ABSTRACT
The recent coronavirus disease 2019 (COVID-19) pandemic has warranted the need to investigate potential therapies or prophylaxis against this infectious respiratory disease. There is emerging evidence about the potential role of nutrients on COVID-19 in addition to using medications such as hydroxychloroquine and azithromycin. This scoping review aims to explore the literature evaluating the effect of immunomodulatory nutrients on the outcomes including hospitalization, intensive care unit admission, oxygen requirement, and mortality in COVID-19 patients. A literature search of databases including Medline, EMBASE, CINAHL, Web of Science, Cochrane, Scopus, and PubMed, as well as hand-searching in Google Scholar (up to 10 February 2021) was conducted. All human studies with different study designs and without limitation on publication year were included except for non-English-language and review articles. Overall, out of 4412 studies, 19 met our inclusion criteria. Four studies examined the impact of supplementation with vitamin C, 4 studies – zinc, 8 studies – vitamin D, and 3 studies investigated the combination of 2 (zinc and vitamin C) or 3 (vitamin D, vitamin B-12, and magnesium) nutrients. Although limited data exist, available evidence demonstrated that supplementation with immune-supportive micronutrients such as vitamins D and C and zinc may modulate immunity and alleviate the severity and risk of infection. The effectiveness of vitamin C, vitamin D, and zinc on COVID-19 was different based on baseline nutrient status, the duration and dosage of nutrient therapy, time of administration, and severity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease. This review indicated that supplementation with high-dose vitamin C, vitamin D, and zinc may alleviate the complications caused by COVID-19, including inflammatory markers, oxygen therapy, length of hospitalization, and mortality; however, studies were mixed regarding these effects. Further randomized clinical trials are necessary to identify the most effective nutrients and the safe dosage to combat SARS-CoV-2.
Keywords: COVID-19, immunomodulatory nutrients, SARS-CoV-2, vitamin C, vitamin D, zinc
Statement of Significance: Current knowledge about the efficacy of nutrients as supplementary therapy for COVID-19 disease has been compiled up to 10 February 2021. This review aims to provide timely, comprehensive, and continuously updated evidence available on the role of immunomodulatory nutrients regarding one of the most important health issues in the world over the past 18 months. To our knowledge, this review is the first attempt that outlines evidence and knowledge gaps recognizing the need for future studies on the role of nutrients in SARS-CoV-2.
Introduction
Coronavirus disease 2019 (COVID-19) has become the most significant infectious disease pandemic since the 1918 Spanish influenza pandemic. COVID-19 disease is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a member of the Coronaviridae family closely related to the severe acute respiratory syndrome coronavirus (SARS-CoV) (1). According to epidemiological studies, those with comorbidities and risk factors, including cardiovascular disease, diabetes, hypertension, history of smoking, chronic lung disease, cancer, chronic kidney disease, BMI (in kg/m2) >40, and liver disease, and those in older age groups and men are at increased risk of severe complications due to COVID-19. An extreme inflammatory response to SARS-CoV-2 termed “the cytokine release syndrome,” is one of the severe complications leading to poor outcomes including respiratory failure and death (2). An overactive immune system plays a pivotal role in the development of complications related to SARS-CoV-2. Monocytes, macrophages, and T lymphocytes are responsible for an overactive immune response, which produces a systemic cytokine storm contributing to pulmonary edema and systemic organ damage (3).
Although COVID-19 has been a major cause of death among populations in 2020 and 2021, definitive treatment has yet to be discovered. Despite the large variation in the mortality rate due to the different time points and various epidemiological perspectives and vaccination status, more than 214 million confirmed cases including almost 5 million deaths have been reported by the WHO as of September 2021 (4). There has been significant progress in vaccination, mainly in developed countries. However, concerns about new variants and potential vaccine resistance exist. Nevertheless, several innovative disease-management approaches have been developed, including a critical care nutrient therapy protocol designed by the Eastern Virginia Medical School (5). It consists of supplement therapy with vitamin D, vitamin C, zinc, quercetin, melatonin, omega-3 fatty acids, vitamin B-1, and magnesium, along with several medications, such as ivermectin and famotidine, that are prescribed based on the stage and severity of COVID-19 (5).
Observational studies showed that nutritional deficiencies, such as vitamin D and zinc deficiency, are associated with an increased risk of infection with COVID-19 and severe complications of the disease and mortality (6, 7). Evidence shows that micronutrient deficiency is among the plausible mechanisms involved in the spread and severity of COVID-19 (8, 9). In addition, risk factors linked to severe COVID-19 illness, such as older age, diabetes mellitus, and cardiovascular disease, are also associated with micronutrient deficiency, such as zinc (10). Proper immune function is dependent on the intake of multiple micronutrients with immunomodulatory roles, and deficiency leads to impaired immunity (11, 12). The immunomodulatory impact of some nutrients—such as vitamins A, D, C, and E; the minerals zinc and selenium; and ɷ-3 fatty acids, as well as their potential role in reducing the risk and severity of COVID-19 infection—has been examined in vivo or in vitro (13–16). These nutrients have pleiotropic roles in supporting innate and adaptive immunity (12). With regard to innate immunity, these vitamins and minerals improve physical barriers, antimicrobial protein activity, innate cell differentiation and motility, neutrophil and macrophage function for phagocytosis and killing, inflammation recovery by producing anti-inflammatory cytokines, and antioxidant activity. In addition, immunomodulatory nutrients also provide adaptive immunity via supporting and regulating antibody and cytokine production, lymphocyte differentiation, and generation of immunological memory cells (17, 18).
In the current pandemic, where there is a significant international effort among researchers to find innovative approaches that may have an impact on COVID-19 prevention and control (19), a comprehensive evaluation of the existing literature on the effect of nutrients on COVID-19 is warranted. However, limited studies have evaluated nutrients as adjunct therapy against the novel coronavirus. Thus, in this scoping review, we aimed to comprehensively identify and summarize available evidence about the role of immunomodulatory nutrients in reducing the complications of COVID-19. We hypothesized that certain nutrients have a potential therapeutic impact on COVID-19.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist from Tricco et al. (20) was used to conduct this scoping review. The following research questions guided the scoping review:
What is the effect of supplementation or treatment with immunomodulatory nutrients including vitamins D, E, A, K, C, B-6, and B-12 and folate; the minerals zinc, copper, and selenium; and ɷ-3 fatty acids on COVID-19 symptoms, outcomes, severity, and mortality?
What dosage of the immunomodulatory nutrients can prevent or therapeutically affect COVID-19–related symptoms or outcomes?
Eligibility criteria
Inclusion criteria were published full-text articles investigating the preventative or therapeutic effect of immunomodulatory nutrients on SARS-CoV-2 using interventional, observational, cross-sectional, and case report studies on humans. There were no limitations on the sample size, publication year, and setting. Studies on infants and pregnant and breastfeeding women, animals and cultured cells, non–English-language articles, and any type of review-style articles were excluded from this review.
Search plan
The search was carried out using 7 electronic databases, including MEDLINE, EMBASE, CINAHL, Web of Science, Cochrane, Scopus, and PubMed, as well as hand-searching in Google Scholar at 3 time ranges—up to 23 June 2020, up to 7 September 2020, and 1 January 2021 to 10 February 2021—to incorporate rapid research updates during the pandemic. The final search strategy conducted in Ovid MEDLINE was applied in searching the rest of the databases.
Study selection and data extraction
Initially, 885 articles were identified via search engine in the first attempt and 494 records remained after removing duplicates. Studies were evaluated according to the inclusion and exclusion criteria in 2 phases. After screening titles and abstracts (by PJ), 199 articles were extracted and those with unrelated research context were excluded. Second, 2 reviewers (PJ and ZH) assessed full-text articles based on eligibility criteria independently. At this stage, studies using animal models or cell lines on COVID-19 and review articles were excluded, and 4 articles met inclusion criteria in the first search. Due to the rapid increase in publications on the topic, the search process in databases was performed 2 more times on different dates in which 15 more relevant articles were found, and altogether, 19 studies were chosen to be included in this review. Any disagreements were discussed to reach a consensus with a third adjudicator (HV). All co-authors finalized the selected articles. Next, important data were extracted from eligible articles, including demographic information such as age, sex distribution, and region; nutrient of interest; any medication along with dosage, duration, and side effects; the outcome of interest; and summary of study results. The pathway applied to derive the final list of eligible articles is shown in Figure 1.
FIGURE 1.
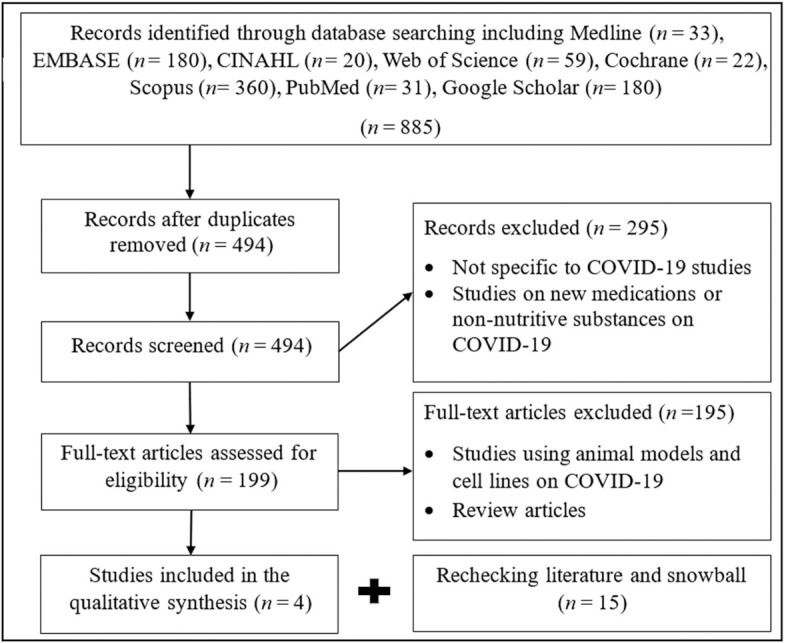
Selection of reported evidence from the primary search in databases (n = 4) and rechecking all databases at 2 other timepoints for collecting more evidence regarding the role of nutrients in COVID-19 patients (n = 15). COVID-19, coronavirus disease 2019.
Predominant themes and keywords of included articles were identified using thematic analysis. A word cloud indicating the most ranked words appearing in the title and abstract of 19 eligible sources was created (Figure 2). The primary themes of this scoping review emphasized by the word cloud were cholecalciferol, zinc, vitamin, HDIVC, ICU, and mortality.
FIGURE 2.
Word cloud indicating the most ranked words appearing in the title and abstract of included article using wordiout.com.
Results
Characteristics of studies
Characteristics of the 19 eligible articles are shown in Table 1 . They were heterogeneous concerning study design, the number of participants, duration of intervention, nutrient of interest, the dose of nutrients, target population, and outcomes. Table 2 summarized the effect of these nutrients on the major complications of COVID-19 disease including inflammation, ICU admission, oxygen requirement, and mortality. To compare these studies, we classified them according to the nutrients of interest and then presented the results based on the study design, dosage, and outcomes, respectively. The study population was from the United States (largest sample sizes), China, Singapore, and Spain. The patients included in these studies were primarily >50 y old, and the number of participants ranged from 1 (21) to 932 (22). There were 6 randomized controlled trials (RCTs) (23–28), 3 non-RCTs (29–31), 4 case reports (21, 32–34), 5 retrospective observational studies (22, 35–38), and 1 cohort study (39), all conducted during 2020–2021.
TABLE 1.
Characteristics and major outcomes of selected studies included in the scoping review evaluating the effect of nutrients on patients with COVID-191
| Study, year; study design (country) (reference) | Sample size (target population) | Exposure, dosage, duration | Control group | Reported outcome | Conclusions |
|---|---|---|---|---|---|
| Anderson et al., 2020; clinical trial (China) (29) | 50 patients with moderate to severe COVID-19 infection | Ascorbic acid: 100 mg/(kg ⸱ d) or 7 g Intravenous continuous infusion of ascorbic acid for 25–28 d | — | No mortality; 3–5 d shorter hospital stays | Adjunctive care of hospitalized COVID-19 patients by ascorbic acid |
| Zhang et al., 2020; randomized controlled clinical trial (Wuhan) (23) | 44 ICU patients: 22 patients in each group Mean age: 67.4 ± 12.4 y, 67% male | Ascorbic acid: 24 g/d HDIVC for 7 d | Placebo: bacteriostatic water for injection | ↓ IL-6; ↓Total bilirubin and ICU mortality of severe patients; ↑PaO2/FiO2 | Protective clinical effect and alternative treatment options for HDIVC |
| Hiedra et al., 2020; clinical trial, a case series (USA) (30) | 17 patients requiring 30% or more FiO2; 64 ± 14 y; 41% females | Ascorbic acid: 3 g daily intravenous vitamin C; Median: 3 d (range: 0–11 d) | — | ↓ Inflammatory markers (ferritin and D-dimer); ↓ FiO2 requirements | The potential therapeutic use of intravenous vitamin C in patients with moderate to severe COVID-19 |
| Zhao et al., 2021; retrospective case series study (China) (34) | 12 patients: 6 patients in each group; Mean age: 56 y in severe disease group and 63 y in the critical group | Ascorbic acid: 162.7 mg/(kg ⸱ d) or 11.39 g for severe patients, 178.6 or 12.50 g for critical patients for 7 d | — | ↓ Significant CRP from day 0 to 3 and 7; The normal level of lymphocyte and CD4 + T-cell counts; ↑PaO2/FiO2SOFA score improvement | Beneficial in terms of the inflammatory response, immune and organ function, especially in severe compared with critical patients |
| Carlucci et al., 2020; retrospective observational study (USA) (22) | Zinc sulfate + medication, n = 411;Medication alone, n = 521 | Zinc sulfate: 220 mg capsule twice daily (50 mg elemental zinc) for 5 d | HCQ (400 mg followed by 200 mg twice daily for 5 d); azithromycin (500 mg once daily) | No effect on the length of hospitalization, ICU duration, and duration of ventilation; ↑ Frequency of being discharged home; ↓ In mortality among patients who did not need ICU | A potential therapeutic as well as the synergistic mechanism of zinc sulfate with HCQ, if used early on in presentation with COVID-19 |
| Yao et al., 2020; retrospective study (USA) (35) | 242 patients: Zinc sulfate group: 196 patient Mean age: 65 y Control group: 46 patients, Mean age: 71 y | Zinc sulfate: Zinc + medication, 440 mg daily (100 mg elemental Zn) for a short period | Medication alone including HCQ, lopinavir/ritonavir, steroids, and IL-6 receptor inhibitors | No significant association between zinc and a change in risk of in-hospital mortality | No association between zinc and the survival of hospitalized patients with COVID-19 |
| Finzi, 2020; case report (USA) (32) | 26-y-old woman | Zinc: 150 mg daily for 14 d | — | Improvement in cough and body aches and fatigue after 1 d; Full recovery after 2 wk | Symptomatic improvement in 4 patients with COVID-19 |
| 41-y-old woman | Zinc: 138 mg daily for 10 d | — | Improvement in PaO2 and fever began 1 d after zinc; Recovery after 10 d | ||
| 57-y-old woman | Zinc: Nine days: one or two 23 mg daily, Day 10: 161 mg, Day 11: 115 mg, Next 10 d: 115 mg daily; For 21 d | — | Gradual improvement in symptoms like dry cough, chest pain, intense neck muscle pain, fever, headache, and shortness of breath at rest | ||
| 63-y-old man | Zinc: Day 1: 69 mg, Day 2: 207 mg, Next 10 d: 184 mg; For 12 + 1 d | — | Improvement in fever, headaches, and muscle pain on the second day; Symptoms continued to improve over the next 10 d | ||
| Abd-Elsalam et al., 2020; randomized, multicenter trial (Egypt) (27) | 96 patients received both HCQ and zinc, 95 received HCQ only | Zinc: 220 mg twice daily for 28 d | HCQ: 400 mg twice daily on the first day, then 200 mg twice daily for 5 d | No significant difference between the 2 groups in terms of the need for MV and the overall mortality rates | Zinc supplements did not enhance the clinical efficacy of HCQ |
| Khan et al., 2020; case report (USA) (21) | Woman, 74-y-old | Zinc sulfate: 220 mg 3 times/d; Continuous intravenous infusion of vitamin C: 11 g per 24 h for 10 d | — | Fewer days on MV (5 d); shorter ICU stay (6 d); Earlier recovery in critical COVID-19 patients | Rapid recovery and shortened length of MV and ICU stay in the patients |
| Thomas et al., 2021; randomized clinical trial (USA) (28) | 214 patients; mean (SD) age of 45.2 (14.6) y and 132 (61.7%) women | 3 groups: Zinc gluconate (50 mg); Ascorbic acid (8000 mg); Both supplements for 10 d | Usual care without supplementation | No significant reduction in primary and secondary endpoints; 50% Symptomatic improvement at a mean (SD) of: 6.7 (4.4) d for the usual care group, 5.5 (3.7) d for the ascorbic acid group, 5.9 (4.9) d for the zinc gluconate group, 5.5 (3.4) d for the group receiving both (overall P = 0.45). | No significant decrease in the duration of symptoms in treatment with high-dose zinc gluconate, ascorbic acid, or a combination of the 2 supplements compared with standard of care |
| Ohaegbulam et al., 2020; clinical case series (USA) (33) | 41-y-old Hispanic man; vitamin D deficient | Ergocalciferol 50,000 IU daily for 5 d | — | On day 5, no fever; ↓CRP; Undetectable concentrations of IL-6; Doubling of serum vitamin D | The higher dose of vitamin D was related to lower lengths of stay and oxygen requirements by day 6 |
| 57-y-old Hispanic woman; vitamin D deficient | Ergocalciferol 50,000 IU daily for 5 d | — | Improvements in the leukocytosis, inflammatory markers; undetectable concentrations of IL-6; more than doubling of vitamin D concentrations | ||
| 74-y-old Hispanic man; vitamin D deficient | Cholecalciferol 1000 IU daily for 5 d | — | Minimal improvement of vitamin D concentrations; ↑CRP, ferritin and ESR concentrations; undetectable IL-6 concentrations; Improved respiratory status | ||
| 53-y-old African-American man; vitamin D deficient | Cholecalciferol 1000 IU daily for 5 d | — | On hospital day 6: minimal increase in vitamin D; a slight decrease in CRP concentrations; ↑Ferritin; Doubling of IL-6 concentrations | ||
| Castillo et al., 2020; pilot randomized clinical study (Spain) (24) | 76 patients (45 men (59%) and 31 women); 50 with 25(OH)D3; 26 without 25(OH)D3; Mean age: 53 ± 10 y | 25(OH)D3: First day: 0.532 mg (21,280 IU) oral; On day 3 and 7: 0.266 mg (10,640 IU), then weekly until discharge or ICU admission | HCQ; azithromycin | 2% of patients in the treatment group required ICU admission with no mortality; all were discharged; 50% of untreated patients required admission to the ICU, 2 patients died and the remaining 11 were discharged | Significant reduction in the need for ICU treatment and disease severity of the patients requiring hospitalization due to proven COVID-19 |
| Annweiler et al., 2020; quasi-experimental study (France) (31) | 77 participants (mean ± SD age: 88 ± 5 y; 49.4% women); Group 1: 29; Group 2: 16; Group 3: 32 | Cholecalciferol: Group 1: 50,000 IU per month, or 80,000 IU or 100,000 IU every 2–3 mo; Group 2: 80,000 IU within a few hours of the diagnosis | Group 3: no vitamin D supplements | Significant longer survival time in group 1 compared with group 3; no difference between groups 2 and 3; ↓ Significant in the risk of OSCI score >5 in group 1 compared with group 3 | Regular bolus vitamin D supplementation was associated with less severe COVID-19 and better survival in frail elderly |
| Giannini et al., 2020; retrospective study (Italy) (36) | Ninety-one patients (aged 74 ± 13 y); 55% were male; 36 patients: cholecalciferol; 55 patients: the best available treatment | Cholecalciferol: 400,000 IU bolus oral cholecalciferol (200,000 IU) in 2 consecutive days | HCQ, glucocorticoids, tocilizumab, lopinavir/ritonavir, azithromycin, and/or other antibiotics | 43 (47.3%) patients experienced the combined endpoint of transfer to ICU and/or death | The positive effect of high-dose cholecalciferol on the combined endpoint was significantly amplified with increasing comorbidity burden |
| Ling et al., 2020; cross-sectional multicenter observational study (UK) (37) | 151 patients received cholecalciferol booster therapy | Cholecalciferol: Different regimens of cholecalciferol booster therapy: 20,000 IU, 40,000 IU, 50,000 IU; for different duration from every 2 wk to daily; for 7 and 14 d | — | Regardless of baseline serum 25(OH)D concentrations, cholecalciferol is associated with a reduced risk of mortality in acute in-patients admitted with COVID-19 | No association between vitamin D status and COVID-19 mortality |
| Rastogi et al., 2020; randomized, placebo-controlled (India) (25) | Forty patients: Intervention group (n = 16), Control group (n = 24) | Cholecalciferol: Daily 60,000 IU cholecalciferol for 7 d | Placebo | Significantly more participants in the intervention group became SARS-CoV-2 RNA negative compared with the placebo group; Significant reduction in fibrinogen unlike other inflammatory biomarkers (D-dimer and CRP) | A greater proportion of vitamin D–deficient individuals turned SARS-CoV-2 RNA negative; Significant decrease in fibrinogen in vitamin D group |
| Murai et al., 2021; multicenter, double-blind, randomized, placebo-controlled trial (Brazil) (26) | 240 hospitalized patients (moderate to severe): supplement group 120; placebo group 120; Mean age: 56.2 ± 14.4 y 43.9% women | Cholecalciferol: A single oral dose of 200,000 IU cholecalciferol | Placebo | No significant difference between the 2 groups in primary and secondary outcomes | A single high dose of cholecalciferol did not make any changes in the supplement group compared with the placebo group. |
| Cereda et al., 2020; Prospective study (Italy) (38) | 324 COVID-19 cases: Vitamin D supplement group 38 (11.7%) | Vitamin D: Mean intake: 58,846 IU/mo | — | — | Supplementation was not associated with either hospitalization or in-hospital mortality; Higher risk of death for supplement users |
| Tan et al., 2020; cohort study (Singapore) (39) | 43 COVID-19 participants aged ≥50 y; 17 patients in DMB; 26 patients in the control group | Cholecalciferol, 1000 IU; oral magnesium, 150 mg; oral vitamin B-12, 500 μg For about 5 d (4–7 d) | — | Fewer patients treated with DMB required initiation of oxygen therapy during their hospitalization compared with the controls | Significant decrease in the proportion of patients with clinical deterioration requiring oxygen support and/or ICU |
Ordered based on the nutrients assessed in the manuscript. COVID-19, coronavirus disease 2019; CRP, C-reactive protein; DMB, vitamin D, magnesium, and vitamin B-12; ESR, erythrocyte sedimentation rate; HCQ, hydroxychloroquine; HDIVC, high-dose intravenous vitamin C; ICU, intensive care unit; MV, mechanical ventilation; OSCI, Ordinal Scale for Clinical Improvement; PaO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, Sequential Organ Failure Assessment; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D3, 25-hydroxyvitamin D3.
TABLE 2.
Summarizing the effect of 3 nutrients on COVID-19 disease focusing on important outcomes including inflammation, ICU admission, oxygen requirement, and mortality1
| Exposure | Inflammatory markers | OR, 95% CI, P value | ICU admission | OR, 95% CI, P value | Oxygen requirement | OR, 95% CI, P value | Mortality | OR, 95% CI, P value |
|---|---|---|---|---|---|---|---|---|
| Vitamin D | FibrinogenD-dimerCRP (25) | P = 0.001P = 0.241 P = 0.507 | Risk estimate for ICU (24) Admission to ICU (26) | OR: 0.03; 95% CI: 0.003, 0.2595% CI: –15.1%, 4.7%; P = 0.30 | Need for mechanical ventilation (26) | 95% CI: –15.1%, 1.2%; P = 0.09 | 14-day mortality (31) Reduced risk of COVID-19 mortality (37)In-hospital mortality (26) | HR: 0.07; P = 0.017 for group 1HR: 0.37; P = 0.28 for group 2ORadj: 0.13; 95% CI: 0.05, 0.35 ; P < 0.001ORadj: 0.38; 95% CI: 0.17, 0.84 ; P = 0.01895% CI: –4.1%, 9.2%; P = 0.43 |
| DMB | — | — | Intensive care support (39) | OR: 0.20; 95% CI: 0.04, 0.93 | Oxygen therapy (39) | OR: 0.13; 95% CI: 0.03, 0.59 | — | — |
| Vitamin C | IL-6 (23)↓D-dimer↓Ferritin (30)CRP (34) | 95% CI:–301.72, –29.79; P = 0.04P = 0.022P = 0.006P = 0.0003 | — | — | ↑PaO2/FiO2 (23)↑PaO2/FiO2(34)IMVFD28 (23) ↓FiO2 (30) | 95% CI: 33.17, 122.47;P = 0.01P = 0.0007HR: 4.8; 95% CI:–2.3, 11.9; P = 0.56P = 0.186 | ICU mortality in HDIVC (23) | HR: 0.22; 95% CI: 0.06, 0.90; P = 0.03 |
| Zinc | — | — | Needed ICU (22) | OR: 0.733;95% CI: 0.471,1.14; P = 0.168 | ↓Needed invasive ventilation (22)Need for mechanical ventilation (27) | OR: 0.804; 95% CI: 0.487, 1.33; P = 0.396P = 0.537 | ↓Mortality or transfer to hospice (22)Risk of in-hospital mortality (35)Mortality rates (27) | OR: 0.449; 95% CI: 0.271, 0.744; P = 0.002HR: 0.66; 95% CI: 0.41, 1.07; P = 0.09 P = 0.986 |
1COVID-19, coronavirus disease 2019; CRP, C-reactive protein; DMB, vitamin D, magnesium, and vitamin B-12; ICU, intensive care unit; IMVFD28, invasive mechanical ventilation free days in 28 days; HDIVC, high-dose intravenous vitamin C; PaO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen.
Vitamin C and COVID-19
Three clinical trials (23, 29, 30) evaluated the effect of vitamin C on COVID-19-related clinical outcomes. The trials were conducted using different dosages including 3 g/d (30), 100 mg/(kg⸱d) (29), and 24 g/d (23), and for various durations. The only RCT was conducted in Wuhan to examine the effect of intravenous high-dose vitamin C (HDIVC), 24 g/d, for 7 d in 22 patients with a mean age of 67.4 ± 12.4 y who were in an intensive care unit (ICU) due to COVID-19. In this study, treatment with the high dosage of vitamin C resulted in significantly lower IL-6 (P = 0.04), decreased total bilirubin (P = 0.03) and mortality among severe patients (P = 0.03), and increased partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) (P = 0.01), but nonsignificant invasive mechanical ventilation (MV)-free days in 28 d (P = 0.57) compared with the placebo group (23). In the case series study by Hiedra et al. (30), in the United States, a daily dose of 3 g intravenous vitamin C for 3 d along with medications including hydroxychloroquine (HCQ), methylprednisolone, and/or tocilizumab in 17 African-American patients aged 64 ± 14 y who were requiring a minimum of 30% FiO2 was investigated. The results showed a 12% mortality rate and 17% rate of intubation and MV in patients. In addition, the concentrations of inflammatory markers, including ferritin (P = 0.006) and fibrin D-dimer (P = 0.022), decreased significantly, and a trend towards decreasing FiO2 requirements (P = 0.186) were identified. In the study by Anderson (29), 50 hospitalized or ICU patients with moderate to severe levels of infection with COVID-19 in China were treated with 100 mg/(kg⸱d) intravenous ascorbic acid for 25–28 d in conjunction with typical hospital/ICU therapies. This resulted in 3–5 fewer days of hospital stays, and improvement without mortality of all patients in the ascorbic acid group compared with patients who only received typical therapies. The report of a case series (n = 12) by Zhao et al. (34), in 2021 in China, also showed a significant reduction in C-reactive protein (CRP) (P = 0.0003), an increase in PaO2/FiO2 (P = 0.0007), and a decrease in Sequential Organ Failure Assessment (SOFA) score (P < 0.0001), from day 0 to 3 and 7, but these beneficial effects of vitamin C were more significant in severe patients than in critical ones (P = 0.01 and P = 0.001, respectively). Thus, intravenous vitamin C was associated with protective and therapeutic impacts on various COVID-19-related outcomes, including inflammatory markers, oxygen therapy, length of hospitalization, and mortality, without any adverse side effects in patients with COVID-19.
Zinc and COVID-19
Two retrospective observational studies (22, 35) reported on the use of zinc supplementation as an adjunctive therapy against COVID-19 in the United States with a significant number of patients and control groups. Both studies used a similar dosage of zinc sulfate (440 mg/d) but reported contradictory results. In the study by Carlucci et al. (22), 411 patients receiving 440 mg Zn for 5 d along with HCQ and azithromycin medications were compared with the control group who only received the 2 medications. The addition of zinc to the medication did not show an impact on the length of hospitalization, duration of ventilation, or ICU duration, but the frequency of being discharged increased (P = 0.008). Moreover, in the zinc group, reduction in mortality or transfer to hospice was significant among patients who did not require an ICU level of care (P = 0.002) compared with those without zinc (22). In contrast, in the study conducted by Yao et al. (35), 440 mg Zn daily for a short period (not specified) along with medications in 196 patients with a mean age of 65 y was not associated with risk of in-hospital mortality (P = 0.09). Similarly, in the study by Abd-Elsalam et al. (27) in Egypt, the same dosage of zinc in 96 patients who received HCQ and zinc compared with 95 patients treated with only HCQ for 28 d indicated no significant differences in terms of MV requirement (P = 0.537) and the overall mortality rates between 2 groups (P = 0.986). In a case series in the United States by Finzi (32), 4 consecutive COVID-19 patients aged 26, 41, 57, and 63 y old took zinc salt lozenges ranging from 23 to 207 mg/d according to the severity of their disease for an average of 14 d (different in each case, ranging from 10 to 21 d). Symptomatic improvement in dry cough, fever, headaches, and muscle pain was observed for all patients who received high-dose zinc lozenges. Together, these inconsistent results do not support a clear conclusion, although there may be a potential therapeutic synergistic mechanism of zinc sulfate while taking medications such as HCQ and azithromycin.
Combination of vitamin C and zinc in COVID-19 treatment
In an RCT by Thomas et al. (28), 50 mg zinc gluconate and/or 8 g ascorbic acid were compared with those without supplementation for 10 d in 214 patients with a mean age of 45.2 y. Primary and secondary endpoints, including 50% symptomatic improvement, hospitalizations, and deaths, were not significant among the 4 groups. Also, in a case report study, a combination of high-dose vitamin C and zinc, 11 g and 660 mg, respectively, per 24 h for 10 d in conjunction with HCQ and azithromycin in a 74-y-old female patient resulted in significant improvements, including fewer days on MV (5 d), shorter ICU stay (6 d), and earlier recovery compared with the average length of MV, disease duration, and ICU stay in critical COVID-19 patients (21). Due to different study designs, these 2 studies are not comparable so we cannot make conclusions about this combination.
Vitamin D and COVID-19
Eight studies investigated different forms and dosages of vitamin D in patients with COVID-19 and 3 of them were RCTs. In an RCT conducted by Castillo et al. (24) in Spain, an intervention with 0.532 mg oral 25-hydroxyvitamin D3 [25(OH)D3] on the first day of admission and 0.266 mg on days 3 and 7 and continuing weekly until discharge in conjunction with HCQ and azithromycin was evaluated in 50 patients aged 53 ± 10 y. Only 1 of the 50 patients treated with 25(OH)D3 needed to be admitted to the ICU (2%), while in the control group, 13 of 26 patients required admission (50%; P < 0.001). No mortality was found among patients treated with 25(OH)D3, and all were eventually discharged without complications. In the control group, the 13 patients (of 26 total) did not need ICU admission and were discharged, although in the remaining 13 ICU-admitted patients, 2 died and the remaining 11 were discharged. In another RCT in India by Rastogi et al. (25), 40 patients were given 60,000 IU cholecalciferol for 7 d, and 62.5% of the intervention group became SARS-CoV-2 RNA negative, whereas this figure in the placebo group was 20.8% (P < 0.018). Supplementation also was associated with a significant reduction in fibrinogen (P = 0.007), unlike other inflammatory biomarkers. In contrast, in the RCT by Murai et al. (26), a single dose of 200,000 IU cholecalciferol in 120 patients with a moderate to severe level of disease was not associated with length of stay (P = 0.59), in-hospital mortality (P = 0.43), admission to the ICU (P = 0.30), or MV requirement (P = 0.09), despite the significant increase in serum concentration of cholecalciferol.
Other study designs mentioned in selected articles in this review include a quasi-experimental study, retrospective, cross-sectional studies, and case series, respectively. In a study by Annweiler et al. (31) in France, 3 groups of patients aged 88 ± 5 y received different cholecalciferol dosages: 50,000 IU/mo over the preceding year in group 1, 80,000 IU after COVID-19 diagnosis in group 2, or no supplementation with cholecalciferol in group 3. Group 1 showed significantly longer survival time and lower risk on the Ordinal Scale for Clinical Improvement (OSCI) score (>5) in comparision with group 3 (P = 0.015 and P = 0.03, respectively). However, no difference between groups 2 and 3 was identified (P = 0.40). Giannini et al. (36) represented that the beneficial effect of high-dose cholecalciferol (400,000 IU) was significantly modified by the comorbidity burden in 36 patients with 2 or more comorbidities. Also, high-dose cholecalciferol booster therapy was conducted by Ling et al. (37) in the United Kingdom with different dosages—20,000 IU, 40,000 IU, and 50,000 IU—and different treatment periods, daily to every 2 wks. Regardless of baseline level of cholecalciferol, cholecalciferol booster therapy was associated with a reduced risk of mortality in patients with acute COVID-19 (P < 0.001) in the primary cohort (first part) and (P =0.018) in the validation cohort study (second part), but cholecalciferol serum concentration was not associated with COVID-19 mortality. Cereda et al. (38) investigated supplementation with a mean intake of 58,846 IU cholecalciferol monthly in 38 hospitalized COVID-19 patients. Unexpectedly, there was a higher risk of mortality among those who were supplemented with cholecalciferol.
In a case series in the United States (33), intake of a high dose of ergocalciferol (50,000 IU/d) for 5 d by a 41-y-old Hispanic man and a 57-y-old Hispanic woman with COVID-19 disease was associated with reduced CRP, undetectable concentrations of IL-6, and more than doubled serum vitamin D. On the other hand, a lower dosage (1000 IU/d cholecalciferol) for 5 d taken by a 74-y-old Hispanic man and a 53-y-old African-American man was associated with minor improvements in serum concentrations of vitamin D but increased CRP, ferritin, and erythrocyte sedimentation rate (ESR) levels, while IL-6 concentrations remained undetectable, and respiratory status improved. The 4 patients were also treated with HCQ. To summarize the effects of vitamin D across all studies, patients who received the higher doses were noted to have a lower need for ICU admission, shorter hospital stay, reduced oxygen requirements, and less severity of the disease and mortality.
Combination of vitamin D, magnesium, and vitamin B-12 in COVID-19 treatment
A cohort study conducted in Singapore (39) treated 17 patients aged 50 y and over with a combination of 1000 IU cholecalciferol/d, 150 mg magnesium/d, and 500 μg vitamin B-12/d for ∼5 d. This study reported that fewer patients in the group treated with a combination of cholecalciferol, magnesium, and vitamin B-12 required oxygen therapy than controls during their hospitalization (17.6% vs. 61.5%; P = 0.006).
Discussion
Overall, the findings of our review are consistent with our hypothesis about vitamin D, vitamin C, and zinc. High-dose vitamin C appears to beneficially impact outcomes related to COVID-19 without adverse effects. Zinc has a potential therapeutic and synergistic role against COVID-19 when administered with HCQ or chloroquine. In addition, high doses of vitamin D reduced the severity of the illness caused by COVID-19. Findings related to combinations of nutrients are not robust, but there are some promising initial results.
Vitamin C and COVID-19
According to the literature, 24 g/d HDIVC (23) for at least 1 wk was found to be useful in clinical outcome of the studied patients and provided protective clinical effects, while lower doses of 3 g and 7 g HDIVC/d for a longer duration were suggested for adjunctive care of inpatients with moderate to severe COVID-19 disease (29, 30). Safety and efficacy and providing a sufficient concentration of vitamin C in blood, which can be preserved only by high doses of vitamin C supplementation due to the fast metabolism of vitamin C in blood, were reasons why 24 g HDIVC for 7 d was used in the trial (23). However, to avoid potential harmful effects of high-dose and long-term intake of vitamin C on renal function, short durations of supplementation were applied in these studies (23, 30, 34), except in the study by Anderson et al. (29). Similarly, recent reviews have concluded that HDIVC can be administered as an alternative and prophylactic treatment for a short period in the early stages of the disease for hospitalized COVID-19 patients (40–42). The evidence in this review indicated that intravenous vitamin C, regardless of different dosages, can significantly reduce the inflammatory markers of CRP, IL-6, D-dimer, and ferritin (23, 30, 34). In addition, the ratio of PaO2/FiO2, known as the Horowitz index or Carrico index, increased significantly with a higher vitamin C dose of 24 g/d and a mean of 170 mg/(kg⸱d) for 7 d in studies by Zhang and Zhao et al. (23, 34). The observed effects are likely the result of vitamin C's anti-inflammatory and immunosuppressive effects. High-dose vitamin C inhibits glyceraldehyde-3-phosphate dehydrogenase, and then decreases ATP and pyruvate production, inducing an energetic crisis and ultimately decreasing immune effector cell activity and associated immunosuppression (41, 43). Intravenous vitamin C's effectiveness in treating sepsis, septic shock, and acute respiratory distress syndrome (ARDS) is likely related to its anti-inflammatory effect (44). Parenteral use of high-dose vitamin C is suggested to act as a pro-oxidant to attenuate the expression of proinflammatory mediators, which improves alveolar fluid clearance and plays an antioxidant role to enhance epithelial cell functions (41, 43, 44). The effectiveness of high-dose oral vitamin C treatment for a critical clinical condition such as ARDS has been confirmed in other studies (45). Although no adverse impact was identified in the reviewed studies, there is still the possibility of renal dysfunction caused by the extremely high dose of intravenous vitamin C, as observed in previous studies (46, 47). This review suggests that intravenous ascorbic acid is safe, cost-effective, and feasible in health care to ameliorate COVID-19 outcomes. However, the effects of vitamin C alone are difficult to discern if it was combined with a number of medications.
Zinc and COVID-19
Evidence on the effectiveness of zinc on COVID-19 patient outcomes is inconsistent. In the study by Carlucci et al. (22), 440 mg zinc sulfate resulted in a nonsignificant reduction in ICU and invasive ventilation requirement but a significant increase in the frequency of being discharged to home and a decrease in mortality or transfer to hospice. In contrast, Yao et al. (35) and Abd-Elsalam et al. (27) did not report a significant association between zinc and mortality and MV requirement despite a similar dosage. This dose was applied based on previous clinical trials. In a case study by Finzi (32), lower-dosage zinc salt lozenges ranging from 23 to 207 mg supplemented daily for ∼14 d led to improved symptoms. Therefore, despite contrasting results, there may be potential benefits with higher doses of zinc. Consistent with these studies, some evidence has demonstrated that zinc ionophores, such as chloroquine, HCQ, and pyrithione, are possible treatments against SARS-CoV-2, especially when administered with zinc supplements (48, 49). It has been suggested that zinc inhibits RNA-dependent RNA polymerase and subsequently hinders the replication of coronavirus in the host cell, whereas zinc ionophores help zinc absorption by the cell (50). This combination has the advantage of being broadly available, affordable, as well as being safe and effective in approved doses for clinically established indications. Moreover, other combinations such as zinc with dimethyl sulfoxide and zinc with curcumin are hypothesized to be secure, effective, and inexpensive as therapy for COVID-19 patients; however, there is insufficient clinical evidence at this time demonstrating the effectiveness of these combinations (51, 52).
Vitamin D and COVID-19
Vitamin D deficiency or insufficiency is prevalent in general, as well as among COVID-19 patients (7). Several investigations suggest that vitamin D deficiency is a potential risk factor for severe outcomes of COVID-19 (53, 54), which may be attributed to the role of vitamin D as an immunocompetence facilitator in innate and adaptive immunity, in addition to its potential role in the cytokine storm linked to serious patient outcomes such as ARDS (18). 1,25-Dihydroxyvitamin D [1,25(OH)2D] can boost innate immunity through several mechanisms, such as inducing cathelicidin production to cause viral clearance via several pathways, recruiting antigen-presenting cells such as macrophages and dendritic cells, and initiating the adaptive immune response. Moreover, calcitriol regulates innate and adaptive immune responses in several ways to prevent destructive cytokine storms (18, 55). The evidence in this review demonstrated that a high dose of 25(OH)D3, 50,000 IU, resulted in a significantly reduced requirement for ICU treatment, shorter lengths of hospital stay, and reduced oxygen requirements, in comparison with a low dosage of cholecalciferol, especially for those who were already vitamin D deficient (24, 33). Although vitamin D in the forms of cholecalciferol and ergocalciferol or 25(OH)D3 seems to decrease the severity of COVID-19, cholecalciferol is thought to be more effective to increase the concentration of 25-hydroxyvitamin D [25(OH)D] (56). The degree of improvement appears to depend on dosage, duration of treatment, and baseline vitamin D status. In this review, some studies indicated the role of supplementation with 10,000 to 60,000 IU cholecalciferol to decrease the inflammatory factors (CRP and fibrinogen) (25, 33), decrease the need for ICU admission (24), increase survival time (31), and lower the risk of mortality (37). However, supplementation with low-dose vitamin D, 1000–2000 IU per day, in COVID-19 patients showed an increase in inflammatory factors and a higher risk of death (33, 38). Treatment with a single high dose of cholecalciferol, 80,000 and 200,000 IU, did not significantly affect outcomes, whereas regular supplementation with vitamin D resulted in better survival and less severe disease (26, 31). Other reviews reported on similar vitamin D treatments for the SARS-CoV-2 infection (57, 58). The studies in this review applied high-dose compared with standard-dose vitamin D on COVID-19 patients as it was shown in previous meta-analyses that high-dose supplementation with vitamin D could reduce the risk of respiratory tract infections (13). Nevertheless, only a few clinical trials have investigated the potential efficacy and safety of supplementation with a high dose of vitamin D to treat COVID-19; therefore, further studies are needed to examine efficacy (59).
Combined nutrient therapy
The review included 3 studies that reported a beneficial effect of a combination of nutrients on COVID-19-related outcomes. The nutrient combination therapy with vitamin D, magnesium, and vitamin B-12 was associated with significantly fewer COVID-19 patients who required oxygen therapy and ICU support (39). Vitamin D with magnesium and vitamin B-12 appears to exert a synergistic effect in this combination, as vitamin D induces an immunomodulatory effect (18), while vitamin B-12 supports a healthy gut microbiome (60, 61), and magnesium serves as an enzyme cofactor involved in vitamin D metabolism (62). The dosages of vitamin D and magnesium were 1000 IU and 150 mg, respectively, which are not high doses of these nutrients. However, the vitamin B-12 dose was 500 μg, which is ∼200 times higher than the RDA, although no adverse effects were noted. In addition to the potential synergistic and preventive effects of vitamin D, vitamin B-12, and magnesium on COVID-19 patient outcomes, these 3 compounds are safe, well tolerated, available, and inexpensive for patients (39). In another study of combined nutrients, Khan et al. (21) highlighted the benefits of 11 g intravenous vitamin C in combination with 660 mg Zn for 10 d for rapid recovery and shortened length of MV and ICU stay. However, a lower dosage of these 2 nutrients, 8 g ascorbic acid with 50 mg Zn, did not show a significant decrease in days to reach a 50% symptomatic improvement compared with a control group (28). Both nutrients play a pivotal role in immune function, modulate infectious agents, and reduce the risk, severity, and duration of infectious diseases (63). Accordingly, treatment with both nutrients has the potential for a synergistic impact to induce a more effective immune reaction against SARS-CoV-2. Evidence supports that combination therapy with zinc and vitamin C, and also vitamin D, magnesium, and vitamin B-12 can reduce the need for ICU and oxygen therapy (39). However, there are insufficient studies investigating the combined effect of these nutrients in COVID-19 patients. Therefore, further research could be undertaken to investigate these combinations of nutrient therapy for COVID-19.
Knowledge Gap and Research Recommendations
Table 3 outlines some gaps in our knowledge and controversial areas about nutrient therapy in COVID-19 and recommendations for future investigations. The findings from limited heterogeneous studies presented in this review do not allow to make a strong conclusive statement about the efficacy of nutrients on COVID-19. This knowledge gap warrants research with stronger study designs and larger sample sizes. Promising findings of the role of immunomodulatory nutrients on COVID-19 were explored in the literature, but the effectiveness of these nutrients on patient outcomes is not completely clear, as nutrient therapy was applied along with medication and, in some studies, without a control group. Therefore, long-term and large-scale RCTs should be designed considering confounders, nutritional status and dietary pattern, any potential side effects, and other nutrients that may affect patient outcomes. In addition, while screening the titles and abstracts, no studies were found regarding pregnant and breastfeeding women with COVID-19 despite the necessity of supplementation for these groups. Therefore, they should be investigated in further research.
TABLE 3.
Knowledge gap and Research recommendations1
| What we know | What we do not know |
|---|---|
| The preventive and therapeutic effects of a high dosage of vitamin D, C, and zinc on patients with COVID-19 were identified. | What is the optimal dosage of nutrients for treatment? |
| How does the practice of regular supplement therapy with these nutrients among the population affect COVID-19? | |
| Studies about the role of supplement therapy with vitamins D and C, and zinc on COVID-19 patients have already been carried out. | What is the effect of other nutrients such as selenium, omega-3, which are suggested to be effective in some studies? |
| A healthy dietary pattern with adequate nutrients plays a key role to improve the immune system against bacterial and viral infection. | How does dietary pattern affect COVID-19 patients either at a preventive or therapeutic level? |
| No side effect has been reported by high dosage of these nutrients. | Is there any long-term adverse effect of high-dose nutrient therapy in COVID-19 patients? |
| The role of vitamin D deficiency in the severity of COVID-19 has been suggested. | What other nutrient deficiencies affect COVID-19 patients? |
| What is the adequate serum concentration of immunomodulatory nutrients to be protective against COVID-19? | |
| The combinations of several nutrients showed a positive effect on complications of COVID-19. | What is the impact of other potential combinations of nutrients? |
| What is the role of combined therapy with nutrients and other compounds such as melatonin? | |
| Only a few randomized clinical controlled trials with a short intervention period and low sample size reported the effectiveness of these nutrients on COVID-19. | How can a more powerful study design with larger sample size and longer intervention help to better understand these effects? |
1COVID-19, coronavirus disease 2019.
A variety of COVID-19 outcomes were reported in included studies assessed in the literature. We classified these outcomes into 2 groups: indicators of symptomatic improvement and clinical outcomes. Only in 3 studies did symptoms evaluated in COVID-19 patients consist of dry cough, chest pain, intense neck muscle pain, fever, headache, and shortness of breath at rest and recovery. Concerning clinical outcomes, out of 19 articles, 8 studies assessed mortality, 7 studies assessed oxygen requirement or MV (PaO2/FiO2), 5 studies reported on inflammatory factors including CRP and IL-6, 4 studies reported on ICU admission, and 3 studies reported on hospital stay. In very few studies were time of recovery or discharge from hospital, ferritin, ESR, D-dimer, fibrinogen and bilirubin, OSCI and SOFA scores evaluated. Overall, the heterogeneity in outcome measures does not allow for a universal assessment of the impact of immune-modulating nutrients in patients affected by COVID-19. Some important clinical outcomes such as PaO2/FiO2, inflammatory markers, hospital stay, and mortality rate should be considered as minimum indicators after supplementation in further investigations.
Strengths and limitations
In this scoping review, a systematic process was applied to collect the related literature using appropriate search terms and specific criteria. A comprehensive literature search was performed up to February 2021 to capture all existing literature. Predominant themes and keywords were identified by qualitative thematic analysis introducing the most ranked words appearing in the title and abstract. Some limitations can be considered due to the nature of the review studies.This study covered only papers published in English language up to February 10, 2021. Due to the nature of pandemic and related research activities, we expect growing publications in different languages in this field that will likely clarify some gaps in research.
Conclusions
This study summarized the evidence supporting the existence of an association between vitamin D, vitamin C, and zinc and COVID-19 patient outcomes while highlighting several knowledge gaps and recommendations for further work. HDIVC served as an adjunctive and alternative agent against SARS-CoV-2 by a significant reduction in inflammatory factors including IL-6 and CRP, fibrin D-dimer, and ferritin; length of hospital stay; ICU mortality; and increased the PaO2/FiO2. Findings related to zinc supplementation are not conclusive because similar dosages resulted in contrary results; however, the intake of high-dose zinc with zinc ionophores, such as HCQ, demonstrated a potential therapeutic impact on COVID-19 consequences including reducing the ICU need, MV, and mortality. High doses of vitamin D in different forms, such as ergocalciferol or cholecalciferol, were effective in reducing the severity of disease by reducing inflammatory factors, the need for ICU treatment, and the risk of mortality. However, to benefit from these promising effects, this treatment should be started early at the first onset of symptoms. These promising preventive and therapeutic impacts of immunomodulatory nutrients depend on the baseline nutritional status of patients, dosage, the chemical form of the vitamins, time of administration (early or late stage), the severity of the disease, and medication treatment. Although these promising results identified the relevance of immunomodulatory nutrients on SARS-CoV-2 patient outcomes, more human RCTs with larger sample sizes and high-dosage vitamin C, vitamin D, zinc, and combined therapy among different populations or various ethnicities merit further investigation.
Supplementary Material
Acknowledgments
PJ was the primary reviewer and the first author for this manuscript. ZH was the secondary reviewer and author for this manuscript. HV was the third adjudicator and corresponding author. All co-authors including PhCh, AJH, JRD, BB, and PP were involved in reviewing and editing the manuscript. All authors have read and approved the final manuscript. We would like to thank Mr. Kevin Read, Associate Librarian at the University of Saskatchewan for his assistance in literature search process.
Notes
The authors reported no funding for this study.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HCQ, hydroxychloroquine; HDIVC, high-dose intravenous vitamin C; ICU, intensive care unit; MV, mechanical ventilation; OSCI, Ordinal Scale for Clinical Improvement; PaO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen; RCT, randomized controlled trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, Sequential Organ Failure Assessment; 25(OH)D3, 25-hydroxyvitamin D3.
Contributor Information
Parisa Jandaghi, College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, Canada.
Zeinab Hosseini, College of Kinesiology, University of Saskatchewan, Saskatoon, Canada.
Philip Chilibeck, College of Kinesiology, University of Saskatchewan, Saskatoon, Canada.
Anthony J Hanley, Department of Nutritional Sciences, University of Toronto, Toronto, Canada.
Jason R Deguire, Centre for Population Health Data, Statistics Canada, Ottawa, Canada.
Brian Bandy, College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, Canada.
Punam Pahwa, Department of Community Health and Epidemiology, College of Medicine, University of Saskatchewan, Saskatoon, Canada.
Hassan Vatanparast, College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, Canada; School of Public Health, University of Saskatchewan, Saskatoon, Canada.
References
- 1. World Health Organization . Naming the coronavirus disease (COVID-19) and the virus that causes it. [Internet]. 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. [Google Scholar]
- 2. McIntosh K .Coronavirus disease 2019 (COVID-19): epidemiology, virology, and prevention. [Internet]. 2020. Available from: https://www.uptodate.com/contents/covid-19-epidemiology-virology-and-prevention. [Google Scholar]
- 3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, Lancet North Am Ed. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . WHO coronavirus (COVID-19) dashboard. [Internet]. 2021. Available from: https://covid19.who.int/. [Google Scholar]
- 5. Marik P. (EVMS) critical care COVID-19 management protocol. Eastern Virginia Medical School: EVMS Medical Group; 2020. [Google Scholar]
- 6. Jothimani D, Kailasam E, Danielraj S, Nallathambi B, Ramachandran H, Sekar P, Manoharan S, Ramani V, Narasimhan G, Kaliamoorthy I. COVID-19: poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020;100:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kara M, Ekiz T, Ricci V, Kara Ö, Chang K-V, Özçakar L. ‘Scientific strabismus’ or two related pandemics: COVID-19 & vitamin D deficiency. Br J Nutr. 2020;124(7):736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhodes JM, Subramanian S, Laird E, Kenny RA. low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51(12):1434–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos DA, Aaseth J. Zinc and respiratory tract infections: perspectives for COVID–19. Int J Mol Med. 2020;46(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system—working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10(4):696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alabboud M, Javadmanesh A. In silico study of various antiviral drugs, vitamins, and natural substances as potential binding compounds with SARS-CoV-2 main protease. DYSONA Life Sci. 2020;1(2):44–63. [Google Scholar]
- 16. Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, Apostolopoulos V, Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19?. Maturitas. 2021;143:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Maria A, Gupta FA, Madhavan MV, Nair N, Babalyan V, Hutchings N. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol. 2020;183(5):R133–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kakodkar P, Kaka N, Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus. 2020;12(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MD, Horsley T, Weeks L. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 21. Khan HMW, Parikh N, Megala SM, Predeteanu GS. Unusual early recovery of a critical COVID-19 patient after administration of intravenous vitamin C. Am J Case Rep. 2020;21:e925521–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carlucci P, Ahuja T, Petrilli CM, Rajagopalan H, Jones S, Rahimian J. Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients. medRxiv[Preprint]. May 8, 2020 [Internet]. Available from:; https://www.medrxiv.org/content/medrxiv/early/2020/05/08/2020.05.02.20080036.full.pdf. [Google Scholar]
- 23. Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, Luo G, Meng Z, De Backer D, Xiang H. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intens Care. 2021;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castillo ME, Costa LME, Barrios JMV, Díaz JFA, Miranda JL, Bouillon R, Gomez JMQ. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, Puri G, Malhotra P. Short term, high-dose vitamin d supplementation for COVID-19 disease: a randomised, placebo-controlled study (SHADE study). Postgrad Med J. 2020; doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 26. Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CS, Silva CB, Franco AS, Macedo MB, Dalmolin HH. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325(11):1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abd-Elsalam S, Soliman S, Esmail ES, Khalaf M, Mostafa EF, Medhat MA, Ahmed OA, Abd El Ghafar MS, Alboraie M, Hassany SM. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine? A randomized, multicenter trial. Biol Trace Elem Res. 2021;199(10):3642–46. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A, Il'Giovine ZJ, Mehra R, McWilliams C, Nissen SE. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Network Open. 2021;4(2):e210369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson PS. Intravenous ascorbic acid for supportive treatment in hospitalized COVID-19 patients. J Orthomol Med. 2020;35(1):1–3. [Google Scholar]
- 30. Hiedra R, Lo KB, Elbashabsheh M, Gul F, Wright RM, Albano J, Azmaiparashvili Z, Aponte GP. The use of IV vitamin C for patients with COVID-19: a case series. Expert Rev Anti Infect Ther. 2020;18(12):1259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Annweiler G, Corvaisier M, Gautier J, Dubée V, Legrand E, Sacco G, Annweiler C. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12(11):3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J Infect Dis. 2020;99:307–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohaegbulam KC, Swalih M, Patel P, Smith MA, Perrin R. Vitamin D supplementation in COVID-19 patients: a clinical case series. Am J Ther. 2020;27(5):e485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao B, Ling Y, Li J, Peng Y, Huang J, Wang Y, Qu H, Gao Y, Li Y, Hu B. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study. Ann Palliative Med. 2021;10(2):1599–609. [DOI] [PubMed] [Google Scholar]
- 35. Yao JS, Paguio JA, Dee EC, Tan HC, Moulick A, Milazzo C, Jurado J, Della Penna N, Celi LA. The minimal effect of zinc on the survival of hospitalized patients with Covid-19: an observational study. Chest. 2021;159(1):108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giannini S, Passeri G, Tripepi G, Sella S, Fusaro M, Arcidiacono G, Torres MO, Michielin A, Prandini T, Baffa V. Effectiveness of in-hospital cholecalciferol use on clinical outcomes in comorbid COVID-19 patients: a hypothesis-generating study. Nutrients. 2021;13(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ling SF, Broad E, Murphy R, Pappachan JM, Pardesi-Newton S, Kong M-F, Jude EB. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study. Nutrients. 2020;12(12):3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cereda E, Bogliolo L, Lobascio F, Barichella M, Zecchinelli AL, Pezzoli G, Caccialanza R. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition. 2021;82:111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, Wong HM, Tern PJW, Chandran M, Chay JWM. Cohort study to evaluate effect of vitamin D, magnesium, and vitamin B12 in combination on severe outcome progression in older patients with coronavirus (COVID-19). Nutrition. 2020;79-80:111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng RZ, Kogan M, Davis D. Ascorbate as prophylaxis and therapy for COVID-19—update from Shanghai and US medical institutions. Glob Adv Health Med. 2020;9:2164956120934768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erol A. High-dose intravenous vitamin C treatment for COVID-19. OSF Preprints. February 28, 2020 [Internet]. Available from:; https://osf.io/p7ex8/?fbclid=IwAR2b67345SBs9r2QfJ23xH_0GEM771Qwww6EPpOSTSpQ7_x2BUu7-5CZE. [Google Scholar]
- 42. Rossetti CA, Real JP, Palma SD. High dose of ascorbic acid used in SARS Covid-19 treatment: scientific and clinical support for its therapeutic implementation. Ars Pharmaceutica. 2020;61(2):145–8. [Google Scholar]
- 43. Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio IIC, Giannopoulou EG, Rago C. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kashiouris MG, L'Heureux M, Cable CA, Fisher BJ, Leichtle SW. The emerging role of vitamin C as a treatment for sepsis. Nutrients. 2020;12(2):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hemilä H, Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. 2019;11(4):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fontana F, Cazzato S, Giovanella S, Ballestri M, Leonelli M, Mori G, Alfano G, Ligabue G, Magistroni R, Cenacchi G. Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney Int Rep. 2020;5(10):1815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5(7):e11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shittu MO, Afolami OI. Improving the efficacy of chloroquine and hydroxychloroquine against SARS-CoV-2 may require zinc additives—a better synergy for future COVID-19 clinical trials. Infez Med. 2020;28(2):192–7. [PubMed] [Google Scholar]
- 49. Derwand R, Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win todays battle against COVID-19?. Med Hypotheses. 2020;142:109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hecel A, Ostrowska M, Stokowa-Sołtys K, Wątły J, Dudek D, Miller A, Potocki S, Matera-Witkiewicz A, Dominguez-Martin A, Kozłowski H. Zinc (II)—the overlooked eminence grise of chloroquine's fight against COVID-19?. Pharmaceuticals. 2020;13(9):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roy A, Sarkar B, Celik C, Ghosh A, Basu U, Jana M, Jana A, Gencay A, Sezgin GC, Ildiz N. Can concomitant use of zinc and curcumin with other immunity-boosting nutraceuticals be the arsenal against COVID-19?. Phytother Res. 2020;34(10):2425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoang BX, Hoang HQ, Han B. Zinc iodide in combination with dimethyl sulfoxide for treatment of SARS-CoV-2 and other viral infections. Med Hypotheses. 2020;143:109866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Annweiler C, Cao Z, Sabatier J-M. Point of view: should COVID-19 patients be supplemented with vitamin D?. Maturitas. 2020;140:24–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ikeagwulonu R, Etukudoh N, Obeta M, Mgbecheta C. Does vitamin D serum levels affect the risk of Covid 19 and its clinical outcomes? A review of literature. EAS J Med Surg. 2020;2(6):146–51. [Google Scholar]
- 55. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. medRxiv[Preprint]. May 18, 2020 [Internet]. Available from:; https://www.medrxiv.org/content/medrxiv/early/2020/05/18/2020.04.08.20058578.full.pdf. [Google Scholar]
- 56. Simonson W. Vitamin D dosing considerations in COVID-19. Geriatr Nurs. 2020;41(5):648–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Verdoia M, De Luca G. Potential role of hypovitaminosis D and vitamin D supplementation during COVID-19 pandemic. QJM. 2021;114(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dofitas BL, Flores-Genuino RNS. Should vitamin D supplements be used in the prevention or treatment of COVID-19?. ASIA PACIFIC CENTER FOR EVIDENCE BASED HEALTHCARE.May 22, 2020 [Internet]. Available from:; https://www.psmid.org/wp-content/uploads/2020/0/VitaminD_Abridged_report_22May2020-v1.2.pdf. [Google Scholar]
- 60. Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014;20(5):769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Negi S, Das DK, Pahari S, Nadeem S, Agrewala JN. Potential role of gut microbiota in induction and regulation of innate immune memory. Front Immunol. 2019;10:2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118(3):181–9. [DOI] [PubMed] [Google Scholar]
- 63. Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


