ABSTRACT
Previous studies have suggested that soy products may be beneficial for cardiometabolic health, but current evidence regarding their effects in type 2 diabetes (T2D) remains unclear. The aim of this systematic review and meta-analysis was to determine the impact of soy product consumption on cardiovascular risk factors in patients with T2D. PubMed, Scopus, Embase, and the Cochrane library were systematically searched from inception to March 2021 using relevant keywords. All randomized controlled trials (RCTs) investigating the effects of soy product consumption on cardiovascular risk factors in patients with T2D were included. Meta-analysis was performed using random-effects models and subgroup analysis was performed to explore variations by dose and baseline risk profile. A total of 22 trials with 867 participants were included in this meta-analysis. Soy product consumption led to a significant reduction in serum concentrations of triglycerides (TGs) [weighted mean difference (WMD): –24.73 mg/dL; 95% CI: –37.49, –11.97], total cholesterol (WMD: –9.84 mg/dL; 95% CI: –15.07, –4.61), LDL cholesterol (WMD: –6.94 mg/dL; 95% CI: –11.71, –2.17), and C-reactive protein (WMD: –1.27 mg/L; 95% CI: –2.39, –0.16). In contrast, soy products had no effect on HDL cholesterol, fasting blood sugar (FBS), fasting insulin, glycated hemoglobin, HOMA-IR, systolic blood pressure (SBP) and diastolic blood pressure, or BMI (all P ≥ 0.05). In subgroup analyses, there was a significant reduction in FBS after soy consumption in patients with elevated baseline FBS (>126 mg/dL) and in those who received higher doses of soy intake (>30 g/d). Moreover, soy products decreased SBP in patients with baseline hypertension (>135 mm Hg). Our meta-analysis suggests that soy product consumption may improve cardiovascular parameters in patients with T2D, particularly in individuals with poor baseline risk profiles. However, larger studies with longer durations and improved methodological quality are needed before firm conclusions can be reached.
Keywords: soy, diabetes, cardiovascular risk, meta-analysis, systematic review
Statement of Significance: Several studies have investigated the effects of soy products on cardiovascular risk, but the findings were conflicting. We conducted a comprehensive systematic review and meta-analysis of published randomized controlled trials to evaluate the effects of soy product consumption on cardiovascular risk factors in patients with type 2 diabetes.
Introduction
Diabetes mellitus is a global health concern, representing the seventh leading cause of death (1), with high morbidity and mortality rates (2). The International Diabetes Federation has estimated that, globally, 425 million adults aged 20–79 y had diabetes in 2015, with approximately half (50%) being undiagnosed. This number is estimated to increase to 629 million by 2045 (3). Type 2 diabetes (T2D) represents over 90% of all diabetes cases (4, 5). Poorly controlled T2D is associated with hyperglycemia, chronic inflammation, and dyslipidemia, which, over time, can lead to microvascular complications (nephropathy, neuropathy, retinopathy) and macrovascular complications including atherosclerosis and cardiovascular diseases (CVDs) (6, 7). People with T2D have a 2-fold excess risk of CVDs and premature mortality from cardiovascular causes (8). The mechanisms of the pathogenesis of CVD in diabetes are linked to epigenetic, genetic, and cell-signaling deficiencies in interrelated metabolic and inflammatory pathways (9). There is strong evidence to suggest that T2D and CVD can be prevented by lifestyle modification, including a healthy diet, physical activity, and avoidance of smoking and alcohol (10–13). Specific ingredients of plant-based foods have also been proposed to have important benefits in relation to cardiometabolic health (14, 15).
Soy foods are the main source of plant protein, dietary fiber, PUFAs, and phytoestrogens (16). Previous studies have reported that soy products may have a positive impact on glucose metabolism, due to soy being a rich source of isoflavones, such as genistein and daidzein, which have well-established antidiabetic effects (17–19). It seems that soy isoflavones can increase serum insulin by enhancing insulin signaling, and eventually improve the glucose uptake (20). Numerous studies have also reported inverse associations between the intake of soy protein and its isoflavones with risk factors for CVD, including hypertension, dyslipidemia, glycemic control, arterial stiffness, and endothelial function (16, 21–24). A study by Hermansen et al. (25) showed that consumption of soy protein for 6 wk in patients with T2D had a positive effect on cardiovascular risk markers, including LDL cholesterol, total cholesterol (TC), LDL- to HDL-cholesterol ratio, apoB100, triglycerides (TGs), and homocysteine. Soy protein may exert the lipid-lowering effect through reducing the activity of lipoprotein lipase (26). Consuming soy products can also decrease postprandial glycemia, inflammatory markers, and other cardiovascular risk factors, although current evidence is inconsistent (27–29). For instance, some studies report that soy consumption exerts positive effects in modulating proinflammatory cytokines (30, 31), whereas others do not (32, 33). Dietary intake of soy protein has also been shown to reduce body weight in overweight and obese individuals, compared with diets using animal protein (34, 35). However, the benefits of soy protein in reducing overall fat mass, abdominal adiposity, and circulating adipokine concentrations remain controversial (36, 37).
Due to the effects of a soy-based diet in reducing TC, LDL cholesterol, TGs, body weight, and postprandial glycemia and in increasing HDL cholesterol, several nutritional recommendations have been suggested to increase the dietary intake of soy products for the prevention and management of T2D (38, 39). However, there is no comprehensive study to investigate the effect of soy products on cardiovascular risk factors in patients with T2D.
Findings of 2 previous meta-analyses in adults indicated that soy protein consumption had a significant effect on serum lipoprotein concentrations (40, 41). In these studies, subgroup analysis was not performed based on participants’ health status. Another recent meta-analysis did not show significant effects of overall soy product consumption on concentrations of C-reactive protein (CRP) in adults (42). However, subgroup analysis indicated that natural soy products may reduce plasma concentrations of CRP. In this research, the effect of soy products on CRP in T2D was not evaluated. Moreover, in a systematic review and meta-analysis of 8 trials published before 2010, it was concluded that soy products have beneficial effects in T2D patients in relation to serum lipids without a significant effect on fasting glucose, insulin, and glycated hemoglobin (HbA1c) (43). In this investigation, only a few databases were searched and comprehensive subgroup analysis was not performed.
Given the discrepancies in the current body of evidence, there is still no clear understanding of the effects of soy products on cardiovascular risk factors in patients with T2D. To address this gap in knowledge, we conducted a comprehensive systematic review and meta-analysis of published randomized controlled trials (RCTs) to evaluate the effects of soy product consumption on cardiovascular risk factors in patients with T2D.
Methods
Search strategy and study selection
The present meta-analysis was designed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (44), with a protocol developed a priori (registered on PROSPERO: CRD42021226508). Two investigators (OA and SPM) performed a systematic search of articles from inception to March 2021 in electronic databases including PubMed, Scopus, Embase, and the Cochrane Library without any date or language restrictions. Databases were searched using the following Medical Subject Heading (MeSH) and non-MeSH terms as outlined in Supplemental Table 1: (Soy OR “Soy protein” OR Isoflavones OR “Soy products”) AND (“Type 2 diabetes” OR “T2DM” OR “diabetes” OR “insulin-independent diabetes” OR “insulin resistance” OR “diabetic patients”). Two independent reviewers (OA and SPM) screened records based on title and abstract to determine eligibility for inclusion; then retrieved all potentially eligible articles and reviewed these articles in full text. In addition, we conducted a manual search via Google Scholar, and reference lists of relevant articles and previous reviews were checked to identify any missing eligible papers.
Eligibility criteria
Studies that met the following criteria were included: 1) RCT design (parallel or crossover), 2) evaluating the effects of soy products (soy protein, isoflavones, soy bean, and/or soy milk) of any dose on cardiovascular risk factors [lipid and glycemic profiles, blood pressure (BP), inflammatory biomarkers, and BMI], 3) with a control or comparator group (as placebo, animal or milk protein, or other diet/dietary components), 4) in adult patients with T2D, 5) with a duration of >1 wk, and 6) with outcomes reported at baseline and at the end of the intervention.
Animal studies, observational studies, reviews, commentaries, or RCTs without a control group were excluded, as were studies that lacked the required data (i.e., CVD risk factors) at baseline or follow-up, studies in children (<18 y old), or those in individuals without T2D.
Data extraction
Data were extracted from eligible full-text articles and included the following: first author's name, publication year, study design, study location, sample size in intervention and control groups, duration and dose of intervention, supplement type, and aggregate baseline and follow-up outcome measurements along with SDs and/or changes (delta values) in outcome measures from baseline to the end of the trial. If a study provided multiple data at different time points, only data from the latest time points were extracted. Also, data from the first period were extracted from crossover trials.
Quality assessment
The quality of studies was assessed using the Cochrane risk-of-bias tool (45) by 2 reviewers (OA and SPM) and any disagreement was resolved through discussion. The assessment consists of 7 criteria to evaluate risk of bias at the study level. These criteria are as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. These domains were classified as low or high risk for bias or unclear. The overall risk of bias of individual studies was regarded as low risk (low risk for bias for all items), moderate (unclear risk of bias for ≥1 key domains), or high risk (high risk of bias for ≥1 key domains).
Data synthesis and statistical analysis
The data were analyzed by using Stata version 12.0 (StataCorp). Mean change and SD of the relevant outcomes were used to estimate the overall effect size. Effect sizes for all variables are reported as weighted mean differences (WMDs) and 95% CIs derived from random-effects models. A random-effects model was selected because there is significant heterogeneity between studies in terms of the study methods, measures, and population characteristics. If the SD change was not reported in studies, we calculated the SD change as per the formula provided by the Cochrane Collaboration (45), which is as follows: SD = square root [(SD baseline) 2 + (SD final) 2 – (2R × SDbaseline × SDfinal)], where the correlation coefficient (R) = 0.8. Statistical heterogeneity between studies was assessed by Cochraneʼs Q test (significance point at P < 0.1) and the I2 index (significance point at I2 > 40%). Publication bias assessment was performed using visual inspection of funnel plots and statistically using Eggerʼs regression test and Begg's test (46). Subgroup analyses were performed on factors presumed to cause variation in outcomes based on previous evidence (47, 48), and these included study duration (<8 or ≥8 wk), soy types (soy protein, isoflavones, soy bean, soy milk), soy dose (≤30 or >30 g/d) and baseline BMI (kg/m2; 25–29.9 or ≥30). Baseline risk profiles were also examined in relation to each outcome [e.g., for meta-analysis of TGs, subgroups with high (>150 mg/dL) and low (<150 mg/dL) TGs at baseline were explored] (46).
Results
Study selection
The process of study selection is shown in Supplemental Figure 1. The primary search yielded 3275 records. Duplicates were removed and 1920 articles remained, which were screened by title and abstract. At this stage, 1873 articles were excluded, and the remaining 47 records were reviewed in full text to confirm eligibility. Of these, 25 articles were excluded due to lack of desired outcome data—that is, CVD risk factors (n = 23) and/or not having a control group (n = 2). In total, 22 trials with 897 participants met the inclusion criteria and were included in this meta-analysis (26, 27, 30, 49–67).
Characteristics of the included studies
The general characteristics of the included studies are summarized in Table 1. Trials were published between 1998 and 2019 and were carried out in Canada (26, 57), China (58), Iran (30, 51, 54, 55, 59, 60, 62, 64, 65, 67), Korea (27), Mexico (56), Qatar (66), the United Kingdom (50, 53, 63) and the United States (49, 52). Out of these 22 RCTs, 9 were of parallel design (27, 30, 54, 58, 63–67) and 13 were randomized crossover trials (26, 49–53, 55–57, 59–62). Follow-up durations ranged from 4 wk to 4 y. Intervention doses varied between 17.8 and 69 g/d. Fourteen studies were performed in both genders (26, 27, 30, 51, 54–57, 59, 60, 64–67), whereas 4 studies were performed only in females (50, 58, 61, 62) and 4 studies were in males (49, 52, 53, 63). Participants used both antidiabetic and antihypertensive medications (30, 55, 65), both antidiabetic and lipid-lowering drugs (66), or antidiabetic drugs only (27, 49, 67). However, 15 trials did not report any information on the use of medications (26, 50–54, 56–61, 63, 64). In the case of the overall diet in the included studies, patients were following a diabetic diet (26, 49, 50, 53, 57, 66), usual diet (27, 52, 56, 58–63), low-calorie diet (54), weight-maintenance diet (65), TLC (Therapeutic Life Style Changes) diet (67), a diet containing 0.8 g/kg protein (30, 51, 55), or a diet containing 55% carbohydrate, 15% protein, and 30% fat (64). Control diets consisted of a habitual diet (67), cow milk (59, 60), bread (61, 62), animal protein (30, 49, 51, 55), soy protein (63, 66), red meat (64, 65), milk protein (26, 57, 58), banana starch (56), low-calorie diet (54), a diabetic diet (27), casein (52), and placebo (cellulose) (50, 53). All the 22 included trials had an appropriate controlled design, and the only difference between the control and treatment groups was the soy product intervention.
TABLE 1.
Characteristics of studies included in a systematic review and meta-analysis of the effects of soy products on cardiovascular risk factors in patients with type 2 diabetes1
| Trial duration, wk | Intervention | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Age, y | Mean BMI (kg/m2) | Treatment | Isoflavone | ||||||||
| (ref) | Country | Study design | Sample, n (M/F) | IG | CG | IG | CG | group | Soy dose | dose | Control | |
| Anderson et al., 1998 (49) | USA | Crossover (R) | 8 MIG: 8 MCG: 8 M | 8 | 64.0 ± 26.0 | 35.1 ± 6.2 | Soy protein | NR | NR | Animal protein | ||
| Jayagopal et al., 2002 (50) | UK | Crossover (R/DB) | 32 FIG: 32 FCG: 32 F | 12 | 62.5 ± 6.8 | 32.2 ± 5.0 | Soy protein + isoflavone | 30 g | 132 mg | Placebo (cellulose) | ||
| Azadbakht et al., 2003 (51) | Iran | Crossover (R) | 14 (10 M/4 F)IG: 14 (10 M/4 F)CG: 14 (10 M/4 F) | 7 | 62.5 ± 12.1 | 26.6 ± 4.0 | Soy protein | 0.8 g/kg | NR | Animal protein | ||
| Teixiera et al., 2004 (52) | USA | Crossover (R) | 14 MIG: 14 MCG: 14 M | 8 | NR | NR | 29.8 ± 0.8 | 29.8 ± 0.8 | Soy protein | 0.5 g/kg | 0.2 mg/g protein | Casein |
| González et al., 2007 (53) | UK | Crossover (R/DB/PC) | 24 MIG: 24 MCG: 24 M | 4 | NR | NR | 30.7 ± 5.5 | 31.0 ± 6.4 | Isoflavone | 25 g | 132 mg | Placebo (cellulose) |
| Noroozi et al., 2008 (54) | Iran | Parallel (R) | 32 (4 M/28 F)IG: 16 (M/F: NR)CG: 16 (M/F: NR) | 4 | 48.7 ± 7.5 | 46.2 ± 7.4 | 30.8 ± 4.1 | 32.7 ± 5.2 | Soy protein + low-calorie diet | 30 g | 45 mg | Low-calorie diet |
| Azadbakht et al., 2008 (30) | Iran | Parallel (R) | 41 (18 M/23 F)IG: 20 (M/F: NR)CG: 21 (M/F: NR) | 208(4 y) | 62.1 ± 12.1 | 62.1 ± 12.1 | NR | NR | Isoflavone | 17 g | 48 mg | Animal protein |
| Chang et al., 2008 (27) | Korea | Parallel (R) | 20 (8 M/ 12 F)IG: 10 (4 M/6 F)CG: 10 (4 M/6 F) | 4 | 56.6 ± 2.9 | 54.9 ± 2.4 | 25.2 ± 1.1 | 24.3 ± 0.6 | Soybean + a diabetes diet | 69 g | NR | Diabetes diet |
| Pipe et al., 2009 (26) | Canada | Crossover (R/DB) | 29 (16 M/13 F)IG: 29 (16 M/13 F)CG: 29 (16 M/13 F) | 4 | 60.1 ± 9.6 | 29.6 ± 9.0 | Soy protein | 40 g | 88 mg | Milk protein | ||
| Azadbakht and Esmaillzadeh, 2009 (55) | Iran | Crossover (R) | 14 (10 M/4 F)IG: 14 (10 M/4 F)CG: 14 (10 M/4 F) | 7 | 62.5 ± 12.1 | NR | Soy protein | 17.8 g | NR | Animal protein | ||
| Liu et al., 2010 (58) | China | Parallel (R/DB) | 120 FIG: 60 FCG: 60 F | 24 | 56.4 ± 4.7 | 55.9 ± 3.8 | 24.1 ± 3.8 | 24.6 ± 3.4 | Soy protein | 15 g | 100 mg | Milk protein |
| Gobert et al., 2010 (57) | Canada | Crossover (R/DB) | 29 (16 M/13 F)IG: 29 (16 M/13 F)CG: 29 (16 M/13 F) | 4 | 60.1 ± 9.6 | 29.6 ± 9.0 | Soy protein | 40 g | 88 mg | Milk protein | ||
| Ble-Castillo et al., 2010 (56) | Mexico | Crossover (R) | 28 (2 M/24 F)IG: 28 (2 M/24 F)CG: 28 (2 M/24 F) | 4 | 51.7 ± 5.6 | 34.9 ± 2.3 | Soy milk | 24 g | 24 g | Native banana starch | ||
| Miraghajani et al., 2012 (59) | Iran | Crossover (R) | 25 (10 M/15 F)IG: 25 (10 M/15 F)CG: 25 (10 M/15 F) | 4 | 51.0 ± 10.0 | 28.6 ± 4.0 | Soy milk | 240 cc | NR | Cow milk | ||
| Miraghajani et al., 2013 (60) | Iran | Crossover (R) | 25 (10 M/15 F)IG: 25 (10 M/15 F)CG: 25 (10 M/15 F) | 4 | 51.0 ± 10.0 | 28.6 ± 4.0 | Soy milk | 240 cc | NR | Cow milk | ||
| Salari Moghaddam et al., 2014 (61) | Iran | Crossover (R) | 30 FIG:30 FCG: 30 F | 6 | 45.7 ± 3.8 | 45.7 ± 3.8 | 28.6 ± 3.6 | 30.2 ± 4.3 | Soybean flour–enriched bread | 120 g | NR | Bread |
| Salari Moghaddam et al., 2014 (62) | Iran | Crossover (R) | 30 FIG: 30 FCG: 30 F | 6 | 45.7 ± 3.8 | 45.7 ± 3.8 | 28.6 ± 3.6 | 30.2 ± 4.3 | Soybean flour–enriched bread | 120 g | NR | Bread |
| Sathyapalan et al., 2016 (63) | UK | Parallel (R/DB) | 200 MIG: 100 MCG: 100 M | 12 | 52.0 ± 5.0 | 52.0 ± 5.0 | 31.8 ± 5.9 | 31.6 ± 5.8 | Soy protein + isoflavone | 15 g | 66 mg | Soy protein |
| Hematdar et al., 2018 (64) | Iran | Parallel (R) | 44 (11 M/33 F)IG: 21 (7 M/14 F)CG: 23 (4 M/19 F) | 8 | 57.0 ± 7.3 | 56.0 ± 7.3 | 25.6 ± 3.9 | 26.7 ± 2.9 | Soybean | 40 g | NR | 2 servings of red meat 3 days per week |
| Sedaghat et al., 2019 (67) | Iran | Parallel (R) | 68 (31 M/35 F)IG: 34 (16 M/19 F)CG: 34 (15 M/19 F) | 8 | 49.9 ± 9.2 | 50.2 ± 8.3 | 29 ± 3.2 | 28.2 ± 3.3 | Soybean | 60 g | NR | Usual diet |
| Hassanzadeh-Rostami et al., 2019 (65) | Iran | Parallel (R) | 44 (12 M/32 F)IG: 21 (7 M/14 F)CG: 23 (5 M/19 F) | 8 | 57.1 ± 7.3 | 56.1 ± 7.2 | 25.7 ± 3.9 | 26.5 ± 3.2 | Soybean | ×2 servings–cooked soybeans | NR | 2 servings of red meat 3 days per week |
| Konya et al., 2019 (66) | Qatar | Parallel (R/DB) | 26 (18 M/8 F)IG: 13 (11 M/12 F)CG: 13 (7 M/6 F) | 8 | 63.5 ± 6.3 | 66.8 ± 8.4 | 31.0 ± 5.6 | 30.9 ± 4.5 | Soy protein + isoflavone | 15 g | 32 mg | Soy protein |
Some data are reported for the overall sample and not by intervention or control group. Values for age and BMI are presented as mean ± SDs. CG, control group; DB, double-blinded; IG, intervention group; NR, not reported; PC, placebo controlled; R, random; ref, reference.
Quality assessment
Based on random-sequence generation, most studies had an unclear risk of bias (26, 27, 30, 49–52, 54–57, 59–62, 64, 67), whereas 5 studies had low risk of bias (53, 58, 63, 65, 66). In relation to allocation concealment, 7 studies demonstrated low risk of bias (50, 56, 58, 63–66), and the rest showed high (26, 49, 52–54, 57, 59, 60, 64) or unclear (27, 30, 51, 55, 59, 60) risk. With the exception of 5 studies (49, 54, 59, 60, 64), all studies had low risk of bias (26, 27, 30, 50–53, 55–58, 61–63, 65–67) for the selective reporting criteria. Only 7 studies (26, 50, 53, 57, 58, 63, 66) reported blinding of participants and personnel, whereas blinding of outcome assessors was noted in 8 studies (26, 50, 53, 57, 58, 63, 64, 66). In nearly all the trials, there was a low risk of incomplete outcome data (26, 27, 30, 49–63, 65) and other sources of bias (26, 27, 30, 49–67). Overall, 18 trials were judged as high risk, 2 had moderate risk, and 2 studies were low risk. The quality assessment of the included studies is presented in Supplemental Table 2.
Meta-analysis
Effect of soy products on lipid profiles
Effect of soy products on TG concentrations
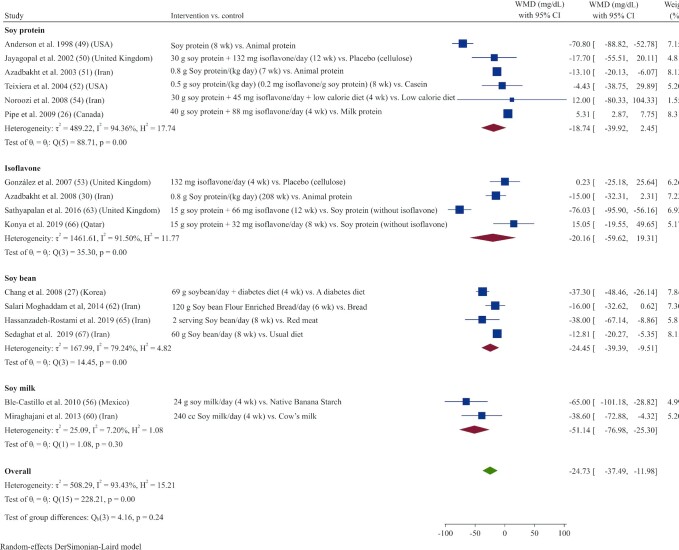
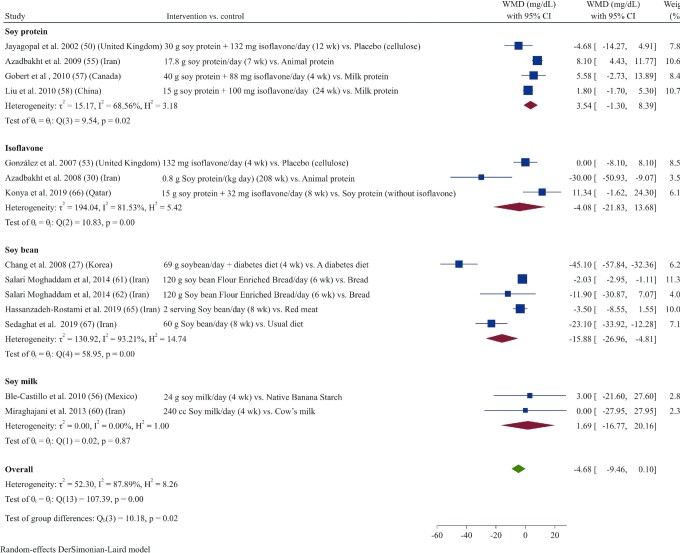
Sixteen studies (26, 27, 30, 49–54, 56, 60, 62, 63, 65–67) reported TGs as an outcome measure. Overall, results from the random‐effects model indicated that consumption of soy products resulted in a significant reduction in TGs (WMD: –24.73 mg/dL; 95% CI: –37.49, –11.97; I2 = 93.4%) (Figure 1). In subgroup analysis, the effect of soy product consumption on TGs was no longer significant in groups that received low doses (<30 g/d) or who were supplemented with soy protein or isoflavones, as well as in subgroups with normal serum TG concentrations at baseline (<150 mg/dL) (Supplemental Table 3).
FIGURE 1.
Forest plot of a random-effects meta-analysis of the effect of soy products on circulating TG concentrations in patients with type 2 diabetes. TG, triglyceride; WMD, weighted mean difference.
Effect of soy products on TC concentrations
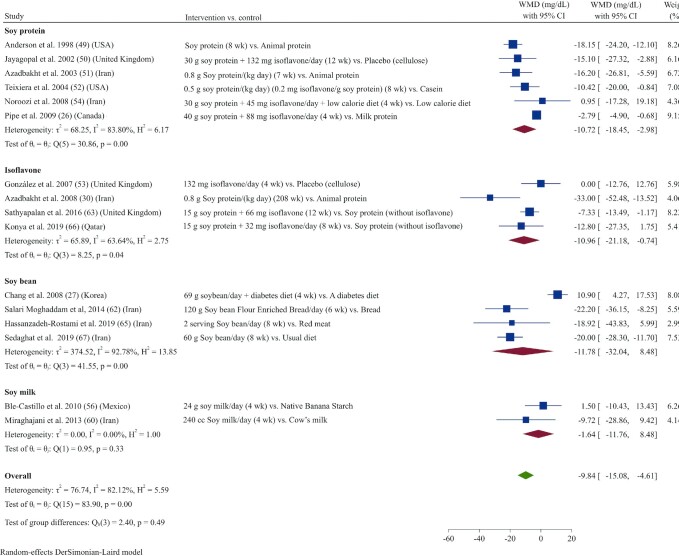
Pooled data from 16 studies (26, 27, 30, 49–54, 56, 60, 62, 63, 65–67) indicated that TC concentrations were reduced significantly in those receiving soy products compared with controls (WMD: −9.84 mg/dL; 95% CI: –15.07, –4.61; I2 = 82.1%) (Figure 2). Subgroup analysis showed that this effect on TC concentrations was not significant in studies with short durations (<8 wk) or in those that used soybean or soy-milk products (Supplemental Table 3).
FIGURE 2.
Forest plot of a random-effects meta-analysis of the effect of soy products on circulating TC concentrations in patients with type 2 diabetes. TC, total cholesterol; WMD, weighted mean difference.
Effect of soy products on LDL-cholesterol concentrations
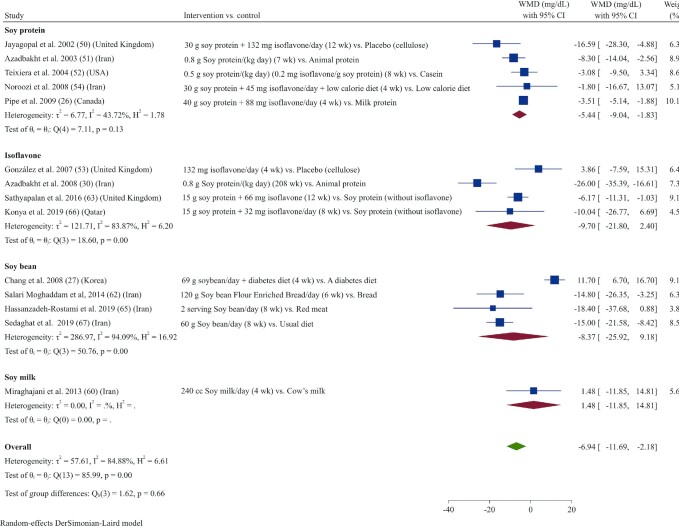
Meta-analysis of data from 14 studies (26, 27, 30, 50–54, 60, 62, 63, 65–67) demonstrated that soy product consumption led to a decrease in LDL-cholesterol concentrations (WMD: –6.94 mg/dL; 95% CI: –11.71, –2.17; I2 = 85.0%) (Figure 3). In subgroup analyses, the effect of soy product consumption on reducing serum LDL cholesterol was only significant in studies with longer durations (≥8 wk) and lower doses (<30 g/d), in studies that used soy protein as the intervention, or in those that included participants with elevated concentrations of LDL cholesterol (>130 mg/dL) at baseline (Supplemental Table 3).
FIGURE 3.
Forest plot of a random-effects meta-analysis of the effect of soy products on circulating LDL-cholesterol concentrations in patients with type 2 diabetes. WMD, weighted mean difference.
Effect of soy products on HDL-cholesterol concentrations
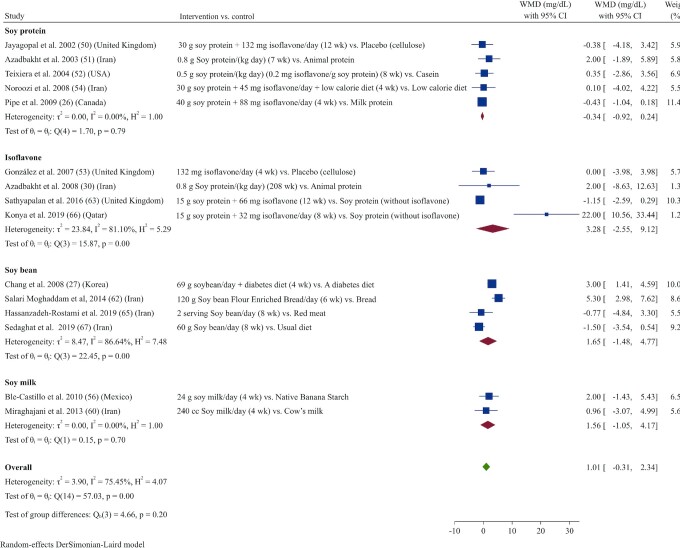
The effects of soy product consumption on HDL cholesterol were evaluated in 15 studies (26, 27, 30, 50–54, 56, 60, 62, 63, 65–67). Combined results indicated that HDL-cholesterol concentrations did not change significantly following soy product consumption compared with control (WMD: 1.01 mg/dL; 95% CI: –0.31, 2.33; I2 = 75.5%) (Figure 4). In subgroup analyses, results remained nonsignificant across all subgroups (Supplemental Table 3).
FIGURE 4.
Forest plot of a random-effects meta-analysis of the effect of soy products on circulating HDL-cholesterol concentrations in patients with type 2 diabetes. WMD, weighted mean difference.
Effect of soy products on glycemic control
Effect of soy products on fasting blood sugar
A total of 14 studies (27, 30, 50, 53, 55–58, 60–62, 65–67) investigated the effects of soy products on fasting blood sugar (FBS) concentrations. Pooled results from the random-effects model indicated that FBS concentrations did not change significantly after soy product consumption (WMD: –4.67 mg/dL; 95% CI: –9.44, 0.09; I2 = 87.9%) (Figure 5). However, subgroup analysis showed that soy products had a significant decreasing effect on FBS concentrations in studies of patients with elevated FBS at baseline (>126 mg/dL) or with an overweight BMI status, as well as in studies that used high soy doses (>30 g/d) or soy milk as the intervention (Supplemental Table 3).
FIGURE 5.
Forest plot of a random-effects meta-analysis of the effect of soy products on circulating FBS concentrations in patients with type 2 diabetes. FBS, fasting blood sugar; WMD, weighted mean difference.
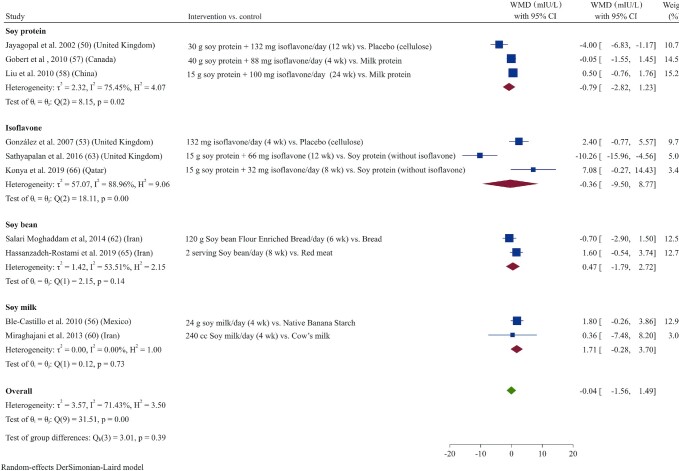
Effect of soy products on fasting insulin
Fasting insulin was reported as an outcome in 10 studies (50, 53, 56–58, 60, 62, 63, 65, 66). Pooled analysis demonstrated that soy product consumption did not significantly affect fasting insulin concentrations in patients with T2D (WMD: –0.04 mIU/L; 95% CI: –1.56, 1.48; I2 = 71.4%) (Figure 6). Subgroup analysis also did not reveal any significant effects of soy product consumption on fasting insulin concentrations compared with control groups (Supplemental Table 3).
FIGURE 6.
Forest plot of a random-effects meta-analysis of the effect of soy products on circulating fasting insulin concentrations in patients with type 2 diabetes. WMD, weighted mean difference.
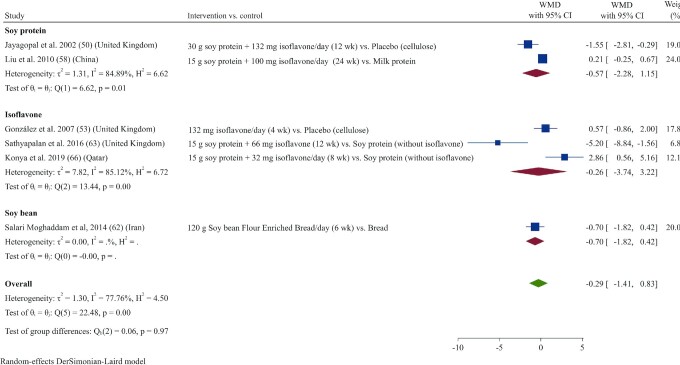
Effect of soy products on HOMA-IR
HOMA-IR did not change significantly following soy product consumption compared with control in a meta-analysis of 6 studies (50, 53, 58, 61, 63, 66) (WMD: −0.29; 95% CI: –1.41, 0.82; 77.8%) (Figure 7). These results remained nonsignificant in subgroup analysis (Supplemental Table 3).
FIGURE 7.
Forest plot of a random-effects meta-analysis of the effect of soy products on HOMA-IR in patients with type 2 diabetes. WMD, weighted mean difference.
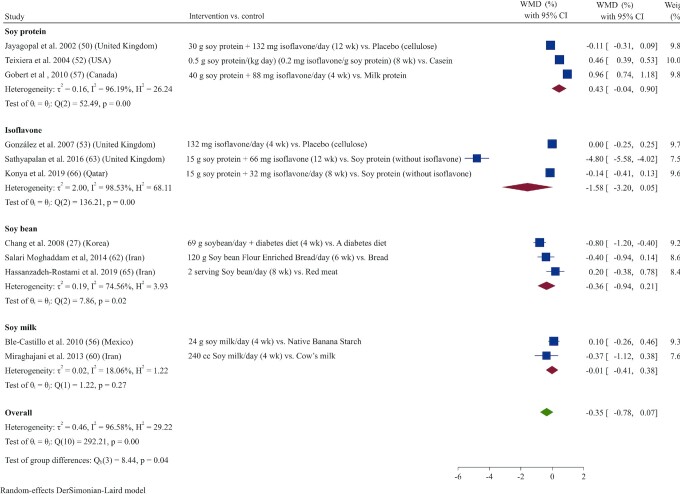
Effect of soy products on HbA1c
There was no effect on HbA1c after soy product consumption based on the overall pooled analysis of 11 studies (27, 50, 52, 53, 56, 57, 60, 62, 63, 65, 66) (WMD: –0.35%; 95% CI: –0.77, 0.06; I2 = 96.6%) (Figure 8). Subgroup analysis revealed that soy product consumption significantly reduced HbA1c in studies that used low doses (≤30 g/d) or that enrolled obese participants (BMI ≥30) or participants with HbA1c >6.5% at baseline (Supplemental Table 3).
FIGURE 8.
Forest plot of a random-effects meta-analysis of the effect of soy products on circulating HbA1c concentrations in patients with type 2 diabetes. HbA1c, glycated hemoglobin; WMD, weighted mean difference.
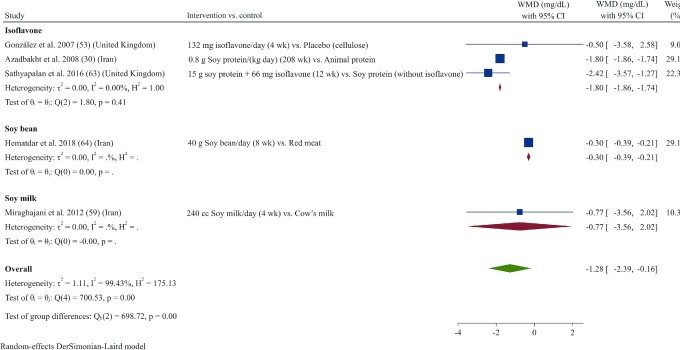
Effect of soy products on CRP
In total, 5 studies (30, 53, 59, 63, 64) evaluated the effects of soy products on CRP. The overall results indicated that intervention with soy products significantly reduced serum concentrations of CRP (WMD: –1.27 mg/L; 95% CI: –2.39, –0.16; I2 = 99.4%) (Figure 9). Subgroup analyses showed that these effects remained significant only in studies that had longer durations (>8 wk), used soybean or isoflavones as the intervention (not soy milk) irrespective of the dose used, or included overweight or obese patients with higher baseline CRP concentrations (>3 mg/L) (Supplemental Table 3).
FIGURE 9.
Forest plot of a random-effects meta-analysis of the effect of soy products on circulating CRP concentrations in patients with type 2 diabetes. CRP, C-reactive protein; WMD, weighted mean difference.
Effect of soy products on BP
BP was assessed in 10 studies (30, 50, 53, 55, 60, 62, 63, 65–67). Combined results indicated that both systolic BP (SBP) and diastolic BP (DBP) did not change significantly following soy product administration (WMD: –2.51 mm Hg; 95% CI: –5.12, 0.08; I2 = 61.6% for SBP; WMD: –1.21 mm Hg; 95% CI: –3.17, 0.75; I2 = 73.6% for DBP) (Supplemental Figures 2 and 3, respectively). In subgroup analysis, SBP was significantly reduced after soy consumption when studies used soy protein or soy milk as the intervention or were conducted in patients with high SBP at baseline (>135 mm Hg). In the subgroup analysis for DBP, a significant reduction in DBP was seen only in studies that used soy milk as the intervention (Supplemental Table 3).
Effect of soy products on BMI
Pooled data from 7 studies (52, 53, 56, 57, 61, 63, 66) did not indicate a significant change in BMI following soy consumption (WMD: 0.21; 95% CI: −0.22, 0.65; I2 = 62.5%) (Supplemental Figure 4) in patients with T2D.
Publication bias
Based on Begg's test, there was no indication of publication bias for TGs (P = 0.26), TC (P = 0.68), LDL cholesterol (P = 0.38), HDL cholesterol (P = 0.23), FBS (P = 0.44), fasting insulin (P = 0.85), HbA1c (P = 0.27), HOMA-IR (P = 0.70), SBP (P = 0.85), DBP (P = 0.47), CRP (P = 0.46), or BMI (P = 0.36). In addition, Egger's regression test showed no significant publication bias for TGs (P = 0.15), TC (P = 0.60), LDL cholesterol (P = 0.43), HDL cholesterol (P = 0.28), FBS (P = 0.51), fasting insulin (P = 0.57), HOMA-IR (P = 0.61), SBP (P = 0.23), DBP (P = 0.79), and CRP (P = 0.06), but there was significant publication bias found for HbA1c (P = 0.008) and BMI (P = 0.03). Funnel plots (Supplemental Figures 5–16) indicated no evidence of asymmetry in the effects of soy product consumption on cardiovascular risk factors, except for HbA1c and BMI.
Sensitivity analysis by study quality
To find out if studies with different qualities affect the overall results, we stratified the studies based on quality (low risk, moderate risk, and high risk) and then analyzed them (Supplemental Table 3). For TGs we found that when low- and high-risk studies were removed, the overall results changed to nonsignificant (WMD: –0.40 mg/dL; 95% CI: –32.44, 31.64). Also, after removing studies with low and moderate risk, the overall effect of soy products on FBS (WMD: –7.49 mg/dL; 95% CI: –13.80, –1.19) and SBP (WMD: –3.39 mm Hg; 95% CI: –6.50, –0.28) significantly changed. With regard to HbA1c, when moderate- and high-risk studies were deleted, the overall effect size significantly changed (WMD: –4.80%; 95% CI: –5.57, –4.02). In relation to CRP, after removing low-risk studies, the result significantly changed (WMD: –0.94 mg/dL; 95% CI: –2.21, 0.32).
Discussion
In this meta-analysis, we evaluated the effects of soy products on CVD risk factors including lipid and glycemic profiles, BP, CRP, and BMI among patients with T2D. According to the results of this study, soy product consumption was associated with reductions in TG, TC, LDL cholesterol, and CRP, without any significant alterations in HDL cholesterol, glycemic responses (FBS, insulin, HbA1c, and HOMA-IR), BP (SBP and DBP), or BMI when compared with a control group. Meanwhile, subgroup analyses based on study duration showed that soy consumption had more favorable effects on lipid profiles when interventions were longer than 8 wk. Moreover, patients with T2D with dyslipidemia at baseline (TGs >150 mg/dL; TC >200 mg/dL; LDL cholesterol >130 mg/dL; and HDL cholesterol <40 mg/dL) were more likely to have improved lipid profiles following soy consumption compared with patients with normal lipid concentrations. Soy products also showed more favorable anti-inflammatory effects in individuals with T2D with a higher baseline CRP concentration (>3 mg/dL), compared with patients with lower concentrations (CRP <3 mg/dL) (68). Similarly, soy consumption decreased FBS and SBP in patients with T2D with less than ideal glycemic control (FBS >126 mg/dL) or with baseline hypertension (SBP >135 mm Hg), respectively, suggesting that soy consumption may be more beneficial in patients with poorer baseline risk profiles.
In recent years, the health benefits of soy products in patients with T2D have been rigorously reported (69). A large number of observational studies showed the positive effects of various soy products on risk factors for diabetes as well as diabetes complications (70–72). Moreover, some studies showed hypolipidemic, hypoglycemic, and anti-inflammatory effects of soy products in patients with T2D. In 2011, a systematic review and meta-analysis of 8 RCTs showed that intake of soy products improves lipid profiles by decreasing TGs, TC, and LDL cholesterol and increasing HDL cholesterol, without any significant effects on FBS, insulin, or HbA1c (43). This is in agreement with the present study, which shows that soy products have favorable effects on lipid profiles, but not on BMI or glycemic profiles. Moreover, our results indicate that soy intake may have potential inflammatory-modulating effects via decreasing CRP concentrations.
Numerous epidemiological studies have reported that dyslipidemia is associated with an increased risk of CVD in patients with T2D (73–75). Current recommendations based on the American Diabetes Association, the European Association for Cardiovascular Prevention and Rehabilitation, and the National Cholesterol Education Program guidelines are to use lipid-lowering agents to prevent CVD in diabetic patients with dyslipidemia (76–78). These hypolipidemic agents have been shown to decrease the risk of CVD events in patients with T2D (79, 80). Our findings revealed that soy products may act as lipid-lowering agents by reducing TGs, TC, and LDL cholesterol in patients with T2D. Our results are consistent with previous meta-analyses showing the hypolipidemic properties of soy products in different nondiabetic populations (40, 81). For example, Anderson and Bush (41) reported that regular consumption of 1 to 2 servings of soy protein daily (15 to 30 g) has a significant favorable impact on serum lipoprotein risk factors for coronary artery disease (41). The mechanisms underlying the lipid-lowering effects of soy products remain unclear. However, we postulate 4 possible reasons for the relation between soy products and lipid profiles in our meta-analysis. First, isoflavones contained in soy products may serve as a natural selective estrogen receptor modulator (SERM) due to structural similarity to 17β-estradiol (82) and could therefore exert an effect on lipid metabolism through their biological similarities to estrogens and estrogen-receptor–dependent gene expressions (83, 84). Second, soy products containing isoflavones may have positive effects on hepatic lipase activity and adipose tissue (85). Hepatic lipase has emerged as a key player in the metabolism of both LDL and HDL cholesterol (86) and its lower activity results in overall improved lipid profile via increasing HDL cholesterol and decreasing LDL cholesterol (86, 87). Third, it has been shown that soy protein peptide chains may upregulate LDL-cholesterol receptors and induce gene expression of several enzymes and proteins important in lipid metabolism (88–93). Finally, studies have reported that soy protein peptides may regulate cholesterol homeostasis in HepG2 cells (94). While these data are intriguing, there is a need for additional studies to confirm and further define the possible mechanisms underlying the effects of different kinds of soy products on lipid profiles in patients with T2D.
It has been previously suggested that soy-rich diets may improve the management of patients with T2D (95–97). Several animal studies demonstrate that consumption of soy protein and its isoflavones improves glucose control in diabetic rats (98–100). It seems that the protective effects of soy-based diets in animals may occur via increasing insulin sensitivity and decreasing insulin requirements (91, 101, 102). However, there remains some controversy regarding the effects of soy products on glycemic profiles in humans. In an RCT by Sedaghat et al. (103), a 60-g soy nut diet as a part of daily protein for 8 wk improved FBS in patients with T2D. In contrast, other intervention studies did not show any beneficial effects (104, 105). For example, a study revealed that an increase in dietary intake of isoflavones (>150 times the standard Western dietary intake) for 4 wk did not lead to any significant improvements in glycemic profiles in postmenopausal women with T2D (106). This is consistent with our pooled analysis of data from up to 14 human RCTs, which showed no significant effects on glycemic profile following soy product interventions. Since all but one of the included studies in our analysis were of ≤3 mo duration, it is possible that this did not allow sufficient time to observe the beneficial effects of soy on glycemic control, if any. Our findings also suggest that soy products may decrease FBS in patients with T2D with less than ideal glycemic control (FBS >126 mg/dL) and when using higher doses of soy intake (>30 g/d). Given the ongoing controversies and sparse data currently available, additional large-scale studies in patients with a diverse range of risk profiles are needed, ideally using higher soy doses for longer durations, to delineate the impact of soy on glycemic profiles in patients with T2D.
Chronic inflammation can exacerbate the CVD events by developing atherosclerotic plaque (107). CRP is one of the most well-documented biomarkers of emerging CVD in patients with T2D (108). Available evidence supports the favorable effects of soy products in moderating proinflammatory cytokines, such as CRP (109). However, a recent meta-analysis in the general population did not show significant effects of overall soy product consumption on concentrations of CRP (42). Yet, their subgroup analyses suggested that natural soya products may have anti-inflammatory properties by reducing CRP concentrations. Based on our findings, it appears that soya products may decrease concentrations of CRP in patients with T2D, with the anti-inflammatory effects of soy products possibly being related to soy isoflavones (107). Beyond isoflavones, soy products are a good source of PUFAs (110) and fiber (111), which may contribute to the anti-inflammatory health benefits of soy-based products (112). Moreover, soy products contain a class of phytoestrogens belonging to the flavonoid family, and the 3 most active isoflavones (daidzein, genistein, glycitein) are structurally similar to mammalian estrogen (113). It has been shown that these isoflavones modulate the production of proinflammatory biomarkers by inhibiting inducible NO synthase (iNOS) gene expression and NO production (114). Furthermore, some animal studies showed that daidzein and genistein reduced inflammation by different mechanisms such as inhibiting NF-κB activation (115–117). The NF-κB pathway is a potent immunomodulatory pathway and its suppression results in decreasing proinflammatory cytokine concentrations such as IL-6. Since IL-6 can induce CRP gene expression, inhibiting the NF-κB/IL-6 pathway decreases circulating concentrations of CRP (118). In another possible mechanism, soy isoflavones may have anti-inflammatory properties via their estrogen-like biological activities (119).
Some studies have reported the possible antihypertensive effects of soy products, attributing these effects to their isoflavone components (120, 121). However, epidemiological and intervention studies have reported mixed effects with regard to BP (122, 123). A meta-analysis of 14 RCTs by Taku et al. (124) showed that soy isoflavone extracts significantly decreased SBP but not DBP in adult humans. In another meta-analysis, Liu et al. (125) revealed that 15 g soy protein for 24 wk had hypotensive properties only in patients with existing hypertension, but not in normotensive subjects. In line with these 2 previous meta-analyses, our results showed that soy products only decreased SBP in hypertensive subjects. It is posited that soy products may reduce the risk for hypertension via targeting mechanisms involving vasodilation and inhibiting key enzymes involved in the regulation of BP (107). In particular, soy may interact with the estrogen-response element of genes related to increasing endothelial NO synthase (eNOS), which can be responsible for enhancing eNOS expression and endogenous NO production, which improves brachial artery flow (126). A study by Colacurci et al. (127) showed that 6 mo of soy isoflavone (30 mg genistein and 30 mg daidzein) treatment may improve endothelium-dependent vasodilation and reduce plasma adhesion molecules in postmenopausal women, such as intercellular adhesion molecule 1, vascular cell adhesion protein 1, and E-selectin. Furthermore, angiotensin-converting enzyme (ACE) inhibitory peptides exist in plant proteins, and soy may reduce BP by acting as an angiotensin II receptor blocker and decreasing its vasoconstrictive effects while enhancing the vasodilatory effects of bradykinin (128). In support of these findings, animal studies have shown that soy isoflavones increase renal blood flow and sodium excretion, and affect the renin-angiotensin-aldosterone system (RAAS) by interacting with estrogen receptors to inhibit ACE activity (129). Importantly, the hypotensive effects of soy isoflavones and their ability to alter endothelial function may be related to the individual capacity to metabolize daidzein into equol (130). We were unable to confirm any hypotensive effects of soy in the present meta-analysis, which may be due to the high heterogeneity between studies or interindividual differences in the study populations. Nevertheless, additional studies are warranted to clarify the effects of soy on BP in patients with T2D and the possible mechanisms underlying these effects.
It has been previously hypothesized that soy products may contribute to improving body weight (131). Previous studies have shown that a diet high in protein (132) and fiber (133) may lead to suppressed appetite and decreased caloric intake. Therefore, due to the high protein and fiber content of soy, soy-based meal replacement formula is used as a weight-loss diet (134–136). Furthermore, it has been revealed that soy isoflavones may decrease food intake in animals (137, 138). However, human studies have generally failed to show significant improvements in body weight and BMI following soy product interventions (139–141), which is consistent with our findings in patients with T2D where we observed no change in BMI after soy consumption compared with controls. It should be noted that this is based on a meta-analysis of 7 trials, with relatively small sample sizes; hence, results should be interpreted with caution.
It is well known that dietary factors such as negative energy balance, macro- and micronutrient intake, as well as dietary fiber are related to cardiovascular risks. From all 22 included studies, 14 studies reported the between-group differences in dietary intakes following interventions (26, 27, 51, 52, 55, 57–60, 62, 64, 65, 103, 142). No significant difference was shown between the interventions regarding the amount of calorie and macronutrient intake, except for carbohydrate, which was significantly lower in the soy-milk group compared with the cow-milk group in the Miraghajani et al. study (60). However, a higher amount of dietary fiber (27, 51, 55, 59, 60, 64, 103, 142) and lower amount of saturated fats (26, 51, 55, 57) have been reported in some included studies. However, some other studies failed to see any between-group differences in the intake of fiber (26, 52, 57, 65) and saturated fats (52, 62, 142). Generally, soy products are low in saturated fat or high in fiber (143). Therefore, higher intake of fiber and lower intake of saturated fat in some included studies can be because of higher soy product intake and replacing animal sources with soy products. Since almost all included studies that reported dietary changes before and after the interventions did not see any significant differences in calorie and macronutrient intake, the cardiovascular risk decrement of soy may not relate to lower calorie and/or different macronutrient intake.
It should be noted that most included studies have high risk of bias. Of the 22 included studies in the present meta-analysis, 9 did not describe the method used to conceal the allocation sequence (26, 49, 52–54, 57, 61, 62, 67); a lack of allocation concealment has been demonstrated to inflate treatment estimates (144). Also, some of the trials have been classified as high risk of bias with respect to selective reporting (49, 54, 59, 60, 64), blinding of participants (27, 30, 49, 51, 52, 54, 55, 59, 60, 65, 67), and blinding of outcome assessment (27, 30, 49, 51, 52, 54, 55, 59, 60, 67). Selective reporting can potentially compromise the validity of a trial and has an impact on the pooled summary in systematic reviews, which can increase/decrease the overall effect (145). In addition, Schulz et al. (144) reported that trials that were not double-blinded produced larger estimates of effects, compared with blinded trials. The randomization, concealment, and blinding for most studies were judged as having high or unclear risk of bias. Previous research has demonstrated that high-risk judgments from the Cochrane risk-of-bias tool are associated with increased effect sizes and exaggerated treatment effect estimates (146, 147). It should be noted that it potentially dilutes the generalizability and impact of this finding (148).
Our present analysis is not without limitations. First, since all but 1 trial lasted less than 3 mo, our analysis is unable to show the long-term effects of soy products on cardiovascular risk factors in patients with T2D. Sample sizes of the included studies were also relatively small, with only 2 studies including >100 participants. Half the studies were performed in Iran, which limits generalizability. Factors such as diabetes duration or smoking status may influence cardiovascular risk but were not included in the analysis due to poor reporting of these variables. Moreover, statistical heterogeneity is apparent in our analysis. This may be attributed to methodological diversity (different study designs) and/or differences in treatment regimens (doses/durations) or the soy products used (soy protein, isoflavone, soybean, and soy milk) (149). Control or nonintervention groups were different in the included trials in this meta-analysis; this might tend to bias the findings toward the null. In the case of the overall diet in the included studies, patients were following different diets. Therefore, some of the changes in CVD markers may be attributed to changed dietary intake, such as micronutrient intake, rather than soy intake.
Notwithstanding these limitations, we conducted a comprehensive review of randomized trials, which are the gold-standard study design for establishing causality. We used a rigorous search with no limits on language or year of publication, and we included multiple endpoints to provide a comprehensive overview of the effects of soy on cardiovascular risk in patients with T2D.
In conclusion, our findings show that soy products may reduce CVD risk in patients with T2D, by decreasing TGs, TC, LDL cholesterol, and CRP, but have no significant effects on HDL cholesterol, glycemic outcomes (FBS, insulin, HbA1c, and HOMA-IR), BP (SBP and DBP), or BMI when compared with a control group. The favorable effects on lipid profiles and inflammation appear to be more prominent when soy interventions last longer than 8 wk and/or when administered to patients with existing dyslipidemia or inflammation (indicated by a CRP >3 mg/dL). Similarly, while there was no overall effect on glycemic outcomes or hypertension, soy products may decrease FBS or SBP when provided in high doses (>30 g/d) to patients with T2D with poor glycemic control (FBS >126 mg/dL) or with existing hypertension (SBP >150 mmHg), respectively. Further studies with adequate sample sizes, diverse risk profiles, and longer durations are needed to confirm our findings and to establish if improvements in these risk factors following soy consumption translate to reduced CVD risk in the long term.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—OA: contributed to the search and screening and to writing the first draft of the manuscript; DA-L: contributed to writing and editing the first draft of the manuscript; AM: contributed to editing and revising the manuscript; MRK: contributed to the search and screening; SPM: contributed to data extraction, quality assessment of studies, and writing the first draft of the manuscript; and all authors: provided intellectual input in line with ICMJE criteria for authorship and read and approved the final manuscript.
Notes
This work received no specific external funding. AM is supported by a Biomedical Research Fellowship provided by the National Health and Medical Research Council (NHMRC) of Australia.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–16 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ACE, angiotensin-converting enzyme; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; eNOS, endothelial NO synthase; FBS, fasting blood sugar; HbA1c, glycated hemoglobin; MeSH, Medical Subject Heading; RCT, randomized controlled trial; SBP, systolic blood pressure; T2D, type 2 diabetes; TC, total cholesterol; TG, triglyceride; WMD, weighted mean difference.
Contributor Information
Omid Asbaghi, Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Damoon Ashtary-Larky, Nutrition and Metabolic Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Aya Mousa, Monash Centre for Health Research and Implementation (MCHRI), School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Mahnaz Rezaei Kelishadi, Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran.
Seyedeh Parisa Moosavian, Department of Clinical Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran.
References
- 1. World Health Organization . The top 10 causes of death [Internet]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en. Accessed September 2020. [Google Scholar]
- 2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Federation . IDF diabetes atlas. Brussels (Belgium): International Diabetes Federation; 2015. [Google Scholar]
- 4. Bruno G, Runzo C, Cavallo-Perin P, Merletti F, Rivetti M, Pinach S, Novelli G, Trovati M, Cerutti F, Pagano G. Incidence of type 1 and type 2 diabetes in adults aged 30–49 years. The population-based registry in the province of Turin, Italy. Diabetes Care. 2005;28(11):2613–19. [DOI] [PubMed] [Google Scholar]
- 5. Holman N, Young B, Gadsby R. Current prevalence of type 1 and type 2 diabetes in adults and children in the UK. Diabet Med. 2015;32(9):1119–20. [DOI] [PubMed] [Google Scholar]
- 6. Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, Virmani R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37(2):191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krishnappa M, Patil K, Parmar K, Trivedi P, Mody N, Shah C, Faldu K, Maroo S, Parmar D. Effect of saroglitazar 2 mg and 4 mg on glycemic control, lipid profile and cardiovascular disease risk in patients with type 2 diabetes mellitus: a 56-week, randomized, double blind, phase 3 study (PRESS XII study). Cardiovasc Diabetol. 2020;19(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brownrigg JR, Hughes CO, Burleigh D, Karthikesalingam A, Patterson BO, Holt PJ, Thompson MM, de Lusignan S, Ray KK, Hinchliffe RJ. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol. 2016;4(7):588–97. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez-Araujo G, Nakagami H. Pathophysiology of cardiovascular disease in diabetes mellitus. Cardiovasc Endocrinol Metab. 2018;7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(8):543–51. [DOI] [PubMed] [Google Scholar]
- 11. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. [DOI] [PubMed] [Google Scholar]
- 12. Herrera MCA, Subhan FB, Chan CB. Dietary patterns and cardiovascular disease risk in people with type 2 diabetes. Current Obesity Rep. 2017;6(4):405–13. [DOI] [PubMed] [Google Scholar]
- 13. Lloyd D, Yuling H, Labarthe D, Mozaffarian L, Appel L, Van Horn K. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2020;121:(4):586–613. [DOI] [PubMed] [Google Scholar]
- 14. Hu FB. Do functional foods have a role in the prevention of cardiovascular disease?. Am Heart Assoc. 2011;124:538–40. [DOI] [PubMed] [Google Scholar]
- 15. Izadi V, Haghighatdoost F, Moosavian P, Azadbakht L. Effect of low-energy-dense diet rich in multiple functional foods on weight-loss maintenance, inflammation, and cardiovascular risk factors: a randomized controlled trial. J Am Coll Nutr. 2018;37(5):399–405. [DOI] [PubMed] [Google Scholar]
- 16. Kwon DY, Daily JW III, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010;30(1):1–13. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen C, Pham N, Do V, Binns C, Hoang V, Dang D, Lee A. Soyfood and isoflavone intake and risk of type 2 diabetes in Vietnamese adults. Eur J Clin Nutr. 2017;71(10):1186–92. [DOI] [PubMed] [Google Scholar]
- 18. Das D, Sarkar S, Bordoloi J, Wann SB, Kalita J, Manna P. Daidzein, its effects on impaired glucose and lipid metabolism and vascular inflammation associated with type 2 diabetes. Biofactors; 2018;44(5):407–17. [DOI] [PubMed] [Google Scholar]
- 19. Behloul N, Wu GJE. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol; 2013;698(1-3):31–8. [DOI] [PubMed] [Google Scholar]
- 20. Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes. 2006;55(4):1043–50. [DOI] [PubMed] [Google Scholar]
- 21. Kwak JH, Kim M, Lee E, Lee S-H, Ahn C-W, Lee JH. Effects of black soy peptide supplementation on blood pressure and oxidative stress: a randomized controlled trial. Hypertens Res. 2013;36(12):1060. [DOI] [PubMed] [Google Scholar]
- 22. Hu X, Gao J, Zhang Q, Fu Y, Li K, Zhu S, Li D. Soy fiber improves weight loss and lipid profile in overweight and obese adults: a randomized controlled trial. Mol Nutr Food Res. 2013;57(12):2147–54. [DOI] [PubMed] [Google Scholar]
- 23. Pase MP, Grima NA, Sarris J. The effects of dietary and nutrient interventions on arterial stiffness: a systematic review. Am J Clin Nutr. 2011;93(2):446–54. [DOI] [PubMed] [Google Scholar]
- 24. Beavers D, Beavers K, Miller M, Stamey J, Messina M. Exposure to isoflavone-containing soy products and endothelial function: a Bayesian meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2012;22(3):182–91. [DOI] [PubMed] [Google Scholar]
- 25. Hermansen K, Søndergaard M, Høie L, Carstensen M, Brock B. Beneficial effects of a soy-based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care. 2001;24(2):228–33. [DOI] [PubMed] [Google Scholar]
- 26. Pipe EA, Gobert CP, Capes SE, Darlington GA, Lampe JW, Duncan AM. Soy protein reduces serum LDL cholesterol and the LDL cholesterol:HDL cholesterol and apolipoprotein B:apolipoprotein A-I ratios in adults with type 2 diabetes. J Nutr. 2009;139(9):1700–6. [DOI] [PubMed] [Google Scholar]
- 27. Chang JH, Kim MS, Kim TW, Lee SS. Effects of soybean supplementation on blood glucose, plasma lipid levels, and erythrocyte antioxidant enzyme activity in type 2 diabetes mellitus patients. Nutr Res Practice. 2008;2(3):152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shahbazian H, Amani R, Siadatan J, Shabbazian H, Latifi M. Beneficial effects of soy protein isoflavones on lipid and blood glucose concentrations in type 2 diabetic subjects. Saudi Med J. 2007;28(4):652–4. [PubMed] [Google Scholar]
- 29. Asbaghi O, Yaghubi E, Nazarian B, Kelishadi MR, Khadem H, Moodi V, Naeini F, Ghaedi E. The effects of soy supplementation on inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Cytokine. 2020;136:155282. [DOI] [PubMed] [Google Scholar]
- 30. Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy. Diabetes Care. 2008;31(4):648–54. [DOI] [PubMed] [Google Scholar]
- 31. Li Z, Hong K, Saltsman P, DeShields S, Bellman M, Thames G, Liu Y, Wang H, Elashoff R, Heber D. Long-term efficacy of soy-based meal replacements vs an individualized diet plan in obese type II DM patients: relative effects on weight loss, metabolic parameters, and C-reactive protein. Eur J Clin Nutr. 2005;59(3):411–18. [DOI] [PubMed] [Google Scholar]
- 32. Nikander E, Metsa-Heikkila M, Tiitinen A, Ylikorkala O. Evidence of a lack of effect of a phytoestrogen regimen on the levels of C-reactive protein, E-selectin, and nitrate in postmenopausal women. J Clin Endocrinol Metab. 2003;88(11):5180–5. [DOI] [PubMed] [Google Scholar]
- 33. Jenkins DJ, Kendall CW, Connelly PW, Jackson C-JC, Parker T, Faulkner D, Vidgen E. Effects of high-and low-isoflavone (phytoestrogen) soy foods on inflammatory biomarkers and proinflammatory cytokines in middle-aged men and women. Metabolism. 2002;51(7):919–24. [DOI] [PubMed] [Google Scholar]
- 34. Allison DB, Gadbury G, Schwartz LG, Murugesan R, Kraker JL, Heshka S, Fontaine KR, Heymsfield SB. A novel soy-based meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial. Eur J Clin Nutr. 2003;57(4):514–22. [DOI] [PubMed] [Google Scholar]
- 35. Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci. 2007;4(2):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takahira M, Noda K, Fukushima M, Zhang B, Mitsutake R, Uehara Y, Ogawa M, Kakuma T, Saku K. Randomized, double-blind, controlled, comparative trial of formula food containing soy protein vs. milk protein in visceral fat obesity. Circ J. 2011;75(9):2235–43. [DOI] [PubMed] [Google Scholar]
- 37. Rebholz C, Reynolds K, Wofford M, Chen J, Kelly T, Mei H, Whelton P, He J. Effect of soybean protein on novel cardiovascular disease risk factors: a randomized controlled trial. Eur J Clin Nutr. 2013;67(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mann JI, De Leeuw I, Hermansen K, Karamanos B, Karlström B, Katsilambros N, Riccardi G, Rivellese AA, Rizkalla S, Slama Get al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus.Nutr Metab Cardiovasc Dis. 2004;14(6):373–94. [DOI] [PubMed] [Google Scholar]
- 39. American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2007;30(Suppl 1):S48–65. [DOI] [PubMed] [Google Scholar]
- 40. Reynolds K, Chin A, Lees KA, Nguyen A, Bujnowski D, He J. A meta-analysis of the effect of soy protein supplementation on serum lipids. Am J Cardiol. 2006;98(5):633–40. [DOI] [PubMed] [Google Scholar]
- 41. Anderson JW, Bush HM. Soy protein effects on serum lipoproteins: a quality assessment and meta-analysis of randomized, controlled studies. J Am Coll Nutr. 2011;30(2):79–91. [DOI] [PubMed] [Google Scholar]
- 42. Khodarahmi M, Jafarabadi MA, Moludi J, Farhangi MA. A systematic review and meta-analysis of the effects of soy on serum hs-CRP. Clin Nutr. 2019;38(3):996–1011. [DOI] [PubMed] [Google Scholar]
- 43. Yang B, Chen Y, Xu T, Yu Y, Huang T, Hu X, Li D. Systematic review and meta-analysis of soy products consumption in patients with type 2 diabetes mellitus. Asia Pac J Clin Nutr. 2011;20(4):593–602. [PubMed] [Google Scholar]
- 44. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 45. Higgins JS. Cochrane handbook for systematic reviews of interventions version 5.0.2. [updated September 2009]. Oxford (UK): The Cochrane Collaboration; 2008. [Google Scholar]
- 46. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Asbaghi O, Fouladvand F, Gonzalez MJ, Ashtary-Larky D, Choghakhori R, Abbasnezhad A. Effect of green tea on glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr: Clin Res Rev. 2020;15:23–31. [DOI] [PubMed] [Google Scholar]
- 48. Asbaghi O, Fouladvand F, Moradi S, Ashtary-Larky D, Choghakhori R, Abbasnezhad A. Effect of green tea extract on lipid profile in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr: Clin Res Rev. 2020;14:293–301. [DOI] [PubMed] [Google Scholar]
- 49. Anderson JW, Blake JE, Turner J, Smith BM. Effects of soy protein on renal function and proteinuria in patients with type 2 diabetes. Am J Clin Nutr. 1998;68(6):1347S–53S. [DOI] [PubMed] [Google Scholar]
- 50. Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25(10):1709–14. [DOI] [PubMed] [Google Scholar]
- 51. Azadbakht L, Shakerhosseini R, Atabak S, Jamshidian M, Mehrabi Y, Esmaill-Zadeh A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. Eur J Clin Nutr. 2003;57(10):1292–94. [DOI] [PubMed] [Google Scholar]
- 52. Teixeira SR, Tappenden KA, Carson L, Jones R, Prabhudesai M, Marshall WP, Erdman JW Jr. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr. 2004;134(8):1874–80. [DOI] [PubMed] [Google Scholar]
- 53. González S, Jayagopal V, Kilpatrick ES, Chapman T, Atkin SL. Effects of isoflavone dietary supplementation on cardiovascular risk factors in type 2 diabetes. Diabetes Care. 2007;30(7):1871–73. [DOI] [PubMed] [Google Scholar]
- 54. Noroozi M, Zavoshy R, Hashemi HJ, Asefzadeh S. The effect of soy protein with low calorie diet on blood lipids in hyperlipidemic type 2 diabetic patients. J Food Lipids. 2008;15(3):398–406. [Google Scholar]
- 55. Azadbakht L, Esmaillzadeh A. Soy-protein consumption and kidney-related biomarkers among type 2 diabetics: a crossover, randomized clinical trial. J Ren Nutr. 2009;19(6):479–86. [DOI] [PubMed] [Google Scholar]
- 56. Ble-Castillo JL, Aparicio-Trápala MA, Francisco-Luria MU, Córdova-Uscanga R, Rodríguez-Hernández A, Méndez JD, Díaz-Zagoya JC. Effects of native banana starch supplementation on body weight and insulin sensitivity in obese type 2 diabetics. Int J Environ Res Public Health. 2010;7(5):1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gobert CP, Pipe EA, Capes SE, Darlington GA, Lampe JW, Duncan AM. Soya protein does not affect glycaemic control in adults with type 2 diabetes. Br J Nutr. 2010;103(3):412–21. [DOI] [PubMed] [Google Scholar]
- 58. Liu ZM, Chen YM, Ho SC, Ho YP, Woo J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: a 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am J Clin Nutr. 2010;91(5):1394–401. [DOI] [PubMed] [Google Scholar]
- 59. Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35(10):1981–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miraghajani MS, Najafabadi MM, Surkan PJ, Esmaillzadeh A, Mirlohi M, Azadbakht L. Soy milk consumption and blood pressure among type 2 diabetic patients with nephropathy. J Ren Nutr. 2013;23(4):277–282.e1. [DOI] [PubMed] [Google Scholar]
- 61. Salari Moghaddam A, Entezari MH, Iraj B, Askari GR, Maracy MR. The effects of consumption of bread fortified with soy bean flour on metabolic profile in type 2 diabetic women: a cross-over randomized controlled clinical trial. Int J Prev Med. 2014;5(12):1529–36. [PMC free article] [PubMed] [Google Scholar]
- 62. Salari Moghaddam A, Entezari MH, Iraj B, Askari G, Sharifi Zahabi E, Maracy MR. The effects of soy bean flour enriched bread intake on anthropometric indices and blood pressure in type 2 diabetic women: a crossover randomized controlled clinical trial. Int J Endocrinol. 2014;2014:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sathyapalan T, Rigby AS, Bhasin S, Thatcher NJ, Kilpatrick ES, Atkin SL. Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: a randomized controlled study. J Clin Endocrinol Metab. 2017;102(2):425–33. [DOI] [PubMed] [Google Scholar]
- 64. Hematdar Z, Ghasemifard N, Phishdad G, Faghih S. Substitution of red meat with soybean but not non- soy legumes improves inflammation in patients with type 2 diabetes; a randomized clinical trial. J Diabetes Metab Disord. 2018;17(2):111–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hassanzadeh-Rostami Z, Hemmatdar Z, Pishdad GR, Faghih S. Moderate consumption of red meat, compared to soy or non-soy legume, has no adverse effect on cardio-metabolic factors in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2019;129:429–37. [DOI] [PubMed] [Google Scholar]
- 66. Konya J, Sathyapalan T, Kilpatrick ES, Atkin SL. The effects of soy protein and cocoa with or without isoflavones on glycemic control in type 2 diabetes: a double-blind, randomized, placebo-controlled study. Front Endocrinol. 2019;10:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sedaghat A, Shahbazian H, Rezazadeh A, Haidari F, Jahanshahi A, Mahmoud Latifi S, Shirbeigi E. The effect of soy nut on serum total antioxidant, endothelial function and cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metab Syndr. Clin Res Rev. 2019;13(2):1387–91. [DOI] [PubMed] [Google Scholar]
- 68. Ridker P. C-reactive protein, inflammation, and cardiovascular disease: clinical update. Tex Heart Inst J. 2005;32(3):384. [PMC free article] [PubMed] [Google Scholar]
- 69. Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: a review. Mol Cell Endocrinol. 2009;304(1-2):30–42. [DOI] [PubMed] [Google Scholar]
- 70. Villegas R, Gao Y-T, Yang G, Li H-L, Elasy TA, Zheng W, Shu X. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr. 2008;87(1):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mueller NT, Odegaard AO, Gross MD, Koh W-P, Mimi CY, Yuan J-M, Pereira M. Soy intake and risk of type 2 diabetes mellitus in Chinese Singaporeans. Eur J Nutr. 2012;51(8):1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nanri A, Mizoue T, Takahashi Y, Kirii K, Inoue M, Noda M, Tsugane S. Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J Nutr. 2010;140(3):580–6. [DOI] [PubMed] [Google Scholar]
- 73. Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, De Boer IH, Deedwania P, Eckel RH, Ershow AG, Fradkin J. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2015;132(8):691–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson A-M, Zethelius B, Miftaraj M, McGuire DK, Rosengren AMet al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. NEJM. 2018;379:633–44. [DOI] [PubMed] [Google Scholar]
- 75. Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, del Cañizo-Gómez F. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength?. World J Diabetes. 2014;5(4):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Haffner SM. Dyslipidemia management in adults with diabetes. Diabetes Care. 2004;27(Suppl 1):s68–71. [DOI] [PubMed] [Google Scholar]
- 77. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corra U, Cosyns B, Deaton C. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 2016;37(29):2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ; Coordinating Committee of the National Cholesterol Education Program . Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Am Coll Cardiol. 2004;44(3):720–32. [DOI] [PubMed] [Google Scholar]
- 79. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HAW, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller J. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–96. [DOI] [PubMed] [Google Scholar]
- 80. Elam MB, Ginsberg HN, Lovato LC, Corson M, Largay J, Leiter LA, Lopez C, O'Connor PJ, Sweeney ME, Weiss Det al. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2(4):370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhan S, Ho S. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81(2):397–408. [DOI] [PubMed] [Google Scholar]
- 82. Rizzo G, Baroni L. Soy, soy foods and their role in vegetarian diets. Nutrients. 2018;10(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Clarkson TB, Anthony M. Phytoestrogens and coronary heart disease. Baillieres Clin Endocrinol Metab. 1998;12(4):589–604. [DOI] [PubMed] [Google Scholar]
- 84. Potter S. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J Nutr. 1995;125(Suppl 3):606S–11S. [DOI] [PubMed] [Google Scholar]
- 85. Nogowski L, Maćkowiak P, Kandulska K, Szkudelski T, Nowak K. Genistein-induced changes in lipid metabolism of ovariectomized rats. Ann Nutr Metab. 1998;42(6):360–6. [DOI] [PubMed] [Google Scholar]
- 86. Zambon A, Deeb SS, Hokanson JE, Brown BG, Brunzell J. Common variants in the promoter of the hepatic lipase gene are associated with lower levels of hepatic lipase activity, buoyant LDL, and higher HDL2 cholesterol. Arterioscler Thromb Vasc Biol. 1998;18(11):1723–9. [DOI] [PubMed] [Google Scholar]
- 87. Busch SJ, Barnhart RL, Martin GA, Fitzgerald MC, Yates MT, Mao S, Thomas CE, Jackson RL. Human hepatic triglyceride lipase expression reduces high density lipoprotein and aortic cholesterol in cholesterol-fed transgenic mice. J Biol Chem. 1994;269(23):16376–82. [PubMed] [Google Scholar]
- 88. Lammi C, Zanoni C, Arnoldi A, Vistoli G. Two peptides from soy β-conglycinin induce a hypocholesterolemic effect in HepG2 cells by a statin-like mechanism: Comparative in vitro and in silico modeling studies. J Agric Food Chem. 2015;63(36):7945–51. [DOI] [PubMed] [Google Scholar]
- 89. Iqbal MJ, Yaegashi S, Ahsan R, Lightfoot DA, Banz W. Differentially abundant mRNAs in rat liver in response to diets containing soy protein isolate. Physiol Genomics. 2002;11(3):219–26. [DOI] [PubMed] [Google Scholar]
- 90. Cho S-J, Juillerat MA, Lee C-H. Cholesterol lowering mechanism of soybean protein hydrolysate.J Agric Food Chem. 2007;55(26):10599–604. [DOI] [PubMed] [Google Scholar]
- 91. Iritani N, Sugimoto T, Fukuda H, Komiya M, Ikeda H. Dietary soybean protein increases insulin receptor gene expression in Wistar fatty rats when dietary polyunsaturated fatty acid level is low. J Nutr. 1997;127(6):1077–83. [DOI] [PubMed] [Google Scholar]
- 92. Tovar AR, Murguía F, Cruz C, Hernández-Pando R, Aguilar-Salinas CA, Pedraza-Chaverri J, Correa-Rotter R, Torres N. A soy protein diet alters hepatic lipid metabolism gene expression and reduces serum lipids and renal fibrogenic cytokines in rats with chronic nephrotic syndrome. J Nutr. 2002;132(9):2562–9. [DOI] [PubMed] [Google Scholar]
- 93. Baum JA, Teng H, Erdman JW Jr, Weigel RM, Klein BP, Persky VW, Freels S, Surya P, Bakhit RM, Ramos E. Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women. Am J Clin Nutr. 1998;68(3):545–51. [DOI] [PubMed] [Google Scholar]
- 94. Lovati MR, Manzoni C, Gianazza E, Arnoldi A, Kurowska E, Carroll KK, Sirtori C. Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. J Nutr. 2000;130(10):2543–9. [DOI] [PubMed] [Google Scholar]
- 95. Kwon DY, Daily JW, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010;30(1):1–13. [DOI] [PubMed] [Google Scholar]
- 96. Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25(10):1709–14. [DOI] [PubMed] [Google Scholar]
- 97. Bhathena SJ, Velasquez M. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76(6):1191–201. [DOI] [PubMed] [Google Scholar]
- 98. Dyrskog SEU, Jeppesen PB, Colombo M, Abudula R, Hermansen KJM. Preventive effects of a soy-based diet supplemented with stevioside on the development of the metabolic syndrome and type 2 diabetes in Zucker diabetic fatty rats. Metabolism. 2005;54(9):1181–8. [DOI] [PubMed] [Google Scholar]
- 99. Harini R, Ezhumalai M, Pugalendi K. Antihyperglycemic effect of biochanin A, a soy isoflavone, on streptozotocin-diabetic rats. Eur J Pharmacol. 2012;676(1-3):89–94. [DOI] [PubMed] [Google Scholar]
- 100. Jeppesen PB, Rolfsen SE, Agger A, Gregersen S, Colombo M, Xiao J, Hermansen K. Can stevioside in combination with a soy-based dietary supplement be a new useful treatment of type 2 diabetes? An in vivo study in the diabetic goto-kakizaki rat. Rev Diabet Stud. 2006;3(4):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lavigne C, Marette A, Jacques H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol Endocrinol Metab. 2000;278(3):E491–500. [DOI] [PubMed] [Google Scholar]
- 102. Vahouny GV, Adamson I, Chalcarz W, Satchithanandam S, Muesing R, Klurfeld D, Tepper SA, Sanghvi A, Kritchevsky DJA. Effects of casein and soy protein on hepatic and serum lipids and lipoprotein lipid distributions in the rat. Atherosclerosis. 1985;56(2):127–37. [DOI] [PubMed] [Google Scholar]
- 103. Sedaghat A, Shahbazian H, Rezazadeh A, Haidari F, Jahanshahi A, Latifi SM, Shirbeigi E . The effect of soy nut on serum total antioxidant, endothelial function and cardiovascular risk factors in patients with type 2 diabetes. Diabetes Metab Syndr. 2019;13(2):1387–91. [DOI] [PubMed] [Google Scholar]
- 104. Liu Z-M, Chen Y-M, Ho SC, Ho YP, Woo J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: a 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am J Clin Nutr. 2010;91(5):1394–401. [DOI] [PubMed] [Google Scholar]
- 105. Gobert CP, Pipe EA, Capes SE, Darlington GA, Lampe JW, Duncan A. Soya protein does not affect glycaemic control in adults with type 2 diabetes. Br J Nutr. 2010;103(3):412–21. [DOI] [PubMed] [Google Scholar]
- 106. González S, Jayagopal V, Kilpatrick ES, Chapman T, Atkin S. Effects of isoflavone dietary supplementation on cardiovascular risk factors in type 2 diabetes. Diabetes Care. 2007;30(7):1871–3. [DOI] [PubMed] [Google Scholar]
- 107. Ramdath DD, Padhi EM, Sarfaraz S, Renwick S, Duncan A. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients. 2017;9(4):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four UK prospective cohort studies. Diabetes Care. 2012;35(2):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Miraghajani MS, Azadbakht L. Can soy products affect on inflammation level? A review on the current evidence. J Isfahan Med Sch. 2011;29(151):1116–28. [Google Scholar]
- 110. Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, Willett WC, Hu F. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134(7):1806–11. [DOI] [PubMed] [Google Scholar]
- 111. King DN. Dietary fiber, inflammation, and cardiovascular disease. Mol Nutr Food Res. 2005;49(6):594–600. [DOI] [PubMed] [Google Scholar]
- 112. Esposito K, Giugliano DJE. Diet and inflammation: a link to metabolic and cardiovascular diseases. Eur Heart J. 2006;27(1):15–20. [DOI] [PubMed] [Google Scholar]
- 113. Barnes S, Prasain J, D'Alessandro T, Arabshahi A, Botting N, Lila MA, Jackson G, Janle EM, Weaver C. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct. 2011;2(5):235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bellik Y, Boukraâ L, Alzahrani HA, Bakhotmah BA, Abdellah F, Hammoudi SM, Iguer-Ouada M. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: an update. Molecules. 2013;18(1):322–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim JW, Jin YC, Kim YM, Rhie S, Kim HJ, Seo HG, Lee JH, Ha YL, Chang KC. Daidzein administration in vivo reduces myocardial injury in a rat ischemia/reperfusion model by inhibiting NF-kB activation. Life Sci. 2009;84(7-8):227–34. [DOI] [PubMed] [Google Scholar]
- 116. Takaoka O, Mori T, Ito F, Okimura H, Kataoka H, Tanaka Y, Koshiba A, Kusuki I, Shigehiro S, Amami Tet al. Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. J Steroid Biochem Mol Biol. 2018;181:125–32. [DOI] [PubMed] [Google Scholar]
- 117. Wan C, Jin F, Du Y, Yang K, Yao L, Mei Z, Huang W. Genistein improves schistosomiasis liver granuloma and fibrosis via dampening NF-kB signaling in mice. Parasitol Res. 2017;116(4):1165–74. [DOI] [PubMed] [Google Scholar]
- 118. Agrawal A, Cha-Molstad H, Samols D, Kushner I. Overexpressed nuclear factor-κB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPβ and signal transducer and activator of transcription-3. Immunology. 2003;108(4):539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yu J, Bi X, Yu B, Chen D. Isoflavones: anti-inflammatory benefit and possible caveats. Nutrients. 2016;8(6):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Grassi D, Desideri G, Croce G, Tiberti S, Aggio A, Ferri C. Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des. 2009;15(10):1072–84. [DOI] [PubMed] [Google Scholar]
- 121. Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88(1):38–50. [DOI] [PubMed] [Google Scholar]
- 122. Azadbakht L, Nurbakhsh S. Effect of soy drink replacement in a weight reducing diet on anthropometric values and blood pressure among overweight and obese female youths. Asia Pac J Clin Nutr. 2011;20(3):383. [PubMed] [Google Scholar]
- 123. Miraghajani MS, Najafabadi MM, Surkan PJ, Esmaillzadeh A, Mirlohi M, Azadbakht L. Soy milk consumption and blood pressure among type 2 diabetic patients with nephropathy. J Ren Nutr. 2013;23(4):277–8.e1. [DOI] [PubMed] [Google Scholar]
- 124. Taku K, Lin N, Cai D, Hu J, Zhao X, Zhang Y, Wang P, Melby MK, Hooper L, Kurzer M. Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. J Hypertens. 2010;28(10):1971–82. [DOI] [PubMed] [Google Scholar]
- 125. Liu X, Li S, Chen J, Sun K, Wang X, Wang X, Hui R. Effect of soy isoflavones on blood pressure: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2012;22(6):463–70. [DOI] [PubMed] [Google Scholar]
- 126. Jackson RL, Greiwe JS, Schwen R. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr Rev. 2011;69(8):432–48. [DOI] [PubMed] [Google Scholar]
- 127. Colacurci N, Chiàntera A, Fornaro F, de Novellis V, Manzella D, Arciello A, Chiàntera V, Improta L, Paolisso G. Effects of soy isoflavones on endothelial function in healthy postmenopausal women. Menopause. 2005;12(3):299–307. [DOI] [PubMed] [Google Scholar]
- 128. De Leo F, Panarese S, Gallerani R, Ceci L. Angiotensin converting enzyme (ACE) inhibitory peptides: production and implementation of functional food. Curr Pharm Des. 2009;15(31):3622–43. [DOI] [PubMed] [Google Scholar]
- 129. Patten GS, Abeywardena MY, Bennett LE. Inhibition of angiotensin converting enzyme, angiotensin II receptor blocking, and blood pressure lowering bioactivity across plant families. Nutrition. 2016;56(2):181–214. [DOI] [PubMed] [Google Scholar]
- 130. Hügel HM, Jackson N, May B, Zhang AL, Xue CCJP. Polyphenol protection and treatment of hypertension. Phytomedicine. 2016;23(2):220–31. [DOI] [PubMed] [Google Scholar]
- 131. Ørgaard A, Jensen L. The effects of soy isoflavones on obesity. Medicine (Baltimore). 2008;233(9):1066–80. [DOI] [PubMed] [Google Scholar]
- 132. Anderson GH, Moore S. Dietary proteins in the regulation of food intake and body weight in humans. J Nutr. 2004;134(4):974S–9S. [DOI] [PubMed] [Google Scholar]
- 133. Tremblay A, Clinchamps M, Pereira B, Courteix D, Lesourd B, Chapier R, Obert P, Vinet A, Walther G, Chaplais E. Dietary fibres and the management of obesity and metabolic syndrome: the RESOLVE study. Nutrients. 2020;12(10):2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fontaine KR, Yang D, Gadbury GL, Heshka S, Schwartz LG, Murugesan R, Kraker JL, Heo M, Heymsfield SB, Allison D. Results of soy-based meal replacement formula on weight, anthropometry, serum lipids & blood pressure during a 40-week clinical weight loss trial. Nutr J. 2003;2(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Allison DB, Gadbury G, Schwartz LG, Murugesan R, Kraker JL, Heshka S, Fontaine KR, Heymsfield S. A novel soy-based meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial. Eur J Clin Nutr. 2003;57(4):514–22. [DOI] [PubMed] [Google Scholar]
- 136. Anderson JW, Fuller J, Patterson K, Blair R, Tabor A. Soy compared to casein meal replacement shakes with energy-restricted diets for obese women: randomized controlled trial. Metabolism. 2007;56(2):280–8. [DOI] [PubMed] [Google Scholar]
- 137. Penza M, Montani C, Romani A, Vignolini P, Pampaloni B, Tanini A, Brandi M, Alonso-Magdalena P, Nadal A, Ottobrini L. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology. 2006;147(12):5740–51. [DOI] [PubMed] [Google Scholar]
- 138. Davis J, Higginbotham A, O'Connor T, Moustaid-Moussa N, Tebbe A, Kim Y-C, Cho KW, Shay N, Adler S, Peterson Ret al. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann Nutr Metab. 2007;51(1):42–52. [DOI] [PubMed] [Google Scholar]
- 139. Kok L, Kreijkamp-Kaspers S, Grobbee DE, Lampe JW, van der Schouw Y. Soy isoflavones, body composition, and physical performance. Maturitas. 2005;52(2):102–10. [DOI] [PubMed] [Google Scholar]
- 140. St-Onge M-P, Claps N, Wolper C, Heymsfield S. Supplementation with soy-protein−rich foods does not enhance weight loss. J Am Diet Assoc. 2007;107(3):500–5. [DOI] [PubMed] [Google Scholar]
- 141. Wagner JD, Jorgensen MJ, Cline JM, Lees CJ, Franke AA, Zhang L, Ayers MR, Schultz C, Kaplan JR. Effects of soy vs. casein protein on body weight and glycemic control in female monkeys and their offspring. Am J Primatol. 2009;71(9):802–11. [DOI] [PubMed] [Google Scholar]
- 142. Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: a longitudinal randomized clinical trial. Diabetes Care. 2008;31(4):648–54. [DOI] [PubMed] [Google Scholar]
- 143. Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8(12):754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–12. [DOI] [PubMed] [Google Scholar]
- 145. Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, Hing C, Kwok CS, Pang C, Harvey I. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14(8):iii, ix–xi, 1-193. [DOI] [PubMed] [Google Scholar]
- 146. Savović J, Weeks L, Sterne JA, Turner L, Altman DG, Moher D, Higgins J. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. 2014;3(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hartling L, Ospina M, Liang Y, Dryden DM, Hooton N, Seida JK, Klassen T. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ. 2009;339:b4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Delaney J, Cui R, Engel A. Risk of bias judgements and strength of conclusions in meta-evidence from the Cochrane Colorectal Cancer Group. Syst Rev. 2019;8(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Impellizzeri FM, Bizzini M. Systematic review and meta-analysis: a primer. Int J Sports Phys Ther. 2012;7(5):493. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.