Abstract
Although substantial progress has been made in the diagnosis and treatment of acute coronary syndromes, cardiovascular disease remains the leading cause of death globally, with nearly half of these deaths due to ischaemic heart disease. The broadening availability of high-sensitivity troponin assays has allowed for rapid rule-out algorithms in patients with suspected non-ST-segment elevated myocardial infarction (NSTEMI). Dual antiplatelet therapy is recommended for 12 months following an acute coronary syndrome in most patients, and additional secondary prevention measures including intensive lipid-lowering therapy (LDL-C <1·4 mmol/L), neurohormonal agents, and lifestyle modification, are crucial. The scientific evidence for diagnosis and management of acute coronary syndromes continues to evolve rapidly, including adapting to the COVID-19 pandemic, which has impacted all aspects of care. This Seminar provides a clinically relevant overview of the pathobiology, diagnosis, and management of acute coronary syndromes, and describes key scientific advances.
Epidemiology
Although substantial progress has been made in the diagnosis and treatment of acute coronary syndromes, cardiovascular disease remains the leading cause of death worldwide, with nearly half of these deaths due to ischaemic heart disease.1, 2 Globally, 12% of disability-adjusted life-years lost annually are attributable to ischaemic heart disease.2, 3, 4 Marked global variation in rates of revascularisation and long-term mortality following acute coronary syndromes exist (panel ).5, 6, 7
Panel. Acute coronary syndromes.
Summary
Cardiovascular disease is the leading cause of death globally, with nearly half of these deaths due to ischaemic heart disease. The proportion of acute coronary syndromes that are ST-segment elevation myocardial infarction (STEMI) is decreasing and the broadening availability of high-sensitivity troponin assays has allowed for so-called rapid rule-out algorithms in patients with suspected non-STEMI (NSTEMI). Dual antiplatelet therapy is recommended for 12 months following acute coronary syndrome in most patients, and additional secondary prevention measures including intensive lipid-lowering therapy, neurohormonal agents, and lifestyle modification, are crucial. Despite important scientific progress in the management of acute coronary syndromes, marked racial and sex-based disparities exist. Furthermore, the burden of cardiovascular disease is extensive in low-income and middle-income countries, with increasing population risk factor exposure and structural impediments to acute coronary syndrome diagnosis and treatment. This Seminar aims to provide a clinically relevant overview of the pathobiology, diagnosis, and management of acute coronary syndromes, and describe major recent scientific advances.
Fast facts
-
•
Cardiovascular disease remains the leading cause of death globally
-
•
The proportion of acute coronary syndromes that are STEMI is decreasing in high-income countries
-
•
Myocardial infarction with no obstructive coronary artery disease and spontaneous coronary artery dissection are two commonly encountered clinical scenarios with evolving evidence bases to guide management
-
•
High-sensitivity troponin (hsTn) assays have allowed for the adoption of rapid rule-out algorithms for NSTEMI, which are now endorsed by major society guidelines
-
•
The timing of invasive coronary angiography in NSTEMI is determined by clinical risk stratification: very high-risk patients (eg, haemodynamic instability, refractory chest discomfort, life-threatening arrhythmia) warrant emergent (<2 h) angiography; patients at high risk (eg, elevated clinical risk score or dynamic ECG changes) should undergo catheterisation within 24 h; and patients at low risk should be submitted to a selective invasive strategy
-
•
In STEMI, emergent revascularisation, ideally by percutaneous coronary intervention when available, is the immediate clinical priority with goal of first medical contact to device time of <60–90 min
-
•
Dual antiplatelet therapy, ideally with ticagrelor or prasugrel in addition to low dose aspirin, is recommended for 12 months following acute coronary syndrome in most patients
-
•
Secondary prevention with intensive lipid-lowering therapy, antithrombotic therapy, neurohormonal agents, and lifestyle modification (including cardiac rehabilitation) is critical after acute coronary syndrome
-
•
Marked racial and sex-based disparities exist in the prevention, diagnosis, and management of acute coronary syndromes
-
•
The burden of cardiovascular disease is extensive in low-income and middle-income countries, with increasing population risk factor exposure, structural impediments to acute coronary syndrome diagnosis and treatment, and high rates of mortality at younger ages
-
•
COVID-19 has complicated diagnosis and treatment of acute coronary syndromes globally, as COVID-19 can cause direct or indirect myocardial inflammation and injury; it predisposes patients to arterial and venous thrombotic events; and it causes societal and health-care system disruptions
The proportion of acute coronary syndromes that are ST-segment elevation myocardial infarction (STEMI) is decreasing in high-income countries (HIC),8 likely in part due to secular trends in patient risk profiles, including declining rates of smoking in western Europe and North America, and in part related to increasingly widespread use of high-sensitivity troponin (hsTn) assays to diagnose non-STEMI (NSTEMI). Nonetheless, rates of in-hospital mortality in patients with STEMI complicated by shock remain high, particularly in the setting of cardiac arrest.9
Pathobiology
At the least severe end of the acute coronary syndrome spectrum is unstable angina, in which clinical symptoms suggest acute coronary syndrome, but there is no biochemical evidence of myocardial infarction. Type 1 myocardial infarction, which is caused by atherothrombotic coronary artery disease, is further classified as NSTEMI or STEMI based on ECG findings and is defined by the 4th Universal Definition of Myocardial Infarction (UDMI) as requiring a rise or fall in cardiac troponin (cTn) level (or another biomarker if cTn is not available), or both, accompanied by clinical evidence of ischaemia (ie, symptoms, ECG changes, supportive ECG or other imaging findings, or evidence of coronary thrombus).10 Beyond atherosclerosis, myocardial injury and infarction can result from numerous other processes, including Takotsubo cardiomyopathy, myocarditis, and supply versus demand mismatch. A framework for defining these events is provided in the 4th UDMI.10 It is important to note that Type 2 myocardial infarction, which results from myocardial oxygen supply versus demand mismatch unrelated to acute atherothrombosis, tends to have a higher mortality rate than Type 1 myocardial infarction.10 Although data are scarce concerning targeted treatments for Type 2 myocardial infarction, intensive lipid-lowering might reduce incidence.11
The previous paradigm of coronary atherosclerotic plaque rupture as the singular cause of STEMI or non-ST-segment elevation acute coronary syndrome (NSTEACS) has been disrupted in recent years, with intracoronary imaging studies showing acute coronary syndrome at times to be caused by plaque erosion rather than rupture, or, less commonly, a calcific nodule leading to thrombus formation.12, 13 Two particular scenarios of interest are: 1) the presence of a clinically diagnosed myocardial infarction with no obstructive coronary artery disease identified on angiography (referred to as MINOCA); and 2) spontaneous coronary artery dissection (SCAD).
Myocardial infarction with no obstructive coronary artery disease
MINOCA, which is seen in a minority of acute coronary syndrome cases and with a predominance in women compared with men (14·9% vs 3·5%, odds ratio [OR] 4·84; 95% CI 3·29–7·13),14 carries significant diagnostic and therapeutic uncertainty. The HARP-MINOCA study, which enrolled 170 women with MINOCA, showed that the combined use of intracoronary optical coherence tomography (OCT) and cardiac MRI resulted in identification of a mechanism for the myocardial infarction in 85% of patients, with an ischaemic aetiology, typically plaque rupture in a mild atherosclerotic lesion (type 1 myocardial infarction), seen in 64%.15 For patients with MINOCA, guidelines recommend establishing a diagnosis where possible, directing further testing and therapy according to an established diagnosis, and treating with standard secondary preventative measures if the diagnosis remains unclear.8
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection (SCAD) refers to an intimal tear (or less commonly vasa vasorum haemorrhage) leading to creation of a false lumen in the arterial wall in the absence of a clear mechanical cause (eg, trauma or catheter manipulation).8 Ensuing compression of the vessel lumen can result in ischaemia in the subtended myocardial territory. Fewer than 5% of all ACS is caused by SCAD, but proportions are higher in certain populations, such as women who are pregnant or post-partum.16 Optimal management of SCAD is uncertain given the absence of randomised trials, but for patients with low-risk anatomy, non-obstructive lesions, and resolution of symptoms, conservative medical management and aggressive secondary prevention, including blood pressure control, is generally preferred.8 The possibility of a primary vascular syndrome such as fibromuscular dysplasia should be considered in these patients.
Diagnosis
Diagnosis of acute coronary syndrome relies on clinical presentation, ECG findings, and biochemical evidence of myocardial injury. The immediate initial branchpoint for a patient with possible acute coronary syndrome is, of course, the presence or absence of diagnostic ST-segment elevations on the 12-lead ECG (table 1 ). Diagnosis of NSTEACS, which encompasses unstable angina and NSTEMI, has evolved significantly with the development of high-sensitivity troponin (hsTn) assays and is discussed in the next section. Adjunctive diagnostic tools, such as ECG or cardiac MRI, can help detect regional wall motion abnormalities and other evidence of myocardial ischemia in patients with suspected ACS.10
Table 1.
ECG criteria for the diagnosis of ST-elevation myocardial infarction
| Women | Men <40 years | Men >40 years | |
|---|---|---|---|
| Leads V2-V3 | >1·5 mm | >2·5 mm | >2 mm |
| Other leads | >1 mm | >1 mm | >1 mm |
ST elevation is measured at the J-point and should be present in at least two contiguous leads. Assess right-sided leads (V3R and V4R) in inferior myocardial infarction and assess posterior leads (V7-V9) in suspected posterior myocardial infarction (ST depressions in V1-V3).
Physical examination
Although physical examination findings are not generally specific for the diagnosis of acute coronary syndromes, careful patient evaluation is critical for immediate risk assessment, recognition of impending haemo-dynamic collapse, and identification of mechanical complications of myocardial infarction. Tachycardia, a narrow pulse pressure, hypotension, and signs of congestion (eg, pulmonary oedema) or inadequate perfusion (eg, cool extremities) are all indicators of high clinical risk. The Killip classification stratifies patients with acute coronary syndromes based on the degree of clinical heart failure, ranging from no evidence of congestion (Class I) to cardiogenic shock (Class IV), and strongly predicts mortality.17 Mechanical complications of myocardial infarction are typically accompanied by abrupt haemodynamic deterioration along with a loud holosystolic murmur in the left parasternal region in the case of acute ventricular septal rupture, an oftentimes soft systolic murmur in the case of acute mitral regurgitation, and signs of tamponade in the case of free wall rupture.18
Evaluation and management of suspected NSTEACS
The evaluation of suspected acute coronary syndromes is challenging given the time-sensitivity, potential under-lying life-threatening pathology, and often non-specific findings on initial assessment. The ECG in NSTEACS may show T-wave inversions or ST-segment depressions, but these findings are commonly absent and are not necessary for diagnosis. Elevated concentration of a circulating marker of myocardial necrosis such as cardiac troponin I or T (cTnI or cTnT) or creatine kinase-myocardial band (CK-MB) differentiates NSTEMI from unstable angina and is typically marked by an early rise, peak, and then fall in biomarker concentration.10
High-sensitivity troponin assays
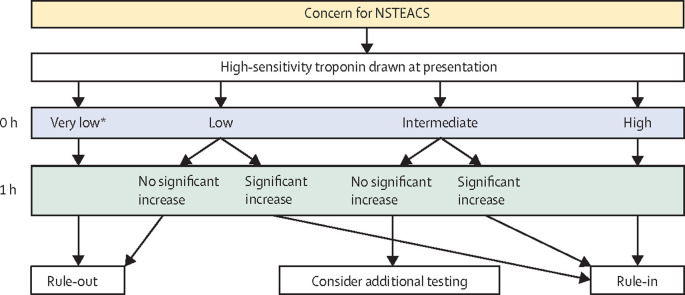
cTn assays are sensitive and reasonably specific and are therefore preferred over other biomarkers, including CK-MB, for the diagnosis of NSTEMI.8 hsTn assays have improved test characteristics relative to standard cTn assays and are more sensitive early after symptom onset. As such, they have allowed for the adoption of so-called rapid rule-out treatment algorithms.8, 19, 20, 21, 22, 23, 24 These algorithms rely on hsTn measurement at presentation (0 h) and at a second time point 1–3 h later, with attention both to the absolute concentration as well as the magnitude of change between samples (figure 1 ). Based on these criteria, patients can be ruled-out of having NSTEMI with very low rates of 30-day major adverse cardiovascular events (MACE), ruled-in for NSTEMI and managed accordingly or fall in an intermediate group where further observation and investigation might be warranted based on overall clinical assessment. A 0 h/1 h algorithm is endorsed by the 2020 European Society of Cardiology ESC guidelines.8 The specific concentrations for the hsTn thresholds are assay specific.8
Figure 1.
Approach to non-ST elevation acute coronary syndromes using a rapid rule-out strategy.
General scheme for a 0 h/1 h rapid rule-out algorithm for patients with suspected NSTEACS. High-sensitivity troponin concentration thresholds are assay-specific. NSTEACS=non-ST-segment elevation acute coronary syndrome. *And symptom duration at least 3 h.
Timing of coronary angiography in NSTEACS
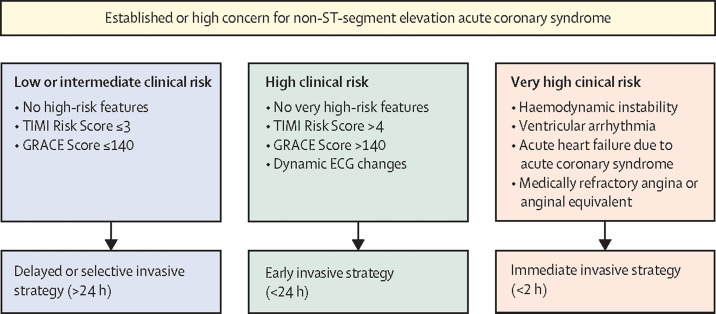
Immediate therapy for patients with NSTEACS includes administration of a loading dose of aspirin and systemic anticoagulation.8 The optimal timing of catheterisation in NSTEACS continues to be debated. Clinical risk stratification is the major determinant of timing, with very high-risk patients (eg, haemodynamic instability, refractory chest discomfort, life-threatening arrhythmia) warranting emergent (<2 h) angiography, patients at high risk (eg, Global Registry of Acute Coronary Events [GRACE] score >140) undergoing catheterisation within 24 h, and patients at low risk being submitted to a selective invasive strategy (figure 2 ). Increasing evidence, such as the VERDICT trial,25 has reinforced the importance of early catheterisation in the highest-risk patients and generally supports the safety of delayed catheterisation in lower-risk patients. P2Y12 inhibitor pretreatment before catheterisation is now recommended against as a routine strategy in patients with NSTEACS and a planned early invasive strategy.8 Non-invasive anatomical assessment via coronary computed tomography angiography (CCTA) might be expanded in the future.26, 27, 28, 29
Figure 2.
Risk stratification for timing of invasive angiography in non-ST segment elevation acute coronary syndrome
Selection of non-ST-segment elevation acute coronary syndrome treatment strategy and timing according to initial risk stratification. GRACE=Global Registry of Acute Coronary Events. TIMI=thrombolysis in myocardial infarction.
Evaluation and management of STEMI
For patients who manifest ST-segment elevation diagnostic for STEMI, emergent reperfusion therapy remains the immediate priority, as emphasised in the most recent European and American guidelines.30, 31, 32 The latest data continue to reinforce the association between prompt (<90 min) reperfusion and more favourable long-term clinical outcomes.33 Patients presenting to a percutaneous coronary intervention (PCI)-capable hospital should undergo immediate coronary angio-graphy with a goal of first medical contact-to-device time of less than 60–90 min.30, 32 For patients presenting to a non-PCI centre, transfer to PCI should be executed if the anticipated time to PCI will be 120 min or less. Alternatively, if PCI within this timeframe is not possible, fibrinolysis should be administered if not contraindicated, and a pharmaco-invasive approach should be considered in which initial fibrinolytic therapy is followed by invasive angiography within 24 h.34
Non-culprit lesions
About half of patients with STEMI have obstructive coronary disease outside the infarct-related artery, and the presence of flow-limiting non-infarct-related artery lesions portends a worse prognosis.35 The COMPLETE trial36 of 4041 patients with hemodynamically stable STEMI and at least one other significant non-culprit lesion found that patients randomised to complete revascularisation of all lesions within 45 days resulted in a lower rate of cardiovascular death or myocardial infarction compared with culprit-only PCI. The COMPLETE trial built upon several smaller trials suggesting potential benefit with complete revascularisation37, 38, 39 and a meta-analysis of complete versus culprit-only revascularisation in haemodynamically stable STEMI found a significantly lower rate of cardiovascular death in patients assigned to complete revascularisation.40
What does this mean for clinicians caring for patients with recent STEMI? Providers should anticipate planned staged revascularisation procedures in many patients discharged after STEMI. These subsequent procedures heighten the importance of antiplatelet therapy adherence and can create further need for follow-up visits and renal function assessment. They do have the additional benefit of more touchpoints in the system, however, which provide further opportunities for optimisation of the secondary prevention regimen.
Importantly, the COMPLETE trial findings cannot necessarily be extended to patients with acute myocardial infarction (STEMI or NSTEMI) complicated by shock. In the CULPRIT-SHOCK trial,41 706 patients with acute myocardial infarction with shock and significant non-culprit coronary lesions were randomised to multivessel PCI at the time of the index procedure or to culprit lesion-only PCI. Patients assigned to immediate multivessel PCI had higher rates of renal failure and death than did those assigned to culprit lesion-only PCI. Based on the CULPRIT-SHOCK trial, routine immediate non-culprit lesion PCI is not recommended in these patients.8 Whether staged revascularisation after resolution of the shock and concomitant end-organ injury may be beneficial is not currently known.
Antiplatelet therapy
Antiplatelet therapy is a critical component of the medical regimen in the acute phase of acute coronary syndromes as well as for secondary prevention following stabilisation (table 2 ). Previous randomised trials have shown that treatment with aspirin plus a P2Y12 inhibitor for at least 12 months following acute coronary syndromes reduces ischaemic events,42 with additional benefit seen with the 3rd generation P2Y12 inhibitors ticagrelor and prasugrel compared to clopidogrel.43, 44 These reductions in ischaemic events come at a cost of increased bleeding, but with an overall favourable net outcome in the pivotal trials. As such, DAPT has typically been recommended for 12 months following acute coronary syndromes in patients not meeting criteria for high bleeding risk (see Bleeding Risk below), with ticagrelor and prasugrel generally preferred over clopidogrel.8, 30, 32
Table 2.
P2Y12 inhibitors
| Route | Loading dose | Maintenance dose | Dose reduction | Notes | |
|---|---|---|---|---|---|
| Clopidogrel | Oral | 300–600 mg | 75 mg daily | NA | .. |
| Ticagrelor | Oral | 180 mg | 90 mg BID | NA | Long-term dose (>12 months after ACS) is 60 mg BID |
| Prasugrel | Oral | 60 mg | 10 mg daily | 5 mg daily for patients <60 kg or >75 years | Avoid if patient has had stroke or TIA; use with caution if age >75 years |
| Cangrelor | IV | 30 μg/kg bolus | 4 μg/kg/min (2 h or duration of PCI, whichever is longer) | NA | .. |
ACS=acute coronary syndrome. BID=twice per day. IV=intravenous. NA=not applicable. PCI=percutaneous coronary intervention. TIA=transient ischaemic attack.
The approach to antiplatelet therapy in the setting of ACS is evolving, however. The major areas of recent new clinically relevant data are: 1) choice of agent; 2) early aspirin cessation; and 3) strategy in patients with an indication for an anticoagulant.
Choice of P2Y12 antagonist
The large randomised trials evaluating prasugrel (TRITON-TIMI 38)43 and ticagrelor (PLATO)44 in acute coronary syndromes compared each agent with clopidogrel. As such, until recently, there have been scant randomised trial data comparing the 3rd generation P2Y12 inhibitors to each other. The ISAR-REACT 5 trial,45 which was a randomised, open-label comparison of prasugrel versus ticagrelor in 4018 patients with acute coronary syndromes and a planned invasive strategy, provides the only major randomised comparison of these agents. There was a higher rate of the composite primary end point (death, myocardial infarction, or stroke) at 1 year among patients randomised to ticagrelor (9·3 vs 6·9%; hazard ratio [HR] 1·36, 95% CI 1·09–1·70) with no significant difference in major bleeding. There were critical limitations to this trial, however, including an open-label design and frequent loss to follow-up. Nonetheless, the 2020 ESC NSTEACS guidelines provide a Class IIa, Level of Evidence B recommendation for prasugrel over ticagrelor in patients with NSTEACS who undergo PCI and are eligible for prasugrel (no prior stroke or transient ischaemic attack).8 As noted previously, these guidelines recommend against routine P2Y12 inhibitor loading before catheteri-sation, regardless of agent chosen, in patients with NSTEACS planned for an invasive strategy.8 The intravenous P2Y12 inhibitor cangrelor is an additional option in the acute phase for patients undergoing PCI who have not been pretreated with an oral P2Y12 inhibitor and who are not receiving a glycoprotein IIb/IIIa inhibitor.8, 46
The potential utility of genetic or platelet function testing to guide P2Y12 inhibitor choice has also been a topic of renewed interest. Clopidogrel is a prodrug which requires biotransformation by the hepatic CYP450 enzyme into its active metabolite. Variations in the CYP2C19 locus impact the metabolism of clopidogrel and it was established over a decade ago that CYP2C19 loss of function carriers who are treated with clopidogrel after acute coronary syndromes are at higher risk for MACE than are patients with typical clopidogrel metabolism.47, 48 Nevertheless, genotype and platelet function-guided treatment strategies have not found widespread clinical uptake or been supported over clinical judgement alone in major society guidelines. As such, clinical assessment of ischaemic and bleeding risk remains the cornerstone of agent selection.
This question was revisited in the POPular Genetics trial.49 Among 2488 patients undergoing primary PCI for STEMI, a genotype-guided strategy with de-escalation to clopidogrel in subjects without loss of function CYP2C19 alleles was non-inferior to standard therapy for ischemic outcomes and had a significantly lower rate of bleeding.50 Conversely, in the TAILOR-PCI trial51 of 5302 patients undergoing PCI for acute coronary syndromes or stable coronary disease, a point-of-care genetic testing strategy had no effect on clinical outcomes at 12 months. Nonetheless, this is a topic of heightened current interest and further evidence paired with evolution in point-of-care genetic or platelet function testing could lead to clinically validated guided platelet inhibition strategies moving forward.52
Early aspirin cessation
Several trials have investigated early aspirin cessation after PCI. The TWILIGHT trial53 randomised 7119 patients (65% of whom had NSTEACS) who had undergone PCI followed by 3 months of treatment with aspirin and ticagrelor to either ticagrelor monotherapy or to continued DAPT with aspirin and ticagrelor. Patients assigned to ticagrelor monotherapy had lower rates at 1 year of the primary bleeding endpoint. There was no difference in rates of ischaemic events such as myocardial infarction and stroke between treatment arms, although the trial was not powered to assess these outcomes. Although TWILIGHT and other related trials54, 55, 56, 57 have been underpowered individually to study the effect of early aspirin discontinuation on ischaemic events, a meta-analysis including data from more than 32 000 patients found no increased risk of MACE with early discontinuation of aspirin, including in the 16 898 patients with acute coronary syndromes.58
It remains important to keep in mind, however, that trials of early aspirin cessation were not designed to evaluate the effect of these strategies on the endpoints the medications are intended to influence (eg, myocardial infarction, stent thrombosis, stroke) and that scarce information is available beyond 1 year. Furthermore, reconciling these findings with separate previous trials showing benefit with extended-duration (>12 months) ticagrelor, prasugrel, or clopidogrel on top of aspirin therapy is not straightforward. Nonetheless, the trial data in aggregate suggest there might not be a major ischaemic risk in most patients with a strategy of de-escalation to P2Y12 inhibitor monotherapy after 3 months of DAPT and the most recent NSTEACS guidelines allow for consideration of such a strategy in patients at high risk for bleeding.8
Of note, although scarce data exist to-date, a strategy of early single antiplatelet therapy (SAPT) with aspirin alone rather than a P2Y12 inhibitor might be a consideration in some patients. In the MASTER DAPT trial,59 among 4434 patients with high bleeding risk undergoing PCI with drug-eluting stents, one month of DAPT was non-inferior to at least 3 months of DAPT in terms of net adverse clinical events. Approximately 30% of patients in the SAPT arm were treated with aspirin alone after 1 month of DAPT.
Concomitant anticoagulant therapy
Approximately 8–10% of patients undergoing PCI have atrial fibrillation or another indication for an oral anticoagulant.60 Understandably, the use of triple antithrombotic therapy in the form of DAPT plus an oral anticoagulant has raised concern for excessive bleeding risk in these patients.
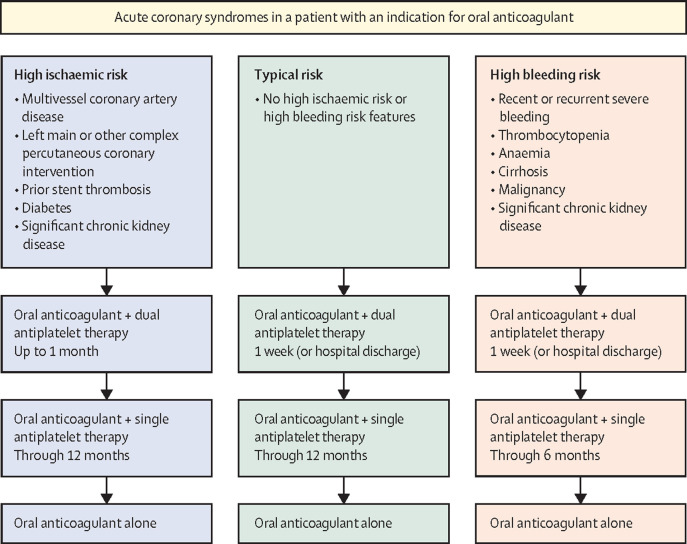
Several trials have tested various strategies of dual versus triple antithrombotic therapy, the majority of which have incorporated asymmetric comparisons; for example, a direct oral anticoagulant (DOAC)-based dual antithrombotic therapy regimen versus warfarin-based triple antithrombotic therapy.61, 62, 63 A meta-analysis of these randomised trials found lower rates of bleeding with DOAC dual antithrombotic therapy than vitamin K agonist triple antithrombotic therapy, but with numerically greater rates of myocardial infarction and stent thrombosis not meeting statistical significance.63 A single, large, symmetric randomised comparison between a DOAC (apixaban) and vitamin K agonist in this setting found lower rates of bleeding with the DOAC.60 The 2020 ESC NSTEACS guidelines recommend 1 week of triple antithrombotic therapy (or until hospital discharge) as a default strategy followed by dual antithrombotic therapy with a DOAC plus P2Y12 inhibitor (typically clopidogrel) until 1 year, at which point DOAC monotherapy can be considered.8 The duration of triple antithrombotic therapy might be extended to 1 month in patients with high ischaemic risk and acceptable bleeding risk. A general approach to antithrombotic therapy after ACS in patients with an indication for anticoagulation is shown in figure 3 .
Figure 3.
Approach to antithrombotic therapy in patients with an indication for oral anticoagulation who have undergone percutaneous coronary intervention for acute coronary syndromes.
The strategy for antithrombotic therapy is guided by assessment of each patient's risk for ischaemic events and for bleeding.
Bleeding risk
Central to the risk versus benefit considerations of anti-thrombotic strategies following acute coronary syndromes is a determination of a patient's risk for clinically significant bleeding. The ESC recommends considering use of the PRECISE-DAPT scale, with a score of 25 or more considered to indicate high bleeding risk, or the ARC-HBR criteria.8 To-date, no high-quality randomised data support the use of any specific bleeding scale in determining antithrombotic strategy.
Secondary prevention
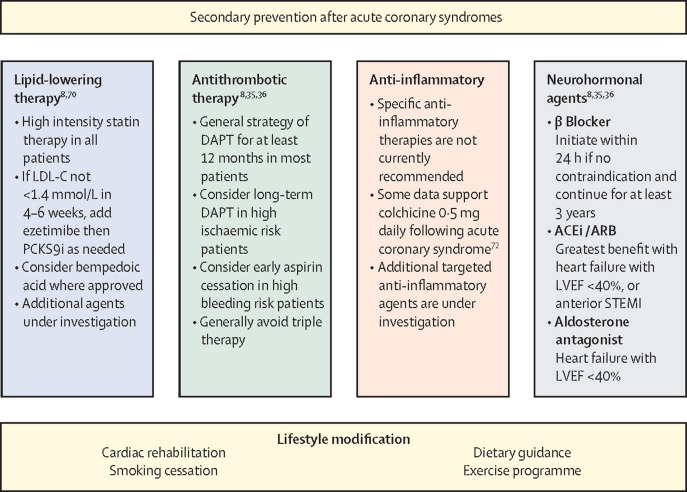
Secondary prevention in patients who have had an acute coronary syndrome is crucial and includes several non-pharmacological interventions such as diet and exercise guidance, smoking cessation, and cardiac rehabilitation (figure 4 ).8, 30 Beta blockade and renin angiotensin aldosterone system (RAAS) modification are long-standing secondary prevention therapies. With respect to recent evolution in secondary prevention medical therapy, there is important new evidence guiding lipid-lowering and anti-inflammatory agents.
Figure 4.
Secondary prevention after acute coronary syndromes
Bempedoic acid is a novel oral inhibitor of cholesterol synthesis which was approved by the European Medicines Agency and the US Food and Drug Administration in 2020 for lipid-lowering, though clinical efficacy to reduce cardiovascular events is not yet proved.64 Numerous additional targets for lipid-lowering therapy, including ANGPTL3, lipoprotein(a), APOC3, lipoprotein lipase, and others, are in development.65, 66 ACEi=angiotensin converting enzyme inhibitor. ACS=acute coronary syndrome. ARB=angiotensin receptor blocker. DAPT=dual antiplatelet therapy. HF=heart failure. LVEF=left ventricular ejection fraction. PCSK9i=proprotein covertase subtilisin/kexin type 9 inhibitor. STEMI=ST-segment elevation myocardial infarction.
Lipid-lowering therapy
Reducing the concentration of circulating atherogenic lipoproteins has a major effect on the risk of adverse cardiovascular events in numerous clinical settings.67 Whereas the prior evidence base for LDL-cholesterol (LDL-C) lowering after acute coronary syndromes was primarily for statins and ezetimibe,68 additional classes of agents are now providing promising new data.
Two monoclonal antibodies against PCSK9, evolocumab and alirocumab, which reduce LDL-C by 50–70%, have shown major reductions in cardiovascular events in high-risk patients, including within 12 months of acute coronary syndromes occurring.69, 70, 71 The safety and feasibility of in-hospital initiation of evolocumab was investigated in the EVOPACS trial,72 which randomised patients with acute coronary syndromes and elevated LDL-C treated with atorvastatin to evolocumab or placebo and found rapid attainment of guideline-recommended LDL-C levels in the evolocumab arm. A recommended step-wise approach is to treat all patients with acute coronary syndromes with high-intensity statin therapy.73 ESC guidelines recommend that patients in whom an LDL-C concentration of less than 1·4 mmol/L is not achieved in 4–6 weeks should be treated additionally with ezetimibe and that if the patient remains above this LDL-C goal on the high-intensity statin and ezetimibe, a PCSK9 inhibitor should be added.8 Furthermore, if the current acute coronary event occurred within 2 years of a previous acute coronary syndrome event, an LDL-C goal <40 mg/dL (<1 mmo/L) might be considered.8 American society guidelines have endorsed a treatment LDL-C threshold of 70 mg/dL (1·8 mmol/L) for high risk patients, above which the addition of non-statin therapy is recommended to further reduce LDL-C.74
Targeting inflammation
Inflammation appears to contribute to the increased risk of recurrent events across vascular territories following acute coronary syndrome,74 but identifying safe and effective anti-inflammation therapy has been challenging. Anti-inflammatory treatment with colchicine after acute coronary syndrome has recently shown some promise, however. Patients within 30 days of a myocardial infarction randomised to colchicine 0·5 mg daily had a 23% lower risk of MACE compared to placebo-treated patients in the COLCOT trial,75 and similar findings were observed among patients with chronic coronary syndrome in the LoDoCo2 trial.76 Even so, there were numerically higher rates of non-cardiovascular death among colchicine-treated patients in these trials and a separate trial of colchicine in patients with acute coronary syndrome, COPS,77 found a significantly increased risk of death with colchicine. To-date, no major society guidelines recommend colchicine following acute coronary syndrome.
Disparities in health-care access and outcomes
Entrenched racial disparities impact the recognition, management, and outcomes of acute coronary syndrome. According to the Heart Disease and Stroke Statistics 2022 Update,3, 4 which compiles epidemiological data from multiple sources, Black men and women in the USA continue to have higher rates of incident myocardial infarction than do White men or women across all age groups, with a nearly two times higher rate for Black men than White men aged 75–84 years (16 vs 9 per 1000 person-years).3, 4 Despite this, Black patients evaluated for chest pain are less likely than other patients to have an ECG ordered in the emergency department (adjusted odds ratio [aOR] 0·82; 95% CI 0·69–0·99),78 have a 30% longer wait time in the emergency department for symptoms suggestive of acute coronary syndrome,4, 79 are less likely to receive mechanical circulatory support in myocardial infarction complicated by shock (0·84; 0·79–0·89),80 are less likely to be referred to cardiac rehabilitation after a myocardial infarction (0·70; 0·53–0·93),4 and have higher rates of heart failure or death following myocardial infarction.4, 81 In an analysis from the REGARDS registry,82 3-year outcomes after myocardial infarction were compared for Black versus White patients. Black patients had a more than 40% higher adjusted risk than White patients of cardio-vascular death, myocardial infarction, stroke, or heart failure hospitalisation after a first myocardial infarction. There were statistically significant contributions from pre-existing comorbidities and myocardial infarction characteristics to these relationships, emphasising the complex and important associations underlying the unequal long-term outcomes after acute coronary syndrome.82
An analysis of racial representation in 460 acute coronary syndrome trials, many of which were multi-national, found that on average 3·7% of participants were Black and that this proportion had not changed significantly from 2001 to 2018.83 Addressing these long-standing inequities in all aspects of cardiovascular disease, from risk exposure to diagnosis, treatment, outcomes, and representation in clinical studies, is crucially important.84
Acute coronary syndrome in women
Sex-based disparities in the evaluation and management of acute coronary syndrome are also pervasive. Women with STEMI tend to present later after symptom onset than men,85 and among patients who have sought medical attention for symptoms before acute coronary syndrome onset, women are more likely to have been reassured that the symptoms were not cardiac in nature (53·4% vs 36·4%; p<0·001).86 Chest pain appears to be present in approximately 90% of patients with an acute myocardial infarction regardless of sex,14 although women tend to present with more diverse symptoms.87 Importantly, commonly recommended hsTn concentration thresholds for NSTEMI diagnosis might be less sensitive in women than in men.88
Women with acute coronary syndrome are less likely than men to undergo revascularisation (adjusted OR for PCI 0·68; 95% CI 0·66–0·70; CABG 0·40; 95% CI 0·39–0·44).86 When PCI for acute coronary syndrome is performed, radial access is less commonly used in women and older women have higher rates of significant post-procedural bleeding than do older men.89, 90
In terms of secondary prevention, women are less likely than men to receive statins, angiotensin converting enzyme inhibitors, or angiotensin receptor blockers at time of discharge (p=0·01).91, 92 These differences in secondary prevention regimens might contribute to the lesser observed reductions in recurrent myocardial infarction rates over time in women than in men.93
Acute coronary syndrome in older patients
Elderly patients comprise an increasing proportion of patients with acute coronary syndrome in HIC, accounting for approximately one-third of patients with acute myocardial infarction and two-thirds of deaths following myocardial infarction.94 Older patients might be more likely to have atypical symptoms of acute coronary syndrome than younger patients and are often at heightened risk for both ischaemic and bleeding events.8, 94 Furthermore, less than 10% of people enrolled in trials of acute coronary syndrome are 75 years or older, indicating an important gap in clinical evidence for this population.94 The open-label POPular AGE trial49 randomised 1002 patients who were at least 70 years old with NSTEACS to clopidogrel versus ticagrelor or prasugrel and found a significantly lower rate of bleeding with clopidogrel with no increase in ischaemic events. The 2020 ESC NSTEACS guidelines recommend that the same diagnostic and interventional strategies should be applied to older patients as well as their younger counterparts and that the choice of antithrombotic agent and secondary prevention measures should take into account renal function and specific contraindications.8 Ongoing studies, such as the SENIOR-RITA trial of coronary angiography versus medical therapy in patients 75 years or older with NSTEMI, might add important evidence to guide clinical management of acute coronary syndrome in these older patients.8
Acute coronary syndrome worldwide
Vast disparities in acute coronary syndrome exist globally, from risk factor prevalence to acute coronary syndrome incidence, treatment availability, and long-term outcomes.7 Whereas aggregate risk factor exposure appears to have been relatively stable from 2010 to 2019 on a global scale, there have been important increases in hyperlipidaemia, hyperglycaemia, high body-mass index, hypertension, and air pollution exposure in LMIC during this timeframe.95 The INTERHEART study, found smoking to be one of the most significant modifiable risk factors for acute myocardial infarction, accounting for approximately 36% of the population attributable risks of acute coronary syndrome globally.96 However, tobacco use has particularly marked regional variation, accounting for more than 15% of lost disability-adjusted life-years (DALYs) in some countries in Asia and eastern Europe but very few lost DALYs in sub-Saharan Africa.95 Furthermore, social determinants of health such as education level, socioeconomic status, dietary patterns, alcohol consumption, and physical activity levels, are increasingly associated with cardiovascular disease risk in LMICs, with notable regional variation.7 Although cardiovascular disease accounts for a smaller portion of the relative disease burden in LMIC as compared with HIC at present, the absolute disease burden is extensive in these countries, is more likely to occur at younger ages, is expected to grow rapidly given rising risk factor prevalence, and soberingly, is associated with significantly higher morbidity and mortality.95, 97, 98, 99
Impediments to the diagnosis and treatment of acute coronary syndrome exist at every step in LMIC, from symptom recognition to availability of an appropriate health-care facility, transportation, and access to disease-specific health-care personnel, medications, medical equipment, and secondary prevention measures.6, 100 Additionally, increasing health-care costs and limited insurance coverage penetration lead to magnified patient and health-system-centred barriers to diagnosis and management of acute coronary syndrome worldwide, particularly in LMIC.98, 99 There are crucial disparities in access to specialised health-care system professionals and cardiovascular care resource capacity in LMIC compared with HIC.97 For example, the majority of countries in sub-Saharan Africa have fewer than five physicians per 10 000 people and nearly a fifth of these countries had no registered cardiologists according to recent surveys.103 In an analysis of 196 acute coronary syndrome admissions to Kenyatta National Hospital in Nairobi, Kenya, only 5% of patients with STEMI received reperfusion therapy and the rate of in-hospital mortality was 17%.103 In the Caribbean, similar acute myocardial infarction in-hospital mortality rates have been reported. In an analysis of 3794 admissions to the University Hospital of the West Indies in Jamaica, acute coronary syndrome accounted for 8% of all medical admissions and there was an impatient acute myocardial infarction mortality rate of 19%.104 A recent consensus document endorsed by several professional societies in Africa, Asia, and the Americas outlined management strategies for STEMI in resource-limited settings along with directions forward.6 This work addressing the large and growing burden of acute coronary syndrome in LMIC is complex and is essential to the health and development of these countries.
COVID-19
The global spread of the novel coronavirus SARS-CoV-2 and its associated clinical disease, COVID-19, has impacted the management of acute coronary syndrome worldwide. COVID-19 has complicated acute coronary syndrome diagnosis and treatment for several reasons: 1) COVID-19 can cause direct or indirect myocardial inflammation or injury,105 which creates diagnostic uncertainty in patients with ST elevations when COVID-19 is known or suspected to be present; 2) COVID-19 predisposes patients to arterial (including coronary) and venous thrombotic events;105 and 3) COVID-19 causes societal and health-care system disruptions that make delivery of timely evidence-based care for prevention and treatment of acute coronary syndromes difficult or impossible in some circumstances.106
As a general approach, consensus guidance recom-mends that for most patients with COVID-19 in whom true STEMI or NSTEMI is suspected, particularly those with shock or malignant ventricular arrhythmia, immediate coronary angiography or reperfusion should be performed when available.107 When circumstances are less clear and clinical stability allows, an initial strategy of non-invasive testing with ECG for regional wall motion assessment or coronary computed tomography angiography or both might be reasonable.
Beyond the acute phase of revascularisation for myocardial infarction, COVID-19 introduces numerous challenges for medical optimisation, secondary prevention (eg, cardiac rehabilitation), and post-discharge monitoring. How these issues will interact with the evolving COVID-19 landscape, challenging logistical and economic realities, and the increasing availability of telemedicine is, of course, unknown, but will bear heavily on the true cardiovascular cost of COVID-19 moving forward.
Conclusion and future directions
The diagnosis and management of acute coronary syndromes continue to evolve. Investigations are underway to refine diagnostic algorithms and risk stratification, integrate intracoronary imaging findings into treatment pathways, identify novel targets for lipid-lowering and secondary prevention, and eliminate structural barriers to healthy lives. Sex, racial, and ethnic disparities in its recognition, management, and outcomes must be addressed. Also important is improvement of its diagnosis and treatment in LMIC. Much has been learned about the identification and management of acute coronary syndrome, and much work remains.
Search strategy and selection criteria
We searched MEDLINE and Embase for “acute coronary syndrome”, “STEMI”, “NSTEMI”, and “NSTEACS” for articles published since inception through May 1, 2021. We also reviewed recent major society guidelines as well as presentations from the European Society of Cardiology, American Heart Association, and American College of Cardiology scientific congresses from the past 5 years.
Declaration of interests
BAB reports grants from Ionis, Abbott Vascular, Pfizer, and AstraZeneca; personal fees from Abiomed, Abbott Vascular, CSI, Quark, Servier, Janssen, Daiichi-Sankyo, and Philips, outside the submitted work. RPG reports grants from Amgen, Ionis, Daiichi-Sankyo, and Anthos; honoraria for Continuing Medical Education Programmes and lectures from Amgen, Centrix, Daiichi Sankyo, Dr. Reddy's Laboratories, Medical Education Resources, Medscape, Menarini, Pfizer, SAJA Pharmaceuticals, Servier, and Voxmedia; and consultant fees from Amarin, Amgen, Bayer, CryoLife, Daiichi Sankyo, Esperion, Gilead, Hengrui, Inari, Pfizer, PhaseBio Pharmaceuticals, St Luke's Hospital (Kansas City, MO, USA), and Sanofi Aventis. All other authors declare no competing interests.
Acknowledgments
Contributors
BAB and RPG contributed to the concept, literature review, manuscript drafting, and critical revision of the study. NM, PAM, and MBL-W contributed to the literature review, manuscript drafting, and critical revision.
References
- 1.Global Burden of Disease Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1242. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;143:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 5.Bueno H, Rossello X, Pocock SJ, et al. In-hospital coronary revascularization rates and post-discharge mortality risk in non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2019;74:1454–1461. doi: 10.1016/j.jacc.2019.06.068. [DOI] [PubMed] [Google Scholar]
- 6.Chandrashekhar Y, Alexander T, Mullasari A, et al. Resource and infrastructure-appropriate management of ST-segment elevation myocardial infarction in low- and middle-income countries. Circulation. 2020;141:2004–2025. doi: 10.1161/CIRCULATIONAHA.119.041297. [DOI] [PubMed] [Google Scholar]
- 7.Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395:785–794. doi: 10.1016/S0140-6736(19)32007-0. [DOI] [PubMed] [Google Scholar]
- 8.Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 9.Omer MA, Tyler JM, Henry TD, et al. Clinical characteristics and outcomes of STEMI patients with cardiogenic shock and cardiac arrest. JACC Cardiovasc Interv. 2020;13:1211–1219. doi: 10.1016/j.jcin.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 11.White HD, Steg PG, Szarek M, et al. Effects of alirocumab on types of myocardial infarction: insights from the ODYSSEY OUTCOMES trial. Eur Heart J. 2019;40:2801–2809. doi: 10.1093/eurheartj/ehz299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libby P, Pasterkamp G, Crea F, Jang IK. reassessing the mechanisms of acute coronary syndromes. Circ Res. 2019;124:150–160. doi: 10.1161/CIRCRESAHA.118.311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safdar B, Spatz ES, Dreyer RP, et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds HR, Maehara A, Kwong RY, et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of MINOCA in women. Circulation. 2021;143:624–640. doi: 10.1161/CIRCULATIONAHA.120.052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiguchi T, Tanaka A, Ozaki Y, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5:263–270. doi: 10.1177/2048872613504310. [DOI] [PubMed] [Google Scholar]
- 17.Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 18.Zeymer U, Bueno H, Granger CB, et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2020;9:183–197. doi: 10.1177/2048872619894254. [DOI] [PubMed] [Google Scholar]
- 19.Neumann JT, Twerenbold R, Ojeda F, et al. Application of high-sensitivity troponin in suspected myocardial infarction. N Engl J Med. 2019;380:2529–2540. doi: 10.1056/NEJMoa1803377. [DOI] [PubMed] [Google Scholar]
- 20.Twerenbold R, Costabel JP, Nestelberger T, et al. Outcome of applying the ESC 0/1-hour algorithm in patients with suspected myocardial infarction. J Am Coll Cardiol. 2019;74:483–494. doi: 10.1016/j.jacc.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 21.Boeddinghaus J, Nestelberger T, Twerenbold R, et al. Direct comparison of 4 very early rule-out strategies for acute myocardial infarction using high-sensitivity cardiac troponin I. Circulation. 2017;135:1597–1611. doi: 10.1161/CIRCULATIONAHA.116.025661. [DOI] [PubMed] [Google Scholar]
- 22.Chew DP, Lambrakis K, Blyth A, et al. A randomized trial of a 1-hour troponin T protocol in suspected acute coronary syndromes: the rapid assessment of possible acute coronary syndrome in the emergency department with high-sensitivity troponin T study (RAPID-TnT) Circulation. 2019;140:1543–1556. doi: 10.1161/CIRCULATIONAHA.119.042891. [DOI] [PubMed] [Google Scholar]
- 23.Chapman AR, Adamson PD, Shah ASV, et al. High-sensitivity cardiac troponin and the universal definition of myocardial infarction. Circulation. 2020;141:161–171. doi: 10.1161/CIRCULATIONAHA.119.042960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrow DA. The fourth universal definition of myocardial infarction and the emerging importance of myocardial injury. Circulation. 2020;141:172–175. doi: 10.1161/CIRCULATIONAHA.119.044125. [DOI] [PubMed] [Google Scholar]
- 25.Kofoed KF, Kelbaek H, Hansen PR, et al. Early versus standard care invasive examination and treatment of patients with non-st-segment elevation acute coronary syndrome. Circulation. 2018;138:2741–2750. doi: 10.1161/CIRCULATIONAHA.118.037152. [DOI] [PubMed] [Google Scholar]
- 26.Abdelrahman KM, Chen MY, Dey AK, et al. Coronary computed tomography angiography from clinical uses to emerging technologies: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:1226–1243. doi: 10.1016/j.jacc.2020.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray A, Roobottom C, Smith JE. Late-breaking science abstracts and featured science abstracts from the American Heart Association's Scientific sessions 2020 and late-breaking abstracts in resuscitation science from the Resuscitation Science Symposium 2020. Circulation. 2020;142:e470–e500. [Google Scholar]
- 28.Gray AJ, Roobottom C, Smith JE, et al. The RAPID-CTCA trial (rapid assessment of potential ischaemic heart disease with CTCA). A multicentre parallel-group randomised trial to compare early computerised tomography coronary angiography versus standard care in patients presenting with suspected or confirmed acute coronary syndrome: study protocol for a randomised controlled trial. Trials. 2016;17:579. doi: 10.1186/s13063-016-1717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linde JJ, Kelbaek H, Hansen TF, et al. Coronary CT Angiography in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2020;75:453–463. doi: 10.1016/j.jacc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 31.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol. 2016;67:1235–1250. doi: 10.1016/j.jacc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:871. doi: 10.1093/eurheartj/ehy855. 65. [DOI] [PubMed] [Google Scholar]
- 33.Loh JP, Tan LL, Zheng H, et al. First medical contact-to-device time and heart failure outcomes among patients undergoing primary percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.004699. [DOI] [PubMed] [Google Scholar]
- 34.Fazel R, Joseph TI, Sankardas MA, et al. Comparison of reperfusion strategies for ST-segment-elevation myocardial infarction: a multivariate network meta-analysis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312:2019–2027. doi: 10.1001/jama.2014.15095. [DOI] [PubMed] [Google Scholar]
- 36.Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381:1411–1421. doi: 10.1056/NEJMoa1907775. [DOI] [PubMed] [Google Scholar]
- 37.Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665–671. doi: 10.1016/s0140-6736(15)60648-1. [DOI] [PubMed] [Google Scholar]
- 38.McCann GP, Khan JN, Greenwood JP, et al. Complete versus lesion-only primary PCI: the randomized cardiovascular MR CvLPRIT Substudy. J Am Coll Cardiol. 2015;66:2713–2724. doi: 10.1016/j.jacc.2015.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. doi: 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]
- 40.Bainey KR, Engstrom T, Smits PC, et al. Complete vs culprit-lesion-only revascularization for ST-segment elevation myocardial infarction: a systematic review and meta-analysis. JAMA Cardiol. 2020;5:881–888. doi: 10.1001/jamacardio.2020.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 42.Investigators CiUAtPRET Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;2001:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 43.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 44.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 45.Schupke S, Neumann FJ, Menichelli M, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381:1524–1534. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368:1303–1313. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- 47.Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 49.Gimbel M, Qaderdan K, Willemsen L, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. 2020;395:1374–1381. doi: 10.1016/S0140-6736(20)30325-1. [DOI] [PubMed] [Google Scholar]
- 50.Claassens DMF, Vos GJA, Bergmeijer TO, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 2019;381:1621–1631. doi: 10.1056/NEJMoa1907096. [DOI] [PubMed] [Google Scholar]
- 51.Pereira NL, Farkouh ME, So D, et al. Effect of genotype-guided oral p2y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. 2020;324:761–771. doi: 10.1001/jama.2020.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galli M, Benenati S, Capodanno D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. 2021;397:1470–1483. doi: 10.1016/S0140-6736(21)00533-X. [DOI] [PubMed] [Google Scholar]
- 53.Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381:1524–1534. doi: 10.1056/NEJMoa1908419. [DOI] [PubMed] [Google Scholar]
- 54.Hahn JY, Song YB, Oh JH, et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the smart-CHOICE randomized clinical trial. JAMA. 2019;321:2428–2437. doi: 10.1001/jama.2019.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vranckx P, Valgimigli M, Juni P, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018;392:940–949. doi: 10.1016/S0140-6736(18)31858-0. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe H, Domei T, Morimoto T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019;321:2414–2427. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim BK, Hong SJ, Cho YH, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323:2407–2416. doi: 10.1001/jama.2020.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Donoghue ML, Murphy SA, Sabatine MS. The safety and efficacy of aspirin discontinuation on a background of a P2Y12 inhibitor in patients after percutaneous coronary intervention: a systematic review and meta-analysis. Circulation. 2020;142:538–545. doi: 10.1161/CIRCULATIONAHA.120.046251. [DOI] [PubMed] [Google Scholar]
- 59.Valgimigli M, Frigoli E, Heg D, et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med. 2021;385:1643–1655. doi: 10.1056/NEJMoa2108749. [DOI] [PubMed] [Google Scholar]
- 60.Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;385:1643–1655. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 61.Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 62.Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 63.Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–1343. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- 64.Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 65.Hegele RA, Tsimikas S. Lipid-lowering agents. Circ Res. 2019;124:386–404. doi: 10.1161/CIRCRESAHA.118.313171. [DOI] [PubMed] [Google Scholar]
- 66.Gaudet D, Karwatowska-Prokopczuk E, Baum SJ, et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J. 2020;41:3936–3945. doi: 10.1093/eurheartj/ehaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 68.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 69.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 71.Gencer B, Mach F, Murphy SA, et al. Efficacy of evolocumab on cardiovascular outcomes in patients with recent myocardial infarction: a prespecified secondary analysis from the FOURIER trial. JAMA Cardiol. 2020;5:952–957. doi: 10.1001/jamacardio.2020.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koskinas KC, Windecker S, Pedrazzini G, et al. Evolocumab for early reduction of LDL-cholesterol levels in patients with acute coronary syndromes (EVOPACS) J Am Coll Cardiol. 2019;74:2452–2462. doi: 10.1016/j.jacc.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 75.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 76.Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 77.Tong DC, Quinn S, Nasis A, Hiew C, et al. Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation. 2020;142:1890–1900. doi: 10.1161/CIRCULATIONAHA.120.050771. [DOI] [PubMed] [Google Scholar]
- 78.Mukhopadhyay A, D'Angelo R, Senser E, Whelan K, Wee CC, Mukamal KJ. Racial and insurance disparities among patients presenting with chest pain in the US: 2009–2015. Am J Emerg Med. 2020;38:1373–1376. doi: 10.1016/j.ajem.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 79.Alrwisan A, Eworuke E. Are discrepancies in waiting time for chest pain at emergency departments between African Americans and Whites improving over time? J Emerg Med. 2016;50:349–355. doi: 10.1016/j.jemermed.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 80.Kim Y, Park J, Essa M, Lansky AJ, Sugeng L. Frequency of management of cardiogenic shock with mechanical circulatory support devices according to race. Am J Cardiol. 2020;125:1782–1787. doi: 10.1016/j.amjcard.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 81.Srivastava PK, Fonarow GC, Bahiru E, Ziaeian B. Association of hospital racial composition and payer mix with mortality in acute coronary syndrome. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blackston JW, Safford MM, Mefford MT, et al. cardiovascular disease events and mortality after myocardial infarction among black and white adults: REGARDS study. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tahhan AS, Vaduganathan M, Greene SJ, et al. Enrollment of older patients, women, and racial/ethnic minority groups in contemporary acute coronary syndrome clinical trials: a systematic review. JAMA Cardiol. 2020;5:714–722. doi: 10.1001/jamacardio.2020.0359. [DOI] [PubMed] [Google Scholar]
- 84.Hill JA, Albert MA, Carnethon MR, Watson KE. Disparities in cardiovascular medicine: Circulation's response. Circulation. 2020;142:1127–1128. doi: 10.1161/CIRCULATIONAHA.120.050670. [DOI] [PubMed] [Google Scholar]
- 85.Meyer MR, Bernheim AM, Kurz DJ, et al. Gender differences in patient and system delay for primary percutaneous coronary intervention: current trends in a Swiss ST-segment elevation myocardial infarction population. Eur Heart J Acute Cardiovasc Care. 2019;8:283–290. doi: 10.1177/2048872618810410. [DOI] [PubMed] [Google Scholar]
- 86.Lichtman JH, Leifheit EC, Safdar B, et al. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction: evidence from the VIRGO study (variation in recovery: role of gender on outcomes of young AMI patients) Circulation. 2018;137:781–790. doi: 10.1161/CIRCULATIONAHA.117.031650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brush JE, Jr., Krumholz HM, Greene EJ, Dreyer RP. Sex differences in symptom phenotypes among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.119.005948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kimenai DM, Janssen E, Eggers KM, et al. Sex-specific versus overall clinical decision limits for cardiac troponin i and t for the diagnosis of acute myocardial infarction: a systematic review. Clin Chem. 2018;64:1034–1043. doi: 10.1373/clinchem.2018.286781. [DOI] [PubMed] [Google Scholar]
- 89.Steitieh DA, Lu DY, Kalil RK, et al. Sex-based differences in revascularization and 30-day readmission after ST-segment-elevation myocardial infarction in the United States. Cardiovasc Revasc Med. 2020;31:41–47. doi: 10.1016/j.carrev.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 90.Nanna MG, Hajduk AM, Krumholz HM, et al. Sex-based differences in presentation, treatment, and complications among older adults hospitalized for acute myocardial infarction: the SILVER-AMI study. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.119.005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DeFilippis EM, Collins BL, Singh A, et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: the Mass General Brigham YOUNG-MI registry. Eur Heart J. 2020;41:4127–4137. doi: 10.1093/eurheartj/ehaa662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jackson AM, Zhang R, Findlay I, et al. Healthcare disparities for women hospitalized with myocardial infarction and angina. Eur Heart J Qual Care Clin Outcomes. 2020;6:156–165. doi: 10.1093/ehjqcco/qcz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peters SAE, Colantonio LD, Dai Y, et al. Trends in recurrent coronary heart disease after myocardial infarction among US women and men between 2008 and 2017. Circulation. 2021;143:650–660. doi: 10.1161/CIRCULATIONAHA.120.047065. [DOI] [PubMed] [Google Scholar]
- 94.Kayani WT, Khan MR, Deshotels MR, Jneid H. Challenges and controversies in the management of ACS in elderly patients. Curr Cardiol Rep. 2020;22:51. doi: 10.1007/s11886-020-01298-x. [DOI] [PubMed] [Google Scholar]
- 95.Global Burden of Disease Collaborators Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 97.Kwan GF, Mayosi BM, Mocumbi AO, et al. Endemic cardiovascular diseases of the poorest billion. Circulation. 2016;133:2561–2575. doi: 10.1161/CIRCULATIONAHA.116.008731. [DOI] [PubMed] [Google Scholar]
- 98.Prabhakaran D, Anand S, Watkins DA, et al. Cardiovascular, respiratory, and related disorders:key messages from Disease Control Priorities, 3rd edition. Lancet. 2018;391:1224–1236. doi: 10.1016/S0140-6736(17)32471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371:818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 100.Yuyun MF, Sliwa K, Kengne AP, Mocumbi AO, Bukhman G. Cardiovascular diseases in sub-Saharan Africa compared to high-income countries: an epidemiological perspective. Global Heart. 2020;15:15. doi: 10.5334/gh.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hertz JT, Kweka GL, Manavalan P, Watt MH, Sakita FM. Provider-perceived barriers to diagnosis and treatment of acute coronary syndrome in Tanzania: a qualitative study. Int Health. 2020;12:148–154. doi: 10.1093/inthealth/ihz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lynch A, Sobuwa S, Castle N. Barriers to the implementation of prehospital thrombolysis in the treatment of ST-segment elevation myocardial infarction in South Africa: an exploratory inquiry. Afr J Emerg Med. 2020;10:243–248. doi: 10.1016/j.afjem.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bahiru E, Temu T, Gitura B, Farquhar C, Huffman MD, Bukachi F. Presentation, management and outcomes of acute coronary syndrome: a registry study from Kenyatta National Hospital in Nairobi, Kenya. Cardiovasc J Afr. 2018;29:225–230. doi: 10.5830/CVJA-2018-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mani KA, Hoo Sang M, Younger-Coleman NO, Ferguson TS. Ischaemic heart disease at the University Hospital of the West Indies: trends in hospital admissions and inpatient mortality rates 2005–2010. West Indian Med J. 2014;63:424–430. doi: 10.7727/wimj.2013.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Atri D, Siddiqi HK, Lang JP, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. J Am Coll Cardiol Basic Trans Science. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 Pandemic. J Am Coll Cardiol. 2020;76:280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chieffo A, Stefanini GG, Price S, et al. EAPCI position statement on invasive management of acute coronary syndromes during the COVID-19 pandemic. Eur Heart J. 2020;41:1839–1851. doi: 10.1093/eurheartj/ehaa381. [DOI] [PMC free article] [PubMed] [Google Scholar]