MSI by NGS was determined to be 46 or more loci with insertions or deletions to generate a sensitivity of > 95% and specificity of > 99%.
Tumor mutational burden (TMB)
TMB was measured by counting all nonsynonymous missense mutations found per tumor that had not been described previously as germline alterations [592 genes and 1.4 megabases (MB) sequenced/tumor]. Potential germline mutations are excluded by comparing data against dbSNP 137 full and 1000 Genomes Phase 3. The threshold to define TMB-high (TMB-H) was greater than or equal to 17 mutations/MB and was established by comparing TMB with MSI by fragment analysis in colorectal cancer cases, based on reports of TMB having high concordance with MSI-H in colorectal cancer. Differences in mean TMB was assessed using Student’s t-test.
Statistical Analysis
Chi-square test was performed for comparative analysis using SPSS v23 (IBM SPSS Statistics), and a statistical significance was defined as p-value < 0.05.
Ethics Statement
All human subjects’ data were de-identified prior to analysis. Thus, this research was determined to be exempt from the requirement for informed consent per the Western Institutional Review Board (WIRB).
RESULTS
Patients and tumors characteristics
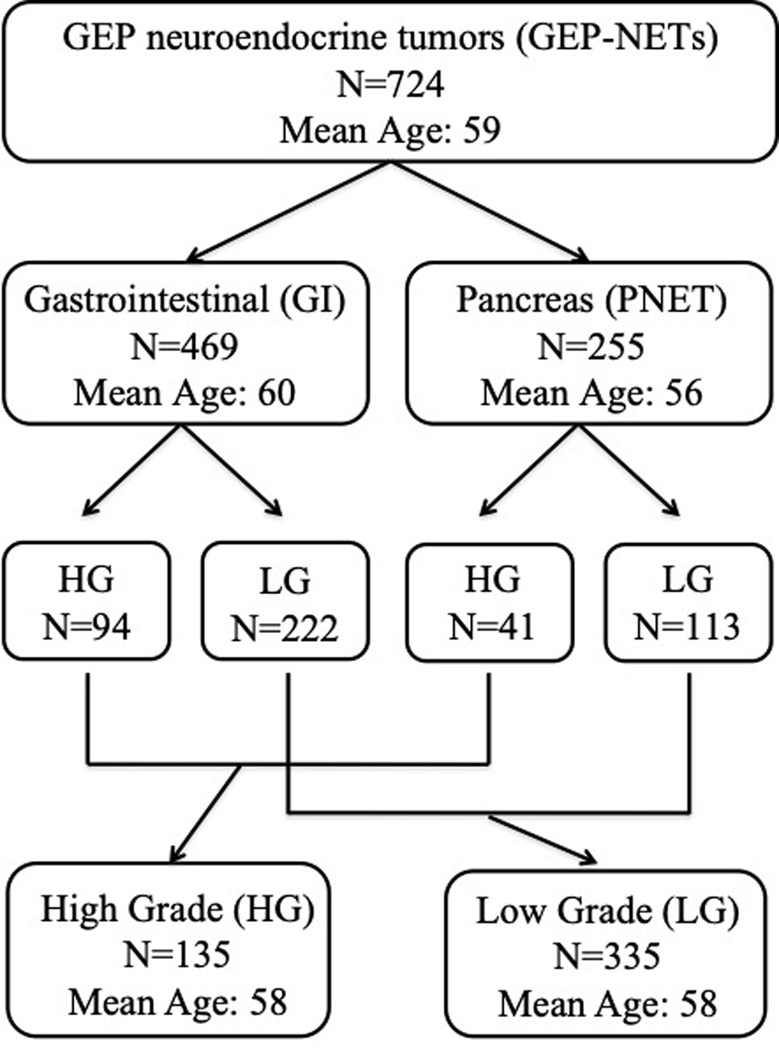
The analyzed cohort consisted of 724 GEP-NENs; mean age of the patients was 59 [range 19–90], 49% were female [N=358] and 51% were male [N=366]. The tumor primary site was in the gastrointestinal tract (GI) in 469 patients (64%), and in the pancreas in 255 patients (36%). Age was significantly higher in GI-NENs compared to PNENs (mean of 59.7 vs 56.8; p < 0.001). Tumor grading was available for 470 out of 724 samples; in the cohorts 135 high grade tumors were identified: 94 in GI-NENs and 41 in PNENs. The remaining 335 samples were classified as low grade: 222 GI-NETs and 113 PNET. (Figure 1)
Figure 1 –
Patient Demographics. NEN from GI (N = 469), PNEN (N = 255), HG (N = 135), and LG (N = 335). Female to male ratio was 49%/51% and the median age was 59.
Molecular landscape of GEP-NENs
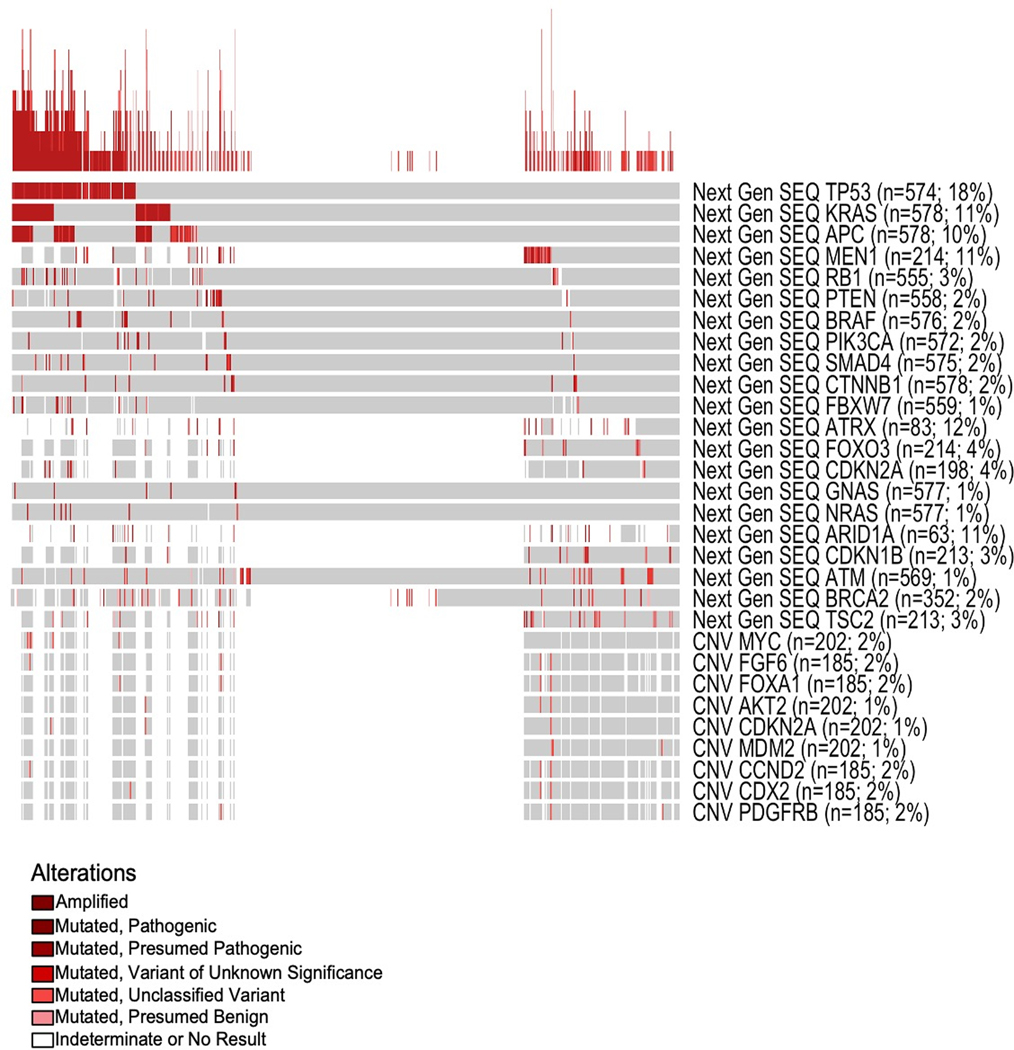
Across the whole cohort the most frequently mutated genes, identified by mean of NGS, were: TP53 (n=574, 18%), ATRX (N=83, 12%), KRAS (n=578, 11%), MEN1 (n=214, 11%) ARID1A (n=63, 11%) and APC (n=578, 10%). Less frequently mutated genes included CDKN2A (n=198, 4%), RB1 (n=555, 3%), BRAF (n=576, 2%), PIK3CA (n=572, 2%) and BRCA2 (n= 352, 2%) (Figure 2). Amplifications events, defined by copy number variation (CNV) were rare; the most frequently amplified genes were MYC (n=202, 2%), FGF6 (n=185, 2%), CCND2 (n=2%) and FOXA1 (n=185, 2%).
Figure 2. Oncoprint.
Comprehensive molecular profile of 724 GEP-NENs. Overall, the most frequently mutated genes were TP53 (18%), ATRX (12%), KRAS (11%), MEN1 (11%), ARID1A (11%), and APC (10%). Gene amplification events, as determined by NGS, were rare across the cohort. The most frequently amplified genes are MYC (2%), FGF6 (2%), CCND2 (2%), and FOXA1 (2%).
Each column is a single patient; gray boxes are those in which no alteration was detected. Samples were arranged by those that harbored a mutation in the genes listed as rows top to bottom (those with TP53 mutation show up on left, then by KRAS mt, then by APC, etc). The stacked graph at the top is a representation of the number of alterations that case had (the higher the line the more alterations). Mutation frequencies were determined on a per gene level excluding cases from that analysis where a particular gene was determined to be indeterminate. The variation of sample size per gene comes from the fact that not all genes within a single case are evaluable.
Immunohistochemistry staining was used to evaluate several biomarkers; the most frequently identified were: TUBB3 expression (n=547, 68%), MGMT methylation (n=595, 37%), TOP2A expression (n= 660, 36%), PGP expression (n=380,20%), PR expression (n=402;15%), EGFR expression (n=165, 12%) and ER expression (n=403,9%).
Less frequently observed were ALK translocations by immunohistochemistry (n=97, 5%) and cMET expression (n=391, 3%). ALK fusions were in frame and potentially targetable via tyrosine kinase inhibitors (TKIs) treatment. No NTRK 1/2/3 fusion event was observed.
Given the molecular landscape of the whole GEP-NENs cohort statistical analysis where performed to assess if grading and tumor primary sites are correlated with significant molecular differences.
Molecular differences between GI vs PNEN
Compared to GI, PNEN carried a significant higher frequency of tumor mutation in MEN1 (25.9 vs 0%, p<0.001), FOXO3 (8.6 vs 0.8%, p<0.005), ATRX (20.6 vs 2%, p=0.007), TSC2 (6.3 vs 0%, p=0.007) but lower frequency in APC mutations (1 vs 13.8%, p<0.001).
PNEN also showed a higher expression of PR (38.8 vs 3.6%, p<0.001) but a lower expression of ER (2.4 vs 12.4%, p=0.001) and of MGMT methylation (31.7 vs 40.7%, p=0.038) (Figure 3). Within the GI cohort, we investigated the molecular differences between upper vs lower GI: upper GI compared to lower showed a higher rate of BRCA2 (7.5 vs 0%, P<.0001), TP53 (33 vs 16%, P=.002), and CTNNB1 mutations (4.7 vs 0%, P<.0001), and lower rate of APC (4.7 vs 16%, P=.018).
Figure 3. Molecular differences between PNEN and GI-NEN.
Compared to GI, PNEN carried significantly higher frequency of MEN1 (25.9% vs 0.0%), FOXO3 (8.6% vs 0.8%), ATRX (20.6% vs 2.0%), and TSC2 (6.3% vs 0.0%), but lower frequency of mutations in APC (1.0% vs 13.8%). PNEN had a significantly higher expression of PR (38.9% vs 3.7%), but interestingly, GI-NEN had higher expression levels of ER (12.5% vs 2.4%).
Molecular differences between GI vs PNET among low grade (LG) tumors.
LG-PNET carried significantly higher frequency of MEN1 (24.3% vs 0%, p<0.001), ATRX (33.3% vs 0%, p=0.001), FOXO3 (12.2 vs 0%, p=0.005) and PTEN (p=0.069) mutations. Conversely, LG GI-NET had a higher, but not significant, mutation rate in APC (1.6% vs 0%, p=0.211). LG-NETs also carried a different pattern of ER and PR expression: LG-PNETs carried a higher expression of PR (56.6% vs 3%, p<0.001) and a lower expression of ER (0 vs 17%, p<0.001) when compared to LG GI-NETs. These results are similar to those of the whole cohort, but with higher percentage of ER and PR expression (Figure 4).
Figure 4. Differences in LG PNET and LG GI-NET.
Molecular differences between LG PNET and LG GI-NET. Compared to LG GI-NET, LG PNET carried significantly higher frequency of MEN1 (24.3% vs 0.0%), ATRX (33.3% vs 0.0%), and FOXO3 (12.2% vs 0.0%). LG GI-NET had a higher mutation rate in APC (1.6% vs 0.0%) and CDKNB1 (4.9% vs 2.5%), although neither was significant. Molecular profiles of LG tumors were similar to those of the entire cohort for each primary location.
Molecular differences between low grade vs high grade GEP-NEN
Tumor grade was associated with significant molecular differences irrespective of the different tumor site. HG GEP-NENs carried a higher frequency of TP53 (51% vs 3.4%, p<0.001), KRAS (29.4% vs 2.6%, p<0.001), APC (27% vs 1.69%, p<0.001), RB1 (11% vs 0%, p<0.001), BRAF (5.4% vs 0%, p<0.001) and PI3KCA (7 vs 0.3%, p<0.001) mutations. On the other hand, LG GEP-NENs showed a higher frequency of MEN1 alterations (9.8% vs 0%, p=0.032), other frequently mutated genes were ATRX (13%), ARID1A (10%). LG GEP-NENs also showed a higher expression of ER (17.9% vs 0, p=0.01) and PR (19.2% vs 7.6%, p=0.029) (Figure 5a).
Figure 5.
Figure 5a – Molecular differences between HG and LG GEP-NEN. Among LG tumors, the most frequently mutated genes were ATRX (13%), ARID1A (10%) and MEN1 (10%). Among HG, TP53 (51%), KRAS (30%), APC (27%), ARID1A (23%) and RB1 (11%). Compared to LG, HG NENs showed a higher mutation rate in BRAF (5.4% vs 0%), KRAS (29.4% v 2.6%) and PI3KCA (7% vs 0.3%), among others.
5b – Differences in immune markers between HG vs LG GEP-NEN. Immune-related biomarkers showed lower prevalence in LG tumors compared to HG: lower mean TML (5.1 mut/MB vs 9.5, P < .0001), MSI-H 0% vs 4% (P = .04), PD-L1 expression 1% vs 6% (P = .03).
Immune-related biomarkers
Immune-related biomarkers were evaluated between HG vs LG GEP-NENs: The cohort of patients with HG-NENs, irrespective of tumor site, had a higher rate of expression of PD-L1 (6% vs 1%, p=0.03), higher mean TMB (9.5 mut/MB vs 5.1, p<0.0001) and higher MSI-H status (4% vs 0%, p=0.04). (Figure 5b)
DISCUSSION
This is one of the largest cohorts of GEP-NENs underwent extensive molecular analyses using NGS. Because of the rarity and heterogeneity of NENs, treatment options had been slow until recent years. Lately, there have been advances both in the characterization of the disease and in the available therapeutic strategies: TKIs, somatostatin analogue (SSA) therapy, mTOR inhibitors, chemotherapy and Peptide Receptor Radionuclide Therapy (PRRT) have substantially improved the management in the advanced disease setting [20]. Nonetheless, treatment decisions remain largely based on tumor stage and grade, despite the observation of significant heterogeneity in tumor biology. There is a well-acknowledged unmet clinical need for novel biomarkers to enable individualized therapeutic strategies [21]. Thus, better understanding of the underlying biology is paramount.
GEP-NENs include two genetically different entities: well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NEC) [22]. In addition, well-differentiated NETs may be high grade (G3 defined as having a mitotic rate >20 per 2 mm2 or Ki67 >20%), but these neoplasms remain well-differentiated genetically and distinct from poorly differentiated NECs. Mutations in MEN1, DAXX and ATRX are entity-defining for well-differentiated NETs, whereas NECs usually have TP53 or RB1 mutations [22]. According to these data, in our cohort, TP53 and RB1 as well as KRAS, APC, BRAF and PI3KCA were frequently mutated in HG but not in LG. On the other hand, LG tumors harbored mutations in MEN1 and ATRX. This underlines the reliability and robustness of our results and the applicability to the most updated classification of NENs to our cohort: the group defined LG may represent the NETs and the HG group may account for NECs. We recognize as a limitation that within NETS, we are not able to distinguish between G1-G2 vs. G3; nor could we distinguish between G3 NET vs G3 NEC according to the novel nomenclature of NENs [23].
GEP-NENs are usually divided in gastrointestinal (GI) and pancreatic (P) NENs because of the different clinical behavior, biology and treatment strategies associated with the two groups of tumors. According to this, our data show that GI and PNET harbor a different molecular profile. Among the LG cohort, PNET carried mutations in MEN1, ATRX, FOXO3 and PTEN which were not found in GI NETs. On the other hand, GI NETs harbored mutations in APC which was not present in PNET. These findings corroborate the fact that these tumors are completely different entities for which different therapeutic approaches are needed, as well as different hereditary syndromes may be predicted
We showed that HG GEP-NENs carried a higher frequency of TP53, KRAS, APC, RB1, BRAF and PI3KCA mutations compared to LG GEP-NENs. According to this, NECs commonly have mutations in TP53 and RB1 and may share mutations in KRAS and SMAD4, genes commonly involved in the pathogenesis of adenocarcinomas, as it has been shown in other series [24–26]. Usually NECs show poor prognosis and platinum-based chemotherapy regimens represent the only treatment usually proposed for these patients. However, considering of the lack of tailored treatments for NECs patients, and of the heterogeneity of response rate to standard chemotherapy, novel potential therapeutic targets may pave the way for more personalized treatment strategies [27].
To date, immunotherapy approaches have led to disappointing results in GEP-NENs. Monotherapy with anti-PD1 (e.g. pembrolizumab) showed no signs of activity in these patients [28–32]. Here, we observed higher expression of PD-L1, higher mean TMB, and higher MSI-H status in high-grade NENs, compared to low-grade, regardless of the site of tumor origin. These findings may help to explain why patients with high-grade tumors benefit more from immunotherapy combination that those with low-grade tumors. Ipilimumab plus nivolumab demonstrated a 44% overall response rate (ORR) in patients with non-pancreatic high-grade neuroendocrine carcinoma, with 0% ORR in low/intermediate grade disease [33]. These results are encouraging and may potentially lead to a novel treatment strategy for high grade NEN patients. However, caution should be taken when interpreting these data since the DART SWOG 1609 study was a prospective, open-label, multicenter phase II clinical trial of ipilimumab plus nivolumab across multiple rare tumor cohorts, reporting results from 32 enrolled patients with the (nonpancreatic) neuroendocrine tumors [33]. Thus, several additional studies are warranted to investigate the molecular biology and eventually predictive biomarkers for immunotherapy in GEP-NENs patients.
We observed that PNET carried a higher expression of PR and a lower expression of ER when compared to GI-NETs, especially in low grade tumors. This is in accordance with other previous studies [34] showing that PR expression might also be a prognostic marker in patients with PNET [35, 36]. Although we could not confirm the prognostic role of PR expression, we observed that high grade tumors, which have a worse prognosis, also express lower levels of PR and ER. Hormone receptor (HR) positivity is used as predictive biomarker for endocrine therapy in breast and other cancers, thus our findings may lead to a novel treatment strategy for GEP-NETs patients. In fact, a single-arm phase II trial (NCT03870399) is currently evaluating the effect of tamoxifen in patients with well differentiated NET and hormone receptor positive expression.
We acknowledge that there are several limitations to our study including the retrospective nature of the analysis, the absence of clinical data correlating our findings to outcomes, the heterogeneity of the study population unselected for tumor stage and the difficulties in grouping samples based on pathology reports and different nomenclatures. Especially regarding nomenclature, as stated above, we were not able to distinguish G3 NET from G3 NEC, which are considered two entities both genetically and clinically, thus further studies to distinguish these populations of patients are warranted.
Studies on biologically targeted therapies in GEP-NETs have, to date, focused primarily on inhibitors of VEGF or mTOR signaling pathways, even though the continuous discoveries in molecular pathways involved in tumor genesis and metastatization are paving the way to the introduction of new drugs [20]. Recently, encouraging results have been shown in patients with NTRK fusion-positive solid tumors, included in neuroendocrine tumors, treated with tropomyosin receptor kinase (TRK) inhibitors [37, 38]. Notably no cases of NTRK 1/2/3 fusion were detected in the patients evaluated in this study.
Finally, we reported that up to 25% of PNEN harbor mutations in MEN1, which is similar to previous reports [15, 19, 39], strengthening our data. However, data on germline mutations are not available, therefore we could not evaluate the rate of germline mutations compared to somatic. However, we underline the importance of genetic testing and counseling in these patients because of the crucial clinical implications in case of hereditary syndrome diagnosis.
The demand of patients for a personalized management and therapy represent a challenging and compelling task for NEN oncologists and scientists. Therefore, most attention and efforts are needed in linking management and therapy to molecular profiling.
CONCLUSION
Our findings demonstrated that several molecular differences are present based on tumor location and grade in a large cohort of GEP-NENs. Additionally, immune-related biomarkers showed lower prevalence in low grade compared to high grade tumors.
Based on these data, we can hypothesize that a deep molecular profiling of GEP-NENs provide new insights, which may lay the basis to a new molecular-based classification of these tumors, biomarker-driven clinical trial design and novel targeted agents development, together with hereditary syndrome prediction.
STATEMENT OF TRANSLATIONAL RELEVANCE.
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are rare diseases but the incidence and prevalence are increasing. Molecular data which may explain their clinical heterogeneity, from indolent to highly aggressive, and divergent treatment responses, are lacking. Here, we present data from one of the largest cohorts of GEP-NENs underwent extensive molecular analyses using NGS. We show that significant molecular differences are present in GEP-NENs by tumor location and grade. Moreover, we provide novel insights, into several mutations in targetable genes which may pave the way to novel therapeutic options. Our results suggest that deep molecular profiling of GEP-NENs is paramount to molecular-based classification of these tumors, biomarker-driven clinical trial design and novel targeted agents development, together with hereditary syndrome prediction.
Acknowledgments
Financial support:
The project described was supported in part by award number P30CA014089 from the National Cancer Institute, The Gloria Borges Wunderglo Foundation, Dhont Family Foundation, Daniel Butler Research Fund. Martin D. Berger received a grant from the Swiss Cancer League (BIL KLS-3334-02-2014) and the Werner and Hedy Berger-Janser Foundation for Cancer Research. Ryuma Tokunaga received a grant from the Uehara Memorial Foundation (201630045)
Footnotes
Conflict of interests:
Drs. K. Poorman, J. Xiu and W. M. Korn are employed by Caris Life Sciences. Dr. J. L. Marshall is a consultant for Caris Life Sciences. Drs. A. F. Shields, A. Seeber and R. M. Goldberg received research and travel support from Caris Life Sciences. Drs. M. E. Salem, and H-J. Lenz received travel support from Caris Life Sciences. Drs. A. Puccini, D. Soldato, M.D. Berger, R. Tokunaga, M. Naseem, F. Battaglin, A. Barzi, S. Iqbal, W. Zhang, S. Soni, J.J. Hwang and P.A. Philip and S. Sciallero declare no potential conflicts of interest.
REFERENCES
- 1.Rindi G, Klimstra DS, Abedi-Ardekani B et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018; 31: 1770–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis MA, Yao JC. Molecular pathology and genetics of gastrointestinal neuroendocrine tumours. Curr Opin Endocrinol Diabetes Obes 2014; 21: 22–27. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma MF, Sadanandam A, Tan P, Vermeulen L. Molecular subtypes in cancers of the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 2017; 14: 333–342. [DOI] [PubMed] [Google Scholar]
- 4.El-Deiry WS, Goldberg RM, Lenz HJ et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J Clin 2019; 69: 305–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girardi DM, Silva ACB, Rego JFM et al. Unraveling molecular pathways of poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: A systematic review. Cancer Treat Rev 2017; 56: 28–35. [DOI] [PubMed] [Google Scholar]
- 6.Banck MS, Kanwar R, Kulkarni AA et al. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest 2013; 123: 2502–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis JM, Kiezun A, Ramos AH et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet 2013; 45: 1483–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebauer N, Schmidt-Werthern C, Bernard V et al. Genomic landscape of pancreatic neuroendocrine tumors. World J Gastroenterol 2014; 20: 17498–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salyers WJ, Vega KJ, Munoz JC et al. Neuroendocrine tumors of the gastrointestinal tract: Case reports and literature review. World J Gastrointest Oncol 2014; 6: 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim ST, Lee SJ, Park SH et al. Genomic Profiling of Metastatic Gastroenteropancreatic Neuroendocrine Tumor (GEP-NET) Patients in the Personalized-Medicine Era. J Cancer 2016; 7: 1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijayvergia N, Boland PM, Handorf E et al. Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: a Fox Chase Cancer Center Pilot Study. Br J Cancer 2016; 115: 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karpathakis A, Dibra H, Pipinikas C et al. Prognostic Impact of Novel Molecular Subtypes of Small Intestinal Neuroendocrine Tumor. Clin Cancer Res 2016; 22: 250–258. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez MJ, Subramaniam PS, Tang LH et al. A precision oncology approach to the pharmacological targeting of mechanistic dependencies in neuroendocrine tumors. Nat Genet 2018; 50: 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raj N, Shah R, Stadler Z et al. Real-Time Genomic Characterization of Metastatic Pancreatic Neuroendocrine Tumors Has Prognostic Implications and Identifies Potential Germline Actionability. JCO Precis Oncol 2018; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao Y, Shi C, Edil BH et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011; 331: 1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 2019; 7: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim ST, Ha SY, Lee S et al. The Impact of PD-L1 Expression in Patients with Metastatic GEP-NETs. J Cancer 2016; 7: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidd M, Modlin I, Oberg K. Towards a new classification of gastroenteropancreatic neuroendocrine neoplasms. Nat Rev Clin Oncol 2016; 13: 691–705. [DOI] [PubMed] [Google Scholar]
- 19.Scarpa A, Chang DK, Nones K et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017; 543: 65–71. [DOI] [PubMed] [Google Scholar]
- 20.Camilli M, Papadimitriou K, Nogueira A et al. Molecular profiling of pancreatic neuroendocrine tumors (pNETS) and the clinical potential. Expert Rev Gastroenterol Hepatol 2018; 12: 471–478. [DOI] [PubMed] [Google Scholar]
- 21.Young K, Starling N, Sadanandam A. The molecular biology of pancreatic neuroendocrine neoplasms: Challenges and translational opportunities. Semin Cancer Biol 2019. [DOI] [PubMed] [Google Scholar]
- 22.Nagtegaal ID, Odze RD, Klimstra D et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020; 76: 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavel M, Oberg K, Falconi M et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 24.Jesinghaus M, Konukiewitz B, Keller G et al. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod Pathol 2017; 30: 610–619. [DOI] [PubMed] [Google Scholar]
- 25.Yachida S, Vakiani E, White CM et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2012; 36: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzi S, Azzoni C, Bassi D et al. Genetic alterations in poorly differentiated endocrine carcinomas of the gastrointestinal tract. Cancer 2003; 98: 1273–1282. [DOI] [PubMed] [Google Scholar]
- 27.Busico A, Maisonneuve P, Prinzi N et al. Gastroenteropancreatic High-Grade Neuroendocrine Neoplasms (H-NENs): histology and molecular analysis, two sides of the same coin. Neuroendocrinology 2019. [DOI] [PubMed] [Google Scholar]
- 28.Vijayvergia N, Dasari A, Deng M et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer 2020; 122: 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehnert JM, Rugo HS, O’Neil BH, et al. : Pembrolizumab for patients with PD-L1–positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. 2017 ESMO Congress. Abstract 427O. Presented September 10, 2017. [Google Scholar]
- 30.Strosberg JR, Mizuno N, Doi T, et al. : Pembrolizumab treatment of advanced neuroendocrine tumors: Results from the phase II KEYNOTE-158 study. 2019 Gastrointestinal Cancers Symposium. Abstract 190. Presented January 18, 2019. [Google Scholar]
- 31.Yao JC, Strosberg J, Fazio N, et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Annals of Oncology 2018; 29 (Supplement 8): viii467–viii478, 2018 doi: 10.1093/annonc/mdy293. [DOI] [Google Scholar]
- 32.Mulvey C, Raj NP, Chan JA et al. Phase II study of pembrolizumab-based therapy in previously treated extrapulmonary poorly differentiated neuroendocrine carcinomas: Results of Part A (pembrolizumab alone). Journal of Clinical Oncology 2019; 37: 363–363.30576267 [Google Scholar]
- 33.Patel SP, Othus M, Chae YK et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Non-Pancreatic Neuroendocrine Tumors. Clin Cancer Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmermann N, Lazar-Karsten P, Keck T et al. Expression Pattern of CDX2, Estrogen and Progesterone Receptors in Primary Gastroenteropancreatic Neuroendocrine Tumors and Metastases. Anticancer Res 2016; 36: 921–924. [PubMed] [Google Scholar]
- 35.Estrella JS, Broaddus RR, Mathews A et al. Progesterone receptor and PTEN expression predict survival in patients with low- and intermediate-grade pancreatic neuroendocrine tumors. Arch Pathol Lab Med 2014; 138: 1027–1036. [DOI] [PubMed] [Google Scholar]
- 36.Arnason T, Sapp HL, Barnes PJ et al. Immunohistochemical expression and prognostic value of ER, PR and HER2/neu in pancreatic and small intestinal neuroendocrine tumors. Neuroendocrinology 2011; 93: 249–258. [DOI] [PubMed] [Google Scholar]
- 37.Sigal DS, Bhangoo MS, Hermel JA et al. Comprehensive genomic profiling identifies novel NTRK fusions in neuroendocrine tumors. Oncotarget 2018; 9: 35809–35812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doebele RC, Drilon A, Paz-Ares L et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol 2020; 21: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbo V, Dalai I, Scardoni M et al. MEN1 in pancreatic endocrine tumors: analysis of gene and protein status in 169 sporadic neoplasms reveals alterations in the vast majority of cases. Endocr Relat Cancer 2010; 17: 771–783. [DOI] [PubMed] [Google Scholar]