Abstract
Objective
The characteristics and outcomes of patients undergoing vascular surgery hospitalised and managed in Lombardy are described with a comparison of patients tested positive for COVID-19 (CV19-pos) vs. those tested negative (CV19-neg).
Methods
This was a multicentre, retrospective, observational cohort study which involved all vascular surgery services in Lombardy, Northern Italy. Data were retrospectively merged into a combined dataset covering the nine weeks of the Italian COVID-19 pandemic phase 1 (8 March 2020 to 3 May 2020). The primary outcome was freedom from in hospital death, secondary outcomes were re-thrombosis rate after peripheral revascularisation, and freedom from post-operative complication.
Results
Among 674 patients managed during the outbreak, 659 (97.8%) were included in the final analysis: 121 (18.4%) were CV19-pos. CV19-pos status was associated with a higher rate of complications (OR 4.5; p < .001, 95% CI 2.64 – 7.84), and a higher rate of re-thrombosis after peripheral arterial revascularisation (OR 2.2; p = .004, 95% CI 1.29 – 3.88). In hospital mortality was higher in CV19-pos patients (24.8% vs. 5.6%; OR 5.4, p < .001;95% CI 2.86 – 8.92). Binary logistic regression analysis identified CV19-pos status (OR 7.6; p < .001, 95% CI 3.75 – 15.28) and age > 80 years (OR 3.2; p = .001, 95% CI 1.61 – 6.57) to be predictors of in hospital death.
Conclusion
In this experience of the vascular surgery group of Lombardy, COVID-19 infection was a marker of poor outcomes in terms of mortality and post-operative complications for patients undergoing vascular surgery treatments.
Keywords: COVID-19, vascular surgery activities, acute limb ischaemia
What this paper adds.
This paper evaluates hospital outcomes and reports independent predictors of in hospital mortality in a large cohort of patients undergoing vascular surgery during “phase 1” of the COVID-19 outbreak experienced in one of the European areas with the initial and highest incidence of viral infection. The analysis reveals that COVID-19 patients had 7.6 fold increase in mortality, 4.5 fold increase in complication rate, and a higher re-thrombosis rate after peripheral arterial revascularisation. These findings will help to stratify the clinical risk during a potential following waves of the COVID-19 infection.
Introduction
Since the pandemic spread of novel coronavirus-19 (COVID-19) infection, respiratory distress syndrome has been described as the dominant clinical manifestation of this severe disease.1 , 2 Following the first official case in Northern Italy on 18 February 2020, the emerging involvement of the cardiovascular system in this disease was soon recognised, but much of the attention was paid to venous thrombo-embolism, and cardiac ischaemic and inflammatory emergencies.3, 4, 5, 6 Furthermore, one of the most important activities of the vascular surgical community has been to concentrate on structural reorganisation of the centres.7, 8, 9, 10, 11, 12, 13 In contrast, little has been described of the epidemiological trend of the vascular pathologies and of the outcomes stratified by COVID-19 infection and type of hospital organisation.14 Although the Italian Government was forced to take extraordinary measures to contain the infection such as quarantine and the closure of major social and economic activities, the number of newly infected patients increased exponentially on a daily base, especially in Lombardy, which is the Italian area which experienced the highest COVID-19 incidence, but it is also the most populous area of the country with more than 10 million inhabitants. In this emergency situation investigators collected clinical data and early outcomes of those patients managed during “phase 1” of the Italian outbreak (8 March to 3 May 2020). This article analyses the data set of the Vascular Surgery Group of the Lombardy region (VSG-RL) and evaluates the mortality and complication rate stratified by COVID-19 infection.
Materials and Methods
Study cohort
This was a multicentre, retrospective, observational cohort study organised following the STROBE guidelines.15 The study involved all referral and tertiary vascular surgery services in Lombardy, Northern Italy.16 All vascular surgery units were queried for data collected in hospital of consecutive vascular patients admitted and managed operatively. Informed consent regarding the specific treatment was obtained from each patient. Approval for this specific study was waived by each local Institutional Review Board, according to the National Policy in the matter of the Privacy Act on retrospective analysis of anonymised data and in consideration of the assembly prohibition policies in relation to this health emergency.
Data collection
The Lombardy healthcare system is based on public and private hospitals, including academic and teaching hospitals. All the hospitals included in the regional health system are free of charge. Vascular surgery units cover the entire needs of patients suffering from arterial and venous diseases, so that there are no independent angiology units. During the COVID-19 outbreak, the re-organisation process planned that both some public hospitals and private hospitals were identified for referral of patients suffering from vascular pathologies. All vascular surgery units were involved in this study, none was excluded a priori. According to the regional health system re-organisation of vascular surgery services, all planned interventions were cancelled and rescheduled only after “phase 1” was officially declared over. Therefore, only those vascular conditions that required urgent/emergency interventions, namely those lifesaving interventions, those to be performed < 24 hours from diagnosis, and those defined as “non-deferrable” (to be treated within 30 days from diagnosis or hospital admission) were operated on during the pandemic “phase 1”.16 Clinical data were collected on admission and during hospitalisation by attending physicians. A single dedicated database was created, and data were merged retrospectively into a combined dataset. Information about demographics, co-morbidities (hypertension, chronic obstructive pulmonary disease, chronic kidney disease, smoking habits, diabetes, left ischaemic heart disease, atrial fibrillation), risk factors (urgency, COVID-19 status), previous vascular surgical history, operative details, and post-operative events during the hospital stay were all registered. These data were obtained from the institutional database of each of the participating centres. Additional collected data during the hospital stay included baseline laboratory blood tests, respiratory parameters, Xrays, computed tomography scans, COVID-19 related treatment, operative treatment, and type of complication and outcomes (including length of hospital stay, death, discharge, and readmission). Data collection covered the “phase 1” period of the COVID-19 pandemic in Italy which started on 8 March 2020, and was declared over on 3 May 2020.17
Definition
“Phase 1” emergency of the CV19 outbreak was officially established by National Government, and included a mandatory requirement to stay at home, social distancing and avoiding gatherings, meticulous and frequent disinfection of hands, and prohibition of moving among municipalities, except for health or food emergencies. Physicians went to work under the condition of a specific signed permission released by the National Government. Patients were considered to have confirmed infection if the initial reverse transcription polymerase chain reaction test on throat or lower respiratory tract swab result was positive, or if the initial test was negative but repeated testing was positive. Generally, tests were repeated if the initial negative test result was judged likely to be a false negative, especially if there was a high clinical probability of COVID-19 based on high resolution chest computed tomography (hrCT). Patients were divided in two groups according to COVID-19 testing: patients who were tested positive for COVID-19 on admission or during hospitalisation, and those who always tested negative for COVID-19. The clinical status of patients with chronic limb threatening ischaemia (CLTI) was defined according to the Rutherford classification.18 Chronic kidney disease was defined in agreement to the Kidney Disease Improving Global Outcomes clinical practice guidelines.19 Pre-existing chronic obstructive pulmonary disease was classified according to the Global initiative for chronic Obstructive Lung Disease (GOLD).20 Acute respiratory distress syndrome was defined according to the Berlin definition.21 Arterial dissection was defined as acute (0 – 7 days), subacute (8 – 30 days), and chronic (≥ 30 days).22 Urgency was considered when intervention was performed ≤ 24 hours from hospital admission and on the first diagnosis of the vascular disease especially in symptomatic patients. All centres had similar protocols regarding the definition for performing urgent intervention in agreement with the guidelines of the Italian Society for Vascular and Endovascular Surgery, and adhered to the treatment guidelines of acute limb ischaemia (ALI) of the European Society For Vascular Surgery.18 , 23 Pharmacological treatment for COVID-19 infection was in agreement with the most recent guidelines.23, 24, 25 Post-operative complications were classified according to the Society for Vascular Surgery (SVS) grading system.26 The analysis was focused on two major outcomes: freedom from in hospital mortality for all vascular treatments, and freedom from post-operative complication.
Database completion
The database was initially shared to all Vascular Surgery units. After single institution collection, each centre returned its single centre dataset which was then collated into a single database, then managed and analysed by two separate authors (G.P., D.B.). In the event of inconsistency, dataset compilers at each single hospital were directly contacted by the authors to discuss any doubts. For result discrepancies, a third author (R.B.) was consulted to resolve any controversies and achieve consensus. This process was done because of bias derived from dataset compilation among centres and to maximise the interpretation of definitions and results between blinded investigators.
Statistical analysis27
Clinical data were recorded and tabulated in a Microsoft Excel (Microsoft Corp., Redmond, WA, USA) spreadsheet: statistical analysis was performed with SPSS 26.0 for Windows (IBM SPSS; Chicago; IL, USA). Categorical (nominal) variables were presented using frequencies and percentages, and continuous (quantitative) variables with mean ± standard deviation (SD), or median with interquartile range (IQR) and ranges according to data distribution. Categorical variables were analysed with the chi square test, and Fisher’s exact test when appropriate. Continuous variables were tested for normal distribution by the Shapiro–Wilk’s test and compared between groups with unpaired Student’s t test for normally distributed values; otherwise, the Mann–Whitney U test was used. Tukey’s honest significance test was used as a single step multiple comparison to find significant differences between means. The Wilcoxon signed rank test was used to evaluate the difference between specific covariate measurements before and after intervention. Univariable analysis was used to identify potential predictors of in hospital death. Associations that yielded a p value < .20 on univariable screen were then included in a binary logistic regression analysis using a Wald model. The strength of the association of variables with mortality was estimated by calculating the odds ratio (OR) and 95% confidence intervals (95% CI) (significance criteria p = .25 for entry, p = .05 for removal). Model discrimination was evaluated by using the area under the receiver operating characteristic (AUROC) curve, with ≥ 0.7 being considered significantly accurate and 0.5 - 0.69 moderately accurate. All reported p values were two sided; p < .050 was considered significant.
Results
General cohort
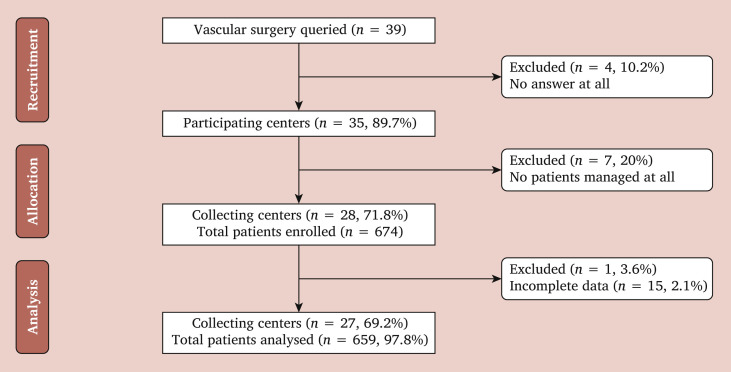
Overall, 35/39 (89.7%) centres responded to the invitation, and data from 31/39 (79.5%) centres were finally included in the study. Six hundred seventy-four patients were managed, and 659 (97.8%) form the cohort for the final analysis as reported in Fig. 1 . The recruitment period during “phase 1” lasted nine weeks. There were 464 (70.4%) males and 193 (29.3%) females. The median of age for the whole cohort was 72 years (IQR, 65 – 81). Table 1 reports the characteristics of the whole cohort with the treatment strategies pursued during “phase 1”. On admission, 269 (40.4%) patients were already taking aspirin, 104 (15.6%) were on dual antiplatelet treatment, and 82 (12.3%) were on oral anticoagulants.
Figure 1.
Consort diagram of patients hospitalised and managed in the vascular surgery units of the Lombardy region during phase one of the COVID-19 outbreak (8 March 2020 to 3 May 2020).
Table 1.
Demographics, comorbidities, and operative strategies of the 659 vascular surgery patients treated in Lombardy, Italy, during the COVID-19 pandemic
| Characteristics | Vascular surgery patients (n = 659) |
|---|---|
| Demographics | |
| Male : Female | 464 : 193 |
| Age – y | 72 ± 12 |
| >80 | 195 (29.6) |
| Comorbidities | |
| Hypertension | 474 (75.2) |
| Diabetes | 231 (37.3) |
| Previous vascular interventions | 169 (34.8) |
| Coronary artery disease | 157 (33.1) |
| Active smoker | 193 (29.3) |
| Previous thrombo-embolism | 17 (23.4) |
| Chronic kidney disease | 143 (23.3) |
| Chronic obstructive pulmonary disease | 129 (21) |
| Atrial fibrillation | 121 (19.7) |
| Obesity; BMI >30 kg/m2 | 76 (13.1) |
| Type of intervention∗ | 607 (92.1) |
| Urgent/Emergency | 372 (61.3) |
| PAOD endovascular | 111 (18.3) |
| Thrombo-embolectomy | 110 (18.1) |
| PAOD open surgical | 86 (14.2) |
| Amputation | 68 (11.2) |
| Carotid endarterectomy | 60 (9.9) |
| Carotid artery stenting | 9 (1.5) |
| EVAR | 30 (4.9) |
| Open AAA repair | 22 (3.6) |
| TEVAR | 16 (2.6) |
| Thoraco-abdominal open | 2 (0.3) |
| Renovisceral | 6 (0.9) |
| Vascular access surgery | 37 (6.1) |
| Miscellaneous | 50 (8.2) |
Data are presented as n (%) or mean ± standard deviation. BMI = body mass index; PAOD = peripheral arterial occlusive disease; EVAR = endovascular abdominal aortic aneurysm repair; AAA = abdominal aortic aneurysm; TEVAR = thoracic endovascular aortic repair.
Proportions of interventions calculated as proportions of total n = 607.
Global surgical activities and early outcomes
Overall, 607 (92.1%) patients underwent operative repair: 434 (71.5%) were urgent/emergency. Fifty-two (7.9%) patients did not receive operative treatment, and terminal illness (n = 44) was the leading cause for non-operative treatment. Analysing the nine weeks of “phase 1” as three weeks tertiles the most frequent indication for hospital admission was peripheral arterial occlusive disease (n = 403, 61.1%): there were 238 (59%) CLTI cases, and 165 (41%) ALI cases: 375 (93.1%) underwent operative intervention. The second most frequent indication for hospital admission was aortic aneurysm (n = 84, 12.7%), and 52 of 63 abdominal aortic aneurysms, and 18 of 21 thoracic/thoraco-abdominal aortic aneurysms were operated on. One case of thoracic aortic dissection was treated with best medical treatment, only. The third most frequent cause of hospital admission was extracranial carotid artery disease, and of the 70 (10.6%) cases managed during this period, 69 (98.6%) were treated operatively. In Table 2 the total numbers of interventions performed for the three main vascular diseases are reported, stratified by urgent/emergency vs. non-deferrable interventions. Overall, 62 (9.4%) patients died in hospital. Complications were observed in 132 (20%) patients, mild in 26 (19.7%), moderate in 53 (40.1%), and severe in 53 (40.1%). The median hospital stay was four days (IQR, 2 – 8).
Table 2.
Numbers of interventions performed for the three main vascular surgery diseases stratified by clinical setting in Lombardy, Italy, during the COVID-19 pandemic
| Intervention | Urgent/Emergent (n = 337) | Non-deferrable (n = 177) |
|---|---|---|
| Aorta | ||
| Abdominal aorta | ||
| Endovascular | 29 (8.6) | 1 (0.6) |
| Open | 21 (6.2) | 1 (0.6) |
| Descending thoracic aorta | ||
| Endovascular | 14 (4.1) | 2 (1.2) |
| Open | n.p. | n.p. |
| Thoraco-abdominal aorta | ||
| Endovascular | n.p. | n.p. |
| Open | 1 (0.3) | 1 (0.6) |
| Carotid | ||
| Carotid endarterectomy | 46 (13.6) | 14 (7.9) |
| Carotid artery stenting | 8 (2.4) | 1 (0.6) |
| PAOD | ||
| Acute limb ischaemia | ||
| Endovascular | 7 (2.1) | n.p. |
| Open | 135 (40.0) | 2 (1.2) |
| Primary amputation | 8 (2.4) | n.p. |
| Chronic limb threatening ischaemia | ||
| Endovascular | 22 (6.5) | 82 (46.3) |
| Open | 22 (6.5) | 37 (20.9) |
| Primary amputation | 24 (7.1) | 36 (20.3) |
Data are presented as n (%). PAOD = peripheral arterial occlusive disease; n.p. = not performed.
Comparative analysis by COVID-19 status
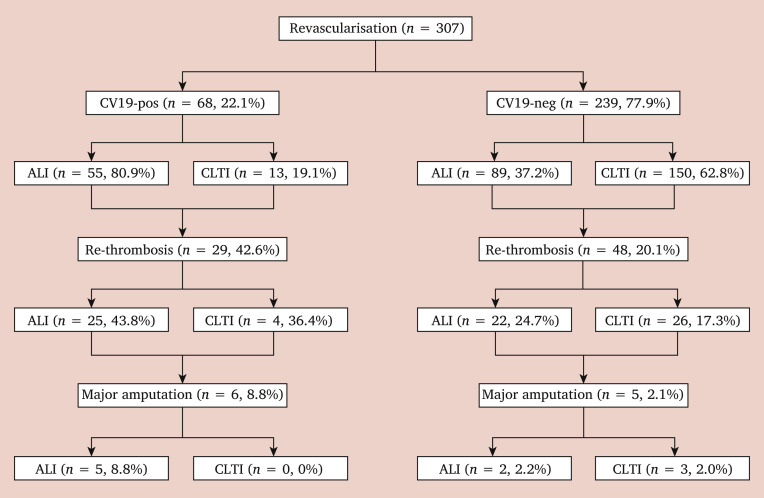
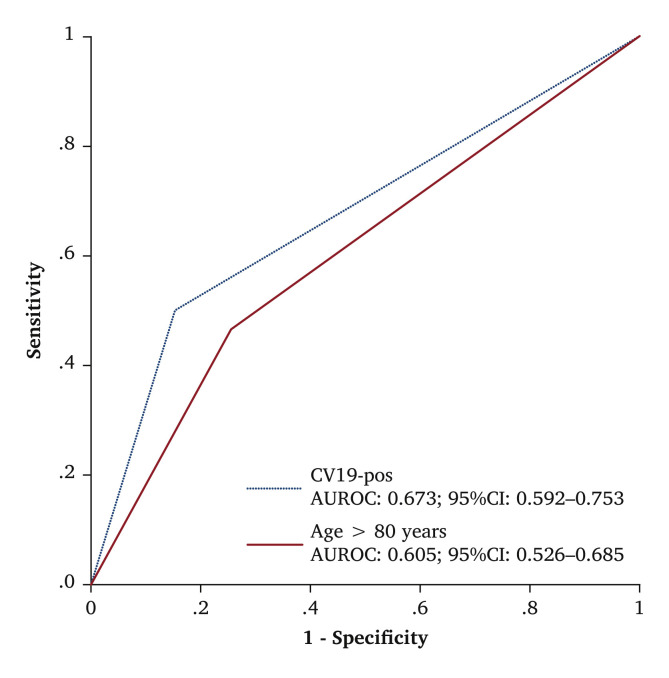
The VSG-RL managed 121 (18.4%) COVID-19 positive patients, and 103 (85.1%) received an operation. The number of COVID-19 positive patients did not differ across the three weeks (W) tertiles (W1 – 3 vs. W4 – 6 vs. W7 – 9, p = .293). Demographic data, comorbidities, and risk factors of the two groups are reported in Table 3 . Ninety-nine (81.8%) COVID-19 positive patients had hrCT signs of COVID-19 related pneumonia on admission. Urgent or emergency clinical onset was higher in COVID-19 positive patients (75.8% vs. 56.6%; OR 2.4, p < .001; 95% CI 1.52 – 3.77). Among vascular diseases, peripheral arterial occlusive disease was the most frequent disease observed in COVID-19 positive patients compared with COVID-19 negative patients (78.5% vs. 56.1%; OR 2.1, p < .001; 95% CI 1.36 – 3.33). Table 4 reports the type of intervention stratified by COVID-19 status. Post-operative complications occurred in 55 COVID-19 positive patients and in 77 COVID-19 negative patients (45.4% vs. 14.3%, p < .001; OR 5.1). COVID-19 positive patients had a higher rate of complications overall (OR 4.5; p < .001, 95% CI 2.64 – 7.84). The type of intervention was also a predictor of post-operative complications (OR 2.6; p < .001, 95% CI 1.50 – 4.58). Of those patients who underwent peripheral revascularisation, 77 (25.1%) developed early re-thrombosis: 10 (3.2%) ended up with major amputation, more frequently after revascularisation for ALI as reported in the flow diagram in Fig. 2 . Among the variables included in the model with re-thrombosis as the endpoint, binary logistic regression analysis identified COVID-19 positive status to be associated with early re-thrombosis (OR 2.2; p = .004, 95% CI 1.29 – 3.88). Hospitalisation was longer in COVID-19 positive patients (median: 7 days vs. 4 days, p = .023). Mortality was higher in COVID-19 positive patients than in COVID-19 negative patients (24.8% vs. 5.6%, p < .001; OR 5.4). There were no deaths in patients < 60 years of age, but there was a different mortality rate within the different decades for those aged > 61 years (p = .002) as shown in Table 5 . Causes of death are reported in Table 6 . COVID-19 positive status (OR 7.6; p < .001, 95% CI 3.75 – 15.28) and age > 80 years (OR 3.2; p = .001, 95% CI 1.61 – 6.57) were predictors of death. The Hosmer–Lemeshow goodness of fit test (χ2 = 4.89; p = .30) and ROC analysis provided a reasonable discrimination for COVID-19 positive status (AUROC, 0.67; 95% CI 0.59 – 0.75) and for age > 80 years (AUROC, 0.61; 95% CI 0.53 – 0.69) as reported in Fig. 3 . Table 7 reports factors affecting post-operative complications and death in the whole study group.
Table 3.
Demographics, comorbidities, and operative risk stratified by COVID-19 (CV19) status in vascular surgery patients in Lombardy, Italy, during the COVID-19 pandemic
| CV19- positive (n = 121) | CV19- negative (n = 538) | p | |
|---|---|---|---|
| Demographics | |||
| Male : Female | 92 : 29 | 366 : 157 | .22 |
| Age – y | 73 ± 12 | 72 ± 12 | .59 |
| >80 | 40 (21.3) | 80 (17.6) | .32 |
| BMI – kg/m2 | 26 ± 4 | 25 ± 4 | .35 |
| >30 | 3 (8.1) | 48 (19) | .16 |
| Comorbidities | |||
| Hypertension | 82 (71.3) | 393 (76) | .29 |
| Coronary artery disease | 27 (31) | 126 (32.4) | .90 |
| Chronic obstructive pulmonary disease | 28 (25.2) | 102 (20.1) | .25 |
| Chronic kidney disease | 14 (12.5) | 131 (25.9) | .002 |
| Atrial fibrillation | 30 (26.8) | 90 (17.9) | .035 |
| Active smoker | 39 (32.2) | 154 (28.6) | .50 |
| Diabetes | 33 (29.2) | 199 (38.9) | .067 |
| Previous interventions | 17 (18.7) | 153 (38.4) | <.001 |
| Vascular | 17 | 150 | |
| Cardiac | 0 | 3 | |
| Previous thrombo-embolism | 14 (15.9) | 97 (24.7) | .093 |
| Risk factors | |||
| Urgent/Emergency | 92 (76.1) | 296 (56.6) | <.001 |
| PaO2 – % | 69 ± 12 | 89 ± 33 | <.001 |
| Leucocytes – 109/L | 11 215 ± 6 299 | 10 292 ± 4 576 | .15 |
| Median D dimer – ng/L∗ | 897 | 2410 | .23 |
| Platelets – 109/L | 246 ± 118 | 246 ± 107 | .99 |
| Median CPK – U/L | 426 | 88.5 | .005 |
| AT-III – % | 81 ± 14 | 85 ± 20 | .50 |
Data are presented as n (%) or mean ± standard deviation unless stated otherwise. CPK = creatine phosphokinase; AT-III = antithrombin-III.
p value of difference between COVID-19 positive patients and COVID-19 negative patients.
Table 4.
Type of operative procedure performed during the “phase 1” period of 8 March to 3 May 2020 stratified by COVID-19 (CV19) status in Lombardy, Italy
| CV19-pos (n = 121) | CV19-neg (n = 538) | p | |
|---|---|---|---|
| PAOD revascularisation∗ | |||
| Open surgical† | 11 (9.1) | 75 (13.9) | <.001 |
| Thrombo-embolectomy | 52 (42.9) | 58 (10.8) | |
| PTA/stent | 4 (3.3) | 68 (12.6) | |
| PTA/stent + planned minor amputation | 1 (0.8) | 38 (7.1) | |
| Aortic | |||
| EVAR | 3 (2.5) | 27 (5.0) | .49 |
| TEVAR | 1 (0.8) | 15 (2.8) | |
| Open AAA repair | 4 (3.3) | 18 (3.3) | |
| Open thoraco-abdominal | 0 (0) | 2 (0.4) | |
| Carotids | |||
| CEA | 1 (0.8) | 59 (10.9) | 1.0 |
| CAS | 0 (0) | 9 (1.7) | |
| Amputation | |||
| Major | 10 (8.3) | 45 (8.4) | .51 |
| Minor | 0 (0) | 13 (2.4) | |
| AV access | |||
| Creation | 1 (0.8) | 25 (4.6) | 1.0 |
| Rescue | 0 (0) | 11 (2.0) | |
| Venous complex | |||
| Open abdominal | 0 (0) | 4 (0.7) | .20 |
| Stenting | 1 (0.8) | 0 (0) | |
| Renovisceral | |||
| Embolectomy | 1 (0.8) | 0 (0) | .33 |
| Endovascular | 0 (0) | 4 (0.7) | |
| Open renal | 0 (0) | 1 (0.2) | |
| Trauma/Miscellaneous | |||
| Embolisation | 9 (7.4) | 2 (0.4) | <.001 |
| Others | 5 (4.1) | 29 (5.4) | |
Data are presented as n (%). AAA = abdominal aortic aneurysm; PAOD = peripheral arterial occlusive disease. EVAR = endovascular abdominal aortic aneurysm repair; TEVAR = thoracic endovascular aortic repair; CEA = carotid endarterectomy; CAS = carotid artery stenting; AV = arteriovenous; PTA = percutaneous transluminal angioplasty.
Sixty-eight primary amputations excluded.
Thrombo-embolectomy/surgical bypass/endarterectomy/hybrid.
Figure 2.
Flow diagram of 307 patients who underwent revascularisation for peripheral arterial occlusive disease patients in Lombardy, Italy, during the COVID-19 (CV19) pandemic. ALI = acute limb ischaemia; CLTI = chronic limb threatening ischaemia.
Table 5.
Mortality rates stratified by patient age interval and COVID-19 (CV19) status
| Age decade – y | CV19-positive (n = 121) |
CV19-negative (n = 538) |
p | ||
|---|---|---|---|---|---|
| Dead | Alive | Dead | Alive | ||
| <60 | 0 (0) | 18 (14.9) | 0 (0) | 80 (14.9) | 1.0 |
| 61–70 | 25 (20.7) | 5 (4.1) | 119 (22.1) | 8 (1.5) | .14 |
| 71–80 | 22 (18.2) | 12 (9.1) | 179 (33.3) | 8 (1.5) | <.001 |
| 81–90 | 24 (19.8) | 11 (9.1) | 106 (19.7) | 10 (1.8) | .002 |
| >91 | 1 (0.8) | 2 (1.6) | 9 (1.7) | 4 (0.7) | .52 |
Data are presented as n (%).
Table 6.
Complications and Society for Vascular Surgery (SVS) severity scoring grade stratified by COVID-19 (CV19) status
| Parameters | CV19-positive (n = 121) | CV19-negative (n = 538) | p |
|---|---|---|---|
| SVS severity | |||
| Mild | 9 (7.4) | 13 (2.4) | .012 |
| Moderate | 19 (15.7) | 22 (4.1) | < .001 |
| Severe | 22 (18.2) | 31 (5.8) | < .001 |
| Type of complication | |||
| Re-thrombosis | 18 (14.9) | 18 (3.3) | |
| ARDS | 10 (8.3) | 7 (1.3) | |
| Renal | 6 (4.9) | 9 (1.7) | |
| Cardiac/PE | 2 (1.6) | 12 (2.2) | |
| Bleeding | 7 (5.8) | 7 (1.3) | |
| Miscellaneous | 1 (0.8) | 5 (0.9) | |
| Sepsis/gangrene | 3 (2.5) | 2 (0.4) | |
| Stroke/SCI | 2 (1.6) | 3 (0.6) | |
| MOF | 1 (0.8) | 3 (0.6) | |
Data are presented as n (%). ARDS = acute respiratory distress syndrome; PE = pulmonary embolism; SCI = spinal cord ischaemia; MOF = multiple organ failure.
Figure 3.
Receiver operating characteristic (ROC) curve analysis for mortality predictors (positivity for COVID-19 [CV19] and age > 80 years) among 659 vascular surgery patients in Lombardy, Italy. AUROC = area under the ROC curve.
Table 7.
Univariable and multivariable analysis for factors affecting post-operative complications and mortality among 659 vascular surgery patients in Lombardy, Italy, during the COVID-19 (CV19) pandemic
| Variable | Post–operative complications |
Death |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable OR (CI) | p | Multivariable OR (CI) | p | Univariable OR (CI) | p | Multivariable OR (CI) | p | |
| Gender | ||||||||
| Age >80 y | 2.44 (1.41–4.20) | .002 | 3.2 (1.61–6.57) | .001 | ||||
| Hypertension | ||||||||
| Obesity; BMI >30 kg/m2 | 0.22 (0.51–0.94) | .024 | 1.69 (0.82–3.48) | .148 | ||||
| Diabetes | 0.57 (0.37–0.87) | .010 | ||||||
| CAD | 1.45 (0.81–2.59) | .225 | ||||||
| Atrial fibrillatio | 2.07 (1.13–3.81) | .022 | ||||||
| CKD | ||||||||
| COPD | ||||||||
| Previous thrombo-embolism | 0.61 (0.34–1.08) | .11 | 0.31 (0.13–0.76) | .007 | ||||
| Previous vascular intervention | 0.68 (0.42–1.10) | .13 | 0.59 (0.32–1.12) | .131 | ||||
| Urgent/Emergency intervention | 2.98 (1.89–4.68) | <.001 | 2.62 (1.50–4.58) | .001 | 2.27 (1.17–4.38) | .014 | ||
| CV19-positive | 4.78 (3.09–7.36) | <.001 | 4.55 (2.64–7.84) | <.001 | 5.04 (2.85–8.92) | <.001 | 7.6 (3.75–15.28) | <.001 |
OR odds ratio; CI = confidence interval; BMI = body mass index; CAD = coronary artery disease
CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease.
Discussion
The analysis of phase 1 of the Italian COVID-19 outbreak showed that COVID-19 positive status was a marker of poor outcomes even in vascular surgery patients. These COVID-19 positive patients had higher mortality and complication rates, with a higher re-thrombosis rate after revascularisation procedures for PAOD, which was the most frequent indication for vascular surgery during this time.
From the beginning COVID-19 outbreak, the primary clinical focus has been COVID-19 related respiratory issues and their impact on overall outcomes.1 , 2 Only few weeks later, did some studies shed light on another worrisome scenario, the association between COVID-19 infection and major cardiovascular events such as venous thrombo-embolism and myocardial injury.4, 5, 6 So far, only Seiffert et al. have investigated the rate of emergency hospital admissions during the pandemic but very few have reported detailed outcomes of vascular treatments.11 , 12 , 14 While hypercoagulable state, thrombophilia, and sepsis induced coagulopathy have been preferentially associated with venous thrombo-embolism, what was unexpected during this outbreak period was the predominant incidence of PAOD among all the vascular diseases, especially acute peripheral arterial thrombo-embolism.14 , 16 , 28 , 29 A hypothetical hypercoagulability status triggered by the SARS-CoV-2 viral infection, may have also contributed to this 62% incidence of peripheral arterial interventions in the COVID-19 positive cohort which was two times higher than the COVID-19 negative cohort.
The reorganisation process dictated by the regional Health Government was an attempt to limit the spread of the outbreak, but it was also the consequence of this outbreak which forced the severe restrictions from a logistic viewpoint.7 , 12 , 16 COVID-19 infection had an important impact on surgical activities; in fact, during this time these decreased > 75% in 97% of the vascular services, with 10 centres not performing vascular interventions at all.9, 10, 11 , 13 , 30, 31, 32 Nearly 72% of the interventions were performed in an urgent/emergency setting, and such data correlate with the decrease in surgical activities. The restrictions imposed by this overwhelming outbreak may have had further possible alarming downsides on the overall cohort of patient. While the main finding of this analysis is that COVID-19 infection was also a predictor of death in patients undergoing vascular surgery, the 6.9% mortality rate in the COVID-19 negative subgroup is relevant as well. There may be different interpretations for these data: first, patient attitude may have contributed to the increased mortality because they may have reached hospital in a worse condition.30 It is reasonable to consider that those who had been examined and planned for an intervention in non-critical conditions may consequently have presented in a more critical clinical stage than the original one. This observation finds support in a large review of the Chinese experience in which, when mortality was analysed in specific cohorts of patients presenting with major adverse cardiovascular events, it was significantly higher.1 Second, the rapidly increasing numbers COVID-19 positive patients also required a reorganisation of personnel, especially in the anaesthetic sector in order to look after the most critical patients. In this “war like” scenario, at full capacity many more intensive care unit beds would have been needed for best management of the patients.
A recent analysis of the Italian national data reported that increased mortality was more consistent for men and women aged 80 – 89 years, for whom the cumulative deaths from 1 January to 30 April 2020 increased by 52% compared with the average for the same period in 2015 – 2019.33 It was not surprising that age > 80 years was a predictor of death in patients undergoing vascular surgery. This observation was even more significant if we consider that mortality was different across every 10-years decade, despite the heterogeneous distribution of age within the entire cohort.
The analysis and other previous experiences showed that peripheral ischaemic events have been the primary vascular emergency of this initial phase of the outbreak. As a consequence, early re-thrombosis of the revascularisation was the secondary outcome to be evaluated.3 , 14 The COVID-19 infection was detected as a marker of poor clinical outcome, and as with mortality, the re-thrombosis rate was significantly higher in COVID-19 positive patients, irrespective of the type of intervention. Notably, this higher than expected re-thrombosis rate after peripheral revascularisation was reported to be quite similar to what happened after percutaneous coronary interventions for myocardial ischaemia.3 , 4 , 6 , 34 Whether this higher re-thrombosis rate can be associated with the acute hypercoagulability states or due to advanced pre-operative condition is currently impossible to determine. Although there are no data to support the fact that this high failure rate was determined by the hypercoagulability status triggered by SARA-CoV-2, some indirect data are available for supporting the current purely speculative observation. First, the higher than previously reported incidence of ALI, which was the first ever vascular disease event also in younger patients.14 Second, the better results obtained in terms of patency rates with the use of a more aggressive heparin treatment.14 , 34 Third, data coming from the ICUs reported better overall outcomes in those patients on anticoagulants.35 Last but not least, the macroscopic aspect of the excised thrombi, that had a peculiarly gelatinous consistency a finding that had previously been described in SARS-CoV-1 outbreak patients.14
In the recent analysis from the COVID-19 surgical collaborative group cohort initiative, merging the surgical experience from 235 hospitals in 24 countries, the Authors showed that post-operative pulmonary complications occurred in half of patients with peri-operative COVID-19 infection and were associated with high mortality.36 Similar observations were seen in vascular patients, where not only was the overall complication rate significantly higher in this COVID-19 positive subgroup, but COVID-19 positive patients experienced much more severe complications, and those COVID-19 positive who had complications had a higher mortality rate compared with COVID-19 negative patients.
Waiting for the potential resurgence of the viral infection, it is believed that this analysis may provide a few but important take home messages to be better prepared in this “war like” scenario. In order to prevent spread of the outbreak, outpatient visits have been closed but the potential to detect early on those who could present later in a more critical situation than expected would be valuable. Indeed, rescheduling those clinically not deferrable patients will again be effective to be better able to focus on the most critical ones: the high mortality rate in COVID-19 negative patients may indirectly support this latter observation. It is necessary to be more careful during revascularisation procedures for PAOD, and the use of heparin seemed to improve overall outcomes, not only primarily vascular ones. Given the hypercoagulable state of the COVID-19 positive patients, the routines for thrombosis treatment are being changed for COVID-19 positive patients presenting with ALI compared with prior to the pandemic: there is a common position favouring continuous intravenous administration of unfractionated heparin starting from the intra-operative period and maintained throughout the post-operative period accompanied by the correction of any eventual antithrombin-III alteration. In addition, the COVID-19 positive pandemic “phase 1”, which expanded in a very tight time window, should cause the health system governors to increase personnel hiring as well as strengthen hospital infrastructure.
Limitations
This study has several limitations. First, the analysis is essentially retrospective in nature. Large registry databases rely solely on accurate site reporting. Thus, it is possible that in such an emergency situation, investigators might have not identified all patients and all variables. However, missing data were not defaulted to negative, and denominators reflect only reported cases. The different type of hospital, as per governmental reorganisation, may have played a role regarding the type of patients and the type of intervention performed in the different vascular operations. Second, this study involves many centres, with patients having any type of surgery, with potential different indications for the different procedures, and operative techniques. Third, only in hospital results were reported: longer term follow up may result in different outcomes and mortality rates. Lastly, according to the reorganisation process and lockdown indications made by the National Government for the prevention of the epidemic spread, elective interventions were rescheduled and delayed. It is reasonable to consider that those who had been examined and planned for an intervention in non-critical conditions may subsequently have presented in a more critical clinical stage than the original one thus worsening the overall results. Consequently, the initial results should be interpreted with caution and are not generalisable.
In conclusion, this experience of the VSG-RL is one of the first studies describing clinical outcomes of a large cohort of patients undergoing vascular surgery, stratified by COVID-19 infection status. This experience showed that in a challenging scenario, the primary vascular surgery emergency was peripheral arterial ischaemia. Allowing for the described shortcomings, COVID-19 infection seemed to be a marker of poor outcomes in vascular diseases requiring operative management: COVID-19 positive patients had higher mortality at each decade interval, higher complication rate and higher rate of re-thrombosis after peripheral arterial revascularisation. COVID-19 infection may have had a negative impact also on COVID-19 negative patient mortality, reasonably explained by the overwhelming pandemic wave that limited both clinical and logistical activities, admitting only patients at higher risk to hospitals for scheduled surgery.
Conflicts of interest
None.
Funding
None.
Acknowledgements
We thank Dr Luigia Scudeller, medical statistician for manuscript review and statistical analyses.
Contributor Information
The Lombardy Covid-19 Vascular Study Group:
Franco Briolini, Pietro Cefali, Roberto Caronno, Aldo Arzini, Domenico Diaco, Vittorio Baratta, Stefano Aiello, Alessandro C.L. Molinari, Francesca Giovannini, Anna Maria Socrate, Matteo Ferraris, Antonino Silvestro, Gianluca Canu, Emidio Costantini, Davide Logaldo, Federico Romani, Alfredo Lista, Cristina Busoni, Marco Setti, Roberto Mezzetti, Piergiorgio Sala, Luca Bassi, Luca Luzzani, Matteo A. Pegorer, Luca Attisani, Claudio Carugati, Monica Vescovi, Piero Trabattoni, Stefano Zoli, Andrea Rignano, Clara Magri, Pierluigi Vandone, Sergio Losa, Efrem Civilini, Giovanni Nano, Daniela Mazzaccaro, Valerio Tolva, Jessica Lanza, Ruggiero Curci, Giovanna Simonetti, Chiara Lomazzi, Viviana Grassi, Daniele Bissacco, Andrea Kahlberg, Daniele Mascia, Raffaello Dallatana, Michele Carmo, Franco Ragni, Enrico M. Marone, Antonio Bozzani, Matteo Tozzi, Marco Franchin, Gianluca Lussardi, Vittorio Segramora, Gaetano Deleo, Matteo Crippa, Tiziano Porretta, Marco Viani, Silvia Stegher, Davide Foresti, and Giovanni Bonalumi
Appendix
Collaborators of the VSG-RL: ASST Bergamo “Papa Giovanni XXIII”: Franco Briolini, Pietro Cefali; ASST Lariana Como “Sant’Anna”: Roberto Caronno; ASST Crema “Ospedale Maggiore”: Aldo Arzini, Domenico Diaco; ASST di Cremona: Vittorio Baratta, Stefano Aiello; ASST Lecco “Alessandro Manzoni”: Alessandro Carlo Luigi Molinari; ASST Mantova “Carlo Poma”: Francesca Giovannini; ASST Ovest Milano Legnano “Civile”: Anna Maria Socrate, Matteo Ferraris; ASST Rhodense: Antonino Silvestro; ASST Valtellina Sondalo: Gianluca Canu; ASST Valle Olona Busto Arsizio: Emidio Costantini, Davide Logaldo; ASST Grande Ospedale Metropolitano Niguarda Milano: Federico Romani, Alfredo Lista; ASST Vimercate: Cristina Busoni; IRCCS Humanitas Gavazzeni Bergamo: Marco Setti; IRCCS Policlinico “San Marco” Zingonia: Roberto Mezzetti; Clinica “Sant’ Anna” Brescia: Piergiorgio Sala, Luca Bassi; Istituto Ospedaliero Fondazione Poliambulanza Brescia: Luca Luzzani, Matteo Alberto Pegorer, Luca Attisani; Clinica “San Carlo” Paderno Dugnano: Claudio Carugati, Monica Vescovi; IRCCS Centro Cardiologico Monzino: Piero Trabattoni, Stefano Zoli; IRCCS Istituto Clinico “Sant’Ambrogio” Milano: Andrea Rignano, Clara Magri; IRCCS Istituto Ortopedico Galeazzi Milano: Pierluigi Vandone; IRCCS Multimedica Sesto San Giovanni: Sergio Losa; IRCCS Humanitas Rozzano: Efrem Civilini; IRCCS Policlinico San Donato: Giovanni Nano, Daniela Mazzaccaro; Ospedale Policlinico Monza: Valerio Tolva; IRCCS Multimedica Castellanza: Jessica Lanza; ASST “Ospedale Maggiore” Lodi: Ruggiero Curci, Giovanna Simonetti; Univerity of Milan School of Medicine – IRCCS Policlinico Fondazione Cà Granda Milano: Chiara Lomazzi, Viviana Grassi, Daniele Bissacco; University “Vita Salute” – IRCCS Istituto “San Raffaele” Milano: Andrea Kahlberg, Daniele Mascia; Ospedale “San Carlo Borromeo” Milano: Raffaello Dallatana, Michele Carmo; Fondazione IRCCS Policlinico San Matteo: Franco Ragni, Enrico Maria Marone, Antonio Bozzani; University of Insubria School of Medicine ASST Settelaghi Varese: Matteo Tozzi, Marco Franchin; University of Brescia School of Medicine – ASST Spedali Civili Brescia: Gianluca Lussardi; ASST Monza Ospedale “San Gerardo”: Vittorio Segramora, Gaetano Deleo; ASST “Santi Paolo e Carlo” Milano: Matteo Crippa; ASST Fatebenefratelli Sacco Milano: Tiziano Porretta, Marco Viani, Silvia Stegher, Davide Foresti; Istituto di Cura Città di Pavia: Giovanni Bonalumi.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccolo R., Bruzzese D., Mauro C., Aloia A., Baldi C., Boccalatte M., et al. Population trends in rates of percutaneous coronary revascularization for acute coronary syndromes associated with the COVID-19 outbreak. Circulation. 2020;141:2035–2037. doi: 10.1161/CIRCULATIONAHA.120.047457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., et al. Lille ICU Haemostasis COVID-19 group Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 6.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bissacco D., Grassi V., Lomazzi C., Domanin M., Bellosta R., Piffaretti G., et al. Is there a vascular side of the story? Vascular consequences during COVID-19 outbreak in Lombardy, Italy. J Card Surg. 2020 doi: 10.1111/jocs.15069. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pini R., Faggioli G., Vacirca A., Gallitto E., Mascoli C., Attard L., et al. Is it possible to safely maintain a regular vascular practice during the COVID-19 Pandemic? Eur J Vasc Endovasc Surg. 2020;60:127–134. doi: 10.1016/j.ejvs.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latz C.A., Boitano L.T., Png C.Y.M., Tanious A., Kibrik P., Conrad M., et al. Early vascular surgery response to the COVID-19 pandemic: results of a nationwide survey. J Vasc Surg. 2020 doi: 10.1016/j.jvs.2020.05.032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiffert M., Brunner F.J., Remmel M., Thomalla G., Marschall U., L'Hoest H., et al. Temporal trends in the presentation of cardiovascular and cerebrovascular emergencies during the COVID-19 pandemic in Germany: an analysis of health insurance claims. Clin Res Cardiol. 2020;4:1–9. doi: 10.1007/s00392-020-01723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng J.J., Gan T.R.X., Niam J.Y., Menon R.K., Ho P., Dharmaraj R.B., et al. Experience from a Singapore tertiary hospital with restructuring a vascular surgery practice in response to national and institutional policies during the COVID-19 pandemic. J Vasc Surg. 2020;72:1166–1172. doi: 10.1016/j.jvs.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellosta R., Bissacco D., Rossi G., Pirrelli S., Lanza G., Frigerio D., et al. Vascular Surgery Group of Regione Lombardia (VSG-RL). Differences in hub and spoke vascular units practice during the novel Coronavirus-19 (COVID-19) outbreak in Lombardy, Italy. J Cardiovasc Surg (Torino) 2020 doi: 10.23736/S0021-9509.20.11564-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Hemingway J.F., Singh N., Starnes B.W. Emerging practice patterns in vascular surgery during the COVID-19 pandemic. J Vasc Surg. 2020;72:396–402. doi: 10.1016/j.jvs.2020.04.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Cossu L.G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 16.Bonalumi G., Giambuzzi I., Barbone A., Ranieri C., Cavallotti L., Trabattoni P., et al. A call to action becomes practice: cardiac and vascular surgery during the COVID-19 pandemic based on the Lombardy emergency guidelines. Eur J Cardiothorac Surg. 2020;58:319–327. doi: 10.1093/ejcts/ezaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministero della Salute. Available at: http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=4449 [Accessed 11 April 11].
- 18.Björck M., Earnshaw J.J., Acosta S., Bastos Gonçalves F., Cochennec F., Debus E.S., et al. European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of acute limb ischaemia. Eur J Vasc Endovasc Surg. 2020;59:173–218. doi: 10.1016/j.ejvs.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Inker L.A., Astor B.C., Fox C.H., Isakova T., Lash J.P., Peralta C.A., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 20.Vestbo J., Hurd S.S., Agustí A.G., Jones P.W., Vogelmeier C., Anzueto A., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson N.D., Fan E., Camporota L., Antonelli M., Anzueto A., Beale R., et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 22.Riambau V., Böckler D., Brunkwall J., Cao P., Chiesa R., Coppi G., et al. Editor’s Choice – Management of descending thoracic aorta diseases: clinical practice guidelines of the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2017;53:4–52. doi: 10.1016/j.ejvs.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P., et al. ESC Scientific Document Group 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 24.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaikof E.L., Blankensteijn J.D., Harris P.L., White G.H., Zarins C.K., Bernhard V.M., et al. Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 27.Hickey G.L., Dunning J., Seifert B., Sodeck G., Carr M.J., Burger H.U., et al. EJCTS and ICVTS Editorial Committees Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery. Eur J Cardiothorac Surg. 2015;48:180–193. doi: 10.1093/ejcts/ezv168. [DOI] [PubMed] [Google Scholar]
- 28.Perini P., Nabulsi B., Massoni C.B., Azzarone M., Freyrie A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395:1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giacomelli E., Dorigo W., Fargion A., Calugi G., Cianchi G., Pratesi C. Acute thrombosis of an aortic prosthetic graft in a patient with severe COVID-19-related pneumonia. Ann Vasc Surg. 2020;66:8–10. doi: 10.1016/j.avsg.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira-Neves A., Rocha-Neves J., Cerqueira A., Fernando-Teixeira J. Management and outcomes of a Vascular Surgery Department with sudden medical staff outbreak of COVID-19. J Vasc Surg. 2020;72:1151. doi: 10.1016/j.jvs.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng J.J., Ho P., Dharmaraj R.B., Wong J.C.L., Choong A.M.T.L. The global impact of COVID-19 on vascular surgical services. J Vasc Surg. 2020;71:2182–2183.e1. doi: 10.1016/j.jvs.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudino M., Chikwe J., Hameed I., Robinson N.B., Fremes S.E., Ruel M. Response of cardiac surgery units to COVID-19: an internationally-based quantitative survey. Circulation. 2020;142:300–302. doi: 10.1161/CIRCULATIONAHA.120.047865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Istituto Superiore di Sanità. Available at: https://www.epicentro.iss.it/coronavirus/pdf/Rapp_Istat_Iss_3Giugno.pdf [Accessed 18 January 2020].
- 34.Bellosta R., Pegorer M.A., Bettari L., Luzzani L., Attisani L., Fossati A., et al. Major cardiovascular events in patients with Coronavirus Disease 2019: experience of a cardiovascular department of Northern Italy. Thromb Res. 2020;197:202–204. doi: 10.1016/j.thromres.2020.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.COVID Surg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]