Abstract
Kenya's vision 2030 partly aims at ensuring adequate health care for all, and the integration of traditional healthcare practices into the national healthcare system would present a more rapid alternative towards the realization of universal health coverage in Kenya. Currently, research on Kenyan medicinal plants with potential antibacterial activity remains vastly fragmented across numerous literature studies and databases; thus, it is imperative to collate and appraise these data for the ease of future research and possible clinical application. Objective. This review aims at exploring and compiling research evidence on medicinal plants used in the management of bacterial infections in Kenya, with a focus on their efficacy and safety. Methodology. A comprehensive web-based systematic review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was executed to highlight the Kenyan medicinal plants used for the management of bacterial infections in Kenya. This review includes studies published until January 2021 from the PubMed, Science Direct, AJOL, and Google Scholar databases. Results. A total of 105 Kenyan medicinal plants belonging to 43 families have their in vitro activity against various human pathogenic bacteria evaluated. Plants from the Lamiaceae, Rutaceae, and Fabaceae families were the most commonly studied. Aloe secundiflora, Toddalia asiatica, Senna didymobotrya, Warbugia ugandensis, Tithonia diversifolia, Fuerstia africana, Olea africana, and Harrisonia abyssinica were the plants frequently evaluated within Kenya. The plants with the strongest antimicrobial activities were Toddalia asiatica, Hagenia abyssinica, Ocimum gratissimum, Harrisonia abyssinica, Senna didymobotrya, Olea Africana, Camellia sinensis, and Tarmarindus indica. Conclusion. Based on a published work, it is evident that traditional medicine is seemingly an acceptable and efficient system among Kenyan communities in the management of bacterial infections. Kenya's rich biodiversity with diverse secondary metabolites presents a promising source of new therapeutic alternatives with possibly different mechanisms of action against bacteria.
1. Introduction
Despite the remarkable investment in health care witnessed over the past decade, microbial infections remain a major threat to human and animal health and are a cause of morbidity and mortality especially in low- and middle-income countries (LMICs). The rising cases of antibiotic resistance present a major health problem globally, and there is an immediate need for strategies to manage it as it relentlessly compromises the effectiveness of antimicrobial therapy and increases the threat of therapeutic failure [1–3]. Due to an inefficient antimicrobial resistance (AMR) surveillance system, the exact liability of AMR in Kenya is indefinite although cases such as reduced susceptibility of community-acquired pneumococci, Vibrio cholera outbreaks, and methicillin-resistant Staphylococcus aureus (MRSA) from hospitalized patients have been reported [4].
Herbalism is the most preferred form of traditional medicine and is highly lucrative in the international market with annual sales ranging from US dollar 5 billion in Western Europe to US dollar 14 billion in China [5]. In Africa, herbal products are available in most markets in the urban centers and rural areas [6]. Irrespective of the accessibility to modern medicines, various communities in Kenya (either deliberately or due to economic limitations) utilize medicinal plants for the management of microbial infections and other diseases; thus, various legislations are actively being formulated to regulate this practice [7]. Presently, there are over 400 plant species used for the management of common diseases in East Africa documented in several ethnobotanical [8–10].
As a developing nation with numerous healthcare challenges such as the high costs of medications, Kenya needs to grow its scientific base and create logical and effective solutions to manage them. Laboratory investigations and various clinical trials have often suggested the positive effects of phytomedicines both in vivo and in vitro; however, there has been little systematic appraisal of their benefits [11]. Due to their unrivaled chemical diversity, plants offer the infinite potential for innovative and effective antimicrobial agents, but there is the scantiness of information in regard to their efficacy and their safety levels [12]. Critical consideration to the prospect of producing pharmaceutical products using local raw materials is a worthy endeavor to ensure the affordability of drugs. In a bid to provide herbal practitioners and consumers with insight, this study primarily aimed at evaluating the bioactivity of Kenyan medicinal plants useful in the management of bacterial infections.
2. Materials and Methods
A comprehensive web-based systematic review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines on identification, screening, eligibility, and inclusion was executed to highlight the medicinal plants used for the management of bacterial infections in Kenya. This review covers published literature from 1994 up to January 2021 obtained from the PubMed, Science Direct, African Journals Online (AJOL), and Google Scholar databases. Grey literature [13] from the local university repositories and conference proceedings were also included in this review [14].
The literature search was performed using search terms identified from previous similar reviews. The Boolean search operators (AND and OR) were used to effectively combine the search terms [15]. The following search terms were used: Kenya AND antimicrobial plants OR Kenyan AND antimicrobial plants. Kenyan AND antibacterial plants OR Kenyan AND antibacterial plants. Kenya AND traditional medicine AND antimicrobial plants OR Kenyan AND traditional medicine AND antimicrobial plants, Kenya AND traditional medicine AND antibacterial plants OR Kenyan AND traditional medicine AND antibacterial plants, Kenya AND ethnopharmacological AND antimicrobial plants OR Kenyan AND ethnopharmacological AND antimicrobial plants and Kenya AND ethnopharmacological AND antibacterial plants OR Kenyan AND ethnopharmacological AND antibacterial plants [16].
Screening of search outputs was performed in two stages. First, the title and abstract of identified journal articles/theses were overviewed based on PICO (Participants Intervention Comparison and Outcomes) and the studies classified as “yes” or “no” based on the information provided by the title and abstract. Thereafter, suitable articles/theses were downloaded and critically assessed for inclusion in the review [16].
The studies eligible for inclusion were limited to the English language. The assessment of eligibility of studies was performed by at least two people, independently, using the Critical Appraisal Skills Programme (CASP) appraisal checklist as a guide [16]. This study excluded research data from papers with poor methodology and retracted studies. The quality of the papers was assessed based on study design, description of the subject, method and assay, variables assessment, control groups, and data collection. To minimize bias, data extraction from selected study reports was independently performed by two reviewers and any disagreements resolved through discussion with the third reviewer [17].
3. Results
The study included research data from the pharmacological assays/ethno-medicinal studies reporting on Kenyan medicinal plants used for the treatment of bacterial infections. The initial database search identified a total of 105, 157 articles. After removing the duplicates (n = 15000), 89857 studies were excluded based on the title and abstract. Three hundred (300) full-text articles were assessed for eligibility, from which 211 were excluded based on scope, methodological approach, and very little/no bioactivity reported. A total of seventy-nine (79) studies regarding the in vitro antibacterial activity of Kenyan medicinal plants were ultimately included in the review. No in vivo studies within Kenya on Kenyan medicinal plants with antibacterial activity were found.
Data collected included herbal plant name, plant family, part of plant used for extraction, extraction/preparation method, concentrations of extracts, bacteria species, data on reported activity, toxicity, exposure time, geographical information, the year of publication, and the first author (Table 1). A total of 105 medicinal plants from 43 families were studied for in vitro activity against various human pathogenic bacteria. Plants from Lamiaceae, Rutaceae, and Fabaceae families were the most common (Table 1).
Table 1.
Systematic review of Kenyan antibacterial medicinal plants.
| Plant | Ethnopharmacological use | Part used | Bioactivity | Assay method used | Collection site in Kenya | Side effects/contraindications/toxicity | References |
|---|---|---|---|---|---|---|---|
| Aloe secundiflora Engl. (Asphodelaceae) | Candidiasis, diarrhea, sore throat, and wound healing | Leaves | The ethanol leaf extract exhibited a ZOI of 17.0 ± 0.8 mm compared to 8.4 ± 0.7 mm (erythromycin) and 8.0 ± 0.8 mm (gentamycin) against Streptococcus pneumoniae | Disk diffusion method | Eastern Kenya | None reported | [18] |
| Wounds, appetizer, and malaria | The methanol leaf extract (100 mg/ml) exhibited a ZOI of 17 ± 1 mm against Staphylococcus aureus, 18 ± 2 mm, Bacillus subtilis, 17 ± 2 mm. K. pneumoniae, and 19 ± 2 mm against E. coli | Agar well assay | Department of Biological Sciences, Egerton University | Not reported | [19] | ||
| Stomachache, polio, malaria, and chest problems | The methanol leaf extract (1 g/ml) exhibited a ZOI (13.0 ± 0.17 mm) compared to (25.0 ± 1.06) ciprofloxacin against S. aureus, ZOI (17.0 ± 1.38 mm) compared to (20 ± 2.47 mm) against E. coli, and ZOI (18 ± 0.35 mm) compared to (22.0 ± 1.06 mm) ciprofloxacin against E. faecalis | Disk diffusion method | Kenyatta University Arboretum | Not reported | [20] | ||
| Stomachache | The methanol leaf extract had an MIC of 9.375 mg/mL against P. aeruginosa compared to amoxicillin 4.687 mg/mL, MIC of 18.75 mg/mL compared to amoxicillin 4.687 mg/mL against E. coli (MIC and MBC of 37.5 mg/mL compared to amoxicillin 4.687 mg/mL against S. aureus and S. typhi) | Broth dilution method | Lake Victoria Region of Kenya | Not reported | [21] | ||
| Wound healing | The methanol leaf extract had an MIC (mg/ml) of 9.1 and an MBC (mg/ml) of 10.4 and exhibited a ZOI of 16 ± 1.27 mm against E. coli compared to ciprofloxacin 17 ± 1.38 mm | Disc diffusion method/broth dilution method | Kenyatta University Arboretum | Not reported | [22] | ||
| Tithonia diversifolia (Hemsl.) A. Gray (Asteraceae) | Constipation, stomach pains, liver pains, indigestion and sore throats and as an antiviral | Leaves | The ethyl acetate leaf extract exhibited a ZOI of 8.0 ± 0.5 mm against Streptococcus pneumoniae, compared to 2.4 ± 0.6 mm (gentamycin) 2.2 ± 0.4 mm (erythromycin) | Disk diffusion method | Eastern Kenya | A 70% ethanol extract of the aerial parts was toxic to the kidney and liver toxicity at the lowest dose tested (400 mg/kg). T. diversifolia should be used with caution as it may be toxic especially in prolonged use at higher doses | [18, 23] |
| Diarrhea | The methanol leaf extract (1 g/ml) exhibited ZOI of 21.6 mm, 19.3, and 18.0 against S. aureus, P. aeruginosa, and K. pneumoniae compared to amoxicillin 23.0 mm, 17.3 mm, and 17. 66 mm, respectively, MIC of 37.5 mg/ml against S. aureus | Agar disc diffusion method/broth dilution | Twiga Region in Central Province | [24] | |||
| Skin infections | The ethyl acetate leaf extract exhibited a ZOI of 18.2 mm against S. typhi compared to chloramphenicol with a ZOI of 23.3 mm and ciprofloxacin with a ZOI of 26.0 mm | Disc diffusion method | Nyamira County | [25] | |||
| Gastrointestinal disorders | The dichloromethane leaf extract (25 mg/mL) exhibited a ZOI of 18 mm against S. aureus and 14 mm against P. aeruginosa | Agar well diffusion method | University of Kabianga Botanical Garden, Kericho County | [26] | |||
| Senna didymobotrya (Fresen.) Irwin & Barneby (Caesalpiniaceae) | Skin diseases, diarrhea, dysentery, laxative, malaria | Roots, Stem barks, leaves | The methanol root extracts exhibited a ZOI of 1.58 cm compared to streptomycin (1.30 cm) against S. aureus | Disk diffusion method | Kibuye, Kisumu County | The methanol and dichloromethane crude root extracts of had an LD50 of 1927 mg/kg after a period of 14 days. the extracts at high concentration and at a high dose tend to be toxic | [27, 28] |
| Malaria, skin conditions, livestock infections | The methanol stem bark extracts (100 mg/ml) had a ZOI of 19.0 mm compared to 30 μg/ml gentamycin (19.0 mm) against S. aureus, ZOI (11.0 mm) compared to 30 μg/ml gentamycin (9.0 mm) against MRSA, ZOI (12.0 mm) compared to 30 μg/ml gentamycin (17.0 mm) against K. pneumoniae | Disk diffusion method | Bomet District | [29] | |||
| Diarrhea | The methanol leaf extracts (1 g/ml) had ZOI (16.0 mm) compared to (60.0 mm) gentamycin (10 μg/ml) against B. subtilis. The methanol extracts (1 g/ml) had ZOI (16.0 mm) compared to (24.0 mm) gentamycin (10 μg/ml) against S. aureus | Disk diffusion method | Rarieda | [30] | |||
| Diarrhea, fevers, abscesses of the skeletal muscles, and venereal diseases | The methanol 2.5% root bark extract and 7.5% stem bark extracts here inhibited the growth of S. aureus, which was also observed in streptomycin (1 g/L) | The area under disease progress stairs (AUDPS) | Siaya, Nakuru, and Nandi counties | [31] | |||
| Oral infections | The ethanol leaf extract (1 mg/mL) exhibited a ZOI of 21.70 ± 0.88 mm, against P. gingivalis and (MIC 0.13 ± 0.00 mg/mL and MBC 0.50 ± 0.00 mg/mL). Amoxicillin had a ZOI of 40.3 mm | Agar well diffusion assay | Borabu Sub-county in Nyamira County | [32] | |||
| Toddalia asiatica L. (Rutaceae) | Food poisoning, malaria, and sore throat | Fruits, stems, barks, roots, leaves | The essential oil (10 μL) exhibited a ZOI (mm) of 21.00 ± 2.08 against E.coli, 22.33 ± 1.67 against MRSA and 19.00 ± 1.16 S. aureus compared to tetracycline 26.00 ± 0.58 against E.coli, 9.00 ± 0.58 against MRSA and 11.67 ± 0.88 S. aureus | Disc diffusion method | Maseno area, Kisumu County | The root extract showed LD50 >1000 mg/kg and CC50 >100 μg/ml | [33, 34] |
| Malaria and diuretic | The stem bark methanol extract (1 g/ml) had a ZOI of 16.67 ± 0.67 mm against S. aureus compared to gentamycin (1.0 μg/disc) 25.33 ± 0.67 | Disc diffusion method | Kakamega Forest | [35] | |||
| TB and measles | The methanol root extract exhibited a ZOI (mm) of 7.0 against E.coli, 6.33 against S. typhi, and 8.66 against S. aureus compared to tetracycline 20.22 against E.coli, 16.00 against S. typhi, and 21.33 S. aureus. MIC and MBC of 9.375 mg/mL against S. typhi and S. aureus | Agar disc diffusion (DD) method broth microdilution technique | Bondo (Alego) | [36] | |||
| Skin infections, bronchial pains, and stomachache | A formulated antiseptic herbal detergent exhibited ZOI of 24.30 ± 0.67 mm, 18.00 ± 0.58 mm, 16.00 ± 0.58 and 19.67 ± 0.67 mm against MRSA, P. aeruginosa. E.coli, and S. typhi, respectively, compared to the commercial hand wash 21.67 ± 0.33 mm, 19.67 ± 0.67 mm, 13.67 ± 0.33 mm and 18.33 ± 0.33 | Disc diffusion method | Slopes of Kajulu Hills, Lake Victoria Basin | [37] | |||
| Malaria and flu | The stem bark DMSO extract exhibited an ZOI of 10 ± 0.3 mm compared to flucloxacillin 10 ± 0.1 mm against MRSA | Agar well diffusion method | Narok | [38] | |||
| Harrisonia abyssinica Oliv. (Simaroubaceae) | Stomachache, abdominal pains, fever, nausea, vomiting, plague, swollen testicles, dysentery, gonorrhea, tuberculosis | Whole plant, leaves, barks, berries | The methanol whole plant extract had MIC (6.25 mg/ml) compared to (>1 mg/ml for antibiotic standards) against S. aureus and P. aeruginosa and MIC (250 mg/ml) against E. coli | Broth dilution method | Meru Central District | The methanol root bark extract had LC50 (μg/ml) of 198.498 and was considered cytotoxic | [39, 40] |
| Pneumonia, malaria, and eye ointment | The methanol leaf extract had an MIC of 100, 15.6, 75, 150 mg/mL against S. aureus B. cereus, P. aeruginosa, and E. coli, respectively, compared to that of 0, 0, 0.25 , 0.25 mg/mL of streptomycin against S. aureus B. cereus, P. aeruginosa, and E. coli, respectively, and benzylpenicillin 0.6, 0.6 against S. aureus and B. cereus | Broth dilution method | Machakos and Kitui | [39] | |||
| Fever, tuberculosis, and snake bite | The methanol-dichloromethane extract (100 mg/ml) had an ZOI of 20.1.6 mm compared to (18.1.2 mm) gentamycin against S. aureus methanol-dichloromethane extract (100 mg/ml) had an ZOI 30.1.7 mm compared to (15.1.3 mm) gentamycin against E. coli | Agar diffusion assay | Bondo District in Nyanza Province | [41] | |||
| Infertility, menstrual problems, and stomach pain menstrual | The crude extracts showed a moderate activity against S. aureus (11 mm), B. subtilis (7.8 mm), P. aeruginosa (7.0 mm), E. coli (8.5 mm) | Disc diffusion method | Chuka, Meru-South District, Tharaka Nithi County | [42] | |||
| Fuerstia africana T. C. E. Fr. (Lamiaceae) | Urinary problems, tongue infections, diarrhea, skin infections | Leaves, aerial parts | The methanol leaf extracts (1 g/ml) exhibited a ZOI of 17.0 mm compared to (26.0 mm) gentamycin (10 μg/ml) against B. subtilis. The methanol extracts (1 g/ml) had a ZOI of 19.0 mm compared to (24.0 mm) gentamycin (10 μg/ml) against S.. aureus, methanol extracts (1 g/ml) had a ZOI of 20.0 mm compared to (26.0 mm) gentamycin (10 μg/ml) against MRSA | Disk diffusion method | Kisii south | Extracts were found to be safe at 5000 mg/kg body weight per day. median lethal dose (LD50) of methanol and DCM extracts is >5000 mg/kg | [30, 43] |
| Eye ailments, toothache | The hexane leaf extract (100 mg/ml) exhibited a ZOI of 10.67 ± 0.33 mm compared to (17.33 ± 0.33) chloramphenicol (30 μg/ml) against S. aureus, ZOI (10.50 ± 0.29 mm) compared to (15.00 ± 0.00) chloramphenicol (30 μg/ml) against MRSA, and ZOI (9.67 ± 0.33 mm) compared to (16.50 ± 0.29) chloramphenicol (30 μg) against P. aeruginosa | Agar well diffusion method | Olenguruone, Nakuru County, and Cheptenye, Kericho County | [43] | |||
| Boils | The methanol extract exhibited ZOI of (17.21 ± 0.22) compared to gentamycin (23.88 ± 0.01) against K. pneumoniae, ZOI of (14.24 ± 0.35) compared to gentamycin (23.88 ± 0.01) against E. coli and ZOI of (15.18 ± 0.42) compared to gentamycin (25.9 ± 0.01) against S. aureus | Agar well diffusion | Magadi, Kajiado District of Kenya | [44] | |||
| Oral infections | The chloroform extract exhibited ZOI (15.88 ± 0.54) compared to chloramphenicol (21.7 ± 0.11) against S. aureus | Agar well diffusion | Vihiga County, Western Kenya | [45] | |||
| Olea africana (Oleaceae) | Sore throat and urinary tract infections | Stem bark, barks, twigs, leaves | The ethanol stem bark extract (1 g/ml) exhibited ZOI (18.5 mm) compared to gentamycin (10 μg/ml) 19.5 mm and a MIC of (62.5 mg/ml) against S. aureus. The methanol extract had a ZOI of 8.3 mm) compared to gentamycin (19 mm) against E. coli and ZOI of 9.8 mm compared to gentamycin (21.0 mm) against P. aeruginosa | Agar well diffusion/broth dilution | Bomet District | The methanol leaf extract had an LD50 value of 3475 mg/kg was; thus, it is nontoxic | [46, 47] |
| Sap used for bone setting (fracture) | The aqueous bark extract (1 g/ml) exhibited a ZOI of 10.2 ± 0.6 mm compared to (18.0 ± 0.1) streptomycin (25 μg/ml) against S. aureus | Disk diffusion method | Mbeere, and Embu-Eastern Province | [48] | |||
| Chewing stick | The methanol extract exhibited a ZOI of 12.4 mm against S. aureus, MIC of 1.5 mg/ml against E.coli and 0.30 mg/ml against S. aureus | Broth dilution method | University of Kabianga Botanical Garden, Kericho County | [49] | |||
| Chewing stick | The methanol leaf extract (25 mg/mL) exhibited ZOI 18 m against Pseudomonas aeruginosa, ZOI 19.20 mm against S. aureus and 17 mm against E. coli | Broth dilution method | University of Kabianga Botanical Garden, Kericho County | [26] | |||
| Carissa edulis Vahl. (Apocynaceae) | Malaise, antiviral, and appetizer | Roots, stem, leaves | The ethanol root extract exhibited a ZOI of 8.0 ± 0.9 mm against S. pneumoniae compared to 7.2 ± 0.1 mm (gentamycin) and 7.8 ± 0.3 mm (erythromycin) | Disk diffusion method | Eastern Kenya | The oral LD50 of the extract was estimated to be >5000 mg/kg. generally safe at doses lower than 1000 mg/kg is in rats () | [18, 50] |
| Kidney problems, pneumonia | MICs and MBCs of 37.50 mg/ml against S. typhi | Broth dilution | Transmara West | [51] | |||
| Gonorrhea, asthma | The methanol extract exhibited a ZOI of 9.00 mm against S. typhi compared to amoxicillin 16.0 mm and both MIC and MBC of 37.5 mg/mL against S. typhi and S. aureus | Agar disc diffusion method/broth microdilution technique | Lake Victoria Region | [36] | |||
| Rhus natalensis Bernh. (Anacardiaceae) | Malaria | Roots, stems, barks and leaves | The methanol root extract had a MIC of 6.25 mg/against both S. aureus and P. aeruginosa and had a moderate activity with inhibition zone diameters of 11.6 mm against S. aureus and P. aeruginosa compared to gentamycin 25.3 mm and 18 mm | Broth dilution method | Kilifi district | The extracts were safe to the mammalian cells | [52] |
| Diarrhea and stomachache | The isolated compound (1)-epicatechin exhibited a ZOI of 15 ± 0.3 mm against S. aureus and (10 ± 0.2 mm) against P. aeruginosa compared to streptomycin 10 μg/disc 22 ± 0.2 mm and 20 ± 0.3 mm, respectively | Disc diffusion method | Kapkonga Iten, Eldoret town | [53] | |||
| Microbial infections | The isolated compound 1 had a ZOI of exhibited ZOI of 21 mm against S. aureus compared to Chloramphenicol 20 mm | Agar diffusion method | Thika River in Gatanga division, Central Kenya | [54] | |||
| 38. Prunus africana (Hoolh f.) Kalkman (Rosaceae) | Arrow poisoning and gonorrhea | Barks, stems | The methanol bark extract showed a moderate activity against S. aureus (11.0 mm), B. subtilis (10.7 mm), P. aeruginosa (9.7 mm), and E. coli (8.0 mm) | Disc diffusion method | Chuka, Meru-South District, Tharaka Nithi County | The bark had an LD50 of 2201 mg/ kg. The stem bark extract was determined to be nontoxic at the therapeutic dose of 500 mg/kg body weight | [42, 55] |
| Diarrhea | The methanol stem bark extract exhibited a ZOI of 20 mm against S. aureus and MIC of 0.073 mg/ml. ZOI of 17 mm with MIC of 0.156 mg/ml against MRSA. ZOI of 15 mm and the MIC of 0.3125 mg/ml against P. aeruginosa. . ZOI of 12 mm and the MIC of 2.50 mg/ml against S. pneumoniae | Disc diffusion assay | Rift Valley Province of Kenya | [56, 57] | |||
| Chest pain and stomach problems | The hydro-methanolic bark extract exhibited a ZOI of 17.33 ± 0.882 mm against S. typhi and ZOI of 12.33 ± 0.333 mm against Escherichia coli compared to penicillin 27.67 ± 1.2 mm and 20.33 ± 0.333 mm | Agar well diffusion method | University of Eastern Africa, Baraton | [58] | |||
| Warbugia ugandensis Sprague (Canellaceae) | Diarrhea, constipation and cough, | Bark, roots, leaves, stem bark | The methanol extract (100 mg/ml) exhibited a ZOI of 15.0 mm compared to (18.0 mm) chloramphenicol against S. aureus, ZOI (14.0 mm) compared to (24.0 mm) chloramphenicol against MRSA. The Dichloromethane extract had a MIC of 3.125 mg/ml against S. aureus and MRSA | Disc diffusion test/broth dilution | Ngong Forest | The extract had a LD50 > 5000 mg/kg body weight. Extract displayed no apparent deleterious toxicity | [55, 59] |
| STIs, diarrhea, and bronchitis | The methanol extract exhibited ZOI of 3.169 ± 0.27 mg/ml against S. aureus | Disk diffusion method | Rift Valley | [60] | |||
| Microbial infections | The methanol extract exhibited a ZOI of 19.33 ± 0.333 mm against S. epidermidis compared to penicillin 26.67 ± 0.333, ZOI 17.00 ± 0.882 mm against B. cereus and ZOI 11.67 ± 0.333 mm against E. coli compared to penicillin 31.33 ± 0.333 | Disc diffusion | Natural Forest around the University of Eastern Africa, Baraton | [61] | |||
| Hepatitis, gonorrhea tuberculosis, bronchitis, and pneumonia | The stem bark extract of hexane exhibited a ZOI of 11.32 mm against S. typhi compared to chloramphenicol 23.3 mm and ciprofloxacin 26.0 mm | Disc diffusion test | Nyamira county | [25] | |||
| Sexually transmitted diseases, throat, and chest infections, diarrhea, and wounds/ulcers | The methanol extracts exhibited a ZOI of 30 mm, 28 mm, and 16 mm for the root, stem‐bark, and leaf extracts, respectively, at 100 μg/ml concentration against E. coli. The ZOI for the water extracts was 20 mm, 18 mm, and 12 mm for the root, stem‐bark, and leaf extracts, respectively, at an equivalent concentration of 100 μg/ml compared to Norfloxacin 23 mm. The extracts had an effective MIC of 42 μg/ml | Disc diffusion method/broth dilution | Jomo Kenyatta University of Agricultural and Technology (JKUAT) Botanical Garden | [62] | |||
| Allium sativum L. (Liliaceae) | Dysentery, spice | Rhizome, bulbs | Garlic juice exhibited a ZOI of 10.0 mm against P. aeruginosa, 11.7 mm against E. coli, 14.7 mm against S. aureus and 17.7 mm for S. typhi. The activity of ampicillin on E. coli and S. typhi was 11.7 mm and 18.7 mm () | Disc diffusion test | Githurai Market, Nairobi | The LD50 was found to be 3034 mg/kg, and maximum tolerated dose was 2200 mg/kg | [63, 64] |
| Infection, colds | Garlic extract (GE) 200 μl/ml/ exhibited a ZOI of 14 mm compared to gentamycin 24 mm against S. aureus | Disc diffusion method | Nakuru Municipal Council Market in Nakuru Town | [65] | |||
| Reduce blood lipids and blood pressure | The methanolic extract of garlic was effective against E. coli, Staphylococcus aureus, and Pseudomonas aeruginosa, with ZOI of 21 mm, 27 mm, and 28 mm, compared to tetracycline 19 mm, 22 mm, and 27 mm, respectively. The garlic methanolic (GM) extract had 0.14 μg/ml against S. aureus and P. aeruginosa | Agar well diffusion method/broth dilution | Kenyatta University | [66] | |||
| Camellia sinensis L. (Theaceae) | Beverage | Leaves | Green tea (0.1 mg/ml) exhibited a ZOI of 21.3 ± 0.33 mm against E.coli compared to gentamicin 22.3 ± 0.50 mm and ZOI of 23.7 ± 0.33 mm against S. aureus compared to gentamicin 23.2 ± 0.28 mm | Agar well diffusion method | Tea Research Foundation, Kangaita Substation in Kirinyaga | There were no observed adverse effects at 2500 mg/kg body weight/day | [67, 68] |
| The aqueous crude green tea extracts 400 mg/ml had a ZOI of 20 ± 0.0 mm against S. aureus and MIC 100 mg/ml compared to streptomycin 20 ± 0.0 mm, ZOI of 18 ± 0.0 mm and MIC of 200 mg/ml against E. coli compared to streptomycin 10 ± 0.0 mm | Agar well diffusion method | Ngere in Murang'a County | [69] | ||||
| Azadirachta indica A. Juss. (Meliaceae) | Udder infections | Leaves, barks, seeds | The neem extract (NE) 200 μl/ml exhibited ZOI 11 mm against S. aureus compared to gentamycin 24 mm | Disc diffusion method | Kisauni in Mombasa County | Not reported | [65] |
| Methanol bark extract exhibited ZOI of 25 mm, 24 mm, and 20 mm against E. coli, P. aeruginosa, and S. aureus, respectively, with MIC (mg/ml) of 15, 17, and 16 | Disc diffusion method | Chumani in Kilifi North Constituency | [70] | ||||
| Tagetes minuta L. (Asteraceae) | Intestinal disorders and stomach problems | Leaves | The methanolic leaf extract exhibited a ZOI of 17 ± 1.94 mm against S. aureus compared to vancomycin 25.0 mm and ciprofloxacin 22.0 mm (MIC 8.9 mg/ml; MBC 10.0 mg/ml) | Disc diffusion test/broth dilution | Kenyatta university arboretum | [20, 71] | |
| Oils may cause irritation to the skin | The methanol extract had a MIC (mg/ml) 8.7 and MBC (mg/ml) 10 and ZOI of 16 ± 1.27 mm against E. coli compared to ciprofloxacin 20 ± 3.11 mm | Disc diffusion method/broth dilution method | Kenyatta University Arboretum | [22] | |||
| Adansonia digitata L. (Bombacaceae) | Opthalmia | Leaves, barks | The synthesized AgNPs exhibited a ZOI of 17.1 ± 0.130 mm against E. coli and 12.9 ± 0.082 mm against S. aureus compared to ciprofloxacin with a ZOI of 33.4 ± 0.443 and 12.9 ± 0.082 mm against E. coli and S. aureus | Disc diffusion technique. | Makueni County | The stem extracts are non-toxic to brine shrimp larvae | [72, 73] |
| Diarrhea, dysentery | The organic extract at 200 mg/ml and 100 mg/ml showed the highest inhibition zones of 14.33 mm and 12 mm, respectively, against MRSA compared to gentamycin 15.5 mm | Disc diffusion technique | Msambweni District | [74] | |||
| Euclea divinorum Hern (Ebenaceae) | Toothbrush, constipation and ulcers | Stems, barks, leaves | The DCM stem bark extract exhibited ZOI (mm) of 10.8 ± 0.26 against P. aeruginosa and 17.0 ± 0.42 against S. aureus compared to Augmentin 10.0 ± 0.02 against P. aeruginosa and 27.0 ± 0.02 against S. aureus | Disc diffusion method | Bunyala (Budalang‟i) district of Busia County | The root extracts have toxic effects and should be used with care; gargling of extracts is recommended instead of swallowing | [75, 76] |
| Dental caries | The ethanolic root bark extract had MIC of 25, 50, 25 and 25 μg/ml for S. pyogenes, S. aureus, E. coli | Broth dilution | Elgeyo Marakwet, Rift Valley | [77] | |||
| Salvadora persica L. var. persica (Salvadoraceae) | Chest problems, stomachache, teeth problems | Roots, stems, barks | The organic root extract had 10 GUs (numerical growth units) at 0.5 mg/ml against M. tuberculosis compared to Isoniazid that had zero GUs at 0.5 mg/ml | BACTEC mgIT™ 960 system | Various conservancies in Samburu | High concentrations >5 g/kg of the mammal's body weight can result in toxicity | [36, 78] |
| Oral thrush and diarrhea | The methanol bark extract exhibited a ZOI of 21.66 mm against S. aureus compared to Amoxicillin 21.3 mm. ZOI of 20 mm against P. aeruginosa compared to amoxicillin 14.33 mm. ZOI of 15 mm against E. coli compared to amoxicillin 23.6 mm | Agar disk diffusion technique | Nkaroni, Wamba Division, Samburu District | [79] | |||
| Plectranthus barbatus Andrews Lamiaceae) | Oral and throat infections | Leaves, roots | The DCM: MeOH crude leaf extract 200 mg/ml and 100 mg/ml exhibited ZOI (mm) of 13 and 10.3, respectively, against MRSA compared to 14 mm for amoxicillin [50 mg/ml]. The lowest MIC values were observed in DCM fraction (40 mg/ml) against MRSA | Disc diffusion technique/broth dilution technique | Various geographical regions of Kenya | Not reported | [80] |
| Stomachache and wounds | The root extracts exhibited a ZOI of 18.67 mm, 20.00 mm, and 25.33 mm in S. aureus, MRSA, and B. cereus compared to streptomycin 39.67 ± 1.76 mm, 39.67 ± 1.76 mm, and 33.00 ± 1.15 mm () | Agar well diffusion method | Msambweni Subcounty, Kwale County | [81] | |||
| Cordia purpurea (Picc.) Aiton (Fabaceae) | Diarrhea | Roots, barks | The methanolic root extract exhibited a ZOI of 15 mm compared to (21.33 mm) amoxicillin against S. aureus and ZOI (23.66 mm) compared to (17.58 mm) amoxicillin against P. aeruginosa. The methanolic extract had a MIC of 18.75 mg/ml compared to 18.75 mg/ml cefpodoxime against S. aureus and 18.75 mg/ml compared to 9.372 mg/ml cefpodoxime against P. aeruginosa | Agar disc diffusion method/broth dilution | Samburu‐Wamba conservancies | Not reported | [79] |
| The methanolic extract exhibited a ZOI of 14.33 mm compared to (21.33 mm) amoxicillin against S. aureus and ZOI (19.66 mm) compared to (17.58 mm) amoxicillin against P. aeruginosa. The methanolic extract had MIC of 37.50 mg/ml compared to 18.75 mg/ml cefpodoxime against S. aureus and 37.50 mg/ml compared to 9.372 mg/ml cefpodoxime against P. aeruginosa | Agar disc diffusion method/broth dilution | Samburu‐Wamba conservancies | [79] | ||||
| Organic bark extract exhibited zero GUs at 0.5 mg/ml against M. tuberculosis and M. kansasii compared to Isoniazid that had zero GUs at 0.5 mg/ml | BACTEC mgIT™ 960 system | Various conservancies in Samburu | [36] | ||||
| Croton macrostachyus Hochst. Ex Delile: (Euphorbiaceae) | Diarrhea, stomach ache | Barks, roots | The ethyl acetate bark extract exhibited ZOI between 10.1 ± 0.6 mm and 16.0 ± 1.2 mm against S. typhi, E. coli and K. pneumoniae | Agar disc diffusion method | Baraton Community in Nandi District of Kenya | The aqueous stem extract does not provoke death until the dose 16 g/kg. There is a wide margin of safety for the therapeutic use of the extract | [82, 83] |
| The methanolic extract exhibited a ZOI of 23.66 mm compared to (21.33 mm) amoxicillin against S. aureus and ZOI (18.0 mm) compared to (17.58 mm) amoxicillin against P. aeruginosa. The methanolic extract had an MIC of 37.50 mg/ml compared to 18.75 mg/ml cefpodoxime against S. aureus and 18.75 mg/ml compared to 9.372 mg/ml cefpodoxime against P. aeruginosa | Agar disc diffusion method/broth dilution | Samburu‐Wamba conservancies | [79] | ||||
| Ocimum gratissimum L. (Lamiaceae) | Ear infections, tooth gargle | Leaves | The essential oil from leaves exhibited ZOI (26.6 ± 5.7 mm) compared to (24.5 ± 0.7 mm) chloramphenicol against S. aureus. ZOI (21.7 ± 2.1 mm) compared to (32.5 ± 2.5 mm) chloramphenicol against E. coli | Agar disc diffusion method | Meru | The oil can cause an inflammatory response | [84, 85] |
| Sore eyes and rectal prolapse | The essential oil from leaves extract had ZOI (21.7 ± 2.1 mm) compared to (28.0 ± 07 mm) chloramphenicol against E. coli. ZOI (26.6 ± 5.7 mm) compared to (23.5 ± 2.1 mm) chloramphenicol against S. aureus | Agar disc diffusion method | Meru District of Eastern Kenya | [86] | |||
| Ocimum suave Wild (Lamiaceae) | Ear infections, cough and disinfectant | Leaves, roots | The methanolic leaf extract had a MIC of (6.25 mg/ml) compared to >1 mg/ml for antibiotic standards against S. aureus and methanolic extract had an MIC of 31.25 mg/ml compared to >1 mg/ml for antibiotic standards against P. aeruginosa and E. coli | Broth dilution method | Meru Central district | The aqueous leaf extract is nontoxic in acute and subchronic intake. No gross abnormalities, teratogenic, or histological changes observed | [40, 87] |
| Stomach ache | The methanol root extract exhibited a mean ZOI of 14 mm against S. aureus compared to amoxicillin 21.3 mm, ZOI of 21 mm against P. aeruginosa compared to amoxicillin 17.5 mm and ZOI of 18 mm against E. coli compared to amoxicillin 23.6 mm | Agar disk diffusion technique | Namunyak, Wamba division, Samburu district | [79] | |||
| Premna resinosa (Hochst.) Schauer (Compositae) | Respiratory-related illnesses | Roots | Dichloromethane root extract had a MIC of 31.25 μg/ml against MRSA, while ethyl acetate fraction had a ZOI of 22.3 ± 0.3 against S. aureus compared to 33.7 ± 0.3 mm (oxacillin 10 μg/disc and gentamycin 10 μg), methanolic extract had a ZOI of 8.7 mm compared to (22 mm) oxacillin 10 μg/disc and Gentamycin 10 μg against S. aureus and ZOI 11.7 mm) compared to (24 mm) oxacillin 10 μg/disc and gentamycin 10 μg against E. coli | Disc diffusion and microdilution techniques | Mbeere community, Kenya | The dichloromethane and ethyl acetate fractions were within the acceptable toxicity limit (CC50 < 90) | [88] |
| Hagenia abyssinica (Bruce) JF Gmel (Rosaceae) | Diarrhea, stomachache, tongue infections, sores | Stem bark, leaves | The dichloromethane/methanol stem bark extract exhibited ZOI of 19.0 mm against S. aureus compared to Erythromycin (0.01 mg/ml) 22 mm, ZOI of 20.0 mm against E. coli compared to Erythromycin 0.01 mg/ml 20.0 mm, ZOI of 18 mm against B. subtilis compared to Erythromycin 0.01 mg/ml 20 mm | Agar well diffusion method | Aberdare ranges, Kiburu Forest Station | The extracts were safe at 5000 mg/kg body weight per day. Median lethal dose (LD50) of methanol and DCM extracts is >5000 mg/kg | [43, 89] |
| The hexane leaf extract (100 mg/ml) had a ZOI of 16.67 ± 0.67 mm compared to (17.33 ± 0.33) chloramphenicol (30 μg/ml) against S. aureus, ZOI (19.33 ± 1.33 mm) compared to (15.00 ± 0.00) chloramphenicol (30 μg/ml) against MRSA and ZOI (13.00 ± 1.00 mm) compared to (16.50 ± 0.29) chloramphenicol (30 μg) against P. aeruginosa | Agar well diffusion method | Olenguruone in Nakuru County and Cheptenye in Kericho County | [43] | ||||
| Clerodendrum myricoides (Hochst.) R. Br. ex Vatke: (Lamiaceae) | Respiratory diseases, tonsillitis, eye infections, gonorrhea | Whole plant | The methanol extracts (1 g/ml) had a ZOI of 14.7 ± 0.3 mm compared to (17.0 mm) gentamycin (10 μg/ml) against E. coli and ZOI (20.3 ± 0.3 mm) compared to (33.0 mm) gentamycin (10 μg/ml) against S. aureus | Disk diffusion method | Mbeere Community, Kenya | The methanol extracts within the acceptable toxicity limit with a CC50 of >500 μg/ml) the LD50 value of 3475 mg/kg and thus is non-toxic | [90] |
| Pneumonia | The aqueous extract (1 g/ml) exhibited ZOI (13.8 ± 0.2 mm) compared to (18.0 ± 0.1) streptomycin (25 μg/ml) against S. aureus | Disk diffusion method | Mbeere, and Embu-Eastern Province | [48] | |||
| Securidaca longipedunculata Var. parvifolia (Polygalaceae) | Infusion reduces swellings | Roots, barks | The aqueous extract (1 g/ml) had a ZOI of 12.5 ± 2.2 mm compared to (18.0 ± 0.1) streptomycin (25 μg/ml) against S. aureus | Disk diffusion method | Mbeere, and Embu-Eastern Province | The extract has an LD50 value of 771 mg/kg body weight and is nontoxic at relatively high concentrations | [48, 91] |
| Sexually transmitted infections | The bark and root extract exhibited ZOI of 20.1 mm and 13.5 mm, respectively, against N. gonorrhoeae, compared to 12 mm and 19.1 mm ciprofloxacin and tetracycline | Disc diffusion method | Bungoma County | [92] | |||
| Tarmarindus indica L. (Fabaceae) | Meat preservative | Fruit paste, bark | The water extract exhibited a ZOI of 34.67 mm, and 24 mm against E. coli and S. aureus, respectively, compared to chloramphenicol 16 mm and 18 mm | Disc diffusion test | Chepararia and Kongelai subcounties of West Pokot County | The pulp extract of Tamarindus indica at 3000 mg/kg and 5000 mg/kg body weight of resulted in no mortality and is practically nontoxic | [93, 94] |
| Diarrhea, typhoid | Bark | The methanol bark extract (1 g/ml) had a ZOI of 14.5 mm compared to (24.0 mm) gentamycin (10 μg/ml) against S. aureus | Disk diffusion method | Rarieda | [30] | ||
| Zanthoxylum chalybeum Engl. (Rutaceae) | Malaria, pneumonia, sore throat | Leaves, roots, barks | The methanol extracts (1 g/ml) had a ZOI of 16.0 mm compared to (26.0 mm) gentamycin (10 μg/ml) against B. subtilis | Disk diffusion method | Kisii South | The acute oral median dose (LD50) of the root bark extract was >6750 mg/kg body weight. Plant is of relatively low toxicity | [30, 95] |
| The organic crude extract exhibited the good inhibition against B. cereus at 200 and 100 mg/ml concentrations with a ZOI of 13.87 mm and 12.167 mm, respectively, compared to gentamycin 15 mm | Disc diffusion technique | Msambweni District | [74] | ||||
| The organic extract exhibited mean inhibition zone values of 24.33 ± 0.33 mm against MRSA compared to streptomycin 39.67 ± 1.76 mm | Agar well diffusion method | Msambweni Kwale County | [81] | ||||
| Lantana camara L. (Verbenaceae) | Skin rashes, boils | Leaves | The methanol leaf extract (1 g/ml) had a ZOI of 17.0 mm compared to gentamycin (10 μg/ml) against S. aureus | Agar well diffusion | Bomet District | For short-term use, the extract exhibited very low toxicity, while long-term exposure results in liver and kidneys. the root extract was the most toxic part | [46, 96] |
| leaves | The organic leaf extracts MICs and MBCs of 37.5 mg/mL against both S. aureus and P. aeruginosa | Broth dilution technique | Around Lake Victoria Region | [36] | |||
| Mangifera indica L. (Anacardiaceae) | Burns, scalds, sores, abscesses, food | Leaves, fruits | The methanol extracts (1 g/ml) had ZOI (18.5 mm) compared to gentamycin 10 μg/ml (19 mm) against S. aureus, ZOI (13.0 mm) compared to gentamycin (20 mm) against E. coli, ZOI (17 mm) compared to 10 μg/ml gentamycin (18.5 mm) against P. aeruginosa | Agar well diffusion | Bomet District | The oral or dermal administration of the extract showed no lethality at the limit doses of 2,000 mg/kg body weight, and no adverse effects were found | [46, 97] |
| The methanol extract exhibited a ZOI of 2.07 ± 0.15) cm against S. aureus compared to norfloxacin at 10 μg 2.95 cm and ZOI 1.93 ± 0.09) compared to Norfloxacin at 10 μg 2.95 cm against E. coli | Disc diffusion method | Makueni and Embu | [98] | ||||
| Terminalia brownii Fresen (Combretaceae) | Diarrhea, ulcers, and sexually transmitted diseases | Bark, leaves, roots | The ethanol extract had ZOI (mm) of 9.2 ± 0.3 compared to 6.8 ± 0.4 (gentamycin) and 6.6 ± 0.2 (erythromycin) against S. pneumoniae | Disk diffusion method | Eastern Kenya | The roots and stem bark extracts exhibited mild cytotoxic activity with LC50 values ranging from 113.75 to 4356.76 and 36.12 to 1458.81 μg/ml | [18, 99] |
| The aqueous leaf extract (1 g/ml) had a ZOI of 18.0 ± 0.8 mm compared to (18.0 ± 0.1) streptomycin (25 μg/ml) against S. aureus, ZOI (11.7 ± 0.5 mm) compared to (16.0 ± 0.2) streptomycin (25 μg/ml) against E. coli, ZOI (12.8 ± 1.0 mm) compared to (15.0 ± 0.3 mm) streptomycin 25 μg/ml against B. subtilis | Disk diffusion method | Mbeere, and Embu-Eastern Province | [48] | ||||
| Markhamia lutea (Benth.) K. Schum. (Bignoniaceae) | Eye infection | Bark | The chloroform extracts had a ZOI of 22.82 mm against E.coli, 18.79 mm against S. aureus and 17.94 mm against P. aeruginosa compared to gentamycin 22.27 mm, 22.52 mm, and 20.17 mm, respectively | Agar well diffusion | Emuhaya Sub-county, Western Kenya | Not reported | [100] |
| Asparagus setaceous Kunth Jessop (Asparagaceae) | Syphilis, gonorrhea | Aerial parts, roots | The MIC values for ethanolic aerial part extract ranged from 3.2 mg/ml for S. aureus, 6.25 mg/ml for E. coli, and 25 mg/ml for B. subtilis, P. aeruginosa, and S. faecalis, while for the ethanolic root extracts of the same plant, MIC ranged from 6.25 mg/ml for S. aureus and B. subtilis to 25 mg/ml for E. coli, P. aeruginosa, and S. faecalis | Broth dilution | Gatundu | Not reported | [101] |
| Caesalpinia volkensii Harm (Caesalpiniaceae) | Bronchitis , pneumonia | Leaves | The MIC values for the ethanolic leaf extract was 6.25 mg/ml for S. aureus, 12.5 mg/ml for B. subtilis and 25 mg/ml for E. coli and P. aeruginosa | Broth dilution | Gatundu | The organic extract had a median lethal dose of >2000 mg/kg body weight, hence is safe | [101, 102] |
| Thylachium africanum Lour. (Capparaceae) | Diarrhea | Bark | The methanol extract exhibited a ZOI of 18.66 mm against S. aureus compared to amoxicillin 21.3 mm, ZOI of 23.33 mm against P. aeruginosa compared to amoxicillin 17.5 mm, ZOI of 15 mm against E. coli compared to amoxicillin 23.6 mm | Agar disk diffusion technique | Namunyak, Wamba Division, Samburu District | Not reported | [79] |
| Alectra sessiliflora (Vahl) Kuntze (Scrophulariaceae) | Diarrhea, sexually transmitted infections, wounds | Whole plant | The methanol extract 50 mg/ml exhibited ZOI of 15.46 mm against S. aureus compared to chloramphenicol 19.23 mm, ZOI of 10.72 mm against P. aeruginosa compared to chloramphenicol 19.22 mm, ZOI of 9.76 mm against E. coli compared to chloramphenicol 19.10 mm | Disk diffusion method | Vihiga county | Not reported | [103] |
| Teclea nobilis (Rutaceae) | Colds and chest problems | Leaves | The DCM extract exhibited a ZOI of 10 mm against S. aureus compared to gentamycin 13 mm and ampicillin 14 mm | Disk diffusion method | Siroch, Keiyo Sub-county, Elgeyo-Marakwet County | Not reported | [104] |
| Ochna thomasiana (Ochnaceae) | Microbial infection | Root, stem barks | The methanolic extract was found effective against S. aureus and B. subtilis, which gave ZOI of 15 mm and 20 mm, respectively, compared to tetracycline 20 mm and 18 mm | Disc diffusion method | Arabuko-Sokoke, forest in Malindi district, Kilifi County | Not reported | [105] |
| Cinnamomum cassia Presl. (Lauraceae) | Food poisoning, flavoring | Fruits | The ethanolic extract of cinnamon was effective against E. coli by with a ZOI of 27 mm and MIC of 0.12 μg/ml | Agar well diffusion method/broth dilution | Kenyatta University | Not reported | [66] |
| Bidens pilosa L. (Asteraceae) | Stomach upsets | Leaves | The stem bark DMSO extract exhibited ZOI of 12 ± 0.1 mm compared to Flucloxacillin 14 ± 0.7 mm against E. coli | Agar well diffusion method | Narok | Extract showed no adverse effects in mice and chickens at a dose of 5% or less of food | [38, 106] |
| Acacia lahai Stead. & HochsLei Benth (Fabaceae) | Skin eruptions | Barks | The methanol extract (200 mg/ml) had a ZOI of 15.00 ± 0.00 mm against B. cereus compared to Gentamicin (40 ug/ml) 15.83 ± 0.76 mm, ZOI of 11.33 ± 0.29 mm against MRSA compared to Gentamicin (40 μg/ml) 15.67 ± 1.04 mm. The acetone extract (200 mg/ml) had a ZOI of 10.33 ± 0.58 mm against P. aeruginosa compared to Gentamicin (40 μg/ml) 15.12 ± 0.63 mm | Disk diffusion method | Mosonik hill, Sotik Sub-county, Bomet County | Not reported | [107] |
| Bridelia micrantha (Hochst.) Baill. (Euphorbiaceae) | Stomachache, diarrhea in children | Leaves | The methanol extract 100 mg/mL exhibited ZOI of 19 mm and 13 mm against S. aureus and S. typhi | Disc diffusion method | Kilifi District | The extract has a wide margin of safety for oral use at doses below 2000 mg/kg | [49, 108] |
| Grewia plagiophylla K. Schum. (Malvaceae) | Dysentery, typhoid | Leaves | The methanol extract 100 mg/mL exhibited ZOI of 20 mm and 17 mm against S. aureus and S. typhi | Disc diffusion method | Kilifi District | Not reported | [49] |
| Vigna subterranea (L.) (Fabaceae) | Traditional food | Nuts | The MIC values for organic extract ranged from E. coli—7.72 ± 0.35 μg/ml, S. aureus—12.5 ± 0.32 μg/ml, and P. aeruginosa—7.95 ± 0.10 μg/ml. At 100 μg/ml, E. coli, S. aureus, and P. aeruginosa showed a ZOI of 27 ± 0.74 mm, 25.3 ± 0.40 mm, and 25.1 ± 0.24 mm, respectively, compared to those of ceftriaxone, which were 37.0 ± 0.5, 41.3 ± 0.9, and 42.3 ± 0.9 mm | Disc diffusion method | Bungoma county | Not reported | [109] |
| Citrus limon (L.) Osbeck (Rutaceae) | Sore throat, chest pain | Rhizomes | Lemon juice inhibited the growth of S. typhi with a ZOI of 11.0 mm and ZOI 11. 0 mm against P. aeruginosa compared to chloramphenicol 20.0 ± 0.0 mm | Disc diffusion test | Githurai market, Nairobi | The juice is considered non–toxic and extremely safe for consumption even at above 80% concentration | [64, 110] |
| Ziziphus abyssinica Hochst (Rhamnaceae) | Meat preservative | Fruit paste | The methanolic extract gave ZOI of 24 mm, and 20 mm against E. coli and S. aureus, respectively, compared to chloramphenicol 16 mm and 18 | Disc diffusion test | Chepararia and Kongelai subcounties of West Pokot county | The acute toxicity (LD50) of the leaf extracts was found to be greater than 5000 mg/kg and is considered relatively safe for use | [94, 111] |
| Mentha spicata L. (Lamiaceae) | Common cold | Leaves | The ZOI in S. aureus varied from 16 ± 0.02 mm in replicate 2 to 18 ± 0.01 mm in replicate 1, E. coli (13 ± 0.02 mm in replicate 2 to 15 ± 0.02 mm in replicate 1), and in K. pneumoniae (20 ± 0.01 mm in replicate 3 to 20 ± 0.02 mm in replicates 1 and 2) | Agar well diffusion method | Egerton University | The LC50 value was 1701 g/ml in brine shrimp lethality assay, indicating that the plant extract is nontoxic | [112, 113] |
| Indigofera lupatana Baker F. (Leguminosae) | Cough, diarrhea, and gonorrhea | Roots | The organic extract showed a highest activity against B. subtilis (28.5 ± 0.3 mm), S. aureus (22.6 ± 1.0 mm), B. cereus (22.0 ± 0.3 mm), E. coli (21.7 ± 0.7 mm), P. aeruginosa (21.5 ± 0.9 mm), S. typhimurium (17.3 ± 0.3 mm), K. pneumoniae (15.3 ± 0.4 mm), and P. mirabilis (12.3 ± 0.5 mm) | Disc diffusion assay | Mbeere District, in the Eastern Province of Kenya | The extract had an LC50 value greater than 1000 μg/ml which is an indication that they are all nontoxic | [114] |
| Momordica charantia L. (Cucurbitaceae) | Diabetes | Fruit | The extracts exhibited a ZOI of 10.66 mm against S. aureus compared to amoxicillin 21.03 mm and MIC and MBC of 37.5 mg/mL , ZOI of 9.33 mm MIC and MBC of 37.5 mg/mL against P. aeruginosa | Agar disc diffusion (DD) method/broth dilution technique | Lake Victoria Region | The LD50 of the ethanolic extract is considered safe to be consumed below 2000 mg/kg | [36, 115] |
| Blighia unijugata Bak (Sapindaceae) | Tonic, anthelminthic | Roots, pods, and leaves | The methanol and chloroform extracts together with the pure compound, friedelin, were active against S. aureus with zones of inhibition of 18.0, 22.0, and 10.0 mm, respectively. Gentamicin (10 μg/rnl) had a ZOI of 26.0 mm against S. aureus | Disc diffusion assay | Kiangwachi, Kirinyaga District | The extract has the LD50 of 5.628 ± 0.29 g/kg b. wt | [116, 117] |
| Moringa oleifera Lam. (Moringaceae) | Antioxidant, spasms | Seeds, stem | The water extracts showed activity against S. aureus with MIC values ranging from 6.25 to 50 mg/ml | Broth microdilution technique | Moringa oleifera is genotoxic at supra-supplementation levels of 3000 mg/kg b.wt. However, intake is safe at levels ≤ 1000 mg /kg b.wt | [118, 119] | |
| Maesa lanceolata Forssk (Myrsinaceae) | Bacterial infections | Roots, leaves, and stem bark | The stem bark extract exhibited a ZOI of 20.70 ± 0.6 mm against S. aureus compared to gentamycin (1.0 μg/disc), 14.30 ± 0.6 and ZOI of 13.00 ± 1.0 mm against P. aeruginosa compared to gentamycin (1.0 μg/disc) 16.00 ± 1.0 mm | Disc diffusion method | Elgeyo Marakwet county | DCM extracts of stem bark and leaves were lowly toxic. No mortality was observed within 24 hours | [120] |
| Satureja biflora Buch-Ham (Lamiaceae) | Antimicrobial | Leaves | The essential oil exhibited a ZOI of (31 ± 0.5 mm), MIC 125 mg/mL against S. typhi and (24 ± 02 mm), 93.8 mg/mL against S. aureus compared to chloramphenicol 10 ± 1.0 mm, MIC 25 mg/mL against S. typhi and 24 ± 1.0 mm, MIC 31 mg/mL against S. aureus | Agar disc diffusion method/broth dilution | Botanical garden of Egerton university | Not reported | [86] |
| Lannea schweinfurthii (Engl.) Engl (Anacardiaceae) | Bacterial infections | An isolated compound epicatechin had zone diameter of growth inhibition of crude extract was (15.05 mm) against S. aureus and (14.02 mm) against B. subtilis compared to tetraycline 18.02 mm against S. aureus and B. subtilis | Disc diffusion method | Bondo, Siaya County | Not reported | [121] | |
| Annanus comosus (Bromeliaceae) | Indigestion | Fruits | The MIC of nanoencapsulated bromelain against Enterobacter spp., Citrobacter spp., Serratia spp., and coagulase-negative Staphylococci was 25 μg/ml, while that of E. coli was 50 μg/ml. The MIC of nanoencapsulated bromelain against Klebsiella spp. and S. aureus was 200 μg/ml. Bromelain was effective against gram-positive and gram-negative bacteria. Streptomycin had a MIC of 22.2 μg/ml | Agar well diffusion method/broth microdilution method | Thika Town | Leaf extract is nontoxic | [122, 123] |
| Helichrysum forskahlii (Asteraceae) | Cough | Whole plant | H. forskahlii had the highest inhibition zone against MRSA of 19.5 and 18.5 mm in agar well and agar disk diffusion respectively. Chloramphenicol had ZOI 24 mm | Disc diffusion method/agar well diffusion method | Losho, Narok County | The brine shrimp lethality test found the plant to be highly toxic with a lethal concentration of 0.009 mg/ml | [124] |
| Citrullus lanatus (Cucurbitaceae) | Food | Fruit | The MIC value of the nanoparticles was 45.00 ± 0.01 mg/ml for S. typhi and 38.50 ± 0.00 mg/ml for E.coli, while the MBC value was 60.00 ± 0.05 mg/ml for S. typhi and 50.00 ± 0.00 mg/ml for E. coli | Disc diffusion method | Wakulima Market, Muthurwa Market, and Githurai Market within Nairobi county | LD50 of EECLS was greater than 2000 mg/kg BW and the no observed adverse effect level (NOAEL) of EECLS was at a dose of 1000 mg/kg in rats | [125, 126] |
| Hyptis spicigera (Lamiaceae) | Stomach ache, pulmonary troubles | Leaves | The methanolic extract (1 g/ml) had a ZOI of 19.3 mm and 19.0 mm against S. aureus and S. typhi, respectively, compared to amoxicillin 23.0 mm and 21.3 mm. The methanolic extract had an MIC of 37.5 mg/ml against S. aureus and S. typhi compared to 18.75 mg/ml for amoxicillin | Agar disc diffusion method | Marera Region in Central Province | Not reported | [79] |
| Crotalaria quartiniana (Fabaceae) | Diarrhoea | Leaves | The methanol extract (1 g/ml) had a ZOI of 21.0 mm, 19.3, 21.0, 20.7, 18.7, and 20.7 against S. aureus, S. typhi, E. coli, P. aeruginosa, and K. pneumoniae, respectively, compared to amoxicillin 23.0, 21.3, 20.2, 17.3, and 17. 66 and an MIC of 37.5 mg/ml against S. aureus, S. typhi, E. coli, P. aeruginosa, and K. pneumoniae compared to 18.75 mg/ml for amoxicillin | Agar disc diffusion method/broth dilution | Tatu region in central province | Not reported | [79] |
| Eurphobia hirta | Diarrhea, asthma | Whole plant | The methanol extract (1 g/ml) had a ZOI of 21.0 mm, 18.66, 19.66, 16.33, 16.33, and 14.33 against S. aureus, S. typhi, E. coli, P. aeruginosa, and K. pneumoniae, respectively, compared to amoxicillin 23.0, 21.3, 20.2, 17.3, and 17. 66 and MIC of 18.75 mg/ml against S. aureus, S. typhi, E. coli, P. aeruginosa, and K. pneumoniae compared to 18.75 mg/ml for amoxicillin | Agar disc diffusion method/broth dilution | Twiga Region in Central Province | The LD50 of this plant is more than 5000 mg/kg | [79, 127] |
| Lippia kituiensis (Verbenaceae) | Diarrhea, chest problems | Leaves | The methanol extract (1 g/ml) had a ZOI of 23.3 mm and 17.6 mm, against S. aureus and P. aeruginosa, respectively, compared to amoxicillin 23.0, and 17.3. The methanol extract (1 g/ml) had an MIC of 37.5 mg/ml against S. aureus | Agar disc diffusion method/broth dilution | Marera Region in Central Province | Not reported | [79] |
| Eurphobia scarlatina (Euphorbiaceae) | Stomach ache, common cold, TB | Stem | The extract exhibited zero GUs at 0.5 mg/ml against M. kansasii and M. tuberculosis compared to Isoniazid that had zero GUs at 0.5 mg/ml | BACTEC mgIT™ 960 system | Various conservancies in Samburu | Not reported | [36] |
| Acacia horrida. (Fabaceae) | Diarrhoea, TB | Barks | A. horrida had appreciable inhibition (257 GUs) against M. tuberculosis (198 GUs) at the concentration of 0.5 mg/ml compared to Isoniazid that had zero GUs at 0.5 mg/m | BACTEC mgIT™ 960 system | Various conservancies in Samburu | Not reported | [36] |
| Phyllanthus urinaria Linn (Phyllanthaceae) | Dysentery, diarrhea, stomach ache | Leaves, roots | MIC and MBC of 18.75 mg/ml and 37.50 mg/ml, respectively, against E. coli | Broth dilution | Transmara West | Not reported | [51] |
| Rhamnus prinoides L'He'r (Rhamnaceae) | Typhoid, stomach ache | Stem, roots | The extract inhibited E. coli with MIC and MBC of 9.37 mg/ml | Broth dilution | Transmara west | Rhamnus prinoides was nontoxic to brine shrimp | [51, 128] |
| Tetradenia riparia (Lamiaceae) | Respiratory problems, stomach ache, diarrhea, antiseptic | Roots, stem | The organic extract inhibited S. epidermidis with a ZOI of 27.67 ± 0.333 mm compared to penicillin 26.67 ± 0.333 and E. coli with a ZOI of 13.33 ± 0.333 mm compared to penicillin 31.33 ± 0.333 | Disc diffusion | Natural forest around the University of Eastern Africa, Baraton | Toxic effect recorded for root and fruit extracts but not for leaf or stem extracts. In mice at dose 1.0 g/kg | [61, 129] |
| Kigelia africana Lam and Benth (Bignoniaceae) | Laxative, gonorrhea, tuberculosis, diarrhea | Fruits, barks | The methanolic extract had a ZOI of 11.3 mm compared to (19 mm) gentamycin against S. aureus and ZOI (10 mm) compared to (9 mm) chloramphenicol against MRSA. The MIC values of acetone extracts were 6.25 mg/ml against MRSA | Disc diffusion | Kaptumo Division, Nandi | Not reported | [130] |
| Conyza sumatrensis (Asteraceae) | Pimples | Leaves/roots | The methanolic extract had a ZOI of 26.85 mm compared to (13.67 mm) chloramphenicol against E. coli and ZOI (27 mm) compared to (15.8 mm) chloramphenicol against B. pumulus | Agar diffusion assay method | Rarieda, Bondo district, of Nyanza province in Kenya | Experiments indicate the methanol extract to be safe even at high and repeated doses in pre-clinical studies | [131, 132] |
| Piliostigma thonningii (Fabaceae) | Cough, colds, chest pains, stomachache, wounds | Stem bark | The methanolic extract had MIC (3.125 mg/ml) compared to (>1 mg/ml for antibiotic standards) against S. aureus, MIC (31.25 mg/ml) compared to (>1 mg/ml for antibiotic standards) against and E. coli and MIC (15.625 mg/ml) against P. aeruginosa | Broth dilution method | Meru central district | Plant extracts had LD50 values >2000 mg/kg bw and were hence deemed to be nontoxic | [40, 133] |
| Erythrina abyssinica (Fabaceae) | Anthrax, syphilis, gonorrhea, burns, body swellings | Root bark | The methanolic extract had an MIC of 3.125 mg/ml compared to >1 mg/ml for antibiotic standards against S. aureus, MIC (250 mg/ml) compared to (>1 mg/ml for antibiotic standards) against and E. coli and MIC (125 mg/ml) against P. aeruginosa | Test tube method | Meru Central District | The extracts are not toxic to the human cell | [40, 134] |
| Rynchosia minima DC. (Fabaceae) | Swelling | Roots | The methanolic extract had a ZOI of 11.5 mm compared to 15 mm gentamycin against S. aureus | Disk diffusion technique | Central Kenya | Not reported | [135] |
| Entada abysinnica (Fabaceae) | Gastrointestinal bacterial infections, bronchitis | Leaves | The methanol leaf extract (100 mg/ml) had ZOI (10. 33 mm) compared to (16.0 mm) zeftazidime against S. typhi | Disk diffusion technique | Bondo (Sakwa) in western Kenya | Not reported | [36] |
| Withania somnifera (Solanaceae) | Microbial infections, cholesterol-lowering | Leaves, roots | The dichloromethane extract (100 mg/ml) had a ZOI of 16.0 mm compared to (18.0 mm) chloramphenicol against S. aureus, ZOI (14.0 mm) compared to (24.0 mm) chloramphenicol against MRSA and MIC of 6.25 mg/ml against S. aureus and 12.5 mg/ml against MRSA | Disc diffusion test/broth dilution | Ngong forest | The extract is relatively safe for use even in dose levels exceeding 200 μg/ml | [59] |
| Thalictrum rhynchocarpum (Ranunculaceae) | Stomach discomfort and bacterial infections | Roots, bark | The root extract had an MIC of 21.5 mg/ml against B. subtilis compared to ciprofloxacin 21.5 mg/ml | Broth dilution | Ngong Forest | Not reported | [136] |
| Hugonia castaneifolia (Linaceae) | Intestinal worms | Roots | The dichloromethane stem bark extract (100 mg/ml) was active against S. aureus (MIC 0.0008 mg/ml, respectively). Hexane stem bark extracts were active against S. aureus at 0.0031 mg/ml gentamicin and had an MIC of 0.5 mg/ml | Broth dilution | Coast Province of Kenya | Not reported | [29] |
| Tabernaemontana stapfiana Britten (Apocynaceae) | STIs and respiratory-tract infections | Stem bark, root bark, fruits and leaves | The ethanolic root extract (100 mg/ml) had a ZOI of 18.0 mm compared to (22.0 mm) chloramphenicol against MRSA and multiple drug-resistant S. aureus (MDRS) and MIC of 3.9 μg/ml | Disc diffusion test/broth dilution | Kaptagat Forest in Keiyo District | Not reported | [137] |
| Rhus vulgaris (Anacardiaceae) | GIT disorders | Leaves, bark | The methanol extract of Rhus vulgaris showed significant antimicrobial activity against MRSA (12.00 ± 0.00 mm; MIC of 0.391 mg/ml; minimum bactericidal concentration of 1.563 mg/ml). Compared to (6.00 ± 0.00 mm) sulfamethoxazole/trimethoprim (23.7:1.25 μg). The methanol extract showed significant antimicrobial activity against S. aureus (19.50 ± 0.71 mm; MIC of 0.391 mg/ml; compared to (24.19 ± 3.60 mm) sulfamethoxazole/trimethoprim (23.7:1.25 μg) | Disc diffusion assay/minimum inhibitory concentration assay | Mwala Sub-County, Machakos County | There were no observable adverse effects from oral administration of the extracts (acute oral toxicity testing) at concentrations of 50 mg/kg, 300 mg/kg, and 2000 mg/kg | [138] |
| Zanthoxylum paracanthum Kokwaro (Rutaceae) | Diarrhoea | Root bark | The CH2Cl2/CH3OH (1 : 1) extract from the root bark had MIC values of 3.91, 1.95, 0.98, and 7.81 μg/mL, against MRSA, E. coli, S. aureus compared to 0.98, 0.49, and 0.98 μg/ml of omacillin | Minimum inhibitory concentration assay | Mrima Hills, Kwale County in Kenya | Not reported | [139] |
| Centella asiatica (Apiaceae) | Bacterial infections, diarrhea, skin lesions, psoriasis, keloids | Leaves | Organic crude extract of the leaf showed the highest activity ZOI of 16.33 ± 0.33 mm against E. coli compared to tetracycline 26.67 ± 0.33 mm | Disc diffusion method | Kisii County | The lethal dose and no observable adverse effect level were 2000 mg/kg and 1000 mg/kg | [32, 140] |
| Aloe vera (Asphodelaceae) | Blood purifier, malaria, skin disease, diabetes | Leaves | The organic extract exhibited a ZOI of 17 ± 2 − 19 ± 2 mm against S. aureus, (18 ± 2 − 20 ± 1 mm against B. subtilis), (17 ± 1 − 19 ± 3 mm) against K. pneumoniae, (16 ± 1 − 20 ± 3 mm) against E. coli | Agar diffusion method | Department of biological sciences, Egerton university | Not reported | [141] |
| Aloe volkensii (Asphodelaceae) | Laxative, burns, wounds and sores | Leaves | The organic extract exhibited activity against S. aureus (19 ± 1 − 20 ± 2 mm), B. subtilis (17 ± 2 − 21 ± 3 mm), K. pneumoniae (18 ± 2 − 19 ± 1 mm), E. coli (18 ± 2 − 19 ± 3 mm | Agar diffusion method | Department of Biological Sciences, Egerton University | Not reported | [141] |
| Senna spectabilis (Fabaceae) | Laxatives | Leaves, pods | The organic leaf extract (100 mg/ml) had a ZOI of 9.6 ± 0.6 compared to chloramphenicol (11.7 ± 2.3) against S. typhi | Agar diffusion method | Mbeere North District, Embu County | Not reported | [142] |
| Maytenus putterlickioides (Celastraceae) | Malaria, emmenagogue, aphrodisiac | Roots | The methanol root extract (100 mg/ml) had a ZOI of 9.2 ± 1.1 compared to chloramphenicol (11.7 ± 2.3) against S. typhi | Agar diffusion method | Mbeere North District, Embu county | Not reported | [142] |
| Olinia usambarensis (Oliniaceae) | Malaria, abscess, cough, measles | Bark, roots, leaves | The methanol leaf extract (100 mg/ml) had ZOI (12.2 ± 0.8) compared to chloramphenicol (11.7 ± 2.3) against S. typhi | Agar diffusion method | Mbeere North District, Embu County | Not reported | [142] |
| Crotalaria goodformis Vatke. (Fabaceae) | The aqueous extract (1 g/ml) had a ZOI of 14.8 ± 0.2 mm compared to (18.0 ± 0.1) streptomycin (25 μg/ml) against S. aureus | Disk diffusion method | Mbeere, and Embu-Eastern Province | Not reported | [48] | ||
| Prosopis juliflora (Sw.) DC (Fabaceae) | Open wounds and dermatological ailments. | Leaves | The ethanolic leaf extract (100 mg/ml) had a ZOI of 20.00 ± 1.00 mm compared to (19.0) erythromycin (15 μg/ml) and (30.0 mm) chloramphenicol (30 μg/ml) against E. coli, ZOI (15.33 ± 0.58 mm) compared to (11.0) erythromycin (15 μg/ml) and (22.0 mm) chloramphenicol (30 μg/ml) against P. aeruginosa | Disk diffusion method | Endao, Marigat District, in Baringo County | The pods are toxic, mainly for cattle and goats, and have piperidine alkaloids and can cause neurotoxicity | [143, 144] |
| Osyris abyssinica (Santalaceae) | Dysentery, typhoid | Roots | The aqueous root extract (1 g/ml) had ZOI (15.2 ± 0.7 mm) compared to (18.0 ± 0.1) streptomycin (25 μg/ml) against S. aureus, ZOI (14.8 ± 0.3 mm) compared to (16.0 ± 0.2) streptomycin (25 μg/ml) against E. coli, ZOI (15.5 ± 0.5 mm) compared to (15.0 ± 0.3 mm) streptomycin 25 μg/ml against B. subtilis | Disk diffusion method | Mbeere, and Embu-eastern province | Not reported | [48] |
| Abrus precatorius (Fabaceae) | Gonorrhea, coughs in children | Leaves, roots | The aqueous leaf extract (1 g/ml) had a ZOI of 15.7 ± 0.5 mm compared to (18.0 ± 0.1) streptomycin (25 μg/ml) against S. aureus. The aqueous bark extract (1 g/ml) had a ZOI of 7.2 ± 0.8 mm compared to (16.0 ± 0.2) streptomycin (25 μg/ml) against E. coli, ZOI (15.5 ± 0.5 mm) compared to (10.7 ± 1.2 mm) streptomycin 25 μg/ml against B. subtilis | Disk diffusion method | Mbeere, and Embu-Eastern Province | It contains abrin, a toxalbumin that inhibits protein synthesis causing cell death. especially seeds | [48, 145] |
| Ormocarpum trichocarpum (Fabaceae) | Bone setting | Roots | The methanol extract (1 g/ml) had a ZOI of 15.5 mm compared to (26.0 mm) gentamycin (10 μg/ml) against B. subtilis | Disk diffusion method | Kisii South | Not reported | [30] |
| Psidium guajava (Myrtaceae) | Wounds, ulcers, cholera | Leaves | The methanol extracts (1 g/ml) had a ZOI of 19.7 mm compared to gentamycin (10 μg/ml) against S. aureus, ZOI (16.0 mm) compared to gentamycin (10 μg/ml) 19 mm against E. coli and, ZOI (16 mm) compared to 10 μg/ml gentamycin (17 mm) against P. aeruginosa | Agar well diffusion | Bomet District | The median lethal dose (LD50) of bark extract is greater than 5000 mg/kg body weight | [46, 145] |
| Cyathula polycephala (Amaranthaceae) | Diabetes, skin infections, pneumonia | Stem barks | The methanol extract 100 mg/ml had a ZOI of 14.2 mm compared to 30 μg/ml gentamycin (19.0 mm) against S. aureus, ZOI (16.0 mm) compared to 30 μg/ml gentamycin (9.0 mm) against MRSA, ZOI (10.0 mm) compared to 30 μg/ml gentamycin (21.0 mm) against P. aeruginosa | Disk diffusion method | Bomet District | The methanol extract was very safe with a CC50 of 100%, while water extract were toxic with CC50 of 23.75% and 31.56% as compared to the positive control Chloroquine with CC50 of 25.28 and 51.94% at concentration 1000 and 100 mg mL-1 | [29] |
| Blumea axillaris (Lam.) DC. (Asteraceae) | Skin disease | Aerial parts | Methanol extracts had a ZOI 16.41 ± 0.31 compared to chloramphenicol (21.7 ± 0.11) against S. aureus | Agar well diffusion | Vihiga County, Western Kenya | None reported | [45] |
| Chamaecrista mimosoides (L.) Greene (Fabaceae) | Respiratory system disorders, dysentery | Aerial parts, roots | The aqueous extracts had a ZOI 30.00 ± 1.46 compared to chloramphenicol (21.7 ± 0.11) against S. aureus | Agar well diffusion | Vihiga County, Western Kenya | Not reported | [45] |
| Lantana trifolia L. (Verbenaceae) | Cough and common colds | Aerial parts | The methanol extracts had a ZOI of 20.59 ± 0.92 compared to chloramphenicol (21.7 ± 0.11) against S. aureus | Agar well diffusion | Vihiga County, Western Kenya | The ethanol extracts of Lantana trifolia (LC50 32.3 μg/ml exhibited mild toxicity and are safe for short-term use (Moshi et al., 2010) | [45] |
| Terminalia kilimandscharica Engl. (Combretaceae) | Cough, sexually transmitted diseases | Barks | The methanolic bark extract had a MIC of 25, 15.6, 37.5, and 150 mg/mL against S. aureus B. cereus, P. aeruginosa, and E. coli, respectively, compared to that of 0, 0, 0.25, 0.25 mg/mL of streptomycin against S. aureus B. cereus, P. aeruginosa. and E. coli, respectively, and benzylpenicillin 0.6 and 0.6 against S. aureus and B. cereus, respectively | Broth dilution | Machakos and Kitui | The methanolic bark extracts had an LC50 of <1000 μg/mL, which is considered relatively nontoxic | [39] |
| Pentas lanceolata (Rubiaceae) | Genital and oral thrush | Roots | The ethyl acetate extract (100 mg/ml) had a ZOI of (10.96 ± 0.08 mm) compared to gentamycin (23.88 ± 0.01 mm) against K. pneumoniae, ZOI of (12.08 ± 0.26 mm) compared to gentamycin (23.88 ± 0.01 mm) against Escherichia coli and ZOI of (11.39 ± 0.6 mm) compared to gentamycin (25.9 ± 0.01 mm) against S. aureus | Agar well diffusion | Magadi, Kajiado District of Kenya | Not reported | [44] |
| Sericocomposis hildebrandtii Schinz (Amaranthaceae) | Purgative | Roots | The ethyl acetate root extract (100 mg/ml) had a ZOI of 11.28 ± 0.09 mm compared to gentamycin (0.1 μg/ml), (23.88 ± 0.01 mm) against K. pneumoniae, ZOI of (10.33 ± 0.06 mm) compared to gentamycin (0.1 μg/ml) (23.88 ± 0.01) against E. coli, and ZOI of (10.66 ± 0.18) compared to gentamycin (0.1 μg/ml) (25.9 ± 0.01) against S. aureus | Agar well diffusion | Magadi, Kajiado District of Kenya | Not reported | [44] |
| Combretum molle R.Br. ex G.Don (Combretaceae) | Tooth brush, stomach ache, and dysentery | Stem bark | The ethanolic stem bark extract (0.5 mg) exhibited ZOI (mm) of 7.6 ± 0.24 against P. aeruginosa, 15.4 ± 0.3 against E. coli, and 2.2 ± 0.4 against S. aureus compared to Augmentin 8.0 ± 0.02 against P. aeruginosa, 19.0 ± 0.03 against E. coli and 17.0 ± 0.02 against S. aureus | Disc diffusion method | Mwingi District in Kitui County | For the acute toxicity test, no death and signs of poisoning were observed in the treated groups. In the subacute study, LD50 in the rats after intraperitoneal administration was 700 mg/kg | [76, 146] |
| Combretum illairii (Combretaceae) | Roots, stems, leaves | The methanol leaf extract (100 mg/ml) had ZOI of 15.60 mm and 17.00 mm against S. aureus and P. aeruginosa, respectively, against gentamycin (30 μg/ml) 25.3 mm and 18 mm | Disc diffusion assay | Kilifi District | The stem bark extracts had neither cytotoxicity nor brine shrimp lethality. plant extracts | [29] | |
| Combretuam tanaense (Combretaceae) | Skin infections, wounds dressings and ointments | Roots | The methanol root extract (100 mg/ml) had a ZOI of 11.50 ± 0.5 mm compared to ciprofloxacin (0.32 μg/ ml) 14.75 ± 0.25 mm against S. aureus and ZOI of 12.25 ± 0.25 mm compared to ciprofloxacin (0.32 μg/ ml) 16.00 ± 0.00 mm against K. pneumoniae | Agar well diffusion assay | Mount Kenya University Botanical Garden, Thika | Not reported | [104] |
| Vernonia brachycalyx (Asteraceae) | Antimalarial, emetic | Stem, leaves | The ethanol extract had a ZOI of 9.5 ± 1.2 mm against S. pneumoniae compared to 8.2 ± 0.6 (erythromycin) 7.2 ± 0.5 (gentamycin 15 μg) | Disk diffusion method | Eastern Kenya | An isolated compound (16,17-dihydrobrachycalyxolide) displayed high toxicity against human lymphocytes | [18] |
| Vernonia amygdalina (Asteraceae) | Stomach discomfort and bacterial infections | Leaves | The methanol leaf extract (1 g/ml) exhibited a ZOI 17.0 mm compared to gentamycin (10 μg/ml) 19 mm against S. aureus | Agar well diffusion | Bomet District | The extract had an LD50 of 288.5 mg/kg body weight. It has relative toxicity [147] | [46] |
| Vernonia glabra (Steetz) Oliv. & Hiern (Asteraceae) | Gastrointestinal problems , snake bites | Leaves, roots | The dichloromethane/methanol leaf extract (1 g/ml) exhibited a ZOI of 1.85 cm against S. aureus compared to streptomycin with a ZOI of 1.30 cm. dichloromethane/methanol extract of flower showed significant activity only against S. aureus, with the lowest MIC of 1.5625 mg/100 μl, compared to streptomycin with a MIC of 6.25 mg/100 μl | Disc diffusion | Machakos | Not reported | [27] |
| Vernonia adoensis (Asteraceae) | Oral health | Stem bark | The methanol stem bark extract (100 mg/ml) exhibited a ZOI of 13.00 ± 0.577 mm against E. aerogenes, 11.00 ± 0.577 mm against S. pyogenes, 9.67 ± 0.333 mm against S. epidermidis, and 9.00 ± 0.577 mm against E. faecalis. The acetone stem bark extract (100 mg/ml) exhibited a ZOI of 16.00 ± 0.577 mm against E. aerogenes, 11.33 ± 0.882 mm against S. epidermidis, and 10.00 ± 0.577 mm against S. faecalis. Penicillin (100 mg/ml) exhibited a ZOI of 38.00 ± 0.577 mm against E. faecalis, 43.33 ± 0.882 against S. pyogenes, 19.33 ± 0.333 against S. epidermidis, and 36.33 ± 0.882 against E. aerogenes | Disc diffusion method | Natural forests around the University of Eastern Africa, Baraton, Nandi County | Not reported | [148] |
| Vernonia hymenolepis (Asteraceae) | Infections, toothache | Leaves | The MBC and MIC values of aqueous leaf extract was 400 mg/ml against S. aureus, while the DCM/methanol leaf extract had MIC and MBC of 400 mg/ml against P. aeruginosa and E. coli, and MIC of 100 mg/ml against S. aureus. Amoxicillin had MIC and MBC of 3.125 mg/ml and 6.25 mg/ml against E. coli, respectively | Broth dilution | Trans Nzoia County | Not reported | [149] |
Aloe secundiflora (5), Toddalia asiatica (5), Senna didymobotrya (5), Warbugia ugandensis (5), Tithonia diversifolia (4), Fuerstia africana (4), Olea africana (4), and Harrisonia abyssinica (4) were the plants frequently evaluated within Kenya. The plants with the strongest antimicrobial activities were Toddalia asiatica, Hagenia abyssinica, Ocimum gratissimum, Harrisonia abyssinica, Conyza sumatrensis, Senna didymobotrya, Aloe secundiflora, Olea Africana, Vernonia glabra, Camellia sinensis, Tetradenia riparia, and Tarmarindus indica as they exhibited high mean inhibition zone values or low minimum inhibitory concentration (MIC) values. The zones of inhibition (ZOIs) were interpreted as low activity (1 mm–6 mm), moderate activity (7 mm–10 mm), high activity (11 mm–15 mm), and very high activity (>16 mm) (Zaidan et al., 2005). The frequently analyzed plant parts were leaves (37%), bark/stem bark (47%), fruits/seeds (5), pods (1%), and roots (24%). Water and methanol were the most used solvents for plant extract preparation, whereas ethanol and dichloromethane were the least utilized solvents used (Table 1).
The reported medicinal plants were commonly used in the treatment of STIs, respiratory diseases, diarrhea, and oral infections (Table 1). (Figure 1).
Figure 1.
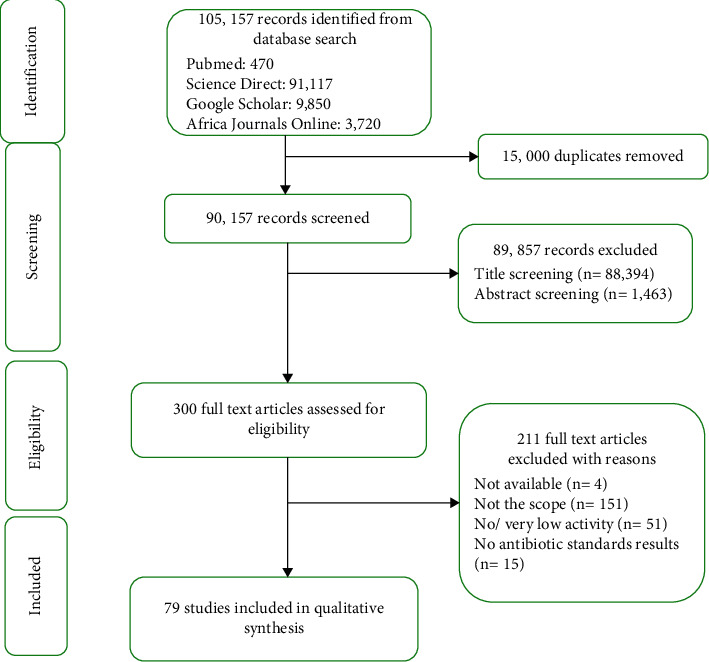
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement of search results.
4. Discussion
Plants generally accumulate diverse bioactive compounds in varying concentrations in the different parts of a plant, and this eventually affects the efficacy of medicinal plants. The leaves (37%), bark/stem bark (47%), and roots (24%) were the most utilized plant parts against bacterial infections. The variances in their antimicrobial activities could be due to the synergistic or antagonistic actions of various secondary metabolites present [60].
4.1. Nutraceuticals
From the review, several common foods/spices are reported to have potential antibacterial benefits. For example, green tea (Camellia sinensis) that is often famed for its antioxidant activity, exhibited good antibacterial activity zone of inhibition (ZOI) of 21.3 Ł} 0.33mm against E.coli and a ZOI of 22.3 ± 0.50 mm against S. aureus compared to gentamicin 22.3 Ł} 0.50 mm (against E. coli) and ZOI 23.7 Ł} 0.33 mm (against S. aureus) at a concentration of 0.1 mg/ml [67]. The aqueous crude green tea extracts at a concentration of 400 mg/ml exhibited ZOI of 20 ± 0.0 mm which was similar to that of streptomycin against S. aureus. The extract also displayed a ZOI of 18 ± 0.0 mm against E. coli compared to ZOI of 10 ± 0.0 mm of streptomycin against E. coli 20 ± 0.0 mm, ZOI of 18 ± 0.0 mm, and MIC 200 mg/ml against E. coli compared to streptomycin 10 ± 0.0 mm [69]. The Bambara nut (Vigna subterranea) had an MIC value of 7.72 ± 0.35 μg/ml for E. coli, 12.5 ± 0.32 μg/ml for S. aureus and 7.95 ± 0.10 μg/ml for P. aeruginosa at 100 μg/ml, and showed zone of inhibition of 27 ± 0.74 mm, 25.3 ± 0.40 mm, and 25.1 ± 0.24 mm E. coli, S. aureus, and P. aeruginosa, respectively [109].
4.2. Complementary Medicine
As has been shown in previous ethnomedical surveys by Omwenga et al., traditional medicine is widely practiced in Kenya and is culturally acceptable. It is estimated that about 75% population in Kenya seeks health care among traditional healers [8–10, 150]. In certain instances, people utilize both traditional and modern medicine simultaneously. Njoroge and Kibunga noted that herbal products were used as complementary therapy in the management of diarrhea by residents in Thika, Kenya [151]. The lack of enquiry about Traditional Complementary and Alternative Medicine (TCAM) use and the conventional healthcare providers' negative attitude towards TCAM were cited as some of the reasons why patients fail to reveal their TCAM use [152].
The regulatory framework for the practice of traditional medicine in Kenya is still underway [153], but several crude drugs or formulated herbal products with reported antibacterial activity are already available in the Kenyan market, for example, the Lifebuoy germ protection antibacterial herbal hand and body soap and the Dettol herbal bar soap. Skin care products (soaps and lotions) formulated from plant extracts (Thevetia peruviana, Tithonia diversifolia, Azadirachta indica, Aloe secundiflora) had antimicrobial properties. Soap made from Tithonia diversifolia plant extract was the most effective against E. coli, while Azadirachta indica soap was the most effective against C. albicans. T. diversifolia soap exhibited the highest activity against E. coli [154].
The ethanolic extract of E. divinorum root bark had a MIC of 25, 50, and 25 μg/ml for Streptococcus pyogenes, Staphylococcus aureus, and Escherichia coli, respectively (Table 1). A herbal toothpaste formulated with the ethanol extract of E. divinorum root bark had a higher antimicrobial activity against the tested microorganisms compared to Colgate herbal toothpaste formulated with fluoride [77]. Also, the formulation containing the aqueous extracts of T. asiatica (50 mg/ml) stem bark exhibited pronounced antimicrobial activity as indicated by zone of inhibition diameters of 24 mm (MRSA) and 22 mm (M. gypseum) compared to 22 mm and 14 mm, respectively, by the commercial hand wash (50 mg/ml). In the model hand washes efficacy experiment, the formulated herbal detergent attained a 78.8% reduction of pathogenic load as compared to 67.9% reduction with the commercial hand wash [37].
Unfortunately, despite the surge in the consumption herbal products and the limited number of standardized herbal products in the Kenyan market, the pharmacovigilance for herbal medicines is nonexistent in Kenya [155]. The increased demand for herbal products has resulted in the market being flooded with adulterated products and false herbal claims on the products' labels for marketing purposes. For instance, 50% of the investigated products by Ngari et al. [75] lacked antimicrobial activity against test bacteria (Staphylococcus aureus, Pseudomonas aeruginosa, Proteus vulgaris, Bacillus subtilis, Candida albicans, Escherichia coli, Streptococcus mutans, Enterococcus faecalis, and Lactobacillus acidophilus). The evaluation of herbal suspensions (used in the management of oral health in Nairobi County, Kenya) by Ngari et al. [75] reported a lack of detectable phytochemicals in the suspensions and noted that this occurrence could be due to very low concentrations of phytochemicals that could not be detected by standard laboratory methods or mineral adulterants might have been used. Studies have demonstrated that antimicrobial properties of natural products can be enhanced by the addition of metal ions [14, 156].
4.3. Polyherbalism
The use of herbs as combinations is common practice with many herbal practitioners and aimed at giving better results as compared to single herbs and also treating more than one ailment [157]. Ngari et al. [75] evaluated herbal pastes and suspensions used in the management of oral health in Nairobi County, Kenya, and various products showed significant antimicrobial activity that is comparable to positive control. For example, product HS4 composed of W. ugandensis, M. piperita, and S. aromaticum had ZOI of 33.1 ± 0.85 mm, 20.3 ± 1.71 mm, and 19.3 ± 1.65 mm against E. coli, S. aureus, and P. aeruginosa, respectively, compared to that of co-trimoxazole of 27.5 ± 0.7 mm, 8.5 ± 0.5 mm, and 10 ± 0.0 mm, respectively. Product HS5 composed of Aloe vera gel, W. ugandensis, and W. sominifera had a ZOI of 25.3 ± 0.25 mm, 20.25 ± 1.26 mm, and 21.5 ± 1.71 mm against E. coli, S. aureus, and P. aeruginosa, respectively, compared toco-trimoxazole 27.5 ± 0.7 mm, 8.5 ± 0.5 mm, and 10 ± 0.0 mm, respectively. This biological activity is attributed to the presence of various secondary metabolites in plants [14].
Mbuthia et al. evaluated the synergistic properties of water-soluble green and black tea extracts with penicillin G. The antimicrobial results showed a marked increase in the inhibition zone diameters on the combination of green tea extracts with penicillin G. The catechins, theaflavins, and thearubigins are the antimicrobial agents present in tea [67].4 Synergistic inhibition by green tea extracts and penicillin G is due to the presence of dual binding sites on the bacterial surface for antibiotic and tea extract [158].
4.4. Bioactivity/Assay Methods
Disc diffusion method was the preferred method to assay for antibacterial activity. A clearing zone of 9 mm or greater for Gram-positive and Gram-negative bacteria was used as the criterion for designating significant antibacterial activity [159]. The in vitro MIC results were classified as described in the study by Pessini et al., 2003: the antimicrobial activity of the extracts that displayed MIC lower than 100 μg/ml was considered very high; 100–500 μg/ml, high; 500–1000 μg/ml, moderate; 1000–4000 μg/ml, low; and anything above this, inactive. The plants with the strongest antimicrobial activities were Toddalia asiatica, Hagenia abyssinica, Ocimum gratissimum, Harrisonia abyssinica, Conyza sumatrensis, Senna didymobotrya, Aloe secundiflora, Olea Africana, Vernonia glabra, Camellia sinensis, Tetradenia riparia, and Tarmarindus indica as they exhibited high mean inhibition zone values or low minimum inhibitory concentration (MIC) values.
Several plants exhibited a high activity superior or comparable to the standard antibiotic drugs (Table 1); the methanol-dichloromethane extract (100 mg/ml) of Harrisonia abyssinica had a ZOI of 20 ± 1.6 mm compared to gentamycin (ZOI of 18 ± 1.2 mm) against S. aureus and a ZOI of 30. ± 1.7 mm against E. coli compared to gentamycin ZOI of 15.1.3 mm against E. coli [41]. The methanolic extract of Croton macrostachyus exhibited ZOI (23.66 mm) compared to (21.33 mm) amoxicillin against S. aureus and ZOI (18.0 mm) compared to (17.58 mm) amoxicillin against P. aeruginosa. The methanolic extract had an MIC of 37.50 mg/ml compared to 18.75 mg/ml cefpodoxime against S. aureus and 18.75 mg/ml compared to 9.372 mg/ml cefpodoxime against P. aeruginosa [79].
Plants such as Toddalia asiatica, Hagenia abyssinica, Senna didymobotrya, Aloe secundiflora, and Camellia sinensis displayed good activities; thus, they may be considered for the assessment of in vivo activity and possibly formulated into different consumable forms. Korir et al. recommended that for plants with very low or no activity, bioactivity on all parts of the plants, for example, root, stem bark, and leaves combined ought to be done against a wide variety of pathogenic bacteria in order to conclusively report that a certain plant is inactive [29].
4.5. Toxicity
Despite herbal remedies being affordable, their lack of efficacy and safety evaluation is a great impediment to their acceptance into mainstream medicine. The safety assessment of herbal remedies remains a challenge as most of the studies of herbal medicines are directed at the toxicological properties of single plant formulations, yet most herbal preparations, especially those used in traditional medicine, contain multiple herbs [160].
From this review, 45% of the plants were relatively safe, 44% of the plants have not been assessed for their safety, and 11% of the plants were reported to have high toxicity (Table 1). The plants with very high toxicity can be further explored for the antitumor activity or as insecticides.
4.6. Plant Conservation Status
Other than W. ugandensis and Prunus africana, most of the plants identified in this review are largely available and are not under any serious threat to become extinct. Since most of them are obtained from wild habitats, sustainable use of the reported medicinal plants against bacterial infections is advised as a conservation measure. The cultivation of wild medicinal plants is an important approach to safeguard the herbal industry. Biotechnological techniques such as plant cell or tissue culture, biochemical conversions, and clonal propagation of indigenous medicinal plants are another potential strategy in improving herbal medicine [161].
5. Conclusion
This review demonstrates the potential of medicinal plants to treat bacterial infections alongside justifying the use of these plant traditional medicine. It may serve as a starting point of research geared towards the clinical application of these plants. There is a need for standardization to improve the acceptance of herbalism by mainstream health practitioners.
6. Recommendation
Further research into the in vivo activity of plants displayed remarkable in vitro activity. Plants exhibiting strong antibacterial activity can be evaluated for their interactions with conventional antibiotics, and those displaying synergistic activity may provide useful leads in antibiotic therapy.
Data Availability
No data were used in the study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Eryilmaz M., Bozkurt M. E., Yildiz M. M., Akin A. Antimicrobial resistance of urinary Escherichia coli isolates. Tropical Journal of Pharmaceutical Research . 2010;9:205–209. [Google Scholar]
- 2.Emacar J., Okemo P., Gatheri G., Kariuki S. Antibiotic resistance patterns of Escherichia coli isolated from HIV-sero positive adults at Mbagathi district hospital, Nairobi, Kenya. Journal of Applied Biosciences . 2010;27:1705–1714. [Google Scholar]
- 3.UNICEF Report. Maternal, Newborn and Child Health Working Paper on Access to Healthcare through Community Health Workers in East and Southern Africa United Nations Children’s Fund (UNICEF) New York, NY, USA: UNICEF; 2014. [Google Scholar]
- 4.Government of Kenya. National Policy for the Prevention and Containment of Antimicrobial Resistance . Nairobi, Kenya: Government of Kenya; 2017. [Google Scholar]
- 5.World Health Organization. WHO Global Report on Traditional and Complementary Medicine . Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 6.Rukangira E. The African herbal Industry: constraints and challenges. Proceedings of the Natural Products and Cosmeceutcals Conference; 2001; Erboristeria, Domani. pp. 1–23. [Google Scholar]
- 7.Wanzala W., Walingo M. K. Ethnomedicines and health management in Kenya: which way forward? Journal of Complementary Medicine and Alternative Healthcare . 2019;10(3) [Google Scholar]
- 8.Omwenga E. O., Hensel A., Shitandi A., Goycoolea F. M. Ethnobotanical survey of traditionally used medicinal plants for infections of skin, gastrointestinal tract, urinary tract and the oral cavity in Borabu sub-county, Nyamira county, Kenya. Journal of Ethnopharmacology . 2015;176:508–514. doi: 10.1016/j.jep.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Kimondo J., Miaron J., Mutai P., Njogu P. Ethnobotanical survey of food and medicinal plants of the Ilkisonko Maasai community in Kenya. Journal of Ethnopharmacology . 2015;175:463–469. doi: 10.1016/j.jep.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Mutie F. M., Gao L. L., Kathambi V., et al. An ethnobotanical survey of a dryland botanical garden and its environs in Kenya: the Mutomo hill plant sanctuary. Evidence-Based Complementary and Alternative Medicine . 2020;2020:22. doi: 10.1155/2020/1543831.1543831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oniyangi O., Cohall D. H. Phytomedicines (medicines derived from plants) for sickle cell disease. Cochrane Database of Systematic Reviews . 2018;2 doi: 10.1002/14651858.CD004448.pub6.CD004448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Njeru S. N., Matasyoh J., Mwaniki C. G., Kobia K. A review of some phytochemicals commonly found in medicinal plants. International Journal of Medicinal Plants Photon . 2013;105:135–140. [Google Scholar]
- 13.Godin K., Stapleton J., Kirkpatrick S. I., Hanning R. M., Leatherdale S. T. Applying systematic review search methods to the grey literature: a case study examining guidelines for school-based breakfast programs in Canada. Systematic Reviews . 2015;4(1):p. 138. doi: 10.1186/s13643-015-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scherer R. W., Saldanha I. J. How should systematic reviewers handle conference abstracts? a view from the trenches. Systematic Reviews . 2019;8(1):p. 264. doi: 10.1186/s13643-019-1188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bramer W. M., de Jonge G. B., Rethlefsen M. L., Mast F., Kleijnen J. A systematic approach to searching: an efficient and complete method to develop literature searches. Journal of the Medical Library Association . 2018;106(4):531–541. doi: 10.5195/jmla.2018.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alebie G., Urga B., Worku A. Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: trends and perspectives. Malaria Journal . 2017;16(1):307–316. doi: 10.1186/s12936-017-1953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huy N. T., Van Giang T., Thuy D. H. D., et al. Factors associated with dengue shock syndrome: a systematic review and meta-analysis. PLoS Neglected Tropical Diseases . 2013;7(9) doi: 10.1371/journal.pntd.0002412.e2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amadi E. K., Kareru P. G., Keriko J. M., Kiptoo J. Antimicrobial activity of selected medicinal plants’ extracts against Streptococcus pneumonia. International Journal of Botany Studies . 2016;1(1):20–22. [Google Scholar]
- 19.Waithaka P. N., Gathuru E. M., Githaiga B. M., Kazungu R. Z. Antimicrobial properties of Aloe vera, Aloe volkensii and Aloe secundiflora from Egerton university. Acta Scientific Microbiology . 2018;1:06–10. [Google Scholar]
- 20.Rachuonyo H. O., Ogola P. E., Arika W. M., Wambani J. R. Efficacy of crude leaf extracts of Aloe secundiflora on selected enteric bacterial pathogens and Candida albicans. Journal of Antimicrobiology . 2016;2:p. 112. [Google Scholar]
- 21.Mariita R. M., Orodho J. A., Okemo P. O., Kirimuhuzya C., Otieno J. N., Magadula J. J. Methanolic extracts of Aloe secundiflora Engl. inhibits in vitro growth of tuberculosis and diarrhea-causing bacteria. Pharmacognosy Research . 2011;3:95–99. doi: 10.4103/0974-8490.81956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opinde H. R., Gatheri G. W., Nyamache A. K. Antimicrobial evaluation of crude methanolic leaf extracts from selected medicinal against Escherichia coli. Journal of Bacteriology and Parasitology . 2016;7:p. 272. doi: 10.4172/2155-9597.1000272. [DOI] [Google Scholar]
- 23.Elufioye T. O., Alatise O. I., Fakoya F. A., Agbedahunsi J. M., Houghton P. J. Toxicity studies of Tithonia diversifolia A. Gray (Asteraceae) in rats. Journal of Ethnopharmacology . 2009;122(2):410–415. doi: 10.1016/j.jep.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Omwenga O. E., Mariita R. M., Alaro L., Okemo P. O. Evaluation of methanolic extracts of six medicinal plants used by herbal practitioners in central province-Kenya. International Journal of Pharmaceutical Sciences and Research. . 2011;2(4):867–874. [Google Scholar]
- 25.Ogoti P. M. Bioprospecting for Effective Antibiotics from Selected Kenyan Medicinal Plants against Four Clinical Salmonella Isolates . Juja, Kenya: Jomo Kenyatta University of Agriculture and Technology; 2017. https://ir.jkuat.ac.ke/handle/123456789/3432 . [Google Scholar]
- 26.Douglas K., Jeruto J. Phytochemistry and antimicrobial activity of extracts from medicinal plants Tithonia diversifolia and Olea africana. British Journal of Pharmaceutical Research . 2016;12(3):1–7. doi: 10.9734/bjpr/2016/26566. [DOI] [Google Scholar]
- 27.Kitonde C. K., Fidahusein D. S., Lukhoba C. W., Jumba M. M. Antimicrobial activity and phytochemical screening of Senna didymobotrya used to treat bacterial and fungal infections in Kenya. International Journal of Educational Research . 2014;2(1) [Google Scholar]
- 28.Nyamwamu L. B., Moses M., Ngeiywa M. M., et al. Cytotoxicity and in vitro antiamoebic activity of senna didymobotrya crude root extracts in comparison with metronidazole against entamoeba histolytica. 2015. https://www.semanticscholar.org/paper/CYTOTOXICITY-AND-IN-VITRO-ANTIAMOEBIC-ACTIVITY-OF-Nyamwamu-Ngeiywa/65a5ea31bca67ec6a9b555ddbf689addfbd3e791 .
- 29.Korir R. K., Mutai C., Kiiyukia C., Bii C. Antimicrobial activity and safety of two medicinal plants traditionally used in Bomet district of Kenya. Research Journal of Medicinal Plant . 2012;6(5):370–382. doi: 10.3923/rjmp.2012.370.382. [DOI] [Google Scholar]
- 30.India J. Efficacy of some medicinal plants used in various parts of Kenya in treating selected bacterial and fungal pathogens. 2015. https://irlibrary.ku.ac.ke/handle/123456789/14965 .
- 31.Jeruto P., Arama P. F., Anyango B., Maroa G. Phytochemical screening and antibacterial investigations of crude methanol extracts of Senna didymobotrya (Fresen.) H. S. Irwin & Barneby. Journal of Applied Biosciences . 2017;114:11357–11367. [Google Scholar]
- 32.Omwenga E. O., Goycoolea F. M., Hensel A., Shitandi A. Antimicrobial, cytotoxicity and preliminary phytochemical determination of commonly used medicinal plants to treat oral cavity, urinary tract and gut infections by inhabitants of Borabu sub-county, Nyamira County, Kenya. Malaysian Journal of Microbiology . 2020;16(4):312–322. doi: 10.21161/mjm.200697. [DOI] [Google Scholar]
- 33.Orwa J. A., Ngeny L., Mwikwabe N. M., Ondicho J., Jondiko I. J. O. Antimalarial and safety evaluation of extracts from Toddalia asiatica (L) Lam. (Rutaceae) Journal of Ethnopharmacology . 2013;145(2):587–590. doi: 10.1016/j.jep.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 34.Gakuubi M. M., Micheni K. N., Wanzala W. In vitro antibacterial activity of essential oil from the fruits of Toddalia asiatica (L) Lam. (Rutaceae) Journal of Biologically Active Products from Nature . 2017;7(1):52–61. doi: 10.1080/22311866.2017.1299590. [DOI] [Google Scholar]
- 35.Onjero I. Antimicrobial activity and interaction of Toddalia asiatica coumarins with two known-drugs. 2020. https://irlibrary.mmust.ac.ke/xmlui/bitstream/handle/123456789/1436/Isaiah%20Onjero.pdf?sequence=1&isAllowed=y .
- 36.Mariita R. M., Ogol C. K. P., Oguge N. O., Okemo P. O. Antitubercular and phytochemical investigation of methanol extracts of medicinal plants used by the Samburu community in Kenya. Tropical Journal of Pharmaceutical Research . 2010;9(4):379–385. doi: 10.4314/tjpr.v9i4.58935. [DOI] [Google Scholar]
- 37.Munyendo W. L. L., Kiprop A. K. Design, preparation and evaluation of germicidal Toddalia asiatica herbal antiseptic detergent. Journal of Applied Pharmaceutical Science . 2016;6(11) [Google Scholar]
- 38.Maima A. O., Munyendo W. L. L. Antimicrobial assay of aqueous extracts of selected ethno-pharmacologic alternatives used by the Maasai community of Narok, Kenya. European Journal of Medicinal Plants . 2019;26(3):1–11. doi: 10.9734/ejmp/2018/46227. [DOI] [Google Scholar]
- 39.Wagate C. G., Gakuya W. D., Nanyingi M. O., Njonge F. K., Mbaria J. M. Antibacterial and cytotoxic activity of Kenyan medicinal plants. Memorias Do Instituto Oswaldo Cruz . 2008;103(7):650–652. doi: 10.1590/s0074-02762008000700004. [DOI] [PubMed] [Google Scholar]
- 40.Musau J. K., Mbaria J. M., Gakuya D. W. The anti-bacterial activity of some medicinal plants used in Meru central district, Kenya. The Kenya Veterinarian . 2011;35(1):18–24. [Google Scholar]
- 41.Mayaka R. K., Langat M. K., Omolo J. O., Cheplogoi P. K. Antimicrobial prenylated acetophenones from berries of Harrisonia abyssinica. Planta Medica . 2012;78:383–386. doi: 10.1055/s-0031-1298143. [DOI] [PubMed] [Google Scholar]
- 42.Madivoli E., Maina E., Kairigo P., et al. In vitro antioxidant and antimicrobial activity of Prunus africana (Hook. f.) Kalkman (bark extracts) and Harrisonia abyssinica Oliv. extracts (bark extracts): a comparative study. Journal of Medicinal Plants for Economic Development . 2018;2(1) [Google Scholar]
- 43.Ngeny L. C., Magiri E., Mutai C., Mwikwabe N., Bii C. Antimicrobial properties and toxicity of Hagenia abyssinica (B.) J. F. Gmel, Fuerstia Africana T. C. E. Fries, Asparagus racemosus (willd.) and Ekebergia capensis sparrm. African Journal of Pharmacology and Theurapeutics . 2013;2(3):76–82. [Google Scholar]
- 44.Matu E. N., Kirira P. G., Kigondu E. V. M., Moindi E., Amugune B. Antimicrobial activity of organic total extracts of three Kenyan medicinal plants. African Journal of Pharmacology and Therapeutics . 2012;1(1):14–18. [Google Scholar]
- 45.Amugune B. K., Mwangi J. W., Thoithi G. N., Kibwage I. O. In vitro screening of ten selected traditionally used medicinal plants in Vihiga county, Kenya for antibacterial and antifungal activity. International Journal of Medicinal Plants and Natural Products . 2017;3(2):37–44. [Google Scholar]
- 46.Cheruiyot K. R., Olila D., Kateregga J. In-vitro antibacterial activity of selected medicinal plants from Longisa region of Bomet district, Kenya. African Health Sciences . 2009;9(Suppl 1):S42–S46. [PMC free article] [PubMed] [Google Scholar]
- 47.Amabeoku G. J., Bamuamba K. Evaluation of the effects of Olea europaea L. subsp. africana (Mill.) P.S. Green (Oleaceae) leaf methanol extract against castor oil-induced diarrhoea in mice. Journal of Pharmacy and Pharmacology . 2010;62(3):368–373. doi: 10.1211/jpp.62.03.0012. [DOI] [PubMed] [Google Scholar]
- 48.Kareru P. G., Gachanja A. N., Keriko J. M., Kenji G. M. Antimicrobial activity of some medicinal plants used by herbalists in eastern province, Kenya. African Journal of Traditional, Complementary, and Alternative Medicines . 2007;5(1):51–55. doi: 10.4314/ajtcam.v5i1.31256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kemboi D., Gitonga A. Antimicrobial activity of Bridelia micrantha and Grewia plagiophylla leaf extracts. Journal of Pharmaceutical Research International . 2016;12(3):1–7. [Google Scholar]
- 50.Ya’u J., Chindo B. A., Yaro A. H., Okhale S. E., Anuka J. A., Hussaini I. M. Safety assessment of the standardized extract of Carissa edulis root bark in rats. Journal of Ethnopharmacology . 2013;147(3):653–661. doi: 10.1016/j.jep.2013.03.064. [DOI] [PubMed] [Google Scholar]
- 51.Nyang’au H. O., Maingi J., Kebira A. The efficacy of some medicinal plants used locally within Transmara west, Narok County, Kenya against selected enterobacteria and candida. IOSR Journal of Pharmacy and Biological Sciences . 2017;12(1):115–122. [Google Scholar]
- 52.Korir R., Kimani F., Gathirwa J., Wambura M., Bii C. In-vitro antimicrobial properties of methanol extracts of three medicinal plants from Kilifi district—Kenya. African Journal of Health Sciences . 2007;20(1):5–10. [Google Scholar]
- 53.Njoroge P. W., Opiyo S. A. Some antibacterial and antifungal compounds from root bark of Rhus natalensis. American Journal of Chemistry . 2019;9(5):150–158. [Google Scholar]
- 54.Mwangi H. M., Mabusela W. T., Abegaz B. M., Martin O. O. Antimicrobial activities of a novel biflavonoid and other constituents from Rhus natalensis. Journal of Medicinal Plants Research . 2013;7(10):619–623. [Google Scholar]
- 55.Karani L. W., Tolo F. M., Karanja S. M., Khayeka−Wandabwa C. Safety of Prunus africana and Warburgia ugandensis in asthma treatment. South African Journal of Botany . 2013;88:183–190. doi: 10.1016/j.sajb.2013.07.007. [DOI] [Google Scholar]
- 56.Tolo F. M., Rukunga G. M., Muli F. W., et al. The anti-viral effect of Acacia mellifera, Melia azedarach and Prunus africana, extracts against herpes simplex virus type 1 infection in mice. Journal of Tropical Microbiology and Biotechnology . 2006;2(1):3–9. doi: 10.4314/jtmb.v2i1.35440. [DOI] [Google Scholar]
- 57.Bii C., Korir K., Rugutt J., Mutai C. The potential use of Prunus africana for the control, treatment and management of common fungal and bacterial infections. Journal of Medicinal Plants Research . 2010;4(11):995–998. [Google Scholar]
- 58.Ngule M. C., Ndiku M. H., Ramesh F. Chemical constituents screening and in vitro antibacterial assessment of Prunus africana bark hydromethanolic extract. Journal of Natural Sciences Research . 2014;4(16):85–90. [Google Scholar]
- 59.Mwitari P. G., Ayeka P. A., Ondicho J., Matu E. N., Bii C. Antimicrobial activity and probable mechanisms of action of medicinal plants of Kenya: Withania somnifera, Warbugia ugandensis, Prunus africana and Plectranthus barbatus. PLoS One . 2013;8(6) doi: 10.1371/journal.pone.0065619.e65619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abuto J. O., Morono D. A. Interaction effects of site, samples, plant parts and solvent types on antimicrobial activity of the Kenyan populations of Warburgia ugandensis (Sprague) Journal of Pharmaceutical, Chemical and Biological Sciences . 2018;6:1–12. [Google Scholar]
- 61.Ngule M., Ndiku M. Antibacterial potency of ethnobotanical plants as alternative remedies to curtail nosocomial infections: a case study of five native plants in Kenya. British Journal of Pharmaceutical Research . 2015;6(4):284–292. doi: 10.9734/bjpr/2015/13684. [DOI] [Google Scholar]
- 62.Njire M. M., Budambula N. L. M., Kiiru J. Antimicrobial activity of Warbugia ugandensis against gram negative multi‐drug resistant bacteria. Journal of Agriculture, Science and Technology . 2014;16(2) [Google Scholar]
- 63.Mikail H. G. Phytochemical screening, elemental analysis and acute toxicity of aqueous extract of Allium sativum L. bulbs in experimental rabbits. Journal of Medicinal Plants Research . 2010;4(4):322–326. [Google Scholar]
- 64.Nyaitondi O. D., Wanjau R., Nyambaka H. N., Hassanali A. Anti-bacterial properties and GC-MS analysis of extracts and essential oils of selected plant product. Biofarmasi Journal of Natural Product Biochemistry . 2018;16:44–58. [Google Scholar]
- 65.Makhulu E. E., Nyaga N. S., Wambugu S., Areba G. Synergistic antimicrobial activity of crude ethanolic extracts of garlic and neem leaves against bovine mastitis pathogens: an in vitro assay. International Journal of Basic and Clinical Pharmacology . 2017;6(9) [Google Scholar]
- 66.Mauti G. O., Mauti E. M., Ouno G., Mabeya B. Antibacterial activity of garlic, tulsi, bitter guard and cinnamon extracts against wound pathogens. Journal of Scientific and Innovative Research . 2015;4(44):178–181. [Google Scholar]
- 67.Mbuthia S. K., Wachira F. N., Koech R. K. In-vitro antimicrobial and synergistic properties of water-soluble green and black tea extracts. African Journal of Microbiology Research . 2014;8(14):1527–1534. [Google Scholar]
- 68.Hsu Y.-W., Tsai C.-F., Chen W.-K., Huang C.-F., Yen C.-C. A subacute toxicity evaluation of green tea (Camellia sinensis) extract in mice. Food and Chemical Toxicology . 2011;49(10):2624–2630. doi: 10.1016/j.fct.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 69.Obwoge J. O., Kinyua J. K., Kariuki D. W., Magoma G. N. Phytochemical screening and antimicrobial studies of green, orthodox and black Kenyan tea. Journal of Agriculture, Science and Technology . 2014;16(3) [Google Scholar]
- 70.Waithaka P. N., Gathuru E. M., Githaiga B. M., Tembo J. K. Synergistic antimicrobial effect of Egerton university cow’s urine and neem tree (Azadirachta indica) crude extracts on selected infectious human and plant pathogenic microbes. International Academy of Engineering and Medical Research . 2016;1(3) [Google Scholar]
- 71.Soule J. A. Tagetes minuta: a potential new herb from South America. In: Janick J., Simon J. E., editors. New Crops . New York, NY, USA: Wiley; 1993. pp. 649–654. [Google Scholar]
- 72.Musila M. F., Dossaji S. F., Nguta J. M., Lukhoba C. W., Munyao J. M. In vivo antimalarial activity, toxicity and phytochemical screening of selected antimalarial plants. Journal of Ethnopharmacology . 2013;146(2):557–561. doi: 10.1016/j.jep.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 73.Njue W., Kithokoi J. K., Mburu J., Mwangi H., Swaleh S. Green sonochemical synthesis of silver nanoparticles using Adansonia digitata leaves extract and evaluation of their antibacterial potential. European Journal of Advanced Chemistry Research . 2020;1(2) doi: 10.24018/ejchem.2020.1.2.5. [DOI] [Google Scholar]
- 74.Kaigongi M. M. Antimicrobial activity, toxicity and phytochemical analysis of four medicinal plants traditionally used in Msambweni District, Kenya. 2014. https://erepository.uonbi.ac.ke/handle/11295/74294?show=full .
- 75.Ngari F. W., Wanjau R. N., Njagi E. M., Gikonyo N. K. Antimicrobial and phytochemical investigation of herbal suspensions used in management of oral health in Nairobi County, Kenya. Journal of Biology, Agriculture and Healthcare . 2014;4(14) [Google Scholar]
- 76.Abiba A. O. Antibacterial efficacy and safety of selected Kenyan medicinal plants. 2013. https://www.semanticscholar.org/paper/Antibacterial-Efficacy-and-Safety-of-Selected-Abiba/9c596064195df743f118378c37e4de8e0b97d219 .
- 77.Mbabazi I., Wangila P., K’Owino I. O. Antimicrobial activity of Euclea divinorum hern (ebenaceae) leaves, tender stems, root bark and an herbal toothpaste formulated from its ethanolic root bark extract. International Journal of Research and Reports in Dentistry . 2020;3:8–16. [Google Scholar]
- 78.Kumar S., Rani C., Mangal M. A critical review on Salvadora persica: an important medicinal plant of arid zone. International Journal of Phytomedicine . 2012;4:292–303. [Google Scholar]
- 79.Omwenga E. O., Okemo P., Mbugua P. In vitro antimicrobial and preliminary phytochemical screening of some Samburu anti‐diarrheal medicinal plants‐Kenya. South Asian Journal of Experimental Biology . 2012;89 [Google Scholar]
- 80.Musila F. M., Nguta J. M., Lukhoba C. W., Dossaji S. F. Antibacterial and antifungal activities of 10 Kenyan Plectranthus species in the Coleus clade. Journal of Pharmacy Research . 2017;11(8):1003–1015. [Google Scholar]
- 81.Nguta J. M., Kiraithe M. N. In vitro antimicrobial activity of aqueous extracts of Ocimum suave Willd, Plectranthus barbatus andrews and Zanthoxylum chalybeum Engl. against selected pathogenic bacteria. Biomedical and Biotechnology Research Journal (BBRJ) . 2019;3(1):30–34. doi: 10.4103/bbrj.bbrj_128_18. [DOI] [Google Scholar]
- 82.Obey J. K., von Wright A., Orjala J., Kauhanen J., Tikkanen-Kaukanen C. Antimicrobial activity of Croton macrostachyus stem bark extracts against several human pathogenic bacteria. Journal of Pathogens . 2016;2016:5. doi: 10.1155/2016/1453428.1453428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maroyi A. Ethnopharmacological uses, phytochemistry, and pharmacological properties of Croton macrostachyus hochst. Ex delile: a comprehensive review. Evidence-Based Complementary and Alternative Medicine . 2017;2017:17. doi: 10.1155/2017/1694671.1694671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orafidiya L. O., Agbani E. O., Iwalewa E. O., Adelusola K. A., Oyedapo O. O. Studies on the acute and sub-chronic toxicity of the essential oil of Ocimum gratissimum L. leaf. Phytomedicine . 2004;11(1):71–76. doi: 10.1078/0944-7113-00317. [DOI] [PubMed] [Google Scholar]
- 85.Matasyoh L. G., Matasyoh J. C., Wachira F. N., Kinyua M. G., Muigai A. W., Mukiama T. K. Antimicrobial activity of essential oils of Ocimum gratissimum L. From different populations of Kenya. African Journal of Traditional, Complementary, and Alternative Medicines . 2008;5(2):187–193. doi: 10.4314/ajtcam.v5i2.31272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matasyoh J. C., Kiplimoa J. J., Karubiu N. M., Hailstorksc T. P. Chemical composition and antimicrobial activity of the essential oil of Satureja biflora (lamiaceae) Bulletin of the Chemical Society of Ethiopia . 2007;21(2):249–254. doi: 10.4314/bcse.v21i2.21204. [DOI] [Google Scholar]
- 87.Tan P. V., Mezui C., Enow-Orock G., Njikam N., Dimo T., Bitolog P. Teratogenic effects, acute and sub chronic toxicity of the leaf aqueous extract of Ocimum suave Wild (Lamiaceae) in rats. Journal of Ethnopharmacology . 2008;115(2):232–237. doi: 10.1016/j.jep.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 88.Njeru S. N., Obonyo M. A., Nyambati S. O., Ngari S. M. Antimicrobial and cytotoxicity properties of the crude extracts and fractions of Premna resinosa (Hochst.) Schauer (Compositae): Kenyan traditional medicinal plant. BMC Complementary and Alternative Medicine . 2015;15(1):p. 295. doi: 10.1186/s12906-015-0811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karumi E. Anthelmintic and antimicrobial activity of Hagenia abyssinica (bruce) J. F. Gmel (rosaceae) East and Central African Journal of Pharmaceutical Sciences . 2013;16(4):77–82. [Google Scholar]
- 90.Njeru S., Obonyo M., Nyambati S., Ngari S., Mwakubambanya R., Mavura H. Antimicrobial and cytotoxicity properties of the organic solvent fractions of Clerodendrum myricoides (Hochst.) R. Br. ex Vatke: Kenyan traditional medicinal plant. Journal of Intercultural Ethnopharmacology . 2016;5(3):226–232. doi: 10.5455/jice.20160416122003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Auwal S. M., Atiku M. K., Alhassan M. W., Mohammed S. S. Phytochemical composition and acute toxicity evaluation of aqueous root bark extract of Securidaca longepedunculata (Linn) Bayero Journal of Pure and Applied Sciences . 2013;5(2):67–72. doi: 10.4314/bajopas.v5i2.12. [DOI] [Google Scholar]
- 92.Wekesa E. N., Ojunga M, Otieno-Ayayo Z. N. (Polygalaceae) against two standard isolates of Neisseria gonorrhoeae. International Journal of Biochemistry Research and Review . 2020;29(6):61–68. [Google Scholar]
- 93.Abukakar M. G., Ukwuani A. N., Shehu R. A. Phytochemical screening and antibacterial activity of Tamarindus indica pulp extract. Asian Journal of Biochemistry . 2008;3(2):134–138. doi: 10.3923/ajb.2008.134.138. [DOI] [Google Scholar]
- 94.Nyaberi MO. Development and phytochemical characterization of a herbal preservative from Tamarindus indica and Ziziphus abyssinica herbs. 2018. https://ir.jkuat.ac.ke/handle/123456789/4792 .
- 95.Bunddotich J. K. Study of acute toxicity of Zanthoxylum chalybeum using mice and brine shrimp experimental models. 2012. https://erepository.uonbi.ac.ke/handle/11295/6806 .
- 96.Pour B. M., Sasidharan S. In vivo toxicity study of Lantana camara. Asian Pacific Journal of Tropical Biomedicine . 2011;1(3):230–232. doi: 10.1016/s2221-1691(11)60033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reddeman R. A., Glávits R., Endres J. R., et al. A toxicological evaluation of mango leaf extract (Mangifera indica) containing 60% mangiferin. Journal of Toxicology . 2019;2019:14. doi: 10.1155/2019/4763015.4763015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mutua J. K., Imathiu S., Owino W. Evaluation of the proximate composition, antioxidant potential, and antimicrobial activity of mango seed kernel extracts. Food Sciences and Nutrition . 2016;5(2):349–357. doi: 10.1002/fsn3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mbwambo Z. H., Moshi M. J., Masimba P. J., Kapingu M. C., Nondo R. S. O. Antimicrobial activity and brine shrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Complementary and Alternative Medicine . 2007;7:p. 9. doi: 10.1186/1472-6882-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Omale J. M. Nairobi, Kenya: University of Nairobi; 2017. Ethnobotany and antimicrobial properties of medicinal plants used in Emuhaya Sub-County, Vihiga County in Western Kenya. Masters thesis. [Google Scholar]
- 101.Aduol M. O., Ogila K. O. Antibacterial activity of dichloromethane, hexane, ethanol and methanol extracts of Asparagus setaceous Kunth and Caesalpinia volkensii harm. Asian Journal of Pharmaceutical and Health Sciences . 2012;2(3):380–383. [Google Scholar]
- 102.Ndile M. M., Mbinda W. M., Mbinda W. M., Ngugi M. P. Caesalpinia volkensii: unexploited natural source of medicine. The Journal of Phytopharmacology . 2018;7(3):288–291. doi: 10.31254/phyto.2018.7310. [DOI] [Google Scholar]
- 103.Amugune B., Thoithi G., Mwangi J., Omosa L., Kibwage I. Antimicrobial activity and bioactive constituents of Alectra sessiliflora (vahl) Kuntze methanol extract. The East and Central African Journal of Pharmaceutical Sciences . 2013;16(3):61–68. [Google Scholar]
- 104.Onyancha J. M., Bibiane A. W., Gervason A. M., Lameck N. A., Japhet K. N. The antibacterial, antioxidant and phytochemical composition of Combretum tanaense (J. Clark) root extracts. European Journal of Medicinal Plants . 2018;23(4):1–8. [Google Scholar]
- 105.Muema M. J. Phytochemical and antimicrobial investigation of Ochna thomasiana engl. & gilg. 2015. https://ir-library.ku.ac.ke/handle/123456789/13322 .
- 106.Liang Y.-C., Lin C.-J., Yang C.-Y., et al. Toxicity study of Bidens pilosa in animals. Journal of Traditional and Complementary Medicine . 2019;10(2):150–157. doi: 10.1016/j.jtcme.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cherotich J. Antimicrobial activity, acute toxicity and phytochemical composition of four medicinal plants traditionally used in sotik sub-county, Kenya. 2015. https://erepository.uonbi.ac.ke/handle/11295/90127?show=full .
- 108.Onoja S., Ukwueze C., Ezeja M., Udeh N. Antinociceptive and antioxidant effects of hydromethanolic extract of Bridelia micrantha stem bark. Journal of Experimental and Integrative Medicine . 2014;4(4):p. 273. doi: 10.5455/jeim.271014.or.114. [DOI] [Google Scholar]
- 109.Wanyama A. W., Orwa J. A., Njenga P. K., Irungu B. N. Evaluation of cytotoxicity, antimicrobial activities and minerals composition of Vigna subterranea (L.) verdc. (Bambara groundnut) extracts. African Journal of Health Sciences . 2017;30(2) [Google Scholar]
- 110.Oyebadejo S. A., Solomon I. P. Acute and sub-acute toxicity study of Citrus limon (L) juice in Sprawgue dawley rats. East African Scholars Journal of Biotechnology and Genetics . 2019;1(2) [Google Scholar]
- 111.Namadina M. M., Nuhu A., Yahuza S., et al. Phytochemical screening, physicochemical properties and acute toxicity study of the leaves of Ziziphus abyssinica hochst. EX A. RICH. (RHAMNACEAE) Chemistry . 2019;3(3):102–108. [Google Scholar]
- 112.Naidu J., Ismail R., Sasidharan S. Acute oral toxicity and brine shrimp lethality of methanol extract of Mentha spicata L (lamiaceae) Tropical Journal of Pharmaceutical Research . 2014;13(1):101–107. doi: 10.4314/tjpr.v13i1.15. [DOI] [Google Scholar]
- 113.Githaiga B. M., Gathuru E. M., Gathuru E. M., Waithaka P. N., Kiarie L. W. Determination of antibacterial activity of essential oils from mint (Mentha spicata) leaves on selected pathogenic bacteria. Journal of Drugs and Pharmaceutical Science . 2018;2(2):8–14. doi: 10.31248/jdps2018.015. [DOI] [Google Scholar]
- 114.Njeru S. N., Josphat C. M., Charles G. M., Charles M. M. Antibacterial activity of methanol root extract of Indigofera lupatana Baker F. Eastern Journal of Medicine . 2012;17:11–16. [Google Scholar]
- 115.Husna R. N., Noriham A., Nooraain H., Azizah A. H., Amna O. F. Acute oral toxicity effects of Momordica charantia in Sprague dawley rats. International Journal of Bioscience, Biochemistry and Bioinformatics . 2013;3(4) [Google Scholar]
- 116.Ongarora D. S. B. Phytochemical investigation and antimicrobial activity of Blighia unijugata bak (sapindaceae) 2009. https://erepository.uonbi.ac.ke/handle/11295/24499 .
- 117.Bléyéré N. M., N’dia K. F., Kouakou K. L., Abo K. J., Ehilé E. E. Acute toxicity in mice and effects of a butanol extract from the leaves of Blighia unijugata bak. (Sapindaceae) on electrocardiogram of rabbits. Scholars Academic Journal of Pharmacy . 2013;2(6):429–435. [Google Scholar]
- 118.Ondicho M., Mutai J., Rukunga C., Oketch G., Bii C. Antimicrobial activity of the root, stem bark and seed extracts of Moringa Oleifera Lam.. Proceedings of the 5th World Congress on Chemical, Biological and Radiological Terrorism; 2009; Dubrovnik, Croatia. [Google Scholar]
- 119.Asare G. A., Gyan B., Bugyei K., et al. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. Journal of Ethnopharmacology . 2012;139(1):265–272. doi: 10.1016/j.jep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 120.Chemweno T., Lizzy M., Richard K., Angela M., Christine B. Antimicrobial activity and safety of Maesa lanceolata for the treatment and management of selected bacterial pathogens. Journal of Advances in Microbiology . 2018;8(3):1–8. [Google Scholar]
- 121.Wamuyu K. R., Machocho A. K., Wafula A. W. Antimicrobial and phytochemical screening of Lannea schweinfurthii (Engl.) Engl. Bioteknologi . 2020;17:1–13. [Google Scholar]
- 122.Dutta S., Bhattacharyya D. Enzymatic, antimicrobial and toxicity studies of the aqueous extract of Ananas comosus (pineapple) crown leaf. Journal of Ethnopharmacology . 2013;150(2):451–457. doi: 10.1016/j.jep.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 123.Precious M., John K., Naomi M. In Vitro Antimicrobial Activity of Nano-Encapsulated Bromelain against Bacteria Isolated from Milk of Dairy Goats with Sub-Clinical Mastitis in Thika East Sub-county, Kenya . 2020. [Google Scholar]
- 124.Chalo D. M., Lukhoba C., Fidahussein D. S., Nguta J. M. Antimicrobial activity, toxicity and phytochemical screening of selected medicinal plants of Losho, Narok county, Kenya. Biofarmasi (Rumphius Journal of Natural Product Biochemistry) . 2017;15:29–43. [Google Scholar]
- 125.Ndikau M., Noah N. M., Andala D. M., Masika E. Green synthesis and characterization of silver nanoparticles using Citrullus lanatus fruit rind extract. International Journal of Analytical Chemistry . 2017;2017:9. doi: 10.1155/2017/8108504.8108504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Belemkar S., Shendge P. N. Toxicity profiling of the ethanolic extract of Citrullus lanatus seed in rats: behavioral, biochemical and histopathological aspects. Bioscience Reports . 2021;41(1) doi: 10.1042/BSR20202345.BSR20202345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ping K. W., Darah I., Chen Y., Sreeramanan S., Sasidharan S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed Research International . 2013;2013:14. doi: 10.1155/2013/182064.18206410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kibet LP. Ethnobotanical Study, toxicity and phytochemical screening of selected medicinal plants of Tinderet district, Nandi county, Kenya. 2013. https://erepository.uonbi.ac.ke/handle/11295/61411 .
- 129.Chagnon M. Inventaire pharmacologique general des plantes medicinales rwandaises. Journal of Ethnopharmacology . 1984;12(3):239–251. doi: 10.1016/0378-8741(84)90053-9. [DOI] [PubMed] [Google Scholar]
- 130.Kimutai N., Njenga E. W., Jeruto P., et al. Antimicrobial activity and cytotoxicity of selected medicinal plants found in Nandi county, Kenya. African Journal of Pharmacology and Therapeutics . 2015;4(3):86–91. [Google Scholar]
- 131.Kamdem Boniface P., Singh M., Kumar Maurya A., Pal A. Acute and sub-chronic toxicity of HPLC fingerprinted extract of Conyza sumatrensis (Retz.) E.H. Walker in rodents. Journal of Ethnopharmacology . 2013;149(3):833–837. doi: 10.1016/j.jep.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 132.Maima A. O., Ndwigah S. N., Thoithi G. N., Kamau F. N., Kibwage O. I. Antimicrobial properties of some medicinal plants of the Luo community of Kenya. African Journal of Pharmacology and Therapeutics . 2014;3(4):112–115. [Google Scholar]
- 133.Olela B., Mbaria J., Wachira T., Moriasi G. Acute oral toxicity and anti-inflammatory and analgesic effects of aqueous and methanolic stem bark extracts of Piliostigma thonningii (schumach.) Evidence-Based Complementary and Alternative Medicine . 2020;2020:10. doi: 10.1155/2020/5651390.5651390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chitopoa W., Muchachaa I., Mangoyi R. Evaluation of the antimicrobial activity of Erythrina abyssinica leaf extract. Journal of Microbial and Biochemical Technology . 2019;11(2):p. 413. [Google Scholar]
- 135.Mwangi J. W., Thoithi G. N., Kibwage I. O., et al. Essential oil of Rynchosia minima DC. from Kenya: composition and antibacterial properties. Journal of Essential Oil Research . 2005;17(2):230–231. doi: 10.1080/10412905.2005.9698885. [DOI] [Google Scholar]
- 136.Mayeku P. W., Hassanali A., Kiremire B. T., Odalo J. O., Hertweck C. Anti-bacterial activities and phytochemical screening of extracts of different parts of Thalictrum rhynchocarpum. African Journal of Traditional, Complementary, and Alternative Medicines . 2013;10(5):341–344. doi: 10.4314/ajtcam.v10i5.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruttoh E. K., Tarus P. K., Bii C. C., Machocho A. K., Karimie L. K., Okemo P. O. Antibacterial activity of Tabernaemontana stapfiana britten (apocynaceae) extracts. African Journal of Traditional, Complementary, and Alternative Medicines . 2009;6(2):186–194. [PMC free article] [PubMed] [Google Scholar]
- 138.Mutuku A., Mwamburi L., Keter L., et al. Evaluation of the antimicrobial activity and safety of Rhus vulgaris (Anacardiaceae) extracts. BMC Complementary Medicine and Therapies . 2020;20(1):p. 272. doi: 10.1186/s12906-020-03063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaigongi M. M., Lukhoba C. W., Yaouba S., Makunga N. P., Githiomi J., Yenesew A. In vitro antimicrobial and antiproliferative activities of the root bark extract and isolated chemical constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae) Plants . 2020;9(7):p. 920. doi: 10.3390/plants9070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thakurdesai P., Deshpande P., Mohan V. Preclinical safety assessment of standardized extract of Centella asiatica (L.) urban leaves. Toxicology International . 2015;22(1):10–20. doi: 10.4103/0971-6580.172251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Waithaka P., Eliud G., Githaiga B. Antimicrobial properties of Aloe vera, Aloe volkensii and Aloe secundiflora from egerton university. Acta Scientific Microbiology . 2018;1:6–10. [Google Scholar]
- 142.Mugweru F. G., Nyamai D. W. In vivo safety of aqueous extracts of Maytemus putterlickoides, Senna spectabilis and Olinia usambarensis on mice models. Journal of Clinical Toxicology . 2016;6(3) [Google Scholar]
- 143.Odhiambo R. S., Kareru G. P., Kutima L. H., et al. Antibacterial activity of ethanolic extracts of Prosopis juliflora against gram-negative bacteria. European Journal of Experimental Biology . 2015;5(11):43–46. [Google Scholar]
- 144.da Silva V. D. A., da Silva A. M. M., E Silva J. H. C., Costa S. L. Neurotoxicity of Prosopis juliflora: from natural poisoning to mechanism of action of its piperidine alkaloids. Neurotoxicity Research . 2018;34(4):878–888. doi: 10.1007/s12640-017-9862-2. [DOI] [PubMed] [Google Scholar]
- 145.Manekeng H. T., Mbaveng A. T., Ntyam Mendo S. A., Agokeng A. D., Kuete V. Evaluation of acute and subacute toxicities of Psidium guajava methanolic bark extract: a botanical with in vitro antiproliferative potential. Evidence-Based Complementary and Alternative Medicine . 2019;2019:13. doi: 10.1155/2019/8306986.8306986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yeo D., Djyh B. N., David N. J., Bouagnon R. Acute and subacute toxic study of aqueous leaf extract of Combretum molle. Tropical Journal of Pharmaceutical Research . 2012;11(2):217–223. [Google Scholar]
- 147.Ibrahim G., Abdurahman E. M., Ibrahim H., Ibrahim N. D. G., Magaji M. G. Toxicity and analgesic effects of Vernonia amygdalina Del. (Asteraceae) leaf extract on mice. International Journal of Advanced Pharmaceutical and Biological Sciences . 2011;1(1):1–4. [Google Scholar]
- 148.Muhindi S. W., Ngule C. M., Ramesh F. Phytochemical and antibacterial potential of Vernonia adoensis stem bark to curb cariogenic microorganisms. American Journal of Phytomedicine and Clinincal Therapeutics . 2016;4(01):019–027. [Google Scholar]
- 149.Okindo R. O. Study of antimicrobial, analgesic and toxic properties of Vernonia hymenolepis (A. Rich) 2014. https://erepository.uonbi.ac.ke/handle/11295/73596?show=full .
- 150.Sandiga I., Chacha C. N., Kanunah M. P. Traditional Medicine in Africa: The Existence and Use of Traditional Medicine in Kenya . Nairobi, Kenya: East African Educational Publishers Ltd; 1995. [Google Scholar]
- 151.Njoroge G. N., Kibunga J. W. Herbal medicine acceptance, sources and utilization for diarrhoea management in a cosmopolitan urban area (Thika, Kenya) African Journal of Ecology . 2007;45(s1):65–70. doi: 10.1111/j.1365-2028.2007.00740.x. [DOI] [Google Scholar]
- 152.James P. B., Wardle J., Steel A., Adams J. Traditional, complementary and alternative medicine use in Sub-Saharan Africa: a systematic review. BMJ Global Health . 2018;3(5) doi: 10.1136/bmjgh-2018-000895.e000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maina N., Kagira J. M., Achila O., Karanja S. M., Ngotho M. Herbal medicines in Kenya: a review of the toxicity and quality control issues. African Journal of Health Sciences . 2013;24(1) [Google Scholar]
- 154.Kareru P. G., Keriko J. M., Kenji G. M., Thiong’o G. T., Gachanja A. N., Mukiira H. N. Antimicrobial activities of skincare preparations from plant extracts. African Journal of Traditional, Complementary, and Alternative Medicines . 2010;7(3):214–218. doi: 10.4314/ajtcam.v7i3.54777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Onyambu M. Nairobi, Kenya: University of Nairobi; 2011. Identification and characterization of microbial contaminants of herbal medicines in Kenya. Research thesis. [Google Scholar]
- 156.Sivasankaran N. M., Selwin J. R. Synthesis and characterization of Co (II), Ni (II), Cu (II), and Zinc (II) complexes of tridentate Schiiff base derived from vanillin and DL-α-aminobutyric acid. Spectrochimica Acta . 2008;70:749–753. doi: 10.1016/j.saa.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 157.Odhiambo J. A., Siboe G. M., Lukhoba C. W., Dossaji S. F. Antifungal activity of crude extracts of selected medicinal plants used in combinations in Lake Victoria Basin, Kenya. Plant Products Research Journal . 2011;13 [Google Scholar]
- 158.Tiwari T. P., Bharti S. K., Kaur H. D., Dikshit R. P., Hoondal G. S. Synergistic antimicrobial activity of tea and antibiotics. Indian Journal of Medical Research . 2005;122:80–84. [PubMed] [Google Scholar]
- 159.Faizi S., Mughal N. R., Khan R. A., et al. Evaluation of the antimicrobial property of Polyalthia longifolia var. pendula: isolation of a lactone as the active antibacterial agent from the ethanol extract of the stem. Phytotherapy Research . 2003;17(10):1177–1181. doi: 10.1002/ptr.1333. [DOI] [PubMed] [Google Scholar]
- 160.Ashafa A. O. T., Orekoya L. O., Yakubu M. T. Toxicity profile of ethanolic extract of Azadirachta indica stem bark in male Wistar rats. Asian Pacific Journal of Tropical Biomedicine . 2012;2:811–817. doi: 10.1016/s2221-1691(12)60234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dubey R. C. A. In: Textbook of Biotechnology . Najar R., editor. New Delhi, India: S. Chand and Company Ltd.; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used in the study.


