Abstract
Background:
Reducing depth of anesthesia and anesthetic exposure may help prevent delirium, but trials have been conflicting. Most studies were conducted under general anesthesia or in cognitively-impaired patients. It is unclear whether reducing depth of anesthesia beyond levels consistent with general anesthesia reduces delirium in cognitively-intact patients. Our objective was to determine whether a bundled approach to reduce anesthetic agent exposure as determined by BIS values (spinal anesthesia with targeted sedation based on BIS values) compared with general anesthesia (masked BIS) reduces delirium.
Methods:
Important eligibility criteria for this parallel-arm randomized trial were patients ≥65 years undergoing lumbar spine fusion. The intervention group received spinal anesthesia with targeted sedation to BIS>60–70. The control group received general anesthesia (masked BIS). The primary outcome was delirium using the Confusion Assessment Method daily through postoperative day 3, with blinded assessment.
Results:
The median age of 217 patients in the analysis was 72 (interquartile range 69,77). Median BIS value in the spinal anesthesia with targeted sedation based on BIS values group was 62 (interquartile range 53,70) and in the general anesthesia with masked BIS values group was 45 (interquartile range 41,50, p<0.001). Incident delirium was not different in the spinal anesthesia with targeted sedation based on BIS values group (25.2%[28/111] vs. the general anesthesia with masked BIS values group (18.9%[20/106], p=0.259) (Relative Risk 1.22 [95%CI 0.85–1.76]). In pre-specified subgroup analyses, the effect of anesthetic strategy differed according to Mini-Mental State Examination, but not Charlson Comorbidity Index or age. Two strokes occurred among patients receiving spinal anesthesia and one death among patients receiving general anesthesia.
Conclusions:
Spinal anesthesia with targeted sedation based on BIS values compared with general anesthesia with masked BIS values did not reduce delirium after lumbar fusion.
INTRODUCTION
Post-operative delirium is common in older adults after surgery, with estimates of 10–50% depending on the type of surgery.1–3 Although previously thought to be transient with few long-term effects, it is now recognized that postoperative delirium is associated with important sequelae, including increased duration of hospitalization,4,5 decreased functional status,6,7 and cognitive decline.8,9 Despite its significance, there are few effective treatment strategies, and so prevention of delirium is paramount.3
In the intensive care unit (ICU), reducing the level of sedation has been associated with less delirium.10 However, in the operating room, it is unclear whether a parallel strategy to reduce depth of anesthesia and anesthetic exposure is effective, as the results of prior trials have been promising, but conflicting.11–16 One limitation is that most prior studies were conducted in patients undergoing general anesthesia with the goal of limiting excessive depth of anesthesia and anesthetic exposure,11–14 and the effectiveness of strategies to avoid general anesthesia and target lighter sedation has not been well studied. Although two additional trials did examine the benefits of lighter sedation during hip fracture surgery under spinal anesthesia, the results may not be generalizable to most older adults undergoing surgery, since a substantial number of patients were cognitively impaired.15,16
Thus, there is a clear need to establish whether reducing depth of anesthesia and anesthetic exposure (beyond levels consistent with general anesthesia) can reduce delirium after surgery in a representative population of older adults. This question is highly applicable since many of the most common surgeries in older adults can be performed using neuraxial/regional approaches.17 Lumbar spine fusion surgery is one such surgery that is among the top 5 most frequent surgeries in older adults,17 with an estimated incidence of postoperative delirium of 10–30%.18–20 Therefore, we conducted a randomized pragmatic trial in older patients undergoing lumbar spine surgery, with the hypothesis that a bundled approach to reduce anesthetic agent exposure as determined by Bispectral Index [BIS] values (spinal anesthesia with targeted light sedation based on BIS values) compared with general anesthesia with masked BIS values would reduce the incidence of postoperative delirium.
MATERIALS AND METHODS
Study Design
The research protocol was approved by the Mercy Medical Center (Baltimore MD) Institutional Review Board (IRB# 2015–45). The trial was registered at ClinicalTrials.gov (NCT03133845, PI Charles Brown). The initial protocol was released by the investigators to ClinicalTrials.gov on 10/23/15. Due to quality control issues (in particular, the specificity of some outcomes, most notable post-discharge secondary outcomes that are not reported in this manuscript) the protocol was not formally registered and released to the public until April 2017, and so the formal registration was retrospective to the start of the trial. The primary aim and outcome as reported in this manuscript have been unchanged since the initial submission to ClinicalTrials.gov on 10/23/15. However, the secondary delirium outcomes (delirium severity and number of days of delirium) were not formally added to the trial registration until April 2017, although these outcomes were collected since the start of the trial as part of the study protocol. Other changes in enrollment criteria and sample size calculation are described below. Participants provided written informed consent. The SHaping Anesthetic techniques to Reduce Postoperative delirium (SHARP) study was conducted as a single-center prospective randomized controlled superiority trial with two parallel groups. The protocol was published near the end of the trial to summarize the conduct of the trial and provide the final statistical plan.21
Participants
Patients were approached prior to scheduled surgery by a research coordinator to evaluate eligibility and obtain informed consent. Inclusion criteria were: 1) age ≥65 years; 2) undergoing lumbar spine fusion; 3) expected surgery duration <3 hours; 4) under the care of a participating surgeon; and 5) ability to understand and comply with study procedures. Exclusion criteria were: 1) contraindications to spinal anesthesia (e.g. severe aortic stenosis, anti-coagulant therapy); 2) Body Mass Index >40 kg/m2; 3) prior L2–5 full lumbar fusion; 4) communication issues precluding baseline assessments; 5) baseline dementia or Mini-Mental State Examination <24; 6) psychiatric disease precluding cooperation with sedation; and 7) surgeon or anesthesiologist preference for either anesthetic approach for any reason due to clinical considerations. Delirium was not formally assessed, although all patients were assessed for capacity to consent. Patients were enrolled between September 2015 to May 2019. Eligibility criteria were expanded after the study began to allow slightly younger patients, a higher body mass index, and longer duration of surgery. The specific criteria that were changed were: a decrease in the lower age limit from 70 y/o to 65 y/o, an increase in the upper limit of body mass index (from 35 kg/m2 to 40 kg/m2), and an increase in the upper limit of anticipated surgery duration (from 2 to 3 hours).
Randomization and Assignment of Intervention
A computer-generated simple randomization list with 1:1 allocation was created by a research nurse before the study. For allocation concealment, assignments were placed in sealed opaque envelopes, which were sequentially handed to clinicians after randomization, before entering the operating room.
Intervention and Control
The intervention group received spinal anesthesia with targeted depth of anesthesia based on BIS values. The BIS monitor is approved to monitor depth of anesthesia and displays a unit-less number (0–100) derived from processed EEG waveforms. BIS values between 40–60 are consistent with general anesthesia.22 In the intervention group, spinal anesthesia was obtained using intrathecal injection of bupivacaine (10–15 mg) or lidocaine. Patients received sedation with propofol (25–150 mcg/kg/minute), targeted to a BIS >60–70. However, the anesthesiologist was instructed to prioritize clinical concerns if depth of sedation needed to be increased.
In the control group, patients received general anesthesia with an endotracheal tube. Anesthesia induction was with propofol (1–2 mg/kg) or etomidate, maintenance with a volatile anesthetic, muscle relaxation with a non-depolarizing muscle relaxant, and analgesia with fentanyl (generally 2–5 mcg/kg titrated) or hydromorphone and/or morphine. Patients on baseline opioids could receive additional opioids based on clinical criteria. For patients under general anesthesia, the anesthetic provider was masked to BIS values unless there was a clinical need.
Masking
Delirium outcome assessors were masked to the intervention. Postoperative data was abstracted from the electronic medical record by staff masked to the intervention. Patients, surgeons, and anesthesiologists were not masked, because it is impossible for the anesthetic technique to be masked to treating physicians or patients. Statisticians and investigators involved in data analysis were masked.
Perioperative Management
Perioperative care was based on established clinical protocols. Patients could receive intrathecal morphine during spinal anesthesia at the discretion of the anesthesiologist, or by direct intraoperative injection at the discretion of the surgeon. Postoperative analgesia was with fentanyl or hydromorphone patient-controlled analgesia, with transition to oxycodone or other oral opioids as tolerated.
Outcomes and other Covariates
Delirium was assessed once-daily during the first three postoperative days in the hospital using the validated Confusion Assessment Method23 (sensitivity 94–100%, specificity 90–95%). For purposes of missing data, daily in-hospital assessments were not considered missing if the patient was discharged from the hospital on that day and not available for assessment. The Confusion Assessment Method assessment included formal tests of cognition (Mini-Mental State Examination,24 Calendar Reverse Months, Shortened Digit Span Forward/Reverse, and Delayed Word Recall tests) as well as questions for nurses, clinicians, and family. Patients who refused an assessment and no delirium assessment could be made were considered to not have delirium for that assessment. The primary outcome was incident delirium as defined by any positive assessment during hospitalization. A chart review for delirium was also conducted using validated methods to supplement in-person assessments.25 Secondary outcomes included delirium duration and severity (Delirium Rating Scale-Revised 98).26 Covariate information was collected from baseline assessments, patient report, and the medical record. Instrumental activities of daily living were measured at baseline.27 Number of surgical levels included the range of involved vertebrae.
Sample Size
At the start of the trial, we assumed a delirium incidence of 40% in the control group (general anesthesia with masked BIS values) and a 50% reduction in the intervention group, based on prior studies.15,18 Further, we assumed a 4–6% dropout or crossover. With these assumptions, 190 patients would be needed to show a difference in incidence of delirium at a 0.05 significance level with a power of 0.8. After the first year of data collection, the delirium incidence was noted to be less than predicted, and so the sample size was increased to at least 218, based on a revised assumption of delirium incidence (40% to 35% in the control arm) and similar assumptions regarding 50% reduction in delirium in the intervention group and 4–6% dropout.
Statistical Analysis
The primary analysis was based on the intention to treat principle (patients included in the group to which randomized). For the primary outcome, incident delirium, both the absolute difference and relative change were computed. The chi-squared test was used to compare proportions with the primary outcome between groups. Secondary outcomes were compared using Wilcoxon rank sum tests. Normally-distributed variables are reported using mean and standard deviation, and non-normally distributed variables are reported using mean and interquartile range. Adjusted analyses were conducted with multivariable logistic regression to account for potential confounding, first with pre-specified adjustment for age, education, and cognitive score28 and second with adjustment for additional variables associated with delirium in bivariate analyses. As-treated analyses were also conducted (patients included in the group to which they received treatment). Standard diagnostics, including goodness of fit, influence, and collinearity, were examined for all regression models. BIS data was downloaded from the monitor after surgery and was analyzed in several ways, including the mean(SD) and minutes below or above clinically relevant cutoffs (BIS<40 and BIS>55), based on the methodology of prior studies.11,16
Pre-specified subgroup analyses were conducted based on stratification by age (<75 vs. ≥75 years old), Charlson Comorbidity Index (0 vs. ≥1), and baseline cognition (Mini-Mental State Examination <27 vs ≥27), with cutoffs chosen based on biological relevance and/or to have anticipated sufficient number of patients in the subgroups.16,29,30 Post-hoc, we examined 4 subgroups identified based on differences in bivariate analyses. Relative risks were calculated within each subgroup, and 95% confidence intervals were generated using the percentile method via a bootstrap procedure (5,000 bootstrap samples). The hypothesis that the intervention would have differential effect based on subgroups was formally tested using a p-value for interaction, without adjustment for other co-variates. SAS v9.4 (Cary, NC) was used. Formal interim analyses were to assess recruitment, safety events, and dropout, but not efficacy, and a Data Safety and Monitoring Board monitored study conduct and safety. There were no pre-specified stopping criteria, and enrollment ceased when target sample size was obtained. In all analyses, p<0.05 was considered significant and all hypothesis testing was two-tailed.
RESULTS
A patient flow diagram is shown in Figure 1. Of 799 patients screened from September 8, 2015 to May 6, 2019, 111 patients were randomized to spinal anesthesia with targeted sedation based on BIS values, and 108 patients were randomized to general anesthesia with masked BIS values. Reasons that patients were not enrolled and randomized are listed in Figure 1. Enrollment was stopped upon accrual of enrollment goals. Among patients randomized to spinal anesthesia with targeted sedation based on BIS values, an adequate level of spinal anesthesia could not be obtained in 7 patients, and these patients crossed-over to receive general anesthesia. Among patients randomized to general anesthesia with masked BIS values, 2 patients withdrew after randomization, and 1 patient crossed-over to receive spinal anesthesia.
Figure 1.
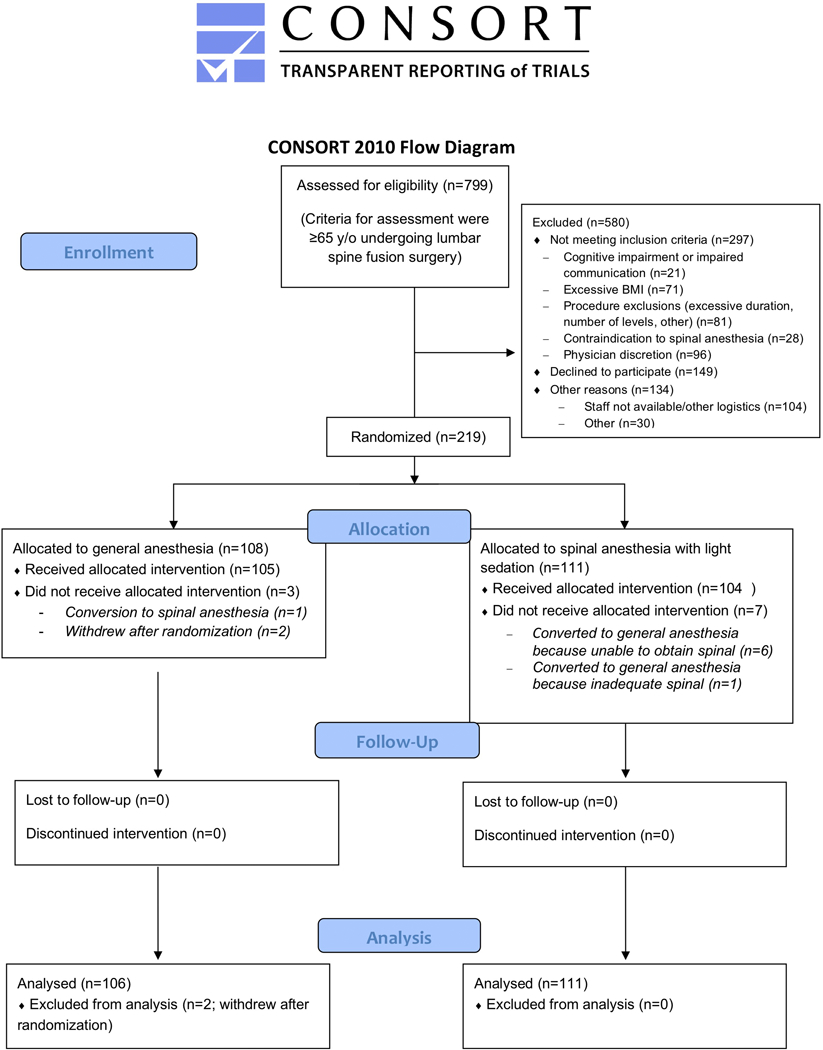
Consort Diagram
A patient flow diagram is shown.
Baseline Patient Characteristics
The median age of patients in this study was 72 (interquartile range [IQR] 69, 77), 38% were male, and the median Mini-Mental State Examination score was 29 (IQR 27, 29). Patients rated their average preoperative pain as a median of 7 (IQR 5, 8) and their current pain as a median of 3 (IQR 1, 6). Patient characteristics were generally similar in the two arms of the study (Table 1). However, Charlson Comorbidity Index was slightly higher and there were more patients with a prior myocardial infarction and atrial fibrillation in the spinal anesthesia with targeted sedation based on BIS values group
Table 1:
Baseline Patient Characteristics
| Total (n=217)a | General Anesthesia with Masked BIS values (n=106) | Spinal Anesthesia with Targeted Sedation Based on BIS Values (n=111) | |
|---|---|---|---|
|
| |||
| Age (years), median (IQR) | 72 (69–77) | 72 (69–76) | 73 (69–78) |
| Male, n (%) | 83 (38.2) | 35 (33.0) | 48 (43.2) |
| Race, n (%) | |||
| Caucasian | 197 (90.8) | 93 (87.7) | 104 (93.7) |
| African-American | 20 (9.2) | 13 (12.3) | 7 (6.3) |
| Education college or higher, n (%) | 104 (47.9) | 49 (46.2) | 55 (49.5) |
| Living arrangement (% at home) | 203 (94.4) | 95 (91.3) | 108 (97.3) |
| Mini-Mental State Examination,b median (IQR) | 29 (27–29) | 28 (27–29) | 29 (27–29) |
| Instrumental Activities of Daily Living,c median (IQR) | 13 (12–14) | 13 (12–14) | 13 (12–14) |
| Comorbidities, n (%) | |||
| Prior Stroke | 3 (1.4) | 2 (1.9) | 1 (0.9) |
| Hypertension | 157 (72.4) | 74 (69.8) | 83 (74.8) |
| Atrial Fibrillation | 12 (5.5) | 2 (1.9) | 10 (9.0) |
| Congestive Heart Failure | 1 (0.5) | 0 (0) | 1 (0.9) |
| Myocardial Infarction | 20 (9.2) | 5 (4.7) | 15 (13.5) |
| Peripheral Vascular Disease | 9 (4.1) | 1 (0.9) | 8 (7.2) |
| Chronic Obstructive Pulmonary Disease | 22 (10.1) | 10 (9.4) | 12 (10.8) |
| Tobacco (prior) | 73 (33.6) | 33 (31.1) | 40 (36) |
| Diabetes | 54 (24.9) | 25 (23.6) | 29 (26.1) |
| Chronic Kidney Disease | 38 (17.5) | 15 (14.2) | 23 (20.7) |
| ASA Classificationd, median (IQR) | 2 (2–3) | 2 (2–3) | 2 (2–3) |
| Charlson Comorbidity Index,e median (IQR) | 1 (0–1) | 0 (0–1) | 1 (0–1) |
| Hemoglobin (g/dL), mean (SD) | 13.5 (1.3) | 13.6 (1.2) | 13.5 (1.4) |
| Baseline Medications | |||
| Aspirin, n (%) | 21 (9.8) | 12 (11.5) | 9 (8.1) |
| Beta Blockers, n (%) | 56 (26) | 21 (20.2) | 35 (31.5) |
| Calcium Channel Blockers, n (%) | 51 (23.7) | 22 (21.2) | 29 (26.1) |
| Angiotensin Converting Enzyme-Inhibitors, n (%) | 43 (20) | 17 (16.3) | 26 (23.4) |
| Angiotensin II-Receptor Blockers, n (%) | 49 (22.8) | 26 (25) | 23 (20.7) |
| Statin, n (%) | 109 (50.7) | 55 (52.9) | 54 (48.6) |
| Selective Serotonin Reuptake Inhibitors or Serotonin and Norepinephrine Reuptake Inhibitors, n (%) | 39 (18.1) | 20 (19.2) | 19 (17.1) |
| Other psychotropic medication, n (%) | 23 (10.7) | 9 (8.7) | 14 (12.6) |
| Short-acting opioids, n (%) | 106 (49.3) | 44 (42.3) | 62 (55.9) |
| Current Pain,f median (IQR) | 3 (1–6) | 3 (1–7) | 3 (0–5) |
| Average Pain,f median (IQR) | 7 (5–8) | 7 (5–8) | 8 (5–8) |
All variables were complete (n=217) except the following: Instrument Activities of Daily Living, ASA score (n=211), current and average pain (n=212), living status, all baseline medications (n=215), hemoglobin (n=216)
Mini-Mental State Examination scores range from 0–30, with higher scores indicating better performance.
Instrumental Activities of Daily Living scores range from 0–14 with higher scores indicating better functional status.
For non-brain dead surgical patients, ASA scores range from 1–5 with higher scores indicating greater co-morbidities.
The Charlson Comorbidity Index ranges from 0–33, with higher scores indicating greater risk of long-term mortality.
Pain is rated on a scale of 0–10, with higher scores indicating more pain.
Perioperative Characteristics and Separation in BIS Values
Intra- and postoperative characteristics are described in Table 2 (intention to treat) and Supplemental Digital Content Table 1 (as-treated). Overall, the median length of surgery was 128 minutes (IQR 106,159), the median number of spinal levels was 3 (IQR 2–4), and the median estimated blood loss was 300 mL (IQR 200–460). In the spinal anesthesia with targeted sedation based on BIS values group, the median dose of bupivacaine was 14 mg (IQR 12.5, 15), and the maximum propofol infusion rate was a median of 80 mcg/kg/min (IQR 75, 100). Among patients who received general anesthesia, desflurane was predominantly utilized. Patients in the general anesthesia with masked BIS values group received more fentanyl and less IV fluids.
Table 2:
Perioperative and Postoperative Characteristics by Randomization Group
| Overall (n=217)a | General Anesthesia with Masked BIS Values (n=106) | Spinal Anesthesia with Targeted Sedation Based on BIS Values (n=111) | P-value | |
|---|---|---|---|---|
|
| ||||
| Intraoperative | ||||
| Duration of Surgery (minutes), median (IQR) | 128 (106–159) | 130 (110–163) | 123 (102–154) | 0.262 |
| Number of Levels, median (IQR) | 3 (2–4) | 3 (2–3) | 3 (2–4) | 0.425 |
| Anesthetic Management | ||||
| Spinal Anesthesia Arm | ||||
| Bupivacaine dose (mg), median (IQR) | 14 (12.5–15) | N/A | 14 (12.5–15) | N/A |
| Maximum propofol infusion (mcg/kg/min), median (IQR) | 80 (75–100) | N/A | 80 (75–100) | N/A |
| General Anesthesia Arm | ||||
| Desflurane, n (%) | 82 (37.8) | 77 (72.6) | N/A | N/A |
| Intrathecal morphine, n (%) | 134 (61.8) | 57 (53.8) | 77 (69.4) | 0.018 |
| Intrathecal morphine (mg), median (IQR) | 0.2 (0.2–0.2) | 0.2 (0.2–0.2) | 0.2 (0.2–0.2) | 0.019 |
| Fentanyl, n (%) | 203 (93.5) | 100 (94.3) | 103 (92.8) | 0.643 |
| Fentanyl (mcg), median (IQR) | 150 (100–250) | 200 (150–250) | 100 (100–100) | <0.001 |
| Hydromorphone, n (%) | 43 (19.8) | 40 (37.7) | 3 (2.7) | <0.001 |
| Hydromorphone (mg), median (IQR) | 1.5 (1–2) | 1.3 (1–2) | 2 (1–2) | 0.449 |
| Midazolam, n (%) | 69 (31.8) | 33 (31.1) | 36 (32.4) | 0.837 |
| Midazolam (mg), median (IQR) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.554 |
| Phenylephrine, n (%) | 50 (23.0) | 23 (21.7) | 27 (24.3) | 0.646 |
| Phenylephrine (mcg), median (IQR) | 300 (200–650) | 300 (50–450) | 250 (150–750) | 0.611 |
| Ephedrine, n (%) | 140 (64.5) | 68 (64.2) | 72 (64.9) | 0.913 |
| Ephedrine (mg), median (IQR) | 20 (10–33) | 25 (13–40) | 20 (10–30) | 0.055 |
| Fluids Administered (mL), median (IQR) | 2000 (1700–2700) | 2000 (1400–2600) |
2050 (1900–2950) | 0.006 |
| Estimated Blood Loss (mL), median (IQR) | 300 (200–460) | 300 (200–500) | 300 (200–400) | 0.648 |
| Packed Red Blood Cell Transfusion, n (%) | 4 (1.8) | 1 (0.9) | 3 (2.7) | 0.622 |
| Lowest MAP (mm Hg), median (IQR) | 59 (52–64) | 59 (51–64) | 60 (52–64) | 0.672 |
| Average BIS, median (IQR) | 51 (44–63) | 45 (41–50) | 62 (53–70) | <0.001 |
| Duration of BIS<40 (minutes), median (IQR) | 22 (1–76) | 68 (22–102) | 3 (0–22) | <0.001 |
| Duration of BIS>55 (minutes), median (IQR) | 31 (16–92) | 20 (13–30) | 87 (34–110) | <0.001 |
| Duration of PACU (minutes), median (IQR) | 119 (75–164) | 119 (75–169) | 118 (75–160) | 0.530 |
| Pain score at PACU discharge, median (IQR) | 4 (2–6) | 5 (3–7) | 4 (1–5) | 0.004 |
| Postoperative | ||||
| ICU admission, n (%) | 4 (1.8) | 0 (0) | 4 (3.6) | 0.122 |
| Duration of hospitalization (days), median (IQR) | 3 (2–3) | 3 (2–3) | 3 (2–3) | 0.087 |
| Maximum pain on postoperative day 1 (0–10), median (IQR) | 8 (7–10) | 8 (7–10) | 8 (7–10) | 0.413 |
| Complications,b n (%) | ||||
| Stroke | 2 (0.9) | 0 (0) | 2 (1.8) | 0.498 |
| Atrial Fibrillation | 1 (0.5) | 0 (0) | 1 (0.9) | 1.000 |
| Congestive Heart Failure | 0 (0) | 0 (0) | 0 (0) | N/A |
| Myocardial Infarction | 1 (0.5) | 0 (0) | 1 (0.9) | 1.000 |
| Sepsis | 0 (0) | 0 (0) | 0 (0) | N/A |
| Pneumonia | 2 (0.9) | 0 (0) | 2 (1.8) | 0.498 |
| Urinary Tract Infection | 18 (8.3) | 9 (8.5) | 9 (8.1) | 0.919 |
| Pulmonary Embolism or Deep Venous Thrombosis | 2 (0.9) | 1 (0.9) | 1 (0.9) | 1.000 |
| Acute Kidney Injury | 1 (0.5) | 0 (0) | 1 (0.9) | 1.000 |
| Fall | 0 (0) | 0 (0) | 0 (0) | N/A |
| Reoperation | 1 (0.5) | 0 (0) | 1 (0.9) | 1.000 |
| In-Hospital Death | 1 (0.5) | 1 (0.9) | 0 (0) | 0.488 |
All variables were complete except bupivacaine and propofol dose in the spinal anesthesia group (n=101), BIS values (n=192), and postoperative day 1 pain (n=216)
Some patients experienced multiple complications, apart from urinary tract infections. One patient in the general anesthesia group had both a pulmonary embolism and died. One patient in the spinal anesthesia group had a stroke, myocardial infarction, and pneumonia.
The average BIS value in the spinal anesthesia with targeted sedation based on BIS values group was higher than in the general anesthesia with masked BIS values group (median of 62 [IQR 53, 70] vs. 45 [IQR 41, 50], p<0.001). The median duration of BIS<40 was substantially lower in the spinal anesthesia with targeted sedation based on BIS values group compared to the general anesthesia with masked BIS values group (3 min [IQR 0, 22] vs. 68 min [IQR 22,102], p<0.001).
Effect of the Intervention on Postoperative Delirium and Other Outcomes
The overall incidence of delirium was 22% (48/217). Out of 544 opportunities for delirium assessments for non-discharged patients, 509 in-person assessments were completed, and 24 assessments were refused by patients. Two patients refused all assessments. In the intention to treat analysis, there was no significant difference in the incidence of delirium in the spinal anesthesia with targeted sedation based on BIS values group (25.2% [28/111]) compared with the general anesthesia with masked BIS values group (18.9% [20/106], p=0.259), absolute difference 6.4% (95%CI −4.6% to 17.4%) and relative risk 1.22 (95% CI 0.85 −1.76). When a chart review delirium method was used to supplement the in-person assessments, there was no significant difference in the incidence of delirium in the spinal anesthesia with targeted sedation based on BIS values group (27.9% [31/111]) compared with the general anesthesia with masked BIS values group (23.6% [25/106], p=0.465). Similarly, there was no difference by group in the incidence of delirium for each individual postoperative day or in maximum delirium severity score (Table 3 [intention to treat], Supplemental Digital Content Table 2 [as-treated]). The incidence of delirium was also not different between groups when adjusted for variables associated with delirium in bivariate analyses (Supplemental Digital Content Table 3).
Table 3:
Effect of the Intervention on Postoperative Delirium
| General Anesthesia with Masked BIS Values (n=106) |
Spinal Anesthesia with Targeted Sedation Based on BIS Values (n=111) |
P-value | |
|---|---|---|---|
|
| |||
| Any Delirium, n (%)a | 20 (18.9) | 28 (25.2) | 0.259 |
| Number of Days of Delirium, among Delirious Patients, median (IQR) | 1 (1–3) | 1 (1–2) | 0.224 |
| Delirium by Postoperative Daya | |||
| Day 1, n (%) | 7 (6.6) | 15 (13.5) | 0.092 |
| Day 2, n (%) | 15 (14.2) | 22 (19.8) | 0.267 |
| Day 3, n (%) | 11 (10.4) | 14 (12.6) | 0.606 |
| Maximum Delirium Severity Score as Measured by Delirium Rating Scale–Revised-98b median (IQR)a | 4 (3–6) | 5 (3–8) | 0.276 |
| Maximum Delirium Severity Score as Measured by Delirium Rating Scale–Revised-98 by Postoperative Daya | |||
| Day 1, median (IQR) | 3 (2–6) | 4 (3–7) | 0.088 |
| Day 2, median (IQR) | 3 (1.5–5) | 3 (2–6) | 0.354 |
| Day 3, median (IQR) | 3 (1–5) | 3 (1–6) | 0.960 |
Out of 544 opportunities for delirium assessments for non-discharged patients at assessment, 509 in-person assessments were completed, and 24 assessments were refused by patients. 215 patients had a postoperative assessment with the Confusion Assessment Method and Delirium Rating Scale-Revised-98. (2 patients refused all assessments and were considered to not have delirium). For each postoperative day, the number of patients with a Confusion Assessment Method and Delirium Rating Scale-Revised-98 evaluation among the number of non-discharged patients at assessment was 199/217 (postoperative day 1), 190/198 (postoperative day 2), and 120/129 (postoperative day 3).
Delirium Rating Scale–Revised-98 severity scores range from 0–39, with higher scores indicating greater severity of delirium.
Duration of recovery in the post-anesthesia care unit (PACU) was similar between the two groups, but pain at PACU discharge was lower in the spinal anesthesia with targeted sedation based on BIS values group compared with the general anesthesia with masked BIS values group (median 4 [IQR 1, 5] vs. median 5 [IQR 3, 7], p=0.003). There were two strokes in the spinal anesthesia with targeted sedation based on BIS values group, and there was 1 death in the general anesthesia with masked BIS values group. Other complications by randomization group are listed in Table 2 (intention to treat) and Supplemental Digital Content Table 1 (as-treated).
Pre-Specified Subgroup Analyses
There were 3 prespecified subgroup analyses, based on cutoffs of Mini-Mental State Examination, Charlson Comorbidity Index, and age, with forest plot results by the primary intention to treat analysis shown in Figure 2. (The forest plot for the as-treated analysis, as well as an expanded description of the numbers of events in each subgroup, are shown in Supplemental Digital Content Figure 1 and Supplemental Digital Content Table 4 respectively). Baseline Mini-Mental State Examination did moderate the effect of the intervention (p-interaction=0.009). Specifically, for patients with Mini-Mental State Examination<27, the incidence of delirium was less in the spinal anesthesia with targeted sedation based on BIS values group compared to the general anesthesia with masked BIS values group (17.7% [3/17] vs. 43.5% [10/23]). On the other hand, for patients with Mini-Mental State Examination≥27, the incidence of delirium was greater in the spinal anesthesia with targeted sedation based on BIS values group vs. the general anesthesia with masked BIS values group (26.6% [25/94] vs. 12.1% [10/83]). There was no difference in the effect of the intervention (i.e. no interaction) based on the other pre-specified subgroups of age strata (<75 vs. ≥75 years old) or Charlson Comorbidity Index (0 vs ≥1). Several other subgroup analyses were chosen post hoc (sex, education, use of short-acting opioids at baseline, and administration of intrathecal morphine during surgery) (Figure 2). Intrathecal morphine did modify the effect of the intervention in the intention to treat analysis (p-interaction=0.029) but not in the as-treated analysis (p-interaction=0.088). Specifically, for patients who did not receive intrathecal morphine, the incidence of delirium in the intention to treat analysis was less in the spinal anesthesia with targeted sedation based on BIS values group compared to the general anesthesia with masked BIS values group (8.8% [3/34] vs. 20.4% [10/49]). On the other hand, for patients who did receive intrathecal morphine, the incidence of delirium was greater in the spinal anesthesia with targeted sedation based on BIS values group vs. the general anesthesia with masked BIS values group (32.5% [25/77] vs. 17.5% [10/57]).
Figure 2.
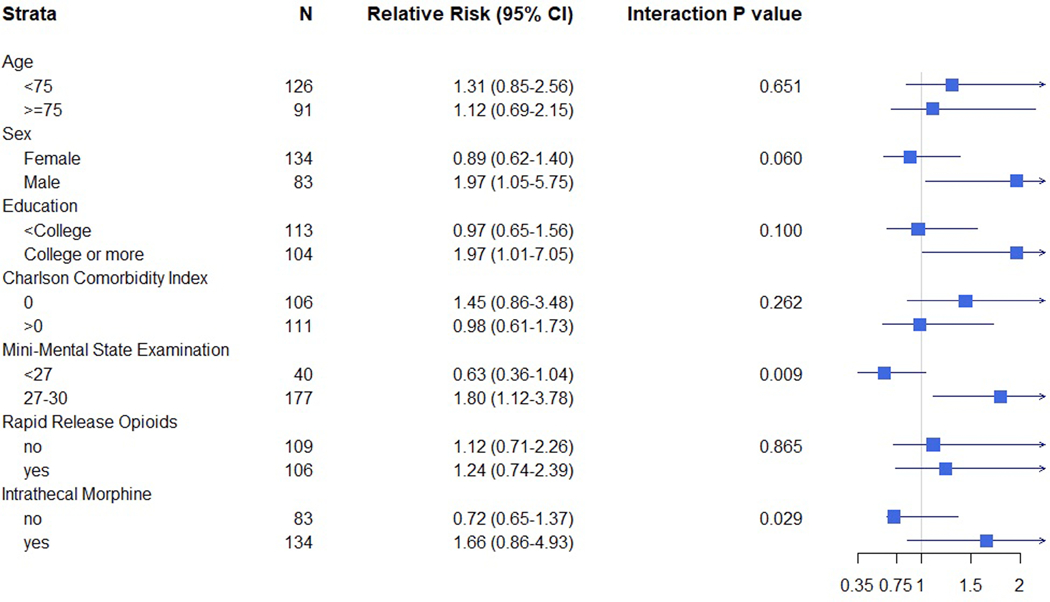
Subgroup Analyses of the Primary Outcome of Incident Delirium
Subgroup analyses based on intention to treat analyses with the primary outcome of incident delirium. Pre-specified subgroup analyses were conducted based on stratification by age, Charlson Comorbidity Index, and baseline cognition. Post-hoc, four subgroups were identified based on differences in bivariate analyses. The effect of anesthetic approach (Relative Risk [95%CI]) is presented separately in each subgroup to define the effect of the intervention in that particular subgroup. The interaction term is a test of significance for whether the effect of anesthetic approach is statistically different between subgroups. Rapid release opioids refer to baseline opioids. Relative Risk <1 favors spinal anesthesia with targeted sedation based on BIS values. Relative Risk >1 favors general anesthesia with masked BIS values.
Risk Factors for Delirium
In bivariate analyses, male sex, lower Mini-Mental State Examination score, Charlson Comorbidity Index score, short-acting opioid medication, anti-depressant medication, longer surgery, and increased postoperative pain were among the variables associated with delirium (Supplemental Digital Content Tables 5 and 6). In adjusted models (Supplemental Digital Content Table 3), only lower Mini-Mental State Examination score remained independently associated with delirium. The administration of intrathecal morphine was also associated with delirium in the adjusted model, but not in the bivariate comparison.
DISCUSSION
The results of this trial demonstrate that spinal anesthesia with targeted sedation based on BIS values compared with general anesthesia with masked BIS values does not reduce the incidence of delirium in lumbar spine surgery patients.
The results of this study add to several studies examining whether titrating depth of anesthesia and anesthetic exposure compared with usual care can reduce delirium. Early trials in general anesthesia patients suggested that a strategy to reduce anesthetic exposure based on BIS values could reduce delirium.11,12 Based on these and other studies, delirium guidelines have recommended depth of anesthesia monitoring may be considered.1 However, the recent large ENGAGES trial reported no difference in delirium in patients randomized to a strategy of avoiding excessive anesthetic exposure and burst suppression on the electroencephalogram.14 Similarly, the results of the current study demonstrate that a bundled approach to reduce anesthetic agent exposure as determined by BIS values does not reduce the incidence of delirium in older adults undergoing lumbar spine fusion surgery.
An important consideration in interpreting prior studies is that in most trials all patients received general anesthesia. The pertinent comparisons were general anesthesia vs. deeper general anesthesia, and the benefits of lighter anesthesia could not be examined. This is an important gap since critical care guidelines recommend that mechanically ventilated patients in the ICU benefit from light sedation,31 a level of consciousness which is substantially more alert than general anesthesia. Two trials in hip fracture surgery patients under spinal anesthesia examined benefits of intraoperative “light” sedation.15,16 However, the results of these two studies were conflicting, and moreover, the elderly, frail, and cognitively-impaired populations may not be generalizable to most older adults undergoing surgery. Thus, there has been a clear need to determine whether reducing depth of anesthesia beyond general anesthesia could reduce delirium in a generalizable population of older adults. This question is highly relevant since many surgeries can be performed with neuraxial or regional approaches. The SHARP study addressed this question in a pragmatic manner and demonstrated no delirium reduction in patients treated with spinal anesthesia with targeted sedation based on BIS values compared with general anesthesia with masked BIS values.
One of three pre-planned subgroup analyses showed different effects of the intervention according to baseline cognition. Specifically, for patients with Mini-Mental State Examination <27, there was less delirium in the spinal anesthesia with targeted sedation based on BIS values group, while for patients with Mini-Mental State Examination ≥27, there was less delirium in the general anesthesia with masked BIS values group. The results of this subgroup analysis are qualitatively similar to a subgroup analysis reported in a trial of depth of sedation in hip fracture surgery patients.16 In this trial in which the median Mini-Mental State Examination score was 24, the subgroup of healthy patients with a CCI score of 0–1 (but not higher) had less delirium with a light vs. deep sedation strategy. Thus, in both the hip fracture trial and the current trial, patients who were relatively healthy but with impaired cognition derived benefit from lighter sedation. These results need to be considered hypothesis-generating since they were subgroup analyses. One potential explanation is that cognitively-impaired patients are more sensitive to anesthetic depth, perhaps due to underlying neurodegenerative disease.32–35 On the other hand, it is not entirely clear why cognitively intact patients benefited from general anesthesia with masked BIS values. The overall risk of delirium was less in these patients, as would be expected. Future studies should examine anesthetic strategies to reduce depth of anesthesia in cognitively impaired older adults, although the logistics of enrolling a sufficient number of eligible patients would be challenging. A post-hoc analysis also showed that the administration of intrathecal morphine was independently associated with delirium and modified the effects of the intervention such that in patients who received intrathecal morphine, there was less delirium in the general anesthesia with masked BIS values group. Prior work has suggested that intrathecal morphine was associated with less postoperative delirium,36 while in our study patients who received intrathecal morphine had more delirium, and the finding of this post-hoc analysis should also be considered exploratory.
In the current study, the strongest and most consistent delirium risk factor was lower Mini-Mental State Examination score. These results are consistent with other studies examining risk factors for delirium,3 and highlight the importance of cognitive testing for risk stratification. Overall, pain and pain treatment were important, with baseline short-acting opioids and maximum postoperative pain being associated with delirium. These results highlight the balance of treating pain while minimizing deliriogenic opioid medication.3,37
There are several strengths of this study. The SHARP trial used a unique study design to compare spinal anesthesia with targeted sedation based on BIS values vs. general anesthesia with masked BIS values in cognitively-intact older adults. The intervention was pragmatic, conducted at a community-based hospital, and achieved a separation in BIS-values. The research group is experienced in assessing postoperative delirium. Although the study sample was older adults undergoing spine surgery, results are likely generalizable to a number of surgeries for which general or neuraxial/regional anesthesia is appropriate.
There are several limitations. The intervention was bundled, and it is unclear which aspect (light sedation, spinal anesthesia, or propofol) was most responsible for the subgroup effect. The doses of propofol that were used were relatively high, the sedation protocol was pragmatic, and a formal observer assessment of sedation was not used. Thus, a number of patients in the spinal anesthesia with targeted sedation based on BIS values group had BIS values below the target of 60–70, and this may have biased the results towards the null. Additionally, BIS may not be an accurate measure of depth of anesthesia in older adults. However, the majority of patients had BIS values that exceeded the upper limit of 55 that has been advocated to prevent awareness during general anesthesia.38,39 The bundled approach also did not permit the use of other sedative agents, such as dexmedetomidine, and future studies are needed to examine potentially beneficial effects of dexmedetomidine in this population. The study was powered for a large effect size, based on a prior study15 and we revised the estimate of delirium incidence due to a lower incidence than originally expected. However, the overall incidence of delirium was still below the expected incidence in the power calculation, and so the study was underpowered. Nevertheless, given the observed effect, it is unlikely that a larger study would demonstrate a benefit in the intervention group. We only assessed delirium once daily, and some cases may have been missed. Thus, imprecision of the outcome assessment and/or misclassification may have biased the results. Patients in the spinal anesthesia with targeted sedation based on BIS values had more cardiac and vascular disease at baseline, although the baseline Mini-Mental State Examination was slightly higher than the general anesthesia with masked BIS values group. Perioperative management aside from the intervention was based on established protocols, and this introduced heterogeneity into the study. There was crossover between study arms in 8 patients, largely due to obtaining adequate spinal anesthesia in patients with degenerative spine disease, and this is a source of bias. However, results were similar in intention to treat and as-treated analyses. The Mini-Mental State Examination is a general screen of cognition and is limited by ceiling effect and educational biases.40 Further the distinction between a Mini-Mental State Examination score above and below 27 may not be clinically meaningful, and so the results of the sub-group analyses should be considered hypothesis-generating. Finally, the trial was not formally registered in ClinicalTrials.gov until 2017 due to quality control issues, although the initial protocol with the aim and primary outcome of this manuscript was submitted in October 2015.
In conclusion, the results of the SHARP study demonstrate that spinal anesthesia with targeted sedation based on BIS values does not reduce delirium in older adults undergoing lumbar spine surgery. Further studies are needed to examine optimal anesthetic strategies in cognitively impaired patients, who are at high risk for delirium.
Supplementary Material
10. Funding:
NIH K76 AG057020 (CB)
International Anesthesia Research Society (CB)
NIH K23 AG051783 (CB)
Footnotes
Conflicts of Interest
CB has consulted for and received grant funding from Medtronic
KJN has received grant funding from Hitachi Inc., and consulted for Merck Inc.
CH has received payment for advisory board membership from Medtronic Inc. (Minneapolis MN) and Edwards Lifesciences (Irvine CA). He serves on a Data Safety Monitoring Committee for Merck Inc. (Kenilworth NJ).
All other authors (CE, CL, LY, ME, YG, RS, DK, RC, NL, SC, ED FS, CD) have no other declaration of interests or conflicts of interests.
Trial Registration:
NCT03133845; PI: Charles Brown; www.clinicaltrials.gov. The initial protocol was released by the investigators to ClinicalTrials.gov on 10/23/2015. Due to quality control issues, the protocol was not formally registered and released to the public until 4/28/2017.
Prior Presentations:
None
Summary Statement:
In a randomized trial of 217 older adults undergoing lumbar spine surgery, spinal anesthesia with targeted sedation based on BIS values did not reduce postoperative delirium compared with general anesthesia with masked BIS values
Contributor Information
Charles H. Brown, IV, Department of Anesthesiology & Critical Care Medicine, Johns Hopkins University School of Medicine, Zayed 6208, 1800 Orleans St. Baltimore MD 21210.
Charles Edwards, II, The Maryland Spine Center at Mercy, 301 St. Paul Place, Baltimore MD, 21202.
Charles Lin, Mercy Anesthesiology Associates, 300 St. Paul Place, Baltimore MD 21202.
Emily Ledford Jones, Department of Anesthesiology & Critical Care Medicine, Johns Hopkins University School of Medicine, Zayed 6208, 1800 Orleans St. Baltimore MD 21210.
Lisa R. Yanek, Department of Medicine, Johns Hopkins University School of Medicine, 1830 E. Monument St. Room 8024, 600 N. Wolfe St, Baltimore, MD 21287.
Melody Esmaili, Mercy Anesthesiology Associates, 300 St. Paul Place, Baltimore MD 21202.
Yara Gorashi, Tufts University School of Medicine, 145 Harrison Ave, Boston, MA 02111.
Richard Skelton, University of Miami Miller School of Medicine, 1600 NW 10th avenue, Miami FL, 33136.
Daniel Kaganov, Wake Forest School of Medicine, 115 N. Sunset Dr. (Apt. C), Winston Salem, NC, 27101.
Ryan Curto, University of Maryland School of Medicine, 655 W Baltimore St, Baltimore MD 21201.
Noah L. Lessing, University of Maryland School of Medicine, 655 W Baltimore St, Baltimore MD 21201.
Stephanie Cha, Department of Anesthesiology & Critical Care Medicine, Johns Hopkins University School of Medicine, Zayed 6208, 1800 Orleans St. Baltimore MD 21210.
Elizabeth Colantuoni, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe St. Baltimore MD, 21287.
Karin Neufeld, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, A4 Center Suite 457, 4940 Eastern Avenue, Baltimore, MD 21224.
Frederick Sieber, Department of Anesthesiology & Critical Care Medicine, Johns Hopkins University School of Medicine, A5W Rm 588, 4940 Eastern Avenue, Baltimore, MD 21224.
Clayton L. Dean, The Maryland Spine Center at Mercy, 301 St. Paul Place, Baltimore MD, 21202.
Charles W. Hogue, Department of Anesthesiology, Northwestern Feinberg School of Medicine, NMH/Feinberg Room 5-704, 251 E Huron, Northwestern Feinberg School of Medicine, Chicago IL 60611.
REFERENCES
- 1.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: Best practice statement from the american geriatrics society. J Am Coll Surg. 2015;220(2):136–48.e1. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: Risk factors and outcomes. Ann Surg. 2009;249(1):173–178. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165. [DOI] [PubMed] [Google Scholar]
- 4.Schubert M, Schurch R, Boettger S, Nunez DG, Schwarz U, Bettex D, Jenewein J, Bogdanovic J, Staehli M, Spirig R, Rudiger A. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - a cohort study. BMC Health Serv Res. 2018;18(1):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown CH, Laflam A, Max L, Lymar D, Neufeld K, Tian J, Shah A, Whitman G, Hogue C. The impact of delirium after cardiac surgical procedures on postoperative resource use. Ann Thorac Surg. 2016;101(5):1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59 Suppl 2:301. [DOI] [PubMed] [Google Scholar]
- 7.Hshieh TT, Saczynski J, Gou RY, Marcantonio E, Jones R, Schmitt E, Cooper Z, Ayres D, Wright J, Travison T, Inouye SK, SAGES Study Group. Trajectory of functional recovery after postoperative delirium in elective surgery. Ann Surg. 2017;265(4):647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt E, Travison T, Saczynski J, Ngo L, Alsop D, Jones R. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown CH, Probert J, Healy R, Parish M, Nomura Y, Yamaguchi A, Tian J, Zehr K, Mandal K, Kamath V, Neufeld K, Hogue C. Cognitive decline after delirium in patients undergoing cardiac surgery. Anesthesiology. 2018;129(3):406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shehabi Y, Bellomo R, Kadiman S,Ti L, Howe B, Reade M, Khoo T, Alias A, Wong Y, Mukhopadhyay A, McArthur C, Seppelt I, Webb S, Green M, Bailey M. Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality: A multinational prospective longitudinal cohort study. Crit Care Med. 2018;46(6):850–859. [DOI] [PubMed] [Google Scholar]
- 11.Chan MT, Cheng BC, Lee TM, Gin T, CODA Trial Group. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25(1):33–42. [DOI] [PubMed] [Google Scholar]
- 12.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110 Suppl 1:98. [DOI] [PubMed] [Google Scholar]
- 13.Whitlock EL, Torres BA, Lin N, Helsten D, Nadelson M, Mashour G, Avidan M . Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. 2014;118(4):809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wildes TS, Mickle AM, Ben Abdallah A, Maybrier H, Oberhaus J, Budelier T, Kronzer A, McKinnon S, Park D, Torres B, Graetz T, Emmert D, Palanca B, Goswami S, Jordan K, Lin N, Fritz B, Stevens T, Jacobsohn E, Schmitt E, Inouye S, Stark S, Lenze E, Avidan M. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: The ENGAGES randomized clinical trial. JAMA. 2019;321(5):473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieber FE, Zakriya KJ, Gottschalk A, Blute M, Lee H, Rosenberg P, Mears S. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieber FE, Neufeld KJ, Gottschalk A, Bigelow G, Oh E, Rosenberg P, Mears S, Stewart K, Ouanes J, Jaberi M, Hasenboehler E, Li T, Wang N. Effect of depth of sedation in older patients undergoing hip fracture repair on postoperative delirium: The STRIDE randomized clinical trial. JAMA Surg. 2018;153(11):987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deiner S, Westlake B, Dutton RP. Patterns of surgical care and complications in elderly adults. J Am Geriatr Soc. 2014;62(5):829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown CH 4th, LaFlam A, Max L, Wyrobek J, Neufeld K, Kebaish K, Cohen D, Walston J, Hogue C, Riley L Delirium after spine surgery in older adults: Incidence, risk factors, and outcomes. J Am Geriatr Soc. 2016;64(10):2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawaguchi Y, Kanamori M, Ishihara H, Abe Y, Nobukiyo M, Sigeta T, Hori T, Kimura T. Postoperative delirium in spine surgery. Spine J. 2006;6(2):164–169. [DOI] [PubMed] [Google Scholar]
- 20.Morino T, Hino M, Yamaoka S, Misaki H, Ogata T, Imai H, Miura H. Risk factors for delirium after spine surgery: An age-matched analysis. Asian Spine J. 2018;12(4):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown CH, Jones EL, Lin C, Esmaili M, Gorashi Y, Skelton R, Kaganov D, Colantuoni E, Yanek L, Neufeld K, Kamath V, Sieber F, Dean C, Edwards C, Hogue C. Shaping anesthetic techniques to reduce post-operative delirium (SHARP) study: A protocol for a prospective pragmatic randomized controlled trial to evaluate spinal anesthesia with targeted sedation compared with general anesthesia in older adults undergoing lumbar spine fusion surgery. BMC Anesthesiol. 2019;19(1):192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2014;(6):CD003843. [DOI] [PMC free article] [PubMed]
- 23.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 25.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr, Leslie DL, Agostini JV A chart-based method for identification of delirium: Validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53(2):312–318. [DOI] [PubMed] [Google Scholar]
- 26.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the delirium rating scale-revised-98: Comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242. [DOI] [PubMed] [Google Scholar]
- 27.Pfeiffer E Multidimensional functional assessment: The OARS methodology. A manual. 2nd ed. Durham NC: Duke University Center for the Study of Aging and Human Development; 1978. [Google Scholar]
- 28.Colantuoni E, Rosenblum M. Leveraging prognostic baseline variables to gain precision in randomized trials. Stat Med. 2015;34(18):2602–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Bryant SE, Humphreys JD, Smith GE, Ivnik R, Graff-Radford N, Petersen R, Lucas J. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65(7):963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitmore RG, Stephen JH, Vernick C, Campbell P, Yadla S, Ghobrial G, Maltenfort M, Ratliff J. ASA grade and charlson comorbidity index of spinal surgery patients: Correlation with complications and societal costs. Spine J. 2014;14(1):31–38. [DOI] [PubMed] [Google Scholar]
- 31.Devlin JW, Skrobik Y, Gelinas C, Needham D, Slooter A, Pandharipande P, Watson P, Weinhouse G, Nunnally M, Rochwerg B, Balas M, van den Boogaard M, Bosma K, Brummel N, Chanques G, Denehy L, Drouot X, Fraser G, Harris J, Joffe A, Kho M, Kress J, Lanphere J, McKinley S, Neufeld K, Pisani M, Payen J, Pun B, Puntillo K, Riker R, Robinson B, Shehabi Y, Szumita P, Winkelman C, Centofanti J, Price C, Nikayin S, Misak C, Flood P, Kiedrowski K, Alhazzani W. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. [DOI] [PubMed] [Google Scholar]
- 32.Xie Z, Dong Y, Maeda U, Moir R, Inouye S, Culley D, Crosby G, Tanzi R. Isoflurane-induced apoptosis: A potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61(12):1300–1306. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z, Dong Y, Wu X, Zhang J, McAuliffe S, Pan C, Zhang Y, Ichinose F, Yue Y, Xie Z. The potential dual effects of anesthetic isoflurane on abeta-induced apoptosis. Curr Alzheimer Res. 2011;8(7):741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Xu J, Wang H, Xu C, Ji C, Wang Y, Feng C, Zhang X, Xu Z, Wu A, Xie Z, Yue Y. Isoflurane-induced spatial memory impairment by a mechanism independent of amyloid-beta levels and tau protein phosphorylation changes in aged rats. Neurol Res. 2012;34(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Z, Dong Y, Maeda U, Alfille P, Culley D, Crosby G, Tanzi R. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104(5):988–994. [DOI] [PubMed] [Google Scholar]
- 36.Koning MV, van der Sijp M, Stolker RJ, Niggebrugge A. Intrathecal morphine is associated with less delirium following hip fracture surgery: A register study. Anesth Pain Med. 2020;10(4):e106076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: The importance of pain and pain management. Anesth Analg. 2006;102(4):1267–1273. [DOI] [PubMed] [Google Scholar]
- 38.Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: The B-aware randomised controlled trial. Lancet. 2004;363(9423):1757–1763. [DOI] [PubMed] [Google Scholar]
- 39.Whitlock EL, Avidan MS. Three blind mice: A tail of discordant trials. Br J Anaesth. 2020;124(2):121–125. [DOI] [PubMed] [Google Scholar]
- 40.Devenney E, Hodges JR. The mini-mental state examination: Pitfalls and limitations. Pract Neurol. 2017;17(1):79–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


