Abstract
Aims
The SYNTAX II study evaluated the impact of advances in percutaneous coronary intervention (PCI), integrated into a single revascularization strategy, on outcomes of patients with de novo three-vessel disease. The study employed decision-making utilizing the SYNTAX score II, use of coronary physiology, thin-strut biodegradable polymer drug-eluting stents, intravascular ultrasound, enhanced treatments of chronic total occlusions, and optimized medical therapy. Patients treated with this approach were compared with predefined patients from the SYNTAX I trial.
Methods and results
SYNTAX II was a multicentre, single-arm, open-label study of patients requiring revascularization who demonstrated clinical equipoise for treatment with either coronary artery bypass grafting (CABG) or PCI, predicted by the SYNTAX score II. The primary endpoint was major adverse cardiac and cerebrovascular events (MACCE), which included any revascularization. The comparators were a matched PCI cohort trial and a matched CABG cohort, both from the SYNTAX I trial. At 5 years, MACCE rate in SYNTAX II was significantly lower than in the SYNTAX I PCI cohort (21.5% vs. 36.4%, P < 0.001). This reflected lower rates of revascularization (13.8% vs. 23.8%, P < 0.001), and myocardial infarction (MI) (2.7% vs. 10.4%, P < 0.001), consisting of both procedural MI (0.2% vs. 3.8%, P < 0.001) and spontaneous MI (2.3% vs. 6.9%, P = 0.004). All-cause mortality was lower in SYNTAX II (8.1% vs. 13.8%, P = 0.013) reflecting a lower rate of cardiac death (2.8% vs. 8.4%, P < 0.001). Major adverse cardiac and cerebrovascular events’ outcomes at 5 years among patients in SYNTAX II and predefined patients in the SYNTAX I CABG cohort were similar (21.5% vs. 24.6%, P = 0.35).
Conclusions
Use of the SYNTAX II PCI strategy in patients with de novo three-vessel disease led to improved and durable clinical results when compared to predefined patients treated with PCI in the original SYNTAX I trial. A predefined exploratory analysis found no significant difference in MACCE between SYNTAX II PCI and matched SYNTAX I CABG patients at 5-year follow-up.
Keywords: Multivessel disease, Percutaneous coronary intervention, SYNTAX score, Coronary physiology, SYNTAX II study
Graphical Abstract
See the editorial comment for this article ‘State of the Art’ PCI: bridging the implementation gap’, by Zaid I. Almarzooq and Robert W. Yeh, https://doi.org/10.1093/eurheartj/ehab855.
Introduction
Percutaneous coronary intervention (PCI) is regarded as the standard of care for patients with non-complex single-vessel coronary artery disease (CAD) who remain symptomatic despite optimal medical therapy. Conversely, in patients with medium or high anatomical complexity, which involves all three vessels (3VD), coronary artery bypass graft (CABG) surgery continues to be the recommended modality of revascularization.1
These recommendations and guidelines are informed by the pivotal SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) trial, which compared CABG to PCI with first-generation drug-eluting stents (DES).2 In the SYNTAX trial, revascularization with PCI was associated with a higher rate of major adverse cardiac or cerebrovascular events (MACCE) at 5 years. Subsequent additional data from the FREEDOM and BEST trials have also suggested the superiority of revascularization with CABG rather than PCI with DES.3 , 4
Of note, over the 15 years elapsed since the design of the SYNTAX trial, the practice of PCI has changed substantially, with multiple developments that have been shown to improve patient outcomes. It is widely acknowledged that the distribution, functional relevance, and anatomical complexity of coronary atheromatous lesions, as well as individual clinical characteristics and comorbidities of patients with complex CAD, impact clinical outcomes of percutaneous and surgical revascularization. The SYNTAX score II, which was based on the 4-year results of the SYNTAX trial, combines the anatomical angiographic score with clinical factors to aid the heart team to undertake objective decision-making between CABG and PCI based on long-term prognosis, allowing a refined triage of patients with multivessel disease for the consideration of PCI.5
The use of intracoronary physiology to set the indication of revascularization on a lesion level has been a transformational change in PCI practice, avoiding unnecessary interventions and improving clinical outcomes. Stent technology has also evolved and the availability of third generation, thin-strut DES with biodegradable polymer together with intravascular imaging to guide stent implantation has improved procedural outcomes. In the SYNTAX trial, revascularization was encouraged as a single procedure, and this contributed to incomplete revascularization in a sizable minority of patients, especially those with chronic total occlusions (CTO).2 Techniques for revascularization of patients with CTO have evolved dramatically, and this potentially should allow more patients to get complete revascularization and theoretically reduce residual ischaemia.6
The main hypothesis of the SYNTAX II study is that the integrated use of these developments, coupled with refined patient selection, could improve outcomes of 3VD patients treated with PCI, compared to the original SYNTAX trial.7 Accordingly, patients with all anatomical complexities including some patients exceeding the low SYNTAX angiographic score group were assessed using the SYNTAX score II, and their projected mortality following revascularization was appraised. Patients where potential equipoise existed after treatment with either PCI or CABG at 4 years were offered PCI as part of a new predefined cohort of PCI patients comprising the SYNTAX II two study cohorts. This study reports the 5-year clinical follow-up of these patients and explores how the integration of new developments in PCI practice potentially impacts upon patient outcomes, compared to those results obtained in the original SYNTAX I trial.
Methods
Study design
The design and rationale of the SYNTAX II study were previously reported.7 In short, SYNTAX II is a multicentre, open-label, single-arm study, which enrolled patients presenting with stable or unstable angina with de novo 3VD with no left main involvement. Between February 2014 and November 2015, patients were included in 22 European catheterization laboratories. SYNTAX II is an investigator-initiated study, sponsored by the European Cardiovascular Research Institute (ECRI, Rotterdam, the Netherlands) with unrestricted research grants from Volcano now Philips Image-Guided Therapy Devices, San Diego, CA, USA, and Boston Scientific Corp, MA, USA. The grant givers were not involved in data collection, data interpretation, or writing the manuscript. The local ethics committee approved the study in all participating sites.
Patients were screened by local heart teams, which included a cardiac surgeon and an interventional cardiologist. Patient selection involved the following sequential steps: (i) anatomical suitability, including the determination of target vessels >1.5 mm requiring treatment; (ii) calculated equipoise between PCI and CABG for 4-year mortality based on the SYNTAX score II estimated by the investigators; and (iii) confirmation of expected equivalent revascularization results with either strategy. Exclusion criteria were minimal, including prior revascularization, ongoing acute myocardial infarction (MI), and concomitant valvular disease requiring surgery.
Eligible patients were treated following the SYNTAX II revascularization strategy, which required an initial hybrid coronary physiology assessment sequentially using instantaneous wave-free ratio (iFR, Volcano Corporation) and fractional flow reserve (FFR) to define revascularization appropriateness based on ischaemia. An iFR <0.86 indicated need for revascularization, an iFR between 0.86 and 0.93 required FFR assessment, and an iFR >0.93 indicated PCI deferral.7 Pre-PCI intravascular ultrasound (IVUS) was left at operator’s discretion. Post-PCI optimization with IVUS was mandatory, according to the modified MUSIC criteria.8 Bifurcation lesions were treated following the European Bifurcation Club consensus.9 Treatment of CTOs was performed by dedicated CTO operators where possible and complete revascularization and staged procedures were encouraged. At least 6-month dual antiplatelet therapy (DAPT) was mandatory with indefinite use of aspirin, following the ACC/AHA/ESC guidelines.10 , 11 Risk factor control, including high-intensity lipid-lowering therapy, was recommended. All lesions were treated with SYNERGY™ (Boston Scientific, Natick, MA, USA), a thin-strut bioresorbable polymer DES.
Control groups
SYNTAX II outcomes were compared to a selected PCI cohort from the SYNTAX I trial.2 SYNTAX I controls were selected based on calculated equipoise between PCI and CABG for 4-year mortality according to the SYNTAX score II, in patients with 3VD.5 The SYNTAX score II (available at http://syntaxscore.org) is a predictive tool that inputs seven clinical parameters (age, creatinine clearance, left ventricular ejection fraction, presence of left main disease, gender, presence of chronic obstructive pulmonary disease, and presence of peripheral artery disease) and the anatomical SYNTAX score. With these data, the SYNTAX score II then calculates a predicted mortality outcome if the patient underwent either PCI or CABG. It then categorizes the patient into a group where, based on this calculation, either PCI is preferred, CABG is preferred, or there is equipoise between the two revascularization strategies. Patients with expected calculated equipoise for PCI or CABG were selected for the historical comparison between SYNTAX I and SYNTAX II. This process yielded 315 patients in the SYNTAX I PCI cohort. Baseline characteristics were deemed comparable, acknowledging the limitations of a historical control. In addition, an exploratory comparison was performed with the CABG population of the SYNTAX I trial, which was selected based on the same criteria and yielded 334 patients. The same selected cohorts from SYNTAX I were used for the analyses at all time points. Long-term clinical status was assessed identically in the SYNTAX I trial and the SYNTAX II study. Ascertainment of events and ongoing reporting was performed by local investigators by reviewing patient charts, and a yearly patient contact via telephone. Source documents were collected centrally for adjudication.
Study endpoints
A composite of MACCE is the primary endpoint defined as all-cause death, any stroke, any MI, or any revascularization, and SYNTAX I and SYNTAX II were adjudicated using the same endpoint definitions, as reported previously.8 Secondary endpoints included a composite of all-cause death, stroke, any MI; individual components and definite stent thrombosis—according to the Academic Research Consortium definitions.12 All endpoints were adjudicated by an independent clinical events committee.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (interquartile range) and compared with the Student’s t-test or Mann–Whitney test as appropriate. Categorical variables are presented as counts and percentages and compared with the χ 2 test. The primary analysis was done for superiority (two-sided alpha of 5%) in the comparison between SYNTAX II and the PCI cohort in SYNTAX I at 1 year and previously reported.8 The full population enrolled in the SYNTAX II study was included in the analysis (i.e. after informed consent). Both SYNTAX I cohorts were selected after randomization and included in the analysis. Kaplan–Meier estimates (tested with log-rank test) and hazard ratios (HRs) (using Cox proportional hazards models and the Wald test) are calculated. Hazard ratios were calculated for those endpoints for which the proportional hazards assumption was met. Censoring of follow-up occurred at 5 years or at the last date of known clinical status, whichever came first. No imputations were performed. Non-inferiority in patient-oriented composite endpoint between SYNTAX II and the CABG cohort of SYNTAX I was also explored at 1 year and previously reported,8 using a non-inferiority margin of 5% with a one-sided alpha of 5%. This report compares rates at the 5-year follow-up time point with no formal testing. A P-value of <0.05 was considered significant. The statistical analyses were performed using the SAS System software, version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
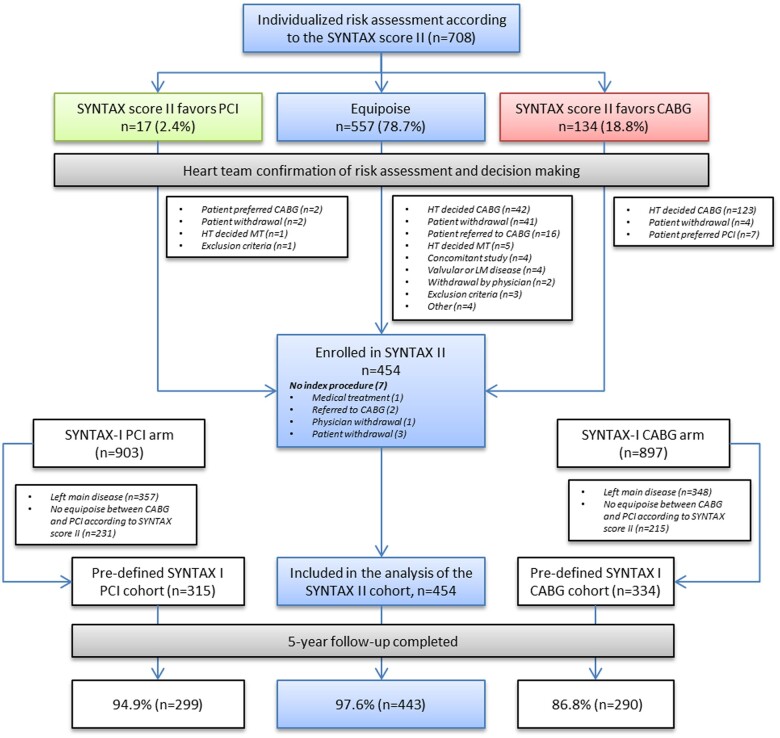
The study flow chart is presented in Figure 1. Out of 454 patients enrolled in SYNTAX II, all but 11 completed the 5-year follow-up visit (98%). Out of 315 selected PCI patients from the SYNTAX I trial, all but 16 completed the 5-year follow-up visit (95%). Similarly, out of the 334 selected CABG patients from the SYNTAX I trial, all but 44 completed the 5-year follow-up visit (87%). Baseline and procedural characteristics have been reported earlier and are provided in the Supplementary material online, Appendix.
Figure 1.
Study patient flow chart. The 5-year clinical outcomes of patients undergoing percutaneous coronary intervention using the SYNTAX II strategy were compared with predefined cohorts of the percutaneous coronary intervention and coronary artery bypass graft arm of the original SYNTAX I trial. CABG, coronary artery bypass graft; HT, heart team; MT, medical treatment; PCI, percutaneous coronary intervention. Adapted from Escaned et al. 8
Medical therapy
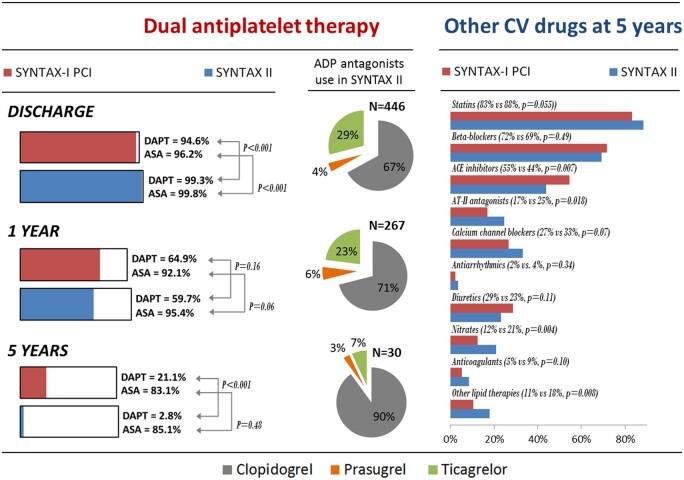
Dual antiplatelet therapy was generally used at discharge (99.3% for SYNTAX II and 94.6% for SYNTAX I, P < 0.001) but by 5 years DAPT was less frequently used in SYNTAX II (2.8% vs. 21.1%, P < 0.001). Among patients taking DAPT in SYNTAX II, ticagrelor or prasugrel was used in 33.2%, 28.8%, and 10% of patients at discharge, 1 year, and 5 years, respectively. Clopidogrel was used in 66.8%, 71.2%, and 90% of patients taking DAPT, respectively. Other cardiovascular medications were similarly used among SYNTAX II and SYNTAX I, with the minor numerical differences summarized in Figure 2 and in the Supplementary material online, Appendix.
Figure 2.
Medical therapy in the SYNTAX II study. ASA, aspirin; CV, cardiovascular; DAPT, dual antiplatelet therapy; PCI, percutaneous coronary intervention.
Primary endpoint and all-cause death
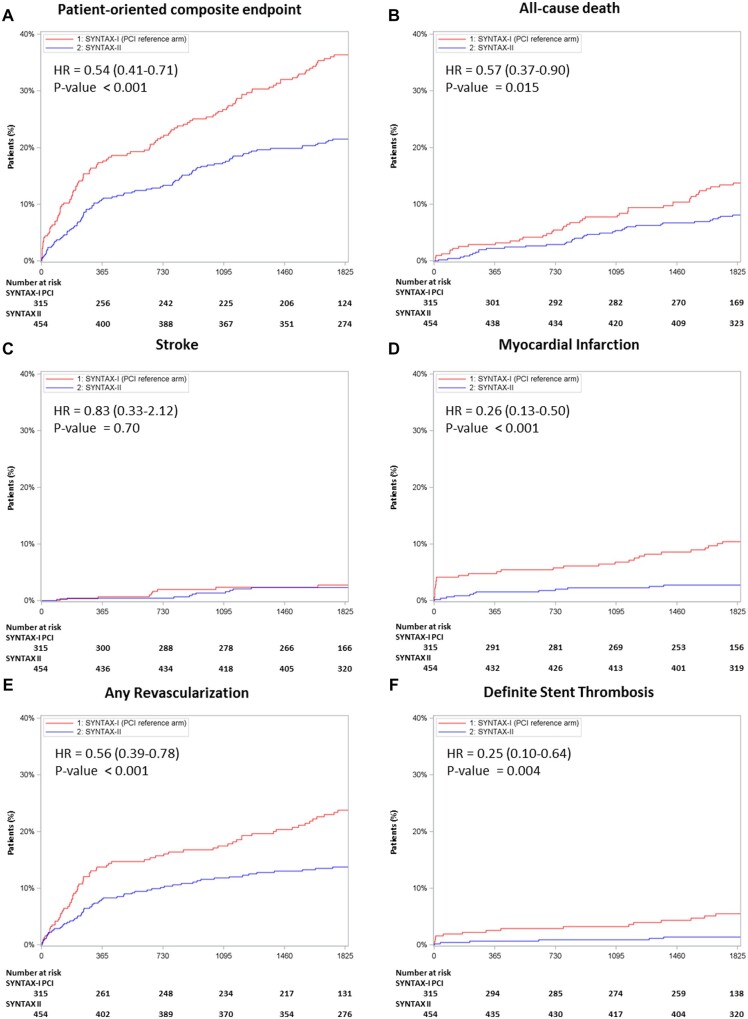
Five-year major adverse cardiac and cerebrovascular events (MACCE) were significantly lower in the SYNTAX II study as compared with the SYNTAX I PCI cohort [21.5% vs. 36.4%, HR 0.54, 95% confidence interval (CI) 0.41–0.71; P < 0.001] (Table 1 and Figure 3). Notably, the 5-year follow-up of the SYNTAX II study showed a lower rate of all-cause death (8.1% vs. 13.8%, HR 0.57, 95% CI 0.37–0.90; P = 0.015), mainly comprised by lower observed rates of cardiac death (2.8% vs. 8.4%, P < 0.001). The adjusted analysis for all-cause death revealed no material change (adjusted HR 0.53, 95% CI 0.33–0.85) relative to the unadjusted HR (see Supplementary material online, Appendix).
Table 1.
Five-year clinical outcomes between the SYNTAX II cohort and the equipoise-derived SYNTAX I percutaneous coronary intervention cohort
| Outcome | SYNTAX II (n = 454) | SYNTAX (PCI control arm) (n = 315) | Log-rank | HR (95% CI) |
|---|---|---|---|---|
| P-valuea | ||||
| POCE | 21.5% (96) | 36.4% (112) | <0.001 | 0.54 (0.41–0.71) |
| Composite of any death, any stroke, any MIb | 10.8% (48) | 21.8% (67) | <0.001 | 0.47 (0.32–0.68) |
| Any death | 8.1% (36) | 13.8% (42) | 0.013 | 0.57 (0.37–0.90) |
| Cardiac death | 2.8% (12) | 8.4% (25) | <0.001 | 0.32 (0.16–0.64) |
| Vascular death | 1.6% (7) | 1.1% (3) | 0.51 | 1.56 (0.40–6.05) |
| Non-cardiovascular death | 4.0% (17) | 4.9% (14) | 0.56 | 0.81 (0.40–1.64) |
| Any stroke | 2.3% (10) | 2.7% (8) | 0.70 | 0.83 (0.33–2.12) |
| Ischaemic | 1.6% (7) | 2.1% (6) | 0.65 | 0.78 (0.26–2.32) |
| Haemorrhagic | 0.9% (4) | 0.7% (2) | 0.74 | 1.34 (0.25–7.31) |
| Any MIb | 2.7% (12) | 10.4% (31) | <0.001 | 0.26 (0.13–0.50) |
| Procedural MI | 0.2% (1) | 3.8% (12) | <0.001 | 0.06 (0.01–0.44) |
| Spontaneous MI | 2.3% (10) | 6.9% (19) | 0.004 | 0.34 (0.16–0.73) |
| Any revascularization | 13.8% (60) | 23.8% (70) | <0.001 | 0.56 (0.39–0.78) |
| CABG | 1.1% (5) | 4.9% (14) | 0.003 | 0.24 (0.09–0.66) |
| PCI | 12.9% (56) | 20.4% (60) | 0.007 | 0.61 (0.42–0.88) |
| Definite stent thrombosis | 1.4% (6) | 5.5% (16) | 0.002 | 0.25 (0.10–0.64) |
| Acute | 0.2% (1) | 0.0% (0) | 0.40 | — |
| Sub-acute | 0.0% (0) | 1.6% (5) | 0.007 | — |
| Late | 0.4% (2) | 1.0% (3) | 0.37 | — |
| Very late | 0.9% (4) | 3.0% (8) | 0.052 | — |
| Probable stent thrombosis | 0.2% (1) | NA | — | — |
The event rates are based on Kaplan–Meier estimates.
CABG, coronary artery bypass graft; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; NA, Not applicable; PCI, percutaneous coronary intervention; POCE, patient-oriented composite endpoint (any death, any stroke, any MI, or any revascularization).
P-values are derived from Kaplan–Meier curves (log-rank).
Although the Cox proportional HR is reported for the outcome, it should be noted that this outcome violated the proportional hazards assumption. The Cox HR is therefore provided for descriptive purposes only.
Figure 3.
Five-year clinical outcomes comparing the SYNTAX II study vs. the equipoise-derived SYNTAX I percutaneous coronary intervention cohort. Kaplan–Meier curves are shown for the SYNTAX II group (blue) and for the SYNTAX I percutaneous coronary intervention cohort (red) for the patient-oriented composite endpoint (A), all-cause death (B), stroke (C), myocardial infarction (D), any revascularization (E), and definite stent thrombosis (F). Although the Cox proportional hazard ratio is reported for myocardial infarction, it should be noted that this outcome violated the proportional hazards assumption. The Cox hazard ratio is therefore provided for descriptive purposes only. HR, hazard ratio; PCI, percutaneous coronary intervention.
Other clinical endpoints
The 5-year composite of any death, any stroke, and any MI was significantly lower in the SYNTAX II study (10.8% vs. 21.8%, HR 0.47, 95% CI 0.32–0.68; P < 0.001) (Table 1 and Figure 3). Similarly, as well as the previously reported lower rate of procedural MI (0.2% vs. 3.8%, P < 0.001), the 5-year results of SYNTAX II patients show a significant reduction in spontaneous MI (2.3% vs. 6.9%, P = 0.004). Five-year revascularization was also significantly lower using the SYNTAX II strategy (13.8% vs. 23.8%, HR 0.56, 95% CI 0.39–0.78; P < 0.001). Furthermore, definite stent thrombosis was significantly lower following treatment with the SYNTAX II strategy (1.4% vs. 5.5%, HR 0.25, 95% CI 0.10–0.64; P = 0.004).
As reported for previous follow-ups, stratification by anatomical SYNTAX score [low (<22) vs. intermediate (22–32) anatomical SYNTAX score] or by diabetes mellitus status did not reveal statistically significant differences in all-cause death in patients enrolled in the SYNTAX II study (Figure 4).
Figure 4.
Five-year all-cause death in the SYNTAX II study population (n = 454). All-cause death Kaplan–Meier curves are shown for the SYNTAX II study population. Panel (A) stratifies patients on the basis of the anatomical SYNTAX score, >22 vs. ≤22. Panel (B) stratifies patients with or without diabetes at baseline.
Exploratory analysis vs. the SYNTAX I coronary artery bypass graft cohort
Five-year MACCE were not statistically different among the SYNTAX II study cohort and the SYNTAX I equipoise-selected CABG cohort (21.5% vs. 24.6%, HR 0.87, 95% CI 0.64–1.17; P = 0.35). Similarly, we observed no significant differences in the occurrence of any death (8.1% vs. 10.8%, HR 0.74, 95% CI 0.46–1.19; P = 0.21), any stroke (2.3% vs. 3.3%, HR 0.68, 95% CI 0.28–1.63; P = 0.39), any MI (2.7% vs. 2.5%, HR 1.04, 95% CI 0.43–2.55; P = 0.93), or any revascularization (13.8% vs. 12.6%, HR 1.14, 95% CI 0.76–1.72; P = 0.53) (see Supplementary material online, Appendix).
Discussion
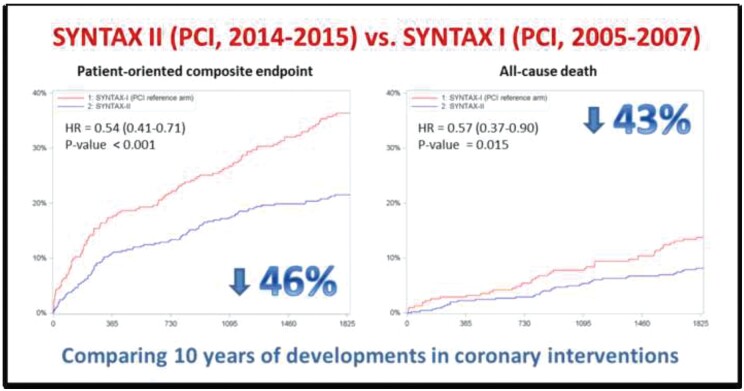
The SYNTAX II study aimed to assess the cumulative benefit of technological advances and guideline recommendations, integrated into a single predefined revascularization strategy, on clinical outcomes of patients with 3VD undergoing PCI. The results of 5-year follow-up show that, compared with patients treated with PCI within the SYNTAX I trial, the use of the SYNTAX II strategy was associated with a significantly lower rate of MACCE, comprising a lower rate of repeat revascularization, spontaneous MI, and lower rate of cardiac death (Graphical abstract). Notably, rates of MACCE at 5 years for patients treated with PCI in SYNTAX II were comparable to rates of MACCE in patients treated surgically in the SYNTAX I CABG cohort.
Graphical Abstract.
Comparison of outcomes after PCI using either the Syntax or Syntax 2 strategies.
A key aspect of the SYNTAX II study is that patient risk stratification based on the SYNTAX score II led to not including patients who, due to a combined effect of clinical and anatomical factors, had an estimated 4-year survival after revascularization with PCI significantly higher than with CABG. The fact that no significant differences were found in 5-year MACCE rates in SYNTAX II between patients with a SYNTAX score ≤22 and those with >22 show eloquently why clinical variables, and not only angiographic data, must be integrated into risk stratification (Figure 4). Overall, we believe that these results support the use of personalized, individual prediction of long-term mortality with either PCI or CABG at the time of performing heart team decisions on coronary revascularization.
The interventional strategy used within the SYNTAX II study was developed to collate different advances in PCI, and the superior outcomes associated with it cannot be attributed to a single diagnostic or therapeutic approach. Yet, it is possible to infer how the elements of the SYNTAX II strategy contributed to improve the quality of PCI: (i) physiological assessment led to deferring treatment in 25% of the interrogated coronary artery stenoses; (ii) systematic use of IVUS triggered stent optimization in 70% of the lesions; and (iii) successful CTO PCI was achieved in 85% of cases, contributing to completeness of revascularization. Furthermore, pre- and post-procedural medical therapy was pre-specified according to available guideline recommendations.7
In the SYNTAX II study, physiological assessment was considered in all stenoses suitable for pressure guidewire interrogation, irrespective of angiographic severity.8 Accordingly, mean iFR in SYNTAX II (iFR 0.77 ± 0.21) was predictably lower than in studies primarily focused on assessing intermediate severity stenoses, e.g. DEFINE FLAIR (0.91 ± 0.09).13 After physiological assessment, on average only 2.75 lesions were treated compared with the angiographically proposed 3.5 lesions per patient. Overall, the 5-year results of the study are highly reassuring regarding the safety and efficacy of revascularization deferral in patients with 3VD in the long term.
The data demonstrating that IVUS-guided PCI optimization reduces the risk of cardiovascular death, MI, target lesion revascularization, and stent thrombosis have continued to evolve since the SYNTAX II study began,14 as well as efforts by scientific societies to systematize and promote its use.15 Likewise, data supporting the safety and efficacy of the thin-strut bioresorbable polymer DES used in the SYNTAX II study (SYNERGY, Boston Scientific) have been gathered over the last 5 years in other high-risk PCI subgroups.16–18
In SYNTAX II, the procedural success rate of CTO recanalization was nearly 90%, compared to 50% in SYNTAX I. Improved procedural CTO success can be explained by the involvement of CTO operators, increased systematization of the procedure, and advances in dedicated PCI hardware. In addition to contribute to the completeness of revascularization and to reduce ischaemic burden, CTO recanalization may reduce spontaneous MI rates during follow-up.19
Optimized medical therapy was a part of the SYNTAX II strategy. The data show an increased maintenance of statins at 5 years following revascularization and it is likely that this improved guideline-directed therapy may be responsible for some of the improved outcomes within the study.20 Interestingly, although more potent antiplatelet medication was prescribed at the beginning of the study, i.e. ticagrelor and prasugrel, rates of ADP antagonist prescription at 5 years were much lower in SYNTAX II than in the original cohort, probably reflecting lower rates of repeat revascularization/MI, lower DAPT dependence of new-generation DES, and global trends in the use of DAPT after PCI. Of note, neither SYNTAX I nor SYNTAX II used bleeding events as endpoints. According to the trial-based evidence, it is plausible that a higher number of bleeding events may potentially have contributed to the documented higher all-cause death rate observed in SYNTAX I patients due to a nearly 10-fold difference in DAPT at 5-year follow-up.20 , 21
The dramatic decrease in periprocedural MI rates in SYNTAX II may be related to several aspects of the SYNTAX II strategy, including mandated pre-procedural loading with statins, deferral of revascularization in non-flow-limiting lesions, use of thin-strut DES, and IVUS-guided stent optimization.7 Lower rates of spontaneous MI during follow-up in SYNTAX II may reflect high revascularization rates of CTO,19 optimal results of stenting, and enforcement of medical treatment according to guideline recommendations.22
Interestingly, the BEST trial also used a new-generation DES to treat patients with multi-vessel disease, but this study lacked a predefined comprehensive strategy equivalent to that applied in SYNTAX II. The 5-year follow-up of the BEST trial4 showed that PCI was inferior to CABG in terms of cumulative incidence of adverse cardiac events (21.1% vs. 14.6%, P = 0.01). Of note, in contrast to SYNTAX II, patients in BEST with an anatomical SYNTAX score >22 had significantly more events than those with lower SYNTAX score values. These findings, supported by a patient level meta-analysis of three major trials comparing PCI vs. CABG,23 highlight the importance of the SYNTAX score II in identifying patients with 3VD in whom treatment with PCI could be a non-inferior strategy compared with CABG. Trials on the impact of FFR measurement upon the long-term results after CABG have contrasted with PCI, showing that revascularization of vessels with a non-ischaemic FFR is not associated with any increase in cardiac events.24 Consequently, restricting stenting to flow-limiting lesions and avoiding unnecessary interventions (thus decreasing the possibility of stent failure) is also a possible explanation for improved PCI outcomes.
The exploratory analysis of SYNTAX II, using surgical revascularization as a comparator, suggests that PCI might have better chances of reaching non-inferiority in a prospective comparison against CABG in treating patients with 3VD, provided that, as outlined above, PCI is performed in patients selected according to the SYNTAX score II and the remaining elements of the SYNTAX II strategy are applied during the procedure. Notably, developments in surgical technique and medical treatments may also have improved the outcomes of patients undergoing CABG since the SYNTAX trial was performed and the SYNTAX surgical and SYNTAX II cohorts were not recruited contemporaneously. Therefore, these observations need cautious interpretation but suggest the need for a properly powered randomized comparison between CABG and PCI of selected patients with 3VD.
Limitations
This study has limitations. Importantly, this is a non-randomized study comparing a contemporary strategy with an historical control group (SYNTAX I). We attempted to minimize this by careful patient matching using the detailed angiographic and clinical SYNTAX score II. Notably, these PCI procedures were undertaken in selected specialist high volume units familiar with the state-of-the-art approach recommended by the study. A hybrid approach was used for the cut-off threshold of iFR with FFR used between 0.86 and 0.93. Consequently, it is possible that these outcomes may not necessarily be replicated in general interventional practice unless similar procedural approaches are employed. It is noteworthy that although the same definition for procedural MI was used in SYNTAX I and SYNTAX II for adjudication, SYNTAX II allowed the use of both creatine kinase-MB and troponins, which were available in up to 85% of procedures. Incomplete availability of biomarkers should be considered when interpreting the results.
Several comparisons described in the results section show no significant differences; however, it should be acknowledged that these comparisons may be underpowered.
Conclusion
At 5 years, clinical outcomes associated with the SYNTAX II strategy were clearly superior to those outcomes observed in predefined patients treated with PCI from the original SYNTAX trial. The exploratory comparison between CABG and PCI suggests that 5-year outcomes following revascularization are similar. These data suggest the need for a randomized clinical trial recruiting appropriately selected patients with multi-vessel CAD comparing outcomes after revascularization with either CABG or PCI using a contemporary strategic approach.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
All authors were given access to summarized trial data and analyses. The principal investigators had unrestricted access to all trial data, prepared the manuscript, and vouch for the completeness and accuracy of the data and analyses and for the fidelity of the trial to the protocol. We would like to thank Chantal Bakker and Maurice Vorage, as representatives of the study team at Cardialysis, Rotterdam, the Netherlands, for their dedication and operational excellence.
Funding
This study was sponsored by the European Cardiovascular Research Institute (ECRI, Rotterdam, the Netherlands) with unrestricted research grants from Volcano Corporation and Boston Scientific. A.P.B. is partially supported by the National Institue for Health Research Biomedical Research Centre, Oxford.
Conflict of interest: A.P.B. declares institutional funding for a fellowship from Boston Scientific and speaker fees from Boston Scientific, Medtronic, and Abbott Vascular. R.J.v.G. declares institutional funding from Abbott Vascular and Infraredx and speaker fees from Abbott Vascular. E.S. declares that the sponsor of the study is the European Cardiovascular Research Institute (ECRI), in which he is a board member, and the research organization executing the study is Cardialysis, in which he is the medical director. P.S. declares consulting fees from Philips/Volcano, SMT, Xeltis, Novartis, Merillife, Sino Medical, Novartis, and Biosensors. S.W. is a consultant to Boston scientific. J.D. is a consultant to Phillips. J.E. declares consulting and speaker fees from Abbott, Boston Scientific, and Philips. N.M.V.M. declares institutional grant support from Abbott Vascular, Boston Scientific, Medtronic, Edwards Lifesciences, Abiomed, and PulseCath BV. All other authors declared no conflict of interest.
Contributor Information
Adrian P Banning, Department of Cardiology, John Radcliffe Hospital, Oxford University Hospitals, Headley Way, Oxford OX3 9DU, UK.
Patrick Serruys, Department of Cardiology, National University of Ireland, Galway, Ireland.
Giovanni Luigi De Maria, Department of Cardiology, John Radcliffe Hospital, Oxford University Hospitals, Headley Way, Oxford OX3 9DU, UK.
Nicola Ryan, Hospital Clinico San Carlos IDISSC and Universidad Complutense de Madrid, Madrid 28040, Spain.
Simon Walsh, Department of Cardiology, Belfast Health & Social Care Trust, Belfast BT8*BH, UK.
Nieves Gonzalo, Hospital Clinico San Carlos IDISSC and Universidad Complutense de Madrid, Madrid 28040, Spain.
Robert Jan van Geuns, Department of Cardiology, Radboudumc, Nijmegen, The Netherlands.
Yoshinobu Onuma, Department of Cardiology, National University of Ireland, Galway, Ireland.
Manel Sabate, Cardiovascular Institute, Hospital Clinic I Provincial de Barcelona, IDIBAPS, Centro de Investigación Biomédica en Red. Enfermedades Cardiovasculares (CIBERCV) CB16/11/00411, Barcelona Spain.
Justin Davies, Department of Cardiology, Imperial College London, Kensington, London SW7 2AZ, UK.
Maciej Lesiak, 1st Department of Cardiology, University of Medical Sciences, Poznan 61-701, Poland.
Raul Moreno, Department of Cardiology, Hospital Universitario la Paz, Paseo de la Castellana, 261, Madrid 28046, Spain.
Ignacio Cruz-Gonzalez, Department of Cardiology, Hospital Universitario de Salamanca, IBSAL, Paseo de San Vicente, 58, Salamanca 37007, Spain.
Stephen P Hoole, Department of Cardiology, Papworth Hospital NHS Foundation Trust, Papworth Everard, Cambridge CB23 3RE, UK.
Jan J Piek, Department of Cardiology, Academic Medical Center of Amsterdam, Amsterdam 1105 AZ, The Netherlands.
Clare Appleby, Liverpool Heart and Chest Hospital, Thomas Dr, Liverpool L14 3PE, UK.
Farzin Fath-Ordoubadi, Manchester Heart Centre, Manchester Royal Infirmary, Central Manchester University Hospitals, Oxford Rd, Manchester M13 9WL, UK.
Azfar Zaman, Department of Cardiology, Freeman Hospital and Newcastle University, High Heaton, Newcastle upon Tyne NE7 7DN, UK.
Nicolas M Van Mieghem, Department of Cardiology, Thoraxcenter, Erasmus MC, ’s-Gravendijkwal 230, 3015 CE Rotterdam, The Netherlands.
Neal Uren, Department of Cardiology, The Royal Infirmary of Edinburgh, 51 Little France Dr, Edinburgh EH16 4SA, UK.
Javier Zueco, Department of Cardiology, Hospital Universitario Valdecilla, Av. Valdecilla, 25, Santander, Cantabria 39008, Spain.
Pawel Buszman, Department of Cardiology, American Heart of Poland (PAK), Sanatoryjna 1, Ustron 43-450, Poland.
Andres Iniguez, Department of Cardiology, Hospital Álvaro Cunqueiro, c/Clara Campoamor 341, Vigo 36213, Spain.
Javier Goicolea, Department of Cardiology, Hospital Puerta de Hierro, C. Joaquín Rodrigo, 1, Majadahonda 28222, Madrid, Spain.
David Hildick-Smith, Department of Cardiology, Brighton & Sussex University Hospitals NHS Trust, Barry Building, Eastern Rd, Brighton BN2 5BE, UK.
Andrzej Ochala, Department of Cardiology, Gornoslaskie Centrum Medycnze, 45/47, Katowice 40-635, Poland.
Dariusz Dudek, Department of Interventional Cardiology, Jagiellonian University, Gołe, bia 24, Krakow 31-007, Poland.
Ton de Vries, Cardialysis BV, Westblaak 98, 3012 KM Rotterdam, The Netherlands.
David Taggart, Department of Cardiology, John Radcliffe Hospital, Oxford University Hospitals, Headley Way, Oxford OX3 9DU, UK.
Vasim Farooq, Manchester Heart Centre, Manchester Royal Infirmary, Central Manchester University Hospitals, Oxford Rd, Manchester M13 9WL, UK.
Ernest Spitzer, Cardialysis BV, Westblaak 98, 3012 KM Rotterdam, The Netherlands; European Cardiovascular Research Institute, Westblaak 98, 3012 KM Rotterdam, The Netherlands.
Jan Tijssen, European Cardiovascular Research Institute, Westblaak 98, 3012 KM Rotterdam, The Netherlands.
Javier Escaned, Hospital Clinico San Carlos IDISSC and Universidad Complutense de Madrid, Madrid 28040, Spain.
References
- 1. Neumann FJ, Sousa-Uva M, Ahlsson A et al. ; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
- 2. Serruys PW, Morice MC, Kappetein AP et al. ; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 3. Farkouh ME, Domanski M, Sleeper LA et al. ; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 4. Park SJ, Ahn JM, Kim YH et al. ; BEST Trial Investigators. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204–1212. [DOI] [PubMed] [Google Scholar]
- 5. Farooq V, van Klaveren D, Steyerberg EW et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013;381:639–650. [DOI] [PubMed] [Google Scholar]
- 6. Brilakis ES, Mashayekhi K, Tsuchikane E et al. Guiding principles for chronic total occlusion percutaneous coronary intervention. Circulation 2019;140:420–433. [DOI] [PubMed] [Google Scholar]
- 7. Escaned J, Banning A, Farooq V et al. Rationale and design of the SYNTAX II trial evaluating the short to long-term outcomes of state-of-the-art percutaneous coronary revascularisation in patients with de novo three-vessel disease. EuroIntervention 2016;12:e224–e234. [DOI] [PubMed] [Google Scholar]
- 8. Escaned J, Collet C, Ryan N et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur Heart J 2017;38:3124–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stankovic G, Lefevre T, Chieffo A et al. ; European Bifurcation Club. Consensus from the 7th European Bifurcation Club meeting. EuroIntervention 2013;9:36–45. [DOI] [PubMed] [Google Scholar]
- 10. Levine GN, Bates ER, Blankenship JC et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions. 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–e122. [DOI] [PubMed] [Google Scholar]
- 11. Wijns W, Kolh P, Danchin N et al. ; European Association for Percutaneous Cardiovascular Interventions (EAPCI). Guidelines on myocardial revascularization: the Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2010;31:2501–2555. [DOI] [PubMed] [Google Scholar]
- 12. Cutlip DE, Windecker S, Mehran R et al. ; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 13. Davies JE, Sen S, Dehbi HM et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med 2017;376:1824–1834. [DOI] [PubMed] [Google Scholar]
- 14. Darmoch F, Alraies MC, Al-Khadra Y et al. Intravascular ultrasound imaging-guided versus coronary angiography-guided percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e013678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raber L, Mintz GS, Koskinas KC et al. ; ESC Scientific Document Group. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2018;39:3281–3300. [DOI] [PubMed] [Google Scholar]
- 16. Baber U, Chandiramani R, Mehta SR et al. Safety and efficacy of the bioabsorbable polymer everolimus-eluting stent versus durable polymer drug-eluting stents in high-risk patients undergoing PCI: TWILIGHT-SYNERGY. Catheter Cardiovasc Interv 2021;97:63–71. [DOI] [PubMed] [Google Scholar]
- 17. Kereiakes DJ, Meredith IT, Masotti M et al. Safety and efficacy of a bioabsorbable polymer-coated, everolimus-eluting coronary stent in patients with diabetes: the EVOLVE II diabetes substudy. EuroIntervention 2017;12:1987–1994. [DOI] [PubMed] [Google Scholar]
- 18. Varenne O, Cook S, Sideris G et al. ; SENIOR Investigators. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet 2018;391:41–50. [DOI] [PubMed] [Google Scholar]
- 19. Schumacher SP, Stuijfzand WJ, de Winter RW et al. Ischemic burden reduction and long-term clinical outcomes after chronic total occlusion percutaneous coronary intervention. JACC Cardiovasc Interv 2021;14:1407–1418. [DOI] [PubMed] [Google Scholar]
- 20. Kawashima H, Serruys PW, Ono M et al. ; SYNTAX Extended Survival Investigators. Impact of optimal medical therapy on 10-year mortality after coronary revascularization. J Am Coll Cardiol 2021;78:27–38. [DOI] [PubMed] [Google Scholar]
- 21. Palmerini T, Benedetto U, Bacchi-Reggiani L et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015;385:2371–2382. [DOI] [PubMed] [Google Scholar]
- 22. Hara H, Serruys PW, Takahashi K et al. ; SYNTAX Extended Survival Investigators. Impact of peri-procedural myocardial infarction on outcomes after revascularization. J Am Coll Cardiol 2020;76:1622–1639. [DOI] [PubMed] [Google Scholar]
- 23. Ahn J-M, Park D-W, Lee CW et al. Comparison of Stenting Versus Bypass Surgery According to the Completeness of Revascularization in Severe Coronary Artery Disease: Patient-Level Pooled Analysis of the SYNTAX, PRECOMBAT, and BEST Trials. JACC Cardiovasc Interv 2017;10:1415–1424. [DOI] [PubMed] [Google Scholar]
- 24. Timbadia D, Ler A, Sazzad F et al. FFR-guided versus coronary angiogram-guided CABG: a review and meta-analysis of prospective randomized controlled trials. J Card Surg 2020;35:2785–2793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.