ABSTRACT
Background
Complementary feeding (CF) provides an opportunity to shape children's future dietary habits, setting the foundation for good nutrition and health.
Objectives
We estimated effects of 3 CF behaviors on early childhood diet quality using inverse probability (IP) weighting of marginal structural models (MSMs).
Methods
Among 1041 children from the Boston-area Project Viva cohort, we estimated effects on the mean Youth Healthy Eating Index (YHEI) score in early childhood of 1) delayed (≥12 mo) compared with early (<12 mo) introduction of sweets and fruit juice; 2) continued compared with ceased offering of initially refused foods; and 3) early (<12 mo) compared with late (≥12 mo) introduction of flavor/texture variety. Mothers reported CF behaviors at 1 y and completed FFQs for children in early childhood (median age: 3.1 y). We estimated average treatment effects (ATEs) using IP weighting of MSMs to adjust for both confounding and selection bias due to censored outcomes and examined effect modification by child sex and breastfeeding compared with formula feeding at 6 mo.
Results
Twelve percent of mothers delayed introducing sweets/fruit juice, 93% continued offering initially refused foods, and 32% introduced flavor/texture variety early. The mean ± SD YHEI score was 52.8 ± 9.2 points. In adjusted models, we estimated a higher mean YHEI score with delayed (compared with early) sweets and fruit juice among breastfeeding children (ATE: 4.5 points; 95% CI: 1.0, 7.4 points), as well as with continued (compared with ceased) offering of refused foods among females (ATE: 5.4 points; 95% CI: 0.8, 9.1 points). The ATE for early (compared with late) flavor/texture variety was 1.7 points (95% CI: 0.3, 3.2 points) overall and stronger (2.8 points; 95% CI: 0.7, 5.1 points) among the formula-fed group.
Conclusions
Delayed introduction of sweets/juice, continued offering of refused foods, and early flavor/texture variety may all result in higher childhood diet quality. Effects may depend on child sex and infant breastfeeding status.
Keywords: complementary feeding, child diet quality, birth cohort, causal inference, taste preferences, Project Viva
Introduction
Diet quality among US children is far from optimal (1). Dietary habits track from early childhood into adolescence and adulthood (2, 3) and independently predict subsequent health outcome markers including obesity and cardiometabolic risk factors (2, 4, 5). Establishing a diet characterized by high intake of nutrient-dense foods such as fruits, vegetables, and whole grains and limited intake of processed and energy-dense foods in early childhood may set the foundation for good nutrition and health throughout the life course.
Infants innately prefer sweet tastes and energy-dense foods and reject bitter tastes (6, 7) from birth, yet the first few months of life are a “sensitive” period during which these preferences can be modified to promote acceptance of nutrient-dense foods (6, 8). Complementary feeding (CF), or introduction of solid foods and liquids other than breast milk or formula (9), is arguably the most important opportunity to override innate preferences and shape children's future dietary habits. Repeated exposure to new foods has consistently been shown to increase acceptance, and early exposure to different textures and flavors may increase acceptance of novel foods and particularly vegetables (6, 10–12).
Prior research shows that fruit and vegetable intake tracks from infancy through early childhood (13, 14). Conversely, high exposure to sweets or other energy-dense foods during the CF period may enhance the innate preference for sweetness (15, 16). At ∼2 y, children may become neophobic and therefore be more resistant to trying new foods and flavors (17). The window for promoting taste preferences for healthier foods is thus short but important. A large cross-sectional study of European children and adolescents (aged 6–16 y) analyzed associations of scores measuring adherence to European dietary guidelines with taste preferences and found that lower diet score was associated with greater preferences for sweet and fatty tastes, whereas higher diet score was associated with greater preference for bitter tastes (18).
Our previous research in the US birth cohort study Project Viva suggested that patterns of CF behaviors thought to increase food acceptance and discourage the innate preference for sweetness (e.g., feeding breast milk at 9–11 mo, delayed sweets and fruit juice introduction, early flavor introduction) may have persistent associations with diet quality (19). However, this previous work assessed patterns, rather than individual CF behaviors. It had descriptive rather than causal goals, and therefore did not adjust for confounding. To our knowledge, there have been no randomized trials of CF behavior interventions examining long-term outcomes, and these may be difficult to implement in practice. In the absence of such trials, well-designed and appropriately analyzed observational studies are needed to provide evidence-based guidance on causal effects (20). The objective of the present study was to estimate effects of 3 potentially beneficial CF behaviors—delayed (compared with early) introduction of sweets and fruit juice, continued (compared with ceased) offering of initially refused foods, and early (compared with late) introduction of flavor/texture variety—on diet quality at ∼3 y. We hypothesized that these behaviors would be associated with higher subsequent diet quality scores after adjustment for measured confounders. We assessed effect modification by child sex or infant breastfeeding status, based on evidence that associations between early taste exposures and later preferences and diet quality may differ according to breastfeeding status (10, 12, 21) and sex (22). In a series of secondary analyses, we examined individual dietary components as outcomes, hypothesizing that delaying introduction of sweets and fruit juice would be associated with higher (more “healthful”) scores on the snack and drink components, whereas continued offering of refused foods and early flavor variety would be associated with higher scores on the fruit, vegetable, and whole grain components of the diet quality score.
Methods
Study participants
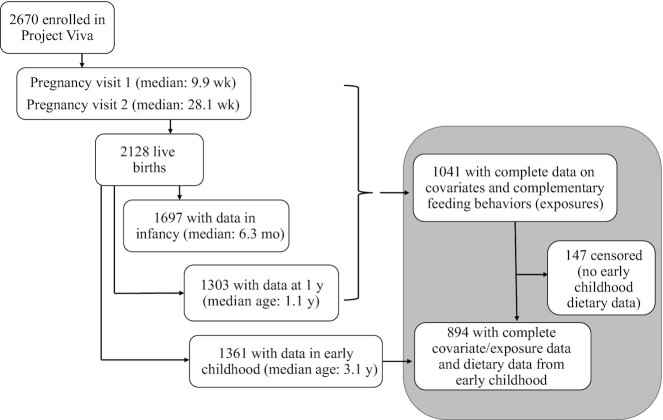
We studied children enrolled in Project Viva, a prospective prebirth cohort study of mother–child pairs. Project Viva recruited mothers in 1999–2002 at their initial obstetric appointment from 8 offices of Atrius Harvard Vanguard Medical Associates, a large multispecialty group practice in eastern Massachusetts. Mothers enrolled their children after delivery. Project Viva collected data on the mothers via in-person study visits in the first and second trimesters of pregnancy and collected data on mothers and children at delivery and in infancy and early childhood at in-person visits. Mothers also provided additional data between in-person visits with annual questionnaires. We have previously described detailed recruitment and retention procedures (23) and show the flow of participant involvement in Figure 1.
FIGURE 1.
Overall flow of participant involvement in Project Viva from enrollment through early childhood. The flow of sample selection for this study is shown on the right side of the figure (gray shading).
Of the 2128 Project Viva children, we included 1041 with mother-reported data on the CF practices of interest from the 1-y questionnaire and complete covariate data. Of these, 894 had dietary data from the early childhood visit. The Harvard Pilgrim Health Care Institutional Review Board approved the study protocols and the study was conducted in accordance with institutional ethical standards. Mothers provided written informed consent for their own participation as well as that of their children.
Exposure: CF behaviors
We measured the timing of introduction of different foods and food groups and other feeding behaviors via a mailed questionnaire completed by the mother when the child was ∼1 y old (median: 1.1 y). For this analysis, we focused on measures of 3 CF behaviors. First, we defined a binary “delayed sweets/fruit juice” measure given the value “1” if the mother reported introduction of both sweets and fruit juice at or after 12 mo of age and the child drank no fruit juice (average daily intake of 0 oz in the past month) at the time of 1-y questionnaire completion, and value “0” if she reported introduction of either sweets or fruit juice before 12 mo of age and/or the child drank at least some daily fruit juice at the time of 1-y questionnaire completion (“early sweets/fruit juice”). Second, we defined a binary “continued offering” measure given the value “1” if the mother responded “agree” or “strongly agree” with the following statement: “If my child refuses to eat a new food, I continue to offer it to him/her at other times.” This measure was given the value “0” if the mother responded “disagree” or “strongly disagree” with this statement (“ceased offering”). Finally, we defined a binary “early flavor/texture variety” measure by timing of introduction of 3 foods: fish, eggs, and peanut butter. These foods were selected for their textures (all are unlikely to be found in commercially prepared baby food or pureed at home) and strong, nonsweet flavors, as well as variable timing of introduction in the study population. We asked mothers, “How old was your child when you first fed him/her…(eggs/peanut butter/fish)?” “Early variety” was given the value “1” if the mother reported introduction of ≥2 of these 3 foods before 12 mo and value “0” if she reported none or only 1 of these foods was introduced before 12 mo (“late variety”). Notably, ≤1% of our sample reported introduction of each of these foods before 6 mo; therefore, this exposure effectively represents introduction between 6 and 12 mo. All 3 exposures were coded as missing if responses to any of their composite questions were missing.
Outcome: Youth Healthy Eating Index in early childhood
We calculated the Youth Healthy Eating Index (YHEI) (24) total and component scores using dietary data reported by the mother in early childhood (median age: 3.1 y; IQR: 3.0–3.2 y) via an FFQ. This FFQ was validated for use in preschool-age children (25) and assessed intake of different foods using questions in the format, “Please check the box that best represents how often your child eats each of the foods listed, on average, in the past month.” For purposes of calculating the score, we assumed that 1 “time” = 1 serving. We obtained information on frequency of fast-food consumption from a questionnaire administered at the early childhood visit. We had data available to calculate 10 of the 13 components of the YHEI in early childhood. The 3 components that were not available were consumption of visible animal fat, eating breakfast, and eating dinner with family, all demonstrated to be minor contributors to variability in the total YHEI score in an external sample used for development of the score (24). In addition, we decided to omit the multivitamin component of the score because we were interested in examining the quality of the child's diet based on food intake. Components contribute ≤10 points each; for example, eating vegetables ≥3 times/d corresponds to the maximum 10 points and eating vegetables 0 times/d corresponds to the minimum score of 0 for that component, with intakes of 1 or 2 times/d given proportional scores of 3.3 and 6.6, respectively, Thus, our total YHEI score included 9 components with a range of possible total scores of 0–80 points, with a higher score indicating a healthier diet. We provide a detailed description of the score components in Supplemental Table 1.
Covariates
We restricted covariates for confounding adjustment to those measured before the exposure period and expected to be associated both with CF practices and with dietary quality in childhood, using our subject-matter knowledge and relevant literature. Because parents or other caregivers (and often mothers) determine both CF practices as well as which foods are offered to children in early childhood, we included covariates indicative of maternal and family diet quality, nutrition knowledge, and food selection practices. The variables selected for consideration included child sex; maternal education (as a proxy for maternal health and nutrition knowledge), prepregnancy BMI, and diet score during pregnancy (as proxies for maternal health and nutrition knowledge and diet quality); household income at enrollment (as a proxy for resources to access more and higher-quality food); and infant breastfeeding status at 6 mo (categorized as partially/fully breastfeeding or formula-fed at 6 mo). Mothers reported their education level, prepregnancy weight, height, and household income at the initial prenatal visit (median: 9.9 weeks of gestation). We calculated maternal prepregnancy BMI from these reports of height and weight, a method that has previously been validated in this cohort (26). We obtained data on infant sex from hospital medical records and infant breastfeeding status from the 6-mo interview (36). We collected comprehensive data on maternal diet during pregnancy, including intake of foods and supplements, from self-administered semiquantitative FFQs completed by mothers during the first and second trimesters. The 166-item FFQ used in Project Viva was based on instruments validated in other cohorts, including the Nurses’ Health Study (27), and modified for use in pregnancy (28). We adjusted individual nutrient estimates for total energy intake using the nutrient residual method (29). We measured maternal dietary quality during pregnancy using the Alternate Healthy Eating Index (AHEI) (30) modified for pregnancy (AHEI-P). A detailed description of the AHEI-P has been published previously (31).
We considered adjustment for mother report of child race or ethnicity as a proxy measure of exposure to structural racism that can affect food access both during CF and in early childhood (32); we conducted a sensitivity analysis that adjusted for mother report of child race or ethnicity and found no difference in results. Given this lack of evidence for this variable being a confounder and our reluctance to include it without clarity about what construct it represents, we did not feel justified in including it in our analysis.
All data collection instruments used in Project Viva are publicly available at https://www.hms.harvard.edu/viva/.
Statistical analysis
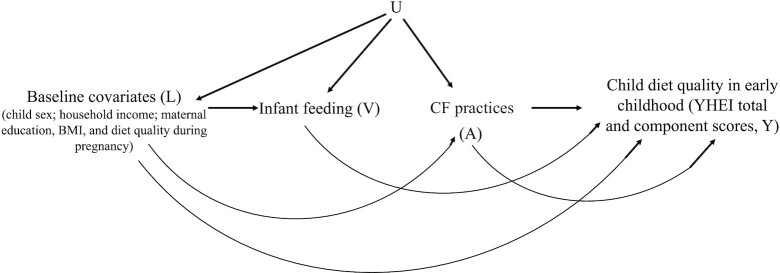
We applied inverse probability (IP) weighting of marginal structural models (MSMs), an approach to estimating average treatment effects (ATEs) in observational data which generally relies on more realistic assumptions than multivariable linear regression in settings where adjustment for a high-dimensional set of confounders is needed. We estimated effects of the following on early childhood YHEI score: 1) delayed (compared with early) introduction of sweets and fruit juice, 2) continued (compared with ceased) offering of initially refused foods, and 3) early (compared with late) introduction of flavor/texture variety. In secondary analyses, we also estimated the effects on individual YHEI component scores. Multivariable linear regression involves conditioning on all assumed confounders in the regression model, which requires assumptions on the presence or absence of effect modification by all assumed confounders; i.e., a linear regression model with no interaction terms presumes no effect modification by any of the confounders. By contrast, IP-weighted estimates do not require a model for the outcome conditional on exposure and all the assumed confounders, rather confounders are accounted for in the weights. To assess effect modification by particular covariates of interest, the IP-weighted analysis may be stratified on only those covariates or an MSM—a model for a mean potential (counterfactual) outcome (33)—may be assumed conditional only on those covariates. IP-weighted estimates require correct specification of a model for 1) probability of exposure, conditional on covariates (a so-called “propensity score”); and 2) probability of censoring, conditional on covariates and exposure (“censoring process”) (34, 35). As in any analysis of observational data, we require assumptions to interpret our IP-weighted estimates in terms of ATEs in the study population and within subgroups of interest. These assumptions include 1) conditional exchangeability, or no unmeasured confounding: the 2 exposure groups are exchangeable (exposure is independent of potential outcomes) conditional on measured covariates; 2) positivity: there is a positive (nonzero) probability of being in each exposure group at all levels of these measured covariates; 3) consistency: for each individual, the potential outcome had we intervened to set exposure to a particular level equals their observed outcome if their observed exposure takes that level; and 4) no interference: each individual's potential outcome depends only on his/her own exposure and is not affected by that of any other individual (36). Figure 2 depicts a causal directed acyclic graph representing an assumption on the underlying causal structure of dependencies between measured variables in Project Viva and possible unmeasured common causes U. The assumption of no unmeasured confounding is represented by the absence of an arrow from U into outcome Y (36).
FIGURE 2.
Directed acyclic graph depicting an assumption on the causal structure of dependence between the exposures, outcomes, and covariates. A represents observed exposures, U represents unmeasured variables, L represents covariates, V represents effect modifiers, and Y represents outcomes. Infant feeding (full/partial breastfeeding compared with formula feeding, represented by V) is included here as a potential effect modifier of the relation of interest between CF practices (exposure, represented by A) and child diet quality in early childhood (outcome, represented by Y). CF, complementary feeding; YHEI, Youth Healthy Eating Index.
Details of IP weighting of MSMs for the estimation of ATEs are described elsewhere (33, 36–38) and in the Supplemental Methods. Briefly, to estimate ATEs in the overall study population, we fit logistic regression models for the propensity score (specific to each exposure) and the censoring process. We then used these fitted models to calculate weights for each individual. The denominator of this weight estimates the product of 1) the probability that an individual received the level of exposure they received conditional on their covariate levels, and 2) the probability of remaining uncensored, conditional on their exposure and covariate levels. The numerator estimates the product of these same probabilities but unconditional on covariates, estimated by sample proportions. In each analysis, CF behaviors that did not act as the exposure were considered confounders: for example, in analyses for the effect of delayed sweets/fruit juice, indicators of continued offering and early variety were included as confounders in the weight denominators. Finally, using these weights, we conducted a weighted linear regression analysis with YHEI score as the dependent variable and exposure (for each exposure-specific analysis) as the only independent variable. The estimated coefficient on exposure in the weighted model is the IP-weighted estimate of the ATE in the study population under no unmeasured confounding and the other assumptions aforementioned.
All 1041 participants with complete exposure and other baseline covariate data were included in estimating weight denominator models, but censored participants (n = 147 of 1041) received a final weight of 0 and therefore only the 894 participants who had an observed outcome were included in the weighted regression analysis. An intuitive justification of this procedure is that, provided the assumptions outlined so far hold, the distribution of outcomes in exposed (and uncensored) individuals is reweighted to represent the distribution of outcomes had everyone been exposed and uncensored—including those who did not fit this pattern in the observed data but were “like” those who did in terms of their measured covariates. Similarly, unexposed (and uncensored) individuals are reweighted to represent what would have happened to the outcomes had everyone been unexposed and uncensored.
We also considered effect modification by child sex and infant breastfeeding status at 6 mo (full or partial breastfeeding compared with no breastfeeding). These analyses differed from those aforementioned in 2 ways: 1) the weighted outcome regression included main terms for the selected effect modifier and an interaction term with exposure and 2) the weight numerator was an estimate of the product of the probability that an individual received the level of exposure they received and the probability of remaining uncensored both conditional on their level of the effect modifier (see Supplemental Methods). We generated 95% CIs using a nonparametric bootstrap with 500 samples by sorting bootstrap estimates with the 2.5th and 97.5th percentiles giving the lower and upper bounds of the interval.
We performed a series of diagnostics to assess balance in the measured confounders across exposure levels before and after weighting. We compared means (for continuous variables) and proportions (for categorical variables), as well as standardized differences and variance ratios before and after weighting for each of the 3 different exposures using the covbal package for Stata (39). All analyses were performed using Stata Statistical Software, release 17 (StataCorp LLC).
Results
Covariate balance before and after weighting
Table 1 displays the 3 CF behaviors, YHEI scores, and proportions censored by category of baseline characteristics in the unweighted sample. Out of the 1041 participants, 11.5% delayed introduction of sweets and fruit juice, 92.7% continued to offer initially refused foods, and 31.7% introduced flavor/texture variety early. Supplemental Figures 1–3 display the absolute standardized differences and variance ratios for the weighted and unweighted samples for each of the 3 exposures. Although there is no universally accepted cutoff to determine imbalance in covariates between exposure groups, a standardized difference of <20% and optimally <10% has been advocated as a reasonable indicator of balance (40). Similarly, variance ratios close to 1 indicate covariate balance, whereas ratios <0.5 and >2 suggest imbalance (41). Dashed lines in Supplemental Figures 1–3 indicate the bounds of values that are cause for concern, whereas solid lines indicate optimal values. In the unweighted sample, the standardized differences and variance ratios indicated meaningful imbalance in the baseline distributions of most covariates between exposed and unexposed groups for ≥1 of the exposures of interest. After weighting, there was no evidence of imbalance between the groups for any of the exposures. The means of the weights were also close to 1, and weight distributions fairly symmetric, for each exposure (see Supplemental Table 2 for a summary of statistics on the stabilized weights for each exposure). This is expected when the weight denominator models reasonably fit the data and in the absence of near positivity violations (individuals with very small chance of a particular exposure given their confounder levels) (42, 43).
TABLE 1.
Complementary feeding behaviors, YHEI scores, and censoring by child and maternal/household characteristics among 1041 Project Viva participants1
| Overall | Delayed sweets and fruit juice | Continues to offer refused foods | Early flavor/texture variety | Censored2 | YHEI score at 3 y, mean ± SD | |
|---|---|---|---|---|---|---|
| Overall | 11.5 | 92.7 | 31.7 | 14.1 | 52.8 ± 9.2 | |
| Child characteristics | ||||||
| Sex | ||||||
| Male | 49.1 | 11.4 | 93.1 | 33.1 | 16.1 | 52.3 ± 9.1 |
| Female | 50.9 | 11.7 | 92.3 | 30.4 | 12.3 | 53.4 ± 9.3 |
| Race/ethnicity | ||||||
| White | 73.8 | 13.2 | 93.7 | 29.7 | 13.8 | 53.1 ± 8.9 |
| Black | 9.1 | 1.0 | 87.4 | 42.1 | 15.8 | 51.4 ± 9.5 |
| Other race/ethnicity | 17.1 | 10.1 | 91.0 | 34.8 | 14.6 | 52.4 ± 10.1 |
| Feeding method at 6 mo | ||||||
| Not breastfeeding (formula only/weaned) | 46.6 | 8.5 | 90.9 | 31.3 | 16.3 | 50.2 ± 8.8 |
| Partially/fully breastfeeding | 53.4 | 14.2 | 94.2 | 32.0 | 12.2 | 55.1 ± 8.9 |
| Maternal/household characteristics | ||||||
| Maternal education | ||||||
| < College degree | 24.0 | 4.8 | 88.8 | 38.8 | 19.6 | 49.5 ± 10.2 |
| College graduate | 76.0 | 13.7 | 93.9 | 29.5 | 12.4 | 53.8 ± 8.7 |
| Household income at enrollment, $ | ||||||
| ≤70,000 | 32.1 | 7.8 | 92.2 | 39.5 | 15.0 | 52.5 ± 9.2 |
| >70,000 | 67.9 | 13.3 | 92.9 | 28.0 | 13.7 | 53.0 ± 9.2 |
| Maternal prepregnancy BMI, kg/m2 | ||||||
| <25 | 65.7 | 13.2 | 93.0 | 32.0 | 13.3 | 53.5 ± 9.1 |
| 25 to <30 | 22.1 | 10.0 | 92.6 | 28.7 | 14.8 | 52.1 ± 9.1 |
| ≥30 | 12.2 | 5.5 | 91.3 | 35.4 | 17.3 | 50.4 ± 9.7 |
| Pregnancy AHEI score | ||||||
| Q1 (37.1–53.8) | 22.7 | 8.5 | 92.4 | 35.6 | 14.4 | 47.4 ± 8.6 |
| Q2 (53.9–60.6) | 25.3 | 10.7 | 92.0 | 31.9 | 13.7 | 52.3 ± 9.0 |
| Q3 (60.7–67.4) | 26.3 | 12.0 | 92.0 | 31.7 | 13.9 | 53.8 ± 8.4 |
| Q4 (67.5–85.8) | 25.7 | 14.6 | 94.4 | 28.0 | 14.6 | 57.2 ± 8.2 |
| Delayed sweets and fruit juice | ||||||
| No | 88.5 | — | 92.9 | 32.9 | 14.7 | 52.4 ± 9.1 |
| Yes | 11.5 | — | 90.8 | 22.5 | 10.0 | 56.1 ± 9.6 |
| Continues to offer refused foods | ||||||
| No | 7.3 | 14.5 | — | 28.9 | 17.1 | 49.5 ± 10.1 |
| Yes | 92.7 | 11.3 | — | 31.9 | 13.9 | 53.1 ± 9.1 |
| Early flavor/texture variety | ||||||
| No | 68.3 | 13.1 | 92.4 | — | 15.2 | 52.5 ± 9.5 |
| Yes | 31.7 | 8.2 | 93.3 | — | 11.8 | 53.5 ± 8.4 |
Values are percentages unless otherwise indicated. Mean YHEI scores were calculated among the 894 participants with dietary data in early childhood. AHEI, Alternate Healthy Eating Index; YHEI, Youth Healthy Eating Index.
Of the participants, 147 had complete exposure and covariate data but did not have observed outcome data (YHEI score in early childhood). These participants were included in estimating the weights but received a final weight of 0, and only the 894 with observed outcome data were included in the weighted regression analysis.
ATEs for delaying sweets and fruit juice introduction on diet quality in early childhood
Total YHEI scores ranged from 21.4 to 76.5 with a mean ± SD score of 52.8 ± 9.2. The mean YHEI total score among the “delayed sweets/fruit juice” group was 56.1, compared with 52.4 for “early sweets/fruit juice.” After confounding adjustment, the IP-weighted estimate of the overall population ATE and individual ATEs among males and females indicated higher YHEI scores with delayed sweets and fruit juice, but the 95% CIs included the null (Table 2). IP-weighted estimates of the ATEs from the weighted regression with interaction terms between exposure and feeding method indicated no effect of this exposure on YHEI total score among children who were not breastfeeding at 6 mo, but a score 4.5 points higher with delayed sweets and fruit juice among children who were partially or fully breastfeeding at 6 mo. The estimated difference in the ATE across feeding methods (from the exposure × feeding method interaction term) was 4.7 (95% CI: −0.8, 10.3). In the secondary analysis of YHEI component scores, the IP-weighted estimate of the ATE for “delayed sweets/fruit juice” (compared with “early sweets/fruit juice”) on the YHEI snack component was 1.3 points (0.6, 1.9 points), indicating lower consumption of snack foods in early childhood with this exposure (Supplemental Table 3). Point estimates for this outcome were in the same direction among males and females and breastfeeding children, although the point estimate for males was slightly smaller and not statistically significant, and it was difficult to infer directionality among nonbreastfeeding children owing to the wide 95% CI. Among children who were partially or fully breastfeeding at 6 mo only, the ATE on the meat ratio component score indicated higher intake of lean protein sources relative to fatty or processed meats with “delayed sweets/fruit juice.” There was no evidence for an effect of delaying sweets and fruit juice on intakes of whole grains, vegetables, whole fruits, nonnutritive drinks, dairy, or fast food in early childhood.
TABLE 2.
Estimated association of delaying sweets and fruit juice to ≥12 mo (compared with <12 mo) with total YHEI score in early childhood among 894 Project Viva participants1
| Full sample overall | Males | Females | Children not breastfeeding at 6 mo | Children fully or partially breastfeeding at 6 mo | |
|---|---|---|---|---|---|
| Delayed sweets and fruit juice, n (%) | 108 (12.1) | 51 (11.9) | 57 (12.3) | 37 (9.1) | 71 (14.6) |
| Linear regression model2 | 3.7 (1.9, 5.5) | 2.2 (−0.5, 4.9) | 5.0 (2.5, 7.6) | 1.1 (−1.9, 4.1) | 4.2 (2.0, 6.4) |
| IP-weighted MSM3 | 2.4 (−0.6, 5.3) | 1.6 (−2.0, 5.2) | 3.3 (−1.0, 8.3) | −0.1 (−4.2, 4.4) | 4.5 (1.0, 7.4) |
Estimates are βs (95% CIs) unless otherwise indicated. IP, inverse probability; MSM, marginal structural model; YHEI, Youth Healthy Eating Index.
No adjustment for covariates.
From IP-weighted MSMs with stabilized weights, adjusted for selection bias due to missing outcome data and for confounding by child sex (except models stratified by sex) and infant breastfeeding status at 6 mo (except models stratified by infant breastfeeding status); maternal education, prepregnancy BMI, and Alternate Healthy Eating Index score during pregnancy; household income at enrollment; continuing to offer refused foods; and early introduction of flavor/texture variety. Estimates for males/females and not breastfeeding/breastfeeding children are from MSMs run on the full sample with exposure-by-effect modifier interaction terms, with the weighted estimates of the coefficients on these terms used to compute effect estimates for each subgroup and the estimate and 95% CI for the interaction term coefficient, an estimate of the difference between the 2 subgroups. 95% CIs generated from 500 bootstrapped samples.
ATEs for continued offering of refused foods on diet quality in early childhood
Mean early childhood YHEI total scores were 53.1 points among children in the “continued offering” group and 49.5 points among children in the “ceased offering” group, but adjustment for confounding attenuated the mean difference in YHEI scores in the overall study population (Table 3). There was no effect of the exposure in males but a YHEI score 5.4 points higher with continued offering among females (β for exposure × sex interaction term: 4.7; 95% CI: −2.3, 11.1). The point estimate for the ATE was higher in breastfeeding children than in nonbreastfeeding children, yet the 95% CIs for both estimates included the null (Table 3) (β for exposure × infant feeding interaction term: 3.0; 95% CI: −4.3, 10.1). In the secondary analyses, the mean scores on the whole grains and fruit components were higher with “continued offering” among females only and the mean score on the dairy component was higher among children not breastfeeding at 6 mo, with no effect overall nor among any other subgroups on any of the other YHEI component scores (Supplemental Table 4).
TABLE 3.
Estimated association of continuing to offer refused foods (compared with no continued offering) with total YHEI score in early childhood among 894 Project Viva participants1
| Full sample overall | Males | Females | Children not breastfeeding at 6 mo | Children fully or partially breastfeeding at 6 mo | |
|---|---|---|---|---|---|
| Continues to offer refused foods, n (%) | 831 (93.0) | 399 (93.0) | 432 (92.9) | 369 (90.9) | 462 (94.7) |
| Linear regression model2 | 3.6 (1.3, 6.0) | 1.1 (−2.3, 4.5) | 5.9 (2.7, 9.2) | 2.2 (−0.8, 5.2) | 3.9 (0.4, 7.4) |
| IP-weighted MSM3 | 2.9 (−0.2, 6.0) | 0.7 (−4.2, 5.4) | 5.4 (0.8, 9.1) | 1.2 (−2.6, 5.4) | 4.2 (−1.6, 9.2) |
Estimates are βs (95% CIs) unless otherwise indicated. IP, inverse probability; MSM, marginal structural model; YHEI, Youth Healthy Eating Index.
No adjustment for covariates.
From IP-weighted MSMs with stabilized weights, adjusted for selection bias due to missing outcome data and for confounding by child sex (except models stratified by sex) and infant breastfeeding status at 6 mo (except models stratified by infant breastfeeding status); maternal education, prepregnancy BMI, and Alternate Healthy Eating Index score during pregnancy; household income at enrollment; delayed introduction of sweets and fruit juice; and early introduction of flavor/texture variety. Estimates for males/females and not breastfeeding/breastfeeding children are from MSMs run on the full sample with exposure-by-effect modifier interaction terms, with the weighted estimates of the coefficients on these terms used to compute effect estimates for each subgroup and the estimate and 95% CI for the interaction term coefficient, an estimate of the difference between the 2 subgroups. 95% CIs generated from 500 bootstrapped samples.
ATEs for early introduction of flavor/texture variety on diet quality in early childhood
The mean YHEI total scores were 53.5 points among children in the “early variety” group and 52.5 points among those with “late variety.” Results from the IP-weighted model indicated an ATE of “early variety” in the sample overall after confounding adjustment (Table 4). The estimated ATEs indicated higher YHEI scores with early variety among both males and females, yet the 95% CI for females included the null (β for interaction term: −1.0; 95% CI: −4.2, 2.0). YHEI scores were nearly 3 points higher with “early variety” among children not breastfeeding at 6 mo, and the point estimate for children who were still partially or fully breastfeeding at 6 mo was smaller and the 95% included the null (β for exposure × feeding method interaction term: −2.0; 95% CI: −4.9, 0.9). In secondary analyses, mean scores on the vegetable and whole fruit components of the YHEI were higher under “early variety.” In addition, among children who were not breastfeeding at 6 mo, the ATE for "early variety” on the meat ratio component score was 0.9 points (95% CI: 0.1, 1.6 points) (Supplemental Table 5).
TABLE 4.
Estimated association of introducing flavor/texture variety at <12 mo (compared with ≥12 mo) with total YHEI score in early childhood among 894 Project Viva participants1
| Full sample overall | Males | Females | Children not breastfeeding at 6 mo | Children fully or partially breastfeeding at 6 mo | |
|---|---|---|---|---|---|
| Introduction of flavor/texture variety <12 mo, n (%) | 291 (32.6) | 145 (33.8) | 146 (31.4) | 133 (32.8) | 158 (32.4) |
| Linear regression model2 | 1.0 (−0.3, 2.3) | 1.7 (−0.1, 3.5) | 0.5 (−1.3, 2.3) | 2.2 (0.4, 4.0) | 0.1 (−1.6, 1.8) |
| IP-weighted MSM3 | 1.7 (0.3, 3.2) | 2.2 (0.2, 4.8) | 1.3 (−0.8, 3.3) | 2.8 (0.7, 5.1) | 0.9 (−1.0, 2.8) |
Estimates are βs (95% CIs) unless otherwise indicated. IP, inverse probability; MSM, marginal structural model; YHEI, Youth Healthy Eating Index.
No adjustment for covariates.
From IP-weighted MSMs with stabilized weights, adjusted for selection bias due to missing outcome data and for confounding by child sex (except models stratified by sex) and infant breastfeeding status at 6 mo (except models stratified by infant breastfeeding status); maternal education, prepregnancy BMI, and Alternate Healthy Eating Index score during pregnancy; household income at enrollment; delayed introduction of sweets and fruit juice; and continuing to offer refused foods. Estimates for males/females and not breastfeeding/breastfeeding children are from MSMs run on the full sample with exposure-by-effect modifier interaction terms, with the weighted estimates of the coefficients on these terms used to compute effect estimates for each subgroup and the estimate and 95% CI for the interaction term coefficient, an estimate of the difference between the 2 subgroups. 95% CIs generated from 500 bootstrapped samples.
Discussion
Using cohort data to estimate causal effects of 3 CF behaviors on diet quality in early childhood, our results suggest that delaying introduction of sweets and fruit juice, continued offering of initially refused foods, and introducing flavor/texture variety before 1 y may all lead to higher diet quality ∼2 y later, with potential effect modification by child sex or infant breastfeeding status. The 2020–2025 Dietary Guidelines for Americans, with newly added recommendations for children < 2 y old, include all 3 of these CF behaviors in their recommendations for infants and children (44). Evidence suggesting that these behaviors are important for developing preferences for nutrient-dense foods over energy-dense, nutrient-poor foods underpins these recommendations, yet there is little research examining longer-term impacts on diet quality. In addition, we identified specific dietary components that seem to be positively affected by these CF behaviors, contributing to higher overall diet quality scores. We do not have data on health benefits corresponding to a specific magnitude of change in YHEI score. However, in children, who require a nutrient-dense diet for optimal health and development (44), even a 2- to 5-point increase in YHEI score (which could correspond to eating fruit or vegetables 1 additional time per day or 1–2 fewer snack foods per day, for example) may translate to clinically meaningful improvement. Recent evidence indicates that the diet quality of US children may be improving; yet it remains poor overall (45, 46).
Among children breastfeeding at 6 mo, delayed (compared with early) introduction of sweets and fruit juice was associated with mean YHEI scores nearly 4.5 points higher. Because breast milk tastes sweet but formula does not, it is possible that infants who are breastfeeding at the start of CF have heightened sensitivity to sweetness, which is enhanced and cemented in children whose diets include more sweet flavors, resulting in lasting preferences for sweet, palatable foods. Regardless of sex or infant breastfeeding status, delayed sweets and fruit juice introduction was associated with lower intake of foods included in the YHEI snack component, including sweets. Interestingly, breastfed children with delayed introduction to sweets and fruit juice had a more favorable ratio of lean:fatty protein sources in their early childhood diet. Children consuming sweets during CF may be exposed to less diverse flavor profiles and therefore retain more innate preferences for sweet and energy-dense foods, whereas children not introduced may have less exposure to other flavors in these foods, such as salty or fat-rich flavors. Contrary to our hypothesis, there was no association with the soda and drinks component, possibly because the overall mean score for this component was 9/10 with little variability, and fruit juice was not included. Delayed sweets and fruit juice was not associated with other dietary components including whole grains, whole fruits, vegetables, or fast food, suggesting that confounding by family dietary habits or nutrition knowledge may not explain these results. Our results are consistent with those of the few other studies examining potential programming of later flavor preferences by exposure to sweets during CF. Among young children in Australia, having tried more nutrient-poor foods by 14 mo predicted preference for and greater intake of these foods at 3.7 y (14). Similarly, higher intake of energy-dense foods in infancy predicted higher intake of these foods at 6 y among children in the United States (13).
Refusal of new foods by children during CF is expected, yet parents may avoid offering foods previously refused owing to concerns about wasting food or time or about ensuring that a child is eating enough. However, research clearly shows that repeated exposure increases acceptance (11, 47, 48). We observed a strong association of continued offering of initially refused foods with later diet quality among females but not males. The literature on sex differences in children's eating behavior suggests that males and females receive different external cues related to eating behaviors, and that females show a heightened response to external cues and potentially less responsivity to internal cues as compared with males (22). Although consistent sex differences have mainly been observed in older children, our finding may reflect heightened sensitivity to continued offering of a food, which could be accompanied by, or interpreted as, an external pressure to taste that food, among females.
There is evidence for programming of taste preferences by CF practices (47, 49) but a lack of research on early introduction of foods other than fruits and vegetables (11). We addressed this research gap by examining the impact of introducing fish, eggs, and peanut butter in the first year. Although we could not quantify the extent of diet variety, we expect that children introduced to these foods early on were exposed to a wider variety of flavors than children not introduced to these foods. These foods are common allergens, and parents may have delayed their introduction (and that of other foods) out of concerns about allergy development. Also, because these foods are all unlikely to be found in commercial baby food preparations, this exposure may capture early introduction of homemade or family foods and thus a variety of texture and sensory properties.
We estimated higher overall diet quality scores in early childhood when fish, eggs, or peanut butter (≥2 of the 3) were introduced during the first year, with higher intake of whole fruits and vegetables regardless of infant breastfeeding status. The effect estimate for total YHEI score was largest among children not receiving any breast milk at 6 mo, supporting the hypothesis that intraindividual differences in response to flavor exposure during CF may be explained by previous exposures from the maternal diet via amniotic fluid or breast milk (11, 50). Breastfed infants are exposed to flavors in the mother's diet, with much more limited flavor exposure among infants fed formula. This may establish later preferences, because breastfed infants prefer foods present in the mother's diet during lactation (6) and formula-fed infants prefer a flavor profile similar to that of their formula (51). Longer breastfeeding duration is associated with higher fruit and vegetable intake at 12 mo (21), and breastfed infants more readily accept new foods (10, 12) and have persistently higher intake of vegetables into childhood than formula-fed infants (47). We found no effect of early variety exposure on later diet quality among those breastfeeding at 6 mo, consistent with evidence that breastfed children are more accepting of new flavors and foods regardless of when and how they are offered (8, 10, 21).
Strengths of our study include examination of 3 well-defined exposures that can easily translate into targets for intervention, a measure of overall diet quality as well as individual diet components, detailed measurement of many covariates, and use of weighting to avoid assumptions about effect modification required by standard linear regression and adjust for confounding and selection bias, 2 major potential sources of bias in observational studies. Our study also has limitations, including potential unmeasured confounding by factors such as family cultural background, which would affect the assumption of conditional exchangeability. We included many key factors associated with both CF practices and diet quality and these were well-balanced between exposure groups after weighting. We acknowledge that early childhood diet quality is heavily influenced by parental selection of foods and family dietary practices, and that socioeconomic status and access to health information and nutritious food affect both decisions about CF and later selection of foods offered to the child. However, several YHEI component scores were unrelated to the exposures, suggesting that our results are not explained by general parental nutrition knowledge or persistence of family dietary practices. We used exposure data from a mailed annual questionnaire, which tends to have lower response rates than in-person visits (23), and had complete exposure and covariate data on about half of our cohort. In addition, our sample has high proportions of high-income households and mothers with college degrees, all of which may limit the generalizability of our findings. Our secondary analyses examining individual YHEI component scores involved many statistical tests, and thus the overall chance of a type I error could be much higher than the usual 5% and results should be interpreted with caution.
Our data rely on accurate reporting of CF behaviors and child diet quality by the mother. In addition, our FFQ did not specify portion sizes and we assumed that each reported “time” eating each food was equivalent to 1 serving. Our estimates of child dietary intake are thus subject to measurement error, yet our FFQ should rank participants accurately. We were also not able to accurately estimate or account for total energy intake. Also, we were able to examine timing of introduction of different foods but not the impact of frequency of exposure to sweet tastes or flavor/texture variety. However, variability in the frequency of exposure within “exposed” groups likely attenuated our estimates, because we expect more frequent exposure to compound the effects of early exposure and although we expect that once foods are introduced they will remain a regular part of the diet, we were not able to control for this variable in our analysis.
In conclusion, our results support the hypothesis that CF practices promoting acceptance of a variety of nutrient-dense foods over sweet and energy-dense foods may result in higher diet quality during childhood. The impact of these behaviors may be modified by child sex or infant breastfeeding status. Future research should examine whether these CF behaviors affect diet quality into later childhood as children become more autonomous. In addition, a randomized trial assessing the effect of CF behaviors on diet quality and health outcomes would improve our ability to provide clear recommendations. Parents and caregivers should receive support in implementing CF practices promoting development of preferences for nutrient-dense foods.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—KMS, EO, and JGY: designed the research; KMS: analyzed the data, wrote the paper, and had primary responsibility for the final content; IMA: provided guidance on methodology and statistical programming; EO and JGY: provided study oversight; VG: provided subject matter expertise; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01 HD034568 (to EO) and NIH grant UH3 OD023286 (to EO). The sources of support had no involvement in the study design; collection, analysis, or interpretation of data; or writing of the manuscript.
Supplemental Tables 1–5, Supplemental Methods, and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI, Alternate Healthy Eating Index; ATE, average treatment effect; CF, complementary feeding; IP, inverse probability; MSM, marginal structural model; YHEI, Youth Healthy Eating Index.
Contributor Information
Karen M Switkowski, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Izzuddin M Aris, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Véronique Gingras, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA; Department of Nutrition, University of Montréal, Montreal, Quebec, Canada; Research Center, Sainte-Justine University Hospital Center (CHU), Montreal, Quebec, Canada.
Emily Oken, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA; Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Jessica G Young, Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA.
Data Availability
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Duffy EW, Kay MC, Jacquier E, Catellier D, Hampton J, Anater AS, Story M. Trends in food consumption patterns of US infants and toddlers from Feeding Infants and Toddlers Studies (FITS) in 2002, 2008, 2016. Nutrients. 2019;11(11):2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saavedra JM, Deming D, Dattilo A, Reidy K. Lessons from the Feeding Infants and Toddlers Study in North America: what children eat, and implications for obesity prevention. Ann Nutr Metab. 2013;62(s3):27–36. [DOI] [PubMed] [Google Scholar]
- 3. Nicklaus S, Remy E. Early origins of overeating: tracking between early food habits and later eating patterns. Curr Obes Rep. 2013;2(2):179–84. [Google Scholar]
- 4. Kaikkonen JE, Mikkilä V, Raitakari OT. Role of childhood food patterns on adult cardiovascular disease risk. Curr Atheroscler Rep. 2014;16(10):443. [DOI] [PubMed] [Google Scholar]
- 5. Jääskeläinen P, Magnussen CG, Pahkala K, Mikkilä V, Kähönen M, Sabin MA, Fogelholm M, Hutri-Kähönen N, Taittonen L, Telama Ret al. Childhood nutrition in predicting metabolic syndrome in adults: the Cardiovascular Risk in Young Finns Study. Diabetes Care. 2012;35(9):1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anzman-Frasca S, Ventura AK, Ehrenberg S, Myers KP. Promoting healthy food preferences from the start: a narrative review of food preference learning from the prenatal period through early childhood. Obes Rev. 2018;19(4):576–604. [DOI] [PubMed] [Google Scholar]
- 7. Mennella JA, Reiter AR, Daniels LM. Vegetable and fruit acceptance during infancy: impact of ontogeny, genetics, and early experiences. Adv Nutr. 2016;7(1):211S–19S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beauchamp GK, Mennella JA. Early flavor learning and its impact on later feeding behavior. J Pediatr Gastroenterol Nutr. 2009;48(Suppl 1):S25–S30. [DOI] [PubMed] [Google Scholar]
- 9. Agostoni C, Decsi T, Fewtrell M, Goulet O, Kolacek S, Koletzko B, Michaelsen KF, Moreno L, Puntis J, Rigo Jet al. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008;46(1):99–110. [DOI] [PubMed] [Google Scholar]
- 10. Maier AS, Chabanet C, Schaal B, Leathwood PD, Issanchou SN. Breastfeeding and experience with variety early in weaning increase infants’ acceptance of new foods for up to two months. Clin Nutr. 2008;27(6):849–57. [DOI] [PubMed] [Google Scholar]
- 11. Spill MK, Johns K, Callahan EH, Shapiro MJ, Wong YP, Benjamin-Neelon SE, Birch L, Black MM, Cook JT, Faith MSet al. Repeated exposure to food and food acceptability in infants and toddlers: a systematic review. Am J Clin Nutr. 2019;109(Supplement_1):978S–89S. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan SA, Birch LL. Infant dietary experience and acceptance of solid foods. Pediatrics. 1994;93(2):271–7. [PubMed] [Google Scholar]
- 13. Rose CM, Birch LL, Savage JS. Dietary patterns in infancy are associated with child diet and weight outcomes at 6 years. Int J Obes. 2017;41(5):783–8. [DOI] [PubMed] [Google Scholar]
- 14. Mallan KM, Fildes A, Magarey AM, Daniels LA. The relationship between number of fruits, vegetables, and noncore foods tried at age 14 months and food preferences, dietary intake patterns, fussy eating behavior, and weight status at age 3.7 years. J Acad Nutr Diet. 2016;116(4):630–7. [DOI] [PubMed] [Google Scholar]
- 15. Silveira PP, Portella AK, Crema L, Correa M, Nieto FB, Diehl L, Lucion AB, Dalmaz C. Both infantile stimulation and exposure to sweet food lead to an increased sweet food ingestion in adult life. Physiol Behav. 2008;93(4–5):877–82. [DOI] [PubMed] [Google Scholar]
- 16. Bertino M, Wehmer F. Dietary influences on the development of sucrose acceptability in rats. Dev Psychobiol. 1981;14(1):19–28. [DOI] [PubMed] [Google Scholar]
- 17. Dovey TM, Staples PA, Gibson EL, Halford JCG. Food neophobia and “picky/fussy” eating in children: a review. Appetite. 2008;50(2–3):181–93. [DOI] [PubMed] [Google Scholar]
- 18. Sina E, Buck C, Jilani H, Tornaritis M, Veidebaum T, Russo P, Moreno LA, Molnar D, Eiben G, Marild Set al. Association of infant feeding patterns with taste preferences in European children and adolescents: a retrospective latent profile analysis. Nutrients. 2019;11(5):1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Switkowski KM, Gingras V, Rifas-Shiman SL, Oken E. Patterns of complementary feeding behaviors predict diet quality in early childhood. Nutrients. 2020;12(3):810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernán MA, Hsu J, Healy B. A second chance to get causal inference right: a classification of data science tasks. Chance. 2019;32(1):42–9. [Google Scholar]
- 21. Beckerman JP, Slade E, Ventura AK. Maternal diet during lactation and breast-feeding practices have synergistic association with child diet at 6 years. Public Health Nutr. 2020;23(2):286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keller KL, Kling SMR, Fuchs B, Pearce AL, Reigh NA, Masterson T, Hickok K. A biopsychosocial model of sex differences in children's eating behaviors. Nutrients. 2019;11(3):682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EMet al. Cohort profile: Project Viva. Int J Epidemiol. 2015;44(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feskanich D, Rockett HRH, Colditz GA. Modifying the Healthy Eating Index to assess diet quality in children and adolescents. J Am Diet Assoc. 2004;104(9):1375–83. [DOI] [PubMed] [Google Scholar]
- 25. Blum RE, Wei EK, Rockett HR, Langeliers JD, Leppert J, Gardner JD, Colditz GA. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Health J. 1999;3(3):167–72. [DOI] [PubMed] [Google Scholar]
- 26. Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol. 2014;24(11):793–800.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 28. Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14(10):754–62. [DOI] [PubMed] [Google Scholar]
- 29. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–8S.; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 30. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–71. [DOI] [PubMed] [Google Scholar]
- 31. Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009;109(6):1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Odoms-Young AM. Examining the impact of structural racism on food insecurity: implications for addressing racial/ethnic disparities. Fam Community Health. 2018;41(S2):S3–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robins JM, Hernán MÁ, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. [DOI] [PubMed] [Google Scholar]
- 34. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 35. Robins JM. Correction for non-compliance in equivalence trials. Stat Med. 1998;17(3):269–302.; discussion 387–9. [DOI] [PubMed] [Google Scholar]
- 36. Hernán M, Robins J. Causal inference: what if. Boca Raton, FL: Chapman & Hall/CRC; 2020. [Google Scholar]
- 37. Chiu Y-H, Rifas-Shiman SL, Kleinman K, Oken E, Young JG. Effects of intergenerational exposure interventions on adolescent outcomes: an application of inverse probability weighting to longitudinal pre-birth cohort data. Paediatr Perinat Epidemiol. 2020;34(3):366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aris IM, Sarvet A, Stensrud M, Neugebauer R, Li L, Hivert M, Oken E, Young JG. Separating algorithms from questions and causal inference with unmeasured exposures: an application to birth cohort studies of early BMI rebound. Am J Epidemiol. 2021;190(7):1414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Linden A. COVBAL: Stata module for producing covariate balance statistics. [Internet]. Statistical Software Components S458188. Boston, MA: Boston College Department of Economics; 2016; [cited 16 September, 2020]. Available from: http://ideas.repec.org/c/boc/bocode/s458188.html. [Google Scholar]
- 40. Linden A, Samuels SJ. Using balance statistics to determine the optimal number of controls in matching studies. J Eval Clin Pract. 2013;19(5):968–75. [DOI] [PubMed] [Google Scholar]
- 41. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(3/4):169–88. [Google Scholar]
- 42. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petersen ML, Porter KE, Gruber S, Wang Y, van der Laan MJ. Diagnosing and responding to violations in the positivity assumption. Stat Methods Med Res. 2012;21(1):31–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. USDA and US Department of Health and Human Services (DHHS) . Dietary Guidelines for Americans, 2020–2025. [Internet]. 9th ed. Washington (DC): USDA and US DHHS; 2020; [cited 16 June, 2021]. Available from: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf. [Google Scholar]
- 45. Thomson JL, Tussing-Humphreys LM, Goodman MH, Landry AS. Diet quality in a nationally representative sample of American children by sociodemographic characteristics. Am J Clin Nutr. 2019;109(1):127–38. [DOI] [PubMed] [Google Scholar]
- 46. Liu J, Rehm CD, Onopa J, Mozaffarian D. Trends in diet quality among youth in the United States, 1999-2016. JAMA. 2020;323(12):1161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maier-Nöth A, Schaal B, Leathwood P, Issanchou S. The lasting influences of early food-related variety experience: a longitudinal study of vegetable acceptance from 5 months to 6 years in two populations. PLoS One. 2016;11(3):e0151356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwartz C, Scholtens P, Lalanne A, Weenen H, Nicklaus S. Development of healthy eating habits early in life. Review of recent evidence and selected guidelines. Appetite. 2011;57(3):796–807. [DOI] [PubMed] [Google Scholar]
- 49. Foterek K, Hilbig A, Alexy U. Associations between commercial complementary food consumption and fruit and vegetable intake in children. Results of the DONALD study. Appetite. 2015;85:84–90. [DOI] [PubMed] [Google Scholar]
- 50. Spahn JM, Callahan EH, Spill MK, Wong YP, Benjamin-Neelon SE, Birch L, Black MM, Cook JT, Faith MS, Mennella JAet al. Influence of maternal diet on flavor transfer to amniotic fluid and breast milk and children's responses: a systematic review. Am J Clin Nutr. 2019;109(Supplement_1):1003S–26S. [DOI] [PubMed] [Google Scholar]
- 51. Mennella JA, Beauchamp GK. Flavor experiences during formula feeding are related to preferences during childhood. Early Hum Dev. 2002;68(2):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.