Abstract
Background
Diabetes is an independent predictor of poor outcomes in patients with COVID-19. We compared the effects of the preadmission use of antidiabetic medications on the in-hospital mortality of patients with COVID-19 having type 2 diabetes.
Methods
A systematic search of PubMed, EMBASE, Scopus and Web of Science databases was performed to include studies (except case reports and review articles) published until November 30, 2021. We excluded papers regarding in-hospital use of antidiabetic medications. We used a random-effects meta-analysis to calculate the pooled OR (95% CI) and performed a sensitivity analysis to confirm the robustness of the meta-analyses.
Main findings
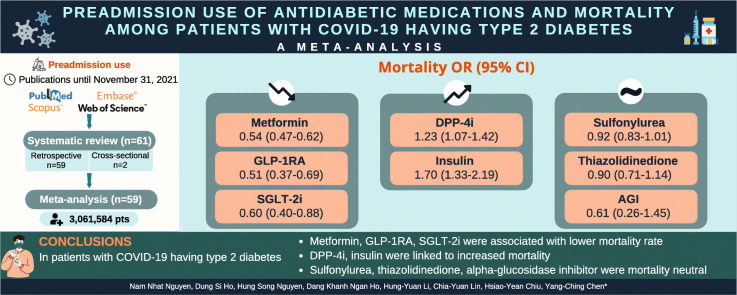
We included 61 studies (3,061,584 individuals), which were rated as having low risk of bias. The OR (95% CI) indicated some medications protective against COVID-related death, including metformin [0.54 (0.47–0.62), I2 86%], glucagon-like peptide-1 receptor agonist (GLP-1RA) [0.51 (0.37–0.69), I2 85%], and sodium–glucose transporter-2 inhibitor (SGLT-2i) [0.60 (0.40–0.88), I2 91%]. Dipeptidyl peptidase-4 inhibitor (DPP-4i) [1.23 (1.07–1.42), I2 82%] and insulin [1.70 (1.33–2.19), I2 97%] users were more likely to die during hospitalization. Sulfonylurea, thiazolidinedione, and alpha-glucosidase inhibitor were mortality neutral [0.92 (95% CI 0.83–1.01, I2 44%), 0.90 (95% CI 0.71–1.14, I2 46%), and 0.61 (95% CI 0.26–1.45, I2 77%), respectively]. The sensitivity analysis indicated that our findings were robust.
Conclusions
Metformin, GLP-1RA, and SGLT-2i were associated with lower mortality rate in patients with COVID-19 having type 2 diabetes. DPP-4i and insulin were linked to increased mortality. Sulfonylurea, thiazolidinedione, and alpha-glucosidase inhibitors were mortality neutral. These findings can have a large impact on the clinicians' decisions amid the COVID-19 pandemic.
Keywords: COVID-19, Type 2 diabetes mellitus, Antidiabetic medication
Graphical abstract
1. Introduction
Since late 2019, SARS-CoV-2 has emerged as a novel pathogenic microbe, resulting in the COVID-19 pandemic. By the end of November 2021, more than 257 million people had been infected with SARS-CoV-2 globally, approximately 5.1 million of whom died [1]. Several risk factors have been linked with the progression and deterioration of COVID-19, such as advanced age, diabetes, hypertension, cardiovascular diseases, and obesity [2]. Diabetes, with its increasing worldwide prevalence, has become major comorbidity in patients with COVID-19 and predisposes them to poor outcomes. Many potential pathways for this have been proposed, including increased inflammatory cascade, immunocompromised status, glucose homeostasis dysfunction, hypercoagulability, alveolar hyperpermeability, and vascular endothelial damage. These pathophysiological changes might lead to acute respiratory distress syndrome, thromboembolism events, and cytokine storms, thereby contributing to increased COVID-19-related deaths [3].
In the past two decades, many drugs have been approved for diabetic patients, leading to a noticeable change in the trend of medication use. Glucose-lowering therapies have also received much critical attention recently as potential host-directed therapies due to their mechanisms of action that may influence the natural course of SARS-CoV-2 infection. Many studies have evaluated whether the preadmission use of certain antidiabetic medications might improve outcomes in those participants. The results have remained controversial, partly because different classes of drugs may differ in their effectiveness and safety against SARS-CoV-2. The gap between preclinical research and real-world data must be bridged. For example, dipeptidyl peptidase-4 inhibitor (DPP-4i) has recently gained much attention due to its safety, cardiovascular neutrality, and potential mechanistic pathways that could alleviate the course of SARS-CoV-2 infection. Although the exact mechanisms underlying the effect of this class on the prognosis of COVID-19 remain unclear, several hypotheses may provide some insights. In addition to glucose homeostasis, DPP-4i inhibits the enzyme DPP-4, which is involved in many events of COVID-19 pathophysiology, including T-cell proliferation, nuclear factor kappa-light-chain-enhancer of activated B (NF-kB) activation, CD86 expression, and inflammatory cytokines production [4]. However, many studies and meta-analyses have indicated no significant benefit of DPP-4i against COVID-19 [5], [6]. Moreover, even for the same drug class, previous small meta-analyses have indicated inconsistent effects regarding the severity or mortality of patients with COVID-19, as in the case of the glucagon-like peptide-1 receptor agonist (GLP-1RA) [5], [7]. Therefore, little is known about their true efficacy in the prognosis of that disease.
In this systematic review and meta-analysis, we (1) summarized the effects of every single antidiabetic medication on the mortality of patients with COVID-19 having diabetes and (2) evaluated the dose-responsiveness of the impacts of medications on mortality. By incorporating much more original papers, our findings would strengthen or reject the evidence for effects of each antidiabetic medication on COVID-19 mortality from inconsistent meta-analyses, and provided novel results regarding the effect of TZD and AGI, and the relationship between dosages and effects, which have not been previously reported.
2. Material and methods
2.1. Population, intervention, comparison, outcomes, and study design (PICOS)
Participants included patients with confirmed COVID-19 who had diabetes and were on prehospital medications extending to the pandemic. A confirmed case of COVID-19 was defined using a positive result on reverse transcription-polymerase chain reaction (RT-PCR) according to the diagnostic procedures of each center. Preexisting diabetes was ascertained through a diabetes diagnosis in medical records. The current use of antidiabetic medications was recorded at the time of recruitment. The interventional therapies considered were one of the following medications: metformin, sulfonylurea (SU), meglitinide (glinide), thiazolidinedione (TZD), alpha-glucosidase inhibitor (AGI), GLP-1RA, DPP-4i, sodium–glucose transporter-2 inhibitor (SGLT-2i), and insulin. Specific-agent users were defined as those who have been on a current prescription. The comparator included nonusers of specific anti-diabetic medications. Our primary outcome was in-hospital mortality or mortality within 90 days, confirmed with the medical record.
We planned to include randomized and nonrandomized controlled trials and observational studies, including prospective and retrospective cohort studies and case-control studies, which were either peer-reviewed or published as abstracts or preprints. If an official publication has already replaced a preprint, the publication was chosen instead of a preprint. We excluded case reports and review articles.
Based on the predetermined inclusion criteria, three independent reviewers (DSH, HSN, and DKNH) searched, screened, reviewed, extracted, and recorded data. In case of discrepancy, a fourth reviewer (NNN) was consulted to reach a final consensus. We verified transparent reporting following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist because we only found observational studies relevant to this topic.
2.2. Systematic review protocol
This systematic review and meta-analysis were registered in the PROSPERO International Prospective Register of Systematic Reviews (ID: CRD42021293064).
2.3. Search strategy and data sources
We systematically searched PubMed/MEDLINE, EMBASE, Scopus, and Web of Science databases for relevant articles up to November 30, 2021, without limiting the language or publication year. The following main keywords and related terms were used: “COVID-19,” “diabetes,” “antidiabetic medication,” or the names of specific classes. The detailed search strategy is presented in Table A.1 (Supplementary appendix). We further identified additional articles through a manual search. We used Endnote (version 20; Clarivate. Philadelphia, PA, USA) to manage studies found.
2.4. Data extraction
The number of events, the number of observations, and other demographic variables, including race/ethnicity, sex, age, HbA1c, diabetes duration, BMI, and percentage of important comorbidities such as hypertension and chronic kidney disease, were documented for each group. OR was also extracted from the papers. The article's corresponding author was contacted through e-mail if raw data were required.
2.5. Data analysis
The risk of bias was assessed by two independent reviewers by using the Newcastle–Ottawa Scale [8].
Effect sizes were calculated as the natural logarithm of ORs. The logOR and standard error of the logOR were used as input for meta-analysis in statistical software. Forest plots were used to display the OR from each original study and the pooled findings. We used Cochran's Q test and I2 statistics to assess heterogeneity between studies [9], [10]. A random-effects model was chosen when the Cochran's Q test p-value of <0.1 or an I2 of >50% was obtained. A fixed-effects model was preferred if there was no evidence of heterogeneity. Publication bias was statistically assessed using Egger's asymmetry test [11]. A publication bias was suspected if the p-value for Egger's test was <0.05. Meta-regression and subgroup analysis were predefined to explore the source of heterogeneity further. We performed meta-regression on a set of prespecified important characteristics, comorbidities, and chronic complications that are commonly found in diabetes patients, including age, gender, race/ethnicity, BMI, hypertension, and chronic kidney disease. We performed sensitivity analysis by outlier removal and trim-and-fill methods and then compared the original results with re-analyzed results to confirm the stability and robustness of our main meta-analyses.
A two-sided p-value of <0.05 was considered statistically significant. We analyzed data by using R software (version 4.0.2; R Foundation for Statistical Computing; Vienna, Austria).
2.6. Ethics
Formal ethics approval is not required because we only collect nonconfidential information from which the patients' identities could not be ascertained.
3. Results
3.1. Literature search and study selection
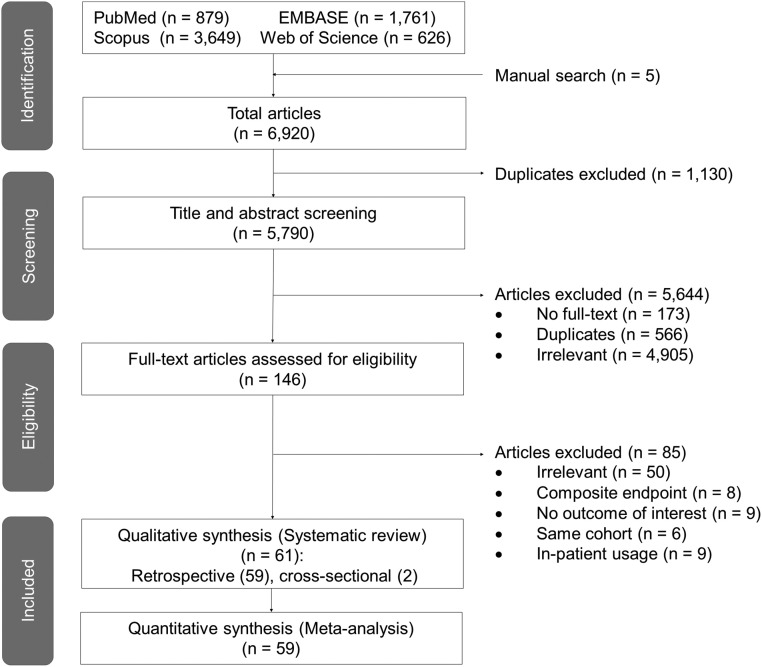
A total of 6920 articles were identified from the databases through a systematic search (Fig. 1 ). Next, 5790 articles remained after deduplication to be screened for their titles and abstracts. Of these articles, 5644 were excluded due to full-text inaccessibility (n = 173), duplication (n = 566), and irrelevancy (n = 4905); thus, 146 papers remained for eligibility assessment. The other 85 publications were further excluded because they did not include the outcome of interest; reported composite endpoint of intensive care unit admission, mechanical ventilation, and death; involved the same cohort; investigated inpatient use of antidiabetic drugs; or were irrelevant to our topic. Finally, 61 studies met our inclusion criteria for a systematic review. However, only 59 articles were pooled in the meta-analyses because one publication reported the hazard ratio instead of odds ratio, and one reported longer-term mortality (7 months) [12], [13].
Fig. 1.
PRISMA flowchart summarizing the study selection process.
3.2. Study and participant characteristics
A total of 3,061,584 participants were recruited from studies [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72]. Most of them were retrospective, except for two cross-sectional studies [26], [52]. The antidiabetic drugs that were investigated included metformin (42 articles), SU (21), TZD (8), AGI (8), GLP-1RA (12), DPP-4i (28), SGLT-2i (13), and insulin (33) (Table 1 ). Only two papers reported glinide-associated mortality in patients with COVID-19 with few users [34], [50]. Therefore, we did not present this drug in our research. The Newcastle–Ottawa assessment results revealed that all studies were rated as having adequate quality (Table A.2). No publication bias was found using Egger's test (Table A.3).
Table 1.
Characteristics of studies (systematic review).
| Study | Country | Number of patients | Race/ethnicity (%) | Male sex (%) | Age (years) | HbA1C (%) | Body mass index (kg/m2) or obesity (%) | Hypertension (%) | CKD (%) | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| Metformin users/nonusers | ||||||||||
| An et al. [15] | Korea | 423/598 | 39.9 | 45.0 ± 19.9 | 18.2 | 0.8 | NS | |||
| Bliden et al. [16] | USA | 34/41 | 8.9 ± 2.3 vs. 8.4 ± 1.8 | NS | ||||||
| Bramante et al. [18] | USA | 2333/3923 | 51.6 vs. 44.6 | 73.0 (66.0–80.0) vs. 76.0 (67.0–84.0) | Obesity: 4.8% vs. 9.0% | 56.3 vs. 60.4 | 6.3 vs. 18.6 | NS | ||
| Cernigliaro et al. [19] | Italy | 82/90 | Decreased | |||||||
| Chen et al. [20] | China | 43/77 | 42.9 | 62.0 (56.0–69.0) vs. 67.0 (57.5–73.0) | 7.7 (6.9–9.1) vs. 8.4 (7.4–10.7) | 61.2 | 10.2 | NS | ||
| Cheng et al. [21] | China | 18/32 | 66.7 vs. 46.9 | 48.0 (40.5–56.5) vs. 58.0 (49.5–66.5) | 25.9 (24.7–26.9) vs. 24.5 (21.3–28.0) | 22.2 vs. 43.8 | 27.8 vs. 37.5 | NS | ||
| Crouse et al. [22] | USA | 76/144 | White: 27.6, African American: 64.9 | 50.6 | 8.0 ± 2.6 vs. 7.0 ± 1.8 | 35.2 ± 9.4 vs. 33.6 ± 8.7 | 91.6 | Decreased | ||
| Dave et al. [23] | Africa | 4084/1624 | 39.3 | 55.0 (46.0–63.0) | 55.5 | 9.2 | Decreased | |||
| Do et al. [25] | Korea | 469/1396 | 51.8 vs. 42.1 | 64.8 ± 11.4 vs. 67.4 ± 12.1 | 73.8 vs. 82.1 | 29.4 vs. 47.4 | NS | |||
| Eliboi et al. [26] | Turkey | 379/53 | 45.6 | 63.3 ± 10.3 | 74.1 | 4.6 | NS | |||
| Ghany et al. [29] | USA | 243/350 | Black: 71.0 vs. 70.0 | 39.0 vs. 41.0 | 70.9 ± 8.9 vs. 71.2 ± 8.9 | 7.7 ± 1.5 vs. 6.4 ± 1.5 | 33.2 ± 7.7 vs. 31.7 ± 9.6 | 60.0 vs. 50.0 | Decreased | |
| Goodall et al. [31] | England | 210/166 | White: 25.5, Black: 13.9, Asian: 37.8 | 64.3 | 69.0 (56.0–80.0) | 49.6 | NS | |||
| Khunti et al. [34] | England | 1,800,005/1,051,460 | White: 64.5, Black: 4.8, Asian: 16.0 | 58.1 | 67.0 (57.0–77.0) | 78.0 | Decreased | |||
| Kim et al. [35] | Korea | 113/122 | 45.1 | 68.3 ± 11.9 | 24.2 ± 3.2 | 62.6 | 7.7 | NS | ||
| Lally et al. [37] | USA | 127/172 | White: 61.4, Black: 30.7 | 98.4 vs. 98.6 | 72.3 ± 8.3 vs. 75.6 ± 9.2 | 7.5 ± 1.4 vs. 6.5 ± 1.3 | 29.7 ± 6.6 vs. 28.0.2 ± 7.0 | 13.4 | Decreased | |
| Li et al. (1) [39] | China | 37/94 | 59.5 vs. 55.3 | 64.6 ± 11.2 vs. 67.7 ± 11.7 | 9.2 ± 4.6 vs.9.0 ± 4.6 | 24.2 ± 3.3 vs. 24.2 ± 3.7 | 62.2 vs. 58.5 | Decreased | ||
| Li et al. (2) [38] | China | 142/245 | 51.1 | 60.0 (49.0–68.0) | 48.6 | 1.0 | NS | |||
| Luk et al. [40] | China | 737/254 | Asian | 55.0 vs. 51.6 | 65.6 (57.7–72.6) vs. 68.9 (61.3–79.7) | 7.3 (6.6–8.5) vs. 6.6 (6.1–7.8) | 24.1 (21.5–27.7) vs. 23.7 (22.2–27.0) | 63.1 vs. 56.7 | 19.5 vs. 37.8 | Decreased |
| Luo et al. (1) [41] | China | 104/179 | 51.0 vs. 57.5 | 63.0 (55.8–68.3) vs. 65.0 (57.5–71.0) | 59.6 vs. 57.0 | Decreased | ||||
| Luo et al. (2) [42] | China | 54/137 | 54.0 vs. 54.0 | 61.0 (56.0–69.0) vs. 61.0 (57.8–68.3) | 8.0 ± 1.9 vs. 6.7 ± 1.9 | 55.5 | 2.0 vs. 2.0 | Decreased | ||
| Ma et al. [43] | USA | 361/995 | White: 72.6, Black: 12.2, Asian: 1.9 | 60.4 vs. 54.1 | 79.5 vs. 85.0 | Decreased | ||||
| Mirani et al. [46] | Italy | 69/21 | 72.5 vs. 71.4 | 69.0 ± 13.0 vs. 75.0 ± 8.0 | Obesity: 47.8% vs. 47.6% | 72.5 vs. 90.5 | 11.6 vs. 42.9 | NS | ||
| Mirsoleymani et al. [47] | Iran | 36/69 | 72.4 | 59.8 ± 17.2 | 37.1 | NS | ||||
| Nafakhi et al. [48] | Iraq | 35/32 | 43.0 | 60.0 ± 10.0 | 29.8 ± 5.0 | 66.0 | Decreased | |||
| Nyland et al. [50] | USA | 5077/24,439 | White: 47.9, African American: 25.5, Asian: 3.1 | 48.2 | 60.9 ± 15.0 | 7.7 ± 2.1 | 32.8 ± 8.9 | 47.7 | 15.5 | Decreased |
| Oh et al. [51] | Korea | 7204/20,289 | 44.7 vs. 38.2 | 3.4 vs. 4.1 | NS | |||||
| Ong et al. [52] | Philippines | 186/169 | 58.6 vs. 52.7 | 61.6 ± 11.6 vs. 63.9 ± 12.8 | 7.0 ± 2.4 vs. 7.6 ± 1.9 | Obesity: 62.0% vs. 65.1% | 73.1 vs. 76.3 | 4.8 vs. 21.9 | Decreased | |
| Orioli et al. [53] | Belgium | 45/23 | 48.0 | 69.0 ± 14.0 | 7.1 (6.6–8.3) | 30.5 ± 5.3 | 80.8 | 34.2 | Decreased | |
| Perez-Belmonte et al. [54] | Spain | 825/663 | 65.7 vs. 57.2 | 74.8 ± 7.9 vs. 77.1 ± 7.1 | Obesity: 26.4% vs. 26.1% | 74.2 vs. 79.5 | 4.7 vs. 29.0 | NS | ||
| Philipose et al. [55] | England | 100/59 | White: 45.5, Afro-Caribbean: 20.2, Asian: 19.1 | 59.0 | 50.2 | NS | ||||
| Ramos-Rincon et al. [56] | Spain | 421/369 | 47.1 | Obesity: 17.7% | 84.3 | 17.2 | NS | |||
| Ravindra et al. [57] | India | 53/313 | 63.2 | 46.7 ± 17.1 | 28.7 | 0.9 | NS | |||
| Saygili et al. [60] | Turkey | 432/154 | 49.8 vs. 50.2 | 65.0 ± 11.2 vs. 68.9 ± 13.5 | 8.0 (6.8–9.9) vs. 7.7 (6.7–10.1) | 67.1 vs. 70.1 | 0.0 | Decreased | ||
| Shetaskova et al. [61] | Russia | 196/113 | Decreased | |||||||
| Silverii et al. [62] | Italy | 76/83 | 54.1 | 73.3 ± 12.7 | Decreased | |||||
| Sourij et al. [63] | Austria | 77/103 | 63.9 | 67.6 ± 14.0 | 6.7 (1.9) | 29.4 ± 5.7 | 77.0 | 23.1 | NS | |
| Tamura et al. [65] | Brazil | 116/72 | 63.5 vs. 61.6 | 62.1 ± 15.1 vs. 68.6 ± 17.3 | 29.2 ± 5.3 vs. 29.4 ± 5.1 | 60.3 vs. 76.4 | 1.7 vs. 12.3 | Decreased | ||
| Wander et al. [66] | USA | 29,685/64,892 | White: 66.0, Black: 27.0, Hispanic: 9.0 | 64.0 | 67.7 | 89.0 | 36.0 | Decreased | ||
| Wang et al. (1) [67] | USA | 9/7 | African American: 23.0, Hispanic: 16.0 | 52.0 | 67 (12.5) | 27.6 | 64.0 | 24.0 | NS | |
| Wang et al. (2) [68] | England | 110/54 | 61.9 vs. 52.0 | 64.8 ± 11.7 vs. 67.8 ± 13.1 | 32.1 ± 6.7 vs. 31.8 ± 6.8 | 59.1 vs. 60.7 | 13.0 vs. 24.2 | NS | ||
| Wargny et al. [69] | France | 1553/1241 | White: 58.1, African: 17.4, Asian: 3.6 | 36.3 | 69.7 ± 13.2 | 7.7 (6.8–9.0) | 28.4 (25.0–32.4) | 76.8 | Decreased | |
| Cheng et al. [12] | China | 678/553 | 53.8 vs. 49.9 | 62.0 (55.0–68.0) vs. 64.0 (58.0–70.0) | 8.1 (7.0–9.9) vs. 7.6 (6.7–8.9) | 24.3 (22.0–25.9) vs. 24.5 (22.6–26.2) | 2.4 vs. 2.6 | NS | ||
| Yuan et al. [72] | China | 73/109 | 52.1 | 62.0 (55.0–70.0) | 8.3 (7.2–9.9) | 23.7 (22.0–25.4) | 52.1 | 0.0 | Decreased | |
| Pazoki et al. [13] | Iran | 177/216 | 56.2 | 65.4 ± 11.6 | 28.0 ± 5.1 | 65.4 | 7.9 | NS | ||
| SU users/nonusers | ||||||||||
| An et al. [15] | Korea | 212/809 | 39.9 | 45.0 ± 19.9 | 18.2 | 0.8 | NS | |||
| Cernigliaro et al. [19] | Italy | 35/137 | NS | |||||||
| Chen et al. [20] | China | 53/67 | 42.9 | 66.0 (60.0–72.5) vs. 65.0 (55.0–73.0) | 8.3 (7.4–9.5) vs. 7.7 (7.1–10.4) | 61.2 | 10.2 | NS | ||
| Dave et al. [23] | Africa | 2110/3598 | 39.3 | 55.0 (46.0–63.0) | 55.5 | 9.2 | NS | |||
| Eliboi et al. [26] | Turkey | 66/366 | 45.6 | 63.3 ± 10.3 | 74.1 | 4.6 | NS | |||
| Khunti et al. [34] | England | 561,290/2,290,175 | White: 63.7, Black: 5.0, Asian: 17.2 | 60.5 | 67.0 (57.0–77.0) | 80.7 | Decreased | |||
| Kim et al. [35] | Korea | 60/175 | 45.1 | 68.3 ± 11.9 | 24.2 ± 3.2 | 62.6 | 7.7 | NS | ||
| Li et al. (1) [39] | China | 22/109 | 56.5 | 66.8 ± 11.6 | 7.9 ± 1.9 | 24.2 ± 3.4 | 59.5 | NS | ||
| Li et al. (2) [38] | China | 91/296 | 51.1 | 60.0 (49.0–68.0) | 48.6 | 1.0 | NS | |||
| Luk et al. [40] | China | 385/679 | Asian | 57.7 vs. 51.5 | 66.0 (58.5–73.1) vs. 65.3 (57.3–73.6) | 7.7 (6.9–9.1) vs. 6.9 (6.4–8.2) | 24.4 (21.8–27.8) vs. 23.5 (21.5–27.0) | 69.4 vs. 48.5 | 25.5 vs. 19.9 | NS |
| Luo et al. [42] | China | 37/154 | 56.5 | 62.7 ± 11.0 | 7.9 (6.3–9.0) | 55.5 | 3.0 | Decreased | ||
| Mirani et al. [46] | Italy | 10/80 | 60.0 vs. 73.8 | 75.0 ± 8.0 vs. 70.0 ± 12.0 | Obesity: 50.0% vs. 47.5% | 80.0 vs. 76.3 | 0.0 vs. 21.4 | NS | ||
| Nyland et al. [50] | USA | 1889/27,627 | White: 47.9, African American: 25.5, Asian: 3.1 | 48.2 | 60.9 ± 15.0 | 7.7 ± 2.1 | 32.8 ± 8.9 | 47.7 | 15.5 | Decreased |
| Oh et al. [51] | Korea | 3680/23,813 | NS | |||||||
| Orioli et al. [53] | Belgium | 19/49 | 48.0 | 69.0 ± 14.0 | 7.1 (6.6–8.3) | 30.5 ± 5.3 | 80.8 | 34.2 | NS | |
| Shetaskova et al. [61] | Russia | 129/180 | NS | |||||||
| Silverii et al. [62] | Italy | 33/126 | 54.1 | 73.3 ± 12.7 | NS | |||||
| Sourij et al. [63] | Austria | 14/166 | 63.9 | 67.6 ± 14.0 | 6.7 (1.9) | 29.4 ± 5.7 | 77.0 | 23.1 | NS | |
| Wander et al. [66] | USA | 12,298/52,594 | White: 66.0, Black: 27.0, Hispanic: 9.0 | 64.0 | 67.7 | 89.0 | 36.0 | NS | ||
| Wargny et al. [69] | France | 782/2012 | White: 58.1, African: 17.4, Asian: 3.6 | 36.3 | 69.7 ± 13.2 | 7.7 (6.8–9.0) | 28.4 (25.0–32.4) | 76.8 | NS | |
| Yuan et al. [72] | China | 43/139 | 55.8 | 67.0 (60.0–73.0) | 8.5 (7.0–9.5) | 23.7 (22.0–25.4) | 48.8 | 0.0 | Decreased | |
| Pazoki et al. [13] | Iran | 72/321 | 56.2 | 65.4 ± 11.6 | 28.0 ± 5.1 | 65.4 | 7.9 | NS | ||
| TZD users/nonusers | ||||||||||
| Cernigliaro et al. [19] | Italy | 10/162 | NS | |||||||
| Eliboi et al. [26] | Turkey | 27/405 | 45.6 | 63.3 ± 10.3 | 74.1 | 4.6 | NS | |||
| Khunti et al. [34] | England | 60,085/2,791,380 | White: 63.5, Black: 3.7, Asian: 18.4 | 63.4 | 67.0 (57.0–77.0) | 80.5 | NS | |||
| Luo et al. [42] | China | 7/184 | 56.5 | 62.7 ± 11.0 | 7.9 (6.3–9.0) | 55.5 | 3.0 | NS | ||
| Nyland et al. [50] | USA | 469/23,714 | White:52.4, African American: 23.2, Asian: 3.5 | 53.3 vs. 48.8 | 63.1 ± 12.5 vs. 60.9 ± 15.3 | 8.2 ± 2.0 vs. 7.5 ± 2.1 | 34.3 ± 9.0 vs. 32.3 ± 8.7 | 52.1 vs. 44.9 | 17.4 vs. 14.9 | NS |
| Oh et al. [51] | Korea | 1264/26,229 | NS | |||||||
| Silverii et al. [62] | Italy | 8/151 | 54.1 | 73.3 ± 12.7 | NS | |||||
| Wander et al. [66] | USA | 2075/62,817 | White: 66.0, Black: 27.0, Hispanic: 9.0 | 64.0 | 67.7 | 89.0 | 36.0 | NS | ||
| AGI users/nonusers | ||||||||||
| An et al. [15] | Korea | 7/1014 | 39.9 | 45.0 ± 19.9 | 18.2 | 0.8 | Increased | |||
| Chen et al. [20] | China | 69/51 | 42.9 | 66.0 (57.5–73.0) vs. 65.0 (56.0–72.0) | 8.4 (7.4–10.3) vs. 7.9 (6.9–9.1) | 61.2 | 10.2 | NS | ||
| Khunti et al. [34] | England | 1665/2,849,800 | White: 56.5, Black: 7.5, Asian: 23.4 | 56.8 | 67.0 (57.0–77.0) | 87.4 | NS | |||
| Li et al. (1) [39] | China | 38/93 | 56.5 | 66.8 ± 11.6 | 7.9 ± 1.9 | 24.2 ± 3.4 | 59.5 | NS | ||
| Li et al. (2) [38] | China | 140/247 | 51.1 | 60.0 (49.0–68.0) | 48.6 | 1.0 | NS | |||
| Luo et al. [42] | China | 77/114 | 65.0 vs. 63.0 | 62.3 ± 9.6 vs. 61.9 ± 9.4 | 7.9 ± 1.8 vs. 8.3 ± 2.0 | 55.5 | 2.2 vs. 0.0 | Decreased | ||
| Nyland et al. [50] | USA | 16/29,500 | White: 47.9, African American: 25.5, Asian: 3.1 | 48.2 | 60.9 ± 15.0 | 7.7 ± 2.1 | 32.8 ± 8.9 | 47.7 | 15.5 | NS |
| Yuan et al. [72] | China | 88/94 | 51.1 | 66.0 (57.0–72.0) | 8.2 (7.0–9.2) | 23.7 (22.0–25.4) | 58.0 | 1.1 | Decreased | |
| GLP-1RA users/nonusers | ||||||||||
| Cernigliaro et al. [19] | Italy | 8/164 | NS | |||||||
| Israelsen et al. [32] | Denmark | 370/558 | 53.0 | 59.0 (51.0–70.0) | Obesity: 29.2% | 56.2 | NS | |||
| Kahkoska et al. [33] | US | 6692/5854 | White: 64.1 | 40.9 | 55.7 ± 12.6 | 8.0 ± 2.0 | 37.2 ± 8.1 | 74.9 vs. 76.0 | 18.5 | Decreased |
| Khunti et al. [34] | England | 100,820/2,750,645 | White: 76.3, Black: 3.3, Asian: 7.9 | 51.7 | 67.0 (57.0–77.0) | 83.1 | NS | |||
| Nyland et al. [50] | USA | 1774/23,714 | White: 52.3, Black: 28.7, Asian: 0.9 | 39.2 vs. 48.8 | 55.0 ± 12.7 vs. 60.9 ± 15.3 | 8.4 ± 2.2 vs. 7.5 ± 2.1 | 37.5 ± 9.3 vs. 32.3 ± 8.7 | 55.9 vs. 44.9 | 12.9 vs. 14.9 | Decreased |
| Orioli et al. [53] | Belgium | 5/63 | 48.0 | 69.0 ± 14.0 | 7.1 (6.6–8.3) | 30.5 ± 5.3 | 80.8 | 34.2 | NS | |
| Ramos-Rincon et al. [56] | Spain | 37/753 | 47.1 | Obesity: 17.7% | 84.3 | 17.2 | NS | |||
| Shetaskova et al. [61] | Russia | 1/308 | NS | |||||||
| Silverii et al. [62] | Italy | 7/152 | 54.1 | 73.3 ± 12.7 | NS | |||||
| Sourij et al. [63] | Austria | 3/177 | 63.9 | 67.6 ± 14.0 | 6.7 (1.9) | 29.4 ± 5.7 | 77.0 | 23.1 | NS | |
| Wander et al. [66] | USA | 4737/60,155 | White: 66.0, Black: 27.0, Hispanic: 9.0 | 64.0 | 67.7 | 89.0 | 36.0 | NS | ||
| Wargny et al. [69] | France | 254/2540 | White: 58.1, African: 17.4, Asian: 3.6 | 36.3 | 69.7 ± 13.2 | 7.7 (6.8–9.0) | 28.4 (25.0–32.4) | 76.8 | NS | |
| DPP-4i users/nonusers | ||||||||||
| An et al. [15] | Korea | 229/792 | 39.9 | 45.0 ± 19.9 | 18.2 | 0.8 | NS | |||
| Cernigliaro et al. [19] | Italy | 13/159 | NS | |||||||
| Chen et al. [20] | China | 20/100 | 42.9 | 66.0 (56.0–73.0) vs. 65.0 (57.0–72.0) | 7.8 (6.8–10.6) vs. 8.3 (7.3–9.5) | 61.2 | 10.2 | NS | ||
| Eliboi et al. [26] | Turkey | 246/186 | 45.6 | 63.3 ± 10.3 | 74.1 | 4.6 | NS | |||
| Emral et al. [27] | Turkey | 6846/26,632 | 42.0 vs. 41.3 | 60.0 (16.0) vs. 52.0 (24.0) | 8.1 (2.7) vs. 6.4 (1.6) | 30.8 (6.7) vs. 29.4 (7.3) | 85.6 vs. 64.0 | Decreased | ||
| Fanidi et al. [28] | Italy | 9/72 | 72.2 ± 12.8 vs. 70.1 ± 13.3 | 7.5 ± 3.3 vs. 7.6 ± 4.3 | 88.9 vs. 67.1 | 11.2 vs. 15.8 | NS | |||
| Israelsen et al. [32] | Denmark | 284/644 | 60.9 | 67.0 (57.0–76.0) | Obesity: 12.3% | 61.6 | NS | |||
| Kahkoska et al. [33] | USA | 3511/8935 | White: 57.4 | 49.9 | 64.1 ± 12.9 vs. 58.6 ± 13.1 | 7.8 ± 1.9 vs. 8.0 ± 1.9 | 36.0 ± 6.2 vs. 35.4 ± 8.2 | 78.7 vs. 76.0 | 31.6 | Increased |
| Khunti et al. [34] | England | 479,555/2,371,910 | White: 65.5, Black: 4.7, Asian: 15.7 | 58.3 | 67.0 (57.0–77.0) | 81.6 | Increased | |||
| Kim et al. [35] | Korea | 85/150 | 45.1 | 68.3 ± 11.9 | 24.2 ± 3.2 | 62.6 | 7.7 | NS | ||
| Kristan et al. [36] | USA | 76/756 | White: 32.7, African American: 52.0, Asian: 1.4 | 51.0 | 62.0 ± 15.0 | 7.9 ± 2.3 | 32.9 ± 8.6 | 78.4 | 21.3 | NS |
| Luk et al. [40] | China | 199/952 | Asian | 59.3 vs. 53.2 | 67.0 (58.4–75.5) vs. 65.1 (56.8–72.2) | 7.6 (6.8–8.9) vs. 7.2 (6.5–8.9) | 25.0 (18.7–27.0) vs. 23.3 (21.6–27.4) | 61.8 vs. 52.3 | 36.2 vs. 17.2 | NS |
| Luo et al. [42] | China | 11/180 | 56.5 | 62.7 ± 11.0 | 7.9 (6.3–9.0) | 55.5 | 3.0 | NS | ||
| Meijer et al. [45] | Netherlands | 28/537 | 60.7 vs. 64.2 | 66.9 ± 12.4 vs. 69.5 ± 12.5 | 29.1 ± 6.0 vs. 29.8 ± 6.3 | 66.7 vs. 70.0 | 25.9 vs. 14.4 | NS | ||
| Mirani et al. [46] | Italy | 11/79 | 90.9 vs. 69.6 | 70.0 ± 13.0 vs. 71.0 ± 12.0 | Obesity: 27.3% vs. 50.6% | 54.6 vs. 79.8 | 18.2 vs. 19.0 | Decreased | ||
| Noh et al. [49] | Korea | 453/133 | 49.2 vs. 55.6 | 21.2 vs. 18.0 | NS | |||||
| Nyland et al. [50] | USA | 2264/23,714 | White: 49.2, African American: 36.6, Asian: 5.1 | 49.1 vs. 48.8 | 64.6 ± 13.5 vs. 60.9 ± 15.3 | 8.0 ± 2.0 vs. 7.5 ± 2.1 | 31.4 ± 8.1 vs. 32.3 ± 8.7 | 55.9 vs. 44.9 | 22.4 vs. 14.9 | Increased |
| Oh et al. [51] | Korea | 4132/23,361 | NS | |||||||
| Orioli et al. [53] | Belgium | 4/64 | 48.0 | 69.0 ± 14.0 | 7.1 (6.6–8.3) | 30.5 ± 5.3 | 80.8 | 34.2 | NS | |
| Perez-Belmonte et al. [54] | Spain | 180/1409 | 59.4 vs. 62.9 | 78.8 ± 7.1 vs. 74.7 ± 8.2 | Obesity: 30.6% vs. 28.1% | 55.6 vs. 56.5 | 32.2 vs. 11.9 | Increased | ||
| Ramos-Rincon et al. [56] | Spain | 266/524 | 47.1 | Obesity: 17.7% | 84.3 | 17.2 | Decreased | |||
| Shetaskova et al. [61] | Russia | 26/283 | NS | |||||||
| Silverii et al. [62] | Italy | 13/146 | 54.1 | 73.3 ± 12.7 | NS | |||||
| Sourij et al. [63] | Austria | 42/138 | 63.9 | 67.6 ± 14.0 | 6.7 (1.9) | 29.4 ± 5.7 | 77.0 | 23.1 | NS | |
| Strollo et al. [64] | Italy | 30/163 | 54.9 | 76.7 ± 11.8 | NS | |||||
| Wander et al. [66] | USA | 5810/59,082 | White: 66.0, Black: 27.0, Hispanic: 9.0 | 64.0 | 67.7 | 89.0 | 36.0 | NS | ||
| Wargny et al. [69] | France | 615/2179 | White: 58.1, African: 17.4, Asian: 3.6 | 36.3 | 69.7 ± 13.2 | 7.7 (6.8–9.0) | 28.4 (25.0–32.4) | 76.8 | NS | |
| Wong et al. [70] | China | 107/1107 | 60.7 vs. 53.7 | 66.3 ± 11.7 vs. 65.1 ± 13.0 | 7.8 ± 2.3 vs. 7.4 ± 2.5 | Obesity: 15% vs. 11.3% | 88.8 vs. 75.2 | 30.8 vs. 11.3 | NS | |
| Pazoki et al. [13] | Iran | 20/373 | 56.2 | 65.4 ± 11.6 | 28.0 ± 5.1 | 65.4 | 7.9 | NS | ||
| SGLT-2i users/nonusers | ||||||||||
| Cernigliaro et al. [19] | Italy | 4/168 | Decreased | |||||||
| Eliboi et al. [26] | Turkey | 56/376 | 45.6 | 63.3 ± 10.3 | 74.1 | 4.6 | NS | |||
| Israelsen et al. [32] | Denmark | 274/654 | 61.8 | 59.0 (52.0–68.0) | Obesity: 15.4% | 49.6 | NS | |||
| Kahkoska et al. [33] | USA | 3665/8781 | White: 33.9 | 55.2 | 57.9 ± 11.7 | 8.2 ± 1.8 | 35.2 ± 7.8 | 77.3 | 16.3 | Decreased |
| Khunti et al. [34] | England | 266,505/2,584,960 | White: 66.8, Black: 3.6, Asian: 15.2 | 60.8 | 67.0 (57.0–77.0) | 75.4 | Decreased | |||
| Kim et al. [35] | Korea | 8/227 | 45.1 | 68.3 ± 11.9 | 24.2 ± 3.2 | 62.6 | 7.7 | NS | ||
| Nyland et al. [50] | USA | 792/28,724 | White: 47.9, African American: 25.5, Asian: 3.1 | 48.2 | 60.9 ± 15.0 | 7.7 ± 2.1 | 32.8 ± 8.9 | 47.7 | 15.5 | Decreased |
| Orioli et al. [53] | Belgium | 4/64 | 48.0 | 69.0 ± 14.0 | 7.1 (6.6–8.3) | 30.5 ± 5.3 | 80.8 | 34.2 | NS | |
| Ramos-Rincon et al. [56] | Spain | 45/745 | 47.1 | Obesity: 17.7% | 84.3 | 17.2 | NS | |||
| Shetaskova et al. [61] | Russia | 13/296 | NS | |||||||
| Silverii et al. [62] | Italy | 4/155 | 54.1 | 73.3 ± 12.7 | NS | |||||
| Sourij et al. [63] | Austria | 24/156 | 63.9 | 67.6 ± 14.0 | 6.7 (1.9) | 29.4 ± 5.7 | 77.0 | 23.1 | NS | |
| Wander et al. [66] | USA | 5542/59,350 | White: 66.0, Black: 27.0, Hispanic: 9.0 | 64.0 | 67.7 | 89.0 | 36.0 | Decreased | ||
| Insulin users/nonusers | ||||||||||
| Agarwal et al. [14] | USA | 531/661 | White: 15.5, African American: 74.5 | 49.3 | 67.9 ± 13.7 | 7.5 ± 2.0 | 30.1 ± 7.5 | 90.9 | 42.5 | Increased |
| Boye et al. [17] | USA | 3461/6070 | Caucasian: 47, African American: 31, Asian: 2, Hispanic: 5 | 46.0 | 71.6 ± 12.5 | 7.2 | 37 | Increased | ||
| Cernigliaro et al. [19] | Italy | 42/130 | NS | |||||||
| Chen et al. [20] | China | 7/49 | 42.9 | 65.0 (57.0–72.0) vs. 65.0 (56.0–73.0) | 8.8 (7.4–10.9) vs. 7.5 (6.8–8.3) | 61.2 | 10.2 | Increased | ||
| Cheng et al. [12] | China | 11/39 | 54.5 vs. 53.8 | 58.0 (54.0–60.0) vs. 52.0 (44.0–65.0) | 24.8 (19.9–25.6) vs. 26.0 (23.8–27.2) | 27.3 vs. 38.5 | 27.3 vs. 35.9 | NS | ||
| Crouse et al. [22] | USA | 87/133 | White: 27.6, African American: 64.9 | 50.6 | Obesity: 74.5% | 91.6 | NS | |||
| Dave et al. [23] | Africa | 2073/3635 | 39.3 | 55.0 (46.0–63.0) | 55.5 | 9.2 | Increased | |||
| Deng et al. [24] | China | 29/56 | 57.6 | 65.0 (34.0–91.0) | 68.2 | 7.1 | NS | |||
| Giorda et al. [30] | Italy | 656/1226 | 50.9 | 84.4 | 60.1 | NS | ||||
| Khunti et al. [34] | England | 350,960/2,500,505 | White: 71.1, Black: 4.7, Asian: 12.3 | 54.5 | 67.0 (57.0–77.0) | 85.2 | Increased | |||
| Kim et al. [35] | Korea | 19/216 | 45.1 | 68.3 ± 11.9 | 24.2 ± 3.2 | 62.6 | 7.7 | NS | ||
| Kristan et al. [36] | USA | 281/551 | White: 32.7, African American: 52.0, Asian: 1.4 | 51.0 | 62.0 ± 15.0 | 7.9 ± 2.3 | 32.9 ± 8.6 | 78.4 | 21.3 | NS |
| Lally et al. [37] | USA | 103/190 | White: 54.4, Black: 40.8 | 97.1 | 73.3 ± 9.4 vs. 75.6 ± 9.2 | 7.7 ± 1.5 vs. 6.5 ± 1.3 | 29.3 ± 3.0 vs. 28.0.2 ± 7.0 | 52.4 | NS | |
| Li et al. (1) [39] | China | 26/105 | 56.5 | 66.8 ± 11.6 | 7.9 ± 1.9 | 24.2 ± 3.4 | 59.5 | NS | ||
| Li et al. (2) [38] | China | 102/285 | 51.1 | 60.0 (49.0–68.0) | 48.6 | 1.0 | NS | |||
| Luk et al. [40] | China | 385/679 | Asian | 57.7 vs. 51.5 | 66.0 (58.5–73.1) vs. 65.3 (57.3–73.6) | 7.7 (6.9–9.1) vs. 6.9 (6.4–8.2) | 22.9 (19.8–25.9) vs. 24.4 (22.2–27.4) | 69.4 vs. 48.5 | 25.5 vs. 19.9 | Increased |
| Luo et al. [42] | China | 88/103 | 56.5 | 62.7 ± 11.0 | 7.9 (6.3–9.0) | 55.5 | 3.0 | NS | ||
| Mansour et al. [44] | Iran | 25/86 | 55.9 | 63.6 ± 13.3 | 28.2 ± 5.6 | 57.7 | 9.0 | NS | ||
| Mirani et al. [46] | Italy | 29/61 | 72.4 vs. 72.1 | 72.0 ± 10.0 vs. 70.0 ± 13.0 | Obesity: 51.7% vs. 45.9% | 79.3 vs. 75.4 | 31.0 vs. 13.1 | Increased | ||
| Nyland et al. [50] | USA | 9149/20,367 | White: 47.9, African American: 25.5, Asian: 3.1 | 48.2 | 60.9 ± 15.0 | 7.7 ± 2.1 | 32.8 ± 8.9 | 47.7 | 15.5 | Increased |
| Oh et al. [51] | Korea | 914/26,579 | NS | |||||||
| Orioli et al. [53] | Belgium | 31/37 | 48.0 | 69 ± 14 | 7.1 (6.6–8.3) | 30.5 ± 5.3 | 80.8 | 34.2 | NS | |
| Perez-Belmonte et al. [54] | Spain | 292/1458 | 77.9 ± 9.0 | Obesity: 20.9% vs. 28.8% | 50.0 vs. 57.8 | Increased | ||||
| Ramos-Rincon et al. [56] | Spain | 225/565 | 47.1 | Obesity: 17.7% | 84.3 | 17.2 | NS | |||
| Riahl et al. [58] | USA | 88/78 | White: 6.0, African American: 71.0 | 52.0 | 66.4 ± 12.7 | 8.6 ± 2.5 vs. 7.0 ± 1.7 | 31.1 ± 8.5 | 91.0 | 25.0 | Increased |
| Satman et al. [59] | Turkey | 3340/15,318 | 42.3 | 53.0 (22.0) | 6.9 (2.3) | 30.0 (7.1) | 66.4 | 18.9 | Increased | |
| Shetaskova et al. [61] | Russia | 115/194 | Increased | |||||||
| Silverii et al. [62] | Italy | 43/116 | 54.1 | 73.3 ± 12.7 | NS | |||||
| Sourij et al. [63] | Austria | 41/139 | 63.9 | 67.6 ± 14.0 | 6.7 (1.9) | 29.4 ± 5.7 | 77.0 | 23.1 | NS | |
| Wander et al. [66] | USA | 18,521/46,371 | White: 66.0, Black: 27.0, Hispanic: 9.0 | 64.0 | 67.7 | 89.0 | 36.0 | Increased | ||
| Wargny et al. [69] | France | 1039/1757 | White: 58.1, African: 17.4, Asian: 3.6 | 36.3 | 69.7 ± 13.2 | 7.7 (6.8–9.0) | 28.4 (25.0–32.4) | 76.8 | Increased | |
| Yan et al. [71] | China | 4/30 | 68.8 | 69.4 ± 9.9 | 7.2 (6.7–8.3) | 50.0 | 0.0 | Increased | ||
| Yuan et al. [72] | China | 76/106 | 47.4 | 66.0 (61.0–72.0) | 8.6 (7.9–10.0) | 23.7 (22.0–25.4) | 57.9 | 2.6 | Increased | |
| Pazoki et al. [13] | Iran | 53/340 | 56.2 | 65.4 ± 11.6 | 28.0 ± 5.1 | 65.4 | 7.9 | NS | ||
Data are presented as mean ± SD or median (IQR).
Abbreviation: AGI, alpha-glucosidase inhibitor; CKD, chronic kidney disease; DPP-4i, dipeptidyl peptidase inhibitor; GLP-1RA, glucagon-like peptide-1 receptor agonist; NS, not significant; SGLT-2i, sodium–glucose transporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
3.3. Main findings
3.3.1. Mortality between medication users and nonusers
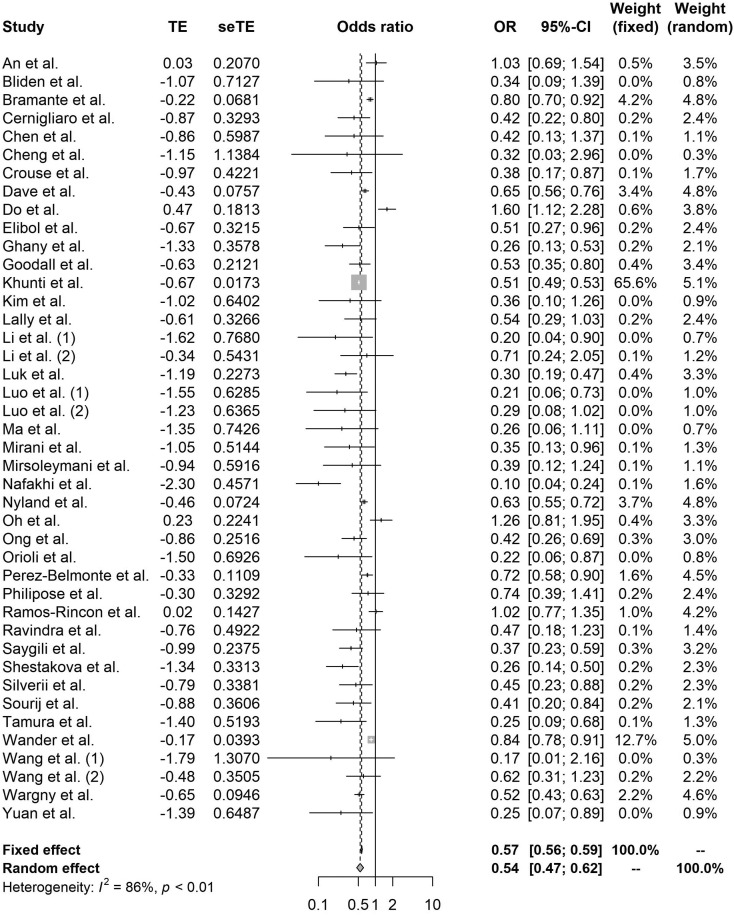
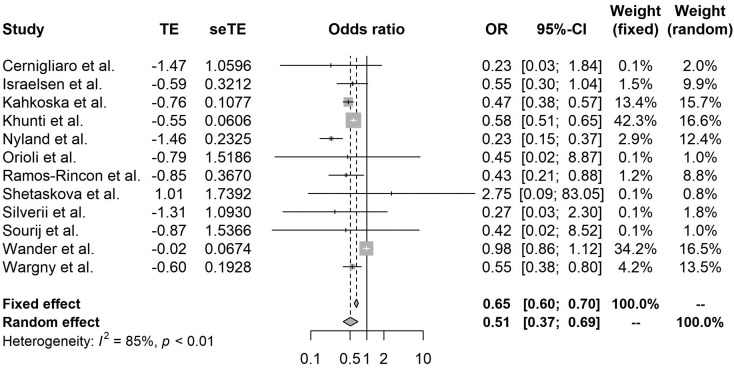
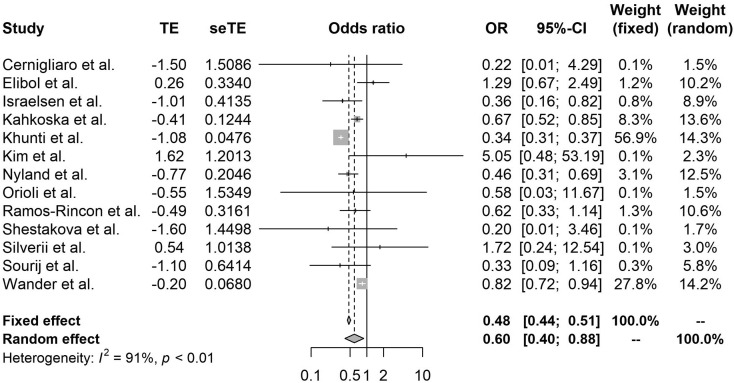
Compared with nonusers, metformin (OR 0.54, 95% CI 0.47–0.62, I2 86%), GLP-1RA (OR 0.51, 95% CI 0.37–0.69, I2 85%), and SGLT-2i (OR 0.60, 95% CI 0.40–0.88, I2 91%) use significantly reduced mortality among patients with COVID-19 with diabetes (Fig. 2, Fig. 3, Fig. 4 ). By contrast, DPP-4i (OR 1.23, 95% CI 1.07–1.42, I2 82%) and insulin (OR 1.70, 95% CI 1.33–2.19, I2 97%) were associated with an increased risk of in-hospital death (Figs. A.1, A.2). SU (OR 0.92, 95% 0.83–1.01, I2 44%), TZD (0.90, 95% CI 0.71–1.14, I2 46%), and AGI (OR 0.61, 95% 0.26–1.45, I2 77%) were mortality neutral (Figs. A.3–A.5).
Fig. 2.
Forest plot of the relationship between metformin and mortality in patients with COVID-19 having type 2 diabetes.
Fig. 3.
Forest plot of the relationship between GLP-1RA and mortality in patients with COVID-19 having type 2 diabetes.
Fig. 4.
Forest plot of the relationship between SGLT-2i and mortality in patients with COVID-19 having type 2 diabetes.
3.3.2. Meta-regression of confounding factors
Using meta-regression, we observed some significant variables that were significantly associated with mortality due to COVID-19, including continent, white race, male sex, age, BMI, HbA1C, hypertension, and CKD (Table 2 ).
Table 2.
Meta-regression analysis on potentially confounding factors.
| Medication | Confounding factor | Estimate | SE | p-Value |
|---|---|---|---|---|
| Metformin | Continent (vs. America) | |||
| Africa | 0.274 | 0.482 | 0.57 | |
| Asia | −0.076 | 0.227 | 0.74 | |
| Europe | 0.096 | 0.235 | 0.68 | |
| White race (%) | 0.004 | 0.006 | 0.53 | |
| Age (years) | −0.003 | 0.013 | 0.81 | |
| Male sex (%) | −0.001 | 0.007 | 0.87 | |
| HbA1C (%) | −0.100 | 0.181 | 0.59 | |
| Body mass index (kg/m2) | 0.043 | 0.037 | 0.26 | |
| Hypertension (%) | −0.001 | 0.006 | 0.87 | |
| Chronic kidney disease (%) | 0.001 | 0.005 | 0.89 | |
| Sulfonylurea | Continent (vs. America) | |||
| Africa | −0.123 | 0.204 | 0.56 | |
| Asia | 0.075 | 0.185 | 0.69 | |
| Europe | 0.076 | 0.158 | 0.64 | |
| White race (%) | 0.017 | 0.003 | 0.02 | |
| Age (years) | 0.015 | 0.007 | 0.03 | |
| Male sex (%) | 0.009 | 0.003 | 0.01 | |
| HbA1C (%) | −0.753 | 0.551 | 0.55 | |
| Body mass index (kg/m2) | −0.045 | 0.030 | 0.19 | |
| Hypertension (%) | 0.006 | 0.002 | 0.01 | |
| Chronic kidney disease (%) | 0.009 | 0.003 | 0.02 | |
| Thiazolidinedione | Continent (vs. America) | |||
| Asia | 0.389 | 0.398 | 0.37 | |
| Europe | 0.182 | 0.350 | 0.62 | |
| White race (%) | 0.071 | 0.026 | 0.22 | |
| Age (years) | 0.099 | 0.063 | 0.19 | |
| Male sex (%) | −0.001 | 0.030 | 0.97 | |
| HbA1C (%) | Insufficient data for analysis | |||
| Body mass index (kg/m2) | Insufficient data for analysis | |||
| Hypertension (%) | 0.025 | 0.008 | 0.05 | |
| Chronic kidney disease (%) | 0.005 | 0.025 | 0.87 | |
| Alpha-glucosidase inhibitor | Continent (vs. America) | |||
| Asia | 0.073 | 1.966 | 0.97 | |
| Europe | 1.452 | 2.234 | 0.54 | |
| White race (%) | Insufficient data for analysis | |||
| Age (years) | −0.078 | 0.067 | 0.28 | |
| Male sex (%) | −0.090 | 0.054 | 0.15 | |
| HbA1C (%) | 1.845 | 1.991 | 0.42 | |
| Body mass index (kg/m2) | 0.108 | 0.174 | 0.65 | |
| Hypertension (%) | −0.007 | 0.027 | 0.81 | |
| Chronic kidney disease (%) | 0.023 | 0.124 | 0.86 | |
| Glucagon-peptide like-1 receptor agonist | Continent (vs. America) | |||
| Asia | 1.707 | 1.459 | 0.27 | |
| Europe | −0.004 | 0.283 | 0.99 | |
| White race (%) | 0.033 | 0.027 | 0.30 | |
| Age (years) | 0.043 | 0.021 | 0.08 | |
| Male sex (%) | 0.032 | 0.010 | 0.01 | |
| HbA1C (%) | −1.000 | 0.361 | 0.07 | |
| Body mass index (kg/m2) | −0.053 | 0.038 | 0.25 | |
| Hypertension (%) | 0.029 | 0.010 | 0.02 | |
| Chronic kidney disease (%) | 0.008 | 0.007 | 0.32 | |
| Dipeptidyl peptidase-4 inhibitor | Continent (vs. America) | |||
| Asia | −0.183 | 0.247 | 0.47 | |
| Europe | −0.260 | 0.243 | 0.30 | |
| White race (%) | −0.003 | 0.018 | 0.90 | |
| Age (years) | −0.005 | 0.014 | 0.74 | |
| Male sex (%) | 0.000 | 0.012 | 1.00 | |
| HbA1C (%) | 0.005 | 0.347 | 0.99 | |
| Body mass index (kg/m2) | 0.087 | 0.030 | 0.02 | |
| Hypertension (%) | −0.001 | 0.006 | 0.87 | |
| Chronic kidney disease (%) | 0.009 | 0.010 | 0.39 | |
| Sodium–glucose transporter-2 inhibitor | Continent (vs. America) | |||
| Asia | 0.675 | 0.381 | 0.11 | |
| Europe | −0.500 | 0.218 | 0.04 | |
| White race (%) | −0.006 | 0.017 | 0.77 | |
| Age (years) | 0.029 | 0.048 | 0.56 | |
| Male sex (%) | −0.031 | 0.023 | 0.21 | |
| HbA1C (%) | 0.565 | 0.128 | 0.05 | |
| Body mass index (kg/m2) | −0.069 | 0.107 | 0.57 | |
| Hypertension (%) | 0.011 | 0.012 | 0.38 | |
| Chronic kidney disease (%) | −0.008 | 0.018 | 0.66 | |
| Insulin | Continent (vs. America) | |||
| Africa | −0.217 | 0.576 | 0.71 | |
| Asia | 0.009 | 0.285 | 0.98 | |
| Europe | −0.221 | 0.280 | 0.44 | |
| White race (%) | −0.000 | 0.011 | 0.98 | |
| Age (years) | −0.032 | 0.020 | 0.12 | |
| Male sex (%) | −0.001 | 0.011 | 0.97 | |
| HbA1C (%) | 0.029 | 0.347 | 0.93 | |
| Body mass index (kg/m2) | 0.115 | 0.061 | 0.08 | |
| Hypertension (%) | −0.011 | 0.008 | 0.19 | |
| Chronic kidney disease (%) | −0.002 | 0.009 | 0.87 | |
Abbreviations: SE, standard error.
3.3.3. Subgroup analysis
We performed subgroup analyses based on confounding factors identified through meta-regression to compare the effects of antidiabetic medications in more homogenous populations. The results of metformin and insulin were consistently confirmed among various groups in terms of vulnerability, including advanced age, high BMI, and high rate of CKD (Figs. A.6–A.8 and A.25–A.27, respectively). Meanwhile, GLP-1RA and SGLT-2i were still beneficial compared to nonusers, albeit less pronounced in populations with a higher rate of comorbidities and older patients, respectively (Figs. A.15, A.17, and A.21). Despite overall mortal neutrality, SU might have mild benefits in younger and less vulnerable populations (Figs. A.9–A.12). In contrast, DPP-4i showed harm or at least no benefit (A.18–A.20).
3.3.4. Sensitivity analysis
We further performed a sensitivity analysis by using two methods. First, we identified outliers by implementing the outlier removal algorithm in the dmetar package to explore the influence of individual studies on pooled effects. After outliers were removed, the pooled OR did not significantly change (all p > 0.05). Next, we conducted the trim-and-fill method to impute missing effects and concluded that our main results were stable after extending additional effects (all p > 0.05; Table 3 ).
Table 3.
Sensitivity analysis.
| Medication | Main meta-analysis |
Sensitivity analysis |
|||||
|---|---|---|---|---|---|---|---|
| Outlier removal method |
Trim-and-fill method |
||||||
| OR (95% CI) | I2 | OR (95% CI) | I2 | p valuea | OR (95% CI) | p valueb | |
| Metformin | 0.54 (0.47–0.62) | 86% | 0.50 (0.45–0.55) | 41% | 0.37 | 0.61 (0.54–0.70) | 0.17 |
| Sulfonylurea | 0.92 (0.83–1.01) | 44% | 0.98 (0.90–1.06) | 18% | 0.31 | 0.93 (0.84–1.04) | 0.80 |
| Thiazolidinedione | 0.90 (0.71–1.14) | 46% | No outlier | 0.88 (0.70–1.11) | 0.91 | ||
| Alpha-glucosidase inhibitor | 0.61 (0.26–1.45) | 77% | 1.13 (0.60–2.11) | 47% | 0.26 | 1.45 (0.57–3.74) | 0.18 |
| Glucagon-like peptide-1 receptor agonist | 0.51 (0.37–0.69) | 85% | 0.54 (0.49–0.60) | 0% | 0.70 | 0.62 (0.45–0.84) | 0.40 |
| Dipeptidyl peptidase-4 inhibitor | 1.23 (1.07–1.42) | 82% | 1.25 (1.14–1.37) | 37% | 0.86 | 1.29 (1.12–1.48) | 0.67 |
| Sodium-glucose transporter-2 inhibitor | 0.60 (0.40–0.88) | 91% | 0.67 (0.52–0.85) | 47% | 0.63 | 0.54 (0.37–0.79) | 0.72 |
| Insulin | 1.70 (1.33–2.19) | 97% | 1.60 (1.41–1.81) | 60% | 0.65 | 2.00 (1.58–2.52) | 0.37 |
Comparison of OR before vs. after removing outliers.
Comparison of OR before vs. after trimming and filling.
3.3.5. Dose-response meta-analysis
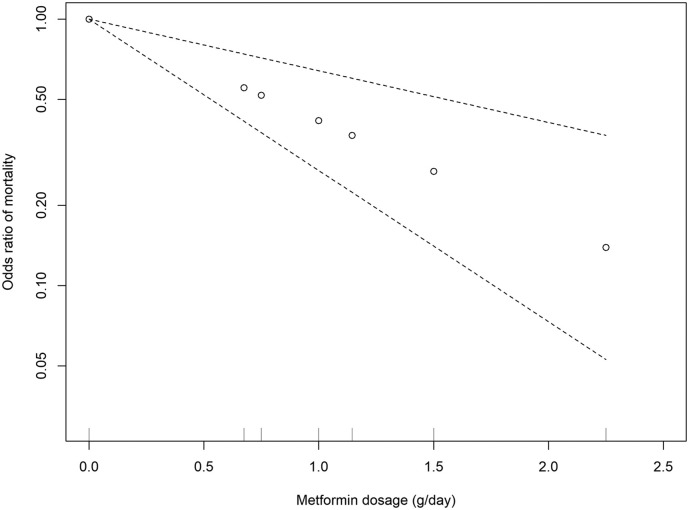
Metformin was the only medication that was reported the daily dosage in these original papers. Therefore, we performed a dose-response meta-analysis for metformin. We observed a significant linear dose-response association between metformin dose and odds ratio of mortality (estimate: −0.88, standard error: 0.22, p < 0.001) and no evidence of heterogeneity among studies (I2 = 0%, p = 0.46; Fig. 5 ).
Fig. 5.
Dose–response meta-analysis between daily metformin dosage and mortality in patients with COVID-19 with diabetes.
3.3.6. Comparison with previous meta-analyses
We next compared our results with those from other publications [4], [5], [6], [7], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88]. No published meta-analysis has analyzed the association between TZD or AGI and COVID-19-related mortality (Table 4 ).
Table 4.
Comparison with previous meta-analyses.
| Medication | Study | Medication use setting | Number of studies | OR/RR | Conclusion |
|---|---|---|---|---|---|
| Metformin | Our current study | Preadmission | 42 | 0.54 (0.47–0.62) | Decreased |
| Han et al. [5] | Preadmission + in-hospital | 20 | 0.62 (0.50–0.76) | Decreased | |
| Hariyanto et al. [74] | Preadmission | 5 | 0.54 (0.32–0.90) | Decreased | |
| Kan et al. [75] | Preadmission + in-hospital | 15 | 0.69 (0.55–0.86) | Decreased | |
| Kow et al. [76] | Preadmission | 5 | 0.62 (0.43–0.89) | Decreased | |
| Li et al. [77] | Preadmission + in-hospital | 19 | 0.66 (0.56–0.78) | Decreased | |
| Lukito et al. [78] | Preadmission | 6 | 0.64 (0.43–0.97) | Decreased | |
| Oscanoa et al. [79] | Preadmission + in-hospital | 22 | 0.56 (0.45–0.68) | Decreased | |
| Poly et al. [82] | Preadmission + in-hospital | 16 | 0.66 (0.54–0.80) | Decreased | |
| Scheen et al. [84] | Preadmission | 4 | 0.75 (0.67–0.85) | Decreased | |
| Schlesinger et al. [85] | ND | 4 | 0.50 (0.28–0.90) | Decreased | |
| Sun et al. [86] | Preadmission | 7 | 0.54 (0.35–0.84) | Decreased | |
| Yang et al. [87] | Preadmission + in-hospital | 17 | 0.63 (0.51–0.79) | Decreased | |
| Sulfonylurea | Our current study | Preadmission | 21 | 0.92 (0.83–1.01) | NS |
| Han et al. [5] | Preadmission + in-hospital | 4 | 0.93 (0.89–0.98) | Decreased | |
| Kan et al. [75] | Preadmission + in-hospital | 5 | 0.80 (0.66–0.96) | Decreased | |
| Schlesinger et al. [85] | ND | 2 | 0.73 (0.49–1.09) | NS | |
| Thiazolidinedione | Our current study | Preadmission | 8 | 0.90 (0.71–1.14) | NS |
| No published meta-analysis | |||||
| Alpha-glucosidase inhibitor | Our current study | Preadmission | 8 | 0.61 (0.26–1.45) | NS |
| No published meta-analysis | |||||
| Glucagon-like peptide-1 receptor agonist | Our current study | Preadmission | 12 | 0.51 (0.37–0.69) | Decreased |
| Han et al. [5] | Preadmission + in-hospital | 3 | 0.92 (0.80–1.04) | NS | |
| Hariyanto et al. [7] | Preadmission | 9 | 0.53 (0.43–0.66) | Decreased | |
| Dipeptidyl peptidase-4 inhibitor | Our current study | Preadmission | 28 | 1.23 (1.07–1.42) | Increased |
| Bonora et al. [73] | Preadmission | 7 | 0.81 (0.57–1.15) | NS | |
| Han et al. [5] | Preadmission + in-hospital | 11 | 0.95 (0.72–1.26) | NS | |
| Hariyanto et al. [6] | Preadmission | 7 | 1.14 (0.87–1.51) | NS | |
| Kan et al. [75] | Preadmission + in-hospital | 8 | 0.72 (0.51–1.51) | NS | |
| Pal et al. [80] | Preadmission | 4 | 1.21 (0.72–2.03) | NS | |
| Patoulias et al. [81] | Preadmission | 8 | 1.14 (0.78–1.66) | NS | |
| Rakhmat et al. [83] | Preadmission + in-hospital | 9 | 0.76 (0.60–0.97) | Decreased | |
| Schlesinger et al. [85] | ND | 2 | 0.90 (0.59–1.36) | NS | |
| Yang et al. [4] | Preadmission + in-hospital | 4 | 0.58 (0.34–0.99) | Decreased | |
| Sodium–glucose transporter-2 inhibitor | Our current study | Preadmission | 13 | 0.60 (0.40–0.88) | Decreased |
| Han et al. [5] | Preadmission + in-hospital | 3 | 1.04 (0.56–1.92) | NS | |
| Insulin | Our current study | Preadmission | 33 | 1.70 (1.33–2.19) | Increased |
| Kan et al. [75] | Preadmission + in-hospital | 7 | 2.20 (1.34–3.60) | Increased | |
| Schlesinger et al. [85] | ND | 5 | 1.75 (1.01–3.03) | Increased | |
| Yang et al. [88] | Preadmission + in-hospital | 12 | 2.10 (1.51–2.93) | Increased |
Abbreviations: ND, not defined; NS, not significant.
4. Discussion
4.1. Summary of main findings
To the best of our knowledge, this timely study has been the most extensive systematic review and meta-analysis confirming that different antidiabetic medications could predispose individuals with COVID-19 to different prognoses. Compared with a previous publication [5], we observed significant roles of GLP-1RA and SGLT-2i, besides metformin, in protecting individuals from COVID-19-related death. Similar to most studies, we also identified a positive association between DPP-4i usage and mortality. Moreover, we are the first to report the pooled effect of TZD and the pooled effect of AGI. Similar to smaller meta-analyses [5], [75], [85], our data also indicated the inconsistent impact of SU. Finally, we are the first to perform a dose-response meta-analysis regarding the daily dose of metformin to predict the magnitude of the effect on mortality in patients with COVID-19 having diabetes. These findings can have a large impact on the outpatient management strategy of diabetes patients amid the COVID-19 pandemic. These results can be helpful for clinicians in terms of choosing proper glucose-lowering regimens and dosage for those patients to reduce the risk of in-hospital death, i.e. by promoting the prescription of metformin, GLP-1RA, and SGLT-2i in the absence of any contraindications. In contrast, caution should be exercised in long-term insulin use.
Metformin might decrease or did not significantly affect COVID-19 death in the original studies. However, when performing meta-analyses, it yielded the most consistent result, even in vulnerable patients. Our study corroborated previous publications highlighting the potential benefits of metformin in patients with COVID-19 and diabetes. Several mechanisms might explain the lower mortality from SARS-CoV-2 infections in individuals taking metformin. First, beyond the hypoglycemic effect, metformin could reduce the release of inflammatory cytokines such as interleukin-6 and tumor necrosis factor-alpha, which play a vital role in COVID-19 pathophysiology [89]. Second, metformin is also involved in other pathways: angiotensin-converting enzyme-2 (ACE-2) modulation through adenosine monophosphate-activated protein kinase, decreased coagulation and thrombosis formation, mast cell stabilization, and improved endothelial function [18], [90]. Therefore, several researchers are currently investigating metformin as a host-directed medication in patients with COVID-19 [91]. Our current study indicated that metformin is effective among different races, sexes, weight status, and levels of glucose control. The dosage of metformin also affected the risk of mortality. First, Cheng et al. indicated that preadmission metformin usage was associated with better outcomes in a dose-response manner. In that study, metformin median dose was 1000 (890–1220) mg/day [21]. Ghany et al. reported that individuals using metformin at a dose of ≥1000 mg/day had lower mortality than those on 500–850 mg/day [29]. Referenced to nonusers, Ong et al. reported the greatest benefit on mortality with the dose from 1000 to <2000 mg/day [52]. Our findings were consistent with these studies. Specifically, every 250 mg/day increase in metformin use was associated with a 19.7% lower odds of mortality. In summary, the minimum metformin dosage that was found beneficial was 500 mg/day, and the higher the dose, the higher the effect. However, notably, the maximum approved dose for metformin is only 2550 mg/day (immediate-release form) and 2000 mg/day (extended-release form).
GLP-1RA and SGLT-2i are two novel classes of antidiabetic medications that have been approved for cardiorenal protection in type 2 diabetes patients. In the COVID-19 scenario, GLP-1RA can help reduce cytokine-induced lung injury by interfering with the NF-kB pathway or exerting anti-inflammatory effects [92], [93]. Meanwhile, when hypoxemia and hypoxia occur, SGLT-2i reverses the acid-base cytokine balance by decreasing lactic acid accumulation, thereby inhibiting the lowering of cytosolic pH and preventing cell damage during COVID-19-induced cytokine storm [94]. These cardiorenal benefits can synergistically offer protection to vital organs to reduce the risk of severity progression and death in the context of SARS-CoV-2 infection. It was not surprised from our findings that SGLT-2i might have more obvious impacts on those with high baseline BMI or history of CKD due to the renal-metabolic benefits of this class. Consistently, SGLT-2i was more beneficial in a subgroup with a history of cardiovascular disease [34]. It should be noted that, however, this benefit might be less pronounced in vulnerable patients.
In contrast to previous smaller meta-analyses reporting that DPP-4i had no significant effect on COVID-19-related death [6], [75], [80], [85], after incorporating a larger number of studies, we observed that preadmission DPP-4i users were associated with higher odds of in-hospital mortality. DPP-4i has yielded both putative protective and harmful effects on the underlying mechanisms of SARS-CoV-2 infection and progression from preclinical studies [4], [95]. Moreover, the controversial results of DPP-4i from various original studies and meta-analyses up to the present might be explained by the fact that the authors could not entirely exclude potential confounders, even with multivariate adjustment or propensity score matching. For example, we observed a trend toward higher use of DPP-4i in older fragile people and in patients with several comorbidities who had a compelling need to minimize hypoglycemia. These characteristics promoted the prescription of DPP-4i and limited the indication of other antidiabetic medications [33], [50], [54]. On the other hand, our subgroup analyses showed that DPP-4i might have little or no benefit among patient groups differed by vulnerability, suggesting that DPP-4i might not be associated with favorable COVID-19-related outcomes. To summarize, higher mortality rates in DPP-4i users should be cautiously interpreted.
The association between insulin treatment and severity or mortality is more complex. This result may still be affected by a confounding factor regarding the late commencement of insulin at an advanced stage of diabetes and the heterogeneous effectiveness of different insulin regimens, such as basal, basal-bolus, or premixed therapies. We speculate that insulin therapy is likely a surrogate indicator of diabetes progression accompanied by beta-cell dysfunction. Therefore, it was not insulin therapy, per se, that was associated with poor prognosis of patients with COVID-19 having type 2 diabetes, but rather that it represented a proxy of severity and duration of diabetes. However, notably, iatrogenic hyperinsulinemia caused by exogenous insulin use might lead to adverse effects, including insulin resistance due to downregulation of insulin receptors, vascular changes, and subsequent adverse cardiovascular outcomes [96]. Moreover, our subgroup analyses as well as those from previous publications controlling for severity markers did not eliminate the association, raising concerns about the actual harmful effects of insulin [17]. Like DPP-4i, the increased risk of death among insulin users should be cautiously interpreted.
Unlike two smaller meta-analyses demonstrating that SU could reduce mortality risk [5], [75], our results indicated that SU was not significantly associated with COVID-19-related mortality. Moreover, our study conducted a meta-analysis of AGI, which has not been reported previously. Traditionally, these drugs were often considered cardiovascular neutral. This characteristic makes them not a first-line treatment in patients with type 2 diabetes in general. Therefore, it is reasonable that they did not affect mortality in the COVID-19 setting, where cardiovascular events caused by hyperinflammation and hypercoagulation were the leading causes of intensive care unit admission, mechanical ventilation, and death. Although TZD could alleviate the long-term progressive atherosclerosis and inhibit the macrophage training, both of which were associated with the development of severe COVID-19, its benefit might be counteracted by the putative harmful effect regarding the fluid retention that could exacerbate pulmonary congestion in acute lung disease [97]. Moreover, evidence has shown that a TZD could downregulate A Disintegrin and Metalloproteinase-17 (ADAM-17), an ACE2 cleaving enzyme in human skeletal muscles [98]. This event, in turn, increased membrane ACE2 and facilitated cellular viral entry, raising concerns about increased susceptibility to SARS-CoV-2 infection. These hypotheses partially explained why TZD did not improve the mortality outcomes of patients with COVID-19 with diabetes in our study.
4.2. Strengths and limitations
Our study has several strengths. Despite the high heterogeneity related to some analyses, the robustness of our findings was confirmed through meta-regression, subgroup analysis, and sensitivity analysis. First, after outliers were identified and removed, the heterogeneity of all remaining studies drastically decreased without a significant change in OR (all p > 0.05). Second, after the trim-and-fill method was performed, the OR did not significantly change (all p > 0.05), indicating that our pooled odds ratio still reflected the actual effect size. In other words, our results were reliable and stable, even in the presence of high heterogeneity. Third, we only included preadmission-usage studies instead of combining both preadmission and in-hospital use like some meta-analyses, leading to a more consistent data interpretation. Moreover, unlike some publications, we updated the most recent and completed data instead of using ongoing data or pooling two studies from the same cohort. Next, we recruited relatively diverse samples from multicenter and multinational cohorts, thus increasing the ability to generalize to a larger population. Finally, we could present a dose-response meta-analysis to predict the effect of daily metformin doses on COVID-19 mortality.
Our study nevertheless has some limitations. First, we could include only observational studies because no randomized controlled trial was conducted on the topic of interest at the time of analysis. Any conclusions, therefore, should be cautiously drawn (considering indication bias). However, we recruited the largest number of participants from various papers of acceptable quality, making our systematic review and meta-analysis have high internal validity. Second, due to the observational nature of the studies, the multidrug issue could not be excluded. An investigation of specific combination therapies was necessary because a large proportion of diabetic patients need two or more glucose-lowering agents (either oral or injectable medications) to achieve glycemic targets. However, it was not feasible to perform such analysis due to limited raw data from original studies, even after we contacted the authors, because of the complexity of current diabetes treatment algorithms that would require additional mining of the original sources. Third, we were also unable to exclude the possibility of using medications beyond the hospital admission. However, our findings still reflected effects received before admission rather than short-term in-hospital effects because several included studies predefined medication users as those who had received a prescription that lasted at least 90–180 days, which is considered enough to exert their long-term effects. Fourth, because the COVID-19 treatment protocol has not been published as an international consensus among medical centers and countries, we lacked standardized severity assessment and concomitant drugs used during hospitalization, both of which are especially critical for mortality modeling. Fifth, it is impossible to completely rule out unmeasured confounders, such as smoking or socioeconomic status, although the original studies tried to adjust for these factors to a certain extent. Therefore, further studies with a strictly controlled design are warranted to confirm the relationships between therapies and mortality among patients with COVID-19 having type 2 diabetes. Last, because of the high publication rate regarding the COVID-19 topic within the past three years, there is a possibility that some studies might have been missed and therefore were not included in our current review. Although it is unavoidable, we minimized that issue by assigning three researchers to systematically search and select studies and another reviewer to be consulted to reach a final decision if needed.
5. Conclusions
The preadmission prescription of glucose-lowering therapies was associated with different outcomes in patients with COVID-19 having type 2 diabetes. Specifically, metformin, GLP-1RA, and SGLT-2i were more likely to be beneficial regarding in-hospital death. By contrast, DPP-4i and insulin were associated with increased mortality. However, SU, TZD, and AGI were mortality neutral.
Abbreviations
- ACE-2
Angiotensin-converting enzyme-2
- ADAM-17
A Disintegrin And Metalloproteinase-17
- AGI
Alpha-glucosidase inhibitor
- CKD
Chronic kidney disease
- COVID-19
Coronavirus disease of 2019
- DPP-4i
Dipeptidyl peptidase-4 inhibitor
- GLP-1RA
Glucagon-like peptide-1 receptor agonist
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SGLT-2i
Sodium–glucose transporter-2 inhibitor
- SU
Sulfonylurea
- TZD
Thiazolidinedione
CRediT authorship contribution statement
NNN conceived of the original idea, performed meta-analyses, meta-regression, sensitivity analyses, interpreted data, and wrote the first manuscript. DSH, HSN, and DKNH performed the systematic search, study selection, risk of bias assessment, and data extraction. HYC and YCC verified the analytical methods, supervised the findings of this work, and contributed to the revisions of the final manuscript. HYL and CYL provided clinical advice on the interpretation of the data and contributed to the revisions of the final manuscript. All authors approved the final manuscript as submitted and have agreed to be accountable for all aspects of the work. YCC is the guarantor of this work.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article to disclose. All authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Acknowledgments
Acknowledgement
This manuscript was edited by Wallace Academic Editing.
We gratefully acknowledge Ngan Khanh Nguyen for her expertise and assistance in designing the graphical abstract.
Data availability
Data were extracted from published research papers, all of which are available and accessible. All datasets generated during the current study are available upon reasonable request from the corresponding authors. The study protocol has been published (PROSPERO ID: CRD42021293064; www.crd.york.ac.uk/PROSPERO/) and is unrestrictedly available.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metabol.2022.155196.
Appendix A. Supplementary data
Supplementary appendix
References
- 1.WHO n.d. Coronavirus disease (COVID-19) pandemic. Updated November 23. Accessed November 30, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Hu J., Wang Y. The clinical characteristics and risk factors of severe COVID-19. Gerontology. 2021;67(3):255–266. doi: 10.1159/000513400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim S., Bae J.H., Kwon H.S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. Jan 2021;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y., Cai Z., Zhang J. DPP-4 inhibitors may improve the mortality of coronavirus disease 2019: a meta-analysis. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0251916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han T., Ma S., Sun C., et al. The association between anti-diabetic agents and clinical outcomes of COVID-19 in patients with diabetes: a systematic review and meta-analysis. Arch Med Res. 2021 doi: 10.1016/j.arcmed.2021.08.002. 2021/12/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hariyanto T.I., Kurniawan A. Dipeptidyl peptidase 4 (DPP4) inhibitor and outcome from coronavirus disease 2019 (COVID-19) in diabetic patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021;20(1):1–8. doi: 10.1007/s40200-021-00777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hariyanto T.I., Intan D., Hananto J.E., Putri C., Kurniawan A. Pre-admission glucagon-like peptide-1 receptor agonist (GLP-1RA) and mortality from coronavirus disease 2019 (Covid-19): a systematic review, meta-analysis, and meta-regression. Diabetes Res Clin Pract. Sep 2021;179 doi: 10.1016/j.diabres.2021.109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells G., Shea B., O'Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 9.Wg C. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 10.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Sterne J.A., Gavaghan D., Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. Nov 2000;53(11):1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 12.Cheng X., Liu Y.M., Li H., et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;32(4):537–547.e3. doi: 10.1016/j.cmet.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pazoki M., Chichagi F., Hadadi A., et al. Association of clinical characteristics, antidiabetic and cardiovascular agents with diabetes mellitus and COVID-19: a 7-month follow-up cohort study. J Diabetes Metab Disord. Nov 8 2021:1–11. doi: 10.1007/s40200-021-00901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal S., Schechter C., Southern W., Crandall J.P., Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care. Oct 2020;43(10):2339–2344. doi: 10.2337/dc20-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An C., Lim H., Kim D.W., Chang J.H., Choi Y.J., Kim S.W. Machine learning prediction for mortality of patients diagnosed with COVID-19: a nationwide Korean cohort study. Sci Rep. Oct 30 2020;10(1) doi: 10.1038/s41598-020-75767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bliden K., Tantry U., Usman A., et al. Abstract 12228: metformin use in patients hospitalized with COVID-19: lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes. Circulation. 2021;144(Suppl_1) doi: 10.1161/circ.144.suppl_1.12228. A12228-A12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boye K.S., Tokar Erdemir E., Zimmerman N., et al. Risk factors associated with COVID-19 hospitalization and mortality: a large claims-based analysis among people with type 2 diabetes mellitus in the United States. Diabetes Ther. Aug 2021;12(8):2223–2239. doi: 10.1007/s13300-021-01110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bramante C.T., Ingraham N.E., Murray T.A., et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Health Longev. Jan 2021;2(1):e34–e41. doi: 10.1016/s2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cernigliaro A., Allotta A.V., Scondotto S. Diabete e trattamento farmacologico con ipoglicemizzanti possono essere considerati fattori di rischio per gli esiti di salute in soggetti COVID-19? I risultati di uno studio nella popolazione residente in Sicilia. Can diabetes and its related hypoglycemic drug treatment be considered risk factors for health outcomes in COVID-19 patients? The results of a study in the population residing in Sicily Region (Southern Italy)Epidemiol Prev. Sep-Dec 2020;44(5-6 Suppl 2):315–322. doi: 10.19191/ep20.5-6.S2.132. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Yang D., Cheng B., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. Jul 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 21.Cheng X., Xin S., Chen Y., et al. Effects of metformin, insulin on COVID-19 patients with pre-existed type 2 diabetes: A multicentral retrospective study. Life Sci. Jun 15 2021;275 doi: 10.1016/j.lfs.2021.119371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crouse A.B., Grimes T., Li P., Might M., Ovalle F., Shalev A. Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front Endocrinol (Lausanne) 2020;11 doi: 10.3389/fendo.2020.600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dave J.A., Tamuhla T., Tiffin N., et al. Risk factors for COVID-19 hospitalisation and death in people living with diabetes: a virtual cohort study from the Western Cape ProvinceSouth Africa. Diabetes Res Clin Pract. Jul 2021;177 doi: 10.1016/j.diabres.2021.108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Y.P., Xie W., Liu T., et al. Association of diabetes with severity and mortality in hospitalized patients with COVID-19 in Wuhan, China: a single-centered, retrospective study. Arch Endocrinol Metab. 2021;65(5):596–608. doi: 10.20945/2359-3997000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Do JY Kim S.W., Park J.W., Cho K.H., Kang S.H. Is there an association between metformin use and clinical outcomes in diabetes patients with COVID-19? Diabetes Metab. Jul 2021;47(4) doi: 10.1016/j.diabet.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elibol A., Eren D., Erdoğan M.D., et al. Factors influencing on development of COVID-19 pneumonia and association with oral anti-diabetic drugs in hospitalized patients with diabetes mellitus. Prim Care Diabetes. Oct 2021;15(5):806–812. doi: 10.1016/j.pcd.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emral R., Haymana C., Demirci I., et al. Lower COVID-19 mortality in patients with type 2 diabetes mellitus taking dipeptidyl peptidase-4 inhibitors: results from a Turkish nationwide study. Diabetes Ther. Nov 2021;12(11):2857–2870. doi: 10.1007/s13300-021-01133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fadini G.P., Morieri M.L., Longato E., et al. Exposure to dipeptidyl-peptidase-4 inhibitors and COVID-19 among people with type 2 diabetes: a case-control study. Diabetes Obes Metab. Oct 2020;22(10):1946–1950. doi: 10.1111/dom.14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghany R., Palacio A., Dawkins E., et al. Metformin is associated with lower hospitalizations, mortality and severe coronavirus infection among elderly medicare minority patients in 8 states in USA. Diabetes Metab Syndr. 2021;15(2):513–518. doi: 10.1016/j.dsx.2021.02.022. Mar-Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giorda C.B., Picariello R., Tartaglino B., et al. From swab testing to health outcomes within the T2DM population: impact of diabetes background on COVID19 progression. Diabetes Res Clin Pract. Oct 2021;180 doi: 10.1016/j.diabres.2021.109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodall J.W., TAN Reed, Ardissino M., et al. Risk factors for severe disease in patients admitted with COVID-19 to a hospital in London, England: a retrospective cohort study. Epidemiol Infect. Oct 13 2020;148 doi: 10.1017/s0950268820002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Israelsen S.B., Pottegård A., Sandholdt H., Madsbad S., Thomsen R.W., Benfield T. Comparable COVID-19 outcomes with current use of GLP-1 receptor agonists, DPP-4 inhibitors or SGLT-2 inhibitors among patients with diabetes who tested positive for SARS-CoV-2. Diabetes Obes Metab. Jun 2021;23(6):1397–1401. doi: 10.1111/dom.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahkoska A.R., Abrahamsen T.J., Alexander G.C., et al. Association between glucagon-like peptide 1 receptor agonist and sodium-glucose cotransporter 2 inhibitor use and COVID-19 outcomes. Diabetes Care. Jul 2021;44(7):1564–1572. doi: 10.2337/dc21-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khunti K., Knighton P., Zaccardi F., et al. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endocrinol. May 2021;9(5):293–303. doi: 10.1016/s2213-8587(21)00050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M.K., Jeon J.H., Kim S.W., et al. The clinical characteristics and outcomes of patients with moderate-to-severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab J. Aug 2020;44(4):602–613. doi: 10.4093/dmj.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kristan M.M., Kim Y.K., Nelson T., et al. Predictors of severe COVID-19 in patients with diabetes: a multicenter review. Endocr Pract. Aug 2021;27(8):842–849. doi: 10.1016/j.eprac.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lally M.A., Tsoukas P., Halladay C.W., O'Neill E., Gravenstein S., Rudolph J.L. Metformin is associated with decreased 30-day mortality among nursing home residents infected with SARS-CoV2. J Am Med Dir Assoc. Jan 2021;22(1):193–198. doi: 10.1016/j.jamda.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F., Cai Y., Gao C., et al. Effects of diabetes and blood glucose on COVID-19 mortality: a retrospective observational study. medRxiv. 2021 doi: 10.1101/2021.01.21.20202119. [DOI] [Google Scholar]
- 39.Li J., Wei Q., Li W.X., et al. Metformin use in diabetes prior to hospitalization: effects on mortality in Covid-19. Endocr Pract. Oct 2020;26(10):1166–1172. doi: 10.4158/ep-2020-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AOY Luk, TCF Yip, Zhang X., et al. Glucose-lowering drugs and outcome from COVID-19 among patients with type 2 diabetes mellitus: a population-wide analysis in Hong Kong. BMJ Open. Oct 20 2021;11(10) doi: 10.1136/bmjopen-2021-052310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo P., Qiu L., Liu Y., et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. Jul 2020;103(1):69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo S.K., Hu W.H., Lu Z.J., et al. Diabetes patients with comorbidities had unfavorable outcomes following COVID-19: a retrospective study. World J Diabetes. 2021;12(10):1789–1808. doi: 10.4239/wjd.v12.i10.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Z., Patel N., Vemparala P., Krishnamurthy M. Metformin is associated with favorable outcomes in patients with COVID-19 and Type 2 diabetes mellitus. medRxiv. 2021 doi: 10.1101/2021.05.20.21257490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mansour A., Sajjadi-Jazi S.M., Kasaeian A., et al. Clinical characteristics and outcomes of diabetics hospitalized for COVID-19 infection: a single-centered, retrospective, observational study. EXCLI J. 2020;19:1533–1543. doi: 10.17179/excli2020-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meijer R.I., Hoekstra T., van den Oever N.C.G. Treatment with a DPP-4 inhibitor at time of hospital admission for COVID-19 is not associated with improved clinical outcomes: data from the COVID-PREDICT cohort study in The Netherlands. J Diabetes Metab Disord. Jun 26 2021:1–6. doi: 10.1007/s40200-021-00833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirani M., Favacchio G., Carrone F., et al. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with Type 2 diabetes with COVID-19: a case series from an academic hospital in Lombardy, Italy. Diabetes Care. Dec 2020;43(12):3042–3049. doi: 10.2337/dc20-1340. [DOI] [PubMed] [Google Scholar]
- 47.Mirsoleymani S., Nekooghadam S.M., Ahmadi Marzaleh M., et al. Assessment of risk factors for severe coronavirus disease 2019 among Iranian patients. Iranian Red Crescent Medical Journal. 2020;22(9) doi: 10.32592/ircmj.2020.22.9.72. 09/30. [DOI] [Google Scholar]
- 48.Nafakhi H., Alareedh M., Al-Buthabhak K., Shaghee F., Nafakhi A., Kasim S. Predictors of adverse in-hospital outcome and recovery in patients with diabetes mellitus and COVID-19 pneumonia in Iraq. Diabetes Metab Syndr. 2021;15(1):33–38. doi: 10.1016/j.dsx.2020.12.014. Jan-Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noh Y., Oh I.S., Jeong H.E., Filion K.B., Yu O.H.Y., Shin J.Y. Association between DPP-4 inhibitors and COVID-19-related outcomes among patients with type 2 diabetes. Diabetes Care. Apr 2021;44(4):e64–e66. doi: 10.2337/dc20-1824. [DOI] [PubMed] [Google Scholar]
- 50.Nyland J.E., Raja-Khan N.T., Bettermann K., et al. Diabetes, drug treatment and mortality in COVID-19: a multinational retrospective cohort study. Diabetes. Sep 27 2021 doi: 10.2337/db21-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh T.K., Song I.A. Metformin use and risk of COVID-19 among patients with type II diabetes mellitus: an NHIS-COVID-19 database cohort study. Acta Diabetol. Jun 2021;58(6):771–778. doi: 10.1007/s00592-020-01666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ong A.N., Tan C.C., Cañete M.T., Lim B.A., Robles J. Association between metformin use and mortality among patients with Type 2 diabetes mellitus hospitalized for COVID-19 infection. Journal of the ASEAN Federation of Endocrine Societies. 2021;36(2):133–141. doi: 10.15605/jafes.036.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orioli L., Servais T., Belkhir L., et al. Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: a retrospective study from an academic center in Belgium. Diabetes Metab Syndr. 2021;15(1):149–157. doi: 10.1016/j.dsx.2020.12.020. Jan-Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pérez-Belmonte L.M., Torres-Peña J.D., López-Carmona M.D., et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. Nov 16 2020;18(1):359. doi: 10.1186/s12916-020-01832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Philipose Z., Smati N., Jefferson Wong C.S., Aspey K., Mendall M. Obesity, old age, and frailty are the true risk factors for COVID-19 mortality and not chronic disease or ethnicity. medRxiv. 2020 doi: 10.1101/2020.08.12.20156257. [DOI] [Google Scholar]
- 56.Ramos-Rincón J.M., Pérez-Belmonte L.M., Carrasco-Sánchez F.J., et al. Cardiometabolic therapy and mortality in very old patients with diabetes hospitalized due to COVID-19. J Gerontol A Biol Sci Med Sci. 2021;76(8):e102–e109. doi: 10.1093/gerona/glab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravindra G., Chitra L., Madhur M., et al. Retrospective assessment of treatments of hospitalized Covid-19 patients. medRxiv. 2021 doi: 10.1101/2021.04.20.21255792. [DOI] [Google Scholar]
- 58.Riahi S., Sombra L.R.S., Lo K.B., et al. Insulin use, diabetes control, and outcomes in patients with COVID-19. Endocr Res. 2021;46(2):45–50. doi: 10.1080/07435800.2020.1856865. Feb-May. [DOI] [PubMed] [Google Scholar]
- 59.Satman I., Demirci I., Haymana C., et al. Unexpectedly lower mortality rates in COVID-19 patients with and without type 2 diabetes in Istanbul. Diabetes Res Clin Pract. Apr 2021;174 doi: 10.1016/j.diabres.2021.108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saygili E.S., Karakiliç E., Mert E., Şener A., Mirci A. Preadmission usage of metformin and mortality in COVID-19 patients including the post-discharge period. Ir J Med Sci. Oct 29 2021:1–7. doi: 10.1007/s11845-021-02823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shestakova M.V., Vikulova O.K., Isakov M., Dedov I.I. Diabetes and COVID-19: analysis of the clinical outcomes according to the data of the Russian diabetes registry. Probl Endokrinol (Mosk) 2020;66(1):35–46. doi: 10.14341/probl12458. [DOI] [PubMed] [Google Scholar]
- 62.Silverii G.A., Monami M., Cernigliaro A., et al. Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr Metab Cardiovasc Dis. 2021;31(2):396–398. doi: 10.1016/j.numecd.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sourij H., Aziz F., Bräuer A., et al. COVID-19 fatality prediction in people with diabetes and prediabetes using a simple score upon hospital admission. Diabetes Obes Metab. Feb 2021;23(2):589–598. doi: 10.1111/dom.14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strollo R., Maddaloni E., Dauriz M., Pedone C., Buzzetti R., Pozzilli P. Use of DPP4 inhibitors in Italy does not correlate with diabetes prevalence among COVID-19 deaths. Diabetes Res Clin Pract. Jan 2021;171 doi: 10.1016/j.diabres.2020.108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamura R.E., Said S.M., de Freitas L.M., IGS Rubio. Outcome and death risk of diabetes patients with Covid-19 receiving pre-hospital and in-hospital metformin therapies. Diabetol Metab Syndr. Jul 13 2021;13(1):76. doi: 10.1186/s13098-021-00695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wander P.L., Lowy E., Beste L.A., et al. Prior glucose-lowering medication use and 30-day outcomes among 64,892 veterans with diabetesand COVID-19. Diabetes Care. Oct 6 2021 doi: 10.2337/dc21-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang B., Van Oekelen O., Mouhieddine T.H., et al. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol. Jul 14 2020;13(1):94. doi: 10.1186/s13045-020-00934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J., Cooper J.M., Gokhale K., et al. Association of Metformin with susceptibility to COVID-19 in people with type 2 diabetes. J Clin Endocrinol Metab. 2021;106(5):1255–1268. doi: 10.1210/clinem/dgab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wargny M., Potier L., Gourdy P., et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. Apr 2021;64(4):778–794. doi: 10.1007/s00125-020-05351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.CKH Wong, DTW Lui, AYC Lui, et al. Use of DPP4i reduced odds of clinical deterioration and hyperinflammatory syndrome in COVID-19 patients with type 2 diabetes: propensity score analysis of a territory-wide cohort in Hong Kong. Diabetes & Metabolism. 2021 doi: 10.1016/j.diabet.2021.101307. 2021/12/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan Y., Yang Y., Wang F., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. Apr 2020;8(1) doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan S., Li H., Chen C., Wang F., Wang D.W. Association of glycosylated haemoglobin HbA1c levels with outcome in patients with COVID-19: a retrospective study. J Cell Mol Med. Apr 2021;25(7):3484–3497. doi: 10.1111/jcmm.16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonora B.M., Avogaro A., Fadini G.P. Disentangling conflicting evidence on DPP-4 inhibitors and outcomes of COVID-19: narrative review and meta-analysis. J Endocrinol Invest. Jul 2021;44(7):1379–1386. doi: 10.1007/s40618-021-01515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. Sep 2020;19 doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kan C., Zhang Y., Han F., et al. Mortality risk of antidiabetic agents for type 2 diabetes with COVID-19: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.708494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kow C.S., Hasan S.S. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: a meta-analysis. J Med Virol. Feb 2021;93(2):695–697. doi: 10.1002/jmv.26498. [DOI] [PubMed] [Google Scholar]
- 77.Li Y., Yang X., Yan P., Sun T., Zeng Z., Li S. Metformin in patients with COVID-19: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.704666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lukito A.A., Pranata R., Henrina J., Lim M.A., Lawrensia S., Suastika K. The effect of metformin consumption on mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):2177–2183. doi: 10.1016/j.dsx.2020.11.006. Nov-Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oscanoa T.J., Amado J., Vidal X., Savarino A., Romero-Ortuno R. Metformin therapy and severity and mortality of SARS-CoV-2 infection: a meta-analysis. Clin Diabetol. 2021;10(4):317–329. doi: 10.5603/DK.a2021.0035. [DOI] [Google Scholar]
- 80.Pal R., Banerjee M., Mukherjee S., Bhogal R.S., Kaur A., Bhadada S.K. Dipeptidyl peptidase-4 inhibitor use and mortality in COVID-19 patients with diabetes mellitus: an updated systematic review and meta-analysis. Ther Adv Endocrinol Metab. 2021;12 doi: 10.1177/2042018821996482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patoulias D., Doumas M. Dipeptidyl Peptidase-4 inhibitors and COVID-19-related deaths among patients with type 2 diabetes mellitus: a meta-analysis of observational studies. Endocrinol Metab (Seoul) Aug 2021;36(4):904–908. doi: 10.3803/EnM.2021.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poly T.N., Islam M.M., Li Y.J., Lin M.C., Hsu M.H., Wang Y.C. Metformin use is associated with decreased mortality in COVID-19 patients with diabetes: evidence from retrospective studies and biological mechanism. J Clin Med. Aug 9 2021;10(16) doi: 10.3390/jcm10163507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rakhmat I.I., Kusmala Y.Y., Handayani D.R., et al. Dipeptidyl peptidase-4 (DPP-4) inhibitor and mortality in coronavirus disease 2019 (COVID-19) - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2021;15(3):777–782. doi: 10.1016/j.dsx.2021.03.027. May-Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scheen A.J. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab. Nov 2020;46(6):423–426. doi: 10.1016/j.diabet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schlesinger S., Neuenschwander M., Lang A., et al. Risk phenotypes of diabetes and association with COVID-19 severity and death: a living systematic review and meta-analysis. Diabetologia. Jul 2021;64(7):1480–1491. doi: 10.1007/s00125-021-05458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun C., Cheng C., Kim K.Y., Ahmed M.A., Manem R. Is metformin use associated with a decreased mortality for COVID-19 diabetic patients? A meta-analysis. Journal of the Endocrine Society. 2021;5(Supplement_1) doi: 10.1210/jendso/bvab048.709. A348-A348. [DOI] [Google Scholar]
- 87.Yang W., Sun X., Zhang J., Zhang K. The effect of metformin on mortality and severity in COVID-19 patients with diabetes mellitus. Diabetes Res Clin Pract. Aug 2021;178 doi: 10.1016/j.diabres.2021.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Y., Cai Z., Zhang J. Insulin treatment may increase adverse outcomes in patients with COVID-19 and diabetes: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.696087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma S., Ray A., Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res Clin Pract. Jun 2020;164 doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou G., Myers R., Li Y., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. Oct 2001;108(8):1167–1174. doi: 10.1172/jci13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Varghese E., Samuel S.M., Liskova A., Kubatka P., Büsselberg D. Diabetes and coronavirus (SARS-CoV-2): molecular mechanism of metformin intervention and the scientific basis of drug repurposing. PLoS Pathog. Jun 2021;17(6) doi: 10.1371/journal.ppat.1009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drucker D.J. Coronavirus infections and Type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev. Jun 1 2020;41(3) doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee Y.S., Jun H.S. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediat Inflamm. 2016;2016:3094642. doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cure E., Cumhur Cure M. Can dapagliflozin have a protective effect against COVID-19 infection?A hypothesis. Diabetes Metab Syndr. 2020;14(4):405–406. doi: 10.1016/j.dsx.2020.04.024. Jul-Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vankadari N., Wilce J.A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9(1):601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Juan C.C., Fang V.S., Kwok C.F., Perng J.C., Chou Y.C., Ho L.T. Exogenous hyperinsulinemia causes insulin resistance, hyperendothelinemia, and subsequent hypertension in rats. Metabolism. Apr 1999;48(4):465–471. doi: 10.1016/s0026-0495(99)90105-1. [DOI] [PubMed] [Google Scholar]
- 97.Erol A. Role of oxidized LDL-induced "trained macrophages" in the pathogenesis of COVID-19 and benefits of pioglitazone: a hypothesis. Diabetes Metab Syndr. 2020;14(4):713–714. doi: 10.1016/j.dsx.2020.05.007. Jul-Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tripathy D., Daniele G., Fiorentino T.V., et al. Pioglitazone improves glucose metabolism and modulates skeletal muscle TIMP-3-TACE dyad in type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled, mechanistic study. Diabetologia. Oct 2013;56(10):2153–2163. doi: 10.1007/s00125-013-2976-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary appendix
Data Availability Statement
Data were extracted from published research papers, all of which are available and accessible. All datasets generated during the current study are available upon reasonable request from the corresponding authors. The study protocol has been published (PROSPERO ID: CRD42021293064; www.crd.york.ac.uk/PROSPERO/) and is unrestrictedly available.