Abstract
The MICs of rabeprazole sodium (RPZ), a newly developed benzimidazole proton pump inhibitor (PPI), against 133 clinical Helicobacter pylori strains revealed a higher degree of activity than the another two PPIs, lansoprazole and omeprazole. Time-kill curve assays of RPZ, when combined with amoxicillin, clarithromycin, or metronidazole, disclosed that synergistic effects were demonstrated in combination with each antibiotic examined. Moreover, no apparent antagonistic effect appeared among all of the strains tested.
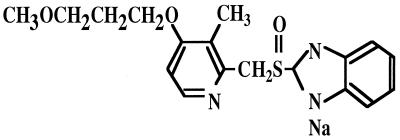
It is well known that Helicobacter pylori is associated with gastric disorders, such as gastritis, and the gastric or duodenal ulcer (2, 5, 8, 15, 21). The combination chemotherapy, i.e., amoxicillin (AMC) plus clarithromycin (CAM) or metronidazole (MNZ) with a proton pump inhibitor (PPI) is now widely recommended for eradication chemotherapy (1, 3, 4, 6, 9, 13, 14, 16). Rabeprazole sodium (RPZ), a benzimidazole PPI, is a new substituted benzimidazole H+, K+ ATPase inhibitor. It acts as an irreversible, noncompetitive inhibitor of the H+, K+ ATPase, and preliminary studies demonstrate that RPZ produces a potent and long-lasting inhibition of gastric acid secretion and a low level of hypergastrinemia (3, 17, 20). A novel RPZ demonstrating a chemical structure of C18H20N3SNa with a molecular weight of 381.43, as shown in Fig. 1, was developed in 1997 and has been proven to be effective against H. pylori strains, like other PPIs, such as lansoprazole (LPZ) and omeprazole (OPZ) (11, 19). It has been demonstrated to act as an irreversible noncompetitive inhibitor of the enzyme urease that is an important virulence factor of pathogenicity of gastric H. pylori (17, 20). The in vivo evaluation study of RPZ has recently been reported (18). However, no in vitro data has been available to date concerning the interaction studies of RPZ in combination with some kinds of antibiotics. Therefore, we tried to evaluate its bactericidal activity when combined with an antibiotic compound against H. pylori strains by the time-kill curve assay (10).
FIG. 1.
Chemical structure of RPZ (C18H20N3SNa; molecular weight, 381.43).
We at first determined the in vitro activities of RPZ and its thioether (TH) derivative, RPZ-TH, together with OPZ and LPZ.
The 133 H. pylori strains tested were recent clinical isolates from different patients with chronic gastritis and gastric and/or duodenal ulcer during the 2 years between April 1996 and March 1998, at the Central Clinical Laboratories, Shinshu University Hospital, Matsumoto, Japan. In addition, two reference strains, H. pylori NCTC 11637 and NCTC 11916, were also included in this study. All of the strains examined were preserved in Microbank (Pro-Lab Diagnostic, Richmond Hill, Ontario, Canada) vials in a deep freezer at −83°C.
The antimicrobials and PPIs used were as follows: AMC from Meiji Seika Kaisha, Ltd., Tokyo, Japan; CAM from Taisho Pharmaceuticals, Co., Ltd., Tokyo, Japan; MNZ from Shionogi & Co., Ltd., Tokyo, Japan; RPZ and its derivative, RPZ-TH, from Eisai Pharmaceuticals Co., Ltd, Tokyo, Japan; LPZ from Takeda Chemical Industries, Ltd., Osaka, Japan; and OPZ from Astra Japan Ltd., Tokyo, Japan.
In determining the MICs, twofold serial dilutions of each drug were made in 50 μl of brucella broth (BBL Microbiology Systems Inc., Cockeysville, Md.) supplemented with 5% horse serum (Irvin Scientific, Santa Ana, Calif.) in microplates with 96 wells (Eiken Chemical Co., Ltd., Tokyo, Japan). The broth dilutions in the wells containing the agent at each concentration were prepared on the day of use.
For a preparation of inocula of H. pylori strains, the cells of H. pylori strains were scraped from 72-h culture lawns on the blood agar plates (Columbia agar base; BBL) containing 5% defibrinated sheep blood) to adjust the density of MacFarland no. 3 standard. Fifty microliters from each broth prepared was applied within 30 min to the wells with 50 μl of drug diluent containing the test compound to make the final concentration approximately 106 CFU/ml. Inoculation into each well was made with a multipoint inoculating device.
The final concentrations prepared in the wells of the microplates were from 0.0078 to 32 μg/ml for AMC, from 0.0078 to 128 μg/ml for CAM and MNZ, and from 0.031 to 64 μg/ml for RPZ, RPZ-TH, LPZ, and OPZ. The results were read after incubation at 35°C for 72 h in the CO2 incubator (Sanyo Co., Ltd., Tokyo, Japan) with 15% CO2 in an atmosphere with high humidity.
The MIC was defined as the lowest concentration of the agent that completely inhibited the growth of the strain examined as detected by the unaided eye.
The time-kill curve assay (10) was performed to determine the behavior of drug interaction of RPZ with another antimicrobial(s), using four representative H. pylori strains, i.e., two reference strains, NCTC 11637 and NCTC 11916, and two clinical isolates, SHP 107 and SHP 133. The medium used was brucella broth (BBL) supplemented with 5% horse serum (Irvin Scientific). The strains tested were inoculated to a final concentration of 5 × 106 CFU/ml and cultured in 10 ml of the medium containing a single agent or a combination of two agents with gentle shaking at 35°C for 24 h. Agent concentrations were based on half the MICs, once the MICs, and twice the MICs of the agent.
The numbers of viable H. pylori cells were calculated as CFU per milliliter. At 0, 3, 6, 12, and 24 h, the samples (100 μl) were removed and 10-fold serial dilutions were performed. An aliquot (100 μl) of each dilution was spotted in duplicate onto blood agar plates (Columbia agar base; BBL) containing 5% defibrinated sheep blood for colony counts. The inoculated plates were grown at 35°C for 7 days. Data obtained were analyzed by determining the number of strains that yielded a Δlog10 CFU per milliliter reduction after 24 h of incubation, compared with the counts at time zero. Synergism was defined as a ≥2-log10 CFU/ml decrease after 24 h of incubation with the drug combination, in comparison with the most active agent alone. Antagonism was defined as a ≥2-log10 CFU/ml increase after 24 h of incubation with the drug combination, compared with the most active agent alone (12).
The MIC distributions of all the agents, including RPZ and RPZ-TH, are shown in Table 1.
TABLE 1.
MICsa (μg/ml) of anti-H. pylori compounds against 133 clinical H. pylori isolates by the microdilution method
| Drug | MIC range | MIC50 | MIC90 |
|---|---|---|---|
| AMC | 0.015–0.5 | 0.031 | 0.031 |
| CAM | ≤0.007–64 | 0.007 | 1 |
| MNZ | 1–128 | 4 | 16 |
| RPZ | 0.063–1 | 0.25 | 0.5 |
| RPZ-TH | 0.031–4 | 0.25 | 0.25 |
| LPZ | 0.25–2 | 1 | 1 |
| OPZ | 0.5–16 | 8 | 16 |
MIC50 and MIC90, MICs at which 50 and 90% of the isolates tested are inhibited, respectively.
Among the three PPIs, RPZ and RPZ-TH are the most active, with almost the same MICs. On the other hand, OPZ is the least active compound among the PPIs.
MICs required by the four H. pylori strains used for the evaluation of the combination effect by the time-kill assay are tabulated in Table 2.
TABLE 2.
MICs (μg/ml) of respective agent to the selective strains tested for the time-kill curve assay
| Antibiotic or PPI | MIC against straina
|
|||
|---|---|---|---|---|
| NCTC 11916 | NCTC 11637 | SHP 107 | SHP 133 | |
| AMC | 0.016 | 0.016 | 0.016 | 0.016 |
| CAM | 0.5 | 0.5 | 0.007 | 8 |
| MNZ | 64 | 0.25 | 4 | 2 |
| RPZ | 0.016 | 0.25 | 0.25 | 0.5 |
| RPZ-TH | 0.031 | 0.13 | 0.25 | 0.25 |
| LPZ | 0.25 | 0.25 | 1 | 1 |
| OPZ | 2 | 8 | 16 | 2 |
SHP, clinical H. pylori strains isolated in Shinshu University Hospital.
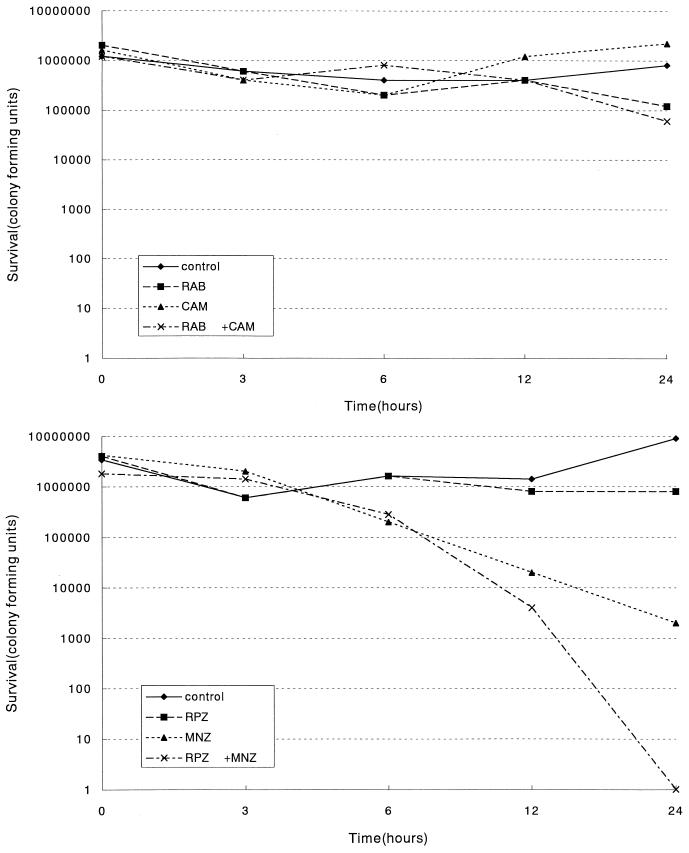
Time-kill curves obtained were almost the same as those obtained with half, once, and twice the MICs. Therefore, among the various plot curves, only the ones representative of once the MIC, demonstrating synergy and indifference, were shown in Fig. 2A and B. Figure 2A demonstrates a representation of indifference in combination with CAM. Figure 2B shows an example of synergy when combined with MNZ. Synergistic effects when combined with CAM were also observed in the NCTC 11637 and NCTC 11916 strains. In combination with MNZ, only the clinical SHP 107 strain exhibited synergy. No apparent antagonistic effect was observed with any combination of a drug.
FIG. 2.
Time-kill curves of H. pylori strains in the presence of the drug(s) at their MICs. The numbers of viable cells per milliliter were plotted versus incubation time. (A) A time-kill curve showing indifference of a clinical strain, H. pylori SHP 133. (B) A time-kill curve showing synergism of a clinical strain, H. pylori SHP 107.
The emergence of synergistic effects (two out of four strains tested for CAM plus RPZ and one out of four strains examined for MNZ plus RPZ) was almost the same as those obtained when combined with LPZ, as previously described (7). Moreover, we observed that RPZ caused no apparent decrease in CFU even after incubation for 24 h. These findings may suggest that the activities of RPZ and RPZ-TH were not bactericidal but bacteriostatic.
We previously demonstrated that synergistic effects were observed only when the strains were sensitive to both of the combined antibiotics investigated, such as AMC and CAM or AMC and MNZ (7). In this study, the NCTC 11916 strain with resistance to MNZ revealed no synergistic effect when determined by applying twice the MIC, thus confirming that synergism did not occur when the strains were resistant to both the agents tested. These findings, thereby, led us to consider that the strains tested revealed different drug interactions.
Notwithstanding the emergence of indifferent and synergistic plot curves observed, it is notably favorable that no antagonistic effect was observed among the four H. pylori strains examined in any combination of RPZ with any of the three antibiotics. These findings were just the same as the previously reported LPZ results (7).
Because of its strong in vitro activity against H. pylori strains, RPZ should be regarded as an additional novel PPI to be administered in combination regimens against H. pylori infections. The fact that clinical H. pylori strains were inhibited in their growth at the lowest MICs of the three PPIs tested is noteworthy and potentially useful information.
Further testing should be done to confirm the activity of RPZ when combined with certain antibiotics using larger numbers of strains. In addition, the accumulation of data concerning clinical efficacy of RPZ appears warranted. Indeed, the highest growth inhibitory activity among the three PPIs tested against H. pylori strains is noteworthy; however, development of novel antimicrobials with specific activity against H. pylori strains is urgently desired.
(The data reported here were presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998.)
REFERENCES
- 1.Bazzoli F, Zagari R M, Fossi S, Pozzato P, Alampi G, Simoni P, Sottili S, Roda A, Roda E. Short-term low-dose triple therapy for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1994;6:773–777. [Google Scholar]
- 2.Blaser M J. Medical significance of H. pylori. In: Clayton C L, Morbley H L T, editors. Helicobacter pylori protocols. New York, N.Y: Humana Press; 1997. pp. 115–124. [Google Scholar]
- 3.Cloud M L, Enas N, Humphries T J, Bassion S. Rabeprazole in treatment of acid peptic diseases: results of three placebo-controlled dose-response clinical trials in duodenal ulcer, gastric ulcer, and gastroesophageal reflux disease (GERD). The Rabeprazole Study Group. Dig Dis Sci. 1998;43:993–1000. doi: 10.1023/a:1018822532736. [DOI] [PubMed] [Google Scholar]
- 4.European Helicobacter pylori Study Group. Current European concepts in the management of Helicobacter pylori infection. Gut. 1997;41:8–13. doi: 10.1136/gut.41.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin C S. Duodenal ulcer, Campylobacter pylori, and the “leaking roof” concept. Lancet. 1988;ii:1467–1469. doi: 10.1016/s0140-6736(88)90942-7. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh A, Kawakami Y, Akahane T, Akamatsu T, Shimizu T, Kiyosawa K, Katsuyama T. Susceptibility of Helicobacter pylori isolates against agents commonly administered for eradication therapy and the efficacy of chemotherapy. Microbiol Immunol. 1997;41:7–12. doi: 10.1111/j.1348-0421.1997.tb01166.x. [DOI] [PubMed] [Google Scholar]
- 7.Gotoh A, Kawakami Y, Akamatsu T, Katsuyama T. Interaction of drugs for eradication therapy against antibiotic-resistant strains of Helicobacter pylori. Microbiol Immunol. 1997;41:865–869. doi: 10.1111/j.1348-0421.1997.tb01942.x. [DOI] [PubMed] [Google Scholar]
- 8.Graham D Y, Go M F. Helicobacter pylori: current status. Gastroenterology. 1993;105:279–282. doi: 10.1016/0016-5085(93)90038-e. [DOI] [PubMed] [Google Scholar]
- 9.Graham D Y, de Boer W A, Tytgat G N J. Choosing the best anti-Helicobacter pylori therapy: effect of antimicrobial resistance. Am J Gastroenterol. 1996;91:1072–1076. [PubMed] [Google Scholar]
- 10.Hindler J. Antimicrobial susceptibility testing. In: Isenberg H D, editor. Clinical microbiology procedure handbook. Vol. 1. Washington, D.C.: American Society for Microbiology; 1995. pp. 5.16.1–33. [Google Scholar]
- 11.Hirai M, Azuma T, Ito S, Kato T, Kohli Y. A proton pump inhibitor, E3810, has antibacterial activity through binding to Helicobacter pylori. J Gastroenterol. 1995;30:461–464. doi: 10.1007/BF02347561. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami Y, Akahane T, Gotoh A, Okimura Y, Oana K, Katsuyama T. Successful development of air-dried microplates (HP-plates) for susceptibility testing against Helicobacter pylori isolates. Microbiol Immunol. 1997;41:703–708. doi: 10.1111/j.1348-0421.1997.tb01914.x. [DOI] [PubMed] [Google Scholar]
- 13.Lind T, Zanten S V, Unge P, Spiller R, Baterdörffer E, O'Moratin C, Bardhan K D, Bradette M, Chiba N, Wrangstadh M, Cederberg M C, Idström J G. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH1 study. Helicobacter. 1996;1:138–144. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 14.Markham A, McTavish D. Clarithromycin and omeprazole as Helicobacter pylori eradication therapy in patients with H. pylori-associated gastric disorders. Drugs. 1996;51:161–178. doi: 10.2165/00003495-199651010-00010. [DOI] [PubMed] [Google Scholar]
- 15.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1314. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 16.Moayyedi P, Sahay P, Tompkins D S, Axon A T R. Efficacy and optimum dose of omeprazole in a new 1-week triple therapy regimen to eradicate Helicobacter pylori. Eur J Gastroenterol Hepatol. 1995;7:835–840. [PubMed] [Google Scholar]
- 17.Park J B, Imamura L, Kobashi K. Kinetic studies of Helicobacter pylori urease inhibition by a novel proton pump inhibitor, rabeprazole. Biol Pharm Bull. 1996;19:182–187. doi: 10.1248/bpb.19.182. [DOI] [PubMed] [Google Scholar]
- 18.Stack W A, Knifton A, Thirlwell D, Cockayne A, Jenkins D, Hawkey C J, Atherton J C. Safety and efficacy of rabeprazole in combination with four antibiotic regimens for the eradication of Helicobacter pylori in patients with chronic gastritis with or without peptic ulceration. Am J Gastroenterol. 1998;93:1909–1913. doi: 10.1111/j.1572-0241.1998.00582.x. [DOI] [PubMed] [Google Scholar]
- 19.Takimoto T, Ido K, Taniguchi Y, Satoh K, Saifuku K, Kihita K, Yoshida Y, Kimura K. Efficacy of lansoprazole in eradication of Helicobacter pylori. J Clin Gastroenterol. 1995;20(Suppl. 2):121–124. doi: 10.1097/00004836-199506002-00033. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya M, Imamura L, Park J B, Kobashi K. Helicobacter pylori urease inhibition by rabeprazole, a proton pump inhibitor. Biol Pharm Bull. 1995;18:1053–1056. doi: 10.1248/bpb.18.1053. [DOI] [PubMed] [Google Scholar]
- 21.Warren J R, Marshall B J. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]