Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) has changed airway management since its introduction into anaesthetic practice just 5 yr ago. Whilst nasal high-flow (NHF) oxygen has been used for more than a decade in critical care to treat patients with hypoxaemic respiratory failure,1 it was not until 2015 when THRIVE was shown to prolong safe apnoea time before desaturation and to reduce the rate of carbon dioxide accumulation that the potential role of NHF oxygen (NHFO) in anaesthesia began to be realised.2
Since 2015, NHFO/THRIVE has been rapidly incorporated into anaesthetic practice worldwide and several key airway guidelines,3 , 4 testament to its utility. The ability of THRIVE to optimise periprocedural oxygenation and prolong safe apnoea time via per-oxygenation (i) increases the margin of safety for securing a definitive airway, improving patient safety; (ii) facilitates safer training in difficult airway management; (iii) provides an extended apnoea ‘tubeless’ technique, for example, in airway/laryngotracheal surgery; and has also led to broader uses of NHFO in anaesthetic practice.
When discussing NHFO therapy, it is important to distinguish between NHFO delivered to a spontaneously breathing patient and THRIVE (NHF therapy) delivered to an apnoeic patient, as the underlying mechanisms differ, resulting in different clinical uses and considerations. In the spontaneously breathing patient perioperative uses of NHFO are: (i) preoxygenation; (ii) sedation, for example during awake tracheal intubation; (iii) general anaesthesia with maintenance of spontaneous breathing; and (iv) postextubation. THRIVE, which we will discuss further here, is used during apnoea to maintain: (i) oxygenation during the intubation process (peri-intubation) and (ii) to facilitate extended apnoea for ‘tubeless’ surgery.
Reproducibility
The THRIVE effect of extended safe apnoea time before desaturation with reduced rate of carbon dioxide accumulation compared with historical studies of ‘classical’ apnoeic oxygenation using low-flow oxygen, has now been reproduced in: (i) many centres worldwide5, 6, 7, 8, 9, 10; (ii) in different patient populations5; (iii) in patients with ‘difficult’ and compromised/stridulous airways2 , 10; (iv) during both peri-intubation6 and extended apnoea application of THRIVE7, 8, 9; and (v) using different methods to measure carbon dioxide (e.g. end-tidal CO2 [ETCO2] on termination of THRIVE, transcutaneous CO2, and PaCO2).
The rate of carbon dioxide increase (0.15 kPa min−1 ETCO2 2; 0.13 kPa min−1 ETCO2 8; 0.21 kPa min−1 PvCO2 7; 0.24 kPa min−1 PaCO2 9) is lower than the 0.35–0.45 kPa min−1 observed in historical apnoeic oxygenation studies with low-flow oxygen.11 , 12 No RCT has compared the rate of carbon dioxide clearance with high-flow vs low-flow apnoeic oxygenation techniques, but results from a current RCT investigating this are awaited.13 It is also unclear whether carbon dioxide clearance, or ‘ventilatory exchange’, occurs in certain populations such as children.14 , 15
Mechanisms
Whilst the mechanisms underlying NHFO in spontaneously breathing patients are well described (high FiO2; positive airway pressure of 0.7 cm H2O per 10 L min−1 flow rate16; pharyngeal dead-space ‘washout’ and improved respiratory mechanics), the physiological mechanisms underlying THRIVE (apnoeic patient) are not fully elucidated. Positive airway pressure was initially considered an important mechanism, but we now realise this is not the case. Positive airway pressure generated during THRIVE is minimal,17 as the patient is apnoeic and therefore does not generate an expiratory flow rate to counter the incoming oxygen stream, which is the predominant mechanism generating high mean positive airway pressures in a spontaneously breathing patient.16
Apnoeic oxygenation and, most importantly, apnoeic ventilation are now considered key. Apnoeic ventilation, or ‘respiration without respiratory movements’, was first described in 1865 when it was appreciated that with closed nose, open glottis, and breath held, ‘air within the respiratory passages moves to and fro in tiny expiratory and inspiratory puffs … with each inspiratory motion coinciding with a cardiac systole, each expiratory puff with a cardiac diastole.‘18 These pulsatile changes in airway gas flow, synchronous with the cardiac cycle, known as cardiogenic oscillations (cardiogenic ‘stroke volume’ 6–40 ml), arise from movement of blood in the pulmonary vessels causing compression and expansion of small airways.19 Cardiogenic oscillations contribute to carbon dioxide removal during apnoea, however this mechanism alone does not explain the increased carbon dioxide removal with high-flow nasal oxygen, as cardiogenic oscillations are present regardless of whether high-flow, low-flow, or no oxygen is applied.
Studies in vivo of apnoeic ventilation mechanisms are challenging because of complex and dynamic interactions of the cardiorespiratory and circulatory systems, so a physical and computational physiological modelling approach has been used.20 , 21 Physical airway models to investigate the fluid dynamics affecting carbon dioxide clearance suggest that enhanced carbon dioxide clearance with THRIVE arises as a result of interaction between entrained, highly turbulent supraglottic flow vortices generated by NHFO (turbulence proportional to THRIVE flow rate), and cardiogenic oscillations.20 This interaction creates a mechanism enhancing carbon dioxide removal from carina to pharynx, whilst also providing a means of increasing oxygen movement from pharynx to carina, an ‘active’ oxygenation component.20 Apnoeic ventilation further increases PA O2 as a consequence of its reciprocal reduction in PA CO2 {alveolar gas equation: PA O2=(FiO2×[Patm–PH2O])–(PA CO2/R)}. Results from computational physiological modelling in adult patients complement these findings.21
Variable apnoea times in different populations
The safe apnoea window provided by THRIVE is shortened in certain patient groups and clinical situations. Factors affecting the rate of oxyhaemoglobin desaturation during apnoea (with airway open or closed, with air or 100% oxygen as the ambient gas) have been extensively modelled, with the models validated against clinical data from human apnoea studies.22, 23, 24 These models did not incorporate high-flow nasal oxygen, which provides a means during apnoea of not only adding oxygen to the reservoir established at the end of preoxygenation via apnoeic oxygenation (as low-flow oxygen also does), but also via apnoeic ventilation.
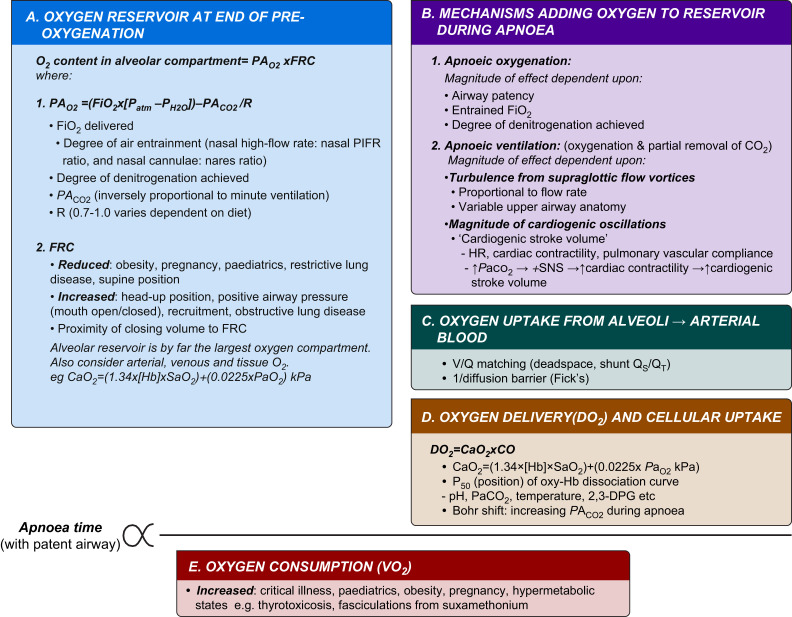
A complex interplay of factors determines apnoea time (Fig. 1 ) in patients undergoing THRIVE: (i) alveolar oxygen content at the end of preoxygenation (FRC×FAO2); (ii) magnitude of effect of mechanisms that will add to the oxygen reservoir during apnoea (i.e. apnoeic oxygenation and apnoeic ventilation) (via both reciprocal clearance of PA CO2 and ‘active’ replacement of oxygen); (iii) factors affecting the oxygen cascade, transport, and cellular uptake; and (iv) metabolic rate of oxygen consumption. These factors differ in their weighted importance, with initial FAO2, alveolar volume and oxygen consumption rate having the greatest effects in low-flow oxygen models.22 , 24 Interestingly, in models, increasing ambient FiO2 from 0.9 to 1.0 almost doubles the time to desaturation, which is a greater effect than when ambient FiO2 is increased from 0.21 to 0.9.23
Fig 1.
Conceptual framework of factors influencing apnoea time with THRIVE. CaO2, arterial O2 content; CO, cardiac output; DO2, O2 delivery; FiO2, fraction of inspired O2; FRC, Functional residual capacity; [Hb], haemoglobin concentration; PACO2, alveolar CO2 partial pressure; PAO2, alveolar partial pressure of O2; Patm, atmospheric pressure; PH2O, standard vapour pressure of water; R, respiratory quotient; SaO2, arterial O2 saturation of haemoglobin; SNS, sympathetic nervous system; V/Q, ventilation/perfusion; PIFR, peak inspiratory flow rate.
The framework shown in Fig. 1 could be incorporated into an equation or App format. The anaesthetist would input known patient parameters to calculate predicted mean apnoea time (standard deviation [sd]) and the likely rate of carbon dioxide increase for the patient. The predicted safe apnoea time would pertain to populations rather than any specific patient, but nonetheless could help guide airway management strategy.
Unanswered questions
Further research is needed to improve our understanding of the mechanisms underlying THRIVE. This knowledge could refine the THRIVE technique, for example by optimising flow rates used and the magnitude of cardiogenic oscillations generated (e.g. by modifying HR or stroke volume), and perhaps even by combining NHFO and oxygen jet ventilation. Better understanding of the variable physiological responses to THRIVE in different patient groups will improve our ability to predict patients in whom the safe apnoea time is likely to be shortened. Furthermore, whether apnoeic ventilation occurs in all patient subgroups, notably children, requires further investigation.
Coronavirus disease 2019
Coronavirus disease 2019 (COVID-19) has changed our lives and has become a defining point for airway management: BC (before COVID-19) and AC (after COVID-19).
In early 2020, the main concern raised with NHFO/THRIVE and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was the potential for the generation and dispersion of aerosols and droplets and consequent influence on infection risk. The evidence base to guide NHFO and aerosolisation risk was limited25; whilst a study suggested low risk of bacterial transmission26 the risk of viral transmission had not been studied. The UK consensus guidelines for managing the airway in patients with COVID-19 were based on limited data and took a precautionary approach, recommending that NHFO should not be used in patients around the time of tracheal intubation.27 Joint UK Critical care guidance for COVID-19 patients also advised against NHFO use in critical care settings.28
Countries and societies worldwide adopted different approaches, with many recommending NHFO therapy for hypoxaemia associated with COVID-19 (e.g. Italy, China).29, 30 The WHO recommended NHFO use in selected patients with hypoxaemic respiratory failure.31 Because of the scale of the pandemic, intensive care resource limitations, and efficacy of NHFO in COVID-19 patients, NHFO has been used on a global scale. Consequently, there is both an increasing body of evidence of NHFO in clinical practice, and simulation and laboratory-based modelling of aerosolisation.
Different particle size thresholds arbitrarily differentiate an aerosol from a droplet (WHO specifies 5 μm diameter threshold32). The critical difference is that aerosols remain temporarily suspended in air (drag forces lifting them upwards equal gravitational forces pulling them to earth), travel over distances and can be inhaled and deposited in the distal airways, leading to airborne spread.41 By contrast, droplets follow a ballistic trajectory falling from patient’s mouth to ground, with droplet mass determining the distance travelled.41 Aerosol and droplet formation occurs when shear forces are generated which are sufficient to overcome surface tension forces between fluid in the respiratory tract and the respiratory tract lining.41 NHFO delivers a high velocity gas flow across the upper respiratory tract epithelium, generating such shear forces.41
The inclusion of NHFO on the current Public Health England list of aerosol-generating procedures is based on expert opinion and consensus, because ‘no evidence of appropriate quality or strength was identified’ for NHFO.33 NHFO use in anaesthesia, in contrast to use in the high-dependency unit setting, forms part of a wider airway management strategy, whether in a peri-intubation or extended apnoea/shared airway context. This involves other processes and techniques, many of which may be aerosol generating. NHFO/THRIVE may generate and disperse aerosols and droplets, but does it produce: (i) more than alternative oxygen delivery devices; (ii) more than other methods of aerosol generation (e.g. coughing); and (iii) increased viral transmission and infection?
NHFO aerosol and droplet dispersion compared with other oxygen delivery devices
In a high-fidelity patient simulator breathing spontaneously with NHFO at 10, 30, and 60 L min−1, mean (sd) exhaled air dispersion distances were 6.5 (1.5) cm, 13.0 (1.1) cm, and 17.2 (3.3) cm (P<0.001), respectively.34 Dispersion distances were lower than historical results of 5 L min−1 O2 via nasal cannula (42 cm), 12 L min−1 non-rebreather mask (<10 cm), noninvasive ventilation (NIV) 18/4 cm H2O (45 to >95 cm, dependent on mask/device).35 Additionally, computational fluid dynamic modelling on manikins suggests 85% of droplets (>5 μm) with NHFO are captured by applying a simple type 1 surgical mask.36
A study of aerosol generation in healthy volunteers during normal breathing found that NHFO did not increase aerosol generation compared with other modes of oxygen delivery (4 L min−1 simple nasal cannula [0.060 particles cm−3], 15 L min−1 face mask [0.059 particles cm−3], NHFO 30 L min−1 [0.046 particles cm−3], NHFO 50 L min−1 [0.041 particles cm−3], NIV 12/5 [0.056 particles cm−3], and NIV 20/10 cm H2O [0.057 particles cm−3]).37
NHFO aerosol and droplet generation compared with coughing and sneezing
Roberts and colleagues38 imaged droplets/aerosols within the exhaled breath of healthy volunteers with violent exhalation (snorting), comparing no NHFO with NHFO at 30 L min−1 or 60 L min−1 . The number of droplets produced was greatest during violent exhalation without NHFO, 43% less with NHFO 60 L min−1 and 56% less with NHFO 30 L min−1.38 Higher gas flow rates generated more droplets, but NHFO actually reduced droplet/aerosol dispersion during sneezing, as the inward gas flow opposed nasal expiration. Gaeckle and colleagues37 reported greater levels of aerosol and droplet generation with coughing, but that NHFO did not increase this. These limited studies suggest NHFO may reduce droplet dispersion during sneezing and does not increase droplet dispersion during coughing compared with other oxygen delivery devices.
Environmental contamination and healthcare worker infection
Environmental SARS-CoV-2 contamination with NHFO has not been assessed, however a study in 19 adult ICU patients with Gram-negative pneumonia found no difference in environmental bacterial contamination between NHFO at 60 L min−1 and standard facemask oxygen, but whether this is translatable to viruses is unclear.26 NHFO has been used for respiratory support in patients with COVID-19, and does not appear to have influenced infectivity rate within cohorted patient groups. An observational study in 28 patients with COVID-19 pneumonitis receiving NHFO wearing a surgical mask, found that all 73 healthcare workers wearing airborne personal protective equipment (PPE) who were exposed for a period of 48 h per person tested negative for SARS-CoV-2 during the study and 14 days afterwards.39
Taken together these findings support recent international expert consensus recommendations that there is currently no convincing evidence that NHFO increases the risk of COVID-19 cross-infection to healthcare workers, with appropriate PPE.40
Conclusions
THRIVE has facilitated a change in terms of the concept of per-oxygenation during airway management. In the future, it might be inconceivable to anaesthetise a patient without adequate per-oxygenation, just as now we would not anaesthetise without adequate pre-oxygenation. THRIVE peri-intubation oxygenation might be used routinely in all patients undergoing anaesthesia, as the positive predictive value of airway assessments to identify a difficult airway is notoriously poor.
In the COVID-19 era, use of NHFO/THRIVE involves balancing the advantages of extending the safe apnoea time to facilitate a less hurried and more-controlled intubation process and having a technique to provide ‘tubeless’ anaesthesia for shared-airway surgery, against the risk of aerosol and droplet formation and potential virus transmission. Since the initial UK guidance for NHFO in patients with COVID-19 was published, there is now experience from many countries using NHFO in COVID-19 patients; adequate protection for healthcare workers with appropriate PPE; evidence that NHFO aerosol dispersion is less than with coughing and comparable with other oxygen delivery devices; and laboratory modelling suggesting limited viral environmental contamination.
Funding
AS has no competing interests to declare. AP has received travel, accommodation, consulting and research funding from Fisher & Paykel Healthcare.
Declarations of interest
The authors declare that they have no conflicts of interest.
References
- 1.Frat J.-P., Thille A.W., Mercat A., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 2.Patel A., Nouraei S.A.R. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70:323–329. doi: 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frerk C., Mitchell V.S., McNarry A.F., et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015;115:827–848. doi: 10.1093/bja/aev371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgs A., McGrath B.A., Goddard C., et al. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018;120:323–352. doi: 10.1016/j.bja.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Wong D.T., Dallaire A., Singh K.P., et al. High-flow nasal oxygen improves safe apnea time in morbidly obese patients undergoing general anesthesia: a randomized controlled trial. Anesth Analg. 2019;129:1130–1136. doi: 10.1213/ANE.0000000000003966. [DOI] [PubMed] [Google Scholar]
- 6.Mir F., Patel A., Iqbal R., Cecconi M., Nouraei S.A.R. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia. 2017;72:439–443. doi: 10.1111/anae.13799. [DOI] [PubMed] [Google Scholar]
- 7.Lyons C., Callaghan M. Apnoeic oxygenation with high-flow nasal oxygen for laryngeal surgery: a case series. Anaesthesia. 2017;72:1379–1387. doi: 10.1111/anae.14036. [DOI] [PubMed] [Google Scholar]
- 8.Waters E., Kellner M., Milligan P., Adamson R.M., Nixon I.J., McNarry A.F. The use of Transnasal Humidified Rapid – insufflation Ventilatory Exchange (THRIVE) in one hundred and five upper airway endoscopies. A case series. Clin Otolaryngol. 2019;44:1115–1119. doi: 10.1111/coa.13408. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson I.-M., Lodenius A., Tunelli J., Ullman J., Jonsson Fagerlund M. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) – a physiological study. Br J Anaesth. 2017;118:610–617. doi: 10.1093/bja/aex036. [DOI] [PubMed] [Google Scholar]
- 10.To K., Harding F., Scott M., et al. The use of transnasal humidified rapid-insufflation ventilatory exchange in 17 cases of subglottic stenosis. Clin Otolaryngol. 2017;42:1407–1410. doi: 10.1111/coa.12921. [DOI] [PubMed] [Google Scholar]
- 11.Frumin M.J., Epstein R.M., Cohen G. Apneic oxygenation in man. Anesthesiology. 1959;20:789–798. doi: 10.1097/00000542-195911000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Eger E.I., Severinghaus J.W. The rate of rise of PACO2 in the apneic anesthetized patient. Anesthesiology. 1961;22:419–425. doi: 10.1097/00000542-196105000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Theiler L., Schneeberg F., Riedel T., Kaiser H., Riva T., Greif R. Apnoeic oxygenation with nasal cannula oxygen at different flow rates in anaesthetised patients: a study protocol for a non-inferiority randomised controlled trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-025442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys S., Lee-Archer P., Reyne G., Long D., Williams T., Schibler A. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. 2017;118:232–238. doi: 10.1093/bja/aew401. [DOI] [PubMed] [Google Scholar]
- 15.Riva T., Pedersen T.H., Seiler S., et al. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: a prospective randomised controlled trial. Br J Anaesth. 2018;120:592–599. doi: 10.1016/j.bja.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Parke R.L., McGuinness S.P. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. 2013;58:1621–1624. doi: 10.4187/respcare.02358. [DOI] [PubMed] [Google Scholar]
- 17.Riva T., Meyer J., Theiler L., et al. Measurement of airway pressure during high-flow nasal therapy in apnoeic oxygenation: a randomised controlled crossover trial. Anaesthesia. 2021;76:27–35. doi: 10.1111/anae.15224. [DOI] [PubMed] [Google Scholar]
- 18.Haycraft J.B., Edie R. The cardiopneumatic movements. J Physiol. 1891;12:426–437. doi: 10.1113/jphysiol.1891.sp000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tusman G., Suarez-Sipmann F., Peces-Barba G., et al. Pulmonary blood flow generates cardiogenic oscillations. Respir Physiol Neurobiol. 2009;167:247–254. doi: 10.1016/j.resp.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Hermez L.A., Spence C.J., Payton M.J., Nouraei S.A.R., Patel A., Barnes T.H. A physiological study to determine the mechanism of carbon dioxide clearance during apnoea when using transnasal humidified rapid insufflation ventilatory exchange (THRIVE) Anaesthesia. 2019;74:441–449. doi: 10.1111/anae.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laviola M., Das A., Chikhani M., Bates D.G., Hardman J.G. Computer simulation clarifies mechanisms of carbon dioxide clearance during apnoea. Br J Anaesth. 2019;122:395–401. doi: 10.1016/j.bja.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Hardman J.G., Wills J.S., Aitkenhead A.R. Factors determining the onset and course of hypoxemia during apnea: an investigation using physiological modelling. Anesth Analg. 2000;90:619–624. doi: 10.1097/00000539-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 23.McNamara M.J., Hardman J.G. Hypoxaemia during open-airway apnoea: a computational modelling analysis. Anaesthesia. 2005;60:741–746. doi: 10.1111/j.1365-2044.2005.04228.x. [DOI] [PubMed] [Google Scholar]
- 24.Farmery A.D., Roe P.G. A model to describe the rate of oxyhaemoglobin desaturation during apnoea. Br J Anaesth. 1996;76:284–291. doi: 10.1093/bja/76.2.284. [DOI] [PubMed] [Google Scholar]
- 25.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung C.C.H., Joynt G.M., Gomersall C.D., et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101:84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faculty Of Intensive Care Medicine, Intensive Care Society . Association of Anaesthetists, Royal College of Anaesthetists; March 2020. Critical care preparation and management in the COVID-19 pandemic.https://icmanaesthesiacovid-19.org/critical-care-preparation-and-management-in-the-covid-19-pandemic (Great Britain) [Google Scholar]
- 29.Harari S., Vitacca M., Blasi F., et al. Italian Thoracic Society; March 2020. Managing the respiratory care of patients with COVID-19.https://ers.app.box.com/s/j09ysr2kdhmkcu1ulm8y8dxnosm6yi0h Available from: [Google Scholar]
- 30.Respiratory Care Committee of Chinese Thoracic Society Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:288–296. doi: 10.3760/cma.j.cn112147-20200304-00239. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization . March 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance.https://apps.who.int/iris/bitstream/handle/10665/331446/WHO-2019-nCoV-clinical-2020.4-eng.pdf Available from: [Google Scholar]
- 32.World Health Organization . 2014. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care: WHO guidelines.http://apps.who.int/iris/bitstream/10665/112656/1/9789241507134_eng.pdf?ua=1 Available from: [PubMed] [Google Scholar]
- 33.New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) and Public Health England (PHE) May 2020. Assessing the evidence base for medical procedures which create a higher risk of respiratory infection transmission from patient to healthcare worker.https://hpspubsrepo.blob.core.windows.net/hps-website/nss/3055/documents/1_agp-sbar.pdf Available from: [accessed )] [Google Scholar]
- 34.Hui D.S., Chow B.K., Lo T., et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53:1802339. doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 35.Hui D.S.C., Chan M.T.V., Chow B. Aerosol dispersion during various respiratory therapies: a risk assessment model of nosocomial infection to health care workers. Hong Kong Med J. 2014;20(Suppl 4):9–13. [PubMed] [Google Scholar]
- 36.Leonard S., Atwood C.W., Walsh B.K., et al. Preliminary findings on control of dispersion of aerosols and droplets during high-velocity nasal insufflation therapy using a simple surgical mask. Chest. 2020;158:1046–1049. doi: 10.1016/j.chest.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaeckle N.T., Lee J., Park Y., Kreykes G., Evans M.D., Hogan C.J., Jr. Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am J Respir Crit Care Med. 2020;202:1115–1124. doi: 10.1164/rccm.202006-2309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts S., Kabaliuk N., Spence C., et al. Nasal high-flow therapy and dispersion of nasal aerosols in an experimental setting. J Crit Care. 2015;30:842. [Google Scholar]
- 39.Vianello A., Arcaro G., Molena B., et al. High-flow nasal cannula oxygen therapy to treat patients with hypoxemic acute respiratory failure consequent to SARS-CoV-2 infection. Thorax. 2020;75:998–1000. doi: 10.1136/thoraxjnl-2020-214993. [DOI] [PubMed] [Google Scholar]
- 40.Wei H., Jiang B., Behringer E.C., et al. Controversies in airway management of COVID-19 patients: updated information and international expert consensus recommendations. Br J Anaesth. 2021;126:361–366. doi: 10.1016/j.bja.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson N.M., Norton A., Young F.P., Collins D.W. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia. 2020;75:1086–1095. doi: 10.1111/anae.15093. [DOI] [PMC free article] [PubMed] [Google Scholar]