Abstract
Background:
Insufficient social support is associated with increased mortality among older adults. Lung cancer is primarily a disease of older adults and is the leading cause of all cancer deaths. We assessed the association of social support with outcomes among older adults with lung cancer.
Materials and Methods:
Adults age 65 and older with lung cancer with a completed geriatric assessment (GA) were assessed. Emotional social support (ES) and tangible (material, instrumental) support (TS) measures and patient characteristics were obtained from the GA. The electronic health record was used to extract clinical variables. Simple linear regression models evaluated the association between social support scales with patient and clinical factors.
Results:
79 adults were assessed. White race was positively associated with ES score (p=.04), while higher BMI (p=.03), depression (p=.03) and anxiety (p=.02) were associated with worse ES. Higher BMI was associated with higher/better TS score (p=.02) while living alone was associated with lower/worse TS score (p=.03). Completion of platinum-based doublet chemotherapy with immunotherapy as planned was associated with higher ES scores (p=.02) and higher TS scores (p=.02). Disease progression was associated with lower ES scores (p=.03).
Conclusion:
Social support may influence clinical outcomes in older adults with lung cancer. As lung cancer often portends to poor prognosis, social support may be an important prognostic indicator.
Keywords: social support, geriatric assessment, older adults, lung cancer
INTRODUCTION
The increased risk of mortality from insufficient social support is comparable to that of smoking or excessive alcohol consumption and potentially more troubling than inadequate levels of physical activity and obesity.1 Older adults are particularly susceptible to the higher mortality-low social support association, as shown in a study of 1,288 patients over the age of 65 that reported a 23% higher 12-year mortality risk associated with decreasing social support levels2. Studies in adults with cancer have shown that patients at high risk for low social support include those who are unmarried3, have depression,3 and have chronic diseases.4 In a systematic review of social influences on clinical outcomes in breast cancer, five out of seven articles reported an association between low social support and cancer progression.5 For example, in a study of 2,264 women with breast cancer who were followed for ten years after they had completed their cancer treatment, women with lower social support had higher overall mortality.6 Similar findings were found in 168 patients with ovarian cancer who were followed from surgery to time of death. Patients with ovarian cancer in the top 75th percentile for social support scores had a 34% lower hazard ratio for death compared to patients in the bottom 25th percentile.7 In a Japanese study following 44,152 people from cancer onset to disease progression, men with the lowest level of social support (baseline questionnaire) had 3.07 times higher risk of colorectal mortality.8 To our knowledge, less is known about the association of social support with clinical outcomes among older adults with lung cancer.
Numerous validated scales exist for measuring social support, including the Medical Outcomes Survey (MOS) Social Support Survey9 which is focused on perceived quality of social support in two domains: tangible and emotional. Tangible social support pertains to the provision of basic needs (assistance with getting to the doctor, preparation of meals, etc.) while emotional support includes the presence of someone who encourages the patient to express their emotions in a meaningful way and offers empathy and comfort.9 Because the consequences of low social support and social isolation can be especially dire in older persons,10 it is important to assess and consider the unique aspects of this variable in the treatment of older persons with cancer.11 In older patients with cancer who have low social support, studies have shown higher rates of unplanned hosital admissions12 and have pointed to the importance of having social networks.13
The median age of cancer diagnosis is 66, with 54% of all new cancer cases diagnosed after the age of 65. 14 Thus, it is fair to characterize cancer as a disease of aging. The median age at diagnosis for lung cancer is 70 years and 70% of new diagnoses are in individuals over the age of 65. 14 Social support may be particularly challenging in this older patient population, as reflected in studies showing particularly high levels of psychological stress associated with this diagnosis.15 Older patients with lung cancer often have decreased mobility as well as comorbid chronic diseases such as Chronic Obstructive Pulmonary Disease(COPD), which may further increase their need for social support.4,16 The positive impact of social support in patients with lung cancer was shown in a study of 289 adults that documented a positive relationship between high quality social support and better psychological function as well as increased patient resilience.17 A second study of 218 patients with lung cancer showed a positive association between their perceived social support and their perception of their health at twelve months follow up.18
An important gap in lung cancer research is the investigation of potential associations between social support, measures of function and quality of life, and clinical outcomes. Within this area of investigation, it is important to consider the unique social support circumstances of older persons with lung cancer. 19 In our study of older adults with lung cancer (age 65 and older), we first investigated the association of emotional and tangible social support with measures included in a brief geriatric assessment (GA) that has been validated in clinical trials20 and clinical practice settings.21 We then investigated the association of social support with clinical outcomes.
MATERIALS AND METHODS
Study Participants
The sample includes adults age 65 or older diagnosed with Small Cell Lung Cancer (SCLC) or Non-Small Cell Lung Cancer (NSCLC) who completed the Cancer and Aging Group (CARG) geriatric assessment tool. The sample came from three studies, all of which included a brief geriatric assessment. NCT01752751 included adults age 65 or older with head/neck or lung cancer and conducted the GA prior to initiation radiotherapy or chemoradiotherapy (2012–2018). NCT01702844 included adults with NSCLC age 65 of older and conducted the GA prior to initiation of nab-paclitaxel treatment; patients had to have completed a single prior treatment regimen (2012–2015). NCT01137825 includes adults diagnosed with any cancer including lung age 65 or older with the GA administered at any time during cancer care – pre-treatment, during, or post-treatment. All participants provided written informed consent (2009–2019). Of the initial sample of 99 patients with lung cancer, eleven were excluded because they had an incomplete oncology history in the electronic medical record (EMR) and nine were excluded because they did not have complete responses for either emotional or tangible social support, leaving a final sample of 79.
Measures
Brief Geriatric Assessment.
The GA assessment used in this study 20 developed by Dr. Hurria and colleagues is available through the Cancer and Aging Research Group (CARG) website (http://www.mycarg.org/). All participants had completed the GA either pre-treatment (N=48, 61%), during treatment (N=17, 22%), or post-treatment (N=14, 17%). Geriatric Assessments were conducted within ten years of our final data collection cut-off date of July 2019. Table 1 provides an overview of GA domains, measures, scoring range, and validated cut-points.
Table 1.
Brief Geriatric Assessment
| Domains | Measures | Score Range | Dichotomized |
|---|---|---|---|
| Research Staff Assessed | |||
| Function | Timed Up and Go (TUG) | Greater number of seconds → Lower function | Time>14 seconds = lower function27,28 |
| Karnofsky Performance Status (KPS) | Range: 0–100 Lower value → lower function | <80 = lower function29,30 | |
| Body Composition | Body Mass Index (BMI) | No range limits | Normal (18.5–24.9), overweight (25–29.9), obese (≥30)31 |
| Patient Reported | |||
| Function | Patient-reported KPS | Range: 30–100 Higher score → better functioning | <80 = lower functioning30 |
| Physical function | Range: 0–20, 20= not limited | <20 = limited32 | |
| Instrumental activities of daily living (IADL) | Range: 0–14 14= no limitations | <14 = limitations33 | |
| Number of falls in last 6 months34 | No range limits | 1 or more | |
| Psychological | Mental Health Index (MHI): Depression | Range 0–43 | Depression score >/= 1235 |
| Mental Health Index (MHI): Anxiety | Range 0–20 | Anxiety score >/=635 | |
| Social | Social Activity Limitation | Range: 0–100 Higher score = more activity | <50 = less activity36 |
| Social support-emotional | Range: 0–100 Higher score = greater support | <50 = lower support9 | |
| Social support-tangible | Range: 0–100 Higher score= greater support | <50 = lower support9 | |
Social Support Scale.
The primary variable of interest is the Social Support Scale9 which is comprised of two subscales, Emotional Support (ES) and Tangible Support (TS). Scoring for each subscale ranges from 0 to 100, and is dichotomized at 50, signifying lower support (<50) as compared to higher support (>=50). The ES subscale inquires about having someone to (1) listen to you when you need to talk, (2) give you good advice about a crisis, (3) give information to help you understand a situation, (4) confide in or talk to about yourself or your problem, (5) whose advice you really want, (6) share your most private worries and fears with, (7) turn to for suggestions about how to deal with a personal problem, and (8) who understands your problems. The TS subscale inquires about someone (1) who can help when you are confined to bed, (2) to take you to the doctor if needed, (3) to prepare your meals if you were unable to do it yourself, and (4) to help you with daily chores if you were sick.
Function and quality of life.
The GA includes self-reported demographic variables – age, race, gender, education, marital status, and living alone. Research staff assessed the Karnofsky Performance Status (KPS) and Timed Up and Go (TUG). All other measures were patient-reported.
Clinical diagnosis, treatment, and outcomes.
Electronic medical records data were extracted retrospectively from lung cancer diagnosis through end of treatment, departure from UNC Health Care, or death. Electronic medical record data included information on lung cancer diagnosis, treatment plan, side effects, adverse events, and treatment response.
Statistical Analysis
Simple linear regression models were used to evaluate the associations between the patient characteristics and the ES and TS subscales. Parameter estimates, standard errors, and p-values are reported in Table 3. The same analyses were conducted for associations of ES and TS with clinical variables (Table 4). When considering complement of regimens as planned, the associations of ES and TS were estimated to compare patients who completed regimens as planned to patients who received but did not complete treatment. All analyses were performed using SAS statistical software v9.4 (Cary, NC). Significance level was set a priori at 0.05.
Table 3.
Univariate associations of Social Support (Emotional and Tangible) with patient characteristics and Geriatric Assessment measures
| Social Support-Emotional Score | Social Support-Tangible Score | |||
|---|---|---|---|---|
| Variable | Estimate | p value | Estimate | p value |
| Age | −0.29 | .45 | −.03 | .94 |
| Race (white) | 13.96 | .04 | 11.95 | .08 |
| Gender (male) | −1.71 | .71 | .86 | .85 |
| Education (high school graduate or less) | −4.88 | .31 | −2.83 | .56 |
| Marital status (married) | 7.35 | .11 | 8.46 | .07 |
| Living Alone – yes | −8.44 | .08 | −11.00 | .03 |
| Body Mass Index (BMI) | .93 | .03 | 1.02 | .02 |
| Karnofsky Performance Status score <80 | −.55 | .92 | 2.15 | .69 |
| Patient-Reported Karnofsky Performance Status score <80 | −2.68 | .59 | 2.33 | .64 |
| Timed Up and Go test > 14 seconds | −5.26 | .25 | −.22 | .96 |
| One or more falls in last 6 months | −5.08 | .37 | 1.44 | .80 |
| Physical Function – low function score <20 | 6.64 | .57 | −15.36 | .21 |
| Instrumental Activities of Daily Living (IADL) – limitations score <14 | −3.26 | .48 | 1.73 | .71 |
| Social Activity Limit Score – lower social activity score <50 | −5.30 | .28 | −1.45 | .77 |
| Social Support-Tangible Score – less tangible support score <50 | −52.64 | <.001 | --- | --- |
| Mental Health Index (MHI) – Depressed | −11.65 | .03 | −8.63 | .14 |
| Mental Health Index (MHI) – Anxious | −11.91 | .02 | −9.66 | .06 |
Social Support subscales Emotional and Tangible range from 0–100, with higher scores signifying higher social support. Positive estimate implies positive association with social support; negative estimate implies inverse association with social support.
Table 4.
Univariate associations of Social Support (Emotional and Tangible) with treatment and outcomes
| Social Support-Emotional N= 77 | Social Support-Tangible N= 78 | |||
|---|---|---|---|---|
| Variable | Estimate | p value | Estimate | p value |
| Number of regimens started | 0.38 | .83 | 1.89 | .30 |
| Received single-agent | −0.01 | 1.00 | 2.49 | .63 |
| Received platinum-based doublet | −9.35 | .06 | −0.68 | .90 |
| Received doublet w/out platinum | 4.22 | .77 | 8.69 | .54 |
| Received platinum-based doublet chemotherapy with biologic | −6.97 | .35 | −14.61 | .05 |
| Received platinum-based doublet chemotherapy with immunotherapy | 14.30 | .02 | 13.73 | .02 |
| Completed planned cycles of chemotherapy | 4.79 | .34 | 2.56 | .60 |
| Completed planned cycles of oral targeted therapy | −6.67 | .26 | −6.35 | .25 |
| Hospitalization – yes | 2.82 | .60 | 2.40 | .68 |
| Dose Delay- yes | −1.95 | .78 | −4.51 | .56 |
| Dose Reductions- yes | −2.30 | .72 | −6.21 | .37 |
| Deceased | 3.90 | .53 | 4.61 | .47 |
| No evidence of disease | 9.46 | .23 | 1.41 | .86 |
| Disease progression | −17.80 | .03 | −10.04 | .25 |
Positive estimate implies positive association between the clinical variables and the outcome – Social Support score.
RESULTS
Sample Characteristics
The final study sample (Table 2) includes 79 patients ranging in age from 65 to 90 years who had completed Social Support scales for at least one type of social support (77 completed ES scales and 78 completed TS scales). GAs were administered between 2009 and 2019. The sample was 87% white, 52% male, 34% high school graduate or less, 51% married, and 32% living alone. Mean Body Mass Index (BMI) was 26 (range 16–49), with 40% overweight (BMI 25–29) and 22% obese (BMI 30 plus).
Table 2.
Patient Characteristics (N=79)
| Variable | Value |
|---|---|
|
| |
| Age – mean (range) | 73 (65–90) |
|
| |
| Race | |
| White | 69(87%) |
| Black or African-American | 8 (10%) |
| Native Hawaiian or Pacific Islander | 1 (1%) |
| Native Indian or Alaskan Native | 1 (1%) |
|
| |
| Gender | |
| Male | 41 (52%) |
| Female | 38 (48%) |
|
| |
| Education | |
| More than high school | 27 (34%) |
| High school graduate or less | 52 (66%) |
|
| |
| Marital Status | |
| Not Married | 38 (49%) |
| Married | 40 (51%) |
| Unknow | 1 |
|
| |
| Living Alone | |
| No | 49 (68%) |
| Yes | 23 (32%) |
| Unknown | 7 |
|
| |
| Body Mass Index (BMI) – mean (range) | 27 (15–49) |
| Underweight (BMI <18.5) | 1 (1%) |
| Normal weight (BMI 18.5 to <25) | 28 (35%) |
| Overweight (BMI 25 to <30) | 31 (39%) |
| Obese (BMI 30 or higher) | 17 (22%) |
| Unknown | 2 (3%) |
|
| |
| Clinical Diagnosis | |
|
| |
| Small Cell Lung Cancer | 5 (6%) |
| Limited | 3 |
| Extensive | 2 |
|
| |
| Non-Small Cell Lung Cancer | 66 (84%) |
|
| |
| Adenocarcinoma | 47 |
|
| |
| Squamous Cell Cancer | 16 |
|
| |
| Large Cell Carcinoma | 1 |
|
| |
| Mixed Histology | 2 |
|
| |
| Missing histology/unspecified histology | 8 (10%) |
|
| |
| Stage at diagnosis | |
| 1 | 11 (14%) |
| 2 | 6 (8%) |
| 3 | 19 (24%) |
| 4 | 32 (41%) |
| Unknown | 11 (14%) |
|
| |
| Metastasis sites | |
| Bone | 11 (14%) |
| Brain | 13 (16%) |
| Both | 4 (5%) |
|
| |
| Assessments and Questionnaires | |
|
| |
| Social Support-Emotional Score (range 0–100) – score <50 (“lower” support) | 5 (6%) |
|
| |
| Social Support-Tangible Score (range 0–100) -- score <50 (“lower” support) | 5 (6%) |
|
| |
| Treatment stage when the Geriatric Assessment was conducted | |
| Pre-treatment | 48 (61%) |
| During treatment | 17 (22%) |
| Post treatment | 14(18%) |
|
| |
| Karnofsky Performance Status (KPS <80) | 19 (24%) |
|
| |
| Timed Up and Go test -- more than 14 seconds | 34 (43%) |
|
| |
| Patient-Reported Karnofsky Performance Status (<80) | 25 (32%) |
|
| |
| Falls in past 6 months -- 1 or more | 18 (23%) |
|
| |
| Physical Function -- <20 (“lower” function) | 73 (96%) |
|
| |
| Instrumental Activities of Daily Living/IADL -- <14 (some limitations) | 42 (54%) |
|
| |
| Social Activity Limit Score (range 0–100) -- <50 (“lower” activity) | 25 (32%) |
|
| |
| Mental Health Index (MHI)-Depressed (range 0–43) – Depressed (score >12) | 19 (28%) |
|
| |
| Mental Health Index (MHI)-Anxious (range 0–20) – Anxious (score >=6) | 26 (35%) |
|
| |
| Treatment | |
|
| |
| Surgery: yes | 34 (43%) |
| Unknown | 8 (10%) |
|
| |
| Radiation: yes | 54 (68%) |
| Unknown | 5 (6%) |
|
| |
| Clinical Trial: Yes | 26 (33%) |
| Unknown | 19 (24%) |
|
| |
| Treatment Goal | |
| Palliative Care | 44 (56%) |
| Curative | 27 (20%) |
| Unknown | 8 (10%) |
|
| |
| Number of Prior Regimens Started | |
| 0 | 17 (22%) |
| 1 | 36 (46%) |
| 2 | 9 (11%) |
| 3 | 8 (10%) |
| 4 | 9 (11%) |
|
| |
| Chemotherapy treatment ever received – can be more than one treatment | |
| Single-Agent Chemotherapy | 26 (42%) |
| Platinum-Based Doublet | 37 (47%) |
| Doublet Therapy without Platinum | 2 (3%) |
| Platinum-Based Doublet with Biologic | 8 (10%) |
| Platinum Doublet with Immunotherapy | 13 (16%) |
|
| |
| Chemotherapy – yes | 62 (78%) |
| Unknown | 5 (6%) |
|
| |
| Completed Planned Cycles of Chemotherapy – yes | 22 (28%) |
| Unknown | 23 (29%) |
|
| |
| Treated with Immunotherapy – yes | 6 (8%) |
| Unknown | 17 (22%) |
|
| |
| Completed Planned Cycles of Immunotherapy -- yes | 3 (50%) |
|
| |
| Given Oral Targeted Therapy – yes | 34 (43%) |
| Unknown | 7 (9%) |
|
| |
| Adverse Events During Treatment | |
|
| |
| Hospitalized -- yes | 36 (46%) |
|
| |
| Dose reduction – yes | 23 (29%) |
|
| |
| Treatment Delay – yes | 19 (24%) |
|
| |
| Early treatment Discontinuation -- yes | 32 (41%) |
|
| |
| Patient Status at the time of Data Entry | |
|
| |
| Deceased | 48 (61%) |
|
| |
| No Evidence of Disease | 8 (10%) |
|
| |
| Disease Progression | 7 (9%) |
|
| |
| Unknown | 16 (20%) |
Geriatric Assessment Scores
For the primary variable of Social Support, both ES and TS subscale scores were very high (range 0–100 with higher scores indicating greater social support). The mean ES score was 83.4 95% CI (78.95– 87.85), with only 6% reporting “lower” emotional support. The mean TS score was 85.4 (95% CI: 80.84–89.99), with only 6% reported “lower” tangible support. Figure 1 illustrates the percent of patients within each decade of subscale units. For example, 22% and 28% of study participants scored TS and ES, respectively, in the 70–80 range.
Figure 1.
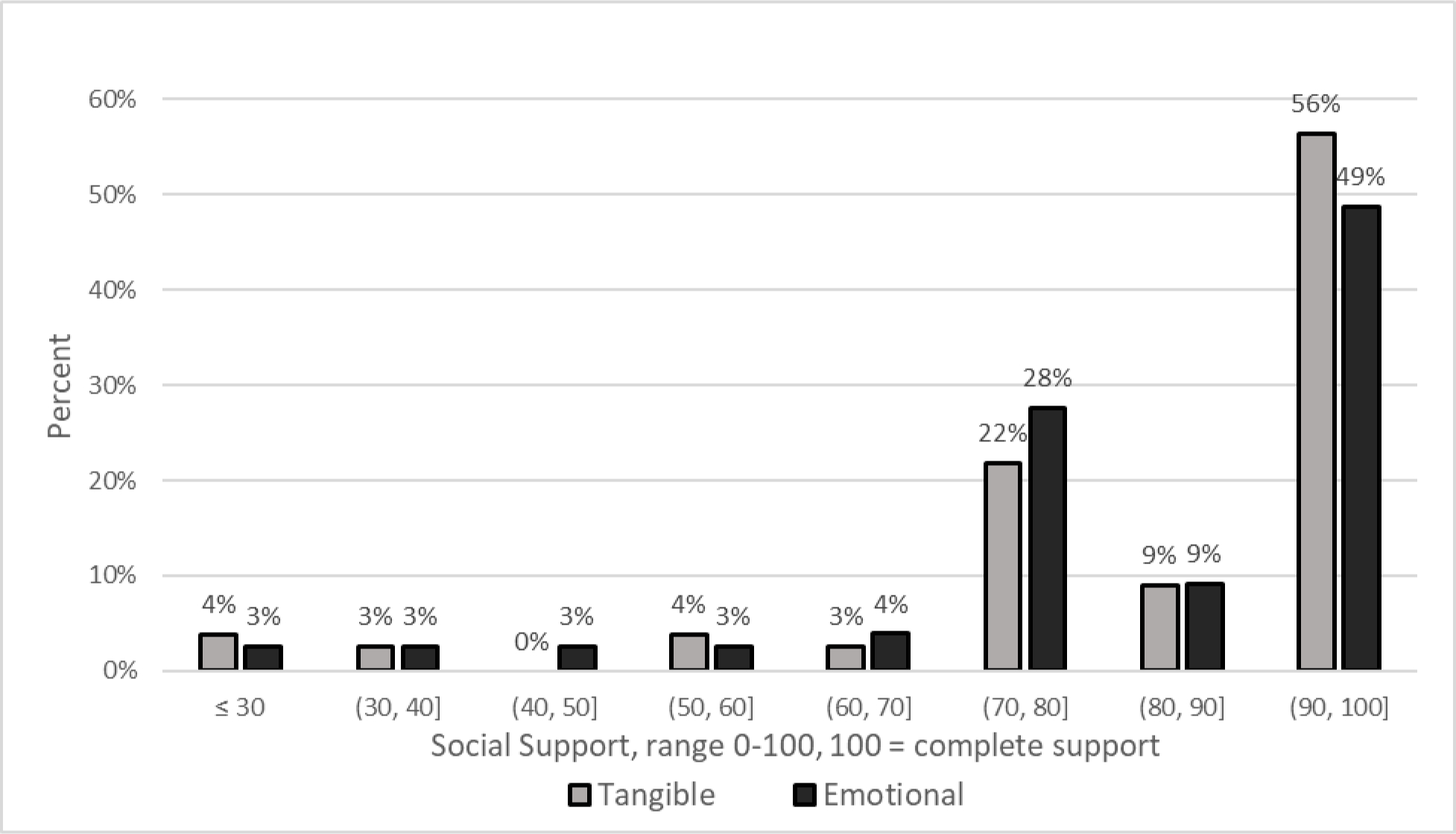
Social Support Scores
Twenty-four percent of study participants had research staff-assessed Karnofsky Performance Status (KPS) scores of less than 80, 43% required more than fourteen seconds to complete the Timed Up and Go test, and 23% had fallen at least once in the last six months. Ninety-six percent scored “lower” function on the Physical Function score, 54% reported some limitations in Instrumental Activities of Daily Living (IADL), and 32% scored “lower activity” on the Social Activities Limitation scale. On the Mental Health Index (MHI), 28% were depressed and 35% were anxious.
Clinical Characteristics and Outcomes
Eighty-five percent of the study sample were diagnosed with NSCLC and 6% with SCLC (Table 2). Almost half of the study sample (47%) had Stage IV cancer, 94% received chemotherapy, 8% immunotherapy, and 43% oral targeted therapy.
Adverse events during the patient’s treatment course included hospitalizations (62%), dose reductions (43%), dose delays (44%), and early treatment discontinuation (61%). Patient status at the time of data entry was deceased (71%), no evidence of disease (12%), and disease progression (17%).
Univariate Associations of Social Support Subscales with Patient Characteristics and Geriatric Assessment Measures
Univariate associations of social support subscales with patient characteristics and geriatric assessment measures are presented in Table 3.
Emotional Support (ES).
Mean ES score was 13.96 points higher (better) in white patients compared to patients of color (p=.04). A one-unit increase in BMI was associated with a .93 increase in the ES score (p=.03). Scoring <50 on the Tangible Support subscale (signifying “lower” support) was associated with 52.64 points lower on the Emotional Support scale (p<.001). MHI Depression was associated with 11.65 lower score on the ES subscale (p<.001), as was MHI Anxiety (decrease of 11.91, p=.02).
Tangible Support (TS).
Living alone was associated with 11.00 points lower TS score (p=.03), and a one-unit increase in BMI was associated with a 1.02 increase in TS (p=.02). There was also borderline negative association with MHI-Anxiety (patients with anxiety had lower TS) (p=.06).
Univariate Associations of Social Support Subscales with Clinical Data
Univariate analysis of associations between social support subscales and clinical data are shown in Table 4. Completion of platinum-based doublet chemotherapy as planned with a biologic was associated with 14.61 points lower TS (p=.05). Completion of platinum-based doublet chemotherapy with immunotherapy as planned was associated with 14.3 points higher ES (p=.02) and 13.73 points higher TS (p=.02). (Among those with metastatic disease, disease progression was associated with 17.8 points lower ES (p=.03).
DISCUSSION
In this study, the social support scale commonly used in a validated geriatric assessment was explored for associations with patient characteristics, measures of function and quality of life, and clinical outcomes among older adults with lung cancer. In univariate analysis, we found a significant association between higher emotional support (ES) with white race, higher BMI, greater tangible support (TS), and less depression and anxiety. Living alone and lower BMI were both significantly associated with lower patient-reported TS. We found a significant association between lower ES and disease progression, and higher ES and TS with the completion of platinum-based doublet with immunotherapy as planned.
A notable finding from this study was that patients with higher social support were more likely to have higher BMI. A serious complication of lung cancer is cancer cachexia. Cachexia is associated with muscle and sometimes fat loss due to a decrease in appetite and the production of inflammatory mediators. These changes lead to a decreased BMI in patients who present with cachexia at the time of diagnosis.22 Recent studies suggest that up to 46% of patients with non-small cell lung cancer already had cachexia at the time of diagnosis and treatment initiation.23 A possible explanation for this difference in BMI is that patients with better social support may have presented earlier in the course of their diagnosis and more likely to present with less cachexia. The higher social support may also reflect having someone to help the patients with cooking and maintaining a healthy diet.
Another noteworthy finding of our study is that patients with lower ES scores were more likely to experience disease progression. As noted earlier, the association of low quality social support with increased risk of mortality has been well studied in other cancers including breast, colon, and ovarian cancer and given that lung cancer is the leading cause of all cancer deaths24 understanding the mechanism by which poor social support leads to an increase in lung cancer progression and mortality is important. One potential molecular mechanism for social support’s influence on cancer progression is the finding of elevated inflammatory mediators in patients with lower levels of social support.25 The same inflammatory mediators (combined with low social support) have been associated with increased cancer-related mortality.25 Other studies have suggested the mechanism of clinical influence of social support may be more multifactorial, focusing on social factors effect on behavioral, treatment, and physiological aspects of cancer.26
Our study has some important limitations. Relatively few patients in our sample reported low levels of emotional or tangible social support. Only 6% of the study sample scored within the range of “lower support” on these subscales. It is unclear if these findings are unique to our specific population of older adults with lung cancer receiving care at our academic cancer center although similar findings of higher mean levels of self-reported social support have been observed in other studies, as well.3,11 GAs were conducted at varying time points during the cancer care continuum as outlined in our methods. This contributed to the heterogeneity of the sample. The GA timeframe was 2009 to 2019, which include a period of substantial advances in the treatment of lung cancer. A combination of a small sample size and low prevalence of “lower support” did not allow multivariate analyses to assess the relative contributions of variables that were significant in our univariate analysis. Further evaluation in a larger sample with a more racially and ethnically diverse patient population is warranted.
Despite these limitations, our findings highlight the presence of significant associations between clinical endpoints (such as disease progression and completion of planned number of cycles) and emotional social support in a sample of older adults with lung cancer. Further research on the role and impact of social support among older patients with lung cancer may ultimately inform interventions that can address the impact of low social support. These interventions may in turn improve cancer outcomes, quality of life, and overall patient satisfaction with care.
All of the authors involved in the production of this manuscript denied any conflict of interest. Study Concepts and study design were completed by Student Doctor Chambers, Dr. Nyrop, Dr. Muss and Dr. Charlot. Data acquisition was completed by Student Doctor Chambers, Quality control of data and algorithms was completed by Student Doctor Chambers, as well as Ms. Damone and Ms. Deal. Statistical analysis was performed by Ms. Damone, Ms. Deal and Mr. Chen. All authors contributed to data analysis and interpretation as well as manuscript preparation, editing and review.
References
- 1.Holt-Lunstad J, Smith T. Social Relationships and Mortality Risk: A Meta-Analytic Review. SciVee. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzella F, Cacciatore F, Della-Morte D, et al. Social Support and Long-Term Mortality in the Elderly: Role of Comorbidity. Archives of Gerontology and Geriatrics. 2010;51:323–328. [DOI] [PubMed] [Google Scholar]
- 3.Thompson T, et al. Perceived Social Support in African American Breast Cancer Patients: Predictors and Effects. Social Science & Medicine. 2017. vol. 223:134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valtorta NHB. Loneliness, isolation and the health of older adults: do we need a new research agenda. Journal of the Royal Society of Medicine. 2012:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gidron YPR, Moss-Morris R, Nausheen B. Social support and cancer progression: a systematic review. J Psychosom Res. 2009:403–415. [DOI] [PubMed] [Google Scholar]
- 6.Kroenke C, Quesenberry C, Kwan M, Sweeney C, Castillo A, Caan B. Social networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the Life After Breast Cancer Epidemiology (LACE) study. Breast Cancer Res Treat. 2013:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutgendorf SK, et al. Social Influences on Clinical Outcomes of Patients With Ovarian Cancer. Journal of Clinical Oncology. 2012:2885–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda A, Kawachi I, Iso H, Iwasaki M, Inoue M, Tsugane S. Social Support and Cancer Incidence and Mortality: the JPHC Study Cohort II. Cancer Causes & Control. 2013;24, no. 5. [DOI] [PubMed] [Google Scholar]
- 9.Sherbourne CDSA. The MOS Social Support Survey. Soc Sci & Med. 1991:705–714. [DOI] [PubMed] [Google Scholar]
- 10.Cornwell EYWL. Measuring social isolation among older adults using multiple indicators from the NSHAP study. J Gerontol B Psychol Sci Soc Sci. 2009:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jatoi A, et al. Social support and its implications in older, early-stage breast cancer patients in CALGB 49907. Psycho-Oncology. 2015;25:441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards B, zhang X, Sun M, et al. Prognostic Significance of Geriatric Assessment. Journal of Clinical Oncology.35. [Google Scholar]
- 13.Yoo G, Levine E, Aviv C, Ewing C, Au A. Older Women, Breast Cancer, and Social Support. Supportive Care in Cancer. 2009;18:1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Stat Facts: Lung and Bronchus Cancer. 2019.
- 15.Hewitt M, Rowland J, Yancik R. Cancer Survivors in the United States: Age, Health, and Disability. The Journals of Gerontology. 2003;58. [DOI] [PubMed] [Google Scholar]
- 16.Takiguchi Y Chronic Obstructive Pulmonary Disease as a Risk Factor for Lung Cancer. World Journal of Clinical Oncology. 2014:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu T, Xiao J, Peng J, Kuang X, He B. Relationship between resilience, social support as well as anxiety/depression of lung cancer patients: A cross-sectional observation study. J Can Res Ther 2018:72–77. [DOI] [PubMed] [Google Scholar]
- 18.Kelley DK, Erin, Litzelman K, Mollica M, Rowland J. Dyadic associations between perceived social support and cancer patient and caregiver health: An actor-partner interdependence modeling approach. Psycho-Oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao A. SN, Gajra A. Management of Lung Cancer in the Elderly. Reckamp K (eds) Lung Cancer. Cancer Treatment and Research. Vol vol 170: Springer, Cham; 2016. [DOI] [PubMed] [Google Scholar]
- 20.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005:104–109. [DOI] [PubMed] [Google Scholar]
- 21.Jolly T, Deal A, Nyrop K, et al. Geriatric Assessment-Identified Deficits in Older Cancer Patients With Normal Performance Status. The Oncologist. 2015;20:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu R, Liu Z, Jiao R. Updates on the pathogenesis of advanced lung cancer-induced cachexia. Thorac Cancer. 2019:1759–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura M, Naito T, Kenmotsu H, al. e. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer. 2015;23(6):1699–1708. [DOI] [PubMed] [Google Scholar]
- 24.Cancer Facts and Figures 2021. Paper presented at: American Cancer Society2021; Atlanta, GA. [Google Scholar]
- 25.Boen CE, Barrow DA, Bensen JT, et al. Social Relationships, Inflammation, and Cancer Survival. Cancer Epidemiology Biomarkers & Prevention. 2018;vol. 27, no. 5:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke CH. A Conceptual Model of Social Networks and Mechanisms of Cancer Mortality, and Potential Strategies to Improve Survival. Translational Behavioral Medicine. 2018;vol. 8, no. 4:629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podsiadlo DRS. The Timed up and Go - a Test of Basic Functional Mobility for Frail Elderly Persons. J Am Geriatr Soc. 1991:142–148. [DOI] [PubMed] [Google Scholar]
- 28.Shumway-Cook ABS, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Physical therapy. 2000:896–903. [PubMed] [Google Scholar]
- 29.Karnofsky DABJ. Evaluation of chemotherapeutic agents in cancer. MacLeod CM, ed. New York: Columbia University Press; 1949:191–205. [Google Scholar]
- 30.Loprinzi CLLJ, Wieand HS, et al. Prospective evaluation of prognostic variables from patient-completed questionnaires. (North Central Cancer Treatment Group.). J Clin Oncol. 1994:601–607. [DOI] [PubMed] [Google Scholar]
- 31.Landi FOG, Gambassi G, et al. Body mass index and mortality among hospitalized patients. Archives of Internal Medicine. 2000:2641–2644. [DOI] [PubMed] [Google Scholar]
- 32.Stewart AL, Kamberg CJ. Physical Functioning Measures. In: Stewart AL, Ware JE Jr, eds. Measuring Functioning and Well-Being: The Medical Outcomes Survey. Durham and London: Duke University Press; 1992. [Google Scholar]
- 33.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS Multidimensional Functional Assessment Questionnaire. J Gerontol. 1981;36(4):428–434. [DOI] [PubMed] [Google Scholar]
- 34.Teno J KD, Mor V. Multiple stumbles: a risk factor for falls in community-dwelling elderly. A prospective study. J Am Geriatr Soc. 1990:1321–1325. [DOI] [PubMed] [Google Scholar]
- 35.Rumpf H-JMC, Hapke U, John U. Screening for mental health: validity of the MHI-5 using DSM-IV Axis I psychiatric disorders as gold standard. . Psychiatry Res. 2001:243–253. [DOI] [PubMed] [Google Scholar]
- 36.CD. S. Social Functioning: Social Activity Limitations Measure. Measuring Functioning and Well-being: The Medical Outcomes Study. Durham and London: Duke University Press; 1991:173–181. [Google Scholar]


