ABSTRACT
Sphingomyelin (SM) is a widely occurring sphingolipid that is a major plasma membrane constituent. Milk and dairy products are rich SM sources, and human milk has high SM content. Numerous studies have evaluated the roles of SM in maintaining cell membrane structure and cellular signal transduction. There has been a growing interest in exploring the role of dietary SM, especially from human milk, in imparting health benefits. This review focuses on recent publications regarding SM content in several dietary sources and dietary SM metabolism. SM digestion and absorption are slow and incomplete and mainly occur in the middle sections of the small intestine. This review also evaluates the effect of dietary SM on gut health and cognitive development. Studies indicate that SM may promote gut health by reducing intestinal cholesterol absorption in adults. However, there has been a lack of data supporting clinical trials. An association between milk SM and neural development is evident before childhood. Hence, additional studies and well-designed randomized controlled trials that incorporate dietary SM evaluation, SM metabolism, and its long-term functions on infants and children are required.
Keywords: sphingolipid, dairy products, milk fat globule membrane, brain development, metabolism, cholesterol
Statement of Significance: Sphingomyelin from food is increasingly recognized as bioactive lipids. This review provides new insights on the dietary sources and metabolism of sphingomyelin, as well as clinical trials on gut health and infant cognitive development.
Introduction
Sphingomyelin (SM), a major sphingolipid (SL) in human and bovine milk, is a critical structural constituent of neurons and lipid bilayers (1). SLs are a class of lipids comprising SM and glycosphingolipids. The predominant SL in plants and mammals is glucosylceramide and SM, respectively. SLs are a biomembrane component of almost all eukaryotes and are involved in cell proliferation and differentiation, stress response, membrane transportation, intracellular and extracellular signal transduction, cell migration, autophagy, and apoptotic cell death. SL metabolites, especially sphingosine (Sph), are involved in cell growth and death (2, 3).
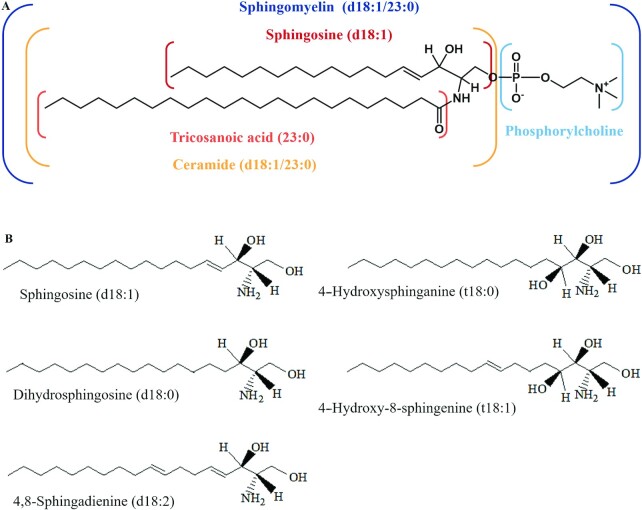
SM is synthesized at the endoplasmic reticulum and the Golgi by sphingomyelin synthases (4). SM is composed of a long-chain Sph base backbone, a fatty acid (FA) that is esterified by an amide group and a phosphorylcholine polar head group attached to the hydroxyl group of the sphingoid base (Figure 1A) (5). The predominant FAs of SM are long-chain or very-long-chain MUFAs or SFAs (6). Most of the existing studies examined sphingoid bases, such as 4-sphingosine (d18:1), whereas other bases, such as dihydrosphingosine (d18:0), 4-hydroxy sphinganine (t18:0), 4,8-sphingadienine (d18:2), and 4-hydroxy-8-sphingenine (t18:1), form a smaller proportion (Figure 1B) (7).
FIGURE 1.
(A) Structure and composition of SM (d18:1/23:0). (B) Naturally occurring sphingoid bases. The prefix d and t indicate the number of hydroxyl groups of the di- and tri-hydroxy base, respectively. SM, sphingomyelin.
SM is generally present at low food levels, and the SM polarity is different from that of glycerophospholipids because of its asymmetric molecular structure as well as extensive hydrogen-bonding capacity (8). Natural SMs (e.g., milk SM) can exhibit important acyl chain heterogeneities in terms of length and saturation, which may affect the mechanical properties and biological functions of the milk fat globule membrane (MFGM) or liposome carriers (9).
This study aims to present a comprehensive overview of the current dietary SM knowledge, including natural sources, concentration, and molecular species, as well as its health benefits. The analytical methods used to identify and quantify food SM are important but are not extensively discussed and were therefore first summarized with the dietary SM contents. Special attention was given to the digestion and metabolism of dietary SM and recent studies on the physiological SM functions, especially on the infant's neural development and intestinal tract maturation. This review focuses on the SM found in dietary phospholipids (PLs) rather than on the combination of SM or glycerophospholipids.
Current Status of Knowledge
Literature search methods
We performed a literature search in PubMed (1965 through 18 June 2021). The following combinations of keywords were used as search terms: “sphingomyelin,” “human breast milk,” “dietary products,” “milk fat globe membrane,” “metabolism,” “absorption,” “digestion,” “intestinal,” “gut,” “inflammation,” “neurodevelopment,” and “clinical trials.” Given that the present article is not a systematic review, we may not have identified all studies, and we must acknowledge a certain publication bias. However, all of the authors conducted the literature search independently (as presented in Supplemental Figure 1).
Dietary sources of SM and its quantification
Milk
Milk fat is an emulsion of natural oil and water, which mainly comprises triglycerides (TGs), PLs, cholesterol, and various lipids (10). Milk-fat globules are surrounded by MFGM derived from the membranes of mammary cells, and most MFGM products applied in studies come from bovine milk. The polar lipids in milk fat are generally localized to the MFGM. The polar lipids in milk mainly comprise SM and glycerophospholipids, such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and phosphatidylinositol (PI) (1, 11). The addition of polar lipid constituents to food systems has currently become a growing area of interest because of their superior emulsion stability and improved rheological properties (12).
Human breast milk
Table 1 summarized the relative SM content in mature human milk reported in different countries and regions over the last 3 decades. The total PL content in human milk is 14.7–42.2 mg/100 mL (3.05–5.06 mg/g total lipid). SM is found to be the most abundant PL in human breast milk (28.4–45.5% of the total PL content), followed by PC, PE, PS, and PI. Most studies that have compared the content and composition of different PLs in human milk have reported the abundance of PLs in the following order: SM > PC > PE (13–17), whereas other studies have reported the PL abundance in the order SM > PE > PC (18–20).
TABLE 1.
Sphingomyelin and phospholipid concentrations in dietary sources and analytical methods used1
| Classes (regions/species) | SM in PL or sample2 | PL in lipid or sample3 | Analytical method | Reference |
|---|---|---|---|---|
| Human breast milk | ||||
| China | Preterm: 7.89–9.15 μmol/100 mL; full-term: 8.15–8.54 μmol/100 mL (s) | Preterm: 20.18–27.47 μmol/100 mL; full-term: 22.57–25.83 μmol/100 mL (s) | 31P NMR | Wei et al. 2019 (42) |
| 227.18 μg/mL (s) | 228.60 μg/mL (s) | HILIC-IT-TOF-MS | Jiang et al. 2018 (47) | |
| 29.3% | 22.5 mg/100 mL(s) | HPLC-ELSD | Yao et al. 2016 (20) | |
| Singapore | — | 8.47 mg/100 mL (s) | HPLC-ELSD | Tavazzi et al. 2018 (23) |
| — | 9.28 mg/100 mL (s) | HPLC-ESI-MS/MS | Tavazzi et al. 2018 (23) | |
| 35.7% | 0.24 mg/g | HPLC-ELSD | Giuffrida et al. 2013 (19) | |
| Denmark | 40.2% | 20.8–24.2 mg/100 mL (s) | HPLC-ELSD | Zou et al. 2013 (16) |
| 38.8% | 5.1 mg/g | HPLC-ELSD | Zou et al. 2012 (17) | |
| Spain | 28.4–36.0% | 26.0–42.2 mg/100 mL (s) | HPLC-ELSD | Claumarchirant et al. 2016 (18) |
| 41% | — | TLC | Sala-Vila et al. 2005 (15) | |
| France | 29.7% | 25.0 mg/100 mL (s) | 31P NMR | Garcia et al. 2012 (13) |
| 36.4–45.5% | 3.1–4.1 mg/g | HPLC-ELSD | Lopez and Ménard 2011 (14) | |
| Other mammalian milk | ||||
| Bovine milk | 24.9% | 8.77 mg/g | HPLC-ELSD | Luo et al. 2018 (28) |
| 25.4% | 22.90 mg/100 mL (s) | HPLC-ELSD | Yao et al. 2016 (20) | |
| 0.054–0.13 mg/mL (s) | — | HILIC-ESI-MS | Liu et al. 2015 (45) | |
| 27.4% | 4.78 mg/g | HPLC-ELSD | Zou et al. 2013 (16) | |
| 11.7 mg/L (s) | 413.4 mg/L (s) | HILIC-ESI-IT-TOF-MS | Russo et al. 2013 (46) | |
| 19.9% | 20.0 mg/100 mL (s), 0.55% | 31P NMR | Garcia et al. 2012 (13) | |
| 26% | 6.25 mg/g | HPLC-ELSD | Lopez et al. 2011 (68) | |
| 20.3% | 0.36% | HPLC-ELSD | Rodríguez-Alcalá and Fontecha 2010 (30) | |
| Yak milk | 33.1% | 370.70 mg/kg (s), 5.13 mg/g | HPLC-ELSD | Luo et al. 2018 (28) |
| Buffalo milk | 31.6% | 3.22 mg/g | HPLC-ELSD | Zou et al. 2013 (16) |
| Mare milk | 22.2% | 7.78 mg/100 mL (s), 1.08% | 31P NMR | Garcia et al. 2012 (13) |
| Sheep milk | 27.8% | 4.30 mg/g | HPLC-ELSD | Zou et al. 2013 (16) |
| Caprine milk | 25% | 28.81 mg/100 mL (s) | HPLC-ELSD | Yao et al. 2016 (20) |
| Ewe milk | 26.1% | 0.38% | HPLC-ELSD | Rodríguez-Alcalá and Fontecha 2010 (30) |
| Goat milk | 23.2% | 0.65% | HPLC-ELSD | Rodríguez-Alcalá and Fontecha 2010 (30) |
| Camel milk | 28.2% | 4.65 mg/g | HPLC-ELSD | Zou et al. 2013 (16) |
| 24.6% | 39.34 mg/100 mL (s), 0.87% | 31P NMR | Garcia et al. 2012 (13) | |
| Donkey milk | 36% | 4.01 mg/g | HPLC-ELSD | Zou et al. 2013 (16) |
| Dairy products | ||||
| Processed milk | 24.1% | — | HPLC-ELSD | Gallier et al. 2010 (29) |
| Cream | 17.7% | — | HPLC-ELSD | Gallier et al. 2010 (29) |
| 20.8% | 0.16% (s), 0.4% | 31P NMR | MacKenzie et al. 2009 (40) | |
| Butter serum | 33.7–34.3% | — | HPLC-ELSD | Lopez et al. 2017 (32) |
| Butter | 20.22% | 0.18% (s), 0.22% | HPLC-ELSD | Rombaut et al. 2007 (31) |
| Buttermilk | 18.6–19.3% | — | HPLC-ELSD | Lopez et al. 2017 (32) |
| 12.8% | 0.09% (s), 21.8% | HPLC-ELSD | Rombaut et al. 2007 (31) | |
| Buttermilk powder | 3.14 g/100 g (s) | — | HPLC-ESI-MS/MS | Tavazzi et al. 2018 (23) |
| 3.19 g/100 g (s) | — | HPLC-ELSD | Tavazzi et al. 2018 (23) | |
| 16.9% | 31.7% | HPLC-ELSD | Rodríguez-Alcalá and Fontecha 2010 (30) | |
| 23.9% | — | HPLC-ELSD | Gallier et al. 2010 (29) | |
| Cheese | 14.2% | 0.28% (s), 2.7% | HPLC-ELSD | Rombaut et al. 2007 (31) |
| 19.4% | 0.03% (s), 24.7% | HPLC-ELSD | Rombaut et al. 2007 (31) | |
| Cheese whey | 17.37% | 8.95 g/100g (s) | 31P NMR | Zhu and Damodaran 2011 (33) |
| Infant formula | 5.45–15.98 μmol/100 mL (s) | 24.60–82.30 μmol/100 mL (s), 0.52–2.14% | 31P NMR | Wei et al. 2019 (42) |
| 11.96–21.13 mg/100 g | — | HPLC-ELSD | Tavazzi et al. 2018 (23) | |
| 17.48–23.82 mg/100 g | — | HPLC-ESI-MS/MS | Tavazzi et al. 2018 (23) | |
| 33.7–177.5 μg/mL | 150.48–2274.33 μg/mL (s) | HILIC-IT-TOF-MS | Jiang et al. 2018 (47) | |
| Egg yolk | ||||
| Hen | 5279 nmol/g (s) | — | UHPLC-ESI-triple TOF-MS | Zhou et al. 2021 (37) |
| 254 mg/100 g (s) | — | HILIC-LC-MS/MS | Xiong et al. 2012 (35) | |
| 195.8 mg/100 g (s) | — | HILIC-LC-MS/MS | Xiong et al. 2012 (35) | |
| 237 mg/100 g (s) | — | 31P NMR | Xiong et al. 2012 (35) | |
| 233.1 mg/100 g (s) | — | 31P NMR | Xiong et al. 2012 (35) | |
| 0.9% | — | HPLC-ELSD | Zhou et al. 2012 (38) | |
| 190 mg/100 g (s) | — | HILIC-LC–MS/MS | Zhao et al. 2011 (34) | |
| Duck | 985 nmol/g (s) | — | UHPLC-ESI-triple TOF-MS | Zhou et al. 2021 (37) |
| Goose | 5090 nmol/g (s) | — | UHPLC-ESI-triple TOF-MS | Zhou et al. 2021 (37) |
| Pigeon | 4504 nmol/g (s) | — | UHPLC-ESI-triple TOF-MS | Zhou et al. 2021 (37) |
| Quail | 584 nmol/g (s) | — | UHPLC-ESI-triple TOF-MS | Zhou et al. 2021 (37) |
| Ostrich | 1016 nmol/g (s) | — | UHPLC-ESI-triple TOF-MS | Zhou et al. 2021 (37) |
| Emu | 387 nmol/g (s) | — | UHPLC-ESI-triple TOF-MS | Zhou et al. 2021 (37) |
ELSD, evaporative light-scattering detection; ESI, electrospray ionization; HILIC, hydrophilic interaction liquid chromatography; IT-TOF, ion trap–time-of-flight; PL, phospholipid; 31P NMR, phosphorus NMR; SM, sphingomyelin; TOF, time-of-flight; UHPLC, ultra-high performance liquid chromatography.
The data that refer to SM in the sample are marked (s) after the data.
The data that refer to PL in the sample are marked (s) after the data.
The PL compositions in human milk have been suggested to vary with the lactation period progression (15) and are classified as colostrum (1–5 d postpartum), transitional (6–15 d postpartum), and mature milk (>15 d postpartum). However, no consensus exists on the variation in PL or SM content in human milk at different lactation periods. The highest PL contents are present in transitional milk with a minimal change in the SM content with lactation period progression (15, 17, 21). However, a marked decreased PL contents between 1 and 12 mo postpartum was reported (18). By contrast, elevated PL concentrations were reported in mature human milk between 2 and 12 mo postpartum (22). Apart from regional, individual, and maternal diets, the reported PL contents (including SM) in human milk also depend on the sensitivity of analytical methods used for quantification.
Figure 2 shows that SM contains more long-chain FAs (LCFAs) compared with glycerophospholipids, especially series 40 and 42, which account for more than 50% of the total SM (23). Supplemental Figure 2 shows the Sph base species and their specific FA composition in breast-milk SM. Sphingosine (d18:1) was the predominant sphingoid base, accounting for 83.6% of breast-milk SM, followed by 4,8-sphingosine (d18:2) (7.2%) and 4-hydroxysphingosine (t18:0) (5.7%). The t18:0 content is an indication of a plant-based diet.
FIGURE 2.
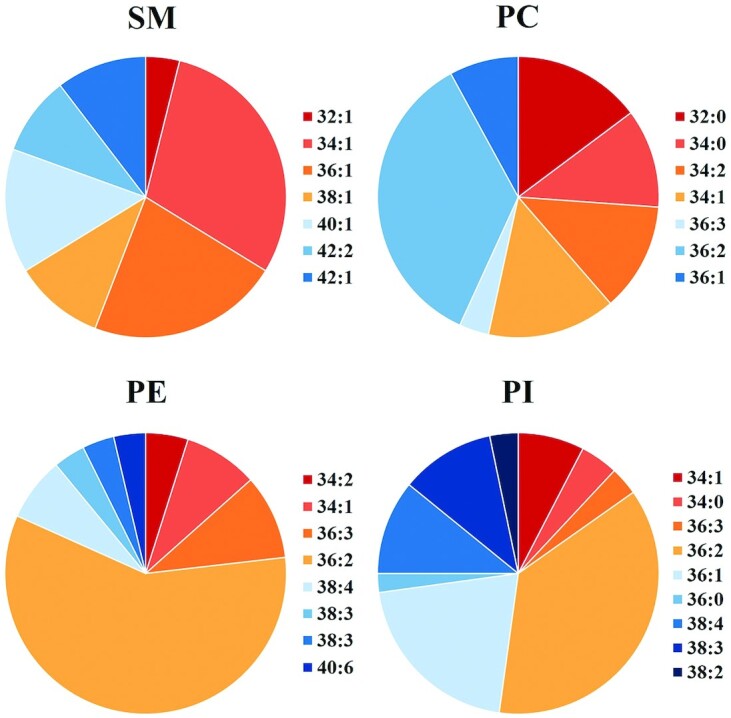
Relative amount of the molecular species of phospholipid classes and SM in human milk. Only species whose content is >3% are shown. PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SM, sphingomyelin. Adapted from reference 23 with permission.
Only a few studies focused on total FAs in SM (7, 20), which may be due to the difficulty in the separation of polar lipids, especially for milk samples. The FA composition was evaluated in milk SM using GC (Figure 3) (24, 25). The predominant FAs in SM had longer chain lengths than the composition of total FAs in human breast milk. In breast-milk SM, the predominant long-chain saturated FAs are hexadecanoic acid (16:0, 5.3–21.3%), octadecanoic acid (18:0, 12.7–13.8%), eicosanoic acid (20:0, 6.3–10.2%), docosanoic acid (22:0, 16.4–20.7%), and tetracosanoic acid (24:0, 8.1–17.5%). Thus, SM has a higher melting temperature that can be easily separated from other PLs at room temperature (16). Among the PLs of breast milk, SM has the lowest PUFA content. The predominant unsaturated FAs in SM of breast milk are 15-tetracosenoic acid (24:1n–9, 9.7–16.1%), 9-octadecenoic acid (18:1n–9), 9,12-octadecadienoic acid (18:2n–6), and 13-docosenoic acid (22:1n–9) (15, 20). The presence of 24:1n–9 in breast milk SM enables its absorption in infants (20).
FIGURE 3.
Composition of fatty acids evaluated in milk sphingomyelin using GC. Adapted from references 24 and 25 with permission.
SM is the primary molecular nervonic acid (24:1n–9) carrier. The lack of permeability of nervonic acid to the placental barrier retains the ability to cross mammary epithelium and intestinal epithelium. It crosses the mammary epithelial barrier where it appears as nervonyl-SM of the MFGM. In addition, nervonic acid crosses the intestinal epithelium, where it is incorporated into the SM of the heart and liver of suckling rats as nervonyl-SM (26). Nervonic acid rapidly accumulates in the fetal brain during 32–37 wk of pregnancy. Moreover, nervonic acid is an essential constituent of the neuronal membrane and plays a crucial role in problems (e.g., early myelination, peroxisomal disorders, and undernourishment) (27).
Other mammalian milk
Table 1 lists the SM content and its proportion in some mammalian milk samples. Similar to human milk, PC, PE, and SM are the predominant PLs of mammalian milk. Most studies on mammalian milk PLs are focused on bovine and goat milk. The PL content in bovine milk accounts for 0.36–0.55% of the total lipid content (4.78–8.77 mg/g of fat and 20.0–22.9 mg/100 mL of milk), and the SM content accounts for 19.9–27.4% of the total PL content. Surprisingly, SM was the predominant PL in mare milk, followed by PC. The PLs in mare milk constitute approximately 1% of the total lipids, which is more than that in other mammalian milk. However, the total lipid content in mare milk is very low (13). Furthermore, the SM contents in yak (33.1%) and donkey (36.0%) milk are markedly greater than that in cow milk, and SM is the predominant PL in yak (28) and donkey milk (16). Hence, yak and donkey milk would be potential raw materials suitable for infant formula.
The differences in SM content listed in Table 1 may be due to various factors (e.g., animal species, lactation period, dietary habits, and environmental and seasonal factors). In addition, very few comparative studies on PL composition in human and other animal milk were noted. Moreover, the studies used different analytical methods to report the phospholipid content. Thus, studies on PLs in mammalian and human milk are not comparable.
Dairy products
Dairy products are essential foods in daily human life. Table 1 summarizes the relative SM content in dairy products. Among the dairy products, PLs are abundant in butter, cheese, and cream. Cheese whey has the highest PL content (8.95% of the sample), whereas the PL content in other dairy products is below 0.30%. Buttermilk powder had the highest PL content in total lipids (31.72% of total lipids). Butter had the highest relative content of SM (33.70–34.30% of the total PLs), followed by cream (13.93–28.60% of the total PLs) and buttermilk powder (16.87–23.90% of total PLs). Some difference exists in the relative SM content between the different varieties of dairy products for the same species (29–32). Previously, dairy-derived ingredients rich in SM (e.g., skimmed-milk powder and whey protein concentrate containing milk fat and MFGM) have been used in infant formulations. The SM levels found in commercially available dairy ingredients used for manufacturing depend on their composition and manufacturing processes, which would affect the SM content of subsequent products that use these ingredients in turn (1, 33). The contents of both PLs and SM in different dairy products can be used as references to estimate the intake or to study the influence of purification and processing methods (31).
Egg yolk
The avian egg yolk (including chicken, duck, goose, turkey, and quail) contains abundant PLs. The most abundant egg yolk PLs are PC and PE, which account for 71.0–87.8% and 10.1–18.0% of the total PLs (34), respectively. The water content in egg yolk, extraction efficiency, and egg type can influence PL content (35). Using analytical methods to efficiently extract and purify PLs is necessary. Using improved analytical techniques, 12 PLs were detected in the egg yolk (i.e., PC, LPC, PE, lysophosphatidylethanolamine, PI, lysophosphatidylinositol, PG, lysophosphatidylglycerol, phosphatidic acid, lysophosphatidic acid, SM, and PS) (35–37).
The total SM content in egg yolks ranges from 190 to 285 mg/100 g, and the SM content accounts for 0.9–1.1% of the total PL content (34, 35, 38). At least 11 SM species were identified in egg yolk by LC-MS (36). The predominant Sph base in egg yolk is d18:1, and the predominant FAs are 14:0, 15:0, 16:0, 16:1n–7, 17:0, 18:0, 18:2n–6, 18:3n–3, 20:0, 20:1n–9, and 22:0.
Quantification and identification of SM
In-depth studies on SM function are affected and limited by detection technology. Table 1 summarizes various analytical methods used for SM characterization from different dietary sources. Thin-layer chromatography (TLC) is a classical method used for the quantification and semiquantification of PLs (39). However, TLC is time-consuming and not suitable for high-throughput analysis (40). The commonly used methods to identify PL classes include HPLC with evaporative light-scattering detection (ELSD) and phosphorus NMR (31P NMR). Hence, removing TGs and other neutral lipids before the quantitative analysis of milk PLs using the HPLC-ELSD method is necessary (41), whereas samples with low levels (1%, wt:wt) of PLs could be detected using the 31P NMR method without the need for polar lipid extraction or longer analysis times and/or a sophisticated instrument (40, 42, 43) shown in Supplemental Figure 3. However, the methods described above cannot meet requirements of molecular information on PL FAs. Since the metabolic of different PL FAs varies from the position in PLs to polar groups (44). LC-MS methods [e.g., hydrophilic interaction LC (HILIC)–tandem MS] have been widely used to obtain molecular SM information and can be used to quantify all the analytes of interest in a single run (35, 45, 46). Furthermore, MS/MS analysis can be used to evaluate the FA composition in PLs (34). The analysis procedure of SM (d18:1/22:0) species, a commonly obtained species when analyzing SM structure via HILIC-electrospray ionization–ion trap–time-of-flight–MS, is shown in Supplemental Figure 4 (47). In recent years, several new chromatography–MS methods have been developed [e.g., supercritical fluid chromatography–MS (48) and ion mobility spectrometry–MS (49)], which can improve characterization efficiency and accuracy. Those studies provided a growing number of analytical methods suitable for SM high-throughput analysis, which enables further SM metabolomic and clinical studies.
Digestion and absorption of SM
The daily SL intake in the common Western diet is approximately 300–400 mg (50). The daily SM consumption among infants is approximately 150 mg (13, 51). The daily consumption of 800 mL of human milk can provide approximately 62 mg SM for a term newborn, whereas the consumption of 170 mL of breast milk can provide 13 mg of SM for a preterm infant (13).
Alkaline sphingomyelinase
Contrary to dietary glycerine, SLs are not degraded by pancreatic enzymes. SM is successively hydrolyzed by alkaline sphingomyelinase (alk-SMase) and neutral ceramidase (N-CDase) to act on the intestinal epithelium brush margin and intestinal cavity. Alk-SMase was identified as a new member of the nucleotide pyrophosphatase/phosphodiesterase (NPP) family and is also called NPP7 (52).
Furthermore, alk-SMase protects the intestinal mucosa from inflammation and tumorigenesis. Alk-SMase can hydrolyze and inactivate the platelet-activating factor (a proinflammatory PL) involved in the pathogenesis of inflammatory bowel disease (53).
SM digestion in adults
Nilsson and Duan (52) summarized that SM hydrolysis takes place in the small intestine and colon. Alk-SMase was detected in the intestinal tract and human bile. High concentrations of alk-SMase were observed in the intestinal tract, and the highest alk-SMase content was found in the middle portion of the small intestine (54). The SM digestion occurs in the small intestine, mainly in the middle and lower sections. This is consistent with the alk-SMase distribution in the intestinal tract, which is important for SM digestion. The N-CDase distribution in the intestinal tract is parallel to the alk-SMase distribution along the intestinal tract. Therefore, alk-SMase and N-CDase have a synergistic effect in SM digestion.
Two sources of alk-SMase in humans are the bile and intestinal mucosa. Figure 4 shows the SM digestion and absorption pathways (54). Recent studies found that, once SM is hydrolyzed, ceramide (Cer) is subsequently broken down by alkaline, neutral, and acid ceramidases, which affects the brush margin of the intestinal epithelium and intestinal lumen. Studies on rats that fed [3H]Sph- or [14C]stearic acid–labeled SM showed that little or no labeled SM was absorbed intact into the chyle (54). SM was sequentially hydrolyzed to Cer and then to sphingosine and free FAs. Similarly, no evidence was obtained for the incorporation of intact dietary Cer into chyle lipoproteins either as Cer or as SM after feeding [3H]palmitoyl-Sph, but the [3H]palmitic acid appears primarily in chyle TGs. Contrary to SM and Cer, free Sph is well absorbed and rapidly metabolized in the mucosal cells. Most of the absorbed Sph is converted to palmitic acid and incorporated into chylomicrons. All 3 hydrolyze the amide linkage to release FA and Sph. Acid ceramidase is located in the lysosome (55), whereas neutral ceramidase is found in the plasma membrane rich in caveolin (56). Alkaline ceramidase is distributed in the Golgi and endoplasmic reticulum and prefers Cer with a very long chain (57). Free Sph is absorbed and rapidly metabolized in mucosal cells. Most of the absorbed Sph is converted into chylomicron palmitic acid, which is catalyzed by sphingosine kinase, sphingosine-1-phosphate lyase, and palmitaldehyde oxidase. These enzymes are enriched in the intestinal mucosa. A small Sph proportion is incorporated into the mucosal Cer and other complex sphingolipids (58). A smaller proportion of the sphingoid bases are reincorporated into mucosal ceramide and more complex sphingolipids, and some of this newly formed Cer appears as chyle Cer rather than chyle SM (54, 59).
FIGURE 4.
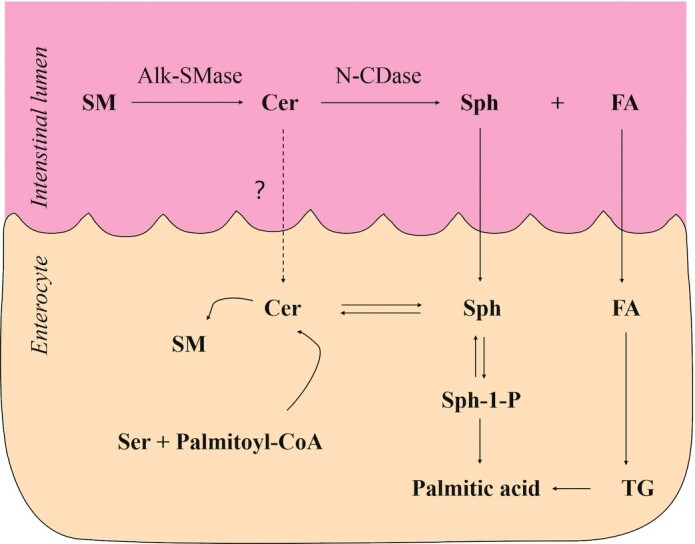
Pathways for the digestion and absorption of SM. Alk-SMase, alkaline sphingomyelinase; Cer, ceramide; FA, fatty acid; N-CDase, neutral ceramidase; Ser, serine; SM, sphingomyelin; Sph, sphingosine; Sph-1-P, sphingosine-1-phosphate; TG, triglyceride. Adapted from reference 54 with permission.
Nevertheless, SM digestion and absorption are slow and incomplete, and a large SM proportion is retained in the intestine (6) and would be co-excreted with cholesterol to reduce intestinal cholesterol absorption (60). Incomplete SM digestion generates Cer and Sph, which can be absorbed by intestinal mucosal cells (61). The intestinal alk-SMase of mice can hydrolyze 8 μmol SM within 1 h in vitro (62). However, in vivo studies suggested that only 7 μmol SM was completely digested in rats within 24 h (63). The presence of cholesterol, glycerophospholipids, TGs, diacylglycerol, and FAs in the intestinal tract can inhibit alk-SMase activity (64). Glycerophospholipid and its hydrolytic product can inhibit alk-SMase activity in the intestinal tract in bile salt presence. The descending order of alk-SMase inhibition by glycerophospholipids is as follows: phosphatidic acid > PS > PI > PC > PE (64). The inhibition caused by other PLs may be due to the competition between these PLs and SM for the enzyme substrate-binding site (65). In addition, the pancreas may influence SM hydrolysis and Cer (62). The high bile salt concentration in the intestinal cavity may also inhibit alk-SMase activity. Moreover, Cer has been found to accumulate in people with obesity or dyslipidemia and alter cellular processes in response to fuel surplus (66), whereas microsomal TGs transfer protein that regulates plasma Cer and SM concentrations by transferring these SLs between vesicles (67).
Maximum bile salt stimulation occurs at the critical micelle concentration (CMC), although bile salt is necessary for alk-SMase activation. Moreover, alk-SMase and N-CDase activity concentrations are reduced when bile salt concentration is higher than CMC. SM digestion is affected at the lower part of the small intestine because bile salt concentration in the upper intestine is higher than that in the CMC under physiological conditions. In addition, most fats and PLs are digested, and bile salt concentration is lowered because of absorption.
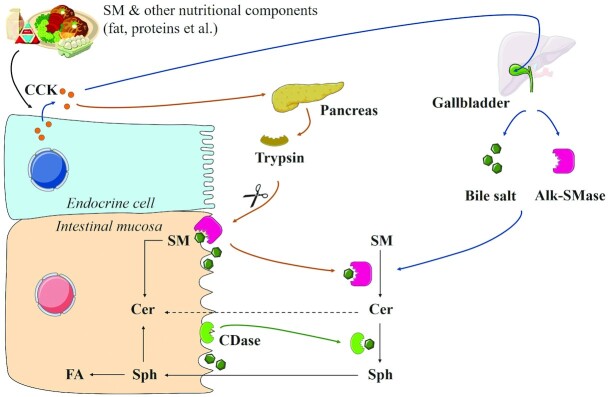
Hence, the SM digestion process and the exposure of the distal small intestine and colon to SM and its metabolites may be influenced by multiple factors (e.g., dietary SM amount, presence of other lipids and bile salt, and the amount of enzyme involved in SM digestion) (68). Figure 5 shows the SM digestion process and regulation in the human intestinal tract (69). Upon meal consumption, cholecystokinin (CCK) stimulates the gallbladder contraction to transfer bile alk-SMase and bile salt into the intestine. Meanwhile, CCK increases pancreatic trypsin secretion. Pancreatic trypsin and bile salt promote the dissociation of both intestinal mucin and N-CDase from the mucosa into the lumen. SM can be successively digested by alk-SMase and N-CDase to form Sph in bile salt presence. Furthermore, Sph is absorbed by epithelial cells and converted into FAs or Cer.
FIGURE 5.
Digestion of dietary SM after meal consumption. Alk-SMase, alkaline sphingomyelinase; CCK, cholecystokinin; CDase, ceramidase; Cer, ceramide; FA, fatty acid; SM, sphingomyelin; Sph, sphingosine. Adapted from reference 69 with permission.
SM digestion in the newborn
A study on gastric and duodenal intubation of 11 breastfed newborns suggested that the milk SM digestion in the stomach and upper duodenum is negligible. Thus, this suggested that the upper digestive tract is not involved in SM digestion. Both alk-SMase and N-CDase are expressed in the intestinal tracts of preterm and term newborns (53). These enzymes can generate bioactive SL messengers to catalyze the hydrolysis of endogenous SM in infants fed human milk and milk SM. Sph that is released after SM digestion is absorbed and phosphorylated into sphingosine-1-phosphate (Sph-1-P), which is converted into palmitic acid by Sph-1-P-lyase in the intestinal mucosa (70). Moreover, elevated NPP7 and N-CDase concentrations were observed in the meconium of both preterm and term human infants. In addition, palmityl sphinganine and Sph (possible products of NPP7 and N-CDase) were observed in meconium (53). Furthermore, in a rat model, they reported that alk-SMase is rapidly expressed before birth and that the enzyme concentrations was stabilized at 4 wk after birth. Thus, they reported that newborn rats gained the ability to digest milk SM early in life.
Roles of SM in gut health and cognitive development
Apart from dietary SM, a previous study reported that adding dihydrosphingomyelin into biological samples as an internal standard enables the actual amount of endogenous SM to be measured. SM concentrations in the brain, kidney, and liver of the Institute of Cancer Research mice were 55.60 ± 0.43, 43.75 ± 0.21, and 22.26 ± 0.14 pmol/μg protein (71). SM is an important cell membrane component and is also involved in regulating cell growth, differentiation, and apoptosis, as well as cholesterol distribution and homeostasis (72–74). The effect of MFGM supplementation rich in SM on the plasma lipidome is correlated with positive cognitive and immunological effects (75). In addition, SM plasma concentrations have been considered to be an indicator or biomarker of some human diseases (e.g., atherosclerosis) (76–78). Dietary SM has proven potential in influencing inflammation by inhibiting intestinal lipid absorption (79), altering gut microbiota (80), and blocking the leakage of LPS across the gut barrier (81, 82). Table 2 summarizes the recent advances in SM effect study based on human models.
TABLE 2.
Results of recent clinical trials of dietary sphingomyelin in children and adults1
| Diet source | Subjects | Treatment and duration | Measurement | Brief summary of the findings | Reference |
|---|---|---|---|---|---|
| Milk polar lipids | 58 Postmenopausal females after a 1-wk run-in period (100 g control cheese/d) | The volunteers were randomly assigned to 3 different groups for a 4-wk intervention period with either cream cheese devoid of polar lipids (n = 19, control group) or enriched with polar lipids (n = 19, 3 g polar lipids group; n = 20, 5 g polar lipids group) (100 g/d) | (1) Total cholesterol, LDL-cholesterol, HDL-cholesterol, TG and glucose concentrations in serum, apoB, and apoA1 in plasma, serum insulin, plasma apoB48 and PCSK9; (2) intestine-derived chylomicron-rich fractions (lipids, hydrodynamic diameter); (3) stools (fecal lipids, gut microbiota, SCFAs); (4) ileal efflux of SM and cholesterol; (5) oleic acid in plasma; (6) exogenous lipid oxidation calculation | Milk polar lipids decreased cholesterol absorption and increased cholesterol-ileal efflux, which can be explained by the observed co-excretion with milk SM in the gut | Vors et al. 2020 (60) |
| MFGM-derived PLs | Male and female adults with MetS | Adults (n = 24; ages 18–65 y) with MetS will receive diet for 2 wk that contains 3 daily servings of an MFGM-enriched bovine milk beverage or a comparator beverage that is formulated with nonfat dairy powder, coconut and palm oils, and soy PLs. After the intervention, participants will ingest a high-fat/high-carbohydrate meal challenge to assess metabolic excursions at 30 min intervals for 3 h | Data collection on day 0, 7, and 14 of each study arm includes anthropometrics, blood pressure, and a fasting blood sample for metabolic assessments. A fecal sample will be collected on day 13 for determination of microbiota composition, fecal SCFAs, and intestinal inflammatory markers. Stool characteristics will also be recorded using a 7-point Bristol stool scale. Participants will collect a complete 24-h urine sample on day 14 to assess gut permeability based on the urinary excretion of sugar probes that are ingested with the test meal challenge | The investigation to provide the first translational evidence in people with MetS of the benefits of MFGM acting at the gut to improve cardiometabolic health | Quarles et al. 2020 (80) |
| Dietary products rich in SM | 54 Males and 34 females under 2 y of age | Subjects were fed product A (n = 39, 28 mg/L SM), product B (n = 28, 71 mg/L SM), or product C (n = 21, 28 mg/L SM) during the first 90 d of life | Subjects received biannual MRI and neurocognitive assessments from age at enrollment until 2 y of age, with annual assessmentsQuantification of SM consumption from products A, B, and C was determined retrospectively | SM concentrations (brain MRI) were associated with myelin content as well as cognitive development. Higher concentrations of SM were significantly associated with higher rates of change in verbal development in the first 2 y of life | Schneider et al., 2019 (84) |
| Milk MFGM | 451 Healthy term infants (265 males and 186 females) born at 37–42 wk of gestation between 10 and 14 d of age | Infants were randomly assigned to a cow-milk–based infant formula (n = 228) or infant formula with an added source of bovine MFGM and lactoferrin (n = 223) through 365 d of age | The Bayley Scales of Infant Development, 3rd edition, cognitive composite score at day 365 was the primary outcome. Secondary outcomes included tolerance measures through day 365, additional neurodevelopmental and language outcomes, weight growth rate, and medically confirmed adverse events through day 545. Parents completed a baseline recall of tolerance and stool characteristics | Infants receiving formula with added bovine MFGM and bovine lactoferrin had an accelerated neurodevelopmental profile as well as fewer diarrhea and respiratory-associated adverse events, and even improved language subcategories | Li et al. 2019 (93) |
| MFGM | 240 Healthy infants, (100 males, 100 females), age <2 mo, gestational age 37–42 wk, birth weight 2500–4500 g | Formula-fed infants were either exclusively standard formula-fed (n = 80) or experimental formula-fed (n = 80), which was modified from standard formula by lowering energy density and protein concentration and adding a bovine MFGM fraction to get identical total energy and protein intakes for 2 formula-fed groups; 80 breast-fed infants were recruited as a reference group | Lipidomic analyses, including TG, DG, MG, PLs, ceramide, were performed on plasma and erythrocyte membranes at 6 mo and on serum at 4 and 12 mo of age | Infants fed a formula supplemented with a bovine MFGM fraction clearly affected their serum/plasma and erythrocyte membrane lipidomes. These changes might reflect or are part of the mechanisms mediating the positive effects on cognitive development and immune defense | Grip et al. 2018 (75) |
| Milk enriched with milk-PL | 62 Men between 50 and 76 y of age, overweight (BMI ≥27 kg/m2), and nonsmokers | The participants consumed daily 200 mL of PL-enriched drink (containing 2 g milk-PL, n = 31) or 200 mL of control drink (containing 2 g milk fat, n = 31) during the 8-wk intervention | Fasting plasma or serum samples were analyzed for routine clinical parameters as well as concentrations of TG, total cholesterol, PLs, HDL cholesterol, LDL cholesterol, glucose, apoA1, apoB, total glutathione, insulin, high-sensitivity C-reactive protein, total homocysteine and activities of γ-glutamyl transferase, aspartate transaminase, and alanine transaminase | Milk-PL as compared with control reduced waist circumference. Activity of γ-glutamyl transferase, a marker of fatty liver, increased in the control but not in the milk-PL group, with a significant intervention effect | Weiland et al. 2016 (98) |
| Bovine MFGM | 160 Infants (80 males and 80 females) <2 mo old and a breastfed reference group with 80 infants (40 males and 40 females) | Infants were randomly assigned to be fed an MFGM-supplemented, low-energy, low-protein experimental formula (n = 73, PL accounting for 70 mg/100 mL), standard formula (n = 62, PL accounting for 30 mg/100 mL), or a breastfed reference group (n = 78) until 6 mo | Cognitive function and growth were assessed by Bayley Scales of Infant and Toddler Development III (Bayley-III), weight, length, head circumference; venous blood was used for plasma insulin, blood urea nitrogen, and plasma amino acid pattern analysis | MFGM-supplemented infant formula bridges the gap in cognitive development between breastfed and formula-fed infants | Timby et al. 2014 (89) |
| Milk SM | 10 Healthy men and women between 18 and 45 y of age | Subjects consumed controlled diets with or without 1 g/d SM for 14 d separated by at least a 4-wk washout period | Serum lipid profile and markers of cholesterol metabolism including cholesterol absorption and synthesis were analyzed | Dietary SM (1 g/d) has no effect on cholesterol absorption, synthesis, and intraluminal solubilization compared with control | Ramprasath et al. 2013 (112) |
| SM-fortified milk | 24 Very-low-birth-weight preterm infants (<1500 g, 11 males and 13 females) who were admitted to the neonatal intensive care unit | 12 were assigned to a test group and fed SM-fortified milk (SM 20% of all PLs in milk) and 12 were assigned to a control group (SM 13% of all PLs in milk) for 18 mo | The composition of the plasma phospholipids and red-cell-membrane fatty acids were analyzed, after which visual evoked potential, Fagan test of infant intelligence, BSID-II, attention and memory tests were performed | Nutritional intervention via administration of SM-fortified milk has a positive association with the neurobehavioral development of low-birth-weight infants | Tanaka et al. 2013 (88) |
| MFGM concentrate | 182 Children (85 males, 97 females) between 2.5 and 6 d of age | Subjects received a daily amount of a 200-mL chocolate formula milk without phospholipids (n = 96) or a 200-mL chocolate formula milk enriched with 500 mg of PLs (n = 86) for 4 mo | The primary endpoints for analysis were the number of days with fever, diarrhea, coughing, or constipation. The secondary endpoints were the number of doctor visits, medication intake, number of missed schooldays, acceptability of the test drinks, and safety | Regular consumption of formula enriched with a concentrated MFGM product by preschool children was safe, well tolerated, and is associated with a significant decrease in the number of short febrile episodes and leads to improved behavioral regulation | Veereman- Wauters et al. 2012 (90) |
| Milk SL | 18 Healthy men between 22 and 65 y of age | Volunteers ingested a high-fat (40 g) standard breakfast together with a 340 mL milk-like formulation (containing 975 mg milk SL) or skim milk (containing 119 mg SL), of which PLs have been replaced by 2/3 of egg phospholipids and 1/3 butter oil for 10–14 d in a randomized crossover design | Postprandial concentrations of TG, total cholesterol, LDL cholesterol, HDL cholesterol, apoAI, apoB, glucose, and insulin were measured 1 to 7 h after the meal | After 1 h there was a trend of lower cholesterol concentrations in large TG-rich lipoproteins after SL-rich buttermilk drink | Ohlsson et al. 2010 (99) |
| SL-enriched butter milk formulation | 48 Healthy adults (33 males and 15 females) between 20 and 65 y of age | Subjects were examined the effects of an SL-enriched butter milk formulation (n = 29, 700 mg SM per day) and an equivalent control formulation (n = 19) in 4 wk | Plasma concentrations of cholesterol, TG, HDL, LDL, apolipoproteins AI and B, and lipoprotein (a) were measured | There was no significant decrease in plasma lipids or lipoprotein concentrations of an SL-enriched formulation containing 2–3 times more SL than the normal dietary intake | Ohlsson et al. 2009 (113) |
1BSID-II, Bayley Scales of Infant Development Second Edition; DG, diglyceride; MetS, metabolic syndrome; MFGM, milk fat globule membrane; MG, monoglyceride; PL, phospholipid; PCSK9, proprotein convertase subtilisin/kexin type 9; SL, sphingolipid; SM, sphingomyelin; TG, triglyceride.
Promoting neural development and intestinal tract maturation in infants
Although very little is known about the importance of SM nutrition during the neonatal period, SM may affect the growth and development of neonatal tissues by regulating cell proliferation and differentiation. Prolonged and exclusive breastfeeding plays an important role in early neurodevelopment and childhood cognitive outcomes (83, 84). A study showed that feeding human milk or infant formula to weanling rats for 28 d can lead to differences in total brain SM concentrations (85). Dietary SM could enhance the DHA concentration in the red cell membrane and promote myelin formation in the central nervous system and brain neuroplasticity, which are 2 imperative processes in neuronal development (86, 87). Tanaka et al. (88) performed a study on humans to investigate the effects of SM on the mental, athletic, and behavioral development of premature infants, which was the first report to evaluate the effect of SM on infant neurodevelopment. They randomly allocated 24 premature infants with birth weights <1500 g into test (fed SM-fortified milk; SM accounts for 20% of PLs) and control (SM accounts for 13% of PLs) groups. Their analysis suggested that the nutritional intervention of SM-fortified milk positively correlated with the neurobehavioral development of infants with low birth weight. However, to assess the impact of SM on long-term development, a detailed study is required. In addition, the cognitive score was higher in the MFGM-supplemented experimental infant milk formula group than in the standard infant milk formula group at 12 mo old but was not significantly different from that of the breastfed infant group (89). Moreover, beneficial effects on behavioral and emotional regulation were reported in preschool children consuming MFGM concentrate in milk for 4 mo (90). However, whether MFGM supplementation benefits from the action of SM or other PLs or a combination of bioactive components is not yet known (91–93).
Only a few studies have examined the SM function in intestinal tract maturation in infants. Motouri et al. (51) used artificially raised mice as a model for intestinal tract maturation in breastfeeding infants to assess the effect of SM. Their study suggested that cow-milk SM played an important role in the intestinal tract maturation of infants during the suckling period. The daily SM consumption estimated to be 50–150 mg in breastfeeding infants could promote digestion in the intestinal tract. Nejrup et al. (94) found that long-chain nonesterified FAs with 10% Sph can increase the relative abundance of bifidobacteria, which are the most abundant bacteria in the gut of breastfed infants in fecal content, according to a 24-h in vitro fermentation study in the feces of healthy infants. The effect on bifidobacteria may mainly rely on the competitive advantage caused by an antagonistic effect of polar lipids on competing species. Sph, a hydrolytic SM product, displays bactericidal activity in vitro among rodent studies. Moreover, Sph has been shown to induce ultrastructural damage to bacteria (e.g., Escherichia coli and Staphylococcus aureus). Sphingoid bases can insert into the outer layers of bacteria and disrupt the normal function of their cytoplasmic membranes or accumulate inside the cells and interfere with normal metabolism (95). This suggests that bovine milk SLs and their metabolic products may potentially exert changes in the intestinal microbiota if regularly consumed (96, 97). Thus, the role of SM in the intestinal tract maturation of infants is still currently an ongoing discussion because several of the studies cited have multiple nutritional interventions (e.g., whey-derived MFGM, cream-derived MFGM, nutrient mixture, and other SM sources).
Intestinal lipid metabolism and obesity complications
Several studies concluded that SM could inhibit the intestinal absorption of dietary lipids (e.g., cholesterol, fat, and other lipids) (98, 99). Moreover, supplementing SM results in decreased development of hepatic steatosis and adipose tissue inflammation in a mouse model (100, 101), and supplementing SM may have the potential to prevent atherosclerosis and protect against diet-induced adiposity by suppressing adipogenesis and promoting brown-like transformation in white adipose tissue (102, 103). Thus, dietary SM mitigates the metabolic complications related to diet-induced obesity. Obesity is characterized by increased LCFA intake and damaged lipid metabolism in hepatocytes (104). In addition, SM has a higher affinity for cholesterol than PC (105). Cholesterol was reported to have a greater affinity for PLs containing SFA chains. Therefore, milk SM is a more potent inhibitor of cholesterol than egg SM, which may be due to milk SM comprising a higher saturation degree and longer chain length of its fatty acyl groups than those in egg SM (79). These could have an effect on emulsification that allowed the SM acyl chains and cholesterol to pack more tightly, slowing the release of micellar lipids to the enterocyte (95). It may inhibit lumen lipolysis, micellar dissolution, and the transfer of micellar lipids to the intestinal cell. SM is incompletely ingested and can act as a long-term inhibitor of cholesterol absorption (106). Furthermore, dietary supplementation with milk polar lipids inhibits the development of obesity without impacting caloric intake but is associated with changes in gut microbiota populations (107).
In addition, researchers demonstrated that milk SM improves lipid metabolism and modulates the intestinal microbiota of mice fed a high-fat diet, suggesting that dietary SLs can modulate the intestinal tract microbiota and lipid absorption (81, 107, 108).
Milk SM, one of the major polar lipids in MFGM, inhibited intestinal lipid absorption and reduced serum and liver lipid concentrations in obese/diabetic KK-Ay mice (109). Milk SM also improved lipid metabolism by reducing hepatic TGs and inducing gene expression associated with cholesterol biosynthesis in high-fat-diet–fed mice (81).
Im et al. (110) recently found that SM concentration positively correlated with cholesteryl esters and waist-to-hip ratio and that SM concentrations were higher in prediabetic men with abdominal obesity. However, dietary milk polar lipids may have limited beneficial effects on gut barrier integrity, systemic inflammation, and lipid metabolism in severe obesity contexts (111).
Conversely, polar lipids and milk SM were also found to have little effect on cholesterol absorption and lower the lipid concentration in other studies. Ramprasath et al. (112) reported that no change was noted in the human blood profile upon daily consumption of 1 g of SM in a small-scale human crossover study. Dietary SM did not affect the absorption, synthesis, or intracavitary dissolution of cholesterol compared with the control group. However, dietary SM enhanced the concentration of HDL cholesterol.
Furthermore, a study by Ohlsson et al. (113) did not demonstrate the lipid-lowering effect of polar lipids in the milk fat that was rich in SLs. In follow-up studies, persons (mean age: 38.2 y; range: 22–50 y) who had undergone a colectomy because of severe ulcerative colitis (no ileal resection) ≥6 mo before the experiment were considered to have well-functioning ileostomies. Similarly, Ohlsson et al. (61) did not observe any changes in the total cholesterol content of ileostomy outputs of subjects consuming different amounts of milk SM (50–200 mg) within a test meal. Although short-term human studies with limited sample sizes showed no effect, more studies should be conducted in humans examining the effects of milk SM on lipid absorption because it is likely that a higher SM dose is required than in mice because alk-SMase is also present in human bile (95).
The effects of SM on cholesterol absorption and obesity differ from the experimental population. The effects were inhibitory in in vivo models (114) and most mouse or rat models, whereas few effects were found in most human models. Existing evidence is far from sufficient to explain the effects of SM on cholesterol absorption and obesity. In addition, a growing concern was noted that laboratory mice may not reflect relevant aspects of the human immune system, which may fail to translate disease treatments from laboratory studies to clinical application (115–118). Laboratory mice live in abnormally hygienic specific pathogen-free barrier facilities. Altering the living conditions of mice greatly impacts the cellular composition of the innate and adaptive immune systems (119). Thus, future studies must be designed considering proper experimental models, including co-ingestion with other components (120), health conditions, sex, and age, to define the precise role of SM in obesity (121, 122).
Colon tumor prevention and suppression
Cell proliferation normalization and cell apoptosis rate other than induced differentiation seem to be the key mechanisms for tumor genesis inhibition by dietary SM. Biologically active products generated through SM digestion regulate cell growth, differentiation, and apoptosis involved in colorectal cancer (3). The number of aberrant colon crypt foci (early markers of colon cancer development) was reduced by 70%, and the number of aberrant crypts at each focus was reduced by 30% in mice fed a diet containing SM (0.1% SM purified from milk) (59). Similarly, dietary SM supplementation decreases colonic inflammation and inflammation-driven colorectal cancer (123, 124). Interestingly, alk-SMase, a key enzyme involved in SM digestion, was found to play a role in colon tumor prevention and suppression. Clinical evidence has shown that the activity of alk-SMase is decreased by 25% and 75% in chronic colitis and colon cancer, respectively (125). The upregulation of colonic alk-SMase plays a positive effect on colon carcinogenesis (126, 127).
However, the effect of dietary SM on colon inflammation remains controversial because several studies observed the exacerbation of colitis in mice fed egg SM (128). More animal or clinical studies are needed before using SM or SLs as a substitute for conventional drugs in cancer-prevention or -treatment strategies. Similarly, conclusions should be drawn conservatively and with rigorous consideration of the evidence when translating disease treatments from laboratory studies to clinical applications.
Conclusions
The current review suggests that milk and dairy products are rich SM sources, especially human milk. SM digestion occurs throughout the small intestine, mainly in the middle sections, which is slow and incomplete. In adults, a large SM proportion is retained in the intestine and would probably be co-excreted with cholesterol to reduce intestinal cholesterol absorption. The SM potential in colon tumor prevention and suppression has been demonstrated in animal studies, whereas it is necessary to thoroughly explore the effect in clinical trials. With regard to newborns and children, SM may promote neural development before childhood. Nevertheless, to evaluate the long-term SM effect on cognitive development, further studies are needed. Only a few studies have focused on the metabolic SM pathway during the digestion of newborns, which still must be studied. Meanwhile, SM biological effects differ from experimental object models. Conclusions should be drawn conservatively and with rigorous consideration of the evidence when translating disease treatments from laboratory studies to clinical applications. In addition, whether MFGM supplementation benefits from the action of SM or other PLs or a combination of bioactive components is not well reported. Thus, more work is required to explore the effects of dietary SM, especially on newborns.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—WW: conceived the review; CJ and XZ: performed the literature search, screened titles and abstracts, and reviewed the full text of articles identified by the literature search; CJ and L-ZC: performed quality assessment; CJ, L-ZC, XZ, and AHA: wrote the first draft of the manuscript; WW and XW: checked the data extraction and edited the final draft of the manuscript; and all authors: read and approved the final manuscript.
Notes
WW is supported by the National Natural Science Foundation of China (31701558). L-ZC is supported by the Natural Science Foundation of Zhejiang Province (LGJ20C200001).
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: alk-SMase, alkaline sphingomyelinase; CCK, cholecystokinin; Cer, ceramide; CMC, critical micelle concentration; ELSD, evaporative light-scattering detection; FA, fatty acid; HILIC, hydrophilic interaction liquid chromatography; LCFA, long-chain fatty acid; MFGM, milk fat globule membrane; N-CDase, neutral ceramidase; NPP, nucleotide pyrophosphatase/phosphodiesterase; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PL, phospholipid; PS, phosphatidylserine; SL, sphingolipid; SM, sphingomyelin; Sph, sphingosine; Sph-1-P, sphingosine-1-phosphate; TG, triglyceride; TLC, thin-layer chromatography; 31P NMR, phosphorus NMR.
Contributor Information
Chenyu Jiang, State Key Lab of Food Science and Technology, Jiangnan University, Wuxi, China; Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, School of Food Science and Technology, Jiangnan University, Wuxi, China.
Ling-Zhi Cheong, Department of Food Science and Engineering, College of Food and Pharmaceutical Sciences, Ningbo University, Ningbo, China.
Xue Zhang, State Key Lab of Food Science and Technology, Jiangnan University, Wuxi, China; Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, School of Food Science and Technology, Jiangnan University, Wuxi, China.
Abdelmoneim H Ali, State Key Lab of Food Science and Technology, Jiangnan University, Wuxi, China; Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, School of Food Science and Technology, Jiangnan University, Wuxi, China.
Qingzhe Jin, State Key Lab of Food Science and Technology, Jiangnan University, Wuxi, China; Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, School of Food Science and Technology, Jiangnan University, Wuxi, China.
Wei Wei, State Key Lab of Food Science and Technology, Jiangnan University, Wuxi, China; Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, School of Food Science and Technology, Jiangnan University, Wuxi, China.
Xingguo Wang, State Key Lab of Food Science and Technology, Jiangnan University, Wuxi, China; Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, School of Food Science and Technology, Jiangnan University, Wuxi, China.
References
- 1. Moloney C, Walshe E, Phelan M, Giuffrida F, Badoud F, Bertschy E, O'Regan J. Sphingomyelin content of dairy protein ingredients and infant formula powders, and identification of bovine sphingomyelin species. Int Dairy J. 2018;78:138–44. [Google Scholar]
- 2. An D, Na C, Bielawski J, Hannun YA, Kasper DL. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc Natl Acad Sci. 2011;108(Suppl 1):4666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50. [DOI] [PubMed] [Google Scholar]
- 4. Bienias K, Fiedorowicz A, Sadowska A, Prokopiuk S, Car H. Regulation of sphingomyelin metabolism. Pharmacol Rep. 2016;68(3):570–81. [DOI] [PubMed] [Google Scholar]
- 5. Norris GH, Blesso CN. Dietary sphingolipids: potential for management of dyslipidemia and nonalcoholic fatty liver disease. Nutr Rev. 2017;75(4):274–85. [DOI] [PubMed] [Google Scholar]
- 6. Fischbeck A, Krüger M, Blaas N, Humpf HU. Analysis of sphingomyelin in meat based on hydrophilic interaction liquid chromatography coupled to electrospray ionization-tandem mass spectrometry (HILIC-HPLC-ESI-MS/MS). J Agric Food Chem. 2009;57(20):9469–74. [DOI] [PubMed] [Google Scholar]
- 7. Blaas N, Schüürmann C, Bartke N, Stahl B, Humpf HU. Structural profiling and quantification of sphingomyelin in human breast milk by HPLC-MS/MS. J Agric Food Chem. 2011;59(11):6018–24. [DOI] [PubMed] [Google Scholar]
- 8. Slotte JP. The importance of hydrogen bonding in sphingomyelin's membrane interactions with co-lipids. Biochim Biophys Acta Biomembranes. 2016;1858(2):304–10. [DOI] [PubMed] [Google Scholar]
- 9. Et-Thakafy O, Delorme N, Guyomarc'h F, Lopez C. Mechanical properties of milk sphingomyelin bilayer membranes in the gel phase: effects of naturally complex heterogeneity, saturation and acyl chain length investigated on liposomes using AFM. Chem Phys Lipids. 2018;210:47–59. [DOI] [PubMed] [Google Scholar]
- 10. Liu Z, Rochfort S, Cocks B. Milk lipidomics: what we know and what we don't. Prog Lipid Res. 2018;71:70–85. [DOI] [PubMed] [Google Scholar]
- 11. Lopez C. Lipid domains in the milk fat globule membrane: specific role of sphingomyelin. Lipid Technology. 2010;22(8):175–8. [Google Scholar]
- 12. Phan TTQ, Le TT, Walle D, Meeren P, Dewettinck K. Combined effects of milk fat globule membrane polar lipids and protein concentrate on the stability of oil-in-water emulsions. Int Dairy J. 2016;52:42–9. [Google Scholar]
- 13. Garcia C, Lutz NW, Confort-Gouny S, Cozzone PJ, Armand M, Bernard M. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: towards specific interest in human health. Food Chem. 2012;135(3):1777–83. [DOI] [PubMed] [Google Scholar]
- 14. Lopez C, Ménard O. Human milk fat globules: polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf B. 2011;83(1):29–41. [DOI] [PubMed] [Google Scholar]
- 15. Sala-Vila A, Castellote AI, Rodriguez-Palmero M, Campoy C, López-Sabater MC. Lipid composition in human breast milk from Granada (Spain): changes during lactation. Nutrition. 2005;21(4):467–73. [DOI] [PubMed] [Google Scholar]
- 16. Zou X, Huang J, Jin Q, Guo Z, Liu Y, Cheong L, Xu X, Wang X. Lipid composition analysis of milk fats from different mammalian species: potential for use as human milk fat substitutes. J Agric Food Chem. 2013;61(29):7070–80. [DOI] [PubMed] [Google Scholar]
- 17. Zou X, Guo Z, Huang J, Jin Q, Cheong LZ, Wang X, Xu X. Human milk fat globules from different stages of lactation: a lipid composition analysis and microstructure characterization. J Agric Food Chem. 2012;60(29):7158–67. [DOI] [PubMed] [Google Scholar]
- 18. Claumarchirant L, Cilla A, Matencio E, Sanchez-Siles LM, Castro-Gomez P, Fontecha J, Alegría A, Lagarda MJ. Addition of milk fat globule membrane as an ingredient of infant formulas for resembling the polar lipids of human milk. Int Dairy J. 2016;61:228–38. [Google Scholar]
- 19. Giuffrida F, Cruz-Hernandez C, Flück B, Tavazzi I, Thakkar SK, Destaillats F, Braun M. Quantification of phospholipids classes in human milk. Lipids. 2013;48(10):1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yao Y, Zhao G, Xiang J, Zou X, Jin Q, Wang X. Lipid composition and structural characteristics of bovine, caprine and human milk fat globules. Int Dairy J. 2016;56:64–73. [Google Scholar]
- 21. MacFarland B, Bettler J, Moloney C, O'Regan J, Giuffrida F, Thakkar S, Lee LY. Sphingomyelin content in breast milk and infant formula: a nutrient that may affect neurodevelopment. Adv Nutr. 2017;8(1):17.28096124 [Google Scholar]
- 22. Ma L, MacGibbon AKH, Mohamed H, Loy S, Rowan A, McJarrow P, Fong BY. Determination of phospholipid concentrations in breast milk and serum using a high performance liquid chromatography–mass spectrometry–multiple reaction monitoring method. Int Dairy J. 2017;71:50–9. [Google Scholar]
- 23. Tavazzi I, Fontannaz P, Lee LY, Giuffrida F. Quantification of glycerophospholipids and sphingomyelin in human milk and infant formula by high performance liquid chromatography coupled with mass spectrometer detector. J Chromatogr B. 2018;1072:235–43. [DOI] [PubMed] [Google Scholar]
- 24. Benoit B, Fauquant C, Daira P, Peretti N, Guichardant M, Michalski MC. Phospholipid species and minor sterols in French human milks. Food Chem. 2010;120(3):684–91. [Google Scholar]
- 25. Sanchez-Juanes F, Alonso JM, Zancada L, Hueso P. Distribution and fatty acid content of phospholipids from bovine milk and bovine milk fat globule membranes. Int Dairy J. 2009;19(5):273–8. [Google Scholar]
- 26. Bettger WJ, DiMichelle-Ranalli E, Dillingham B, Blackadar CB. Nervonic acid is transferred from the maternal diet to milk and tissues of suckling rat pups. J Nutr Biochem. 2003;14(3):160–5. [DOI] [PubMed] [Google Scholar]
- 27. Yu J, Yuan T, Zhang X, Jin Q, Wei W, Wang X. Quantification of nervonic acid in human milk in the first 30 days of lactation: influence of lactation stages and comparison with infant formulae. Nutrients. 2019;11(8):1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo J, Huang Z, Liu H, Zhang Y, Ren F. Yak milk fat globules from the Qinghai-Tibetan Plateau: membrane lipid composition and morphological properties. Food Chem. 2018;245:731–7. [DOI] [PubMed] [Google Scholar]
- 29. Gallier S, Gragson D, Cabral C, Jiménez-Flores R, Everett DW. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. J Agric Food Chem. 2010;58(19):10503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodríguez-Alcalá LM, Fontecha J. Major lipid classes separation of buttermilk, and cows, goats and ewes milk by high performance liquid chromatography with an evaporative light scattering detector focused on the phospholipid fraction. J Chromatogr A. 2010;1217(18):3063–6. [DOI] [PubMed] [Google Scholar]
- 31. Rombaut R, Dewettinck K, Camp JV. Phospho- and sphingolipid content of selected dairy products as determined by HPLC coupled to an evaporative light scattering detector (HPLC–ELSD). J Food Compos Anal. 2007;20(3–4):308–12. [Google Scholar]
- 32. Lopez C, Blot M, Briard-Bion V, Cirié C, Graulet B. Butter serums and buttermilks as sources of bioactive lipids from the milk fat globule membrane: differences in their lipid composition and potentialities of cow diet to increase n-3 PUFA. Food Res Int. 2017;100(Pt 1):864–72. [DOI] [PubMed] [Google Scholar]
- 33. Zhu D, Damodaran S. Composition, thermotropic properties, and oxidative stability of freeze-dried and spray-dried milk fat globule membrane isolated from cheese whey. J Agric Food Chem. 2011;59(16):8931–8. [DOI] [PubMed] [Google Scholar]
- 34. Zhao Y, Xiong Y, Curtis JM. Measurement of phospholipids by hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry: the determination of choline containing compounds in foods. J Chromatogr A. 2011;1218(32):5470–9. [DOI] [PubMed] [Google Scholar]
- 35. Xiong Y, Zhao Y, Goruk S, Oilund K, Field CJ, Jacobs RL, Curtis JM. Validation of an LC-MS/MS method for the quantification of choline-related compounds and phospholipids in foods and tissues. J Chromatogr B. 2012;911:170–9. [DOI] [PubMed] [Google Scholar]
- 36. Ali AH, Zou X, Lu J, Abed SM, Yao Y, Tao G, Jin Q, Wang X. Identification of phospholipids classes and molecular species in different types of egg yolk by using UPLC-Q-TOF-MS. Food Chem. 2017;221:58–66. [DOI] [PubMed] [Google Scholar]
- 37. Zhou L, Yang F, Zhao M, Zhang M, Liu J, Marchioni E. Determination and comparison of phospholipid profiles in eggs from seven different species using UHPLC-ESI-Triple TOF-MS. Food Chem. 2021;339:127856. [DOI] [PubMed] [Google Scholar]
- 38. Zhou L, Zhao M, Ennahar S, Bindler F, Marchioni E. Liquid chromatography-tandem mass spectrometry for the determination of sphingomyelin species from calf brain, ox liver, egg yolk, and krill oil. J Agric Food Chem. 2012;60(1):293–8. [DOI] [PubMed] [Google Scholar]
- 39. Cilla A, Quintaes KD, Barberá R, Alegría A. Phospholipids in human milk and infant formulas: benefits and needs for correct infant nutrition. Crit Rev Food Sci Nutr. 2016;56(11):1880–92. [DOI] [PubMed] [Google Scholar]
- 40. MacKenzie A, Vyssotski M, Nekrasov E. Quantitative analysis of dairy phospholipids by 31P NMR. J Am Oil Chem Soc. 2009;86(8):757–63. [Google Scholar]
- 41. Furse S, Koulman A. The lipid and glyceride profiles of infant formula differ by manufacturer, region and date sold. Nutrients. 2019;11(5):1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei W, Yang J, Yang D, Wang X, Yang Z, Jin Q, Wang M, Lai J, Wang X. Phospholipid composition and fat globule structure I: comparison of human milk fat from different gestational ages, lactation stages, and infant formulas. J Agric Food Chem. 2019;67(50):13922–28. [DOI] [PubMed] [Google Scholar]
- 43. Zhu D, Hayman A, Kebede B, Stewart I, Chen G, Frew R. 31P NMR-Based phospholipid fingerprinting of powdered infant formula. J Agric Food Chem. 2019;67(36):10265–72. [DOI] [PubMed] [Google Scholar]
- 44. Zhang Y, Wu G, Zhang Y, Wang X, Jin Q, Zhang H. Advances in exogenous docosahexaenoic acid-containing phospholipids: sources, positional isomerism, biological activities, and advantages. Compr Rev Food Sci Food Safety. 2020;19(4):1420–48. [DOI] [PubMed] [Google Scholar]
- 45. Liu Z, Moate P, Cocks B, Rochfort S. Comprehensive polar lipid identification and quantification in milk by liquid chromatography-mass spectrometry. J Chromatogr B. 2015;978-979:95–102. [DOI] [PubMed] [Google Scholar]
- 46. Russo M, Cichello F, Ragonese C, Donato P, Cacciola F, Dugo P, Mondello L. Profiling and quantifying polar lipids in milk by hydrophilic interaction liquid chromatography coupled with evaporative light-scattering and mass spectrometry detection. Anal Bioanal Chem. 2013;405(13):4617–26. [DOI] [PubMed] [Google Scholar]
- 47. Jiang C, Ma B, Song S, Lai OM, Cheong LZ. Fingerprinting of phospholipid molecular species from human milk and infant formula using HILIC-ESI-IT-TOF-MS and discriminatory analysis by principal component analysis. J Agric Food Chem. 2018;66(27):7131–38. [DOI] [PubMed] [Google Scholar]
- 48. Lísa M, Holčapek M. High-throughput and comprehensive lipidomic analysis using ultrahigh-performance supercritical fluid chromatography-mass spectrometry. Anal Chem. 2015;87(14):7187–95. [DOI] [PubMed] [Google Scholar]
- 49. Paglia G, Astarita G. Metabolomics and lipidomics using traveling-wave ion mobility mass spectrometry. Nat Protoc. 2017;12(4):797–813. [DOI] [PubMed] [Google Scholar]
- 50. Vesper H, Schmelz EM, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Alfred H, Merrill J. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr. 1999;129(7):1239–50. [DOI] [PubMed] [Google Scholar]
- 51. Motouri M, Matsuyama H, Yamamura JI, Tanaka M, Aoe S, Iwanaga T, Kawakami H. Milk sphingomyelin accelerates enzymatic and morphological maturation of the intestine in artificially reared rats. J Pediatr Gastroenterol Nutr. 2003;36(2):241–7. [DOI] [PubMed] [Google Scholar]
- 52. Nilsson Å, Duan RD. Pancreatic and mucosal enzymes in choline phospholipid digestion. Am J Physiol Gastrointest Liver Physiol. 2019;316(4):G425–G45. [DOI] [PubMed] [Google Scholar]
- 53. Duan R-D, Cheng Y, Jönsson BA, Ohlsson L, Herbst A, Hellström-Westas L, Nilsson A. Human meconium contains significant amounts of alkaline sphingomyelinase, neutral ceramidase, and sphingolipid metabolites. Pediatr Res. 2007;61(1):61–6. [DOI] [PubMed] [Google Scholar]
- 54. Nilsson A, Duan RD. Absorption and lipoprotein transport of sphingomyelin. J Lipid Res. 2006;47(1):154–71. [DOI] [PubMed] [Google Scholar]
- 55. Park JH, Schuchman EH. Acid ceramidase and human disease. Biochim Biophys Acta Biomembranes. 2006;1758(12):2133–8. [DOI] [PubMed] [Google Scholar]
- 56. Romiti E, Meacci E, GiudittaTanzi BL, Mitsutake S, Farnararo M, Ito M, Bruni P. Localization of neutral ceramidase in caveolin-enriched light membranes of murine endothelial cells. FEBS Lett. 2001;506(2):163–8. [DOI] [PubMed] [Google Scholar]
- 57. Xu R, Jin J, Hu W, Sun W, Bielawski J, Szulc Z, Taha T, Obeid LM, Mao C. Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. FASEB J. 2006;20(11):1813–25. [DOI] [PubMed] [Google Scholar]
- 58. Schmelz EM, Crall KJ, Larocque R, Dillehay DL, Alfred H, Merrill J. Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J Nutr. 1994;124(5):702–12. [DOI] [PubMed] [Google Scholar]
- 59. Schmelz EM, Dillehay DL, Webb SK, Reiter A, Adams J, Alfred H, Merrill J. Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 1996;56(21):4936–41. [PubMed] [Google Scholar]
- 60. Vors C, Joumard-Cubizolles L, Lecomte M, Combe E, Ouchchane L, Drai J, Raynal K, Joffre F, Meiller L, Barz MLet al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: towards a gut sphingomyelin-cholesterol interplay. Gut. 2020;69(3):487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ohlsson L, Hertervig E, Jönsson BA, Duan RD, Nyberg L, Svernlöv R, Nilsson A. Sphingolipids in human ileostomy content after meals containing milk sphingomyelin. Am J Clin Nutr. 2010;91(3):672–8. [DOI] [PubMed] [Google Scholar]
- 62. Wu J, Liu F, Nilsson Å, Duan RD. Pancreatic trypsin cleaves intestinal alkaline sphingomyelinase from mucosa and enhances the sphingomyelinase activity. Am J Physiol Gastrointest Liver Physiol. 2004;287(5):G967–G73. [DOI] [PubMed] [Google Scholar]
- 63. Nyberg L, Nilsson Å, Lundgren P, Duan RD. Localization and capacity of sphingomyelin digestion in the rat intestinal tract. J Nutr Biochem. 1997;8(3):112–8. [Google Scholar]
- 64. Liu JJ, Nilsson Å, Duan RD. Effects of phospholipids on sphingomyelin hydrolysis induced by intestinal alkaline sphingomyelinase: an in vitro study. J Nutr Biochem. 2000;11(4):192–7. [DOI] [PubMed] [Google Scholar]
- 65. Duan R-D, Nilsson Å. Purification of a newly identified alkaline sphingomyelinase in human bile and effects of bile salts and phosphatidylcholine on enzyme activity. Hepatology. 1997;26(4):823–30. [DOI] [PubMed] [Google Scholar]
- 66. Summers SA, Chaurasia B, Holland WL. Metabolic messengers: ceramides. Nat Metab. 2019;1(11):1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Iqbal J, Jahangir Z, Al-Qarni AA. Microsomal triglyceride transfer protein: from lipid metabolism to metabolic diseases. In: Jiang X, editor. Lipid transfer in lipoprotein metabolism and cardiovascular disease. Singapore: Springer; 2020. p. 37–52. [DOI] [PubMed] [Google Scholar]
- 68. Lopez C, Briard-Bion V, Ménard O, Beaucher E, Rousseau F, Fauquant J, Leconte N, Robert B. Fat globules selected from whole milk according to their size: different compositions and structure of the biomembrane, revealing sphingomyelin-rich domains. Food Chem. 2011;125(2):355–68. [Google Scholar]
- 69. Duan RD. Alkaline sphingomyelinase: an old enzyme with novel implications. Biochim Biophys Acta Mol Cell Biol Lipids. 2006;1761(3):281–91. [DOI] [PubMed] [Google Scholar]
- 70. Nilsson Å. Role of sphingolipids in infant gut health and immunity. J Pediatr. 2016;173:S53–59. [DOI] [PubMed] [Google Scholar]
- 71. Lee S, Lee YS, Choi KM, Yoo KS, Sin DM, Kim W, Lee YM, Hong JT, Yun YP, Yoo HS. Quantitative analysis of sphingomyelin by high-performance liquid chromatography after enzymatic hydrolysis. Evid Based Complementary Altern Med. 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fernández-Arroyo S, Hernández-Aguilera A, de Vries MA, Burggraaf B, Evd Z, Pouw N, Joven J, Cabezas MC. Effect of vitamin D3 on the postprandial lipid profile in obese patients: a non-targeted lipidomics study. Nutrients. 2019;11(5):1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Slotte JP. Biological functions of sphingomyelins. Prog Lipid Res. 2013;52(4):424–37. [DOI] [PubMed] [Google Scholar]
- 74. Ortega-Anaya J, Jimenez-Flores R. Symposium review: the relevance of bovine milk phospholipids in human nutrition—evidence of the effect on infant gut and brain development. J Dairy Sci. 2019;102(3):2738–48. [DOI] [PubMed] [Google Scholar]
- 75. Grip T, Dyrlund TS, Ahonen L, Domellöf M, Hernell O, Hyötyläinen T, Knip M, Lönnerdal B, Orešič M, Timby N. Serum, plasma and erythrocyte membrane lipidomes in infants fed formula supplemented with bovine milk fat globule membranes. Pediatr Res. 2018;84(5):726–32. [DOI] [PubMed] [Google Scholar]
- 76. Huang Y, Huang T, Zhen X, Li Y, Mo M, Ye D, Cheng N. A selective sphingomyelin synthase 2 inhibitor ameliorates diet induced insulin resistance via the IRS-1/Akt/GSK-3beta signaling pathway. Pharmazie. 2019;74(9):553–8. [DOI] [PubMed] [Google Scholar]
- 77. Witt MJL, Ramón-Krauel M, Samino S, Llobet M, Cuadras D, Jimenez-Chillaron JC, Yanes Ó, Lerin C. Untargeted metabolomics identifies a plasma sphingolipid-related signature associated with lifestyle intervention in prepubertal children with obesity. Int J Obes. 2018;42(1):72–8. [DOI] [PubMed] [Google Scholar]
- 78. Taniguchi M, Okazaki T. The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration—from cell and animal models to human disorders. Biochim Biophys Acta Mol Cell Biol Lipids. 2014;1841(5):692–703. [DOI] [PubMed] [Google Scholar]
- 79. Noh SK, Koo SI. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J Nutr. 2004;134(10):2611–6. [DOI] [PubMed] [Google Scholar]
- 80. Quarles WR, Pokala A, Shaw EL, Ortega-Anaya J, Hillmann L, Jimenez-Flores R, Bruno RS. Alleviation of metabolic endotoxemia by milk fat globule membrane: rationale, design, and methods of a double-blind, randomized, controlled, crossover dietary intervention in adults with metabolic syndrome. Curr Dev Nutr. 2020;4(9):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Norris GH, Jiang C, Ryan J, Porter CM, Blesso CN. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J Nutr Biochem. 2016;30:93–101. [DOI] [PubMed] [Google Scholar]
- 82. Norris GH, Porter CM, Jiang C, Blesso CN. Dietary milk sphingomyelin reduces systemic inflammation in diet-induced obese mice and inhibits LPS activity in macrophages. Beverages. 2017;3(4):37. [Google Scholar]
- 83. Deoni S, DD III, Joelson S, O'Regan J, Schneider N. Early nutrition influences developmental myelination and cognition in infants and young children. Neuroimage. 2018;178:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schneider N, Hauser J, Oliveira M, Cazaubon E, Mottaz SC, O'Neill BV, Steiner P, Deoni SCL. Sphingomyelin in brain and cognitive development: preliminary data. eNeuro. 2019;6(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Su M, Subbaraj AK, Fraser K, Qi X, Jia H, Chen W, Reis MG, Agnew M, Day L, Roy NCet al. Lipidomics of brain tissues in rats fed human milk from Chinese mothers or commercial infant formula. Metabolites. 2019;9(11):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sonnino S, Prinetti A. The role of sphingolipids in neuronal plasticity of the brain. J Neurochem. 2016;137(4):485–8. [DOI] [PubMed] [Google Scholar]
- 87. Fil JE, Fleming SA, Chichlowski M, Gross G, Berg BM, Dilger RN. Evaluation of dietary bovine milk fat globule membrane supplementation on growth, serum cholesterol and lipoproteins, and neurodevelopment in the young pig. Front Pediatr. 2019;7:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tanaka K, Hosozawa M, Kudo N, Yoshikawa N, Hisata K, Shoji H, Shinohara K, Shimizu T. The pilot study: sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013;35(1):45–52. [DOI] [PubMed] [Google Scholar]
- 89. Timby N, Domellöf E, Hernell O, Lönnerdal B, Domellöf M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr. 2014;99(4):860–8. [DOI] [PubMed] [Google Scholar]
- 90. Veereman-Wauters G, Staelens S, Rombaut R, Dewettinck K, Deboutte D, Brummer R-J, Boone M, Ruyet PL. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition. 2012;28(7–8):749–52. [DOI] [PubMed] [Google Scholar]
- 91. Lopez C, Cauty C, Guyomarc'h F. Unraveling the complexity of milk fat globules to tailor bioinspired emulsions providing health benefits: the key role played by the biological membrane. Eur J Lipid Sci Technol. 2018;121(1):1800201. [Google Scholar]
- 92. Zheng L, Fleith M, Giuffrida F, O'Neill BV, Schneider N. Dietary polar lipids and cognitive development: a narrative review. Adv Nutr. 2019;10(6):1163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li F, Wu SS, Berseth CL, Harris CL, Richards JD, Wampler JL, Zhuang W, Cleghorn G, Rudolph CD, Liu Bet al. Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: a randomized, controlled trial. J Pediatr. 2019;215:24–31, e8. [DOI] [PubMed] [Google Scholar]
- 94. Nejrup RG, Bahl MI, Vigsnæs LK, Heerup C, Licht TR, Hellgren LI. Lipid hydrolysis products affect the composition of infant gut microbial communities in vitro. Br J Nutr. 2015;114(1):63–74. [DOI] [PubMed] [Google Scholar]
- 95. Norris GH, Milard M, Michalski MC, Blesso CN. Protective properties of milk sphingomyelin against dysfunctional lipid metabolism, gut dysbiosis, and inflammation. J Nutr Biochem. 2019;73:108224. [DOI] [PubMed] [Google Scholar]
- 96. Anto L, Warykas SW, Torres-Gonzalez M, Blesso CN. Milk polar lipids: underappreciated lipids with emerging health benefits. Nutrients. 2020;12(4):1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gong H, Yuan Q, Pang J, Li T, Li J, Zhan B, Chang R, Mao X. Dietary milk fat globule membrane restores decreased intestinal mucosal barrier development and alterations of intestinal flora in infant-formula-fed rat pups. Mol Nutr Food Res. 2020;64(21):e2000232. [DOI] [PubMed] [Google Scholar]
- 98. Weiland A, Bub A, Barth SW, Schrezenmeir J, Pfeuffer M. Effects of dietary milk- and soya-phospholipids on lipid-parameters and other risk indicators for cardiovascular diseases in overweight or obese men—two double-blind, randomised, controlled, clinical trials. J Nutr Sci. 2016;5:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ohlsson L, Burling H, Duan RD, Nilsson Å. Effects of a sphingolipid-enriched dairy formulation on postprandial lipid concentrations. Eur J Clin Nutr. 2010;64(11):1344–9. [DOI] [PubMed] [Google Scholar]
- 100. Chung RWS, Kamili A, Tandy S, Weir JM, Gaire R, Wong G, Meikle PJ, Cohn JS, Rye KA. Dietary sphingomyelin lowers hepatic lipid levels and inhibits intestinal cholesterol absorption in high-fat-fed mice. PLoS One. 2013;8(2):e55949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Norris GH, Porter CM, Jiang C, Millar CL, Blesso CN. Dietary sphingomyelin attenuates hepatic steatosis and adipose tissue inflammation in high-fat-diet-induced obese mice. J Nutr Biochem. 2017;40:36–43. [DOI] [PubMed] [Google Scholar]
- 102. Millar CL, Norris GH, Vitols A, Garcia C, Seibel S, Anto L, Blesso CN. Dietary egg sphingomyelin prevents aortic root plaque accumulation in apolipoprotein-E knockout mice. Nutrients. 2019;11(5):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li T, Gao J, Du M, Song J, Mao X. Milk fat globule membrane attenuates high-fat diet-induced obesity by inhibiting adipogenesis and increasing uncoupling protein 1 expression in white adipose tissue of mice. Nutrients. 2018;10(3):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Charytoniuk T, Harasim-Symbor E, Polak A, Drygalski K, Berk K, Chabowski A, Konstantynowicz-Nowicka K. Influence of resveratrol on sphingolipid metabolism in hepatocellular carcinoma cells in lipid overload state. Anticancer Agents Med Chem. 2019;19(1):121–9. [DOI] [PubMed] [Google Scholar]
- 105. Erpecum Kv, Petruzzelli M, Groen AK, Moschetta A. Relevance of interactions between sphingomyelin and cholesterol in biliary and intestinal tract. Eur J Lipid Sci Technol. 2007;109(10):982–6. [Google Scholar]
- 106. Zhang P, Chen Y, Cheng Y, Hertervig E, Ohlsson L, Nilsson Å, Duan RD. Alkaline sphingomyelinase (NPP7) promotes cholesterol absorption by affecting sphingomyelin levels in the gut: a study with NPP7 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2014;306(10):G903–G8. [DOI] [PubMed] [Google Scholar]
- 107. Milard M, Laugerette F, Durand A, Buisson C, Meuniere E, Loizon E, Louche-Pelissier C, Sauvinet V, Garnier L, Viel Set al. Milk polar lipids in a high-fat diet can prevent body weight gain: modulated abundance of gut bacteria in relation with fecal loss of specific fatty acids. Mol Nutr Food Res. 2019;63(4):e1801078. [DOI] [PubMed] [Google Scholar]
- 108. Millar CL, Jiang C, Norris GH, Garcia C, Seibel S, Anto L, Lee JY, Blesso CN. Cow's milk polar lipids reduce atherogenic lipoprotein cholesterol, modulate gut microbiota and attenuate atherosclerosis development in LDL-receptor knockout mice fed a Western-type diet. J Nutr Biochem. 2020;79:108351. [DOI] [PubMed] [Google Scholar]
- 109. Yamauchi I, Uemura M, Hosokawa M, Iwashima-Suzuki A, Shiota M, Miyashita K. The dietary effect of milk sphingomyelin on the lipid metabolism of obese/diabetic KK-A(y) mice and wild-type C57BL/6J mice. Food Funct. 2016;7(9):3854–67. [DOI] [PubMed] [Google Scholar]
- 110. Im SS, Park HY, Shon JC, Chung IS, Cho HC, Liu KH, Song DK. Plasma sphingomyelins increase in pre-diabetic Korean men with abdominal obesity. PLoS One. 2019;14(3):e0213285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhou AL, Ward RE. Milk polar lipids modulate lipid metabolism, gut permeability, and systemic inflammation in high-fat-fed C57BL/6J ob/ob mice, a model of severe obesity. J Dairy Sci. 2019;102(6):4816–31. [DOI] [PubMed] [Google Scholar]
- 112. Ramprasath VR, Jones PJH, Buckley DD, Woollett LA, Heubi JE. Effect of dietary sphingomyelin on absorption and fractional synthetic rate of cholesterol and serum lipid profile in humans. Lipids Health Dis. 2013;12(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ohlsson L, Burling H, Nilsson Å. Long term effects on human plasma lipoproteins of a formulation enriched in butter milk polar lipid. Lipids Health Dis. 2009;8(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Eckhardt ERM, Wang DQH, Donovan JM, Carey MC. Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology. 2002;122(4):948–56. [DOI] [PubMed] [Google Scholar]
- 115. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy Let al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci. 2013;110(9):3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Shay T, Jojic V, Zuk O, Rothamel K, Puyraimond-Zemmour D, Feng T, Wakamatsu E, Benoist C, Koller D, Regev Aet al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci. 2013;110(8):2946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci. 2015;112(4):1167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6(2):114–8. [PMC free article] [PubMed] [Google Scholar]
- 119. Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim Aet al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Morifuji M, Higashi S, Oba C, Ichikawa S, Kawahata K, Yamaji T, Itoh H, Manabe Y, Sugawara T. Milk phospholipids enhance lymphatic absorption of dietary sphingomyelin in lymph-cannulated rats. Lipids. 2015;50(10):987–96. [DOI] [PubMed] [Google Scholar]
- 121. Torretta E, Barbacini P, Al-Daghri NM, Gelfi C. Sphingolipids in obesity and correlated co-morbidities: the contribution of gender, age and environment. Int J Mol Sci. 2019;20(23):5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Breij LM, Abrahamse-Berkeveld M, Vandenplas Y, Jespers SNJ, de Mol AC, Khoo PC, Kalenga M, Peeters S, van Beek RHT, Norbruis OFet al. An infant formula with large, milk phospholipid-coated lipid droplets containing a mixture of dairy and vegetable lipids supports adequate growth and is well tolerated in healthy, term infants. Am J Clin Nutr. 2019;109(3):586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mazzei JC, Zhou H, Brayfield BP, Hontecillas R, Bassaganya-Riera J, Schmelz EM. Suppression of intestinal inflammation and inflammation-driven colon cancer in mice by dietary sphingomyelin: importance of peroxisome proliferator-activated receptor gamma expression. J Nutr Biochem. 2011;22(12):1160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang X, Kong X, Qin Y, Zhu X, Liu W, Han J. Milk phospholipids ameliorate mouse colitis associated with colonic goblet cell depletion via the Notch pathway. Food Funct. 2019;10(8):4608–19. [DOI] [PubMed] [Google Scholar]
- 125. Hertervig E, Nilsson Å, Nyberg L, Duan RD. Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer. 1997;79(3):448–53. [PubMed] [Google Scholar]
- 126. Zhang P, Li B, Gao S, Duan RD. Dietary sphingomyelin inhibits colonic tumorigenesis with an up-regulation of alkaline sphingomyelinase expression in ICR mice. Anticancer Res. 2008;28(6A):3631–5. [PubMed] [Google Scholar]
- 127. Guerin J, Burgain J, Gomand F, Scher J, Gaiani C. Milk fat globule membrane glycoproteins: valuable ingredients for lactic acid bacteria encapsulation?. Crit Rev Food Sci Nutr. 2019;59(4):639–51. [DOI] [PubMed] [Google Scholar]
- 128. Leucht K, Fischbeck A, Caj M, Liebisch G, Hartlieb E, Benes P, Fried M, Humpf HU, Rogler G, Hausmann M. Sphingomyelin and phosphatidylcholine contrarily affect the induction of apoptosis in intestinal epithelial cells. Mol Nutr Food Res. 2014;58(4):782–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.