ABSTRACT
Almost 40% of the adult population in the USA will be diagnosed with cancer in their lifetime. Diet is a modifiable factor which is known to affect cancer risk and recurrence. Yet, little is known about how diet influences cancer treatment outcomes. Intermittent fasting, characterized by periods of abstaining from foods and beverages alternated with periods of ad libitum intake, when adopted in the context of chemotherapy, has shown promise in preclinical models resulting in decreased vomiting, diarrhea, visible discomfort, and improved insulin sensitivity and efficacy of chemotherapeutic treatment. Although intermittent fasting during receipt of chemotherapy has been well-established in preclinical models, limited numbers of human studies are now being reported. This review aims to survey the current data examining the effect of intermittent fasting on chemotherapy efficacy, patient treatment outcomes, patient centered outcomes, and circulating biomarkers associated with cancer. Available data show that periodic fasting, a form of intermittent fasting, may hold potential to improve the effectiveness of chemotherapy, decrease treatment-related side effects and cancer-promoting factors such as insulin, while ameliorating treatment-related decreases in quality of life and daily functioning. Larger controlled periodic fasting trials, including exploration of alternate forms of intermittent fasting, are needed to better elucidate the effect of intermittent fasting on treatment and patient outcomes during chemotherapy.
Keywords: intermittent fasting, fasting mimicking diet, short-term fasting, cancer, chemotherapy
Statement of Significance: This article reviews the most current data in intermittent fasting during chemotherapy, elucidates current gaps in clinical research, and introduces future directions for utilizing fasting as adjunct intervention during chemotherapy.
Introduction
It is estimated annually in the USA over 1.8 million people will receive a new cancer diagnosis and >600,000 will not survive the disease (1). Almost 40% of men and women will be diagnosed with cancer at some point in their lifetime. It is estimated that smoking, excess body weight, physical inactivity, excess alcohol consumption, and poor nutrition accounts for ∼18% of new cancer cases and 16% of cancer deaths annually in the USA (2). Maintaining a healthy body weight, being physically active, and adopting a primarily plant-based diet low in red and processed meats, simple sugars, refined carbohydrates, while limiting alcohol can positively influence cancer prevention (2–4). However, there is little evidence on how diet may affect patient outcomes and chemotherapy efficacy. Yet, diet may be a key nonpharmacological intervention to improve cancer treatment efficacy and patient outcomes.
Standard recommendations during chemotherapy from the European Society for Clinical Nutrition and Metabolism suggest regular nutrient intake (25–30 kcal/[kg·d] and 1–1.5 g protein/[kg·d]) for all cancers (5). This current approach is thought to avoid cancer cachexia, the irreversible loss of fat and lean tissue, which results in a reduced ability to fight infection, withstand toxicity of cancer treatment, and is associated with poor prognosis (6). However, current standard care may be outdated for specific types of cancers such as breast, ovarian, and prostate, because these cancers have low risk of cachexia and often result in weight gain during and after treatment (7, 8). Furthermore, in preclinical models, a caloric deficit extended longevity, decreased overall tumor incidence, decreased tumor cell proliferation and metastasis, and increased tumor cell death in mammary, prostate, brain, hepatic, and pancreatic cancers by means of improved glucose regulation and reduced growth factors (9–12). Thus, nonpharmacological diet management may be important in improving survivability for some cancers.
Other preclinical evidence suggests that interventions targeting caloric intake during chemotherapy may influence treatment outcomes and treatment-related side effects (9, 13–16). These outcomes and side effects, including quality of life, fatigue, weight gain, glucoregulation, inflammation, and growth factor biomarkers, are relevant to both prognosis and survival (Figure 1). Intermittent fasting is an emerging nonpharmacological diet intervention that combines periods of caloric fasting with periods of ad libitum eating. Periodic fasting is one type of intermittent fasting that can be broken into 2 paradigms: short-term fasting (STF; subjects abstain from food before and after treatment for a total of 24–120 h) and the fasting mimicking diet (FMD; very low-calorie low-protein diet 1-wk per mo). Periodic fasting in in vitro and in vivo murine models demonstrates that this type of fasting may protect normal cells from oxidative stress, while decreasing cancer proliferation and increasing tumor cell death during chemotherapy treatment (17–24). It is hypothesized that this protection is due to a phenomenon termed differential stress resistance (DSR) in which intermittent fasting results in a state of nutrient deprivation that is thought to suppress tumor growth and proliferation while enabling an intact “stress response” in healthy cells (19, 24) (Figure 2). This stress response boosts metabolic pathways toward maintenance, decreasing the accumulation of cellular damage, and enhancing reproductive fitness in normal healthy cells (24). Cancer cells, however, are unable to induce the same stress resistance response therein selectively increasing the effectiveness of chemotherapy (24). The aim of this review is to examine the state of the science regarding intermittent fasting as an adjunct intervention during chemotherapy for various cancer types. Specifically, we will examine current data on treatment- and patient-related outcomes, possible underlying mechanisms of effect, and discuss directions for future research.
FIGURE 1.
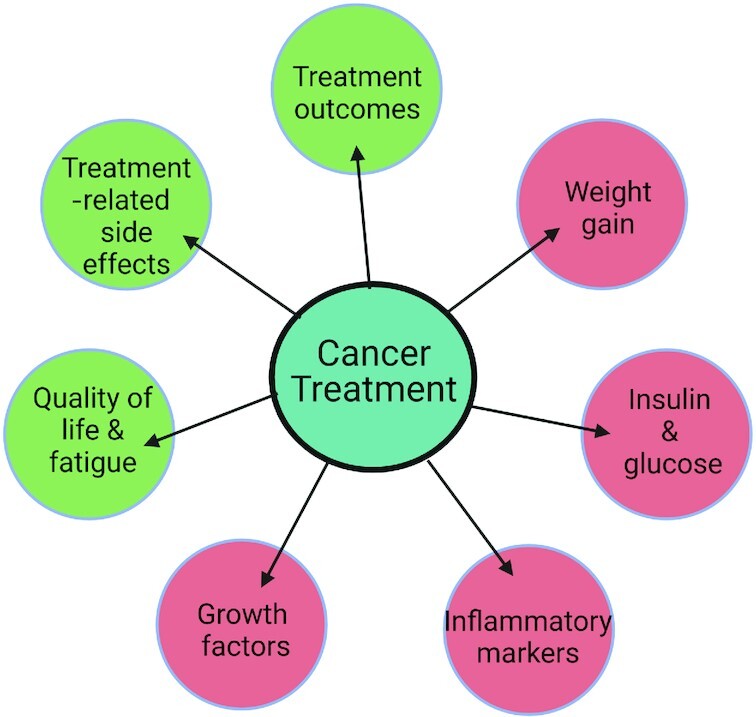
Possible treatment effects of intermittent fasting during chemotherapy. Green circles will potentially increase with fasting. Red circles will potentially decrease with fasting.
FIGURE 2.

Differential Stress Resistance model. The possible mechanisms associated with the differential stress resistance theory. AKT, protein kinase B; IGF-1, insulin-like growth factor 1; IGFBP, insulin-like growth factor binding protein; mTOR, mechanistic target of rapamycin; RAS, rat sarcoma virus.
Methods
A PubMed search was conducted using the following key words: “chemotherapy,” “chemotherapy treatment,” “cancer,” “cancer treatment,” “fasting,” “short-term fasting,” “time restricted eating,” “time restricted feeding,” “intermittent fasting,” “fasting mimicking diet,” “periodic fasting,” “alternate day fasting,” “alternate day modified fasting,” “5:2 diet,” “intermittent energy restriction,” “clinical trial,” “human.” Inclusion criteria for research articles were as follows: 1) adult male and female participants, 2) fasting during cancer treatment, 3) endpoints that included changes in treatment-related outcomes, patient-related outcomes, circulating biomarkers for insulin sensitivity, or cancer-promoting growth factors. The following exclusion criteria were applied: 1) cohort and observational studies, 2) fasting performed as a religious practice (Ramadan or Seventh Day Adventist). Our search revealed 8 human trials (Table 1) in intermittent fasting as an adjunct to chemotherapy, 2 of these studies examined the fasting mimicking diet and 6 examined short-term fasting. The majority of the studies in this emerging area are feasibility and pilot studies; as such, we will also mention a case series report to present the full breadth of data currently available. Due to the novelty of this area of research, most of the studies presented are underpowered to make conclusions about the true effect of fasting during chemotherapy, therefore, this review aims to present a clear picture of what is currently known and future directions for this emerging therapy.
TABLE 1.
Characteristics of current studies examining the effect of intermittent fasting during chemotherapy in patients with cancer1
| Author | Sample size and allocation | Type of cancer and treatment | Fasting treatment and trial design | Age, y | BMI | Menopausal status | Adherence |
|---|---|---|---|---|---|---|---|
| Short-term fasting | |||||||
| Bauersfeld et al., 2018 (40) | n = 34 female block randomization with sealed envelope allocation, allocation nonblinded | breast or ovarian cancers various forms of chemotherapy 6 cycles of chemotherapy | A. STF (36 h before, 24 h after) then normocaloric (n = 18)B. Normocaloric then ST (n = 16)randomized crossover trial | A. 49.8B. 53.6 | A. <25: 925 to <30: 7≥30: 2B. <25: 1025 to <30: 6≥30: 0 | A. Pre: 72.2% Post: 27.8%B. Pre: 68.7%Post: 31.3% | 102 cycles fasted 74 cycles normocaloric 5 patients didn't want to switch to normocaloric after first arm |
| de Groot et al., 2015 (41) | n = 13 femalerandomized | HER2-negative stage II/III breast cancer receiving neoadjuvant TAC (docetaxel/doxorubicin/cyclophosphamide)dexamethasone use | A. STF (24 h before and after) (n = 7)B. Control (n = 6)randomized control trial | A. 51B. 52 | A. 25.5B. 23.8 | — | 2 participants dropped out in the STF group due to neutropenia and pyrosis, did not resolve with normal diet |
| Dorff et al., 2016 (29) | n = 20 male and female | cancer diagnosis prescribed platinum-based chemotherapy without radiation, curative or palliative dexamethasone use and 5HT3 inhibitors | A. 24 h STF (n = 6)B. 48 h STF (n = 7)C. 72 h STF (48 h before/24 h after) (n = 7)nonrandomized, feasibility trial, no control | 61 | ≥20.5 | A. 4/6 compliantB. 5/6 compliantC. 4/7 compliant | |
| Riedinger et al., 2020 (30) | n = 20 femalenonblinded randomized | Gynecologic cancers with ≥6 cycles of chemotherapyMultiagent chemotherapy (bevacizumab, carboplatin, cisplatin, docetaxel, doxorubicin, gemcitabine, and paclitaxel), ≥2 participants neoadjuvant therapy | A. STF (24 h before and after) (n = 10)B. Control (n = 10) – balanced, normocaloric dietRandomized control trial | A. 59.5B. 59.0 | A. 27.69B. 29.06 | 9% dropout rate in the fasting group. No compliance data given. Serum glucose tested to indicate adherence | |
| Safdie et al., 2009 (23) | n = 10 male and female | Neoplasia of breast, esophagus, prostate, lung, uterus, ovary | Voluntarily fasted (48–140 h prior to and 5–56 h following treatment) during treatmentCase series report | 61 | — | — | — |
| Zorn et al., 2020 (36) | n = 30 femaleselectively assigned (nonrandomized) | Breast, endometrial, ovarian, or cervical cancer, first diagnosis/recurrence all stages, neo or adjuvant chemotherapy, ≥4 cycles | A. mSTF/NC (n = 7)B. NC/mSTF (n = 9)C. KD + mSTF/NC (n = 1)D. NC/KD + mSTF (n = 13)Crossover2–3 cycles of CTX of intervention and control | 54 | 26 | — | 41% dropout rate71.4% of fasting participants had physiological blood ketosis detected“difficult”: 1 “quite difficult”: 11 “quite easy” or “easy”: 9 |
| Fasting mimicking diet | |||||||
| de Groot et al., 2020 (22) | n = 129 femaleblock randomization | HER-2 negative stage II/III breast cancer, neoadjuvant FEC-T or AC-T chemotherapy. Dexamethasone dc in FMD group | A. FMD (n = 65)B. Control (n = 64)Diet was 3 d prior to and the day of treatment. Randomized control trial, observer blind | 49 | 25.7 | Pre/peri: 41.5%Post: 58.5% | All cycles: 21.5%4 cycles: 33.8%2 cycles: 50%First cycle: 81.5%Used ITT/per-protocol analysis |
| Lugtenberg et al., 2020 (37) | n = 129 femaleblock randomization | HER-2 negative stage II/III breast cancer, neoadjuvant FEC-T or AC-T chemotherapy | A. FMD (n = 65)B. Control (n = 64) Randomized control trial, observer blindDiet was 3 d prior to and the day of treatment | 49 | 25.7 | Pre/peri: 58.5%Post: 41.5% | All cycles and surgery: 15.4%All cycles: 21.5%Half of cycles: 33.8%First cycle: 81.5% |
AC-T, Adriamycin Cyclophosphamine Taxol regimen; CTX, Cyclophosphamide; FEC-T, Fluorouracil-Epirubicin-Cyclophosphamide-Docetaxel; FMD, fasting mimicking diet; ITT, intention to treat; KD + STF, ketogenic diet combined with short-term fasting (KD 6 d prior to STF); mSTF, modified short-term fasting (<25% of energy needs); NC, normocaloric diet; STF, short-term fasting; TAC, Taxotere-Adriamycin-Cytoxan regimen
Intermittent Fasting and Efficacy of Cancer Treatment
In cell culture, neuroblastoma, breast, ovarian, mesothelioma, lung, and colorectal cancers cells exposed to cycles of fasting had delayed growth and progression of the cancer cells as well as increased effectiveness of the chemotherapeutic drugs (24, 25). Preclinical in vitro models reported that fasting had antiproliferative effects, delaying the progression of tumors, and increasing the effectiveness of chemotherapeutic drugs in melanoma, glioma, colorectal, and breast cancers (24, 26). In preclinical murine models of breast cancer, glioma, and neuroblastoma, healthy cells were protected from oxidative stress, DNA damage, and treatment-related endometrial hyperplasia during high-dose chemotherapy when combined with fasting (24, 26–28). Additionally, in some in vitro and in vivo mouse models, when fasting was combined with chemotherapy, sensitivity to and efficacy of chemotherapeutic drugs in breast cancer and neuroblastoma cells resulted in increased longer-term cancer-free survival (17, 24, 26–28).
The effect of short-term fasting on cancer treatment efficacy has been examined in 2 clinical studies (29, 30) (Table 2). Dorff et al. (29) aimed to determine the optimal length of a short-term fasting window for a future larger scale investigation. Men and women with urothelial, nonsmall cell lung cancer, uterine, breast, or ovarian cancer diagnosis prescribed neoadjuvant or adjuvant platinum-based chemotherapy, were randomly assigned into 3 different fasting groups: 1) 24 h prior to treatment, 2) 48 h prior to treatment, or 3) 72 h surrounding treatment (48 h prior to and 24 h following). No control group was included. After completion of the adjunct fasting protocol 2 patients had complete responses (no cancer cells present), 6 had partial radiographic responses (>30% decrease in sum of all target lesions), 3 had stable disease, and 8 were nonevaluable based on RECIST (Response Evaluation Criteria in Solid Tumors [31]). However, after the completion of the full treatment plan, 5 participants had a pathological complete response of which 4 were from the 72 h fasting group and the other was from the 48 h fasting group (29). Riedinger et al. (30) recruited women with gynecologic cancers with a treatment plan consisting of ≥6 cycles of neoadjuvant or adjuvant chemotherapy and randomly assigned to a short-term fasting group (24 h before and 24 h after treatment) or a standard care control group. There were no differences in partial or complete response between groups. It is possible that the cancer type may impact the degree of recovery in these studies as both gynecological and breast cancer have high survival rates (65% and 90%, respectively [1, 32]). However, these are currently the safest sample to examine the effect of periodic fasting during treatment due to low cancer cachexia risk. Limitations of these initial clinical studies are small sample size and a heterogenous group of cancer diagnosis all with variable clinical outcomes.
TABLE 2.
Treatment efficacy and side effects of intermittent fasting during chemotherapy in patients with cancer1
| Author | Subjects | Treatment and design | Treatment efficacy | GI adverse effects | Neurological adverse effects | Quality of life |
|---|---|---|---|---|---|---|
| Short-term fasting | ||||||
| Bauersfeld et al., 2018 (40) | n = 34 females with gynecologic cancers, various forms of chemotherapy | A. Group A: STF (36 h before, 24 h after)/ControlB. Group B: control/STFrandomized crossover trial, 6 cycles of chemotherapy | A. ↑ FACT-G, FACIT F, FACIT-F TOI during fasting cycles. ↑QOL during fasting and decreased during control cyclesB. Ø between cycles Ø difference between fasting cycles | |||
| de Groot et al., 2015 (41) | n = 13 HER2-negative stage II/III breast cancer (histologically confirmed) receiving neoadjuvant TAC (docetaxel/doxorubicin/cyclophosphamide) | A. STF (24 h before and after)B. Control (follow healthy nutrition guidelines with a minimum of 2 pieces of fruit per day) | Within-group change γ-H2AX intensity↑CD45 + CD13- myeloid cells (30 min) in control only*↑CD45 + CD3 + lymphocytes (7 d) in control only*Treatment-related side effects in STF↑ FT4*↑ Erythrocyte counts (D7, D21) *↑ Thrombocyte (D7) * | Ø differences between groups in grade I/II or III/IV grade toxicities using the CTCAE | Ø differences between groups in grade I/II or III/IV grade toxicities using the CTCAE | |
| Dorff et al., 2016 (29) | n = 20 males and females with cancer diagnosis prescribed platinum-based chemotherapy w/out radiation, curative or palliative | A. 24 h STF (n = 6)B. 48 h STF (n = 7)C. 72 h STF (48 h before/24 h after) (n = 7)Nonrandomized, no control | For all groups after chemotherapy: 2 pathological complete response, 6 partial radiographic response, 3 stable diseaseØ difference in olive moments (indicating DNA damage) based on Comet assay | ↓ grade I/II nausea and vomiting (test for trend)**Toxicity graded using CTCAE | ||
| Riedinger et al., 2020 (30) | n = 20 females with gynecologic cancers with ≥6 cycles of chemotherapy Multiagent chemotherapy (bevacizumab, carboplatin, cisplatin, docetaxel, doxorubicin, gemcitabine, and paclitaxel), ≥2 participants neoadjuvant therapy | A. STF (24 h before and after)B. ControlRandomized control trial | Ø difference in complete or partial response between groups**↑ Platelet count in the STF group at week 18** | Ø difference in treatment-related toxicities via CTCAE** | Ø difference in treatment-related toxicities via CTCAE** | Ø difference between groups in mean NCCN-FACT FOSI-18 score **↑ QOL# in fasting group from first to last cycle in the fasting group compared to control |
| Zorn et al., 2020 (36) | n = 30; females with gynecologic cancers, all stages, neo or adjuvant chemotherapy | A. mSTF or KD + mSTFB. ControlCrossover, 2–3 cycles of CTX of intervention and control | Treatment-related side effects in STF↓MCH*↓MCV*↓Sodium*↑Uric acid*↓ fT3*↑Ft4* | mSTF and KD + mSTF had↓frequent grade I/II stomatitis*↓headaches* *↓total toxicities* measured by CTCAE | Ø score difference between group on EORTC QLQ-C30, EORTC QLQ-CIPN20 or FACIT-F | |
| Fasting mimicking diet | ||||||
| de Groot et al., 2020 (22) | n = 129 patients with HER-2 negative stage II/III breast cancer, neoadjuvant FEC-T or AC-T chemotherapy | A. FMD (n = 65)B. Control (n = 64)Diet was 3 d prior to and the day of treatment. Randomized control trial | Ø difference in toxicity of treatment↑ radiological complete or partial response in pts following FMD*Miller and Payne 4/5 pathological response more likely in those compliant (per-protocol) FMDCD45 + CD3 + lymphocytes (7 d) ↑ in control and ↓ in FMD compliant (per protocol)* | Ø score difference between group on EORTC QLQ-C30 | ||
| Lugtenberg et al., 2020 (37) | n = 129 patients with HER-2 negative stage II/III breast cancer | A. FMD (n = 65)B. Control (n = 64)Diet was 3 d prior to and the day of treatment. Randomized control trial | Ø differences between groupsPer-protocol analysis: less fatigue, nausea, and insomnia in those adherent to FMD* | Ø in QLQ-C30 or QLQ-BR23 between groupsPer-protocol analysis: improved physical, role, emotional, cognitive, and social functioning in those adherent to FMD*↓fatigue & dyspnea # in the FMD complaint 6 mo after treatment | ||
Ø no change, *Significant change within group, P <0.05; **significant difference between groups at endpoint, no time interaction, P <0.05; #significant group × time interaction, P <0.05. AC-T, Adriamuycin Cyclophosphamine Taxol regimen; CIPN, Chemotherapy-induced peripeheral neuropathy; CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; CTX, Cyclophosphamide; EORTC, European Organisation for Research and Treatment of Cancer; FACIT-F, Functional assessment of chronic illiness therapy fatigue; FACIT-G, Functional assessment of chronic illiness therapy general; FEC-T, Fluorouracil-Epirubicin-Cyclophosphamide-Docetaxel; FMD, fasting mimicking diet; KD + STF, ketogenic diet combined with short-term fasting (KD 6 d prior to STF); MCH, Mean corpuscular hemoglobin; MCV, mean corpuscular volume; mSTF, modified short-term fasting (<25% of energy needs); NCCN - FACIT FOSI 18, National comprehensive cancer network functional treatment of cancer therapy, Ovarian cancer symptom index; QOL, quality of life; QLQ, Quality of life questionaire; STF, short-term fasting; TAC, Taxotere-Adriamycin-Cytoxan regimen; TOI, Trial outcome index.
One study has evaluated the effect of the fasting mimicking diet on treatment efficacy during chemotherapy (22) (Table 2). The DIRECT (Dietary Restriction as an Adjunct to Neoadjuvant ChemoTherapy for HER2 Negative Breast Cancer) trial by de Groot et al. (22) examined the fasting mimicking diet as an adjunct to neoadjuvant chemotherapy in women with HER2-negative stage II/III breast cancer. In the per-protocol analysis, participants that were adherent to the fasting mimicking diet protocol had a significantly increased rate of complete or partial radiological response. Moreover, a 4/5 Miller and Payne pathological response (>90% loss of tumor cells/no invasive carcinoma) was also more likely in those that were adherent to the fasting mimicking diet than those who were not adherent or the controls. De Groot et al. (22) also reported a decrease in chemotherapy-induced DNA damage to normal cells in the fasting mimicking diet group. Current trials lack a homogeneous large sample size in order to expound the effect on treatment outcomes. Therefore, larger studies controlling for cancer and treatment type, dose intensity, and adherence to the protocol are needed to determine the true effect of short-term fasting or the fasting mimicking diet on chemotherapy.
Intermittent Fasting and Treatment-related Adverse Effects
Side effects are common in patients receiving chemotherapy for cancer treatment. A prospective cohort study by Pearce et al. (33) examined the incidence and severity of self-reported side effects among 478 patients receiving adjuvant chemotherapy for breast, colorectal, or lung cancer. The authors reported that 86% of participants experienced ≥1 adverse effect during the study period with fatigue, diarrhea, and constipation reported most frequently (33). In murine models, fasting in the context of chemotherapy is associated with reduced occurrence of treatment-related side effects including weight loss, decreased activity, diarrhea, leukopenia, and other visible signs of discomfort compared with nonfasting mice (34, 35). This work indicates an area of opportunity for the use of fasting to mitigate side effects related to cancer treatment.
Three studies have examined the effect of short-term fasting on gastrointestinal and neurological adverse effects in the context of cancer treatment with chemotherapy (23, 29, 36) (Table 2). In a 2009 case series of cancer patients who had voluntarily fasted for 48–140 h prior to and 5–56 h following chemotherapy treatment, gastrointestinal adverse effects such as nausea, vomiting, diarrhea, abdominal cramps, and mucositis were nearly absent (23). Furthermore, the investigators reported that patients who both fasted and followed standard care for their specific malignancy (a diet to prevent or reverse nutrient deficiency and preserve lean body mass), reported reduced weakness and fatigue when fasting (23). However, this evidence is anecdotal at best and the reduced vomiting and diarrhea could have been a result of the lack of foodstuffs in the gut. In a feasibility trial by Dorff et al. (29), all participants in the 24 h group reported grade 1 or 2 nausea, 87% in the 48 h group, and 43% in the 72 h group. Grade 1 and 2 vomiting was reported by 83% of the 24 h group, 43% in the 48 h group, and 0% in the 72 h group (29). Constipation was experienced by 50% of the 24 h group, 28% of the 48 h group, and 43% of the 72 h group. However, the 24 h group reported 33% grade 1–2 diarrhea, none was reported in the 48 h group, and 57% reported in the 72 h group. There was only one report of grade 3 diarrhea, which was in the 48 h group. A trial by Zorn et al. (36) reported a decrease in frequency and severity of stomatitis, headaches, weakness, and total toxicities with a 96 h modified short-term fast (<25% of energy needs daily for 96 h, 72 h before and 24 h after chemotherapy treatment) in women with gynecological cancer (36). Additionally, participants in the trial by Zorn et al. (36) experienced better chemotherapy tolerance, reflected by fewer chemotherapy postponements in the modified short-term fasting group.
One study examined the effect of the fasting mimicking diet on gastrointestinal and neurological adverse effects associated with chemotherapy treatment (37) (Table 2). In the per-protocol analysis, Lugtenberg et al. (37) reported that patients who were adherent to the fasting mimicking diet had less fatigue over time when compared with the control and nonadherent group. The per-protocol analysis also reported a between-group difference between those who were adherent to the fasting protocol and the control or those not adherent for self-reported nausea and insomnia (37). However, the intention-to-treat analysis did not report any differences between the control and fasting mimicking diet groups. Due to the high prevalence of treatment-related side effects associated with chemotherapy, future fully powered studies should explore if intermittent fasting may ameliorate common treatment-related side effects.
Intermittent Fasting, Quality of Life, and Daily Functioning During Cancer Treatment
It is possible that the positive effects of intermittent fasting on treatment-related side effects translate to improved patient quality of life and daily functioning while receiving chemotherapy. Four studies have examined the effect of short-term fasting or the fasting mimicking diet during chemotherapy on quality of life. Validated self-report questionnaires were utilized in all studies presented. Quality of life and function were measured by validated questionnaires from The European Organization for Research and Treatment of Cancer Core 30 (EORTC QLQ-30 [38]) or the Functional Assessment of Chronic Illness Therapy (FACIT [39]).
Three studies have examined the effect of short-term fasting on quality of life in those undergoing chemotherapy for cancer (36, 40) (Table 2) but report conflicting findings. Zorn et al. (36) reported no improvement in patient-reported quality of life (EORTC QLQ-C30), chemotherapy-induced polyneuropathy (EORTC-QLQ-CIPN20), or fatigue scores (FACIT-f) within the short-term fasting group over time, when compared with standard care, or when combined with 6 d of a ketogenic diet prior to the fasting period. Bauersfeld et al. (40) performed a 2 × 2 crossover trial in women with breast and ovarian cancers. Participants either followed a 60 h short-term fast (36 h prior to and 24 after treatment) or a normocaloric diet for the first 3 cycles of chemotherapy, then alternating to the other diet protocol the following 3 cycles. Group A performed short-term fasting in the first 3 cycles and normocaloric diet the last 3 cycles. Overall quality of life (FACIT-G) and fatigue (TOI FACIT-F) scores were statistically higher during the fasting cycles than the normocaloric cycles in Group A. No differences in quality of life and fatigue scores were reported between diet protocols in Group B, which fasted second. Although authors report no carryover effect, this discrepancy may suggest that order of intervention or temporal intensity of chemotherapy may act as confounding variables for quality of life and fatigue scores. When analyzing all participants together, independent of diet sequence, significant differences in quality of life were observed with 60 h short-term fasting compared with the normocaloric diet, in which the latter group was observed to have a decrease in quality of life. Both Groups A and B reported smaller increases in fatigue from baseline during fasting cycles compared with larger increases in fatigue during the normocaloric diet period. Lastly, Riedinger et al. (30) measured a disease-related symptom profile, treatment side effects, general function, and well-being using NCCN-FACT-OSI-18 (National Comprehensive Cancer Network Functional Assessment of Cancer Therapy, Ovarian Cancer Symptom Index). They reported no difference in any outcome from the first to the last cycle when the fasting group was compared with the control. Adequately powered randomized control trials are needed to determine if short-term fasting may ameliorate the decreases in treatment-related quality of life and fatigue while considering timing of treatment-related adverse effects, the degree of fasting (both total hours and caloric allowances), as well as the impact of different chemotherapeutic drugs.
One study by Lugtenberg et al. (37) examined the effect of the fasting mimicking diet on quality of life during chemotherapy treatment of HER-2 negative stage II/III breast cancer (Table 2). No change in quality of life was reported via the EORTC QLQ-30 for general cancer patients or EORTC QLQ-BR23 specifically for breast cancer (37). However, in the per-protocol analysis, among those who were adherent to the diet, scores for physical, role, emotional, cognitive, and social functioning were improved over time (37). Only fatigue and dyspnea were improved 6 mo after surgery in those who were adherent to the fasting mimicking diet group versus the normal diet control over time. Although periodic fasting does not seem to increase baseline quality of life scores during treatment, it may ameliorate the decrease in quality of life and daily functioning during treatment, a common patient-related outcome. However, more data are needed to determine how fasting affects quality of life, fatigue, and daily functioning among cancer patients during chemotherapy treatment.
Intermittent Fasting and Impact on Cancer-promoting Biomarkers During Treatment
The DSR hypothesis (Figure 2) is thought to be driven by improved glucose control and a decrease in growth factors such as IGF-1 (insulin-like growth factor). Further, IGFBP (insulin-like growth factor binding protein) is increased which aids in the sequestration of these proliferative growth promoters. In preclinical murine models of liver-specific IGF-1-deficient mice or hormone receptor positive mice with breast cancer, short-term fasting and the fasting mimicking diet reduced glucose, insulin, and IGF-1 significantly (27, 28). Reductions in c-peptide and leptin, associated with reduced insulin and breast cancer growth, respectively, are also reported in murine models during 72 h short-term fasting and the fasting mimicking diet. Furthermore, during chemotherapy, adiponectin concentrations are increased in fasted mice which is believed to inhibit cancer cell growth and metastasis via the activation of the AMP-activated protein kinase (AMPK) pathway (28).
Glucose regulation
Four studies have examined the effect of short-term fasting on glucose and/or insulin (29, 30, 36, 41) during chemotherapy (Table 3). In a study by de Groot et al. (41), the effect of a 24 h fast before and after chemotherapy infusion among HER 2-negative stage II/III breast cancer patients was examined. Glucose increased significantly in both the short-term fasting and control groups, yet, insulin increased in the control group only (41). However, at baseline, circulating insulin concentrations in the control group were extremely low and increased to within normal range; it is unclear if this is the result of control treatment or reporting error (41). Zorn et al. (36) did not measure glucose but did report a decrease in insulin in the modified short-term fasting group (participants were permitted 25% or less of their daily energy needs on the fasting days). Conversely, Riedinger et al. (30) reported a decrease in glucose but no change in insulin in the short-term fasting group (24 h before and after chemotherapy infusion). Dorff et al. (29) examined the effect of different lengths of fasting during platinum-based neoadjuvant and adjuvant chemotherapy on glucose and insulin. Although the 24 h, 48 h, and 72 h short-term fasting regimens did not alter glucose, fasting insulin decreased by 56%, 27%, and 42%, respectively, in the 3 groups. One study examined the effect of the fasting mimicking diet on glucose and insulin during neoadjuvant chemotherapy for HER2-negative stage II/III breast cancer. In the per-protocol analysis, de Groot et al. (22) reported a decrease in both fasting glucose and fasting insulin in participants that were adherent to the diet compared with those that were nonadherent. There was no change in glucose or insulin between groups over time in the intention-to-treat analysis. The efficacy of short-term fasting and the fasting mimicking diet varies across current pilot data likely due to the small sample sizes, variation in length of the fasting period, as well as differences in energy intake during the fasting period. From these preliminary data, intermittent fasting may indeed improve circulating insulin and glucose. However, whether intermittent fasting can improve insulin sensitivity, supporting the DSR hypothesis, has yet to be confirmed. Therefore, more research is needed utilizing indirect insulin sensitivity markers such as glycosylated hemoglobin (HbA1C) or HOMA-IR calculation as well as direct measures of insulin sensitivity such as the Matsuda index, intravenous glucose tolerance test, or hyperinsulinemic euglycemic clamp.
TABLE 3.
Effect of intermittent fasting on circulating biochemical markers during chemotherapy in patients with cancer1
| Author | Subjects | Treatment | Glucose | Insulin | IGF-1 | IGFBP |
|---|---|---|---|---|---|---|
| Short-term fasting | ||||||
| de Groot et al., 2015 (41) | n = 13 HER2-negative stage II/III breast cancer receiving neoadjuvant TAC | A. STF (24 h before and after)B. Control | A. ↑*B. ↑* | A. ØB. ↑* | A. ↓*B. Ø | A. ØB. Ø |
| Dorff et al., 2016 (29) | n = 20 men and women with cancer diagnosis prescribed platinum-based chemotherapy | A. 24 h STF (n = 6)B. 48 h STF (n = 7)C. 72 h STF (48 h before/24 h after) (n = 7) | A. Ø | A. ↓ 56%B. ↓27%C. ↓42%No significant between-group differences | A. ↓ 30%B. ↓33%C. ↓8%No significant between-group differences | A. ↑23%B. ↑10%C. ↑117% |
| Riedinger et al., 2020 (30) | n = 20 women with gynecologic cancers with ≥6 cycles of chemotherapy | A. STF (24 h before and after)B. ControlRandomized control trial | A. ↓** at time of chemotherapy | NA | NA | NA |
| Zorn et al., 2020 (36) | n = 30; women with gynecologic cancers, all stages, neo or adjuvant chemotherapy | A. mSTF OR KD + mSTFB. Control Crossover, 2–3 cycles of CTX of intervention and control | NA | A. ↓** | A. ↓** | NA |
| Fasting mimicking diet | ||||||
| de Groot 2020 (22) | n = 129 patients with HER-2 negative stage II/III breast cancer | A. FMD (n = 65)B. Control (n = 64)Diet was 3 d prior to and the day of treatment. Randomized control trialDexamethasone dc in FMD group | Ø differences between groupsPer-protocol: A. ↓ in compliant only* | Ø differences between groupsPer-protocol:A. ↓ in compliant only* | Ø differences between groupsPer-protocol: A. ↓ in compliant only* | |
Ø, no; NA, did not measure; *Significant change within group, P <0.05; **significant difference between groups at endpoint, no time interaction, P <0.05; #significant group × time interaction, P <0.05. CTX, Cyclophosphamide; FMD, fasting mimicking diet; IGF-1, insulin like growth factor 1; IGFBP, insulin like growth factor binding protein; KD + STF, ketogenic diet combined with short-term fasting (KD 6 d prior to STF); mSTF, modified short-term fasting (<25% of energy needs); STF, short-term fasting; TAC, Taxotere-Adriamycin-Cytoxan regimen.
Cancer biomarkers
Three studies have examined the effect of short-term fasting on biomarkers associated with cancer promotion (29, 36, 41) (Table 3). Although IGFBP did not appear to be altered after short-term fasting compared with standard care, both de Groot et al. (41) and Zorn et al. (36) reported a significant decrease in IGF-1 in the short-term fasting group with no change in the standard care group. Dorff et al. (29) reported changes in both IGF-1 and IGFBP in cancer patients prescribed neoadjuvant or adjuvant platinum-based chemotherapy who were assigned to 24 h, 48 h, or 72 h short-term fasting. IGF-1 decreased 30% in the 24 h group, 33% in the 48 h group, and 8% in the 72 h group (29). IGFBP increased 23% in the 24 h group, 10% in the 48 h group, and 117% in the 72 h group (29). Dorff et al. (29) also measured the ketone β-hydroxybutyrate, as ketone bodies are elevated during the absence of food and have been associated with the inhibition of colon and breast cancer cells (42). They reported an increase in β-hydroxybutyrate in both the 48 h and 72 h fasting groups and a decrease in the 24 h group (29).
One study examined the effect of the fasting mimicking diet during neoadjuvant chemotherapy on cancer-promoting biomarkers in women with HER2-negative stage II/III breast cancer (22) (Table 3). In the per-protocol analysis, de Groot et al. (22) reported a decrease in IGF-1 in participants that were adherent to the diet. It is currently unclear if this decrease was due to the caloric restriction, the low-protein diet prescription, or both. It is possible that fasting may either directly decrease growth factors during treatment or indirectly decrease growth factors by increasing IGFBP, which sequesters IGF-1. However, low-protein diets, such as that of the macronutrient distribution of the fasting mimicking diet, have also been associated with decreases in IGF-1 and increases in IGFBP. Future studies should consider the possible confounding effect of nutrient intake during the eating period of the fasting protocol.
Limitations and Future Directions
Although data is limited, short-term fasting and the fasting mimicking diet do show promise as an emerging nutrition therapy during chemotherapy for cancers with a low risk of cancer cachexia. The most notable limitations for the adoption of these diets as medical nutrition therapy are limited efficacy data, a low patient adherence, and high dropout rate (Table 1) (22, 29, 40). Improvements in patient and treatment outcomes are dependent on diet adherence. For instance, de Groot et al. (22) reported patient adherence to the fasting mimicking diet for each of the 4 fasting/treatment cycles. In the first round 82% of participants were adherent to the diet, however, this decreased rapidly with each subsequent cycle with only 20% of participants adherent to all 4 cycles (22). In their intention-to-treat analysis, significant differences between the fasting mimicking diet group and the standard care group were only seen for radiological response (22). However, in the per-protocol analysis, Miller and Payne score, glucose, insulin, IGF-1, and DNA damage were significantly improved in participants adherent to the fasting mimicking diet (22). In a follow-up with 24 participants from a trial by Zorn et al. (36), 1 participant declared the diet was “too difficult,” 11 declared “quite difficult,” and 9 as “easy” or “quite easy.” Further, only about half said they would fast again during chemotherapy (36). An additional limitation of periodic fasting for treatment during chemotherapy is that nutrition interventions where food is absent for multiple days increases the patient burden and may have other untoward adverse side effects such as weakness, hypoglycemia, hyponatremia, and hypotension (29, 40). Fully powered studies should continue to determine if periodic fasting may benefit patients with cancers with low risk of cachexia and increased risk of weight gain such as breast, ovarian, and prostate cancer. Further, the utilization of continuous glucose monitors and at home blood pressure cuffs could be applied to maximize safety and monitor possible diet-related adverse effects. It is also currently unclear what participants ate outside the fasting window in any of the aforementioned studies. Food intake data would account for the confounding effect of dietary intake and quality, outside of fasting, on outcome measures. Larger clinical trials utilizing both short-term fasting and the fasting mimicking diet should be examined to assess their feasibility, acceptability, if these diets can improve patient quality of life or the effectiveness of chemotherapeutic drugs despite their current low adherence rates and potential of increased burden to the patient. Further, all current data examines the effect of short-term fasting and the fasting mimicking diet surrounding each chemotherapy infusion, a follow-up period after treatment has yet to be explored. Future studies should also examine if periodic fasting could be adopted during survivorship to impact cancer recurrence.
As previously stated, most studies were conducted as pilot studies and thus, sample size was low and statistical power minimal. However, some studies were able to show some outcomes with statistical significance (Tables 2 and 3). Several studies used rigorous methods to eliminate bias, randomly assigning participants, sealed envelope allocation, and blinding when possible (Table 1). Objective outcomes were often measured using validated questionnaires such as the FACT (Functional Assessment of Cancer Therapy) and CTCAE (Common Terminology Criteria for Adverse Events) for toxicity measures. Overall, there was heterogeneity among cancer type, stages, and treatment therapy within the studies, a limitation adequately noted by study authors. However, in women, breast cancer was well represented across all studies evaluated, lending a more robust picture to the fasting interventions at this cancer site.
Time-restricted eating (TRE) is a popular form of intermittent fasting where individuals extend the overnight fasting period (14–20 h) and shorten the daily eating window (4–10 h). Prolonged nightly fasting has been associated with decreased risk of cancer recurrence and decreased inflammatory markers, a known risk pathway for several cancers (43–45). Additionally, although there are no restrictions on types or quantity of food, TRE appears to naturally decrease daily energy intake by 20% with current data suggesting a 2–4% decrease in body weight after 12–16 wk of 8–10 h TRE (46–49). This form of intermittent fasting may hold the potential to improve treatment efficacy and improve patient-centered outcomes during chemotherapy treatment all while maintaining a high adherence. Further, TRE is accessible with no cost to the patient and unlike periodic fasting, allows the individual to eat daily. Clinical trials in healthy adults with overweight and obesity report high adherence with TRE (47, 50–52) unlike forms of periodic fasting. Limited data is available for the long-term effects of TRE on body weight, however, a recent year-long study in healthy low-income women with obesity resulted in the same weight change as an isocaloric hypocaloric control (53). Yet, body fat and waist circumference decreased significantly in the TRE group when compared with the control diet group over time (53). Further, Gill and Panda (47) reported weight loss retention of 4% a year after a 16 wk 10 h TRE intervention in healthy participants with overweight. This may be another added benefit of intermittent fasting as obesity can impair treatment outcomes, increase cancer recurrence, and weight gain is common during and after treatment in certain cancers such as breast, ovarian, and prostate. Additionally, in a crossover study from Jamshed et al. (54) early TRE (intake between 08:00 and 14:00) participants (n = 11) with overweight and obesity reduced mean 24 h glucose concentrations, increased LC3A and ATG12 expression (genes that may enhance autophagy) over 5 d. Mechanistic target of rapamycin (mTOR), a regulatory pathway for cell growth, increased in the evening only, which may be associated with increases in insulin. This increase in both markers may be due to the completion of the eating window at 14:00 or as part of the protective effect upregulated by healthy cells in order to decrease autophagy. Although both IGF-1 and IGFBP decreased, it was not statistically significant, however, the study was not powered a priori on these parameters. TRE offers another alternative form of intermittent fasting for investigation during chemotherapy and should be examined in the context of cancer care (55–57).
Conclusion
Preliminary human studies exploring the potential of intermittent fasting as a nutrition therapy during chemotherapy to improve treatment and patient outcomes are limited yet promising. Short-term fasting and the fasting mimicking diet may decrease treatment-related side effects, improve quality of life, and decrease insulin compared with the current standard care. Further, if the changes in insulin promote improved insulin sensitivity these periods of nutrient deprivation may improve the efficacy of chemotherapy treatment. Fully powered trials of short-term fasting and the fasting mimicking diet are needed and should be tested within the context of different medication protocols and within specific cancer types. Furthermore, other forms of intermittent fasting such as TRE should be rigorously evaluated as a possible adjunct intervention during cancer chemotherapy to improve treatment- and patient-related outcomes.
Acknowledgments
The authors’ responsibilities were as follows—KG and KC: designed and wrote the manuscript; VG, KV, and LT-H: wrote the manuscript; and all authors: read and approved the final manuscript. Graphical Figures 1 and 2 were created with BioRender.com; Agreement number ZW2399HPRZ and WF2399HIJN.
Notes
This work was supported by NIH R01CA257807 (LT-H and KV), R01CA250390 (LT-H), R01CA204808 (LT-H), and R01DK119783 (KV). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author disclosures: Krista Varady received author fees from Hachette Book Group for “The Every Other Day Diet”.
Abbreviations used: DSR, differential stress resistance; EORTC QLQ-C30, The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; FACIT, Functional Assessment of Chronic Illness Therapy; IGF-1, insulin-like growth factor 1; IGFBP, insulin-like growth factor binding protein; TRE, time-restricted eating.
Contributor Information
Kelsey Gabel, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, IL, USA.
Kate Cares, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, IL, USA.
Krista Varady, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, IL, USA.
Vijayakrishna Gadi, Department of Medicine, University of Illinois at Chicago, Chicago, IL, USA; University of Illinois Cancer Center, Chicago, IL, USA.
Lisa Tussing-Humphreys, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, IL, USA; University of Illinois Cancer Center, Chicago, IL, USA.
References
- 1. Institute NC. 27 April, 2018. [Internet]. Available from: https://seer.cancer.gov/statfacts/html/breast.html(accessed 19 March, 2019).
- 2. Rock CL, Thomson C, Gansler T, Gapstur SM, McCullough ML, Patel AV, Andrews KS, Bandera EV, Spees CK, Robien Ket al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245–71. [DOI] [PubMed] [Google Scholar]
- 3. Bail J, Meneses K, Demark-Wahnefried W. Nutritional status and diet in cancer prevention. Semin Oncol Nurs. 2016;32(3):206–14. [DOI] [PubMed] [Google Scholar]
- 4. Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: a systematic review. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Hutterer E, Isenring E, Kaasa Set al. ESPEN practical guideline: clinical nutrition in cancer. Clin Nutr. 2021;40(5):2898–913. [DOI] [PubMed] [Google Scholar]
- 6. Mahan LK, Escott-Stump S, Raymond JL. Krause's Food and the Nutrition Care Process. 13 ed.St. Louis, Missouri: Elsevier Saunders, 2012. [Google Scholar]
- 7. Harvie MN, Campbell IT, Baildam A, Howell A. Energy balance in early breast cancer patients receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2004;83(3):201–10. [DOI] [PubMed] [Google Scholar]
- 8. Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12(4):282–94. [DOI] [PubMed] [Google Scholar]
- 9. Tannenbaum A, Silverstone H. The influence of the degree of caloric restriction on the formation of skin tumors and hepatomas in mice. Cancer Res. 1949;9(12):724–7. [PubMed] [Google Scholar]
- 10. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JWet al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–54. [DOI] [PubMed] [Google Scholar]
- 12. Lv M, Zhu X, Wang H, Wang F, Guan W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PLoS One. 2014;9(12):e115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31(2):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheney KE, Liu RK, Smith GS, Leung RE, Mickey MR, Walford RL. Survival and disease patterns in C57BL/6J mice subjected to undernutrition. Exp Gerontol. 1980;15(4):237–58. [DOI] [PubMed] [Google Scholar]
- 15. Cheney KE, Liu RK, Smith GS, Meredith PJ, Mickey MR, Walford RL. The effect of dietary restriction of varying duration on survival, tumor patterns, immune function, and body temperature in B10C3F1 female mice. J Gerontol. 1983;38(4):420–30. [DOI] [PubMed] [Google Scholar]
- 16. Klurfeld DM, Welch CB, Davis MJ, Kritchevsky D. Determination of degree of energy restriction necessary to reduce DMBA-induced mammary tumorigenesis in rats during the promotion phase. J Nutr. 1989;119(2):286–91. [DOI] [PubMed] [Google Scholar]
- 17. Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TBet al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, Cacciottolo M, Martin-Montalvo A, de Cabo R, Wei Met al. Fasting mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell. 2016;30(1):136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brandhorst S, Harputlugil E, Mitchell JR, Longo VD. Protective effects of short-term dietary restriction in surgical stress and chemotherapy. Ageing Res Rev. 2017;39:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA. 2008;105(24):8215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Groot S, Lugtenberg RT, Cohen D, Welters MJP, Ehsan I, Vreeswijk MPG, Smit V, de Graaf H, Heijns JB, Portielje JEAet al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat Commun. 2020;11(1):3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY). 2009;1(12):988–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino Aet al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4(124):124ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA. Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. BMC Cancer. 2012;12:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caffa I, D'Agostino V, Damonte P, Soncini D, Cea M, Monacelli F, Odetti P, Ballestrero A, Provenzani A, Longo VDet al. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget. 2015;6(14):11820–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70(4):1564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caffa I, Spagnolo V, Vernieri C, Valdemarin F, Becherini P, Wei M, Brandhorst S, Zucal C, Driehuis E, Ferrando Let al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. 2020;583(7817):620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dorff TB, Groshen S, Garcia A, Shah M, Tsao-Wei D, Pham H, Cheng CW, Brandhorst S, Cohen P, Wei Met al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. 2016;16:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riedinger CJ, Kimball KJ, Kilgore LC, Bell CW, Heidel RE, Boone JD. Water only fasting and its effect on chemotherapy administration in gynecologic malignancies. Gynecol Oncol. 2020;159(3):799–803. [DOI] [PubMed] [Google Scholar]
- 31. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MCet al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. [DOI] [PubMed] [Google Scholar]
- 32. Faubion SS, MacLaughlin KL, Long ME, Pruthi S, Casey PM. Surveillance and care of the gynecologic cancer survivor. J Womens Health (Larchmt). 2015;24(11):899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pearce A, Haas M, Viney R, Pearson SA, Haywood P, Brown C, Ward R. Incidence and severity of self-reported chemotherapy side effects in routine care: a prospective cohort study. PLoS One. 2017;12(10):e0184360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huisman SA, Bijman-Lagcher W, Uzermans JNM, Smits R, de Bruin RW. Fasting protects against the side effects of irinotecan but preserves its anti-tumor effect in apc15lox mutant mice. Cell Cycle. 2015;14(14):2333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huisman SA, de Bruijn P, Ghobadi Moghaddam-Helmantel IM, Uzermans JNM, Wiemer EA, Mathijssen RH, de Bruin RW. Fasting protects against the side effects of irinotecan treatment but does not affect anti-tumour activity in mice. Br J Pharmacol. 2016;173(5):804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zorn S, Ehret J, Schauble R, Rautenberg B, Ihorst G, Bertz H, Urbain P, Raynor A. Impact of modified short-term fasting and its combination with a fasting supportive diet during chemotherapy on the incidence and severity of chemotherapy-induced toxicities in cancer patients - a controlled cross-over pilot study. BMC Cancer. 2020;20(1):578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lugtenberg RT, de Groot S, Kaptein AA, Fischer MJ, Kranenbarg EM, Carpentier MD, Cohen D, de Graaf H, Heijns JB, Portielje JEAet al. Quality of life and illness perceptions in patients with breast cancer using a fasting mimicking diet as an adjunct to neoadjuvant chemotherapy in the phase 2 DIRECT (BOOG 2013-14) trial. Breast Cancer Res Treat. 2021;185(3):741–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Groenvold M, Klee MC, Sprangers MA, Aaronson NK. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol. 1997;50(4):441–50. [DOI] [PubMed] [Google Scholar]
- 39. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon Jet al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9. [DOI] [PubMed] [Google Scholar]
- 40. Bauersfeld SP, Kessler CS, Wischnewsky M, Jaensch A, Steckhan N, Stange R, Kunz B, Bruckner B, Sehouli J, Michalsen A. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study. BMC Cancer. 2018;18(1):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Groot S, Vreeswijk MP, Welters MJ, Gravesteijn G, Boei JJ, Jochems A, Houtsma D, Putter H, van der Hoeven JJ, Nortier JWet al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cui W, Luo W, Zhou X, Lu Y, Xu W, Zhong S, Feng G, Liang Y, Liang L, Mo Yet al. Dysregulation of ketone body metabolism is associated with poor prognosis for clear cell renal cell carcinoma patients. Front Oncol. 2019;9(1422):1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marinac CR, Nelson SH, Breen CI, Hartman SJ, Natarajan L, Pierce JP, Flatt SW, Sears DD, Patterson RE. Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol. 2016;2(8):1049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, Arredondo E, Patterson RE. Prolonged nightly fasting and breast cancer risk: findings from NHANES (2009-2010). Cancer Epidemiol Biomarkers Prev. 2015;24(5):783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marinac CR, Sears DD, Natarajan L, Gallo LC, Breen CI, Patterson RE. Frequency and circadian timing of eating may influence biomarkers of inflammation and insulin resistance associated with breast cancer risk. PLoS One. 2015;10(8):e0136240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, Varady KA. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4(4):345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chow LSea. Time-restricted eating effects on body composition and metabolic measures in humans with overweight: a feasibility study. Obesity (Silver Spring). 2020;28(5):860–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda Set al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92–104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gabel K, Hoddy KK, Varady KA. Safety of 8-h time restricted feeding in adults with obesity. Applied Physiology, Nutrition, and Metabolism. 2018;44(1):107–109. [DOI] [PubMed] [Google Scholar]
- 51. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, Varady KA. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32(3):366–378.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martens CR, Rossman MJ, Mazzo MR, Jankowski LR, Nagy EE, Denman BA, Richey JJ, Johnson SA, Ziemba BP, Wang Yet al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. Geroscience. 2020;42(2):667–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Oliveira Maranhão Pureza IR, da Silva Junior AE, Silva Praxedes DR, Lessa Vasconcelos LG, de Lima Macena M, Vieira de Melo IS, de Menezes Toledo Florêncio TM, Bueno NB. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: a 12-month randomized clinical trial. Clin Nutr. 2021;40(3):759–66. [DOI] [PubMed] [Google Scholar]
- 54. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levesque S, Pol JG, Ferrere G, Galluzzi L, Zitvogel L, Kroemer G. Trial watch: dietary interventions for cancer therapy. Oncoimmunology. 2019;8(7):1591878. [DOI] [PMC free article] [PubMed] [Google Scholar]


